ABSTRACT
Rho-associated kinase 1 (ROCK1) and ROCK2 are activated by Rho GTPase and control cytoskeleton rearrangement through modulating the phosphorylation of their down-stream effector molecules. Although these 2 isoforms share more than 90% homology within their kinase domain the question of whether ROCK proteins function identically in different cell types is not clear. By using both pharmacological inhibition and genetic knockdown approaches recent studies suggest that the ROCK2 isoform plays an exclusive role in controlling of T-cell plasticity and macrophage polarization. Specifically, selective ROCK2 inhibition shifts the balance between pro-inflammatory and regulatory T-cell subsets via concurrent regulation of STAT3 and STAT5 phosphorylation, respectively. Furthermore, the administration of an orally available selective ROCK2 inhibitor effectively ameliorates clinical manifestations in experimental models of autoimmunity and chronic graft-vs.-host disease (cGVHD). Because ROCK2 inhibition results in the suppression of M2-type macrophages while favoring polarization of M1-type macrophages, ROCK2 inhibition can correct the macrophage imbalance seen during age-related macular degeneration (AMD). In summary, the exclusive role of ROCK2 in immune system modulation argues for the development and testing of isoform-specific ROCK2 inhibitors for the treatment of inflammatory disorders.
KEYWORDS: age-related macular degeneration, autoimmunity, chronic graft-versus-host disease, immunological balance, inflammation, macrophages, ROCK1, ROCK2, T cells
Rho-associated coiled-coil kinases (ROCKs) play central roles in the actin cytoskeleton organization and regulate a wide range of fundamental cellular functions, such as contractility, adhesion, migration and phagocytosis.1-4 The two isoforms ROCK1 and ROCK2 are activated by Rho family GTPases and promote actin-myosin mediated contractile force generation via serine-threonine phosphorylation of numerous down-stream targets including myosin light chain (MLC),5 myosin binding subunit of myosin phosphatase (MYPT),6 ezrin/radixin/moesin (ERM) proteins7 and LIM kinase (LIMK).8 Although ROCK1 and ROCK2 exhibit 65% overall identity and 92% within the kinase domain9 the question of whether these 2 isoforms have redundant functions remains controversial and is dependent on the cellular system where they are expressed. Using RNA interference, ROCK1 was reported to be critical for stress fiber formation in fibroblasts, whereas ROCK2 controls cortical contractility and phagocytosis.10 ROCK1 and ROCK2 play distinct roles in the regulation of keratinocyte differentiation and cell detachment.11 However, extensive study recently published by Kumper et al. demonstrated that ROCK1 and ROCK2 act redundantly in cell cycle progression and tumorigenesis.12 Therefore, the activity of each ROCK isoforms needs to be evaluated in a cell type- and stimulus-specific manner. Herein, we discuss the role of ROCK1 and ROCK2 in regulation of immune cell function and the potential therapeutic implication of isoform-specific ROCK inhibitors.
Adaptive immune system cells: T-cells and B-cells
ROCK signaling is critical in the coordination and balancing of T-cell-mediated immune responses, including cellular movement, T-cell receptor (TCR) signaling and the acquisition of the appropriate T-cell effector program.13-16 While increased ROCK activity has been associated with autoimmunity through its capacity to regulate cytoskeletal proteins,14,16,17 only the ROCK2 isoform was shown to be physiologically activated in CD4+ T-cells under T-helper cells producing IL-17 (Th17) skewing, specifically implicated in regulating of pro-inflammatory cytokines, such as IL-21 and IL-17, and development of autoimmunity in mice.18 In humans, oral administration of the selective ROCK2 inhibitor KD025 to healthy subjects attenuates the ability of T-cells to secrete both IL-21 and IL-17 in response to stimulation ex vivo.19 KD025 is ATP competitive small molecule inhibitor, which is 100-fold more selective for the ROCK2 over ROCK1 isoform and effectively down-regulates MLC phosphorylation in human T-cells.20,21 