Summary
Chikungunya virus (CHIKV) and related alphaviruses cause epidemics of acute and chronic musculoskeletal disease. To investigate mechanisms underlying the failure of immune clearance of CHIKV, we studied mice infected with an attenuated CHIKV strain (181/25) and its pathogenic parent strain (AF15561), which differ by five amino acids. Whereas AF15561 infection of wild-type mice resulted in viral persistence in joint tissues, 181/25 was cleared. In contrast, 181/25 infection of μMT mice lacking mature B cells resulted in viral persistence in joint tissues, suggesting that virus-specific antibody is required for clearance of infection. Mapping studies demonstrated that a highly conserved glycine at position 82 in the A domain of the E2 glycoprotein impedes clearance and neutralization of multiple CHIKV strains. Remarkably, murine and human antibodies targeting E2 domain B failed to neutralize pathogenic CHIKV strains efficiently. Our data suggest that pathogenic CHIKV strains evade E2 domain B neutralizing antibodies to establish persistence.
eTOC Blurb
Hawman et al. find that a highly conserved glycine at E2-82 promotes CHIKV persistence in joints and impairs neutralization by antibodies targeting E2 domain B. Mutation of E2-82 to arginine allows viral clearance and enhances neutralization, providing a structural basis for how chronic CHIKV joint infection evades B cell-mediated clearance.
Introduction
Chikungunya virus (CHIKV) is a mosquito-transmitted positive-sense, enveloped RNA virus in the Alphavirus genus of the Togaviridae. CHIKV has caused epidemics of unprecedented scale within the past decade in many regions of the world (Weaver and Forrester, 2015). Beginning in 2004, CHIKV re-emerged from Africa and spread to islands in the Indian Ocean and South Pacific and countries in southern Asia. In October of 2013, local transmission of CHIKV occurred on the Caribbean island of Saint Martin (Leparc-Goffart et al., 2014). Since that time, the virus has spread throughout much of the Americas resulting in at least 1.8 million confirmed and suspected cases in 45 countries or territories (PAHO, 2016).
CHIKV infection typically presents with a sudden onset of high fever and severe pain in peripheral joints (Borgherini et al., 2007; Staples et al., 2009). These signs and symptoms can resolve in a few weeks. However, in up to 50% of patients, musculoskeletal abnormalities, including joint swelling, joint stiffness, and tenosynovitis, endure for months to years after infection (Borgherini et al., 2008; Couturier et al., 2012; Gerardin et al., 2011; Schilte et al., 2013; Sissoko et al., 2009). Infection by related alphaviruses such as Mayaro, o’nyong’nyong, Ross River, and Sindbis viruses also can lead to chronic musculoskeletal disease in humans (Suhrbier et al., 2012).
Mechanisms by which alphaviruses cause chronic musculoskeletal disease remain elusive. Limited studies of human patients suggest that arthritogenic alphaviruses establish persistent infections within the host (Hoarau et al., 2010; Ozden et al., 2007; Soden et al., 2000). Persistent CHIKV infection also has been detected in experimentally infected mice and non-human primates. In cynomolgus macaques, infectious CHIKV or CHIKV RNA is present in synovial tissue, muscle, lymphoid organs, and the liver for weeks after infection (Labadie et al., 2010). Similarly, CHIKV RNA is present in the spleen of aged rhesus macaques for at least five weeks after initial infection (Messaoudi et al., 2013). Infection of wild-type (WT) C57BL/6 mice with CHIKV also results in chronic synovitis and persistence of CHIKV RNA specifically within joint-associated tissues (Hawman et al., 2013). Consistent with these data, long term persistence of positive- and negative-strand CHIKV RNA persists in the feet of CHIKV-inoculated mice (Poo et al., 2014), and mice infected with a recombinant CHIKV strain encoding a luciferase gene display gene activity even 60 days post-infection (Teo et al., 2013). Collectively, these data suggest that CHIKV persists for weeks to months in some tissues, and that the chronic musculoskeletal disease experienced by CHIKV patients may be due to persistent infection or inflammation.
Here, we show that the attenuated CHIKV strain 181/25, in contrast to its pathogenic parental strain AF15561, is cleared from joint-associated tissues within 4 weeks of infection. We took advantage of these disparate outcomes to investigate the viral and host determinants of CHIKV clearance and persistence. Our findings revealed that clearance of the attenuated 181/25 strain is impaired in Rag1−/− mice, B cell-deficient μMT mice, and B cell receptor transgenic mice that cannot produce virus-specific antibody, and that the persistence of 181/25 in joint tissue of Rag1−/− mice occurred without the acquisition of adaptive mutations that confer persistence in WT mice. The amino acid at position 82 of the CHIKV E2 glycoprotein, which varies between 181/25 and AF15561, is a major determinant of CHIKV clearance and the potency of neutralization of multiple epidemic CHIKV strains by both murine and human immune sera. Notably, neutralization of AF15561 by monoclonal antibodies (MAbs) specifically targeting E2 domain B was inefficient relative to 181/25. Overall, our data suggest that virus-specific antibody responses contribute to the clearance of 181/25 infection, and that AF15561 evades this response by minimizing the impact of E2 B domain neutralizing antibodies to establish persistent infection.
Results
CHIKV 181/25 is Cleared From Sites of Dissemination in WT C57BL/6 Mice
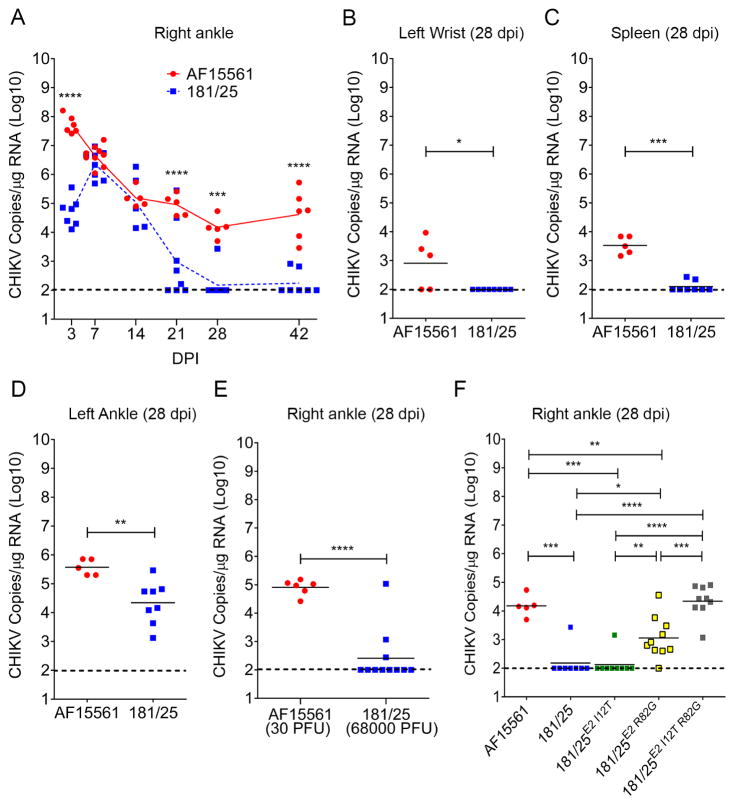
Pathogenic CHIKV strains establish persistent infections in joint tissues of immunocompetent WT C57BL/6 mice (Hawman et al., 2013; Poo et al., 2014). To test whether an attenuated CHIKV strain also establishes persistent infection in mice, we measured viral loads in joint tissues of WT C57BL/6 mice infected with the attenuated CHIKV strain 181/25 and its pathogenic parental strain AF15561 (Levitt et al., 1986), which vary at five amino acids. WT mice were inoculated in the left rear footpad with AF15561 or 181/25, and viral loads in the contralateral right ankle were quantified by RT-qPCR (Figure 1A). At 3 days post-inoculation (dpi), 181/25-infected mice had reduced viral RNA levels in the right ankle compared with AF15561-infected mice. These data are consistent with previous reports demonstrating that CHIKV strain 181/25 has diminished capacity to disseminate in mice from the site of inoculation (Ashbrook et al., 2014; Gardner et al., 2012). At 7 dpi, levels of viral RNA in the contralateral right ankle of mice inoculated with 181/25 were increased compared with levels at 3 dpi (P < 0.001), suggesting that 181/25 replicated at that site. Viral loads in the right ankle at 7 and 14 dpi were comparable in mice infected with 181/25 or AF15561 (Figure 1A). By 21 dpi, however, levels of viral RNA in the right ankle of 181/25-infected mice were reduced 95-fold relative to those in AF15561-infected mice. Similarly, at 28 dpi, high levels of viral RNA were maintained in the contralateral right ankle of AF15561-infected mice, whereas viral RNA was below the limit of detection in 7 of 8 mice infected with 181/25. Viral RNA in the right ankle of AF15561-infected mice remained detectable for at least 6 weeks post-infection (Figure 1A). Similar to the right ankle, viral RNA levels in the wrist (Figure 1B) and the spleen (Figure 1C) of mice infected with 181/25 were below the limit of detection at 28 dpi whereas viral RNA remained detectable at those sites in AF15561-infected animals. In contrast, viral RNA was detectable at 28 dpi in the ipsilateral left ankle, which is proximal to the site of inoculation (left rear footpad), of mice infected with 181/25 (Figure 1D), suggesting that the kinetics, or the influence of the local microenvironment on viral RNA clearance are distinct at that site.
