Abstract
Objective
In Mexico cervical cancer (CC) is the most common cause of death from neoplasia in women. Study aimed to analyze the current distribution of Human papillomavirus (HPV) types in women from Nayarit, Mexico, with Squamous intraepithelial lesions (SIL) and Cervical cancer (CC).
Methodology
Between January 2011 and July 2013, cervical samples were collected from female residents of the Mexican state of Nayarit and were analyzed by means of a LINEAR ARRAY® HPV genotyping test. Data analyses were performed using Stata ver. 8.0 statistical software.
Results
Of the samples analyzed, 91.2%, HPV DNA was detected. Of these positive samples, 82% were High-risk (HR) viral types. The most prevalent HPV genotypes identified were 16, 58, 31, 18, and 70. Forty two percent of participants had a single infection, while 23 and 26% of participants were infected with two or more HPV genotypes, respectively. HPV 16 was the most prevalent genotype identified and was frequently present as a co-infection with HPV types 18, 51, 52, 59, 66, or 70.
Conclusion
Women <20 years of age were most often infected with HPV, and the HPV Quadrivalent vaccine (types 16, 18, 6, and 11), currently available in Mexico, no confers protection against a subset of the HPV genotypes identified in the present study (58, 31, 70, and 35). Thus, it is important evaluate the geographical distribution of specific HPV genotypes in all health of center across Mexico in order to implement a successful vaccination program and to diagnose CC in its early stages.
Introduction
Cervical cancer (CC) is the third most common cancer in women and an estimated 530,000 new cases were diagnosed in 2008. (1) More than 80 % of deaths due to CC occur in developing countries, especially in the poorest regions of Southern Asia, sub-Saharan Africa, and Latin America. (2) In Mexico, the risk of dying from CC has remained higher in low income populations; and this is particularly evident in states with a high level of marginalization, including Chiapas, Durango, Guerrero, Nayarit, Oaxaca, Puebla, and Veracruz. (3) Mortality rates for CC in 2008 were as follows: Chiapas, 21.8; Durango, 13.5; Guerrero, 21.2; Nayarit, 13.4; Oaxaca, 21.6; Puebla, 16.1, and Veracruz, 21.6. In addition to sociodemographic and cultural characteristics, others risk factors for CC include first sexual intercourse at an early age, use of oral contraceptives, cervical trauma, certain nutritional factors, and infection with some types of Human Papilloma Virus (HPV). (4)
Molecular evidence indicates that HPV is the main etiologic factor for CC, with HPV detected in 99.7 % of CC cases. (5, 6) Of the almost 200 different HPV types identified to date, 40 have been commonly found in anogenital lesions. (7) On the basis of their oncogenic potential, most of these genital HPV types have been classified according to their risk for causing CC. The high-risk types of HPV are 16, 18, 31, 33, 35, 45, and 58, and these have been implicated in most invasive cancers. (5, 8, 9) The low-risk types of HPV are 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108, and these have been found to cause genital warts, and under certain conditions, mild cervical dysplasia. (10)
The prevalence of cervical infection with HPV varies greatly worldwide and different populations may harbor various HPV genotypes in the genital tract. (11–15) The distribution of HPV types in relation to CC cases has been studied in other countries. However, epidemiological data for specific HPV types in the states of Mexico are limited. In studies that have been published, the following HPV types have been identified: HPV 16; 31; 35; 52, and 33 in San Luis Potosí, Guanajuato, Durango, Guerrero, and the Mexico City Federal District, Veracruz, the State of Mexico, and Morelos. (16–21) It is hypothesized that determining the prevalence of specific HPV types in a region is an important step towards preventing CC. In 2005, among all the states of Mexico, Nayarit had the highest mortality rate for CC, 13.4 %. (22) Since three of the 20 municipalities of Nayarit are registered as having urban poverty, a high, or very high, degree of marginalization, and a high mortality rate from CC, it is important to determine the prevalence of specific HPV types in this state. Therefore, the purpose of this study was to analyze the current distribution of HPV types in women from Nayarit, Mexico with squamous intraepithelial lesions and cervical cancer (CC).
Methods
Ethical aspects
This study was approved by the institutional ethical committee (Register no. 283, Hospital Antonio González Guevara of Tepic). The purpose and procedures of the study were explained to all of the participants, and a written informed consent was obtained from each participant.
Study population
Between January 2011 and July 2013, an HPV prevalence study was conducted among the female residents of Nayarit, Mexico who attended the Hospital Antonio González Guevara of Tepic, Nayarit. This hospital is one of three health institutions in the state where women with CC are treated, and they accept referrals from both public and private health services.
