Abstract
Background
Cement dust contains heavy metals like nickel, cobalt, lead and chromium, pollutants hazardous to the biotic environment, with adverse impact for vegetation, human and animal health and ecosystems.
Objective
To investigate if long term exposure to cement dust can affect the periodontal health and affect the outcome of non-surgical periodontal therapy.
Methods
A total of sixty subjects were included in this study. Forty patients with chronic periodontitis were grouped into; Group I comprised of 20 patients with chronic periodontitis working in the Portland Cement Company and Group II comprised of 20 patients with chronic periodontitis who does not work in cement factories nor live near any of them. Twenty healthy subjects were included in this study as healthy control group (Group III). Clinical parameters including gingival index (GI), plaque index (PI), pocket depth (PD) and clinical attachment loss (CLA) were scored for all patients before and after periodontal therapy. All patients received non-surgical periodontal therapy together with strict oral hygiene program for one month. Gingival crevicular fluid (GCF) samples were collected from both groups at baseline and one month after periodontal therapy. Real time PCR (RT-PCR) was used to analyze the GCF samples for detection and assessment of the levels of IL-1β and TNFα.
Results
The two studied groups responded well to non-surgical periodontal treatment and there was no significant difference between GI and GII (P>0.05). The levels of TNFα was higher in GI than in GII before and after periodontal therapy (P<0.05). The levels of IL-1β did not show any significant difference between the two groups at base line (P>0.05), but represented with a highly significant difference between G1 and GII after periodontal therapy (P<0.001). A significant positive correlation was found between the levels of both IL-1β and TNFα and all the clinical parameters in GI before and after periodontal therapy and in GII before periodontal therapy (P<0.05).
Conclusion
It seems that long term exposure to cement dust does not affect the clinical outcome of non-surgical periodontal treatment but it affects the levels of the pro-inflammatory mediators leading to more periodontal tissue destruction.
Keywords: Portland cement, Chronic periodontitis, Non-surgical treatment
Introduction
Studies have shown that adverse respiratory health effects were seen in people exposed to cement dust, exemplified in increased frequency of respiratory symptoms and decreased ventilatory function and is most commonly observed among cement workers and could not be explained by age, body mass index (BMI) or smoking, thus are likely to be caused by exposure to cement dust. (1)
Portland Cement is a mixture of Calcium oxide (CaO) (62% – 66%), Silicon oxide (SiO2) (19% – 22%), Aluminum tri-oxide (AL2O3) (4%–8%), Ferric oxide (Fe2O3) (2% – 5%), Magnesium oxide (MgO) (1% – 2%) also in addition to Selenium, Thallium and other impurities.(2) Cement dust also contains heavy metals like nickel, cobalt, lead and chromium, pollutants hazardous to the biotic environment with adverse impact for vegetation, human and animal health and ecosystems. (3)
The population most exposed to cement dust pollution includes workers and managers in cement plants and factories, families of workers and managers living in staff houses of factories and other neighborhood habitations. Children studying in the schools situated in proximity to factories are particularly prone to cement dust exposure. Cement dust irritates skin, mucous membrane of the eyes and the respiratory system. Its deposition in the respiratory tract causes a basic reaction leading to increased pH values that irritates the exposed mucous membranes. (4)
Occupational cement dust exposure has been associated with an increased risk of liver abnormalities, pulmonary disorders and carcinogenesis. Decreased antioxidant capacity and increased plasma lipid peroxidation have been posed as possible causal mechanisms of disease. (5) Chronic exposure to Portland cement dust has been reported to lead to a greater prevalence of chronic respiratory symptoms and a reduction of ventilatory capacity. The seriousness of pulmonary function impairment and respiratory disease has not been consistently associated with the degree of exposure. (6)
Inhalable dust concentrations in cement production plants, especially during cleaning tasks, are usually considerably higher than at the construction site. (7) People at cement dust zone area are usually badly affected by respiratory problems; gastrointestinal diseases etc. (6) The observed acute respiratory health effects among the workers are most likely due to exposure to high concentrations of irritant cement dust. The results also highlight the usefulness of the questionnaire for health surveillance of the acute respiratory health effect. (8)
The chromosomal damage was more pronounced in the cement workers who are also smokers when compared with the non-smokers both in control and exposed groups. A significant increase in the frequency of chromosomal aberrations was also observed with increase in age in both control and exposed subjects. (9) There is good evidence for cement dust exposure acting similarly to tobacco, alcohol and asbestos as independent risk factor for laryngeal carcinoma. (10)
The main route of entry of cement dust particles into the body is the respiratory tract and/or the gastrointestinal tract by inhalation or swallowing respectively. Both routes, especially the respiratory tract, are exposed to numerous potentially harmful substances in the cement mill environment. (11) Struzak and Bozyk (12) observed the condition of the oral mucosa in workers of cement plant, where clinical examination demonstrated features of mechanical trauma and oral mucosal inflammation in all workers exposed to cement dust.
Tuominen (13) observed that the effect of cement and stone dust on teeth, the tooth surface loss was higher (72.2%) in exposed workers than controls (48.4%). In both the maxillae and the mandible, the amount of tooth surface loss was greater in exposed workers than in the controls and both anterior and posterior teeth were affected. These findings indicated that tooth surface loss caused by work-related dust should be considered as an occupational hazard.