Figure 1. CHIKV 181/25 is Cleared From Sites of Dissemination in WT mice.
(A–D) WT C57BL/6 mice were inoculated in the left rear footpad with 1,000 PFU of the pathogenic CHIKV strain AF15561 or the attenuated CHIKV strain 181/25. At the time points shown, CHIKV RNA in tissues was quantified by RT-qPCR.
(E) WT C57BL/6 mice were inoculated in the left rear footpad with 30 PFU of AF15561 or 68,000 PFU of 181/25. At 28 dpi, CHIKV RNA in the right ankle was quantified by RT-qPCR.
(F) WT C57BL/6 mice were inoculated in the left rear footpad with 1,000 PFU of the virus shown. At 28 dpi, CHIKV RNA in the right ankle was quantified by RT-qPCR. Horizontal bars indicate the mean values. The dashed lines indicate the limit of detection. Data are pooled from two or more independent experiments. P values were determined by two-way ANOVA with Bonferroni’s multiple comparison test (A), the Mann-Whitney-test (B–E), or one-way ANOVA with a Tukey’s multiple comparison test (F). *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001.
AF15561 has a higher ratio of genome copies to plaque-forming units (PFU) than 181/25 (Ashbrook et al., 2014; Silva et al., 2014). To determine whether differences in the number of CHIKV particles injected into mice contributed to differences in viral RNA persistence or clearance, we inoculated WT mice with 6.8 × 104 BHK cell PFU of 181/25, an equivalent number of genomes as contained in 1,000 BHK cell PFU of our stock of AF15561 (as determined by RT-qPCR, data not shown). Reciprocally, we also inoculated WT mice with 30 PFU of AF15561. Higher levels of viral RNA were detectable at 28 dpi in the right ankle of mice inoculated with 30 PFU of AF15561 than mice inoculated with 6.8 × 104 BHK cell PFU of 181/25, as the latter were below the limit of detection in 8 of 11 mice (Figure 1E). Thus, differential clearance of CHIKV strains AF15561 and 181/25 at sites of dissemination is not attributable to differences in the amount of viral RNA between the two strains at the time of inoculation.
Viral Determinants of CHIKV Persistence in Mice
Two mutations, I12 and R82, in the E2 glycoprotein of CHIKV 181/25 are responsible for as attenuation of acute disease in mice (Ashbrook et al., 2014; Gorchakov et al., 2012). To determine whether these mutations influenced the persistence of viral RNA in joint tissues, we inoculated mice with 181/25 containing E2 residue 12 reverted to a WT threonine (181/25E2 I12T), E2 residue 82 reverted to a WT glycine (181/25E2 R82G) or both revertant mutations together (181/25E2 I12T R82G) and quantified viral RNA levels in the right ankle at 28 dpi (Figure 1F). In comparison to mice infected with 181/25, infection of mice with 181/25E2 I12T did not alter viral RNA levels in the right ankle at 28 dpi (Figure 1F). However, reversion of E2 residue 82 to a glycine (181/25E2 R82G) resulted in 9 of 10 mice having detectable viral RNA in the right ankle at 28 dpi (Figure 1F), albeit at lower levels than detected in the right ankle of mice inoculated with AF15561. Inoculation of mice with the double revertant 181/25E2 I12T R82G restored viral RNA levels in the right ankle at 28 dpi to those detected in AF15561-infected mice (Figure 1F). Although 181/25E2 I12T was cleared from the right ankle at 28 dpi, the level of viral RNA in the right ankle of mice infected with 181/25E2 I12T R82G was higher compared with 181/25E2 R82G -infected mice, suggesting that a threonine at E2 residue 12 influences viral clearance when paired with the E2 R82G mutation.
CHIKV 181/25 Persists in Rag1−/− Mice
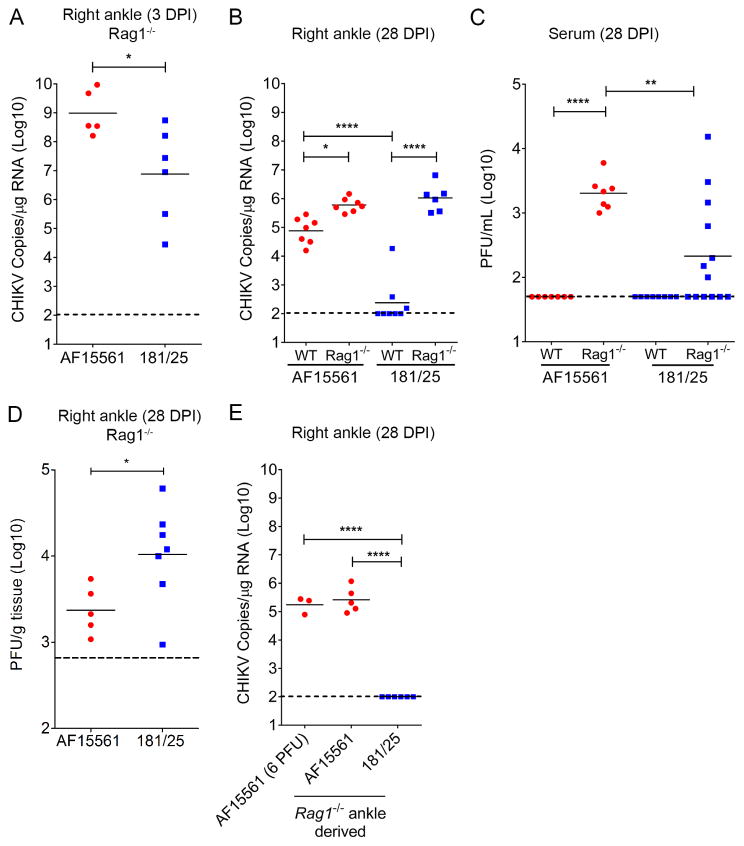
Based on the kinetics of CHIKV 181/25 clearance from the right ankle of WT mice (Figure 1A), we hypothesized that adaptive immune responses prevented persistence of 181/25 infection. To test this hypothesis, WT mice or Rag1−/− mice, which lack mature B and T cells, were inoculated with either AF15561 or 181/25 and viral RNA in the right ankle at 3 dpi (Figure 2A) and 28 dpi (Figure 2B) was quantified by RT-qPCR. Similar to WT mice (Figure 1A), viral RNA levels in the contralateral right ankle of Rag1−/− mice at 3 dpi were reduced in 181/25-infected mice in comparison to AF15561-infected mice (126-fold, P < 0.05) (Figure 2A) suggesting that the lower viral loads of 181/25 in this tissue at this time point are not due to the functions of B or T lymphocytes. AF15561-infected Rag1−/− mice had higher viral RNA levels in the right ankle at 28 dpi in comparison to AF15561-infected WT mice (26-fold; P < 0.001) (Figure 2B), indicating that B and/or T cell responses (or both) contribute to the control of AF15561 infection but fail to mediate clearance. In comparison, viral RNA levels in the right ankle of 181/25-infected Rag1−/− mice at 28 dpi were ~8,000-fold higher than those in 181/25-infected WT mice (P < 0.0001) (Figure 2B). In addition, viral RNA levels in the right ankle of 181/25- and AF15561-infected Rag1−/− mice at 28 dpi were similar (Figure 2B). To confirm these findings, we measured the amounts of infectious virus present in the serum and right ankle at 28 dpi. 181/25- and AF15561-infected Rag1−/− mice had detectable viremia (Figure 2C) although 181/25-infected Rag1−/− mice had reduced levels relative to AF15561-infected mice (9.5-fold; P < 0.001). However, 181/25-infected Rag1−/− mice had increased titers of infectious virus in the right ankle at 28 dpi compared with the same tissue of AF15561-infected mice (4.4-fold, P < 0.05) (Figure 2D). These data suggest that CHIKV strains 181/25 and AF15561 exhibit a similar capacity to persist in joint-associated tissues in the absence of adaptive immune responses.
