Abstract
Background
The prevalence of gastroparesis in type 2 diabetes mellitus (T2DM) vary widely. Our aim is to estimate the prevalence of clinical symptoms of gastroparesis among patients with type 2 diabetes mellitus and explore the relationship between gastroparesis and other risk factors.
Methods
A cross-sectional study evaluating 147 type 2 diabetics using the Gastroparesis Cardinal Symptoms Index (GCSI). A GCSI Total Score ≥ 1.90 were chosen as having definite symptoms of gastroparesis. All patients completed a demographic questionnaire and interviewed to complete the. Demographic Data, disease duration, Medication, comorbidities, recent blood glucose and HbA1C were collected and investigated.
Results
The prevalence of clinical symptoms of gastroparesis among type 2 diabetics was 10.8%. Clinical symptoms of gastroparesis were significantly correlated to HbA1c (p=0.001), blood glucose (p= 0.003), duration of diabetes (p= 0.02) and comorbidities (p=0.009). The most common symptoms were bloating, stomach fullness and early satiety (63.94%, 55.1% and 48.3% respectively). In logistic regression analysis, female gender emerged as significant independent predictors of the presence of at least one symptom.
Conclusions
The prevalence of clinical symptoms of gastroparesis observed in the Saudi patientsdiagnosedwithtype2 diabetes was 10.8% and is independently associated with poor controlled diabetes, hyperglycemia, and long duration of diabetes and history of Co-morbid conditions.
Introduction
Diabetes Mellitus (DM) has now become a worldwide problem. In Saudi Arabia, the figures of prevalence are quite high at about 30% in one study. (1) The relationship between GI dysfunction and diabetes has been noted for almost 70 years. (2) In 1958, Kassander (3) describe the condition of gastric retention observed in six asymptomatic diabetics patients as “gastroparesis diabeticorum.” Gastroparesis is a clinical syndrome characterized by delayed gastric emptying in the absence of mechanical obstruction of the stomach. (4) Symptoms of gastroparesis are variable but may include bloating, nausea, vomiting, early satiety, and upper abdominal pain. Diabetic gastroparesis can result in many consequences such as impaired glucose regulation, hypoglycemia, decrease drug absorption, nutritional compromise, and a high rate of hospitalizations and poor quality of life. (5–7)
The prevalence of gastroparesis in type 2 diabetes mellitus (T2DM) varies widely. In specialized centers, 10% to 30% of patients with T2DM have gastroparesis. (8–10) In 2012, Kristoffer K reported that the prevalence of diabetic gastroparesis was 9.8%. (11) Dickman et. Al. in his paper published in 2014 found that the prevalence of diabetic gastroparesis symptoms was significantly higher in females than in males. (12) Research in this area is still deficient. Many studies show that the prevalence of gastroparesis in T1DM is more than in T2DM. (13) Current literature suggests that upper GI symptoms are common in T2DM, but in light of inconsistent evidence, (14) this notion remains controversial. T2DM represents about 90–95% of all diabetics and it’s quite prevalent in Saudi Arabia (1) so we decided to limit our study to only T2DM.
However, studies assess gastroparesis in DMare hence of great interest and up to our knowledge this is the first study assess the prevalence of clinical symptoms of gastroparesis in Saudi T2DM. Thus, we aimed in this study to estimate the prevalence of clinical symptoms of diabetic gastroparesis in T2DM patients and explore its association with various risk factors.
Methodology
A cross-sectional survey of 148T2DM patients treated at the diabetic clinics in Buraydah Central Hospital which provides Secondary health care to patients. Patients were selected randomly in routine visits by odd number of patients list from July 2015 to October 2015. Participants, who were younger than 18 years or older than 75 years in March, 2015 were excluded. An informed consent from each study participant was obtained. The study was approved by Institutional Review Board of College of Medicine, Qassim University.
All patients were interviewed by trained medical students to complete the Gastroparesis Cardinal Symptoms Index (GCSI). (15) Data regarding disease duration, prescribed medications, comorbidities, most recent fasting blood glucose and HBA1C level were collected from the patient’s medical records. Height, weight and blood pressure were recorded, and body mass index (BMI) were calculated as weight (kg)/height (M2).
