Abstract
This study investigated the suspension of poly(ε-caprolactone) nanoparticles as an ocular delivery system for flurbiprofen (FB-PεCL-NPs) in order to overcome the associated problems, such as stability, sterility, tolerance, and efficacy, with two different FB-PεCL-NP formulations. The formulations were stabilized with poloxamer 188 (1.66% and 3.5%) and submitted individually for freeze-drying and γ-irradiation with polyethylene glycol 3350 (PEG3350) and d-(+)-trehalose (TRE). Both formulations satisfied criteria according to all physicochemical parameters required for ocular pharmaceuticals. The FB-PεCL-NP formulations showed non-Newtonian behavior and sustained drug release. Ex vivo permeation analysis using isolated ocular pig tissues suggested that the presence of PEG3350 results in a reduction of FB transcorneal permeation. Moreover, TRE improved the penetration of FB across the cornea, especially after γ-irradiation. In addition, both formulations did not show a significant affinity in increasing FB transscleral permeation. Both formulations were classified as nonirritating, safe products for ophthalmic administration according to hen’s egg test-chorioallantoic membrane and Draize eye test. Furthermore, an in vivo anti-inflammatory efficacy test showed that irradiated FB-PεCL-NPs prepared with PEG3350 (IR-NPsPEG) have longer anti-inflammatory effects than those presented with irradiated FB-PεCL-NPs prepared with TRE (IR-NPsTRE). IR-NPsPEG showed a suitable physical stability after an aqueous reconstitution over >30 days. This study concludes that both formulations meet the Goldman’s criteria and demonstrate how irradiated nanoparticles, with innovative permeation characteristics, could be used as a feasible alternative to a flurbiprofen solution for ocular application in clinical trials.
Keywords: nanoparticles, flurbiprofen, polyethylene glycol 3350, d-(+)-trehalose, freeze-drying, γ-irradiation
Introduction
Inflammation of the ocular surface has the highest incidence in the ophthalmology consultation, following injury, infection, or from chronic conditions.1 Nonsteroidal anti-inflammatory drugs such as flurbiprofen (FB) have been used to inhibit intraoperative miosis during cataract surgery to reduce the risk of cystoid macular edema and postoperative inflammation of the anterior segment of the eye. FB, 2-(2-fluoro-4-biphenylyl) propionic acid, exerts its anti-inflammatory action by inhibiting the cyclooxygenase enzymes.2,3 The most common FB pharmaceutical presentation is an eye drop solution. However, it has many disadvantages, for example, the solution’s rapid elimination through the precorneal barriers (ie, eye blinking and tear flow), resulting in a reduced duration of drug effect and consequently an increased regimen requiring a proportionately large volume of the administered eye drop.4,5
Polymeric nanoparticles (NPs) enhance the ocular bioavailability of topically administered drugs, thus making a more suitable alternative. This colloidal system is well known to be highly adhesive to the ocular surfaces and forms a depot from which the drug is slowly delivered to the affected area. This not only reduces administration frequency but also directs the drug to a specific site.4,6 In this context, FB NPs suspension allows a direct FB permeation to the ocular tissue.
The development of NPs for ophthalmic preparations, as well as other pharmaceutical ocular presentations, must satisfy the Goldmann’s criteria (stability, sterility, tolerance, and efficacy). Therapy efficacy is the most important criterion for ophthalmic preparation as it depends not only on the stability and tolerance of the preparation but also on the ocular permeability of the active ingredients.7
One major obstacle limiting the use of NPs is their instability in aqueous mediums. Freeze-drying is an industrially suitable method for the improvement of stability of NPs as it causes minimal changes in the product’s physicochemical properties.8 As sterility is essential, eye formulations must satisfy this necessity. γ-Irradiation is commercially available and mainly used for the sterilization of pharmaceuticals; however, it has been suggested that this method can change polymer properties and release kinetics, hence the essentiality of product efficacy.9,10
In order to evaluate the safety and efficacy of materials that may be in contact with the eye, the permeability across ocular tissues has been evaluated using the in vivo and in vitro eye models over many years. This key factor assists in the formulation of candidate selection for in vivo clinical studies.11,12 Porcine ocular tissues offer a good model system because they are the closest to human beings after primate; this is due to the absence of the tapetum layer in porcine eyes, which is present in many other animals such as cows, sheep, and rabbits. Research into other mammalian corneas, mainly those of farmed animals, could also aid the research of veterinary ophthalmic formulation development.13–15 Other notable similarities between human and porcine eyes are the retinal pigment epithelium, photoreceptor cells, and water content. In addition, biomechanical studies into scleral thickness also show similarities between porcine sclera and human sclera.16–18
In this research, an attempt was made to determine the effect of freeze-drying and γ-irradiation sterilization on parameters involved in topical ophthalmic formulation made from FB-loaded poly(ε-caprolactone) nanoparticles (FB-PεCL-NPs). Freeze-drying was carried out using trehalose and polyethylene glycol 3350 (PEG3350) as protectants. After physicochemical characterization, in vitro release and ex vivo permeation were studied. Additionally, rheology properties, physical stability, ocular tolerance tests, and anti-inflammatory efficacy tests were used to determine the most appropriate ophthalmic formulation.
Materials and methods
Materials
FB and poly(ε-caprolactone) with a molecular weight of ~10,000–14,000 from Sigma-Aldrich Co. (St Louis, MO, USA) as well as d-(+)-trehalose (TRE) and PEG3350 were purchased. Poloxamer 188 (P188; Lutrol® F68) was sourced from BASF (Barcelona, Spain). Double distilled water was used after filtration using a Millipore® system (EMD Millipore, Billerica, MA, USA). All other reagents were of analytical grade.
Production and characterization of FB-PεCL-NPs
FB-PεCL-NPs stabilized with P188 were prepared by the solvent displacement technique described by Fessi et al.19 Briefly, an organic solution of 49.5 mg of PεCL in 30 mL of acetone, containing FB (15 mg/mL), was added dropwise into 60 mL of an aqueous P188 solution (8.3 mg/mL or 17.5 mg/mL of P188) at pH 3.5 under moderate magnetic stirring. Finally, using a rotary evaporator (R-144; Buchi, Flawil, Switzerland), the acetone was evaporated at 35°C under reduced pressure and the suspension of FB-PεCL-NPs was concentrated to 15 mL to obtain a final concentrated suspension of FB-PεCL-NPs with 1 mg/mL of FB.
The mean particle size (Zav) and polydispersity index (PI) were determined by PCS using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25°C. Samples were previously diluted with ultrapurified water. Zeta potential (ZP) was calculated from electrophoretic mobility as is described elsewhere.4
Entrapment efficiency (EE) of FB-PεCL-NPs was estimated by indirectly quantifying the amount of nonencapsulated FB in the dispersion medium.9 Briefly, the amount of nonentrapped FB was separated by filtration/centrifugation technique using centrifugal filter devices (EMD Millipore) at 14,000 rpm for 15 minutes. Prior to filtration/centrifugation, each sample was diluted with MilliQ water (1:20) to avoid deposition of free FB (possibly crystallized in the aqueous phase) on the surface of NPs and assessed by reversed-phase high-performance liquid chromatography (RP-HPLC; Waters, Milford, CT, USA) applying the following equation:
| (1) |
The detection wavelength was set at 247 nm in the UV detector, reversed-phase C18 column 4.6×150 mm, using a mobile phase composed of water:acetonitrile (35:60, v:v) acidified with orthophosphoric acid (pH 2.5). A flow rate of 1 mL/min was used, and the retention time of the drug was at 3.2 minutes. Data analysis was done with the Empower Chromatography Software (Milford, MA, USA).
Freeze-drying process
The freeze-drying process was carried out in a Telstar Lyo-Beta freeze dryer (Telstar, Barcelona, Spain) equipped with Pirani and capacitance vacuum gauges. TRE or PEG3350-loaded FB-PεCL-NPs were prepared by adding 15 mL of protectant agent solution (TRE at 10% [w:v] or PEG3350 at 16% [w:v]) to the 15 mL FB-PεCL-NPs formulations. Then an aliquot of 3.0 mL was transferred to an 8 mL flat-bottom screw cap glass vial.