Moreover, ROCK2-dependent regulation of Th17 pathway was mediated through down-regulation of STAT3 phosphorylation, an inducer of pro-inflammatory cytokine responses, as demonstrated by either pharmacological or siRNA-mediated inhibition of ROCK2 expression in human T-cells. Importantly, a recent study by Flynn et al. demonstrated that targeted inhibition of ROCK2 reversed the clinical and immunologic symptoms of an autoimmune-like syndrome, chronic graft-versus-host disease (cGVHD), a complication of allogeneic haematopoietic cell transplantation, in 2 distinct murine models characterized by an immune-mediated fibrosis.22 These studies further validated a common mechanism of KD025-mediated downregulation of STAT3 phosphorylation in vivo.22 In addition to the Th17 pathway, STAT3 signaling is critical for development and function of T follicular helper (Tfh) and germinal B-cells, which in the context of cGVHD and secondary lymphoid organs such as the spleen. These two cell subsets cooperate to induce secretion of auto-antibodies that are deposited in tissues and can lead to fibrosis.23-25 Indeed, the in vivo inhibition of STAT3 phosphorylation by a selective ROCK2 inhibitor in cGVHD mice leads to robust decrease in the percentage of both Tfh and germinal center B-cells, accompanied by reduced splenic cell expression of interferon regulatory factor-4 (IRF4) and RAR-related orphan receptor (RORγt) transcription factors that regulate differentiation of naïve T-cells into T effector cells.22 Interestingly, although both ROCK1 and ROCK2 are expressed in T-cells, only ROCK2, but not ROCK1 siRNA down-regulates protein levels of pSTAT3, IRF4 and RORγt, supporting the exclusive role of ROCK2 isoform in regulation of pro-inflammatory T-cell responses.19 Both ROCK1 and ROCK2 siRNAs efficiently inhibited the phosphorylation of the known ROCK target MLC.19 Further experiments using pharmacological or siRNA-mediated inhibition of ROCK1 and ROCK2 in B-cells are required to define the role of each isoform in regulation of pro-inflammatory B-cell function.
In non-haematopoietic cells, ROCK proteins have been implicated in TGF-β signaling pathway26 that plays instrumental role in regulation of both pro-inflammatory Th17 cells and an anti-inflammatory T-cell subset that possesses immune suppressive capabilities, regulatory Foxp3+ T cells (Tregs).27-29 TGF-β induces the activation of ROCK2 in human CD4+ T-cells in a SMAD2/3-independent manner as a part of a non-canonical TGF-β signaling pathway.19 ROCK2 inhibition leads to the up-regulation of STAT5 phosphorylation that provides critical survival factors for Treg development, expansion and function, followed by a twofold increase in the percentage of Foxp3+ T-cells.19 Therefore, targeted inhibition of ROCK2 shifts the balance between Th17 cells and Tregs toward a more regulatory/immunosuppressive environment through concurrent regulation of STAT3 and STAT5 phosphorylation.30 The increase in regulatory T cell subset and STAT5 phosphorylation also was detected in KD025-treated mice in experimental models of autoimmunity and cGVHD.19,22 Thus, the ROCK2 isoform controls the balance between pro-inflammatory and anti-inflammatory signaling pathways in T cells.
Innate immune cells: Monocytes and macrophages
Monocyte migration and infiltration into inflamed tissue requires a coordinated remodeling of the actin cytoskeleton.31 By using a pan-ROCK inhibitor Y27632, it was demonstrated that both ROCK proteins are essential in regulation of monocytes trans-endothelial migration32 as well as being implicated in controlling of monocyte phagocytosis-dependent IL-1β secretion.33 Selective ROCK1 ablation leads to increased recruitment and migration of macrophages in both in vitro and in vivo settings via mechanism that involves regulation of the phosphorylation and stability of phosphatase and tensin homolog (PTEN).34 Interestingly, enhanced migration resulting from ROCK1 deficiency was observed despite normal expression of ROCK2 and a significant reduction in overall ROCK activity. Also, ROCK activity is required for induction of PTEN phosphatase activity and regulation of PTEN intracellular localization