Samples were collected from women with cervical high-grade lesions or CC which were observed by colposcopy. Diagnosis was confirmed by histopathology. Biopsies were evaluated by the Pathologist at each clinic, according to Bethesda Diagnostic Criteria. (23)
Cervical samples were collected with a cytobrush during gynecological examinations. This was inserted into the endocervical canal and then placed into the transport medium (PreservCyt solution; Hologic, Bedford, MA, USA) and stored at 4°C until DNA extraction. After receiving informed consent, the participants received questionnaires to gather information regarding sociodemographic characteristics, anthropometric characteristics, level of education, occupation, lifestyle, family history of cancer, and obstetric and gynecological history.
Clinical specimens
The 55 cervical cytology specimens collected were analyzed for the presence of DNA of Human papillomavirus (HPV). At the time of sample collection for the HPV test samples, cytological examinations were also performed in all women. Lesions were classified as normal cytology, A typical squamous cells of undetermined significance (ASCUS), Low-grade squamous intraepithelial lesion (LSIL), and High-grade squamous intraepithelial lesion (HSIL). All cervical brushings were rinsed into vials containing PreservCyt™ transport medium prior to testing for HPV genotypes.
Linear Array HPV genotyping test
The Linear Array® (LA) HPV Genotyping Test is registered for use in the European Union for detecting 37 high- and low-risk HPV genotypes. The test is based on four major processes: 1) DNA extraction by the AmpliLute Liquid Media Extraction Kit; 2) PCR amplification of target DNA using HPV primers; 3) hybridization of the amplified products into oligonucleotide probes (Linear Array HPV Genotyping Test), and 4) detection of probe-bound amplified products by colorimetric determination (Linear Array Detection Kit). (24, 25) Briefly, using PGMY09/11 primers, a region approximately 450 base pairs in length within the L1 gene of the HPV genome was amplified by PCR. This assay simultaneously amplified a region within the human β globin gene as a control for cell adequacy, nucleic acid extraction, and PCR efficiency. PCR assays were performed in a reaction volume of 100 μl, using 50 μl of LA HPV master mix (Roche Molecular Systems) and 50 μl of DNA. The amplification parameters were: 2 min at 50 °C and 9 min at 95 °C; 40 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min; followed by a final extension at 72 °C for 5 min or until samples were collected. Nucleic acid hybridization using a reverse line blot system was then performed. Briefly, the PCR amplicons were denatured with the addition of 100 μl of LA denaturation reagent (Roche Molecular Systems). After 10 min at room temperature, the denatured amplicons (100 μl) were hybridized and detected using the recommended LA protocol. Thirty-seven anogenital HPV genotypes were detected simultaneously, including 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51–56, 58, 59, 61, 62, 64, 66–73, 81–84, IS39, and CP6108. The LA HPV genotyping strips were visually read using the HPV reference guide provided.
Statistical analysis
Descriptive statistics included mean, median, Standard deviation (SD) and range. Skewness/Kurtosis tests were employed for normal distribution. Frequency, prevalence, distribution, and comparisons among HPV genotypes, injury type, and age relatedness were evaluated by the Mann-Whitney U test. Differences between age relatedness and the virus and lesion types were evaluated by the Kruskal-Wallis test. P values <0.05 were considered statistically significant. Statistical analyses were conducted using the Stata ver. 8.0 statistical software program (Stata Corporation, College Station, TX, USA).
Results
Sociodemographic and gynecologic data
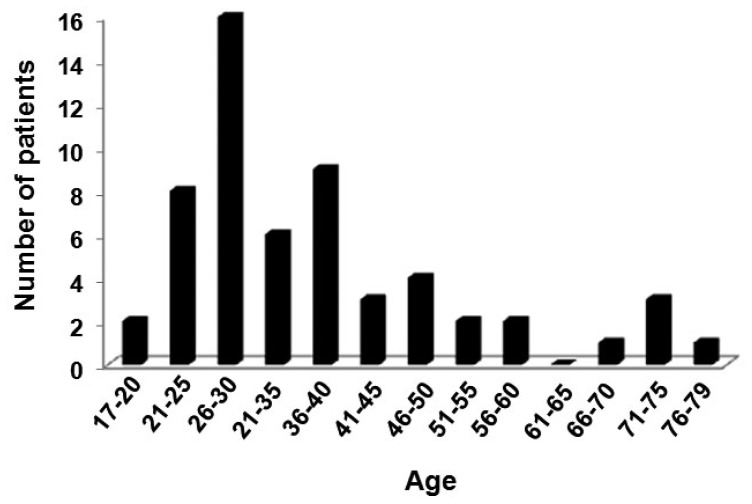
Twenty-six (47.3%) patients with atypical squamous cells of uncertain significance (ASCUS), 24 (43.6%) patients with low-grade squamous intraepithelial lesion (LSIL) and 5 (9.1%) patients with high grade (HSIL) were enrolled in this study. These patients ranged in age from 16 to 75 years (geometric mean = 34), and the age group from 26–30 years had the greatest number of cases (Figure 1).
Figure 1.