The freeze-drying cycle for FB-PεCL-NPs prepared with TRE was as follows: holding in precooling shelf at +10°C for 1 hour, a freezing at −50°C for 4 hours, a primary drying at −3°C/0.14 mbar for 12 hours, and a secondary drying at 42°C for 10 hours. The FB-PεCL-NPs prepared with PEG3350 were freeze-dried using the following cycle: holding in precooling shelf at 10°C for 1 hour, a freezing at −50°C for 4 hours, a primary drying at 5°C/0.14 mbar for 12 hours, and a secondary drying at 45°C for 10 hours. After freeze-drying, the samples did not show any sign of collapse and all the freeze-dried matrix was white and easily rehydrated by manual shaking. They were aqueous reconstituted in the initial volume (volume before addition of protectant solution) in order to recover the initial FB concentration.
Table 1 describes the composition parameters of the optimized freeze-dried NP formulations. The component’s amounts were selected according to experiments satisfying the demands required for eye drops in terms of low Zav, low PI, high EE, appropriate osmolality, and high ZP.
Table 1.
Composition of the freeze-dried optimized NPs formulation
| FB-PεCL-NPs | cFB (mg/mL) | cPεCL (mg/mL) | cP188 (mg/mL) | cPA (mg/mL) |
|---|---|---|---|---|
| FD-NPsTRE | 1.0 | 3.3 | 16.6 | 100 |
| FD-NPsPEG | 1.0 | 3.3 | 35.0 | 160 |
Notes: NPsTRE, formulation prepared with trehalose as a protectant agent; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: c, concentration; NPs, nanoparticles; FB, flurbiprofen; PεCL, poly(ε-caprolactone); P188, poloxamer 188; PA, protectant agent; FD, freeze-dried condition.
Osmolality
The osmolality of ~50 µL of each FB-PεCL-NPs formulation was measured by means of Advanced® Model 3320 Micro-Osmometer (Advanced® Instruments, Inc., Norwood, MA, USA).
γ-Irradiation sterilization
Freeze-dried FB-PεCL-NP powders were γ-irradiated using 60Co as irradiation source (Aragogamma, Barcelona, Spain) and received a dose of 25 kGy. Although recent studies suggest the possibility to use lower irradiation dose previous to validation,20 according to the European Pharmacopoeia, 25 kGy represents the adequate absorbed dose for the purpose of sterilizing pharmaceutical products when bioburden is not known.9,20 Furthermore, it is considered a standard γ-irradiation dose recommended for terminal sterilization of medical products that maintain a valid sterility assurance level of 10−6.21
Rheological studies
Rheological properties of FB-PεCL-NPs suspension were evaluated at 25°C using a rotational rheometer HAAKE RheoStress 1 (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a fixed lower plate and an upper cone plate, 2° (Haake C60/2° Ti, 6 cm diameter). Viscosity curves and flow curves were recorded for 3 minutes during the ramp-up period from 0 seconds−1 to 100 seconds−1, 1 minute at 100 seconds−1 (constant share rate period), and finally, 3 minutes during the ramp-down period from 100 seconds−1 to 0 seconds−1. All measurements were performed in triplicate.
Stability studies
The physical stability of the FB-PεCL-NPs suspension was assessed after 1 day, 7 days, 15 days, 21 days, and 30 days of storage at 4°C in a TurbiScanLab® (Formulaction, L’Union, France). This instrument is able to detect destabilization, without dilution of the sample, much earlier than the operator’s naked eye.5,7 Each formulation (15 mL) was placed in a cylindrical glass measuring cell that was completely scanned by a pulsed near-infrared light source (λ=880 nm) with two synchronous optical detectors. The transmission detector (T) receives the light transmitted through the sample (0° from the incident radiation) and the backscattering (BS) detector receives the light backscattered by the sample (135° from the incident radiation) every 40 µm at 25°C for a period of 60 minutes.
In this study, only BS profile was used to evaluate physical stability of FB-PεCL-NPs due to the opacity of the formulations. The obtained profile characterizes the sample’s stability (no variation of BS and T), particle migration (local peaks of variation of BS or T), and particle size variation (global variation of BS or T on the whole height). If the BS profiles have a deviation of ≤±2%, it can be considered that there are no significant variations in particle size. Variations more than ±10% indicate unstable formulations.22
In vitro release
In vitro release study of FB from FB-PεCL-NP formulations was performed in amber glass Franz-type diffusion cells with a diffusion area of 0.64 cm2 for 40 hours, keeping sink conditions for the entire experiment at 32°C under 600 rpm stirring. These cells consist of a donor and a receptor chamber between which a dialysis membrane is positioned.23 The dialysis membrane (MWCO 12,000–14,000 Da, Visking Dialysis Tubing; Medicell International Ltd., London, UK) was hydrated for 24 hours before being mounted in the Franz cell. In all, 400 µL of the test formulation was applied to the membrane in the donor chamber and the receptor chamber of the cell was filled with 6 mL of phosphate buffer solution (PBS) at pH 7.4. The FB-PεCL-NP formulations were compared with the free drug (1 mg/mL) dissolved in PBS at pH 7.4. At selected time intervals, 300 µL of bulk solution was analyzed by RP-HPLC to determine the concentration of the released FB. The samples withdrawn were replaced by 300 µL of PBS maintaining sink conditions.
Four different kinetic models (zero order, first order, Higuchi, and hyperbola) were used to fit the experimental data obtained from drug release experiments.24 Model parameters were calculated using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). The coefficient of determination (r2) and the Akaike’s information criterion (AIC), which is a discrimination model parameter, were determined in order to select a model that best fits the release of each sample. A lower AIC indicated the best data-adjusted model. The AIC was calculated by the equation:
| (2) |
where n is the number of dissolution data points (Q/t), p is the number of parameters of the model, and WSSR is the weighed sum of square of residues.25
Ex vivo FB permeation across isolated pig cornea and sclera
The ex vivo FB permeation from FB-PεCL-NP formulations was evaluated using isolated pig cornea and sclera using Franz-type diffusion cells. Fresh pig eyes were obtained from adult male pigs (Landrace and Large White hybrids) weighing 45–60 kg. The pig ocular balls were recycled and supplied by the Faculty of Medicine at Barcelona University, Spain. All experiments were performed according to the statement of Association for Research in Vision and Ophthalmology on the Use of Animals in Ophthalmic and Vision Research. They were also approved by the Ethical Committee of the University of Barcelona (number 7428) and the committee of Animal Experimentation of the Regional Autonomous Government of Catalonia, Spain (Law 32/2007 of November 7, 2007, and “Real Decreto 1201/2005”, October 10, 2005).
The pigs were sedated with neck intramuscular administration using ketamine (3 mg/kg) + xylazine (2.5 mg/kg) + midazolam (0.17 mg/kg). The animals were euthanized by an overdose of sodium thiopental (100 mg/kg) administered through the marginal ear vein under deep anesthesia using propofol (1 mg/kg). Eyes were carefully removed and immediately excised. Ocular tissues were kept moist by placing them in Hank’s balanced salt solution in order to maintain the viability of the cells.26 The excised tissue (cornea or sclera) was fixed between clamped donor and receptor compartments of the perfusion with a diffusion area of 0.64 cm2. In all, 200 µL of the test formulation was applied to the tissue surface in the donor compartment, and the receptor compartment of the cell was filled with 6 mL of PBS. A constant temperature of 32°C±0.5°C and 37°C±1°C for cornea and sclera, respectively, was kept by a circulating water jacket and stirring at 600 rpm. A total of 300 µL of the test formulation was withdrawn from the receptor solution at predetermined intervals. It was replaced by an equal volume of fresh buffer after each sample collection. All experiments were carried out under sink conditions. Samples were analyzed in triplicate by RP-HPLC.