Patients were asked to rate clinical symptoms of the gastroparesis through GCSI. The GCSI consists of three subscales of the Patients Assessment of Upper Gastrointestinal Disorders-Symptom Severity Index PAGI-SYM, selected to measure important symptoms related to gastroparesis, that is nausea/vomiting, postprandial symptoms/early satiety, and bloating. The nausea/vomiting subscale includes the following three items: Nausea, retching, and vomiting. The postprandial fullness/early satiety subscale examine stomach fullness, inability to finish a normal-sized meal, feeling excessively full after meals, and loss of appetite. The bloating subscale is comprised of the following two items: bloating, and visibly larger stomach or belly after meals. (15) Overall the GCSI includes 9 questions and each question is rated by the responder according to severity of symptoms from 0 to 5(0=No symptoms to 5=Severe symptoms). Total Scores of Patients’ GCSI were entered into a database and values ≥ 1.90 were chosen as definite symptoms of gastroparesis. The cutoff at 1.90 was chosen in conformity with data from previous literatures. (11,16)
Data entry and analysis was carried out using Statistical Package of Social Studies (SPSS, windows version 18.0). It was done by a statistician to ensure blind assessment of the result. Normally distributed continuous variables are presented as mean ± SD and non-normally distributed as median [range]. Categorical variables are described using frequency distributions and are presented as frequency (%). Mean values of age, duration of diabetes, systolic, diastolic blood pressure, height, weight, BMI, FBG, HbA1c and GCSI scores was compared between groups: “Diabetic gastroparesis” and “Not diabetic gastroparesis” using t-test or the Mann–Whitney U. Chi Square was used to assess associations between categorical variables for presence of symptoms and gender, co-morbid conditions and type of treatment. The presence of any symptom of gastroparesis was modeled using logistic regression analysis. Odds ratios were estimated with 95% confidence intervals. A two-tailed p-value<0.05 was considered statistically significant.
Result
A total of 147 patients completed GCSI questionnaire. The characteristics of the subjects are presented in Table 1. Study subjects aged 53.47 ± 12.37 and 65.25% of the patients were female. The mean duration of diabetes is 8.5 ± 7.5 and most of the patients used metformin (71.4%). The mean BMI was 30.08 ± 5.5 Kg/m2. Over 47.1% were obese, while 35.5% were overweight and only 17.4% had normal BMI. The most recent blood glucose levels were 165 ± 62.53 mg/dl and HbA1C 9.1% ± 6.1%. Hypertension affected 36 (24.5%), 29 (19.7%) of the participants had Dyslipidemia and 22 (14.9%) had both hypertension and dyslipidemia.
Table 1.
Demographic Characteristics of study population:
| Demographic | Total N (%) | Diabetic gastroparesis N (%) | Not Diabetic gastroparesis N (%) | P value |
|---|---|---|---|---|
|
| ||||
| N (%) | 147 (100%) | 10.89% | 89.11% | |
|
| ||||
| Age | 53.4±12.37 | 54.12±14.5 | 53.39±12.1 | NS* |
|
| ||||
| Gender: | ||||
| Male | 37.4% | 10.2% | 89.8% | NS |
| Female | 62.6% | 11.9% | 88.1% | |
|
| ||||
| Weight | 76.8±13.6 | 75.1±11.6 | 77.04±13.9 | NS |
|
| ||||
| Height | 160.6±9.9 | 159.1±8.42 | 160.7±10 | NS |
|
| ||||
| Body mass Index: | 30.07±5.5 | 30.32±5.8 | 30.1±5.5 | NS |
|
| ||||
| BP systolic (mmhg) | 142±20.4 | 142±17.8 | 142±20.8 | NS |
|
| ||||
| BP diastolic (mmhg) | 80±13.2 | 77±12 | 81±13.4 | NS |
|
| ||||
| Duration of Diabetes | 8.5±7.58 | 12.6±7.4 | 7.96±7.5 | 0.02 |
|
| ||||
| Fasting plasma glucose (mg/dl): | 165.15±62.5 | 207.81±66 | 159.7±60.1 | 0.003 |
|
| ||||
| Glycosylated haemoglobin (%) | 9.1±6.1 | 10.1±2.2 | 8.98±6.4 | 0.001 |
|
| ||||
| Type of treatment: | ||||
| Metformin | 105 (71.4%) | 7.6% | 92.4% | NS |
| Insulin | 40 (27.2%) | 22.5% | 77.5% | 0.009 |
|
| ||||
| History of Co-morbid conditions: | ||||
| Hypertension | 44% | 12% | 88% | NS |
| Dyslipidemia | 36% | 17% | 83% | |
NS: not significant P-value.
