Abstract
Flavin-containing monooxygenases (FMOs) are important in detoxication but generally are considered not to be inducible by xenobiotics. Our recent microarray studies revealed induction of FMO2 and FMO3 mRNAs by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver of mice with wild-type aryl hydrocarbon receptor (AHR) but not in Ahr-null mice. The aim of the present study was to delineate mechanisms of FMO regulation. In adult male mice, basal FMO3 mRNA is low but was induced 6-fold at 4 h and 6000-fold at 24 h. The ED50 was approximately 1 μg/kg for FMO2 and FMO3, similar to that for the classic AHR-regulated gene, Cyp1a1. In adult female mice basal FMO3 mRNA is high and was not induced at 4 h but was elevated 8-fold at 24 h. FMO5 mRNA was significantly down-regulated by TCDD in both male and female adult mice. Juvenile mice show no sex difference in response to TCDD; FMO3 was induced 4 to 6-fold by TCDD in both sexes. Chromatin immuno-precipitation demonstrated recruitment of AHR and aryl hydrocarbon nuclear translocator proteins to Fmo3 regulatory regions, suggesting that induction by TCDD is a primary AHR-mediated event. Although FMO2 and FMO3 mRNAs were highly induced by TCDD in adult males, overall FMO catalytic activity increased only modestly. In contrast to the striking up-regulation of FMO2 and FMO3 in mouse liver, TCDD has little effect on FMO mRNA in rat liver. However, FMO2 and FMO3 mRNAs were highly induced in transgenic mice that express wild-type rat AHR, indicating that lack of induction in rat is not due to an incompetent AHR in this species.
Flavin-containing monooxygenases (FMOs) are microsomal enzymes that convert lipophilic substrates to oxygenated metabolites that are more polar and hence more readily excreted. Substrates include a wide range of nitrogen-containing and sulfur-containing drugs and xenobiotics (for review, see Krueger and Williams, 2005). FMOs are important for metabolism and detoxication of numerous therapeutic agents and environmental toxicants. However, FMOs can, in some circumstances, bioactivate particular substrates into reactive metabolites that cause toxicity. There are relatively few known endogenous substrates for FMOs, and their physiological functions are poorly understood (Krueger and Williams, 2005; Cashman and Zhang, 2006).
The human genome contains genes encoding five functional FMO proteins (FMO1–5) along with six pseudogenes (FMO6P–11P) (Hernandez et al., 2004; Cashman and Zhang, 2006). FMO3 is the best-studied human FMO and is expressed at high levels in human liver (Cashman and Zhang, 2006; Phillips et al., 2007). FMO3 has been identified as the enzyme responsible for oxidation of trimethylamine; mutations in the human FMO3 gene are responsible for the disease known as trimethylaminuria (fish odor syndrome) (Dolphin et al., 1997; Treacy et al., 1998; Motika et al., 2007; Yeung et al., 2007).
There is considerable variation in tissue distribution and species distribution of the different FMOs. In mice there are nine Fmo genes, Fmo1–6 and Fmo9, 12, and 13 (Hernandez et al., 2004). FMO expression is affected by exogenous factors such as nutritional status (Fu et al., 2004) and by endogenous factors such as age, gender, and hormonal status. Mouse hepatic FMO activity typically is higher in adult females than in adult males, primarily because testosterone suppresses expression of Fmo1 and Fmo3 genes (Falls et al., 1997). In female mice, constitutive hepatic FMO1, FMO3, and FMO5 mRNA levels peak at approximately 5 weeks of age then decline somewhat with further aging. In male mice FMO3 mRNA is maximal at 3 weeks and then is barely detectable after onset of sexual maturity (Janmohamed et al., 2004). Several hormonal and other factors that regulate developmental expression and tissue-specific expression of FMO mRNAs have been identified (Luo and Hines, 2001; Hines, 2006).
Although FMO expression is subject to extensive regulation by endogenous factors, FMOs generally have been considered not to be inducible by xenobiotic chemicals (Krueger and Williams, 2005; Cashman and Zhang, 2006; Hines, 2006). Recent microarray studies in our laboratory revealed substantial induction of FMO2 and FMO3 mRNAs by TCDD in livers of C57BL/6J mice that express wild-type AHR but not in Ahr-null mice (Tijet et al., 2006). Patel et al. (2007) also detected induction of FMO3 mRNA by the AHR agonist, β-naphthoflavone, in mouse liver. In contrast to mouse, our expression array experiments in rat liver do not show significant FMO induction by TCDD (I. D. Moffat, P. C. Boutros, R. Pohjanvirta, and A. B. Okey, manuscript in preparation).