during leukocyte chemotaxis.35 Moreover, a recent study has demonstrated that ROCK1 and ROCK2 play different roles in regulation of macrophages polarization into classical pro-inflammatory IL-12 producing macrophage type 1 (M1) and alternative anti-inflammatory, IL-10 and TGFβ producing, pro-fibrogenic macrophage type 2 (M2) subtypes in age-related macular degeneration (AMD).36 Selective ROCK2 inhibition decreased M2-like macrophages that are accumulated in and associated with pathogenesis of AMD. In turn, ROCK2, but not dual ROCK1/2 inhibition increased M1 markers and furthered M1 polarization that is beneficial to preserve retinal health. Although ROCK2 targeting (pharmacological and genetic knockdown) does not interfere with overall macrophage recruitment, selective ROCK2 inhibition reduced AMD pathology and restored the immunological balance between M1 and M2 macrophages that prevails in the normal young eye.36
Therapeutic implications of isoform-specific ROCK targeting
Since ROCKs play a central role in the organization of the actin cytoskeleton, it might be anticipated that the complete inhibition of both isoforms could cause adverse events in patients.37 Therefore, selective ROCK2 inhibition could have a greater specific therapeutic index over the dual inhibition of ROCK1 and ROCK2. Indeed, a placebo-controlled, randomized, phase1 clinical study showed that the the oral administration of the selective ROCK2 inhibitor, KD025, was well tolerated, without significant adverse events in healthy human subjects.19 The maximum concentration of the drug in peripheral blood was linear and dose proportional at doses of 40-500 mg. Moreover, an analysis of peripheral blood mononuclear cells revealed that KD025 effectively down-regulated the secretion of pro-inflammatory cytokines, IL-21 and IL-17 during stimulation ex vivo, which is consistent with the exclusive role of ROCK2 isoform in regulation of Th17 pathway. In addition, the antagonism of ROCK1 and ROCK2 isoforms in the regulation of macrophage polarization suggests that more efficacy and fewer side effects are possible with selective ROCK2 targeting compared to dual ROCK1/2 inhibition in immune-cell mediated pathologies, such as autoimmunity, cGVHD and AMD (Table 1).
Table 1.
Outcomes of isoform-specific ROCK targeting in immune cells.
| Cell type | ROCK isoform | Cell function/effect of ROCK inhibition | Preclinical model/outcomes | Ref. |
|---|---|---|---|---|
| T cells | ROCK1/2 | Adhesion and Migration/Inhibition | SLE patients-derived T cells | 14,16 |
| T cells | ROCK1/2 | Proliferation/Inhibition | Increased survival after allogeneic heart transplantation in mice | 15 |
| Th17 cells | ROCK2 | IL-17 and IL-21 secretion, pSTAT3/Inhibition | Collagen-Induced arthritis and cGVHD model/Inhibition | 18,19,22 |
| Tfh cells | ROCK2 | Percentage and function of CXCR5+PD1+ Tfh cells/Inhibition | cGVHD and sclerodermatous GVHD model/Inhibition | 22 |
| Treg cells | ROCK2 | Percentage of Foxp3, pSTAT5, Treg function/Increase | Collagen-Induced arthritis and cGVHD model/Inhibition | 19,22 |
| Macrophages | ROCK2 | M2 polarization/Inhibition M1 polarization/Increase | Restoration of normal macrophage balance in AMD | 36 |
| Macrophages | ROCK1/2 | Migration in MCP-1 implanted corneas/Inhibition | Restoration of normal macrophage balance in AMD | 36 |
| Monocytes | ROCK1/2 | Asbestos-induced IL-1b secretion/Inhibition | In vitro stimulation of THP-1/Inhibition | 33 |
| Macrophages | ROCK1 | Migration/Increase | Wound-healing in vitro assay and cell migration in vivo/Increase | 34 |
In conclusion, we are at an early stage of evaluating the unique role of ROCK1 and ROCK2 isoforms in the regulation of immune cell function both in steady state as well as pathological conditions. Whereas dual ROCK1/2 inhibition has a profound effect on cytoskeletal proteins and leukocyte migration, selective ROCK2 inhibition targets cell plasticity and efficiently restores the balance between pro-inflammatory and regulatory phenotype of immune cells (Fig. 1). Thus, targeted ROCK2 inhibition represents a novel therapeutic approach for treatment of inflammatory disorders.
Figure 1.