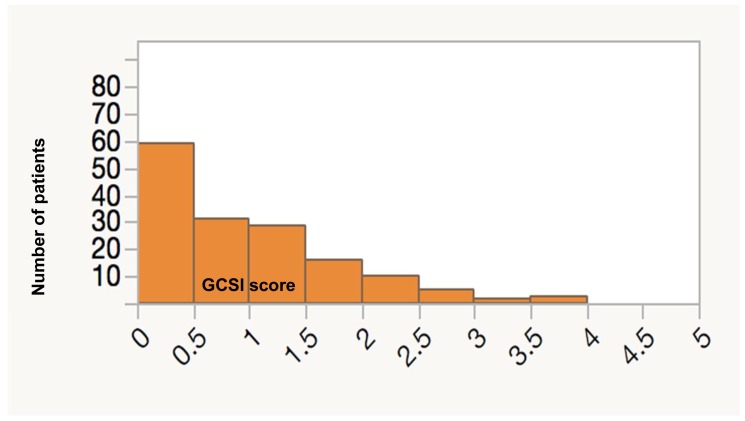
The screening results revealed that overall prevalence of symptoms compatible with gastroparesis as assessed by GCSI was 10.88%. The most common symptoms were bloating, stomach fullness and early satiety (63.94%, 55.1% and 48.3% respectively) Table 2. The distribution of GCSI scores is skewed as shown in Figure 1. Median GCSI score is 0.67 (range: 0.0 to 3.56).
Table 2.
Symptoms suggestive of gastroparesis:
| Symptoms | N = 16 (% of N) |
|---|---|
| Nausea | 55 (37.41%) |
| Retching | 27 (18.37%) |
| Vomiting | 7 (4.76%) |
| Stomach fullness | 81 (55.1%) |
| Early satiety | 71 (48.3%) |
| Feeling excessively full after meals | 59 (40.14%) |
| Loss of appetite | 63 (42.86%) |
| Bloating | 94 (63.94%) |
| Stomach or belly visibly larger | 67 (45.58%) |
Figure 1.
Number of patients according to GCSI Scores.
Symptoms suggestive of gastroparesis was significantly correlated to HbA1c (p=0.001), blood glucose (p= 0.003), duration of diabetes (p= 0.02) and comorbidities (p=0.009). The two groups were similar concerning age, sex and BMI.
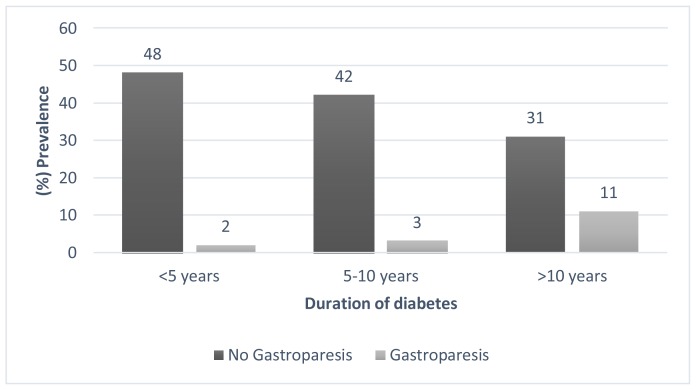
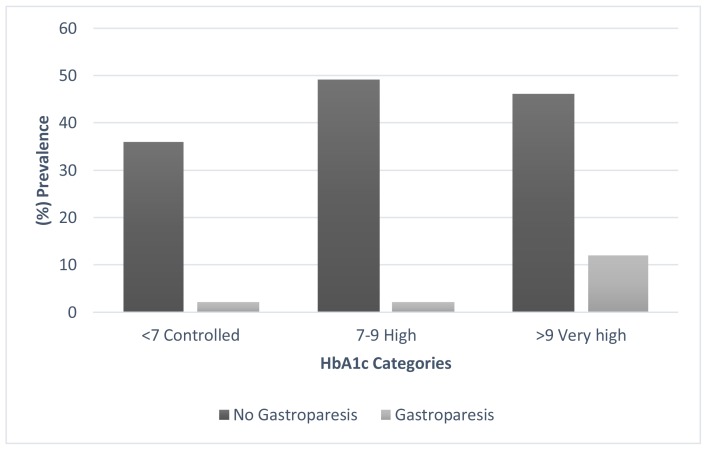
Gastroparesis was significant more common in patient who have diabetes for more than 10 years then who have diabetes for less than 10 years (26.19% and 5,26%, respectively p=0.001) Figure 2. Furthermore, the gastroparesis was significant higher in patient with very high HbA1c than in patients with high HbA1c or controlled HbA1c (20, 69%, 3.92%, 5.26%, p 0.008) Figure 3.