Number of patients according to age group. *P = 0.0001, value obtained by Mann-Whitney U test.
Demographic and gynecologic data for the present cohort are summarized in Table 1. The average body mass index (BMI) value was 26.5 ± 4.3, according to the BMI standards proposed by the World Health Organization (WHO).(26) Overall, 52% of the patients were classified as pre-obese or obese, and only 35% of the patients reported performing some type of exercise for an average of 5.7 h a week (range = 1–14).
Table 1.
Demographic and gynecologic characteristics of the study population.
| Characteristics | n | % |
|---|---|---|
| Education level | ||
| Illiterate | 8 | 14 |
| Elementary - Incomplete | 3 | 5 |
| Elementary - Complete | 10 | 18 |
| Middle School | 25 | 43 |
| High School | 6 | 11 |
| University/Professional | 5 | 9 |
| Occupation | ||
| Housewife | 41 | 72 |
| Student | 2 | 4 |
| Employed | 11 | 19 |
| Other | 3 | 5 |
| Civil Status | ||
| Single | 9 | 16 |
| Married | 19 | 33 |
| Widow | 2 | 4 |
| Divorced | 3 | 5 |
| Co-habitating | 24 | 42 |
| Alcoholism | ||
| Yes | 19 | 33 |
| No | 38 | 67 |
| Smoker | ||
| Yes | 13 | 23 |
| No | 44 | 77 |
| First menstruation | 12.9 ± 1.43 y | |
| First intercourse | 16.8 ± 2.5 y | |
| Body Mass Index | 26.5 ± 4.3 y | |
| Sexual partners | 2.5 ± 2.3 y | |
| Pregnancies | 4 ± 3 | |
| Abortions | ||
| Yes | 19 | 33 |
| No | 38 | 67 |
| Papanicolaou test | ||
| Yes | 48 | 79 |
| No | 12 | 21 |
The average age of menarche was 12 years. The mean age at first sexual intercourse was 16 years (range = 13–27), the mean number of sexual partners was 2.5 (range = 1–15), and the average age at first pregnancy was 18 years (range = 14–29). The use of hormonal contraceptives was reported by 60% of the patients. The most commonly used contraceptive was injections of progesterone, and these were maintained for more than a year in some cases. Thirty-three percent of patients used a condom during intercourse. While 45 (79%) patients reported having had a previous Pap smear performed, the frequency of these screenings was found to be once every 1.5 years. There were 2 (4.4%) patients that reported only having a Pap smear test once in 6 years.
Statistical analysis do not show positive correlation between type lesion type and average age at menarche, first sexual intercourse, number of sexual partners, age at first pregnancy, and use of hormonal contraceptives.
HPV Genotyping
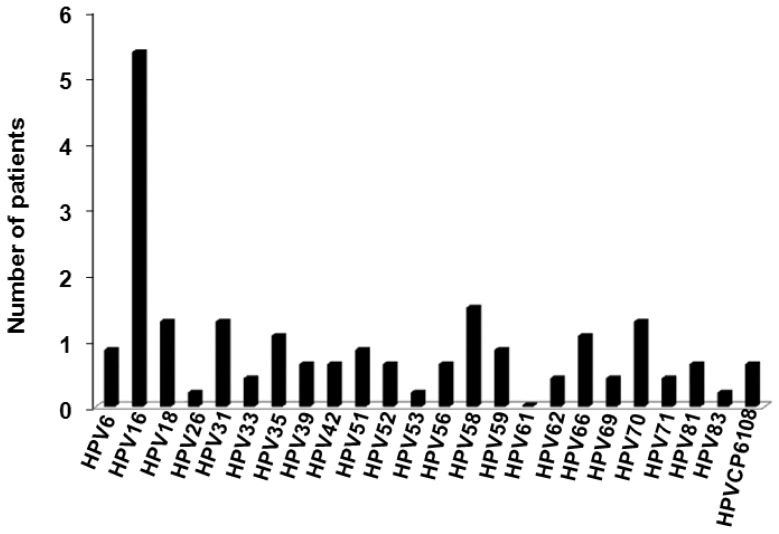
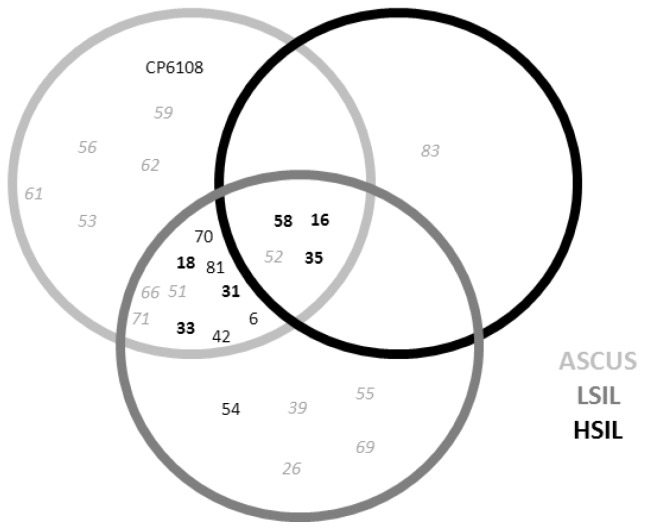
In 92.73% of the samples analyzed, HPV DNA was detected. Moreover, 72.73% of these HPV-positive samples included High-risk (HR) viral types (Figure 2). Women aged 26–30 years had the highest percentage of viral infection, followed by women aged 36–40 years and women 21–25 years of age. As depicted in Figure 2, the most prevalent HPV genotypes identified were 16, 58, 31, 18, and 70. HPV 16 was present in 43.6% of the samples analyzed, while 16, 35, 58, and 52 were present in all lesion types (Figure 3).