Bozyk and Owczarek (14) demonstrated that the intensity of the periodontal disease was greater in workers exposed to cement dust than in that in controls and that a very high incidence of deep periodontitis was noted in young workers of the cement plant. Petersen and Henmar (15) evaluated the oral health condition of workers in the stone work industry and described the prevalence and severity of dental diseases, where they reported that workers exposed to dust revealed a high prevalence of decayed, missing and filled surfaces (DMF), along with poor periodontal conditions. They also reported cases of teeth with severe gingivitis, calculus and pockets deeper than 5 mm. The prevalence of dental abrasion was 100% in particular and abrasions were observed on the front teeth. However, the severity of abrasions and the affection ratio increased by exposure duration to dust.
Periodontitis represents a chronic oral infection that leads to gingival inflammation, destruction of tooth supporting tissues, namely periodontal ligament and alveolar bone and eventual exfoliation of teeth. The etiological agents for periodontitis are the Gram negative, anaerobic micro-organisms present within the dental plaque. These micro-organisms produce endotoxins in the form of lipopolysaccharides (LPS) that are instrumental in generating a host-mediated immune response. When LPS gain access to the gingival tissue they stimulate an inflammatory response characterized by infiltration of neutrophils, lymphocytes, macrophages and mast cells. (16) The net effect of this stimulation is the production of Interleukin-I α and β (IL-I α and β), Tumor Necrosis Factor α (TNF- α), IL-6 (all of which can stimulate bone resorption) and matrix metalloproteinases (a group of calcium & zinc dependent enzymes) which digest collagen. Thus, it is this host-mediated immune response that eventually leads to periodontal tissue destruction. (17)
The objective of this study was to explore if long term exposure to cement dust can affect the periodontal health and affect the outcome of non-surgical periodontal therapy.
Methodology
1. Subjects
This study was conducted on 60 adult males suffering from moderate chronic periodontitis, twenty of them were selected from the workers of the Portland Cement Company – Helwan – Cairo – Egypt with minimum of 5 years working period in the company. These subjects were grouped into two groups. The selection and grouping of the subjects was based on the classification of periodontal disease by the international workshop for classification of periodontal diseases and conditions in 1999. (18) Group I comprised of 20 patients suffering from chronic periodontitis and working in the Portland cement company, Group II, comprised of 20 patients suffering from chronic periodontitis that did not work or even live near any cement company. Twenty healthy volunteer subjects not working in cement factories were also included in the study as a control group (Group III).
2. Inclusion Criteria
1) Presence of at least 10 periodontal pockets with minimum depth of 5mm (for chronic periodontitis patients), 2)Non-smokers, 3)No history of antibiotic therapy during the preceding 6 months, 4) Patients should be systemically free and 5) Age of the patients ranges from 27–51 years.
3. Informed Consent
All patients were informed about the nature and objectives of the study and their full signed consent were obtained prior to inclusion into the study. The study complied with the rules set by the International Conference on Harmonization of Good Clinical Practice Guidelines, and the Declaration of Helsinki.
4. Clinical Parameters
For each patient the following parameters were recorded: Plaque Index (PI), (19) Gingival index (GI), (20) Probing pocket depth (PD), Measuring the clinical loss of attachment (CLA) and Radiographic Examination (periapical radiographs) using long cone parallel technique was taken for each patient to detect the degree of bone loss.
5. GCF samples collection and analysis
For each patient and control subject Gingival Crevicular Fluid (GCF) samples were collected. The samples were pooled from 3 periodontal sites with attachment loss 4mm or more (in three different quadrants). The sampling area was isolated with cotton rolls and carefully cleaned supra-gingivally with sterile cotton pellets. A sterile paper point was inserted into the gingival crevice or pocket until resistance was felt and the paper point was allowed to remain in place for 30 seconds after that the paper point was transferred to a vial containing 100μL of distilled water and vigorously mixed. The paper points were removed and the samples were centrifuged and washed twice with distilled water after that the supernatant pellet was re-suspended in 0.4mL of distilled water and stored at −70ºC for PCR analysis.
6. Periodontal therapy
For each subject, full mouth supra and subgingival scaling and root planning were performed using ultrasonic scaler and periodontal curettes. Debridement was completed on three sessions over 10-days period. As a maintenance program every patient was instructed to use soft toothbrush (Formula system) (1) two times daily with regular tooth paste (2) and the use of interdental tooth picks. (3) Patients were instructed to use chlorhexidine mouthwash (Hexitol) (4) and antibiotic regimen in the form of a combination of 500 mg Amoxicillin5and 250 mg metronidazole (Flagyl) (4) 3 times/day for 7 days. All patients of GI and GII were followed up at 2 weeks interval. The sampling procedures were repeated for each patient one month after periodontal therapy.
7. Real Time-PCR (RT-PCR) analysis
A - RNA extraction
RNA was extracted by the use of denaturing solution (solution D) formed of the following reagents: −250g Guanidiniumthiocyanate dissolved in 293 ml Water, 17.6 ml of 0.75 M sodium citrate (pH 7), 26.4ml 10% sarcosyl and 0.36 ml of 2-mercaptoethanol. Phenol water was saturated at 4°C. The sample was defreezed to room temperature and suspended in 1 ml of solution D in disposable Eppendorf tube and the following reagents was added: 0.1 ml of 2 M sodium acetate (pH4), 1 ml of water saturated phenol and 0.2 ml of chloroform-isoamayl alcohol mixture (49:1). Thorough mixing was performed by inversion after the addition of each reagent. The final suspension was shaken vigorously for 10 Seconds and cooled on ice for 15 minutes; then the samples were centrifuged at 10000g (14000 R P M) for 20m at 4°C (in MIKRO 22 R centrifuge). After centrifugation three phases appeared in the tube; an aqueous phase (containing RNA), phenol phase (containing phenol) and the interphase (containing DNA, proteins and other solid ingredients).