Figure 2. CHIKV 181/25 Persists in Rag1−/− Mice.
(A–D) WT or Rag1−/− mice were inoculated with 1,000 PFU of AF15561 or 181/25. At (A) 3 dpi of Rag1−/− mice or (B) 28 dpi of WT or Rag1−/− mice, viral RNA in the right ankle was quantified by RT-qPCR. Infectious virus in the (C) serum or (D) right ankle was quantified by plaque assay.
(E) WT mice were inoculated in the left rear footpad with clarified ankle tissue homogenate containing 6 PFU of virus from (D) AF15561- or 181/25-infected animals or 6 PFU of CHIKV strain AF15561. At 28 dpi, CHIKV RNA in the right ankle was quantified by RT-qPCR. Horizontal bars indicate mean values. Dashed lines indicate the limit of detection. Data are from two or more independent experiments. P values were determined by Mann-Whitney test (A and D) or one-way ANOVA with a Tukey’s multiple comparison test (B, C, and E). *, P < 0.05, ***, P < 0.001, ****, P < 0.0001.
Reversion of attenuating mutations occurs in certain tissues of 181/25-infected WT mice (Ashbrook et al., 2014; Gorchakov et al., 2012). Therefore, we next determined whether persistence of 181/25 in Rag1−/− mice was associated with reversion of attenuating mutations or the acquisition of other adaptive mutations. Consensus sequencing of the E2 gene in viral RNA isolated from the right ankle of 181/25-infected Rag1−/− mice did not reveal reversion of the mutations at E2 residues 12 or 82 (data not shown). This sequencing strategy does not exclude the presence of compensatory mutations outside the region sequenced or low-frequency variants that could act in a trans-complementing manner to enhance virulence (Vignuzzi et al., 2006). To test whether persisting virus in ankle tissue of 181/25-infected Rag1−/− mice acquired the capacity to persist in WT mice, WT C57BL/6 mice were inoculated with 6 PFU of CHIKV from right ankle tissue homogenates (based on the virus titer, 6 PFU was the amount present in 20 μl of homogenate) from AF15561- or 181/25-infected Rag1−/− mice at 28 dpi. As a control, mice were inoculated with a similar dose of AF15561 stock virus. At 28 dpi, high levels of viral RNA were detected in the right ankle of mice infected with Rag1−/− ankle-derived AF15561 or 6 PFU of stock AF15561 (Figure 2E). In contrast, viral RNA levels in the right ankle of all WT mice inoculated with Rag1−/− ankle-derived 181/25 remained below the limit of detection. Additionally, WT mice inoculated with Rag1−/− ankle-derived AF15561 and 181/25 had high levels of CHIKV-specific IgG in the serum (1:8,000 to 1:64,000) at the time of harvest (data not shown), indicating that all mice had become infected. These results suggest that in the absence of B and T cell immunity, CHIKV strain 181/25 establishes a persistent infection without acquiring adaptive mutations that alter the capacity for viral persistence in WT mice.
B Cells and Virus-Specific Antibody Are Required For Clearance of CHIKV 181/25
To determine whether B cells are required for clearance of CHIKV 181/25 from joint tissues, we inoculated WT mice or μMT mice (deficient in mature B cells) with either AF15561 or 181/25 and quantified viral loads in the right ankle at 28 dpi (Figure 3A). Although not statistically significant (P > 0.05), we found that similar to Rag1−/− mice, viral loads in AF15561-infected μMT mice trended higher compared with WT animals. Moreover, μMT mice were unable to control 181/25 infection, with 10 of 10 mice having persistent viral RNA in the right ankle in comparison with 181/25-infected WT mice (155-fold, P < 0.0001). Furthermore, the levels of viral RNA in the right ankle of μMT mice infected with AF15561 or 181/25 were similar. These findings suggest that B cells are required for clearance of 181/25 infection in joint tissues.
Figure 3. Virus-Specific Antibody is Required to Control 181/25 Infection.
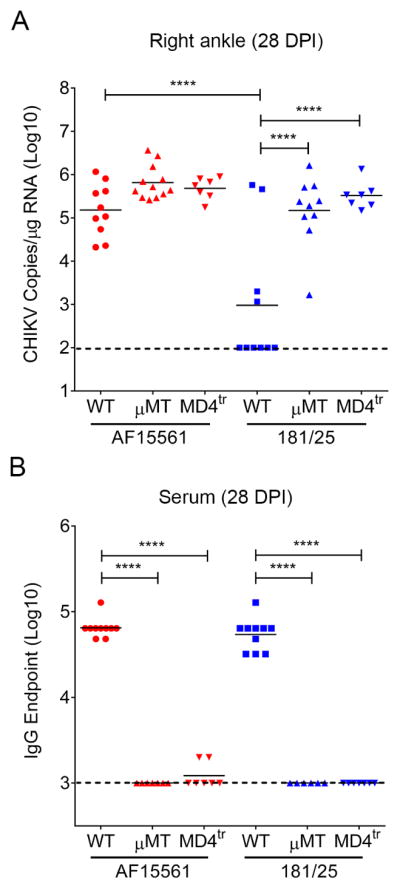
WT, B cell deficient μMT, or BCR transgenic MD4tr C57BL/6 mice were inoculated in the left rear footpad with 1,000 PFU of AF15561 or 181/25. At 28 dpi, (A) CHIKV RNA in the right ankle was quantified by RT-qPCR, and (B) CHIKV-specific IgG in the serum was quantified by a whole virion-based ELISA. Data are from two or more independent experiments. P values were determined by one-way ANOVA with a Tukey’s multiple comparison test. ****, P < 0.0001.
As μMT mice may have altered T cell responses because of the antibody-independent functions of mature B cells (Homann et al., 1998), we repeated these experiments using MD4 transgenic mice (MD4tr), which express a B cell receptor (BCR) specific for hen egg lysozyme (HEL) on a WT C57BL/6 background. In these mice, greater than 90% of B cells express the HEL-specific BCR due to allelic exclusion (Goodnow et al., 1988). Similar to μMT mice, infection of MD4tr mice with CHIKV strain AF15561 resulted in modestly elevated (6-fold) levels of viral RNA in the right ankle at 28 dpi compared with WT mice, although this difference was not statistically significant. In contrast, levels of viral RNA in the right ankle at 28 dpi in 181/25-infected MD4tr mice were higher in comparison to 181/25-infected WT mice (350-fold, P < 0.0001) (Figure 3A) and closely matched the levels observed in μMT mice. Moreover, levels of viral RNA in the right ankle of 181/25- and AF15561-infected MD4tr mice at 28 dpi were similar (P > 0.05) (Figure 3A). Analysis of the CHIKV-specific antibody response in these mice by ELISA confirmed markedly diminished or absent CHIKV-specific IgG in AF15561- and 181/25-infected MD4tr and μMT mice (Figure 3B). These results suggest that CHIKV-specific antibody responses are required for clearance of 181/25 from joint tissue, but the persistent AF15661 strain evades these clearance mechanisms.