The AHR is a ligand-activated transcription factor and a member of the basic-helix-loop-helix period-aryl hydrocarbon nuclear translocator (ARNT) single-minded family. Essentially all biochemical and toxic responses to TCDD and related halogenated aromatic hydrocarbons are mediated by the AHR (for review, see Okey, 2007). Upon binding ligand the AHR translocates to the nucleus where it dimerizes with ARNT. The AHR/ARNT complex binds DNA sequences known as aryl hydrocarbon response elements (AHREs) and activates expression of AHR target genes (for review, see Hankinson, 2005; Okey, 2007). The goal of the present work was to further define the AHR-mediated mechanism by which TCDD induces FMOs in mice.
Materials and Methods
Chemicals and Reagents
TCDD used in studies in C57BL6/N mice was from Wellington Laboratories (Guelph, ON, Canada). TCDD used in studies in transgenic mice was purchased from the Ufa Oil Institute (Ufa, Russia) and was >99% pure as determined by gas chromatography-mass spectrometry. 5,5′-Dithiobis(2-nitrobenzoate) (DTNB), methimazole (MMI), dithiothreitol (DTT), and NADPH were from Sigma-Aldrich (St. Louis, MO). Mouse anti-AHR antibody (SA-210) was from BIOMOL Research Laboratories (Ply-mouth Meeting, PA); human anti-ARNT and rabbit anti-IgG antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Power SYBR Green PCR Master Mix, TaqMan Universal PCR Master Mix, and 96-well real-time polymerase chain reaction (PCR) plates were from Applied Biosystems (Foster City, CA). All other reagents were of the highest quality available from commercial sources.
In Vivo Experimental Design
Juvenile (3 weeks old) or adult (8 weeks old) male and female C57BL6/N mice were obtained from Charles River Laboratories (Montreal, QC, Canada) and were housed under a 12-h light/dark cycle and given food and water ad libitum. Care and treatment of the mice were in compliance with the guidelines of the Canadian Council on Animal Care, and the protocol was approved by the Hospital for Sick Children Animal Care Committee (Toronto, ON, Canada). Mice received i.p. injections of TCDD (30 μg/kg b.wt.), dissolved in corn oil vehicle (Tijet et al., 2006) or corn oil alone and exposed for 2, 4, or 24 h. In the dose-response experiment adult male mice received injections of 0.1, 1.0, or 10 μg/kg TCDD and were exposed for 24 h. Animals were sacrificed by cervical dislocation, and livers were snap-frozen and stored at −80°C for chromatin immunoprecipitation (ChIP) assays and enzyme activity assays, or liver samples were cut into smaller pieces and stored in RNAlater for RNA isolation and real-time PCR analysis.
Development of Transgenic Mice
Constructs for wild-type rat AHR ligated to an EF-1α1 promoter were isolated from plasmids and microinjected into fertilized mouse oocytes with the C57BL/6JOlaHsd strain as recipient. The endogenous mouse AHR was then eliminated by selective crossing with Ahr-null mice. Expression of the transgene was confirmed by measurement of its mRNA (by real-time RT-PCR) and protein (by immunoblotting). The rat AHR transgene fully supports induction by TCDD of the classic AHR-regulated mouse genes, Cyp1a1 and Cyp1a2, in liver and is expressed there at levels some 5 times higher than the endogenous wild-type AHR in C57BL/6 mice. The procedure will be described in detail in a forthcoming article (R. Pohjanvirta, manuscript in preparation).
Real-Time RT-Quantitative PCR
Probes and primers for mouse FMO1 (NM_010231), FMO2 (NM_018881), FMO3 (NM_008030), FMO4 (NM_144878), and FMO5 (NM_010232) were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (Rozen, 2000). Liver total RNA was isolated using a Spin Mini kit (GE Healthcare, Chalfont St. Giles, UK). Real-time RT-PCR was performed using 1 μg of total RNA and random hexamer primers by a standard procedure. Two microliters of the RT reaction was used for each real-time quantitative PCR reaction. Each reaction mixture (25 μl) contained optimized probe and primer concentrations as well as TaqMan Universal Master Mix. Expression levels of target mRNA were normalized to levels of 18S rRNA and analyzed using the comparative CT (ΔΔCT) method. Primer sequences are given in Supplemental Table 1.