Figure 2.
The prevalence of Gastroparesis in different diabetes duration subgroups.
Figure 3.
The prevalence of Gastroparesis in different HbA1c subgroups.
With respect to type of treatment 71.4% of the patients were prescribed Metformin and 27.2% were on insulin. GCSI scores were significantly higher in patients treated with insulin (p<0.001).
A logistic regression model was made to predict any symptoms suggestive of gastroparesis. Age, gender, duration of diabetes, FBG, HbA1c and BMI were involved in the model. The model was significant (p = 0.023) and correctly categorized 85.7% of study participants as to the presence of at least one gastroparesis symptom. Gender emerged as significant independent predictors of the presence of at least one symptom. Specifically, the risk of having at least one gastroparesis symptom in the female is 5 times greater than the male patient (OR 5.68, 95% CI 1.4–29.1, p = 0.015).
Discussion
This is the first study that assessed the prevalence of clinical symptoms of gastroparesis among Saudi with T2DM. In a review by Kenneth L. & Jorge in 2015, it’s estimated that up to 40% of T1DM have gastroparesis and 10–30 % of T2DM have gastroparesis. Our result showed that 10.88% of Saudi T2DM have a clinical symptoms of gastroparesis, on screening patients with GCSI questionnaire. It is comparable to the estimated prevalence reported by Kristoffer K. (9.8%) and also corresponding well to previous estimates. These variations in the prevalence may be related to difference in selection of study population, methods of diagnosis or differences in definition of gastroparesis. Also this may reflects the quality of care and management.
In this study, there was a linear increase in the prevalence of clinical symptoms of gastroparesis with increase duration of diabetes Fig. 2. Which is previously confirmed by similar study. (17,18) This suggests either a common pathogenetic mechanism like poor glycemic control, neuropathy or delayed gastric emptying. (19) Generally, our cross-sectional study is not designed to evaluate causal relationships but merely associations.
With regard to glycemic control status, this survey revealed that most Saudi T2DM in our diabetes clinics had poor glycemic control. The proportion of patients achieved target values of HbA1c was one quarter (25.8%) as showed by the previous study. (20) A comparison of clinical symptoms of gastroparesis among different HbA1c subgroups illustrated that the prevalence in patients with very high HbA1c was much higher than those with controlled HbA1c (20.69% & 5.26%, p 0.008) respectively) Fig. 3. Furthermore, our study showed significant correlation between gastroparesis and hyperglycemia. This strong association of the presence of clinical symptoms of gastroparesis with high level of glycated hemoglobin and hyperglycemia confirm what is known already that poor glycemic control and hyperglycemia is an important risk factor for gastroparesis. (1, 9, 21, 22) This confirm the importance of tighter glycemic control in diabetic patients to improve gastroparesis and clinical symptoms. (23)
Our results concurs with the literature, (13) indicate that female gender emerged as significant predictor of symptoms suggestive of gastroparesis. The underlying mechanism for this phenomenon is not fully understood. Other studies have also reported that female gender was associated with delayed gastric emptying in patients with diabetes. (17,24) Estrogen levels changes might explain this variation as delayed gastric emptying more common with premenopausal women on oral contraceptives and postmenopausal women receiving hormone therapy. (25–27) another possible explanation is a difference in health-care seeking behavior. Additionally, most of functional gastrointestinal disorders are more common in women than in men. (28)
The gold standard technique for evaluating gastric emptying rate (GER) is by gastric scintigraphy. (4) However, this technique requires specialized expensive equipment and imposes a low but measurable radiation exposure. Thus, it is reasonable to select patients for gastric scintigraphy based on symptoms. We used a validated symptom severity instrument, the GCSI, which is based on the upper gastrointestinal symptoms a patient experience during the prior two weeks. Furthermore, GCSI known to be a reliable, accurate and validated score. The other limitation in our study was that GCSI has its inherited intra- and inter-individual variation as any other bedside clinical test. However, we trained our investigator on applying GCSI to minimize this individual variation.