The aqueous phase was transferred to a new fresh tube using micropepittors and mixed with double volume of isopropanol and placed at −20°C for One hour to precipitate RNA. Sedimentation of RNA was performed by centrifugation of the cooled aqueous material for 20 minutes at 4°C. The sedimented RNA pellet was again dissolved in 0.3 ml of solution D and transferred into a new 1.5 Eppendorf tube and recentrifuged for 10 minutes at 4°C to precipitate RNA pellet again. The formed RNA pellet was suspended in 75% ethanol and vacuum dried. The sedimented pellet was dissolved in 500μl H2O. At this point the RNA preparation could be used for PCR assay.
B - PCR Assay
10x - PCR reaction buffer (Tris-HCl 100mM - KCl 500mM - MgCl2 15mM) The pH of the buffer was adjusted to 8.3 (at 25°C), the Gel loading buffer ( 0.2 % Bromophenol blue − 0.2 % Xylene Cyanol-50% Glycerol), deoxynucleotide Tri- Phosphate mix (dNTP) (10mM from dATP, dCTP, dGTPanddTTP), DNA polymerase (AmpliTaq).
8. Primer Sequence:-
The specific primers were prepared (Clinilab). (6) The sequence of PCR primers for IL-1β were 5′CTCAGGTGTCCTCGAAGAAATCAAA3′, and 5′GCTT-TTTTGCTGTGAGTCCCG3′ with expected PCR product size of 194 bp.
The sequence of PCR primers for TNF-α were 5′AGCACAGAAGCATGATCCG3′ and 5′TTTGCTACGACGTGGGCTAC5′ with expected PCR product size of 286 bp. PCR was carried out in a total volume of 50 μl, containing 10 μl of solution DNA, Pre-mix buffer (50mM KCl, 10mM Tris-HCl pH 8.4, 0.1% Triton X-100, 1.5mM MgCl2, deoxynucleoside triphosphates, TaqDNA polymerase) and 20pmol/reaction of the PCR primers. The temperature settings of the PCR reaction for IL-1β were; denaturation at 94°C for 3 min followed by 35 cycles of annealing at 94°C for 30 sec, extension at 54°C for 35 sec and 72°C for 30 sec. The run was terminated by final extension period at 72°C for 5 min.
The temperature settings of the PCR reaction for TNF-α were denaturation at 94oC for 3 min followed by 35 cycles of annealing at57oC for 5 sec, extension at 72°C for 12 sec and 88.5°C for 30 sec. The run was terminated by final extension period at 72°C for 5 min Amplification was performed in a PTC-100-60 thermocycler6. The PCR products were digested with 5U of TaqI DNA polymerase at 65°C for 4 hours. 0.5 μL of DNA primers at the same base position were included as a positive control used in a separate micro centrifuge tube. PCR performed without GCF sample (distilled water only) was used as a negative control.
The detection of the PCR product was performed by using agarose gel electrophoresis. 20 μL of sample (PCR product) was loaded in each Well for electrophoresis. The agarose gel containing 1.5% agarose and 0.5μg/ml ethidium bromide with 0.5X Tris- acetate EDTA buffer. Electrophoresis was conducted at 4Volt/cm3 of the gel (60–80Volt for the total gel piece) for 1–1.5hrs. Running within the gel, a molecular DNA size marker for matching of the position of the base-pairs (bp) of the positive PCR assays. The DNA was photographed under 312 nanometer (nm) Ultraviolet light transilluminator.
After detection of the PCR product 5μl of the positive samples was used for the quantitative analysis of IL-1β and TNF-αin the form of picogram/μl. Quantitation was carried out by the use of the Bright–GloTM Luciferase assay system developed by Promega (7) in TD- 20/20 – Luminometer. Luciferase is an enzyme used for assessment of the quantity of marker (in culture and serum), amplified DNA and RNA by giving a luminescence signal that can be counted by the use of Luminometer. The temperature of the Bright–Glo reagent was held to the room temperature which was near the optimum temperature of luciferase. Then 100μl of Bright-Glo Luciferase was added to 5μl of the sample to be used in quantitation and 5μl of luciferase assay buffer was added to the tube. The mixture was left at room temperature for 2 minutes then measured in the luminometer. All data were tabulated and used for statistical analysis using Statistical Package for Software System (SPSS) version 11.
The clinical parameters (GI, PI, PD and CLA) were scored from each patient before and after periodontal therapy. The mean values of clinical parameters were computed and evaluated before and after periodontal therapy with paired t–test and levels at P<0.05 were considered significant. The frequency of mRNA expression for IL-1β and TNF-α per group, as well as differences from baseline to one month after periodontal therapy was evaluated by Chi-square test and levels at p<0.05 were considered significant. Differences in the mean levels of IL-1β and TNFα mRNA expression from baseline to one month after periodontal therapy was evaluated by paired t – test. Perasons’ correlation analysis was used to study the correlation between clinical parameters and the mean levels of IL-1β and TNFα at baseline and one month after periodontal therapy. Levels at P<0.05 were considered significant.