Figure 2.
Viral Human papillomavirus (HPV) types in samples from patients with cervical lesion.
Figure 3.
Human papillomavirus (HPV) genotypes present according to cytologic results. ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion.
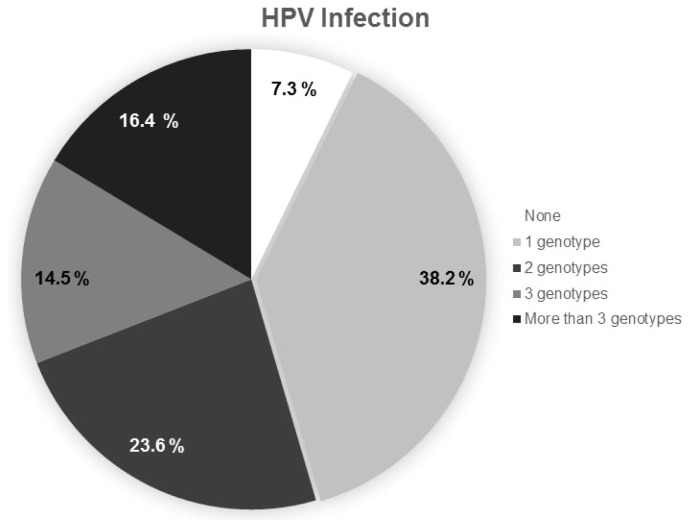
Furthermore, 38.2% of participants presented with a single HPV infection, while 23.6% of the patients exhibited co-infection with two HPV genotypes, while 30.9% of patients were infected with three or more HPV genotypes (Figure 4). Furthermore, a marginal relationship was observed between lesion type and age (p = 0.08).
Figure 4.
Percentage of samples infected with 1, 2, or 3 or more Human papillomavirus (HPV) genotypes (n = 55).
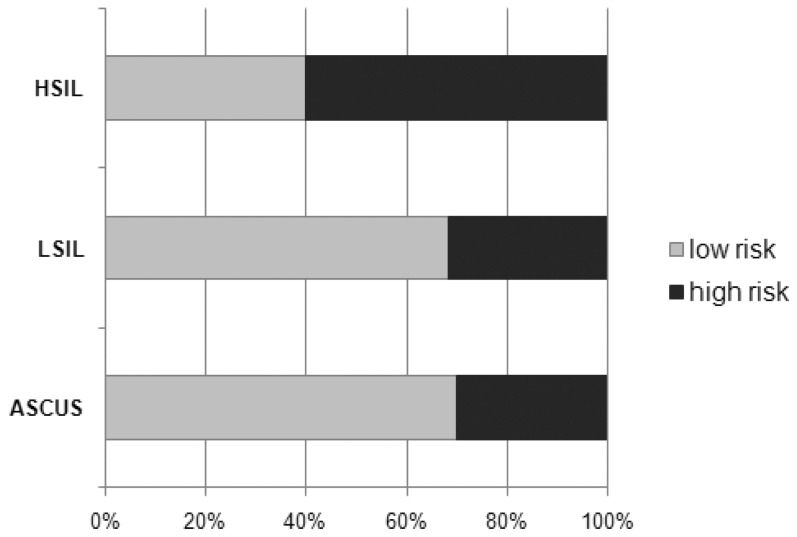
Percentages of an HR genotype HPV with lesion type revealed 30% of women with ASCUS, 31.6% with LISIL, and 60% of women with HSIL (Figure 5).
Figure 5.
Percentage of High (HR)- and Low-risk (LR) Human papillomavirus (HPV) genotypes present according to cytologic results. ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion. *P = 0.4366 = value obtained by Mann-Whitney U test.