The cumulative amounts of FB (mg) that had penetrated per surface area of the ocular membrane (cm2) were corrected for the sample removal and plotted versus time (hour). The permeation profiles were analyzed on the basis of a diffusion model for a finite dose condition.
Flux (J) through the ocular tissue was calculated by plotting the cumulative amount of permeated FB against time in steady state and determining the slope of the linear portion of the curve by linear regression analysis. In this plot, the lag time (TL) is the intercept with the x-axis (time). The apparent permeability coefficients (Kp, cm/h) were calculated according to the following equation:
| (3) |
where J (µg/min) is the flux across the ocular tissue, A is the exposed tissue surface area (cm2), C0 (µg/cm3) is the initial amount of formulation tested in the donor compartment, and 60 is taken as the factor to convert minute into hour. Experimental data were processed using GraphPad Prism and compared using an application of a parametric statistical assay (the analysis of variance [ANOVA] test) followed by Tukey’s multiple comparison test (P<0.05).
At the end of the permeation study, the tissue (cornea or sclera) was removed from the Franz cell. It was then cleaned with gauze soaked in a 0.05% solution of sodium lauryl sulfate and washed with distilled water. The permeation area of the tissue was excised and weighed; its FB content was extracted with acetonitrile:water (50:50, v:v) under sonication for 15 minutes using an ultrasound bath and then analyzed by RP-HPLC.
The corneal hydration levels were investigated by measuring total water content through desiccation (gravimetric method). Each corneal sample was carefully removed from the scleral ring and weighed (Wa). It was then desiccated at 100°C for 6 hours to determine the corresponding dry corneal weight (Wb). The percentage of corneal hydration level (HL%) was defined as:
| (4) |
In vitro ocular tolerance test
The potential ocular irritation of FB-PεCL-NP formulations was determined using Hen’s egg test-chorioallantoic membrane (HET-CAM) bioassay. Fertilized hen’s eggs weighing 50–60 g were obtained from Llorens SA poultry farm (Tarragona, Spain). The egg’s shell and the inner membrane were previously removed; therefore, the choroiallantoic membrane (CAM) separating the embryo from the air chamber became visible.
In order to obtain a baseline of the test, to ensure the assay conditions do not provide an incorrect result, a positive control (0.3 mL of 1% sodium dodecyl sulfate and 0.1 N NaOH) and a negative control (0.3 mL of 0.9% NaCl solution) were performed. In all, 0.3 mL of FB-PεCL-NPs were placed over the CAM and effects such as hemorrhage, lysis, and coagulation were documented within 5 minutes of application.27 The ocular irritation index (OII) was calculated by the following expression:
| (5) |
where h (seconds) is the time of the first appearance of blood hemorrhages, l (seconds) is the time of the first appearance of vessel lysis, and c (seconds) is the time of the first appearance of protein coagulation on CAM. The following classification was used: OII ≤0.9, slightly irritating; 0.9< OII ≤4.9, moderately irritating; 4.9< OII ≤8.9, irritating; and 8.9< OII ≤21, severely irritating.
In vivo ocular tolerance test
In vivo eye irritation was evaluated in pigs (Large White–Landrace) by the Draize eye test, which is the official technique for the evaluation of cosmetic and pharmaceutical products for ocular instillation by the interpretation of Kay and Calandra.28 A single instillation of 50 µL of each sample was instilled in one eye using the untreated contralateral eye as a control, and then readings were performed 1 hour after sample application and then after 1 day, 2 days, 3 days, 4 days, and 7 days. The opacity (cornea), congestion and swelling (iris), and redness and discharge (conjunctiva) were graded on a scale from 0 to 4, 0 to 2, and 0 to 3, respectively, after evaluation by slit lamp. The Draize score was determined by visual assessment of changes in these ocular structures. The mean total score (MTS) was calculated as follows:
| (6) |
where x1(n), x2(n), and x3(n) are the cornea, conjunctiva, and iris scores, respectively, and n is the number of pigs included in the assay.
Ocular anti-inflammatory effect
The anti-inflammatory efficacy of FB-PεCL-NP formulations was tested in pigs by instillation of a single dose of 50 µL of sample tested or 0.9% (w/v) isotonic saline solution (control) in the conjunctival sac of the left eye. The contralateral eye was used as an untreated control. After 60 minutes, 50 µL of 0.5% (w/v) sodium arachidonate solution (SAS) in PBS (pH 7.4) was instilled in the left eye. Inflammation was quantified from 30 minutes to 150 minutes after application of SAS according to a modified Draize scoring system.11 Ocular changes were graded according to the MTS aforementioned.
Results and discussion
FB-PεCL-NP particles characterization
Chemical parameters of FB-PεCL-NPs freeze-dried with TRE (FD-NPsTRE) and FB-PεCL-NPs freeze-dried with PEG3350 (FD-NPsPEG) maximize the desirable physicochemical properties of NPs under certain freeze-drying conditions. It is important to highlight that both formulations are different in terms of protectant agent type, protectant agent concentration, and P188 concentration as shown in Table 1. No significant difference was observed in Zav of FD-NPsTRE and FD-NPsPEG, 191.7±1.9 nm and 190.4±1.3 nm, respectively. PI values of FD-NPsPEG were in the range of monodisperse systems (PI <0.1), whereas FD-NPsTRE showed PI values >0.1. Both formulations had similar negative surface charge, as indicated ZP values. Furthermore, the EE for both formulations reached 85%. These results are summarized in Table 2.
Table 2.
Physicochemical properties and EE of FB-PεCL-NPs
| FB-PεCL-NPs | Zav (nm) | PI | EE (%) | ZP (mV) | Osmolality (mOsm/kg) |
|---|---|---|---|---|---|
| F-A | 171.8±1.7 | 0.091±0.014 | 86±1 | −13.10±0.57 | 6±1 |
| NPsTRE | 170.6±3.2 | 0.090±0.011 | 87±1 | −12.70±0.32 | 315±3 |
| FD-NPsTRE | 191.7±1.9 | 0.139±0.012 | 85±1 | −12.00±0.42 | 296±3 |
| IR-NPsTRE | 187.5±1.2 | 0.131±0.015 | 86±0 | −13.20±0.17 | 305±1 |
| F-B | 175.9±0.0 | 0.078±0.006 | 85±1 | −17.70±0.11 | 9±1 |
| NPsPEG | 190.2±1.2 | 0.069±0.017 | 86±0 | −18.40±0.72 | 310±2 |
| FD-NPsPEG | 190.4±1.3 | 0.087±0.014 | 85±1 | −15.50±0.83 | 316±1 |
| IR-NPsPEG | 192.5±2.0 | 0.091±0.028 | 85±1 | −15.30±0.37 | 318±1 |
Notes: F-A, nanoparticles’ suspension with P188 at 1.66%; NPsTRE, formulation prepared with trehalose as a protectant agent; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsPEG, formulation prepared with PEG3350 as a protectant agent. Data presented as mean ± standard deviation.
Abbreviations: EE, entrapment efficiency; FB, flurbiprofen; PεCL, poly(ε-caprolactone); NPs, nanoparticles; Zav, mean particle size; PI, polydispersity index; ZP, zeta potential; FD, freeze-dried condition; IR, irradiated condition.
Since these formulations have a therapeutic goal, the powders of FD-NPsTRE and FD-NPsPEG were γ-irradiated (IR-NPsTRE and IR-NPsPEG). To have a complete study on the effects of these processes on FB release and ocular permeation profile, we decided to produce FB-PεCL-NPs before freeze-drying and γ-irradiation sterilization. In this way, FB-PεCL-NP suspensions with the protectant agent (NPsTRE and NPsPEG) and without the protectant agent (F-A and F-B) were prepared and their physicochemical properties were evaluated as shown in Table 2.