Results
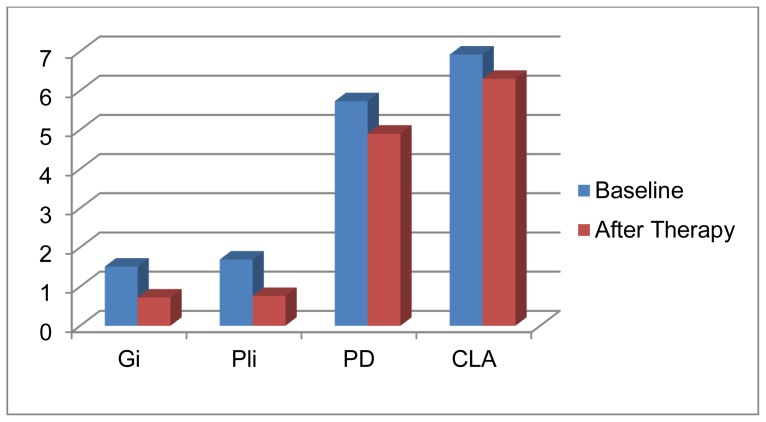
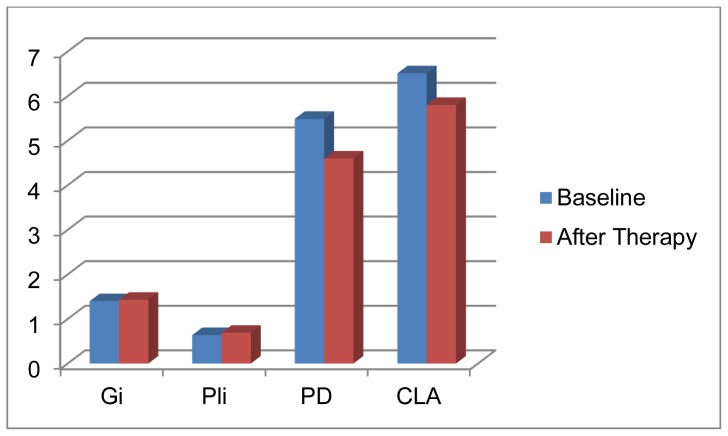
The data and clinical parameters at baseline as well as the changes in the clinical parameters after conservative periodontal therapy of the two studied patients groups are summarized in tables 1 & 2. The data and clinical parameters of the healthy control group are summarized in table (3). Group I (chronic periodontitis patients working in the cement company) was comprised of 20 male patients, with age range 27.5 – 49 years and the mean age was 29.5 ± 4.37 years. At baseline, the mean GI scores were1.51 ± 0.21 and the mean PI scores were1.69 ± 0.29. The mean PD scores were5.73±0.55 with mean loss of attachment of 6.92 ± 0.60mm. One month after periodontal therapy both GI and PI scores were reduced below 1 (P<0.001) with 0.83mm reduction in PD and 0.62mm gain in clinical attachment (P<0.05). Table (1) Group Π (chronic periodontitis patients not working in cement companies) was comprised of 20 male patients with age range 39–51 years and a mean age of 45.75 ± 3.32 years. At baseline the mean GI scores were1.40 ± 0.23 and the mean PI scores were1.43 ± 0.24. The mean pocket depths were 5.49 ± 0.55mm and the mean loss of clinical attachments were 6.51 ± 1.45mm. After periodontal therapy, there was obvious reduction in PI and GI scores (P<0.001) and statistically significant reduction in pocket depth (0.89mm) and gain in clinical attachment (0.71mm) (P<0.05). Tables (2).
Table (1).
Changes in the mean scores of clinical parameters at baseline and after periodontal therapy in group I.
| Before therapy X±SD | After therapy X±SD | Mean difference X±SD | t value | P Value | |
|---|---|---|---|---|---|
| GI | 1.51 ± 0.21 | 0.72 ± 0.21 | 0.79 ± 0.26 | 9.7 | <0.001 |
| PI | 1.69 ± 0.29 | 0.76 ± 0.23 | 0.93 ± 0.33 | 13.60 | <0.001 |
| PD | 5.73 ± 0.55 | 4.9 ± 0.54 | 0.83 ± 0.45 | 4.12 | <0.05 |
| CLA | 6.92 ± 0.60 | 6.3 ± 0.62 | 0.62 ± 0.52 | 3.59 | <0.05 |
Table (2).
Changes in the mean scores of clinical parameters at baseline and after periodontal therapy in group II.
| Before therapy X± SD | After therapy X± SD | Mean difference X± SD | t Value | P Value | |
|---|---|---|---|---|---|
| GI | 1.40 ± 0.23 | 0.64 ± 0.13 | 0.76 ± 0.16 | 12.8 | <0.001 |
| PI | 1.43 ± 0.24 | 0.69 ± 0.15 | 0.74 ± 0.14 | 9.65 | <0.001 |
| PD | 5.49 ± 0.55 | 4.6 ± 0.55 | 0.89 ± 0.35 | 3.5 | <0.05 |
| CLA | 6.51 ± 1.45 | 5.8 ± 1.4 | 0.71± 0.22 | 3.76 | <0.05 |
Table (3).