Antibody Responses to CHIKV AF15561 and 181/25 Infection Are Distinct
The magnitude of the CHIKV-specific IgG response was similar at day 28 p.i. in AF15561- and 181/25-infected WT mice (Figure 3B). We next investigated whether differences in clearance of CHIKV AF15561 and 181/25 infection from joint tissue of WT mice were associated with differences in the kinetics or quality of the anti-CHIKV antibody response. Using a virion-based ELISA to quantify CHIKV-specific IgM and IgG present in the sera of WT mice, we found that virus-specific IgM responses to both AF15561 and 181/25 infection developed rapidly, with IgM titers peaking at 5 to 7 dpi and declining thereafter (Figure 4A). Levels of anti-CHIKV IgM were higher in AF15561-infected mice at both 5 dpi (10-fold, P < 0.0001) and 7 dpi (2.5-fold, P < 0.0001) compared with those in 181/25-infected mice (Figure 4A). CHIKV-specific IgG responses also developed rapidly, with titers detected at 7 dpi with either strain (Figure 4B). Again, the levels of anti-CHIKV IgG in AF15561-infected WT mice at 7 dpi were higher than those detected in 181/25-infected mice (12-fold, P < 0.0001). By 14 dpi and after, CHIKV-specific IgG titers were equivalent in AF15561 and 181/25-infected mice (Figure 4B) as were the individual CHIKV-specific IgG subtypes at 28 dpi (Figure 4C). By these analyses, the principal differences in virus-specific antibody responses during AF15561 or 181/25 infection occurred at early times post-infection; higher antibody levels were detected in AF15561-infected animals, which likely reflects the greater levels of viral replication and antigen in these mice.
Figure 4. Virus-Specific Antibody Responses in WT Mice Infected with AF15561 or 181/25.
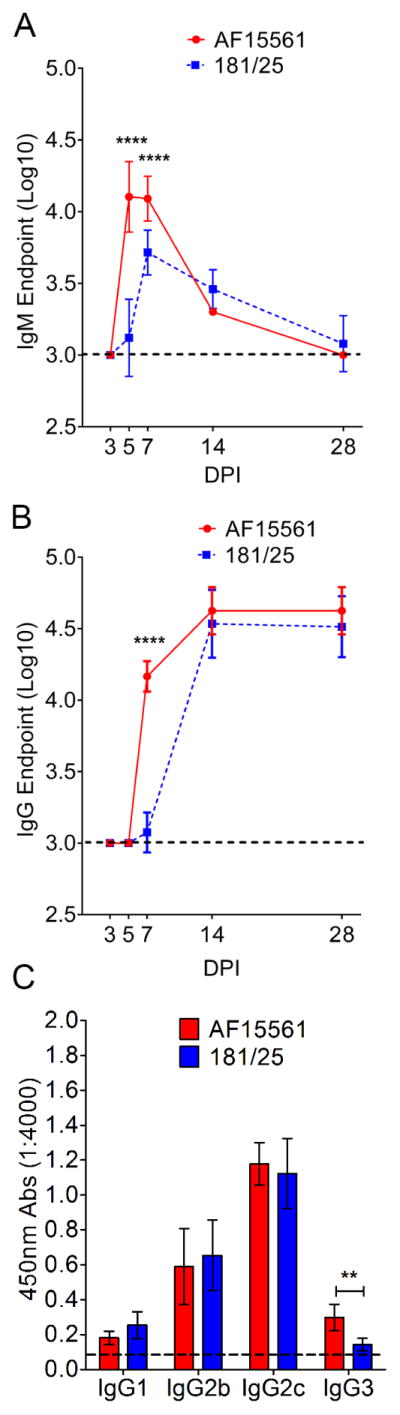
WT C57BL/6 mice were inoculated in the left rear footpad with 1,000 PFU of AF15561 or 181/25 (n = 5–8 mice per group). At the time points shown, a virion-based ELISA was used to quantify the serum endpoint titers of CHIKV-specific (A) IgM or (B) IgG.
(C) IgG subtype-specific detection antibodies were used to quantify IgG subtypes present in serum at 28 dpi (n = 5–6 mice per group). Data are from two or more independent experiments. P values were determined by two-way ANOVA with a Bonferroni’s multiple comparison test (A, B) and Student’s t-test (C). *, P <0.05, **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
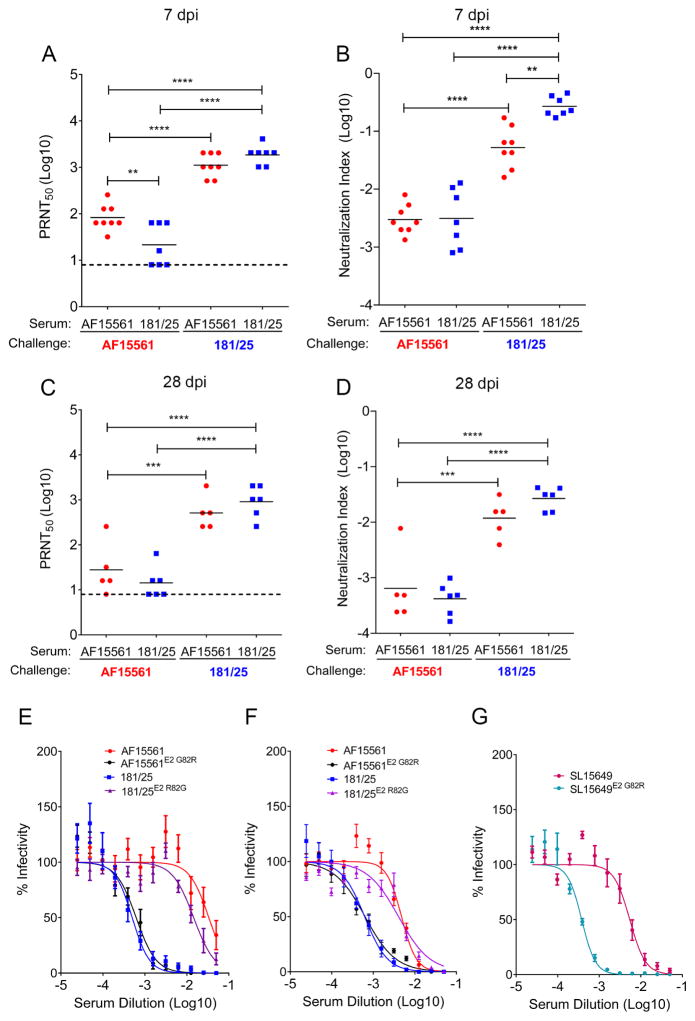
Although early virus-specific IgM and IgG responses were greater after infection with AF15561 compared with 181/25, this pattern did not correlate with clearance of AF15561 and 181/25 from joint tissue of WT mice. Given this lack of correlation, we investigated the qualitative activity of the virus-specific antibody response by measuring the neutralization capacity using a plaque reduction neutralization test (PRNT50) with 181/25 or AF15561 as the challenge viruses. When serum was collected at 7 dpi from AF15561- or 181/25-infected mice and tested against the homologous virus, serum from 181/25-infected mice displayed greater neutralizing activity compared with serum from AF15561-infected mice (22-fold, P < 0.0001) (Figure 5A). However, serum from AF15561-infected mice displayed neutralizing activity against 181/25 that was comparable to serum from 181/25-infected mice (Figure 5A), despite the differences in IgM and IgG ELISA binding titers at this time point (Figure 4A and 4B). By combining the ELISA and neutralization data, we observed a lower (5.2-fold, P < 0.01) serum neutralization index (Suthar et al., 2010) in AF15561-infected mice compared with 181/25-infected mice (Figure 5B), suggesting that the early virus-specific antibody response qualitatively differed during infection with acutely cleared (181/25) or persistent (AF15561) CHIKV strains. Serum collected from 181/25-infected mice at 7 dpi exhibited reduced neutralizing activity (Figure 5A) and a reduced neutralization index (Figure 5B) against AF15561 compared with 181/25. Although we detected similar neutralization activity against 181/25 or AF15561 virus in serum collected at 28 dpi from AF15561- or 181/25-infected mice, the neutralizing activity of sera from mice infected with either virus was consistently reduced against AF15561 compared with 181/25 (Figure 5C and 5D). Accordingly, and similar to 7 dpi, we observed a lower serum neutralization index for sera from AF15561- or 181/25-infected mice against AF15561 (Figure 5D).
Figure 5. Serum Neutralization of CHIKV Strains.
(A–D) WT C57BL/6 mice were inoculated in the left rear footpad with 1,000 PFU of AF15561 or 181/25. At (A) 7 dpi or (C) 28 dpi, the neutralization capacity of serum was quantified using a plaque reduction neutralization test 50 (PRNT50) with AF15561 or 181/25 as the challenge virus. (B and D) A neutralization index was calculated using the formula (PRNT50)/(IgG + IgM ELISA endpoint).
(E–G) The neutralizing activity of pooled serum derived from (E) 181/25-, (F) AF15561- or (G) SL15649-infected mice at 28 dpi was determined using the indicated viruses. Horizontal bars indicate mean values. Data are from two or more independent experiments. Dashed lines indicate the limit of detection. P values were determined by one-way ANOVA with a Tukey’s multiple comparison test. *, P <0.05, **, P < 0.01 ***, P < 0.001, ****, P < 0.0001.