In conclusion, this is to our knowledge the first study assesses the prevalence of clinical symptoms of gastroparesis in Saudi T2DM. However, the prevalence of clinical symptoms of diabetic gastroparesis was 10.88%. Poor glycemic control, hyperglycemic and high duration of diabetes were the significant associated risk factors.
References
- 1.Tung CF, Chang CS, Chen GH, Kao CH, Wang SJ. Comprehensive gastric emptying study for type-II diabetes mellitus dyspeptic patients. Scand J Gastroenterol. 1997;32(9):884–7. doi: 10.3109/00365529709011196. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Malik RA. Diabetic neuropathy. [Review] [125 refs] Med Clin North Am. 1998;82(4):909–29. doi: 10.1016/s0025-7125(05)70029-8. [DOI] [PubMed] [Google Scholar]
- 3.PK ASYMPTOMATIC GASTRIC RETENTION IN DIABETICS (GASTROPARESIS DIABETICORUM) Ann Intern Med. 1958 Apr 1;48(4):797. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 4.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: Diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127(5):1589–91. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull. 2013;105(1):213–30. doi: 10.1093/bmb/ldt003. [DOI] [PubMed] [Google Scholar]
- 6.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22(3):503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 7.Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med. 2002;113(6):449–55. doi: 10.1016/s0002-9343(02)01228-7. [DOI] [PubMed] [Google Scholar]
- 8.Intagliata N, Koch KL. Gastroparesis in type 2 diabetes mellitus: prevalence, etiology, diagnosis, and treatment. Curr Gastroenterol Rep. 2007;9(4):270–9. doi: 10.1007/s11894-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32(3):151–9. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 10.Koch KL. Diabetic Gastroparesis. Gastroenterol Clin NA. Elsevier Inc. 2015;44(1):39–57. doi: 10.1016/j.gtc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Kofod-Andersen K, Tarnow L. Prevalence of gastroparesis-related symptoms in an unselected cohort of patients with Type 1 diabetes. J Diabetes Complications. Elsevier Inc. 2012;26(2):89–93. doi: 10.1016/j.jdiacomp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Dickman R, Wainstein J, Glezerman M, Niv Y, Boaz M. Gender aspects suggestive of gastroparesis in patients with diabetes mellitus: a cross-sectional survey. BMC Gastroenterol. BMC Gastroenterology. 2014;14(1):34. doi: 10.1186/1471-230X-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung H-K, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maleki D, Locke GR, Camilleri M, Zinsmeister aR, Yawn BP, Leibson C, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160(18):2808–16. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 15.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: The Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18(1):141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13(4):833–44. doi: 10.1023/B:QURE.0000021689.86296.e4. [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of Delayed Gastric Emptying in Diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 18.Samsom M, Bharucha A, Gerich JE, Herrmann K, Limmer J, Linke R, et al. Diabetes mellitus and gastric emptying: questions and issues in clinical practice. Diabetes Metab Res Rev. 2009;25(6):502–14. doi: 10.1002/dmrr.974. [DOI] [PubMed] [Google Scholar]
- 19.Phillips WT, Schwartz JG, McMahan Ca. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. J Nucl Med. 1992;33(8):1496–500. [PubMed] [Google Scholar]
- 20.Al-Elq AH. Current practice in the management of patients with type 2 diabetes mellitus in Saudi Arabia. Saudi Med J. 2009;30(12):1551–6. [PubMed] [Google Scholar]
- 21.MacGregor IL, Gueller R, Watts HD, Meyer JH. The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology. 1976;70(2):190–6. [PubMed] [Google Scholar]
- 22.Annese V, Bassotti G, Caruso N, De Cosmo S, Gabbrielli A, Modoni S, et al. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol. 1999;29(2):171–7. doi: 10.1097/00004836-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Sogabe M, Okahisa T, Tsujigami K, Okita Y, Hayashi H, Taniki T, et al. Ultrasonographic assessment of gastric motility in diabetic gastroparesis before and after attaining glycemic control. J Gastroenterol. 2005;40(6):583–90. doi: 10.1007/s00535-005-1592-1. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13(9 Suppl 5):S16–22. [PubMed] [Google Scholar]
- 25.Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Annals of Internal Medicine. 1993:366–75. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, et al. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92(6):968–75. [PubMed] [Google Scholar]
- 27.Datz F, Christian P, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204–7. [PubMed] [Google Scholar]
- 28.Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110(4):1036–42. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]