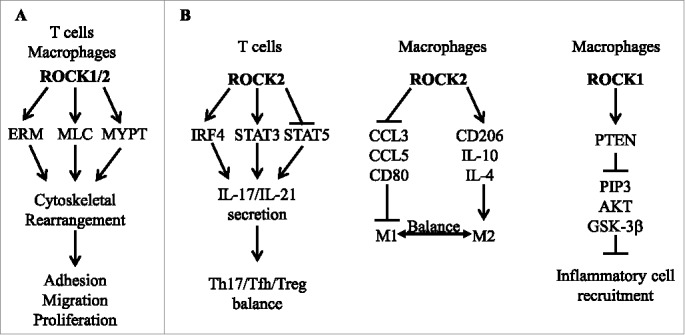
Typical (A) and isoform-selective (B) signaling pathways downstream of ROCK proteins. ERM, ezrin/radixin/moesin; MLC, myosin light chain; MYPT, Myosin binding subunit of myosin phosphatase; IRF4, IFN regulatory factor 4; STAT3/5, signal transducer and activator of transcription 3/5; Th17, T helper 17; Tfh, T follicular helper; Treg, regulatory T cell; M1 and M2, M1-type and M2-type of macrophages; PTEN, phosphatase and tensin homolog; PIP3, phosphatidylinositol 3,4,5-triphosphate; AKT, protein kinase B; GSK-3β, Glycogen synthase kinase 3 β.
Disclosure of potential conflicts of interest
Alexandra Zanin-Zhorov is an employee of Kadmon; Samuel D. Waksal is founder of Kadmon.
References
- [1].Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature reviews. Mol Cell Biol 2003; 4:446-56; PMID:12778124. [DOI] [PubMed] [Google Scholar]
- [2].Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med 1999; 5:221-5; PMID:9930872; http://dx.doi.org/ 10.1038/5587 [DOI] [PubMed] [Google Scholar]
- [3].Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metast 2009; 26:273-87; PMID:18498004; http://dx.doi.org/ 10.1007/s10585-008-9174-2 [DOI] [PubMed] [Google Scholar]
- [4].Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol 2013; 48:301-16; PMID:23601011; http://dx.doi.org/ 10.3109/10409238.2013.786671 [DOI] [PubMed] [Google Scholar]
- [5].Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 1997; 272:12257-60; PMID:9139666; http://dx.doi.org/ 10.1074/jbc.272.19.12257 [DOI] [PubMed] [Google Scholar]
- [6].Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 1999; 274:37385-90; PMID:10601309; http://dx.doi.org/ 10.1074/jbc.274.52.37385 [DOI] [PubMed] [Google Scholar]
- [7].Hebert M, Potin S, Sebbagh M, Bertoglio J, Bréard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol 2008; 181:5963-73; PMID:18941185; http://dx.doi.org/ 10.4049/jimmunol.181.9.5963 [DOI] [PubMed] [Google Scholar]
- [8].Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998; 393:809-12; PMID:9655398; http://dx.doi.org/ 10.1038/31735 [DOI] [PubMed] [Google Scholar]
- [9].Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 1996; 392:189-93; PMID:8772201; http://dx.doi.org/ 10.1016/0014-5793(96)00811-3 [DOI] [PubMed] [Google Scholar]
- [10].Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 2005; 170:443-53; PMID:16043513; http://dx.doi.org/ 10.1083/jcb.200412043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PloS One 2009; 4:e8190; PMID:19997641; http://dx.doi.org/ 10.1371/journal.pone.0008190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kumper S, Mardakheh FK, McCarthy A, Yeo M, Stamp GW, Paul A, Worboys J, Sadok A, Jørgensen C, Guichard S, et al.. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. eLife 2016; 5 pii:e12203; PMID:26765561; http://dx.doi.org/ 10.7554/eLife.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol 2009; 9:630-44; PMID:19696767; http://dx.doi.org/ 10.1038/nri2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol 2007; 178:1938-47; PMID:17237445; http://dx.doi.org/ 10.4049/jimmunol.178.3.1938 [DOI] [PubMed] [Google Scholar]
- [15].Tharaux PL, Bukoski RC, Rocha PN, Crowley SD, Ruiz P, Nataraj C, Howell DN, Kaibuchi K, Spurney RF, Coffman TM. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol 2003; 171:96-105; PMID:12816987; http://dx.doi.org/ 10.4049/jimmunol.171.1.96 [DOI] [PubMed] [Google Scholar]