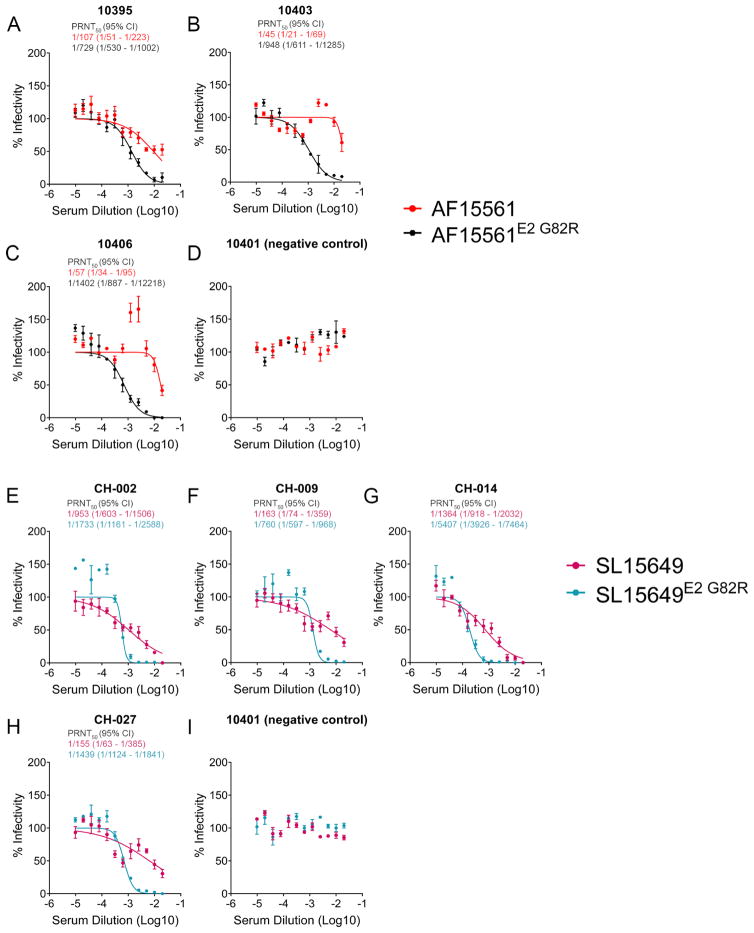
To determine whether amino acid differences between 181/25 and AF15561 explained the differences in serum neutralization capacity, we performed PRNT50 assays using sera from 181/25-infected mice against AF15561, AF15561E2 G82R, 181/25, and 181/25E2 R82G viruses. In comparison to neutralization of 181/25, the neutralizing activity of sera collected from 181/25-infected mice was reduced against AF15561 and 181/25E2 R82G but not AF15561E2 G82R (Figure 5E). Similar results were observed using sera collected from AF15561-infected mice (Figure 5F). Introduction of the E2 G82R mutation into the genome of another epidemic CHIKV strain (SL15649) (Morrison et al., 2011) also enhanced neutralization of the virus by serum collected from SL15649-infected mice (Figure 5G). Furthermore, the neutralizing activity of human sera collected from individuals who acquired CHIKV infection in the Caribbean (Figure 6A–6C) (Miner et al., 2015) or Sri Lanka (Figure 6E–6H) was reduced against AF15561 (an Asian genotype virus with genetic similarity to CHIKV strains circulating in the Caribbean) and SL15649 (an East, Central, South Africa genotype virus isolated during 2006 outbreak in Sri Lanka) in comparison with AF15561E2 G82R and SL15649E2 G82R. Control human sera had no effect on CHIKV infection (Figure 6D and 6I). Thus, the amino acid at E2 residue 82 influences the neutralization of epidemic Asian and East, Central, South Africa (ECSA) genotype CHIKV strains by both murine and human immune sera.
Figure 6. E2 Residue 82 Influences Neutralization of CHIKV with Human Immune Sera.
(A–C and E–H) The neutralizing activity of human sera from CHIKV-immune subjects was quantified using PRNT50 analysis against the indicated viruses. (D and I) Non-immune human sera was included as a control. The serum dilutions at which 50% of AF15561 (red text), AF15561E2 G82R, SL15649 (grey text), or SL15649E2 G82R (blue text) were neutralized (PRNT50) were determined by non-linear regression (95% CI). Each graph represents the mean and standard deviation (SD) from two independent experiments.
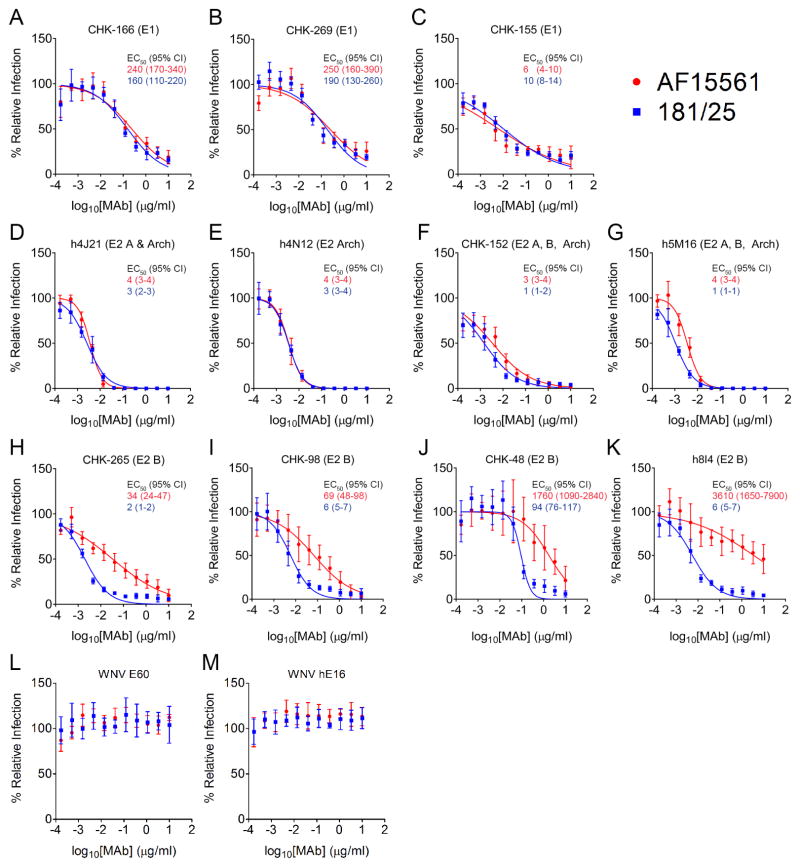
To define the structural basis for differences in neutralization, we tested a panel of recently characterized CHIKV-specific mouse and human monoclonal antibodies (MAbs) with mapped epitopes in E1 and E2 (Fox et al., 2015; Long et al., 2015; Smith et al., 2015; Sun et al., 2013) for their capacity to neutralize AF15561 and 181/25. MAbs recognizing the CHIKV E1 glycoprotein (CHK-166, CHK-269, and CHK-155) or E2 domain A or Arch region (h4J21 and h4N12) (see Figure S1 for domain organization of E2) had similar neutralizing activity against AF15561 and 181/25 (Figure 7A–E). The neutralizing activity of MAbs that bind epitopes spanning E2 domains A and B [CHK-152 (Pal et al., 2013; Sun et al., 2013) and 5M16 (Long et al., 2015)] was reduced modestly against AF15561 compared with 181/25 (2-fold and 3-fold, respectively) (Figure 7F–G). In contrast, mouse and human MAbs recognizing epitopes within the E2 B domain (CHK-265, CHK-98, CHK-48, and h8I4) neutralized AF15561 much less efficiently than 181/25 (17-fold, 11.5-fold, 19-fold and 600-fold respectively) (Figure 7H–K) even though polymorphic E2 residues 12 and 82 are located within the E2 A domain and not within the binding footprint of CHK-265, CHK-98, CHK-48, or h8I4 (Fox et al., 2015; Smith et al., 2015). As expected, West Nile virus-specific mouse and human polyclonal antibodies had no effect on virus infection (Figure 7L–M). These findings, along with the serum neutralization data, suggest that E2 residue 82 influences neutralization of CHIKV, with pathogenic strains evading the inhibitory activity of antibodies targeting the B domain of the E2 glycoprotein.