In Vivo ChIP Assay
ChIP assays were performed according to Matthews et al. (2005) except that 100 to 120 mg of snap-frozen liver was homogenized and cross-linked in 1% formaldehyde, and the DNA was isolated using a PCR purification kit (BioBasic, Inc., East Markham, ON, Canada) and eluted in 50 μl. ChIP DNA (1 μl) was amplified by PCR with primers 5′AAGCCAAGC-CAGAAAATCAA3′ and 5′TCGAGGAACAGAGTGCAATG3′ for the mouse Fmo3 AHRE (−3183 to −3260 relative to the transcriptional start site). For real-time PCR, Power SYBR Green PCR Master Mix was used to amplify the DNA fragments.
Preparation of Hepatic Microsomes
Liver samples (100 mg) were homogenized with 5 volumes of ice-cold homogenization buffer containing 0.1 mM potassium phosphate, pH 7.5, with 0.1 mM EDTA and 1.15% KCl. The homogenate was centrifuged at 10,000g for 15 min at 4°C in a refrigerated centrifuge. The supernatant was centrifuged at 100,000g for 60 min at 4°C. The firmly packed microsomal pellets were resuspended by homogenization in 0.1 mM potassium phosphate, pH 7.5, with 0.1 mM EDTA and 0.25 M sucrose. Microsomes were stored at −80°C until use. The microsomal protein concentration was measured using the method of Bradford (1976) (BioRad kit; Bio-Rad Hercules, CA) with bovine serum albumin as the standard protein.
Enzyme Activity Assay
FMO activity was measured by monitoring MMI oxidation as described by Dixit and Roche (1984). Sample and reference cuvettes contained a 1-ml mixture of 0.1 M Tricine-KOH, pH 8.5, 0.1 mM EDTA, 0.06 mM DTNB (in 0.1 M calcium phosphate buffer, pH 8.0), 0.025 mM DTT, 0.1 mM NADPH, 100 μg of microsomal proteins, and 2.0 mM MMI. In brief, Tricine-EDTA buffer, NADPH, and microsomes were mixed and incubated at room temperature for 40 s. DTT, DTNB, and methimazole (in the sample cuvette only) were added, and the reaction was incubated at 37°C in the chamber of a recording dual-beam spectrophotometer (DU-65; Beckman Coulter, Inc., Fullerton, CA). Methimazole was added to the sample cuvette, and absorbance was recorded for 10 min at 412 nm. The reaction mixture excluding methimazole was used as reference.
Statistics
Statistical significance was tested using two-tailed unpaired t tests with Welch’s adjustment for heteroscedasticity (Excel 2002; Microsoft, Redmond, WA).
Results
Recent microarray studies from our laboratories showed induction of FMO2 and FMO3 mRNAs in livers of C57BL/6J mice exposed to TCDD but not in livers from AHR knockout (Ahr−/−) mice (Tijet et al., 2006). To determine the breadth of the FMO response to TCDD, in the current project we measured mRNA levels for the five most prominent FMOs in mouse liver. As recently reported by Siddens et al. (2008), the most abundant hepatic mRNA under basal (untreated) conditions in adult C57BL/6J mice is FMO5. At a dose of 30 μg/kg for 24 h, TCDD significantly suppressed FMO5 mRNA levels in both male (Fig. 1) and in female mice (Fig. 2). In contrast, mRNA levels for FMO1, FMO2, and FMO3 are low in untreated male mice, but each of these FMOs was highly induced by TCDD; indeed, the magnitude of mRNA induction for FMO3 in adult males was similar to that of the prototypical TCDD-inducible/AHR-regulated gene, Cyp1a1 (Fig. 1). In female mice FMO1 and FMO2 mRNA levels were unaffected by 24-h exposure to TCDD, whereas FMO3 mRNA was significantly induced and FMO4 was significantly suppressed by TCDD (Fig. 2).