The results obtained demonstrate that γ-irradiation did not seem to have any effect on Zav, PI, ZP, and EE of the optimized freeze-dried formulations. It can be observed that FD-NPsTRE and IR-NPsTRE showed significantly different PI values than FD-NPsPEG and IR-NPsPEG as evaluated by the statistical ANOVA (P>0.05). PI values of formulations prepared with TRE after freeze-drying and sterilization revealed less homogenous suspension than the other samples. It is well known that a smaller Zav and a low PI involve a higher and closer contact with the drug-loaded particles and the biological tissue. This helps to obtain a more efficient permeation of the drug into the tissue.29,30
It is widely known that higher ZP values, either positive or negative, allow greater long-term stability.31 In our case, all samples showed a negative ZP value ranging from −12.00 mV to −18.40 mV. This can be attributed to the presence of lactone residues on the polymeric matrix surface.32 Thus, all samples being assayed were considered to be satisfactory NP suspensions.
Hypotonic solution may cause corneal edema.33 Hypertonic solution causes lachrymation, a burning sensation, and cell desquamation.34,35 As expected, F-A and F-B (suspension without therapeutic goal) showed hypo-osmolality values. Other samples displayed appropriate osmolality. The EE in the FB-PεCL-NPs was found to be 86%. No significant differences were observed in the EE values for all samples.
Rheological studies
Rheological behavior of IR-NPsTRE, IR-NPsPEG, FD-NPsTRE, FD-NPsPEG, and their individual basic formulations (F-A and F-B) were analyzed.
All samples presented viscosity curves that were nearly constant with increasing shear rate corresponding to Newtonian behavior. F-A, FD-NPsTRE, and IR-NPsTRE showed 1.225±0.043 mPa/s, 1.608±0.043 mPa/s, and 1.648±0.038 mPa/s, respectively, as viscosity values. Likewise, F-B, FD-NPsPEG, and IR-NPsPEG showed 1.664±0.056 mPa/s, 5.425±0.043 mPa/s, and 6.594±0.033 mPa/s, respectively, as viscosity values. Viscosity was increased in freeze-dried formulations due to the addition of protectant agents. FD-NPsTRE and IR-NPsTRE showed an extremely low viscosity in comparison with formulations prepared with PEG3350. IR-NPsPEG showed the greatest viscosity (P<0.05). A low viscosity benefits redispersion after aqueous reconstitution and makes easy dispensing of the eye drop. Likewise, systems with low viscosity allow good tolerance with little blinking pain.26
In vitro drug release
An in vitro release study of the FB from the protectant agent-free formulations (F-A and F-B), formulations with the protectant agent (NPsTRE and NPsPEG), optimized freeze-dried formulations (FD-NPsTRE and FD-NPsPEG), sterilized freeze-dried formulations (IR-NPsTRE and IR-NPsPEG), and free drug solution (FB, dissolved in PBS) was conducted.
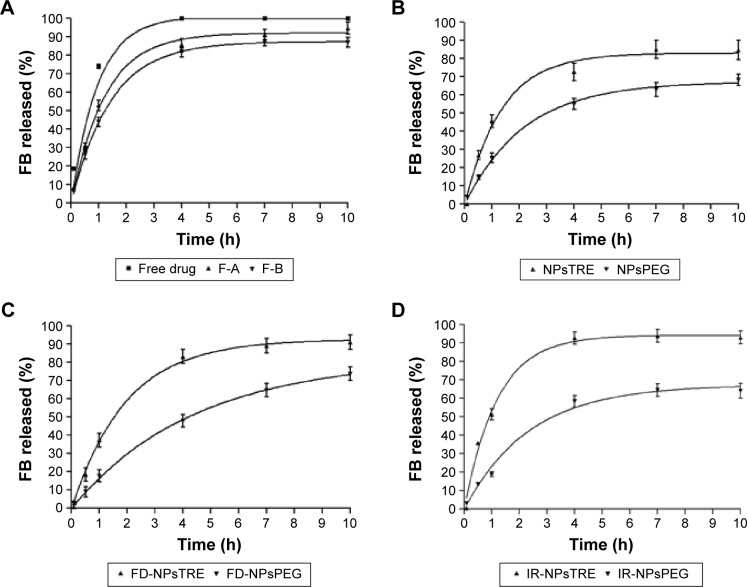
As represented in Figure 1A, the release profile of FB from the free drug solution exhibited faster and complete release behavior after 4 hours. F-A displayed a very similar release profile to the F-B (P>0.05), more prolonged than exhibited by free drug. Similar results were obtained by Vega et al6 for the in vitro drug release with different amounts of P188.6
Figure 1.
In vitro FB release profiles of (A) F-A and F-B, (B) NPsTRE and NPsPEG, (C) FD-NPsTRE and FD-NPsPEG, and (D) IR-NPsTRE and IR-NPsPEG, compared with free drug solution (mean ± SD, n=3).
Notes: F-A, nanoparticles’ suspension with P188 at 1.66%; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsTRE, formulation prepared with trehalose as protectant agent; NPsPEG, formulation prepared with PEG3350 as protectant agent.
Abbreviations: FB, flurbiprofen; FD, freeze-dried condition; IR, irradiated condition; h, hours; SD, standard deviation; NPs, nanoparticles.
However, as seen in Figure 1B, FB release decreased when protectant agents were added to their respective basic formulation. It was more evident in NPsPEG than NPsTRE, which reached 62% and 82% after 10 hours of drug release, respectively. This trend was maintained in the following FB-PεCL-NPs states (freeze-dried and sterilized). These results could be attributed to the fact that an increase in the viscosity of the dispersion medium can decrease the rate of particle sedimentation (according to the equation of Stokes’ law).36 Consequently, we can expect a slower rate of descent to ocular tissue in freeze-dried NPs with PEG3350 after aqueous reconstitution, thus allowing an extended dosage in the same proportion. The presence of Newtonian behavior ensures that blinking should have no effect on viscosity.37
Also, the effective control of FB released either in the burst effect or over 10 hours in FD-NPsPEG and IR-NPsPEG can be related to the compact structure of the polymeric matrix obtained by the addition of PEG3350 that reduces FB diffusion rate, especially when water is removed during sublimation.38
Comparative to the formulations using PEG, the samples with TRE added achieved a higher released amount of FB. Approximately 92%–94% of FB amount was released in the first 10 hours from FD-NPsTRE and IR-NPsTRE, respectively, as shown in Figure 1C and D. TRE was reported as a protectant agent that induces or increases the porosity of NPs.39 It may open a pathway for a faster FB release from NPs and for its possible degradation by external factors. Two mechanisms of pore formation have been postulated, and it is probable that both contribute to the higher FB release rate. The surface of NPs and porosity of the final dried cake are strongly influenced by the freezing stage (freeze-drying process) due to the elimination of the ice crystals by sublimation, creating an open network of pores that may affect the morphological characteristics of NPs. Furthermore, TRE is able to form more flexible hydrogen bonds with NPs; thus, it is removed more easily from the surface of NPs and FB is released in a higher amount.39,40
The literature reported the effect of γ-irradiation on PεCL structure; upon irradiation, the polymer displays cross-linking between polymeric chains or even between surfactant and PεCL chains.41 As can be seen in Figure 1D, the release profile of NPs did not significantly change after γ-irradiation. Only the intrinsic viscosity of FB-PεCL-NPs using PEG added increased from 5.425 mPa/s to 6.594 mPa/s after irradiation. This leads to the assumption that the release was slightly modified after sterilization, although, the γ-irradiation at 25 kGy did not determine cross-linking or modification reactions in the matrix components. Probably, this effect was reduced by the higher amounts of PEG3350 contained in the dried NPs. On the other hand, IR-NPsTRE displayed a very similar release rate than the free drug solution (P>0.05). Besides their rheological properties and the γ-irradiation effects on PεCL aforementioned, this may be because of easy breakdown of TRE coat by sterilization, facilitating a direct exposition of NP. Some authors reported that TRE increases drug release, which corroborated the profiles obtained.20,42,43
Here, it can be suggested that PEG3350 protected FB-PεCL-NPs more successfully than TRE at the studied concentration, although γ-radiation may influence the drug release kinetics.