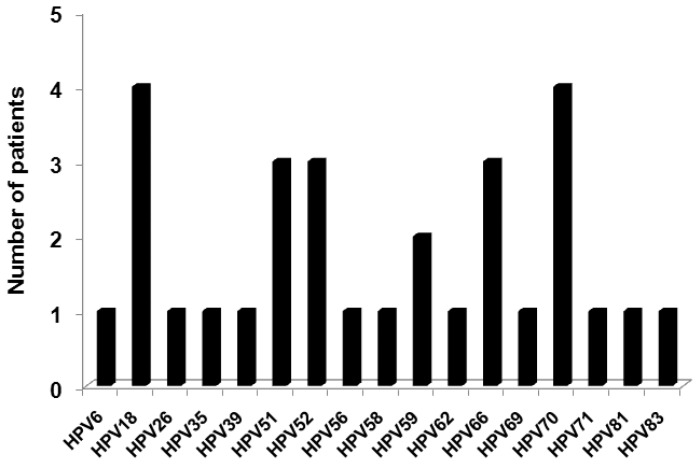
Because HPV 16 was the most prevalent genotype identified, we analyzed which HPV genotypes were more frequently present as co-infections with HPV 16. These genotypes included 18, 51, 52, 59, 66, and 70 (Figure 6).
Figure 6.
Specific Human papillomavirus (HPV) types present in those co-infected with HPV 16 in samples from patients (n = 28).
Discussion
CC is the second most frequent cancer affecting women worldwide. Cervical cancer remains the number one cause of mortality due to malignant neoplasm among 20 to 40-year-old women in Latin America, and the third most common cause of cancer death in females. (27) In Mexico, the incidence and mortality of CC has grown in recent decades, and CC has remained the leading cause of death from neoplasia for women over 25 years of age. (28–31) CC predominantly affects lower social classes. Ward et al. reported the incidence of various cancer types for both men and women, the 5-year survival rates for those in more affluent tracts were 10 percentage points higher than the rates observed for individuals living in poorer census tracts. (32) In Mexico, the incidence of CC has been linked to lack of an effective screening program and to delays in the time to diagnosis. Limited access to screening tests and healthcare services in general are additional considerations for women living in rural areas of Mexico. (32, 40)
In the present study, 92.8 % of the patients started having sexual intercourse before the age of 20 years, and 49 % used hormonal contraceptives for more than a year. In other studies, the start of sexual intercourse at an early age was associated with a high rate of HPV infection because these individuals have a longer exposure time to HPV and the transformation zone of the cervical epithelium is still immature. The transformation zone has been found to be more proliferative during adolescence and to be more susceptible to infections. (17, 33–36) In addition, the use of hormonal contraceptives for five or more years has been found to be a cofactor that increases the risk of CC up to 4-fold among women who are carriers of HPV infection. (5) Epidemiologic studies have showed that HPV infection is a major risk factor for CC, with the relationship between CC and HPV infection being more evident than that between lung cancer and smoking. (37–40) Based on this relationship, it is important to identify the specific types of HPV that are present in various geographical regions in order to implement effective programs for CC prevention.
The prevalence of HPV infection in the present study was 92.73%, and it was highest among women younger than 20 years of age. These results are consistent with those of other studies (13–15, 24–25) and with the natural history of HPV infection which is characterized by higher infection rates after sexual initiation.
In the State of Yucatán in southeastern Mexico, HPV 58 is the most frequent type of HPV detected, followed by HPV types 16, 33, and 18. (41) Similarly, in the states of San Luis Potosí and Guanajuato, and in the Mexico City Federal District, HPV 16 is most frequently detected. (19, 41, 42) Interestingly, HPV 58 was one of the most common HPV types identified in the current study, and it has also been identified in others populations; (43, 32) however, this genotype is uncommon and is not frequently reported. In addition, the percentage of cases involving HPV co-infection was 49 %, and these cases typically involved HPV types 16, 18, 31, 35, 58, and 70. It remains unclear whether co-infection with multiple types of HPV increases the risk of progression to cancer. (44–46)
Epidemiological data related to the prevalence of specific HPV types in the states of Mexico currently includes only six types (16, 31, 18, 35, 52, and 33), and these were detected by universal PCR primers and hybrid capture. (16–21) To our knowledge, this is the first study to investigate the specific type of HPV infections that are present in women from Nayarit, Mexico. Linear Array HPV genotyping was used and this technique has a high sensitivity and specificity for distinguishing 37 different genotypes of HPV. Thus, Linear Array HPV genotyping is a valuable technique for epidemiological studies and clinical trials that monitor HPV infections in cervical samples. (24) Using this diagnostic method, it is also possible to determine whether multiple HPV infections are present in a single sample, and whether recurrence or persistence is associated with a given genotype. Linear Array HPV genotyping can also be used to evaluate whether persistence of high-risk HPV is an important predictor of CC. (25, 47, 48)
The present results and those of previous studies suggest that the current quadrivalent HPV vaccines (directed against HPV 16, 18, 6, and 11) that are provided by health service institutions in Mexico should be equally applicable to all Mexican women. However, this vaccine confers no protection against the other HPV genotypes that were found to have a high prevalence in women from Nayarit in the present study (HPV 58, 31, 70, and 35). Therefore, it is necessary to determine the geographical distribution of specific HPV types among all of the states of Mexico in order to implement a successful vaccination program and for the effective development of new vaccines.