Fig. 1.
Male adult mice: FMO mRNA levels in livers of TCDD-treated versus control. Mice received injections of TCDD (30 μg/kg), and livers were removed 24 h later. mRNA levels for FMO1–5 were measured by real-time RT-PCR as described under Materials and Methods mRNA levels are expressed relative to the level of FMO3 mRNA in male control mice set at 1.0. **, p < 0.01 compared with vehicle control. Plotted bars in all figures represent mean ± S.D.; n = 4 mice per group.
Fig. 2.
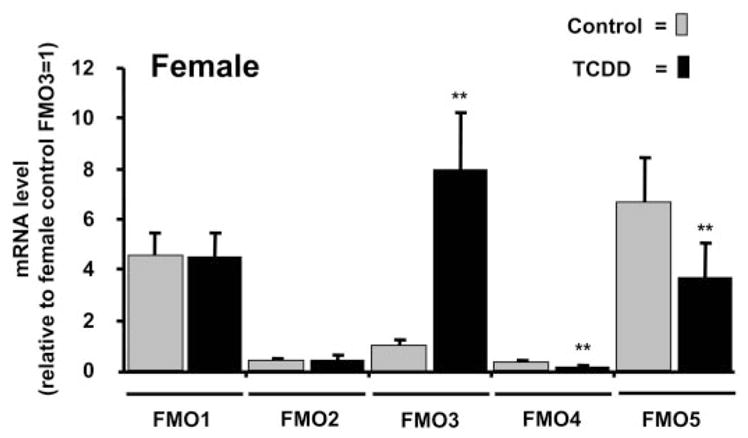
Female adult mice: FMO mRNA levels in livers of TCDD-treated versus control. Female mice received injections of TCDD (30 μg/kg), and livers were removed 24 h later. mRNA levels were measured as described for male mice in the legend to Fig. 1 and are expressed relative to the level of FMO3 mRNA in female control mice set at 1.0. **, p < 0.01 compared with vehicle control; n = 4 mice per group.
To further characterize induction of FMOs we obtained dose-response curves for TCDD induction of FMO2 and FMO3 mRNAs and found that the approximate ED50 values for induction of FMO2 and FMO3 are similar to that for CYP1A1 in male mice (Fig. 3). In livers of male mice, induction of FMO2 and FMO3 mRNAs is significant within 2 h after TCDD injection and the magnitude of induction increases further at 4 h (Fig. 4). In male mice the magnitude of FMO3 induction is dramatically elevated at 24 h, achieving mRNA levels approximately 6000-fold higher than those in control mice (Fig. 5A).
Fig. 3.

Dose-dependent induction of mRNAs for FMO2, FMO3, and CYP1A1. Adult male mice received injections of TCDD at doses of 0.1, 1.0, or 10 μg/kg. Liver was harvested 24 h later, and mRNA levels were measured by real-time RT-PCR. Results are plotted as a percentage of the maximal induced response for that particular mRNA. Error bars represent S.D.; n = 3 mice per group. Curves were fitted with GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA).
Fig. 4.
Time-dependent induction of FMO2 and FMO3 mRNAs in male mice. Mice were exposed to TCDD (30 μg/kg) for 2 or 4 h. mRNA levels were measured by real-time RT-PCR and are expressed relative to the mRNA level in control mice set at 1.0. **, p < 0.01; n = 3 mice per group.
Fig. 5.
Sex-dependent induction of FMO3 mRNA. Adult male and female mice were exposed to TCDD (30 μg/kg) for 4 h (A) or for 24 h (B). FMO3 mRNA was measured by real-time RT-PCR, and levels are expressed relative to the level in control male mice set at 1.0. Note the difference in scales of the y-axes for male versus female mice and that the induction of FMO3 after TCDD exposure in females at 4 h is not significant (n.s.) relative to the female control set at 1.0. **, p < 0.01 compared with vehicle control (n = 4).