The clinical parameters of the healthy control group (Group III).
| Age | GI | PI | Crevice depth | CLA | |
|---|---|---|---|---|---|
| Mean±SD | 25.71±5.23 | 0.21±0.11 | 0.19±0.11 | 1.93±0.75 | 0.0 |
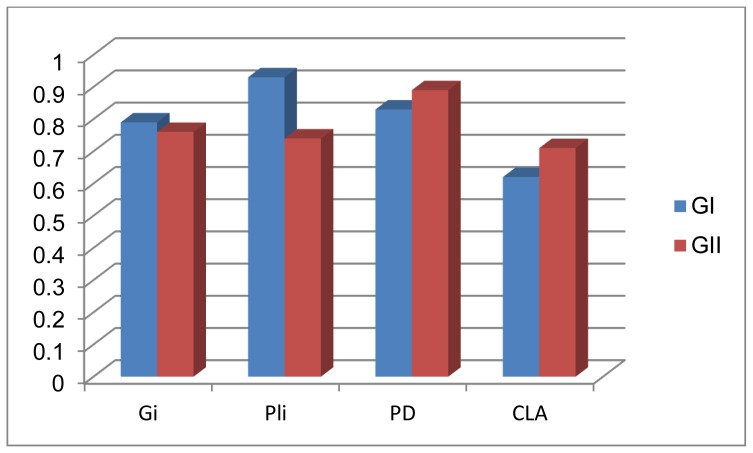
On comparing the response to periodontal therapy there was a non-significant difference between the two studied patients groups (P>0.05) where the two groups responded well to the non-surgical treatment.
The healthy control group (Group III) was comprised of 20 male subjects, with their ages ranging from 23–45 years and the mean age was 25.74 ± 5.23 years. The mean GI and PI scores were below 1 and the mean crevice depth was 1.93 ± 0.75 with no loss of attachment. Table (3).
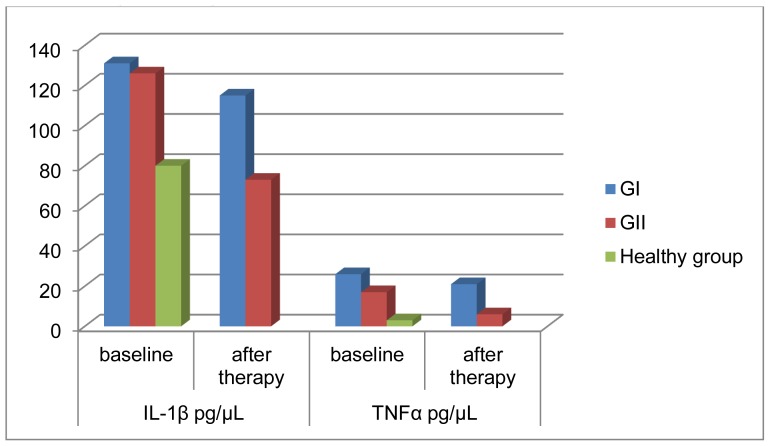
The PCR assay results for IL-1β and TNFα in the studied groups I and II before and after periodontal therapy as well as in the healthy control group (Group III) was shown in table (4) concerning the levels and frequency of detection of IL-1β (fig 4) at baseline there was a statistically non-significant difference between GI and GII (p>0.05) but there was a statistically highly significant difference between the two groups after periodontal therapy regarding the PCR results of TNFα (fig 5) there was a statistically significant difference between the studied two groups at baseline (p<0.05) and a highly significant difference after periodontal therapy (P<0.001). The studied two patients groups reported a statistically highly significant difference in the PCR assay results of the two markers with the healthy control group at baseline (P<0.001).
Table (4).
Levels of IL-1β and TNFα mRNA expression in both studied patient groups (GI and GII) and healthy control group (GIII).
| Study | IL-1β pg/μL | TNFα pg/μL | ||||
|---|---|---|---|---|---|---|
| groups | Baseline | After Therapy | P | Baseline | After therapy | P |
| GI | 131.35±54.23 | 115.45±67.12 | >0.05 | 26.65±23.67 | 21.54±68.13 | >0.05 |
| GII | 126.45±76.16 | 73.17±61.25 | <0.001 | 17.78±11.04 | 6.71±2.34 | <0.001 |
| P | >0.05 | <0.001 | _ | <0.005 | <0.001 | __ |
| Healthy group (Group III) | 80.01±12.76 | __ | _ | 3.20±1.39 | __ | __ |
| P | <0.001 | __ | _ | <0.001 | __ | __ |
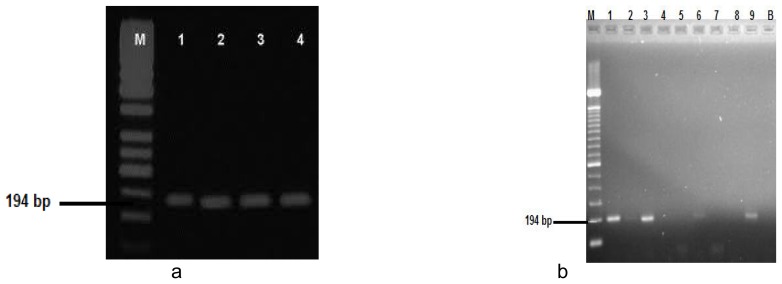
Fig (4). Gel electrophoresis for IL-1β mRNA.
Fig (4): (a) lanes 1, 3 and 9 stands for positive PCR results for patients from GI at baseline, lanes 2 and 6 are positive PCR assay results for 2 patients from GII at baseline, lanes 4, 5 and 7 healthy subjects, lane 8 represents a negative PCR assay results for patient in GI after periodontal therapy. (b) Lanes 1 and 3 represent a positive PCR assay results for two patients from GI after periodontal therapy, lanes 2 and 4 represent a positive PCR assay results for two patients from GI before periodontal therapy. M lane is DNA size marker, B lane is a negative GIII controls.