Currently, the Pap smear technique is routinely employed for a diagnosis of CC, with a colposcopy providing confirmation of the diagnosis. However, even with the high prevalence of HPV infection in cases of CC, both cytology and HPV-DNA tests should be performed for the screening of HPV-related lesions. Currently, DNA sequencing is considered the gold standard for detecting HPV types, although it does not detect the presence of multiple infections because only the dominant genotype is identified. (38, 24, 25) Thus, PCR-based techniques need to be complemented with more specific routine molecular tests which will not only detect the presence of HPV, but will also determine the type or types of HPV that are present. These changes have the potential to improve our ability to provide a diagnosis with direct clinical and public health implications.
A limitation of the present study is that the sample population studied may not be entirely representative of Nayarit’s females because the women who participated in the study were those that were seen at the Antonio González Guevara Hospital of Tepic, Nayarit. In addition, another limitation may have been a small sample size, and this may have affected the HPV genotyping frequency observed.
In summary, this preliminary study concerning HPV types present in a subpopulation of women residing in Nayarit needs to be expanded to include a larger sample size population, and this would be of great value to the Health Secretary of Mexico. However, the present results should encourage the implementation, strengthening, and promotion of CC screening programs specifically in Nayarit, where coverage, particularly in some rural areas, are still lower than in the rest of the country.
Conclusion
Women <20 years of age were most often infected with HPV, and the HPV Quadrivalent vaccine (types 16, 18, 6, and 11), currently available in Mexico, no confers protection against a subset of the HPV genotypes identified in the present study (58, 31, 70, and 35). Thus, it is important evaluate the geographical distribution of specific HPV genotypes in all health of center across Mexico in order to implement a successful vaccination program and to diagnose CC in its early stages.
Acknowledgements
We thank all the patients who participated in our study and all physicians involved in the enrollment of patients.
Footnotes
Conflict of Interest
The Authors declare that there are no conflicts of interest.
References
- 1.International Agency of Research in Cancer (IARC) Estimated Cancer incidence, mortality and prevalence WorldWide in 2012. [On line. Accessed on 12.11.2014]. From http://globocan.iarc.fr.
- 2.International Agency of Research in Cancer (IARC) Cervical Cancer. [On line. Accessed on 13.12.2014]. From http://screening.iarc.fr/cervicalindex.php.
- 3.Dirección General de Evaluación del Desempeño. Desempeño de los sistemas de salud. [On line. Accessed on 10.11.2014]. From: http://www.dged.salud.gob.mx/contenidos/dedss/rcs.html.
- 4.Tovar-Guzmán V, Hernández-Girón C, Lazcano-Ponce E, Romieu I, Hernández Avila M. Breast cancer in Mexican women: an epidemiological study with cervical cancer control. Rev Saude Publica. 2000;34:113–19. doi: 10.1590/s0034-89102000000200003. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piña-Sánchez P, Hernández-Hernández DM, López-Romero R, Vázquez-Ortíz G, Pérez-Plasencia C, Lizano-Soberón M, et al. Human papillomavirus–specific viral types are common in Mexican women affected by cervical lesions. Int J Gynecol Cancer. 2006;16:1041–47. doi: 10.1111/j.1525-1438.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 7.De la Cruz-Hernández E, García-Carrancá A, Mohar-Betancourt A, Dueñas-González A, Contreras-Paredes A, Pérez-Cardenas E, et al. Differential splicing of E6 within human papillomavirus type 18 variants and functional consequences. J Gen Virol. 2005;86:2459–68. doi: 10.1099/vir.0.80945-0. [DOI] [PubMed] [Google Scholar]
- 8.Walboomers JMM, Jacobs MV, Manos MM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Dalstein V, Riethmuller D, Prétet JL, Le Bail Carval K, Sautière JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 10.Salih MM, Safi ME, Hart K, Tobi K, Adam I. Genotypes of human papilloma virus in Sudanese women with cervical pathology. Infect Agent Cancer. 2010;5:26. doi: 10.1186/1750-9378-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illades-Aguiar B, Alarcón-Romero LC, Antonio-Véjar V, Zamudio-López N, Sales-Linares N, Flores-Alfaro E, et al. Prevalence and distribution of human papillomavirus types in cervical cancer, squamous intraepithelial lesions, and with no intraepithelial lesions in women from Southern Mexico. Gynecol Oncol. 2010;117:291–96. doi: 10.1016/j.ygyno.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Deluca GD, Basiletti J, González JV, Díaz Vásquez N, Lucero RH, Picconi MA. Human papilloma virus risk factors for infection and genotype distribution in aboriginal women from Northern Argentina. Medicina (B Aires) 2012;72:461–66. [PubMed] [Google Scholar]