In adult mice there is a notable sex difference in constitutive expression of FMO3 mRNA in liver in which mRNA levels in untreated animals are approximately 500 to 2300-fold higher in females than in males (Fig. 5, A and B). The reason that the constitutive level is 2300-fold higher in females in the experiment shown in Fig. 5A but only 500-fold higher in the experiment shown in Fig. 5B is not known. However, the experiment shown in Fig. 5B was conducted several months after the experiment shown in Fig. 5A; thus, the difference may reflect the sensitivity of basal FMO expression to many factors such as season and nutritional factors. The difference is not due to diurnal variation in basal expression because tissues in all experiments were harvested at a similar time of day—late morning. After 4 h of exposure to TCDD, there is no significant induction of FMO3 mRNA in females (Fig. 5B), but FMO3 mRNA is induced approximately 6-fold in males (Fig. 5A). After 24 h of exposure to TCDD, FMO3 mRNA in female mice does respond with an approximately 8-fold induction (Fig. 5B). In juvenile mice (3 weeks old) basal levels of FMO3 mRNA are 15-fold higher in females than in males, but males and females exhibit a similar magnitude of FMO3 induction, approximately 4-fold, at this age (Fig. 6). Constitutive levels of FMO3 mRNA are higher in juveniles than in adults for both males (36-fold) and females (26-fold) (data not shown).
Fig. 6.

Lack of sex difference in magnitude of FMO3 induction in juvenile mice. Three-week-old male and female mice were exposed to TCDD (30 μg/kg) for 4 h. FMO3 mRNA was measured by real-time RT-PCR, and levels are expressed relative to the level in control male mice set at 1.0. Note the difference in scales of the y-axes for male versus female mice. *, p < 0.05; **, p < 0.01 compared with vehicle control (n = 4).
As described above, our previous experiments in Ahr-null mice indicated that induction of FMO2 and FMO3 was AHR-dependent (Tijet et al., 2006). To gain further insight into the mechanism of induction, we performed ChIP assays to determine whether TCDD could evoke binding of the AHR and its dimerization partner, ARNT, to an enhancer region in the Fmo3 gene. Within 2 h after TCDD injection, both the AHR protein and the ARNT protein were recruited to the region −3260 to −3184 (relative to the transcription start site) that contains an AHRE; this TCDD-induced signal persisted at least up to 4 h (Fig. 7). There were no obvious differences in recruitment of AHR or ARNT between male and female mice (Fig. 7).
Fig. 7.
Recruitment of AHR and ARNT to the Fmo3 enhancer region (AHRE) after TCDD treatment. Adult male and female mice were exposed to TCDD (30 μg/kg) or the corn oil vehicle control (CO) for 2 h (A) or 4 h (B) before harvesting of liver. ChIP assays were performed as described under Materials and Methods with antibodies to AHR and ARNT as well as IgG (as a negative control). Primer pairs were designed to encompass the AHRE-containing region (−3183 to−3260) shown in the diagram. Results are reported as percentage enrichment of AHR and ARNT binding to this region of the total input. *, p < 0.05; **, p < 0.01 compared with vehicle control (n = 4).
Although mRNAs for FMO1, FMO2, and FMO3 are highly up-regulated by TCDD in mice, as shown in our current study and previous study (Tijet et al., 2006), we have found that in liver of TCDD-sensitive male rats carrying wild-type AHR, TCDD has negligible effects on FMO2 and FMO3 (I. D. Moffat, P. C. Boutros, R. Pohjanvirta, and A. B. Okey, manuscript in preparation). There are substantial differences in AH receptor structure between C57BL/6J mice and wild-type rat AHR (Moffat et al., 2007b; Okey, 2007). To determine whether these differences underlie the lack of FMO induction in rats we measured FMO2 and FMO3 mRNA levels in TCDD-treated transgenic mice that express wild-type rat AHR in a C57BL/6J background. As shown in Fig. 8, the wild-type rat AHR fully supports induction of the prototypical AHR-regulated gene, Cyp1a1, as well as FMO2 and FMO3 mRNAs in these transgenic animals. This result demonstrates that the failure of TCDD to up-regulate FMOs in rats is not the result of an inherent inability of wild-type rat AHR to regulate FMO genes.
Fig. 8.
FMO induction in mice that express a rat wild-type AHR transgene. Mice with the rat wild-type AHR transgene were treated with TCDD (5 μg/kg), and liver was harvested 24 h later. mRNA levels for FMO2, FMO3, and CYP1A1 are given relative to the level in control mice for that particular gene set at 1.0. Bars for control FMO3 and control CYP1A1 are not visible when plotted on this scale. **, p < 0.01 compared with vehicle control (n = 4).