The evaluation of FB degradation after irradiation was performed by RP-HPLC. The irradiated sample’s chromatograms were similar to the non-irradiated sample’s chromatograms and did not reveal any evidence of the drug radiolysis in the tested irradiation dose.
Furthermore, as shown in Table 3, all samples were adjusted to various kinetic models, such as zero order, first order, Higuchi, and hyperbola. In this case, the most of the formulations followed the first-order model, which was also confirmed by the coefficient of determination, r2 (highest value), except for NPsPEG and FD-NPsPEG that followed a hyperbola kinetic model. As FB is homogenously distributed in the polymeric matrix, the drug release occurs by drug diffusion mechanism. Moreover, the low molecular weight of FB (244.25 Da) improves the diffusion mechanism.6
Table 3.
Parameters for kinetic models of FB-PεCL-NP formulations and free drug solution
| Models | Parameters | Units | F-A | NPsTRE | FD-NPsTRE | IR-NPsTRE | F-B | NPsPEG | FD-NPsPEG | IR-NPsPEG | Free drug |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zero order | AIC | – | 48.176 | 47.546 | 48.385 | 50.614 | 47.643 | 41.204 | 37.034 | 43.442 | 51.021 |
| r2 | 0.757 | 0.762 | 0.792 | 0.684 | 0.762 | 0.866 | 0.946 | 0.819 | 0.639 | ||
| First order | AIC | – | 22.546 | 29.845 | 23.318 | 27.804 | 13.193 | 18.028 | 11.809 | 26.027 | 38.677 |
| r2 | 0.997 | 0.988 | 0.997 | 0.993 | 0.999 | 0.997 | 0.999 | 0.990 | 0.954 | ||
| Kf | minute−1 | 0.820 | 0.736 | 0.510 | 0.839 | 0.703 | 0.455 | 0.225 | 0.424 | 1.054 | |
| SD | 0.051 | 0.097 | 0.040 | 0.079 | 0.022 | 0.033 | 0.013 | 0.062 | 0.217 | ||
| Q∞ | µg | 91.90 | 82.81 | 92.40 | 94.17 | 87.22 | 67.02 | 81.70 | 67.25 | 100.90 | |
| SD | 1.484 | 2.819 | 1.970 | 2.287 | 0.716 | 1.384 | 1.954 | 2.864 | 5.405 | ||
| t1/2 | minute | 0.845 | 0.942 | 1.358 | 0.827 | 0.986 | 1.524 | 3.079 | 1.634 | 0.657 | |
| SD | 0.031 | 0.064 | 0.127 | 0.093 | 0.099 | 0.008 | 0.385 | 0.095 | 0.039 | ||
| Hyguchi | AIC | – | 42.607 | 41.874 | 42.256 | 46.046 | 41.950 | 32.594 | 24.548 | 37.685 | 47.500 |
| r2 | 0.904 | 0.907 | 0.931 | 0.853 | 0.908 | 0.968 | 0.993 | 0.931 | 0.799 | ||
| Hyperbola | AIC | – | 22.875 | 27.638 | 31.732 | 33.046 | 26.227 | 12.467 | 7.930 | 30.817 | 40.655 |
| r2 | 0.996 | 0.991 | 0.987 | 0.983 | 0.993 | 0.999 | 0.999 | 0.978 | 0.936 |
Notes: F-A, nanoparticles’ suspension with P188 at 1.66%; NPsTRE, formulation prepared with trehalose as a protectant agent; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsPEG, formulation prepared with PEG3350 as a protectant agent; r2, determination coefficient, Kf, release rate constant; Q∞, maximum amount of dissolved drug; t1/2, drug half-life.
Abbreviations: FB, flurbiprofen; PεCL, poly(ε-caprolactone); NPs, nanoparticles; FD, freeze-dried condition; IR, irradiated condition; AIC, Akaike’s information criterion; SD, standard deviation.
FB release from the irradiated samples depended on chemical properties provided by the protectant agent even though the FB release profiles were influenced by freeze-drying. The release pattern was not affected by the P188 amount – even by size homogeneity of NPs in suspension (PI).
In order to help us to predict in vivo release behavior and elucidate the detailed release mechanism of this colloidal system, studies on drug release kinetics are fundamental. The corresponding biopharmaceutical parameters were determined to confirm the results observed in Figure 1. Although there were not statistically differences between F-A and F-B, it could be observed that samples with PEG (NPsPEG, FD-NPsPEG, and IR-NPsPEG) showed a constant weak release rate (Kf) against formulations containing TRE (NPsTRE, FD-NPsTRE, and IR-NPsTRE). The maximum concentration of released drug (Q∞) from IR-NPsTRE and IR-NPsPEG was 379.20±19.44 µg and 267.10±20.06 µg, respectively (P<0.05). It could be seen that PEG3350 increased drug half-life in comparison with TRE that maintained a drug half-life similar to the free drug solution as evaluated by the statistical ANOVA (P>0.05).
Ocular permeation study
The ocular permeability of drugs is clinically important as it is one of the major factors that determine the efficacy of topically applied preparations. An ex vivo permeation study was carried out to compare the permeation profile of FB from the optimized freeze-dried formulations (FD-NPsTRE and FD-NPsPEG), sterilized formulations (IR-NPsTRE and IR-NPsPEG), FB-PεCL-NPs suspensions with and without protectant agent (NPsTRE, NPsPEG, F-A, F-B), and free drug solution. Table 4 shows the permeation parameters of the formulations and the amount of drug retained (Qr). The results of the permeation studies were compared using a parametric statistical assay (the ANOVA test), followed by Tukey’s multiple comparison test (P<0.05).
Table 4.
FB corneal and scleral permeation parameters from FB-PεCL-NP formulations and free drug solution
| FB-PεCL-NPs | Cornea
|
Sclera
|
||||
|---|---|---|---|---|---|---|
| TL (h) | Kp×10−2 (cm/h) | Qr (%/cm2·g) | TL (h) | Kp×10−2 (cm/h) | Qr (%/cm2·g) | |
| F-A | 0.008±0.098 | 1.156±0.144 | 17.96±0.38 | 2.045±0.164 | 1.045±0.045 | 34.15±1.03 |
| NPsTRE | 0.936±0.112 | 1.616±0.150 | 18.78±1.34 | 2.478±0.125 | 0.931±0.073 | 25.25±1.44 |
| FD-NPsTRE | 1.249±0.095 | 1.668±0.118 | 12.49±0.94 | 1.700±0.832 | 0.289±0.061 | 19.82±1.78 |
| IR-NPsTRE | 0.965±0.127 | 2.368±0.132 | 13.95±1.43 | 2.605±0.821 | 0.525±0.092 | 11.31±0.34 |
| F-B | 1.190±0.108 | 1.624±0.095 | 15.52±2.03 | 2.533±0.184 | 1.026±0.075 | 21.75±1.82 |
| NPsPEG | 0.194±0.092 | 1.077±0.168 | 14.76±1.79 | 1.626±0.173 | 0.657±0.057 | 14.51±2.18 |
| FD-NPsPEG | 1.554±0.132 | 1.134±0.128 | 11.18±2.45 | 1.748±0.184 | 0.410±0.088 | 13.30±1.63 |
| IR-NPsPEG | 1.025±0.117 | 0.841±0.131 | 33.95±0.99 | 1.632±0.192 | 0.392±0.037 | 15.08±1.85 |
| Free drug | 0.223±0.243 | 2.048±0.192 | 25.45±1.89 | 1.787±0.274 | 1.824±0.836 | 27.10±1.93 |
Notes: TL, Drug lag time; Kp, permeability coefficient; Qr, amount of drug retained; F-A, nanoparticles’ suspension with P188 at 1.66%; NPsTRE, formulation prepared with trehalose as a protectant agent; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: FB, flurbiprofen; PεCL, poly(ε-caprolactone); NP, nanoparticle; h, hour; FD, freeze-dried condition; IR, irradiated condition.