Figure 7. Differential Neutralization of AF15561 and 181/25 by MAbs Targeting E2 Domain B.
(A–M) MAbs were incubated with 100 FFU of AF15561 (red) or 181/25 (blue) viruses for at 37°C for 1 h. MAb-virus mixtures were added to Vero cells and incubated for 18 h. Virus-infected foci were stained and counted. Wells containing MAbs were compared with wells without MAbs to determine relative infection. (L) WNV E60 and (M) hE16 MAbs were included as isotype control MAbs. The concentrations at which 50% of AF15561 (red text) or 181/25 (blue text) were neutralized (EC50) were determined by non-linear regression and are displayed in ng/ml (95% CI). Each graph represents the mean and standard deviation (SD) from at least two independent experiments.
Discussion
Long-term clinical sequelae are a common outcome of infection with CHIKV and several related alphaviruses (Suhrbier et al., 2012). In this study, we found that in contrast to pathogenic CHIKV strain AF15561, attenuated CHIKV strain 181/25 was cleared from most tissues of infected WT mice within 4 weeks of infection. We reasoned that comparative studies of CHIKV AF15561 and 181/25 infection would provide insight into mechanisms that influence clearance and persistence of CHIKV infection.
Viral Determinants Influence CHIKV Clearance and Persistence
Genomes of CHIKV strains AF15561 and 181/25 differ at five non-synonymous and five synonymous nucleotides positions (Gorchakov et al., 2012). Mutations resulting in amino acid changes in 181/25 are in nsP1 (T301I), E2 (T12I and G82R), 6K (C42P), and E1 (A404V). We found that reversion of the arginine to a glycine at E2 position 82 (181/25E2 R82G) yielded increased viral loads in the contralateral ankle at 28 dpi, suggesting that an arginine at E2 residue 82 restricts CHIKV persistence. Arginine 82 in E2 enhances the affinity of CHIKV particles for glycosaminoglycans (GAGs) in vitro (Silva et al., 2014), which may limit the capacity of CHIKV to disseminate from early sites of primary replication in vivo (Ashbrook et al., 2014; Gardner et al., 2012; Gardner et al., 2014). The extent to which altered interactions with GAGs influences CHIKV clearance remains to be determined, but our data suggest that an arginine at position 82 promotes CHIKV clearance by facilitating neutralization of infectious viral particles by antibodies. An arginine at position 82 also was found to impair cell-to-cell spread of CHIKV in vitro, a mechanism by which CHIKV may evade antibody mediated neutralization; however, this mechanism has not been explored in vivo (Lee et al., 2011). We also found that the amino acid at E2 position 12 contributes to persistence of CHIKV in joint tissue, as mice infected with 181/25 encoding both revertant mutations together (181/25E2 I12T R82G) had increased levels of persistent viral RNA in joint tissue at late times post-infection compared with mice infected with either 181/25E2 R82G or 181/25E2 I12T. Although the mechanism by which a threonine at E2 position 12 enhances viral burden during the persistent phase of infection requires further study, E2 position 12 may stabilize the residue at E2 position 82 (Gorchakov et al., 2012). In addition, for Semliki Forest virus, a related alphavirus, a threonine at E2 position 12 increased the pH threshold of viral fusion (Glomb-Reinmund and Kielian, 1998), which might enhance replication efficiency.
CHIKV 181/25 Persists in Mice Lacking the Capacity to Produce Virus-Specific Antibodies
Pathogenic strains of CHIKV persist in joint tissue of mice, suggesting that adaptive immune responses fail to clear primary infection (Hawman et al., 2013; Poo et al., 2014). In contrast, adaptive immune responses efficiently clear attenuated strain 181/25. Rag1−/− mice infected with 181/25 exhibited a nearly 8,000-fold increase in viral load in joint tissue distal to the site of inoculation at 28 dpi relative to 181/25-infected WT mice. In addition, levels of viral RNA and infectious virus in the contralateral joint tissue of 181/25-infected Rag1−/− mice were similar or greater than those detected in AF15561-infected Rag1−/− mice. Increased titers of infectious virus in joint tissues of 181/25-infected Rag1−/− mice may be due to the enhancement of viral replication within tissues by efficient interactions with GAGs, as has been described for a GAG-binding strain of Sindbis virus (Ryman et al., 2007). Infectious virus recovered from joint tissue of 181/25-infected Rag1−/− mice at 28 dpi had not gained the capacity to establish persistence in WT mice, and reversion of the mutations in the E2 glycoprotein was not detected. Therefore, in mice lacking adaptive immune responses, AF15561 and 181/25 establish persistence in joint tissues with comparable efficiency.
Our findings that Rag1−/− mice infected with 181/25 had detectable viremia at 28 dpi contrast with a previous study in which older 8–10 week old Rag1−/− C57BL/6 mice infected with 181/25 did not have detectable viremia (Seymour et al., 2015). Additionally, we consistently detected infectious virus at 28 dpi in joint tissues of Rag1−/− mice infected with 181/25. In contrast, Seymour et al. did not detect infectious virus at late times post-infection (28–56 dpi) in various tissues of 181/25-infected Rag1−/− mice although joint tissues were not examined. It is possible that the age of mice and specific tissues evaluated in the two studies accounts for the disparate findings. Absence of detectable genetic or phenotypic reversion of 181/25 virus after 4 weeks of viral persistence in the joint tissue of Rag1−/− mice also differs from studies of 181/25-infected WT mice in which rapid reversion of the attenuating mutations in virus present in the circulation and the spleen was detected (Ashbrook et al., 2014; Gorchakov et al., 2012). Deep sequencing of CHIKV from the serum, brain, or kidney of persistently infected Rag1−/− mice revealed small numbers of mutations, suggesting that CHIKV is under less selective pressure in Rag1−/− than WT mice (Poo et al., 2014; Seymour et al., 2015). Collectively, these data suggest that reversion of the attenuating mutations in 181/25 in WT mice likely is driven, at least in part, by adaptive immune responses. Similar to our findings with Rag1−/− mice, 181/25 infection of B cell deficient μMT mice or BCR transgenic mice that have normal B cell numbers but are incapable of producing CHIKV-specific antibody, resulted in persistence of viral RNA in joint tissue at levels similar to those in AF15561-infected WT mice. These findings suggest that CHIKV-specific antibody responses are essential for clearance of 181/25 from joint tissue, and that the pathogenic AF15561 strain evades these clearance mechanisms.
Antibody Responses to Pathogenic and Attenuated CHIKV Strains are Distinct
Characterization of the humoral immune response of WT mice to AF15561 and 181/25 infection revealed differences in the kinetics, magnitude, and quality of the antiviral antibody response. Similar to previous studies (Her et al., 2015; Lum et al., 2013), the anti-CHIKV IgM response peaked at day 5 after AF15561 infection. In comparison, the anti-CHIKV IgM response in 181/25-infected mice was of lower magnitude and peaked slightly later (7 dpi). AF15561 infection of WT mice also induced a rapid CHIKV-specific IgG response, with high antibody levels detected at 7 dpi. In contrast, 181/25-infected mice did not achieve similar CHIKV-specific IgG titers until 14 dpi. Thus, the kinetics and magnitude of the early virus-specific IgM and IgG response did not correlate with relative clearance of AF15561 and 181/25 in joint tissue of WT mice. In addition, characterization of the neutralizing antibody response in AF15561 and 181/25-infected mice revealed three important findings: (1) The neutralization capacity of sera from AF15561-infected and 181/25-infected mice differed when homologous virus was used in the in vitro neutralization assay, with lower neutralizing titers detected in sera from AF15561-infected mice at both 7 and 28 dpi. Given the higher ELISA titers detected in these mice, this difference resulted in a diminished neutralization index; (2) When day 7 sera from AF15561-infected mice was tested against the heterologous 181/25 virus, we detected a lower neutralization index in comparison to day 7 sera from 181/25-infected mice. These findings indicate that the higher levels of CHIKV-specific IgM and IgG antibodies in sera from AF15561-infected mice do not translate to a higher CHIKV-neutralizing capacity, particularly at early times post-infection. Differences in the quality of CHIKV-specific antibody responses could contribute to the difference in clearance of the two viruses in WT mice. Early neutralizing antibody responses in humans protect against acute disease (Yoon et al., 2015), and the appearance of neutralizing IgG3 antibodies in CHIKV-infected patients correlates with reduced chronic CHIKV-induced musculoskeletal disease (Kam et al., 2012); and (3) The neutralization capacity of sera from 181/25-infected mice against AF15561 was lower in comparison to the neutralization capacity of the same sera against the homologous virus. Although sera from AF15561-infected mice did not neutralize 181/25 as efficiently as sera from 181/25-infected mice, sera from AF15561-infected mice neutralized 181/25 to a greater extent than it did AF15561. These findings suggest that differences in the display of neutralizing epitopes in AF15561 and 181/25 virions may contribute to differential inhibition by serum. Consistent with this hypothesis, the neutralization capacity of sera collected from CHIKV-infected mice or humans in Sri Lanka and the Caribbean was enhanced against mutated epidemic CHIKV strains encoding an arginine at E2 position 82.