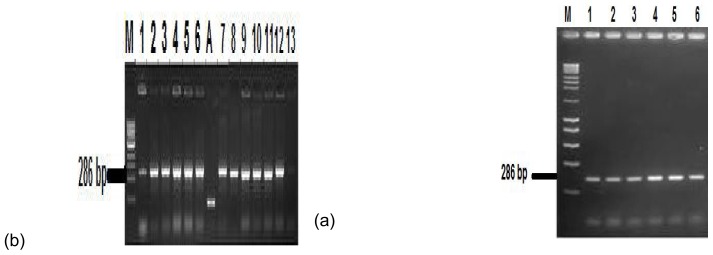
Fig (5). Gel electrophoresis for TNF-α mRNA.
Fig (5): (a) lanes 1and 13 stands for positive PCR results for healthy control subjects (GIII), lanes 2, 4, 5 and 6 are positive PCR assay results for patients from GI before periodontal therapy, lanes 3, 7 and 8 represents positive PCR results for patients from GI after periodontal therapy, lanes 9, 10 and 12 represents a positive PCR assay results for patient in GII before periodontal therapy, lane 11 represent a positive PCR assay results for patient from GII after periodontal therapy. (b) lanes 1, 3, 5 and 6 represent a positive PCR assay results for patients from GI before periodontal therapy, lanes 2 and 4 represents the positive PCR assay results for GI patients after periodontal therapy. M lane is DNA size marker, A lane is a positive control and B lane is a negative control,
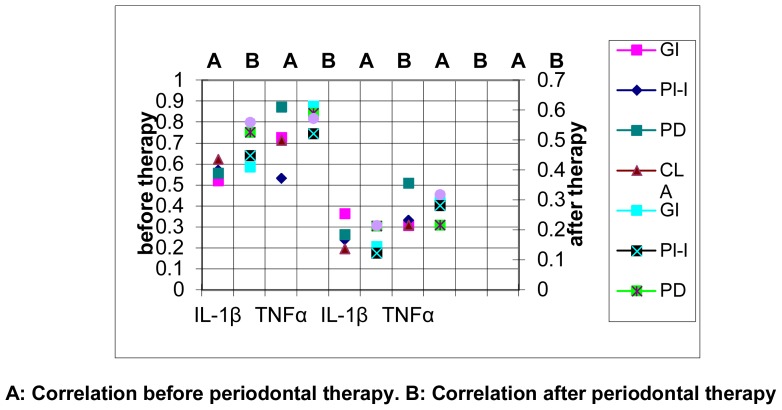
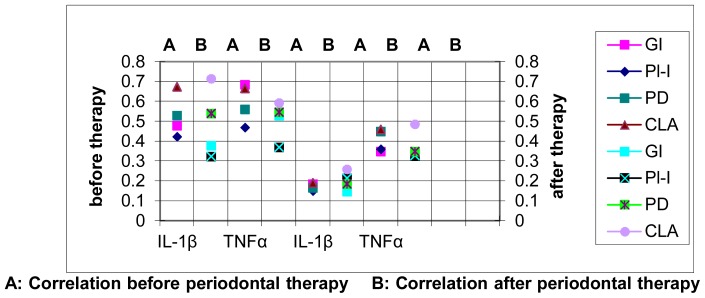
The correlation of the levels of mRNA for IL-1β and TNFα with the clinical parameters in group I (Cement workers with chronic periodontitis) and group II (non-cement workers) before and after periodontal therapy are summarized in tables (6&7). There was a significant correlation between the levels of mRNA for IL-1β and TNFα with the clinical parameters at baseline and one month after periodontal therapy in group I (P<0.05). Considering group II there was a significant correlation between the levels of mRNA for IL-1β and TNFα with all the clinical parameters before periodontal therapy (P<0.05). Similarly significant correlation was demonstrated with the levels of TNFα one month after periodontal therapy (P<0.05), but the levels of IL-1β mRNA did not have any significant correlation with any of the clinical parameters (P>0.05). Figs (7 & 8)
Fig (7).
Correlation Coefficient (r) between the mean levels of IL-1β mRNA and TNFα mRNA with the scores of clinical parameters before and after periodontal treatment in group I.
A: Correlation before periodontal therapy. B: Correlation after periodontal therapy
Fig (8).
Correlation Coefficient (r) between the mean levels of IL-1β mRNA and TNFα mRNA with the scores of clinical parameters before and after periodontal treatment in group I.
A: Correlation before periodontal therapy B: Correlation after periodontal therapy
Discussion
The objective of this study was to explore if long term exposure to cement dust can affect the periodontal health and affect the outcome of non-surgical periodontal therapy. Various factors such as environmental factors, occupational factors, dietary factors, pathologic factors and oral hygiene practices affect the oral health of an individual. Lack of medical health care facilities in the factory premises, especially oral health care facilities, reflects the factory employees’ poor health condition.