- 13.Zohoncon TM, Bisseye C, Djigma FW, Yonli AT, Compaore TR, Sagna T, et al. Prevalence of HPV High-Risk Genotypes in Three Cohorts of Women in Ouagadougou (Burkina Faso) Mediterr J Hematol Infect Dis. 2013;5(1):e2013059. doi: 10.4084/MJHID.2013.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghshenas M, Golini-Moghaddam T, Rafiei A, Emadeian O, Shykhpour A, Ashrafi GH. Prevalence and type distribution of high-risk human papillomavirus in patients with cervical cancer: a population-based study. Infect Agent Cancer. 2013;8:1–6. doi: 10.1186/1750-9378-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezechi OC, Pettersson KO, Okolo CA, Ujah IA, Ostergren PO. The association between HIV infection, antiretroviral therapy and cervical squamous intraepithelial lesions in South Western Nigerian women. PLoS One. 2014;14:1–10. doi: 10.1371/journal.pone.0097150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrillo A, Mohar A, Meneses A, Frías-Mendivil M, Solorza G, Lizano M. Utilidad en la Combinación de Oligonucleótidos Universales para la Detección del Virus del Papiloma Humano en Cáncer Cervicouterino y Lesiones Premalignas. Sal Púb Mex. 2004;46:7–15. doi: 10.1590/s0036-36342004000100002. [DOI] [PubMed] [Google Scholar]
- 17.Tirado-Gómez LL, Mohar-Betancourt A, López-Cervantes M, García-Carrancá A, Franco-Marina F, Borges G. Factores de riesgo de cáncer cérvicouterino invasor en mujeres mexicanas. Sal Púb Mex. 2005;47:342–50. doi: 10.1590/s0036-36342005000500004. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Anguiano LF, Alvarado-Esquivel C, Reyes-Romero MA, Carrera-Rodríguez M. Human papillomavirus infections in women seeking cervical Papanicolaou cytology of Durango, Mexico: prevalence and Genotypes. BMC Infect Dis. 2006;6:27. doi: 10.1186/1471-2334-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Revilla R, Martínez-Contreras LA, Sánchez-Garza M. Prevalence of high-risk human papillomavirus types in Mexican women with cervical intraepithelial neoplasia and invasive carcinoma. Infec Agents and Cancer. 2008;3:3. doi: 10.1186/1750-9378-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illades-Aguiar B, Cortés-Malagón EM, Antonio-Véjar V, Zamudio-López N, Alarcón-Romero LC, Fernández-Tilapa G, et al. Cervical carcinoma in Southern Mexico: Human papillomavirus and cofactors. Cancer Detect Prev. 2009;32(4):300–7. doi: 10.1016/j.cdp.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.López-Rivera MG, Medel-Flores MO, Villalba-Magdaleno JDA, Sánchez-Monroy V. Prevalence of Human Papillomavirus in Women from Mexico City. Infect Dis Obstet Gynecol. 2012;2012:384758. doi: 10.1155/2012/384758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sistema Nacional de Información en Salud. Principales causas de mortalidad en mujeres (SINAIS) [On line. Accessed on 12.11.2014]. From: http://www.sinais.salud.gob.m.
- 23.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–19. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 24.Stevens MP, Rudland E, Garland SM, Tabrizi SN. Assessment of MagNA pure LC extraction system for detection of human papillomavirus (HPV) DNA in PreservCyt samples by the Roche AMPLICOR and LINEAR ARRAY HPV tests. J Clin Microbiol. 2006;44:2428–433. doi: 10.1128/JCM.02608-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshiol J, Dunn ST, Walker JL, Zuna RE, Schiffman M, Sherman ME, et al. Reproducibility of linear array for human papillomavirus genotyping. J Clin Microbiol. 2013;51:625–28. doi: 10.1128/JCM.03036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organización mundial de la salud (WHO) Global status report on noncommunicable diseases 2010. [On line. Accessed on 17.10.2014]. From: http://www.who.int/nmh/publications/ncd_report_full_en.pdf.
- 27.International Agency for Research on Cancer (IARC) Cancer Incidence and Mortality Worldwide (GLOBOCAN 2012) [On line. Accessed on 12.11.2014]. From http://globocaniarc.fr/
- 28.Capote Negrín LG. Epidemiology of cervical cancer in Latin America. Ecancer medicalscience. 2015 doi: 10.3332/ecancer.2015.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Instituto Nacional de Estadística y Geografía (INEGI) InfoCancer. 2009 and 2013. [On line. Accessed on 12.11.2014]. From: http://www.inegi.org.mx/inegi/contenidos/espanol/prensa/./2013/cancer0.doc.