Corneal permeation study
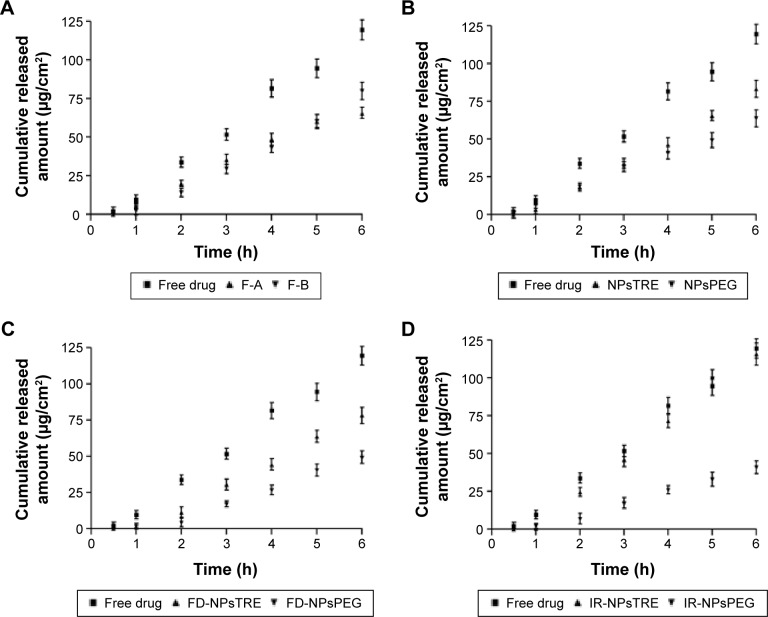
The transcorneal permeation profile of FB is shown in Figure 2. F-A showed a smaller Kp than F-B. It is well known that transcorneal permeation of a lipophilic drug, like FB, is higher than hydrophilic drugs. The higher amount of P188 in F-B probably reinforced the penetration of FB in the cornea as shown in Figure 2A. However, as seen in Figure 2B, the performance changed when a protector was added.
Figure 2.
Ex vivo corneal permeation profile of FB from (A) F-A and F-B, (B) NPsTRE and NPsPEG, (C) FD-NPsTRE and FD-NPsPEG, and (D) IR-NPsTRE and IR-NPsPEG, compared with free drug solution after 6 h (mean ± SD, n=3).
Notes: F-A, nanoparticles’ suspension with P188 at 1.66%; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsTRE, formulation prepared with trehalose as a protectant agent; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: FB, flurbiprofen; FD, freeze-dried condition; IR, irradiated condition; h, hours; SD, standard deviation; NPs, nanoparticles.
In freeze-dried NPs, TRE had a stronger influence on the permeation properties. In spite of FD-NPsPEG and IR-NPsPEG presenting better homogeneity in morphometric characteristics, FD-NPsTRE and IR-NPsTRE reached a significantly higher Kp. Noticeably, IR-NPsPEG displayed the smallest Kp and the largest amount of drug retained in the cornea, 33.95%±0.99%/cm2⋅g.
It can be seen from Figure 2C and D an increase in Kp from FD-NPsTRE to IR-NPsTRE (P<0.05), while Kp from FD-NPsPEG to IR-NPsPEG was maintained (P>0.05). Only the IR-NPsTRE reached similar amount of FB permeated through the corneal tissue to free drug solution, 116.03 µg/cm2 and 119.70 µg/cm2. They represented 58.02%±2.45% and 59.85%±1.95% of total exposed sample amount without significant differences. As was aforementioned, besides TRE lends to expose drug directly through pore formation, sterilization could help to break TRE core; thus, TRE enhances cornea permeation of FB. Furthermore, in spite of their intrinsic viscosity, it was noted that IR-NPsTRE and IR-NPsPEG took a similar time to fill the stratum corneum, TL (P>0.05).
The results indicate that the inclusion of FB in the PεCL matrix with TRE as an additive can help considerably in the penetration of the drug across the cornea after γ-irradiation. Moreover, Qr from IR-NPsPEG was higher than Qr from IR-NPsTRE – this is in accordance with the low FB release profile presented by IR-NPsPEG.
HL is a parameter frequently used to evaluate the ideal cornea conditions. The normal cornea has an HL of 76%–80%. A hydration level that is 3%–7% above the normal value denotes the epithelium or endothelium damage.44 The HL was maintained within the accepted range (78.79%–81.35%) for all formulations and corroborated the lack of damage on the corneal tissue.
Scleral permeation study
Scleral is a fairly leaky tissue that has 20 times greater surface area (potential drug depot) than the cornea tissue. The transscleral delivery route offers advantages over the corneal route, such as metabolic inactivity and high permeability to macromolecules.45,46 So far, FB permeation has been studied in the sclera.
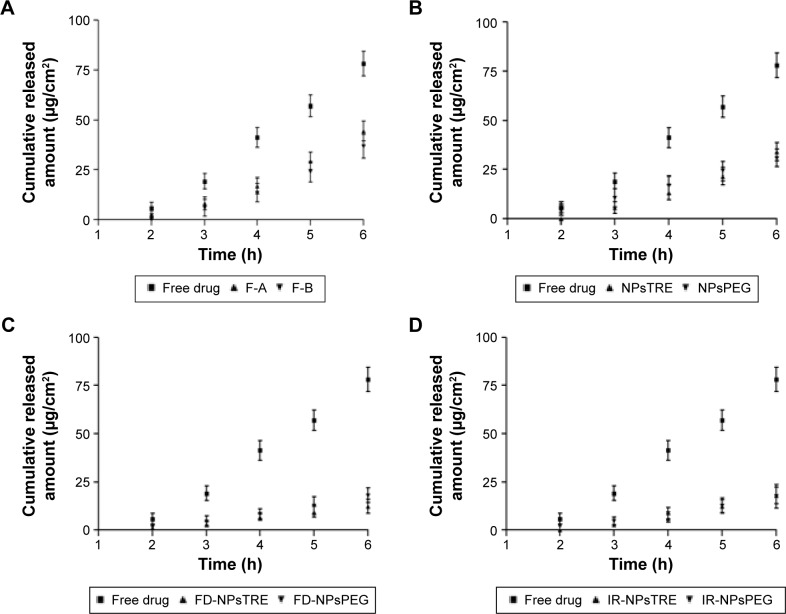
The ex vivo permeation of FB after 6 hours through the scleral tissue can be seen in Figure 3. FB was able to traverse across sclera in spite of the transscleral pathway fairly permeable just to hydrophilic molecules.47 The largest Kp, 1,824±0.836×10−2 cm/h was displayed by the free drug solution, while Kp corresponding to F-A and F-B was similar (P>0.05).
Figure 3.
Ex vivo sclera permeation profile of FB from (A) F-A and F-B, (B) NPsTRE and NPsPEG, (C) FD-NPsTRE and FD-NPsPEG, and (D) IR-NPsTRE and IR-NPsPEG, compared with free drug solution after 6 h (mean ± SD, n=3).
Notes: F-A, nanoparticles’ suspension with P188 at 1.66%; F-B, nanoparticles’ suspension with P188 at 3.5%; NPsTRE, formulation prepared with trehalose as a protectant agent; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: FB, flurbiprofen; FD, freeze-dried condition; IR, irradiated condition; h, hours; SD, standard deviation; NPs, nanoparticles.
However, a Kp decrease in samples with the addition of the protective agent was observed. The results showed that the additive influenced reduced scleral permeability. The freeze-drying process and γ-irradiation did not have any effect on the Kp parameter (P>0.05). Moreover, FB from all samples took similar times to permeate the sclera (lag time). The Qr in sclera from IR-NPsTRE and IR-NPsPEG was similar and considerably less than Qr from the free drug solution.