In the present study the treatment protocol consisted of scaling and root planning in conjunction with antibiotic combination of amoxicillin and metronidazole. The goal of root debridement was to remove microbiologically contaminated cementum and to eliminate and reduce the number of pathogenic bacteria in the periodontal pocket below their disease inducing levels. The rational for the use of systemic antibiotics was to rapidly suppress target microbial species and faster the establishment of a host compatible microflora. Metronidazole has a narrow spectrum and works specifically on anaerobic microorganisms associated with periodontal diseases. Amoxicillin appears very effective against most periodontal pathogens. (21) Both of the studied two patients groups responded well to the non-surgical periodontal therapy in conjunction with antibiotic combination this was consistent with previous studies. (22, 23)
Wilton et al. (24) found that the mean level of GCF IL-1β in chronic periodontitis was 34.16±29.45 pg/μL. Also Yavuzyilmaz et al. (25) found that the mean level of GCF IL-1β in aggressive periodontitis was 38.45±13.99 pg/μL and the mean GCF TNFα was 3.20±1.39pg/μL. The levels detected by the two authors was different from those reported in the present study, and this may be due to the difference in techniques used because the two authors used the ELISA technique while we used PCR analysis in the present study as it is considered to be more sensitive since it counts the mRNA transcripts of the marker present in the sample. The second explanation for the difference between the two studies is that the prolonged exposure to cement dust in group I may have resulted in increased expression of the pro inflammatory cytokines.
Bulut et al. (26) had assessed the levels of IL-1β in diabetic patients with adult periodontitis and in healthy controls. The levels reported by the authors were almost consistent with the levels reported by the present study. This may give an indication that the effect of prolonged exposure to cement dust has nearly the same effect on the periodontium in diabetes mellitus patients.
The present study showed that non-surgical periodontal treatment did not affect the levels of GCF IL-1β and TNFα in the cement workers (group I) although there was improvement in the clinical conditions as reported by changes in the scores and measurements of the clinical parameters
Conclusion
It seems that long term exposure to cement dust does not affect the clinical outcome of non-surgical periodontal treatment, but it affects the levels of the pro inflammatory mediators which lead to more periodontal tissue destruction.
Recommendations
Further researches are required to rule out the underlying mechanism by which the cement dust exposure can affect the levels of the pro inflammatory cytokines in periodontal tissues and in gingival crevicular fluid.
Fig (1).
Changes in the clinical parameters from baseline to one month after periodontal therapy in GI.
Fig (2).
Changes in the clinical parameters from baseline to one month after periodontal therapy in group II.
Fig (3).
Mean differences from baseline scores in the clinical parameters after non-surgical periodontal therapy in the two studied patients groups (Group I & Group II).
Fig (6).
Levels of IL-1β and TNFα mRNA expression in both studied patients groups (GI and GII) and healthy control group (GIII).
Table (5).
Correlation Coefficient (r) between the mean levels of IL-1β mRNA and TNFα mRNA with the scores of clinical parameters before and after periodontal treatment in group I.
| Date | Mean levels of IL-1 and TNF mRNA in Pg/μL | GI X ±SD 1.51 ± 0.21 |
PI X±SD 1.69 ± 0.29 |
PD X ± SD 5.73 ± 0.55 |
CLA X± SD 6.92± 0.60 |
|
|---|---|---|---|---|---|---|
| Before periodontal Therapy | IL-1β | 131.35±54.23 | 0.523 | 0.572 | 0.560 | 0.624 |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | ||
| TNFα | 26.65±23.67 | 0.730 | 0.534 | 0.875 | 0.715 | |
| P value | <0.01 | <0.01 | <0.001 | <0.01 | ||
| After Periodontal Therapy | IL-1β | 115.45±67.12 | 0.412 | 0.451 | 0.528 | 0.561 |
| P value | <0.05 | <0.05 | <0.01 | <0.01 | ||
| TNFα | 21.54±68.13 | 0.615 | 0.523 | 0.592 | 0.574 | |
| P value | <0.01 | <0.05 | <0.01 | <0.01 | ||
Table (6).
Correlation Coefficient (r) between the mean levels of IL-1β mRNA and TNFα mRNA with the scores of clinical parameters before and after periodontal treatment in group II.
| Mean levels of IL-1 and TNF mRNA in Pg/μL | GI X ±SD 0.72 ± 0.21 |
PI X±SD 0.76 ± 0.23 |
PD X ± SD 4.9 ± 0.54 |
CLA X ± SD 6.3 ± 0.62 |
||
|---|---|---|---|---|---|---|
| Before periodontal therapy | IL-1β | 126.45±76.16 | 0.412 | 0.451 | 0.528 | 0.561 |
| P value | <0.05 | <0.05 | <0.01 | <0.01 | ||
| TNFα | 17.78±11.04 | 0.615 | 0.523 | 0.592 | 0.574 | |
| P value | <0.01 | <0.05 | <0.01 | <0.01 | ||
| After periodontal therapy | IL-1β | 73.17±61.25 | 0.146 | 0.125 | 0.215 | 0.216 |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | ||
| TNFα | 6.71±2.34 | 0.295 | 0.284 | 0.280 | 0.320 | |
| P value | <0.05 | <0.05 | <0.05 | <0.05 | ||
References
- 1.Al-Neaimi YI, Gomes J, Lloyd OL. Respiratory illnesses and ventilatory function among workers at a cement factory in a rapidly developing country. Occupational Medicine. 2001;51(6):367–373. doi: 10.1093/occmed/51.6.367. [DOI] [PubMed] [Google Scholar]
- 2.Short S, Petsonk EL. Non-fibrous inorganic dusts. In: Harber Philip, Schenker Marc B, Balmes John R., editors. Occupational and environmental respiratory disease. London: Mosby; 1996. p. 356. [Google Scholar]