181/25 is Neutralized More Efficiently by Anti-CHIKV Antibodies Targeting E2 Domain B
To determine the molecular basis underlying differences in antibody-mediated neutralization of 181/25 and AF15561, we performed neutralization assays using a panel of MAbs targeting epitopes in distinct domains of the CHIKV E1 and E2 glycoproteins. MAbs recognizing epitopes in the E1 glycoprotein, E2 domain A, or E2 Arch region had equivalent neutralizing activity against AF15561 and 181/25. Importantly, this finding suggests that differences in PFU-to-antigen ratios of the viruses do not explain the differential neutralization of the two viruses by sera from virus-infected mice. In contrast, several mouse and human MAbs targeting E2 domain B exhibited reduced neutralization capacity against AF15561 compared with 181/25. These findings are consistent with reports that examined the mechanism of neutralization by the E2 B domain specific anti-CHIKV MAb 5F10 (Lee et al., 2011; Porta et al., 2015). Passage of a CHIKV isolate containing an arginine at E2 position 82 under selective pressure of MAb 5F10 selected for a glycine at position 82 (Lee et al., 2011), and structural studies suggested that 5F10 neutralizes CHIKV encoding an arginine at E2 position 82 by locking domain B into a rigid conformation and preventing exposure of the E1 fusion loop (Porta et al., 2015). Although 5F10 binds to CHIKV strains possessing a glycine at E2 position 82, the binding does not inhibit fusion. This mechanism of locking domain B and preventing fusion appears to be common to both mouse and human neutralizing antibodies targeting E2 domain B (Fox et al., 2015; Long et al., 2015; Sun et al., 2013). In our studies, MAb CHK-265, which neutralizes CHIKV by cross-linking E2 domain B to E2 domain A of an adjacent spike (Figure S2) (Fox et al., 2015), exhibited a substantial difference in EC50 values between AF15561 and 181/25. The mutations in 181/25 at positions 12 and 82 of E2 are located in domain A away from the CHK-265 footprint, suggesting that these mutations do not directly influence the capacity of CHK-265 to recognize the virion. Our data support a model proposed by Porta et al. in which pathogenic CHIKV strains possessing the highly conserved E2 glycine 82 retain domain B flexibility despite bound antibody, thus permitting viral fusion and entry (Porta et al., 2015). In contrast, an arginine at E2 residue 82, located within the core of the E2/E1 trimeric spike, introduces repulsive forces that alter the spatial conformation of E2 domain B (Figure S2). This structural perturbation may allow antibodies to lock domain B into a rigid conformation that impedes exposure of the E1 fusion loop and entry of the virus. As the E2 domain B appears to be a dominant immune target in humans and mice (Weger-Lucarelli et al., 2015), the near uniform conservation of E2 glycine 82 in pathogenic strains suggests a general mechanism by which CHIKV evades neutralizing antibody responses. Based on our findings, we conclude that this immune evasion mechanism contributes to the development of viral persistence. In addition, these findings have implications for the selection of virus strains used for vaccine development and for the evaluation of vaccine-induced protective humoral immune responses.
Experimental Procedures
Viruses
Plasmids encoding infectious cDNA clones of CHIKV strains SL15649, AF15561, and 181/25 have been described (Ashbrook et al., 2014; Morrison et al., 2011). Virus stocks were prepared from cDNA clones (Morrison et al., 2011). Viral titers were determined by plaque assays using BHK-21 and Vero cells as described (Hawman et al., 2013).
Mouse Experiments
Animal experiments were performed with the approval of the Institutional Animal Care and Use Committee at the University School of Medicine (Assurance Number: A3269-01). WT, Rag1−/−, and μMT C57BL/6J mice were obtained from the Jackson Laboratory. Tg(IghelMD4)4Ccg/J (MD4tr) C57BL/6J mice, which encode a B cell receptor specific for hen egg lysozyme, were provided by John C. Cambier (University of Colorado School of Medicine). Three-to-four week-old mice were used for all studies. Mice were inoculated in the left rear footpad. Animal husbandry and experiments were performed in accordance with all University of Colorado School of Medicine Institutional Animal Care and Use Committee guidelines. All mouse studies were performed in an animal Biosafety Level 3 laboratory.
Quantification of Viral RNA
Viral RNA in tissues and cell culture supernatants was quantified by RT-qPCR as previously described (Hawman et al., 2013).
Enzyme-Linked Immunosorbent Assay
CHIKV-binding antibodies in mouse sera, were quantified using a virion-based enzyme-linked immunosorbent assay (ELISA). Concentrated virus was adsorbed to a 96-well Immulon 4HBX plate (Thermo Scientific). Serial dilutions of serum were added to the plate, and bound antibody was detected using biotin-conjugated goat anti-mouse IgM or IgG antibodies (Southern Biotech), followed by streptavidin conjugated to horseradish peroxidase (Southern Biotech). Binding was detected using 3,3′,5,5′-tetramethylbenzidine liquid substrate (Sigma). Endpoint titers were defined as the reciprocal of the last dilution to have an absorbance two times greater than background. Blank wells receiving no serum or serum from naive mice were used to quantify background signal.
Plaque and Focus Reduction Neutralization Tests
Neutralizing activity of murine and human serum was quantified using a plaque reduction neutralization test (PRNT). Serum was heat-inactivated and serially diluted. Diluted serum samples were incubated with 50 PFU of challenge virus for at 37°C for 1 hr. Following incubation, remaining infectious virus was quantified by plaque assay using Vero cells. The PRNT50 value was defined as the reciprocal of the last dilution to exhibit < 50% infectivity. MAbs were tested for the capacity to neutralize CHIKV using a focus reduction neutralization test as described previously (Pal et al., 2013).
Statistical Analyses
All data were analyzed using GraphPad Prism 6 software. Data were evaluated for significant differences using either a two-tailed, unpaired t test, a Mann-Whitney test, a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, or by a two-way ANOVA followed by Bonferroni post-test analysis. A P value < 0.05 was considered statistically significant. All differences not indicated as significant had P > 0.05.
Supplementary Material
Highlights.