- 30.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 31.Anaya-Ruiz M1, Vincent AK, Perez-Santos M. Cervical cancer trends in Mexico: incidence, mortality and research output. Asian Pac J Cancer Prev. 2014;15:8689–92. doi: 10.7314/apjcp.2014.15.20.8689. [DOI] [PubMed] [Google Scholar]
- 32.Palacio-Mejía LS, Lazcano-Ponce E, Allen-Leigh B, Hernández-Avila M. Diferencias regionales en la mortalidad por cáncer de mama y cérvix en México entre 1979 y 2006. Sal Pub Mex. 2009;51:208–19. doi: 10.1590/s0036-36342009000800011. [DOI] [PubMed] [Google Scholar]
- 33.Castañeda-Iñiguez MS, Toledo-Cisneros R, Aguilera-Delgadillo M. Factores de riesgo para cáncer cervicouterino en mujeres de Zacatecas. Sal Pub Mex. 1998;40:330–38. [PubMed] [Google Scholar]
- 34.Muñoz N. Human papillomavirus and cancer: the epidemiological evidence. J ClinVirol. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 35.Yang EJ, Quick MC, Hanamornroongruang S, Lai K, Doyle LA, et al. Microanatomy of the cervical and anorectal squamocolumnar junctions: a proposed model for anatomical differences in HPV-related cancer risk. Mod Pathol. 2015;28:994–1000. doi: 10.1038/modpathol.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michala L, Argyri E, Tsimplaki E, Tsitsika A, Bakoula C, Antsaklis A, Panotopoulou E. Human Papilloma Virus infection in sexually active adolescent girls. Gynecol Oncol. 2012;126:207–10. doi: 10.1016/j.ygyno.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Priya R, Prabhu D, Jayalekshmi, Radhakrishna P. Lung Cancer and Human Papilloma Viruses (HPVs): Examining the Molecular Evidence. Review Article. Journal of Oncology. 2012. ID 750270. http://dx.doi.org/10.1155/2012/750270. [DOI] [PMC free article] [PubMed]
- 38.Akcali S, Goker A, Ecemis T, Kandiloglu AR, Sanlidag T. Human papilloma virus frequency and genotype distribution in a Turkish population. Asian Pac J Cancer Prev. 2013;14:503–6. doi: 10.7314/apjcp.2013.14.1.503. [DOI] [PubMed] [Google Scholar]
- 39.Estudio Internacional de Biología de Cáncer Cervical. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;9:1–16. doi: 10.1016/j.vaccine.2008.05.064. 2003 From: http://ntp-server.niehs.nih.gov./ntp/newhomeroc/roc11/HPV_RG2_Public.pdf. [DOI] [PubMed] [Google Scholar]
- 40.Deleré Y, Remschmidt C, Leuschner J, Schuster M, Fesenfeld M, Schneider A, et al. Human Papillomavirus prevalence and probable first effects of vaccination in 20 to 25 year-old women in Germany: a population-based cross-sectional study via home-based self-sampling. BMC Infect Dis. 2014;14:87. doi: 10.1186/1471-2334-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-Losa MR, Rosado-López I, Valdez-González N, Puerto-Solís M. High prevalence of human papillomavirus type 58 in Mexican colposcopy patients. J Clin Virol. 2004;29:202–5. doi: 10.1016/S1386-6532(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 42.Torroella-Kouri M, Morsberger S, Carrillo A, Mohar A, Meneses A, Ibarra M, et al. HPV prevalence among Mexican women with neoplastic and normal cervixes. Gynecol Oncol. 1998;70:115–20. doi: 10.1006/gyno.1998.5055. [DOI] [PubMed] [Google Scholar]
- 43.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 44.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–59. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 45.Ermel A, Qadadri B, Morishita A, Miyagawa I, Yamazaki G, Weaver B, et al. Human papillomavirus detection and typing in thin prep cervical cytologic specimens comparing the Digene Hybrid Capture II Assay, the Roche Linear Array HPV Genotyping Assay, and the Kurabo GeneSquare Microarray Assay. J Virol Methods. 2010;169:154–61. doi: 10.1016/j.jviromet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Coutlée F, Rouleau D, Petignat P, Ghattas G, Kornegay JR, Schlag P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J Clin Microbiol. 2006;44:1998–2006. doi: 10.1128/JCM.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safaeian M, Solomon D, Castle PE. Cervical cancer prevention-cervical screening: science in evolution. Obstet Gynecol Clin North Am. 2007;34:739–60. doi: 10.1016/j.ogc.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinchai T, Chansaenroj J, Junyangdikul P, Swangvaree S, Karalak A, Niruthisard S, et al. Comparison between direct sequencing and INNO-LiPA methods for HPV detection and genotyping in Thai Women. Asian Pac J Cancer Prev. 2011;12:989–94. [PubMed] [Google Scholar]