- 3.Baby S, Singh NA, Shrivastava P, Nath SR, Kumar SS, Singh D, Vivek K. Impact of dust emission on plant vegetation of vicinity of cement plant. Environmental Engineering and Management Journal. 2008;7(1):31–35. [Google Scholar]
- 4.Zeleke Z, Moen B, Bratveit M. Cement dust exposure and acute lung function: A cross shift study. BMC Pulmonary Medicine. 2010;10(1):19. doi: 10.1186/1471-2466-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydin S, Aydin S, Croteau G, Sahin Í, Citil C. Ghrelin, Nitrite and Paraoxonase/Arylesterase Concentrations in Cement Plant Workers. Journal of Medical Biochemistry. 2010;29(2):78–83. [Google Scholar]
- 6.Adak MD, Adak S, Purohit KM. Ambient air quality and health hazards near mini cement plants. Pollution Research. 2007;26(3):361–364. [Google Scholar]
- 7.Peters S, Thomassen Y, Fechter-Rink E, Kromhout H. Personal exposure to inhalable cement dust among construction workers. Journal of Environmental Monitoring. 2009;11(1):174–180. doi: 10.1039/b812357h. [DOI] [PubMed] [Google Scholar]
- 8.Mwaiselage J, Moen B, Bråtveit M. Acute respiratory health effects among cement factory workers in Tanzania: an evaluation of a simple health surveillance tool. International Archives of Occupational and Environmental Health. 2006;79(1):49–56. doi: 10.1007/s00420-005-0019-x. [DOI] [PubMed] [Google Scholar]
- 9.Fatima SK, Prabhavathi PA, Padmavathi P, Reddy PP. Analysis of chromosomal aberrations in men occupationally exposed to cement dust. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2001;490(2):179–186. doi: 10.1016/s1383-5718(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 10.Dietz A, Ramroth H, Urban T, Ahrens W, Becher H. Exposure to cement dust, related occupational groups and laryngeal cancer risk: Results of a population based case-control study. International Journal of Cancer. 2004;108(6):907–911. doi: 10.1002/ijc.11658. [DOI] [PubMed] [Google Scholar]
- 11.Green GM. The J. Burns Amberson lecture. In defense of lung. Am Rev Rep Dis. 1970;102:691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- 12.Struzak-Wysokinska M, Bozyk A. Condition of the oral mucosa in cement plant workers. WiadLek. 1989;42:641–4. [PubMed] [Google Scholar]
- 13.Tuominen M, Tuominen R. Tooth surface loss and associated factors among factory workers in Finland and Tanzania. Community Dent Health. 1992;2:143–50. [PubMed] [Google Scholar]
- 14.Bozyk A, Owczarek B. Incidence of parodontal diseases in workers of the Chelm Cement Plant exposed to cement dust. Czas Stomatol. 1990;43:375–80. [PubMed] [Google Scholar]
- 15.Petersen PE, Henmar P. Oral conditions among workers in the Danish granite industry. Scand J Work Environ Health. 1988;5:328–31. doi: 10.5271/sjweh.1911. [DOI] [PubMed] [Google Scholar]
- 16.De Nardin E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann Periodontol. 2001;6:30–40. doi: 10.1902/annals.2001.6.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Tandon Shruti, Dhingra Mandeep Singh, Lamba Arundeep Kaur, Verma Mahesh, Munjal Akshay, Faraz Farrukh. Effect of Periodontal Therapy on Serum Lipid Levels. Indian Journal Of Medical Specialties. 2010;1(1):19–25. [Google Scholar]
- 18.Armitage CG. Development of a classification system for periodontal diseases and conditions. Ann periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Silness J, Loe N. periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal conditions. Acta Odontologica Scandinivica. 1964;24:747–759. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 20.Löe H, Silness J. Periodontal disease in pregnancy I. prevalence and severity. Acta Odontologica Scandinavica. 1965;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 21.Walker CB, Pappas JD, Tyer KZ, Cohen S, Gordon JM. Antibiotic susceptibilities of periodontal bacteria. In vitro susceptibilities to eight antimicrobial agents. J Periodontol. 1985;56:67–74. doi: 10.1902/jop.1985.56.11s.67. [DOI] [PubMed] [Google Scholar]
- 22.Ezzo Paul J, Cutler Christopher W. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003;32:1–24. doi: 10.1046/j.0906-6713.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- 23.Pavicic MJAMP, Van Winkelhoff AJ, de Graaf J. synergestic effects between amoxicillin, metronidazole and its hydroxymetabolite against A. actinomycetem-comitans. Antimicrob Agents Chemother. 1991;35:961–966. doi: 10.1128/aac.35.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilton JM, Bampton JL, Griffith GS, Curtis MA, Life JS, Johnson NW, Powell JR, Harrap GJ, Critchely P. interleukin-1 beta (IL-1beta) levels in gingival crevicular fluid from adults with previous evidence of destructive periodontitis. A cross sectional study. J Clin Periodontol. 1992;19(1):53–57. doi: 10.1111/j.1600-051x.1992.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 25.Yavuzyilmaz E, Yamalik N, Bulut S, Ersoy F, Saatci U. The gingival crevicular fluid interleukin-1 beta and tumor necrosis factor-alpha levels in patients with rapidly progressive periodontitits. Aust Dent J. 1995;40(1):46–49. doi: 10.1111/j.1834-7819.1995.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 26.Bulut U, Develioglu H, Taner IL, Berker E. interleukin-1 beta levels in gingival crevicular fluid in type 2 diabetes mellitus and adult periodontitis. J Oral Sci. 2001;43(3):171–177. doi: 10.2334/josnusd.43.171. [DOI] [PubMed] [Google Scholar]