As illustrated in Figure 3D, IR-NPsTRE and IR-NPsPEG reached a similar cumulative permeated FB amount through the scleral tissue, 18.20 µg/cm2 and 17.70 µg/cm2, respectively. They represented 9.10%±0.86% and 8.85%±0.57% of total exposure sample amount, indicating no therapeutic efficacy difference (P>0.05). Moreover, the amount of FB permeated through sclera from the free drug solution was not as high as permeated from the cornea. The amount was 78.20 µg/cm2 and represented only 39.10% of the total exposure sample amount. This result showed that morphometric characteristic (PI) and viscosity of formulations were less important than FB chemical affinity in the target tissues. It is clear that free drug solution showed upper permeation efficacy; however, this assay did not consider the rapid elimination of solution through the blinking of the eye and drainage of tear flow – nasolacrimal, which reduces its bioavailability to ~75%.48
In vitro and in vivo tolerance studies
After application of 0.3 mL of irradiated FB-PεCL-NPs on the CAM, no effect of hemorrhage, lysis, or coagulation was observed. An OII of 0.02±0.08 and 0.07±0.06 was obtained in IR-NPsTRE and IR-NPsPEG, respectively. Therefore, these formulations were classified into the OII of “non-irritatant”, which indicates optimal ocular tolerance. According to Draize eye test, no sign of ocular inflammation, congestion, swelling, or lacrimation was observed (scores were zero in both cases). These results are in accordance with those obtained by the HET-CAM and thus can be classified as non-irritating, safe products for ophthalmic administration.
Efficacy of ocular anti-inflammatory effect
As seen in Figure 4, the ocular anti-inflammatory activity of irradiated FB-PεCL-NPs demonstrated a decrease in the ocular inflammation caused by instillation of SAS. During the first 60 minutes, no statistically significant differences were observed between IR-NPsTRE, IR-NPsPEG, and the free drug solution. After 90 minutes, IR-NPsPEG exhibited significant differences when compared to the free drug solution. Finally, after 120 minutes, IR-NPsPEG exhibited statistically higher anti-inflammatory effect than IR-NPsTRE, which correlated directly with the Qr values in the ocular tissues shown in Table 4.
Figure 4.
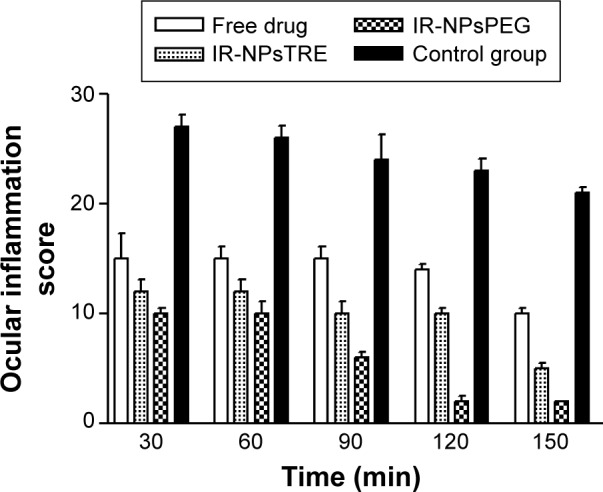
Anti-inflammatory activities of FB from the IR-NPsTRE and IR-NPsPEG formulations, free drug solution, and control (SAS) mean ± SD, n=3.
Notes: NPsTRE, formulation prepared with trehalose as a protectant agent; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: FB, flurbiprofen; IR, irradiated condition; SAS, sodium arachidonate solution; min, minutes; SD, standard deviation; NPs, nanoparticles.
Although the free drug solution showed high Qr values of FB in the cornea and sclera, it performed a lower anti-inflammatory efficacy compared to the NP formulations after 90 minutes. This anti-inflammatory efficacy of longer duration can be explained by the formation of FB depot by adhesive effect that promotes a slow drug release and consequently a continuous pharmacological action.
Stability studies
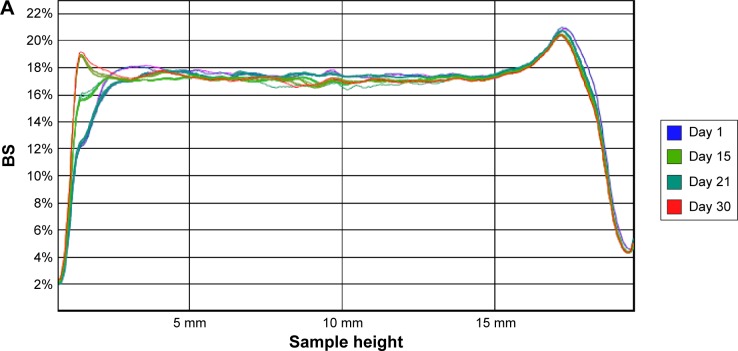
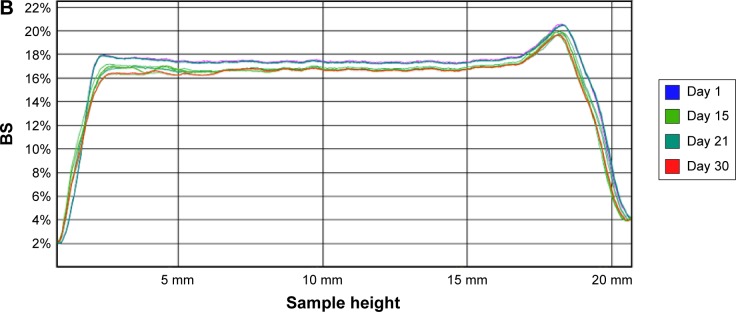
The recorded transmission profiles of IR-NPsTRE and IR-NPsPEG gave relevant information regarding the intrinsic suspension stability. After 15 days, a variation of BS on the right side of the IR-NPsTRE fingerprint (bottom vial) indicated a sedimentation process (Figure 5A), considered a reversible physical process. On the other hand, in IR-NPsPEG, the presence of creaming, sedimentation, or flocculation was undetected for >30 days (Figure 5B). These results may be related to the strong PEG3350 influence over increased viscosity to stabilize this colloidal system than the steric stabilization given by P188. The higher ZP of IR-NPsPEG also has a better positive impact on the system stability than IR-NPsTRE.
Figure 5.
BS profiles of (A) IR-NPsTRE and (B) IR-NPsPEG analyzed at different days during 30 days of storage at 4°C.
Notes: NPsTRE, formulation prepared with trehalose as a protectant agent; NPsPEG, formulation prepared with PEG3350 as a protectant agent.
Abbreviations: BS, backscattering; IR, irradiated condition; NPs, nanoparticles.
The slight changes in the bottom and top are attributed to the meniscus of the samples forming contact with the glass. In parallel with these studies, possible changes in the mean particle size were monitored by photon correlation spectroscopy analysis. There were no significant differences in stability during the monitored time.
Conclusion
A Goldmann’s criteria analysis was performed on FB-loaded polycaprolactone NPs prepared with trehalose and PEG3350 as protective agents. In vitro release profiles showed that both additives, trehalose and PEG3350, gave inherent characteristics to their basic formulation components, which could promote or hinder FB release. Such characteristics have a stronger effect than the NP morphometrical characteristics in the permeation rate. Regarding transcorneal permeation, freeze-drying and γ-irradiation hindering FB release from formulations PEG added, while these conditions have an adverse effect on added formulations of TRE. However, these processes did not influence transscleral permeation.
Ocular irritating effects were absent in both in vitro and in vivo tests. In summary, both formulations could be employed as a controlled release formulation in preclinical studies; however, out of the two formulations, it must be noted that the PEG3350 formulation has a greater potential based on longer satisfactory anti-inflammatory effects.