- [16].Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispin JC. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J Biol Chem 2013; 288:26775-84; PMID:23918926; http://dx.doi.org/ 10.1074/jbc.M113.483743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Isgro J, Gupta S, Jacek E, Pavri T, Duculan R, Kim M, Kirou KA, Salmon JE, Pernis AB. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum 2013; 65:1592-602; PMID:23508371; http://dx.doi.org/ 10.1002/art.37934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest 2010; 120:3280-95; PMID:20697158; http://dx.doi.org/ 10.1172/JCI42856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, Depoil D, Rao N, Liu B, Wei J, et al.. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A 2014; 111:16814-9; PMID:25385601; http://dx.doi.org/ 10.1073/pnas.1414189111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, et al.. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagulation Fibrinolysis: Int J Haemost Thrombosis 2008; 19:709-18; PMID:18832915; http://dx.doi.org/ 10.1097/MBC.0b013e32830b2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee JH, Zheng Y, von Bornstadt D, Wei Y, Balcioglu A, Daneshmand A, Yalcin N, Yu E, Herisson F, Atalay YB, et al.. Selective ROCK2 inhibition in focal cerebral ischemia. Ann Clin Trans Neurol 2014; 1:2-14; PMID:24466563; http://dx.doi.org/ 10.1002/acn3.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Flynn R, Paz K, Du J, Reichenbach DK, Taylor PA, Panoskaltsis-Mortari A, Vulic A, Luznik L, MacDonald KK, Hill GR, et al.. Targeted Rho-associated kinase 2 (ROCK2) inhibition decreases clinical and immune pathology of murine and human chronic GVHD through Stat3-dependent mechanism. Blood 2016; 127(17):2144-54; PMID: 26983850; http://dx.doi.org/ 10.1182/blood-2015-10-678706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chou WC, Levy DE, Lee CK. STAT3 positively regulates an early step in B-cell development. Blood 2006; 108:3005-11; PMID:16825489; http://dx.doi.org/ 10.1182/blood-2006-05-024430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, et al.. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 2009; 31:941-52; PMID:20064451; http://dx.doi.org/ 10.1016/j.immuni.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, et al.. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012; 119:3997-4008; PMID:22403255; http://dx.doi.org/ 10.1182/blood-2011-11-392985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell 2002; 13:902-14; PMID:11907271; http://dx.doi.org/ 10.1091/mbc.01-08-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235-8; PMID:16648838; http://dx.doi.org/ 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- [28].Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008; 9:641-9; PMID:18454151; http://dx.doi.org/ 10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009; 30:646-55; PMID:19464987; http://dx.doi.org/ 10.1016/j.immuni.2009.05.001 [DOI] [PubMed] [Google Scholar]
- [30].Zanin-Zhorov A, Waksal SD. ROCKing cytokine secretion balance in human T cells. Cytokine 2015; 72:224-5; PMID:25649044; http://dx.doi.org/ 10.1016/j.cyto.2014.12.025 [DOI] [PubMed] [Google Scholar]
- [31].Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukocyte Biol 2000; 68:593-602; PMID:11073096. [PubMed] [Google Scholar]
- [32].Honing H, van den Berg TK, van der Pol SM, Dijkstra CD, van der Kammen RA, Collard JG, de Vries HE. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukocyte Biol 2004; 75:523-8; PMID:14634067; http://dx.doi.org/ 10.1189/jlb.0203054 [DOI] [PubMed] [Google Scholar]
- [33].Kanno S, Hirano S, Chiba S, Takeshita H, Nagai T, Takada M, Sakamoto K, Mukai T. The role of Rho-kinases in IL-1beta release through phagocytosis of fibrous particles in human monocytes. Archives Toxicol 2015; 89:73-85; PMID:24760326; http://dx.doi.org/ 10.1007/s00204-014-1238-2 [DOI] [PubMed] [Google Scholar]
- [34].Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood 2010; 115:1785-96; PMID:20008297; http://dx.doi.org/ 10.1182/blood-2009-08-237222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, et al.. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 2005; 7:399-404; PMID:15793569; http://dx.doi.org/ 10.1038/ncb1236 [DOI] [PubMed] [Google Scholar]
- [36].Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, et al.. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep 2015; 10:1173-86; PMID:25704819; http://dx.doi.org/ 10.1016/j.celrep.2015.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol 2008; 20:242-8; PMID:18282695; http://dx.doi.org/ 10.1016/j.ceb.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