A highly conserved glycine at E2 residue 82 promotes CHIKV persistence in joints
E2-R82 promotes viral clearance from joints of WT mice but not B cell deficient mice
E2-R82 enhances CHIKV neutralization by human and mouse polyclonal antibodies
Pathogenic CHIKV strains are resistant to neutralization by E2 B domain antibodies
Acknowledgments
This research was supported by Public Health Service grants R01 AI08725 (T.E.M.), R01 AI114816 (J.E.C. and M.S.D.), R01 AI089591 (M.S.D.), and R01 AI123348 (T.S.D., M.S.D., and T.E.M.) from the National Institute of Allergy and Infectious Diseases. D.W.H. and J.M.F were supported by Public Health Service grant T32 AI052066 and T32 AI007172, respectively, from the National Institute of Allergy and Infectious Diseases. A.W.A. was supported by Public Health Service grant T32 HL07751 from the National Heart, Lung, and Blood Institute. We thank Aruna Dharshan De Silva for providing human sera. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Author Contributions
Conceptualization, D.W.H, T.E.M, M.S.D., R.M.T.; Methodology, D.W.H., J.M.F., T.E.M.; Investigation, D.W.H., J.M.F, N.A.M.; Resources, A.W.A., K.S.S., M.S.D., R.M.T., T.S.D., J.E.C; Writing - Original Draft, D.W.H., M.S.D., T.S.D., T.E.M.; Writing – Review and Editing, D.W.H., A.W.A., R.M.T., T.S.D., J.E.C., M.S.D., T.E.M.; Supervision, T.E.M., M.S.D.; Funding acquisition, T.E.M., M.S.D., T.S.D., J.E.C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashbrook AW, Burrack KS, Silva LA, Montgomery SA, Heise MT, Morrison TE, Dermody TS. Residue 82 of the Chikungunya virus E2 attachment protein modulates viral dissemination and arthritis in mice. Journal of virology. 2014;88:12180–12192. doi: 10.1128/JVI.01672-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, Wengling C, Michault A, Paganin F. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:1401–1407. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- Couturier E, Guillemin F, Mura M, Leon L, Virion JM, Letort MJ, De Valk H, Simon F, Vaillant V. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatology. 2012;51:1315–1322. doi: 10.1093/rheumatology/kes015. [DOI] [PubMed] [Google Scholar]
- Fox JM, Long F, Edeling MA, Lin H, van Duijl-Richter MK, Fong RH, Kahle KM, Smit JM, Jin J, Simmons G, et al. Broadly Neutralizing Alphavirus Antibodies Bind an Epitope on E2 and Inhibit Entry and Egress. Cell. 2015;163:1095–1107. doi: 10.1016/j.cell.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CL, Burke CW, Higgs ST, Klimstra WB, Ryman KD. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology. 2012;425:103–112. doi: 10.1016/j.virol.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CL, Hritz J, Sun C, Vanlandingham DL, Song TY, Ghedin E, Higgs S, Klimstra WB, Ryman KD. Deliberate attenuation of chikungunya virus by adaptation to heparan sulfate-dependent infectivity: a model for rational arboviral vaccine design. PLoS neglected tropical diseases. 2014;8:e2719. doi: 10.1371/journal.pntd.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin P, Fianu A, Malvy D, Mussard C, Boussaid K, Rollot O, Michault A, Gauzere BA, Breart G, Favier F. Perceived morbidity and community burden after a Chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC Med. 2011;9:5. doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomb-Reinmund S, Kielian M. fus-1, a pH shift mutant of Semliki Forest virus, acts by altering spike subunit interactions via a mutation in the E2 subunit. Journal of virology. 1998;72:4281–4287. doi: 10.1128/jvi.72.5.4281-4287.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gorchakov R, Wang E, Leal G, Forrester NL, Plante K, Rossi SL, Partidos CD, Adams AP, Seymour RL, Weger J, et al. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. Journal of virology. 2012;86:6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. Journal of virology. 2013;87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her Z, Teng TS, Tan JJ, Teo TH, Kam YW, Lum FM, Lee WW, Gabriel C, Melchiotti R, Andiappan AK, et al. Loss of TLR3 aggravates CHIKV replication and pathology due to an altered virus-specific neutralizing antibody response. EMBO molecular medicine. 2015;7:24–41. doi: 10.15252/emmm.201404459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. Journal of immunology. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- Homann D, Tishon A, Berger D, Weigle W, Von Herrath M, Oldstone MB. Evidence for an Underlying CD4 Helper and CD8 T-Cell Defect in B-Cell-Deficient Mice: Failure To Clear Persistent Virus Infection after Adoptive Immunotherapy with Virus-Specific Memory Cells from uMT/uMT Mice. Journal of virology. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Renia L, Leo YS, Ng LF. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. The Journal of infectious diseases. 2012;205:1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. The Journal of clinical investigation. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Kam YW, Fric J, Malleret B, Koh EG, Prakash C, Huang W, Lee WW, Lin C, Lin RT, et al. Chikungunya virus neutralization antigens and direct cell-to-cell transmission are revealed by human antibody-escape mutants. PLoS pathogens. 2011;7:e1002390. doi: 10.1371/journal.ppat.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Lupton HW. Development of an Attenuated Strain of Chikungunya Virus for Use in Vaccine Production. Vaccine. 1986;4:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- Long F, Fong RH, Austin SK, Chen Z, Klose T, Fokine A, Liu Y, Porta J, Sapparapu G, Akahata W, et al. Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:13898–13903. doi: 10.1073/pnas.1515558112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum FM, Teo TH, Lee WW, Kam YW, Renia L, Ng LF. An essential role of antibodies in the control of Chikungunya virus infection. Journal of immunology. 2013;190:6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Vomaske J, Totonchy T, Kreklywich CN, Haberthur K, Springgay L, Brien JD, Diamond MS, Defilippis VR, Streblow DN. Chikungunya virus infection results in higher and persistent viral replication in aged rhesus macaques due to defects in antiviral immunity. PLoS neglected tropical diseases. 2013;7:e2343. doi: 10.1371/journal.pntd.0002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Aw Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, Kim AH, Diamond MS, Lenschow DJ, Yokoyama WM. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. The American journal of pathology. 2011;178:32–40. doi: 10.1016/j.ajpath.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden S, Huerre M, Riviere JP, Coffey LL, Afonso PV, Mouly V, de Monredon J, Roger JC, El Amrani M, Yvin JL, et al. Human muscle satellite cells as targets of Chikungunya virus infection. PloS one. 2007;2:e527. doi: 10.1371/journal.pone.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO. 2016 http://wwwpahoorg/hq/indexphp?option=com_topics&view=article&id=343&Itemid=40931.
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, et al. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS pathogens. 2013;9:e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo YS, Rudd PA, Gardner J, Wilson JA, Larcher T, Colle MA, Le TT, Nakaya HI, Warrilow D, Allcock R, et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS neglected tropical diseases. 2014;8:e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta J, Mangala Prasad V, Wang CI, Akahata W, Ng LF, Rossmann MG. Structural Studies of Chikungunya Virus-Like Particles Complexed with Human Antibodies: Neutralization and Cell-to-Cell Transmission. Journal of virology. 2015;90:1169–1177. doi: 10.1128/JVI.02364-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman KD, Gardner CL, Burke CW, Meier KC, Thompson JM, Klimstra WB. Heparan sulfate binding can contribute to the neurovirulence of neuroadapted and nonneuroadapted Sindbis viruses. Journal of virology. 2007;81:3563–3573. doi: 10.1128/JVI.02494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, Albert ML, Lecuit M, Michault A. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS neglected tropical diseases. 2013;7:e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour RL, Adams AP, Leal G, Alcorn MD, Weaver SC. A Rodent Model of Chikungunya Virus Infection in RAG1 −/− Mice, with Features of Persistence, for Vaccine Safety Evaluation. PLoS neglected tropical diseases. 2015;9:e0003800. doi: 10.1371/journal.pntd.0003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LA, Khomandiak S, Ashbrook AW, Weller R, Heise MT, Morrison TE, Dermody TS. A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. Journal of virology. 2014;88:2385–2397. doi: 10.1128/JVI.03116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS neglected tropical diseases. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, et al. Isolation and Characterization of Broad and Ultrapotent Human Monoclonal Antibodies with Therapeutic Activity against Chikungunya Virus. Cell host & microbe. 2015;18:86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, La Brooy J. Detection of Viral Ribonucleic Acid and Histological Analysis of Inflamed Synovium in Ross River Virus Infection. Arthritis and rheumatism. 2000;43:365–369. doi: 10.1002/1529-0131(200002)43:2<365::AID-ANR16>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses--an overview. Nature reviews Rheumatology. 2012;8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- Sun S, Xiang Y, Akahata W, Holdaway H, Pal P, Zhang X, Diamond MS, Nabel GJ, Rossmann MG. Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. Elife. 2013;2:e00435. doi: 10.7554/eLife.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, et al. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS pathogens. 2010;6:e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Renia L, Ng LF. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. Journal of immunology. 2013;190:259–269. doi: 10.4049/jimmunol.1202177. [DOI] [PubMed] [Google Scholar]
- Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Forrester NL. Chikungunya: Evolutionary history and recent epidemic spread. Antiviral research. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Weger-Lucarelli J, Aliota MT, Kamlangdee A, Osorio JE. Identifying the Role of E2 Domains on Alphavirus Neutralization and Protective Immune Responses. PLoS neglected tropical diseases. 2015;9:e0004163. doi: 10.1371/journal.pntd.0004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IK, Alera MT, Lago CB, Tac-An IA, Villa D, Fernandez S, Thaisomboonsuk B, Klungthong C, Levy JW, Velasco JM, et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS neglected tropical diseases. 2015;9:e0003764. doi: 10.1371/journal.pntd.0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.