Acknowledgments
The authors would like to thank the Spanish Ministry of Science and Innovation (grants MAT2011-26994 and MAT2014-59134R). GR Ramos Yacasi would also like to acknowledge the kind help of Doctor Sasha Nikolic from Reig Jofre Group of Barcelona, Spain, for his useful advice regarding the freeze-drying process.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mueller JB, McStay CM. Ocular infection and inflammation. Emerg Med Clin North Am. 2008;26(1):57–72. doi: 10.1016/j.emc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10(2):229–241. doi: 10.1208/s12248-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasconcelos A, Vega E, Pérez Y, Gómara MJ, García ML, Haro I. Conjugation of cell-penetrating peptides with poly (lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int J Nanomedicine. 2015;10:609–631. doi: 10.2147/IJN.S71198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vega E, Gamisans F, García ML, Chauvet A, Lacoulonche F, Egea MA. PLGA nanospheres for the ocular delivery of flurbiprofen: drug release and interactions. J Pharm Sci. 2008;97(12):5306–5317. doi: 10.1002/jps.21383. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Mira E, Egea MA, Souto EB, Calpena AC, García ML. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2011;22(4):045101. doi: 10.1088/0957-4484/22/4/045101. [DOI] [PubMed] [Google Scholar]
- 6.Vega E, Egea MA, Valls O, Espina M, Garci ML. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J Pharm Sci. 2006;95(11):2393–2405. doi: 10.1002/jps.20685. [DOI] [PubMed] [Google Scholar]
- 7.Furrer P, Delie F, Plazonnet B. Ophthalmic drug delivery. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane ME, editors. Modified-Release Drug Delivery Technology. Raleigh, NC: Pharmaceu Tech, Inc; 2008. pp. 59–84. [Google Scholar]
- 8.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Vega E, Egea M, Calpena A. Role of hydroxypropyl-β-cyclodextrin on freeze-dried and gamma-irradiated PLGA and PLGA–PEG diblock copolymer nanospheres for ophthalmic. Int J Nanomedicine. 2012;7(1):1357–1371. doi: 10.2147/IJN.S28481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroll C, Borchert H, Kissel T. Tetracycline-HCl-loaded poly (D,L-lactide-co-glycolide) microspheres prepared by a spray drying technique: influence of γ-irradiation on radical formation and polymer degradation. J Control Release. 1999;59(1):23–32. doi: 10.1016/s0168-3659(98)00170-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelmus KR. The Draize eye test. Surv Ophthalmol. 2015;45(6):493–515. doi: 10.1016/s0039-6257(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 12.Vinardell MP, Mitjans M. Alternative methods for eye and skin irritation tests: an overview. J Pharm Sci. 2008;97(1):46–59. doi: 10.1002/jps.21088. [DOI] [PubMed] [Google Scholar]
- 13.Mohanty B, Mishra S, Majundar D. Effect of formulation factors on in vitro transcorneal permeation of voriconazole from aqueous drops. J Adv Pharm Technol Res. 2013;4(4):210–216. doi: 10.4103/2231-4040.121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3(2):275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Ederra J, García M, Hernández M, et al. The pig eye as a novel model of glaucoma. Exp Eye Res. 2005;81(5):561–569. doi: 10.1016/j.exer.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Nicoli S, Ferrari G, Quarta M, et al. Porcine sclera as a model of human sclera for in vitro transport experiments: histology, SEM, and comparative permeability. Mol Vis. 2009;15:259–266. [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability: effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci. 1995;36(9):1893–1903. [PubMed] [Google Scholar]
- 18.Olsen TW, Sanderson S, Feng X, Hubbard WC. Porcine sclera: thickness and surface area. Invest Ophthalmol Vis Sci. 2002;43(8):2529–2532. [PubMed] [Google Scholar]
- 19.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nano-capsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–R4. [Google Scholar]
- 20.Bozdag S, Dillen K, Vandervoort J, Ludwig A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D,L-lactide-glycolide) nanoparticles. J Pharm Pharmacol. 2005;57(6):699–707. doi: 10.1211/0022357056145. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski JB, Aoshuang Y, Tallentire A. Radiation sterilization Ð evaluation of a new approach for substantiation of 25 kGy. Radiat Phys Chem. 1999;58(2000):77–86. [Google Scholar]
- 22.Celia C, Trapasso E, Cosco D, Paolino D, Fresta M. Turbiscan lab expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf B Biointerfaces. 2009;72(1):155–160. doi: 10.1016/j.colsurfb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Franz TJ. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol. 1975;64(3):190–195. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- 24.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 25.Alvarado HL, Abrego G, Souto EB, et al. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: in vitro, ex vivo and in vivo characterization. Colloids Surf B Biointerfaces. 2015;130:40–47. doi: 10.1016/j.colsurfb.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 26.Parra A, Mallandrich M, Clares B, et al. Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: ocular anti-inflammatory applications. Colloids Surf B Biointerfaces. 2015;136:935–943. doi: 10.1016/j.colsurfb.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Tavaszi J, Budai P. The use of HET-CAM test in detecting the ocular irritation. Commun Agric Appl Biol Sci. 2007;72(2):137–141. [PubMed] [Google Scholar]
- 28.Kay J, Calandra J. Interpretation of eye irritation tests. J Soc Cosmet Chem. 1962;13:281–289. [Google Scholar]
- 29.Liu CH, Wu CT. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf A Physicochem Eng Asp. 2010;353(2–3):149–156. [Google Scholar]
- 30.Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Feng S, Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J Control Release. 2001;71(1):53–69. doi: 10.1016/s0168-3659(00)00364-3. [DOI] [PubMed] [Google Scholar]
- 32.Ravi PR, Vats R, Dalal V, Gadekar N, Aditya N. Design, optimization and evaluation of poly-ε-caprolactone (PCL) based polymeric nanoparticles for oral delivery of lopinavir. Drug Dev Ind Pharm. 2015;41(1):131–140. doi: 10.3109/03639045.2013.850710. [DOI] [PubMed] [Google Scholar]
- 33.Ludwing A, Van Ooteghem M. The influence of the osmolality on the precorneal retention of ophthalmic solutions. J Pharm Belg. 1987;42(4):259–266. [PubMed] [Google Scholar]
- 34.Motolko M, Breslin CW. The effect of pH and osmolarity on the ability of tolerate artificial tears. Am J Ophthalmol. 1981;91(6):781–784. doi: 10.1016/0002-9394(81)90012-x. [DOI] [PubMed] [Google Scholar]
- 35.Holly FJ, Lamberts DW. Effect of nonisotonic solutions on tear film osmolality. Invest Ophthalmol Vis Sci. 1981;20(2):236–245. [PubMed] [Google Scholar]
- 36.Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater. 2015;2015:13. [Google Scholar]
- 37.Alvarado HL, Abrego G, Garduño-Ramirez ML, Clares B, Calpena AC, García ML. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomedicine. 2015;11(3):521–530. doi: 10.1016/j.nano.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Dorati R, Genta I, Montanari L, et al. The effect of γ-irradiation on PLGA/PEG microspheres containing ovalbumin. J Control Release. 2005;107(1):78–90. doi: 10.1016/j.jconrel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 39.Fonte P, Soares S, Costa A, et al. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter. 2012;2(4):329–339. doi: 10.4161/biom.23246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–263. doi: 10.1016/j.ejpb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Narkis M, Sibony-Chaouat S, Siegmann A, Shkolnik S, Bell JP. Irradiation effects on polycaprolactone. Polymer (Guildf) 1985;26(1):50–54. [Google Scholar]
- 42.Singh KK, Shegokar R. Conversion of stavudine lipid nanoparticles into dry powder. Int J Pharma Bio Sci. 2011;2(1):443–457. [Google Scholar]
- 43.Bartolotta A, D’Oca MC, Campisi M, et al. Effects of gamma-irradiation on trehalose-hydroxyethylcellulose microspheres loaded with vancomycin. Eur J Pharm Biopharm. 2005;59(1):139–146. doi: 10.1016/j.ejpb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Schoenwald R, Huang H. Corneal penetration behavior of beta-blocking agents I: physicochemical factors. J Pharm Sci. 1983;72(11):4–10. doi: 10.1002/jps.2600721108. [DOI] [PubMed] [Google Scholar]
- 45.Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004;21(10):1797–1804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- 46.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 47.Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev. 2001;52(1):37–48. doi: 10.1016/s0169-409x(01)00193-4. [DOI] [PubMed] [Google Scholar]
- 48.Tangri P, Khurana S. Basics of ocular drug delivery systems. Int J Res Pharm Biomed Sci. 2011;2(4):1541–1552. [Google Scholar]