We attempted to determine whether the large elevation of FMO mRNAs in TCDD-treated mice led to a corresponding increase in FMO proteins by performing immunoblotting with antibodies that had been raised against human FMO3. Because of a lack of specificity and high cross-reactivity with other proteins, it was not possible to distinguish FMO3 from other protein bands (data not shown). Unfortunately, suitable antibodies are currently not available for immunological characterization of mouse FMO proteins (Siddens et al., 2008). Thus, as an alternative index to the effect of TCDD on FMO protein, we measured FMO catalytic activity with methimazole, a substrate that is metabolized by multiple species of FMO (Zhang et al., 2007), Metabolism of methimazole was modestly but significantly increased in liver microsomes from TCDD-treated male mice but not in microsomes from female mice (Table 1).
TABLE 1. FMO enzyme activity in TCDD-treated versus control mice.
FMO catalytic activity was measured in liver microsomes from TCDD-treated (30 μg/kg; 24 h) or control mice using methimazole as substrate as described under Materials and Methods. Results are reported as mean ± S.D.
| Control | TCDD | |
|---|---|---|
|
| ||
| μmol/min/mg | ||
| Male | 3.75 ± 0.93 | 5.13 ± 0.99* |
| Female | 11.42 ± 2.34 | 11.14 ± 4.06 |
p < 0.05 compared with control.
One possible explanation for the modest increase in methimazole metabolism despite very high mRNA induction is that TCDD might inhibit catalytic function of FMO enzymes by occupying active sites on the enzyme proteins. There is precedent for such a mechanism in the cytochrome P450 field in which TCDD has been shown to bind extensively to CYP1A2 protein and inhibit CYP1A2-mediated enzyme activity (Diliberto et al., 1997; Chen et al., 2003; Staskal et al., 2005). Thus, we tested the possibility that TCDD might inhibit FMO activity by adding TCDD directly to microsomal preparations in the methimazole metabolism experiments. At a concentration of 1 μM, TCDD had no effect on metabolism of methimazole (data not shown). Therefore, the low magnitude of the increase in FMO activity in TCDD-treated animals cannot be attributed to an inhibitory effect of TCDD on FMO enzyme function.
In addition to liver, we also assessed possible induction by TCDD (30 μg/kg for 24 h) of FMO2 and FMO3 mRNA in lung, kidney, small intestine, brain, and thymus of adult male mice. We did not observe significant induction in any of these tissues (data not shown).
Discussion
FMOs are subject to regulation by hormones (Falls et al., 1997; Janmohamed et al., 2004) and nutritional status (Fu et al., 2004) but usually are considered not to be inducible by xenobiotic chemicals (Krueger and Williams, 2005; Cashman and Zhang, 2006; Hines, 2006; Phillips et al., 2007). However, we found that in mouse liver, especially in males, TCDD causes very large increases in mRNA levels for FMO1, FMO2, and FMO3. The AHR plays an essential role in this up-regulation as evidenced by our current ChIP assays and our previous experiments in Ahr-null versus wild-type mice, which showed that the AHR is required for induction of FMO2 and FMO3 mRNAs (Tijet et al., 2006).
Prolonged feed restriction has been reported to increase FMO3 mRNA levels in mice (Fu et al., 2004). Because TCDD is well known to reduce food intake in dioxin-susceptible rodents (Pohjanvirta and Tuomisto, 1994), it might be argued that FMO up-regulation is a secondary response to reduced food intake rather than a direct, AHR-mediated event. Our current study indicates that up-regulation of FMO3 mRNA is direct, via a rapid TCDD-induced recruitment of the AHR and ARNT proteins to an AHRE-containing enhancer region between −3260 to −3184 in the Fmo3 gene. Induction of FMO3 mRNA follows a pattern very similar to that of the classic AHR-regulated gene Cyp1a1 in that 1) the dose-response curves for their induction by TCDD are similar, 2) their induction is abolished in Ahr-null mice, 3) their induction is accompanied by recruitment of AHR and ARNT proteins to AHREs in upstream enhancer regions, and 4) their induction occurs within a few hours after TCDD administration in vivo.
The dramatic elevation of FMO mRNA, particularly of FMO3 in male mice, is accompanied by only a modest increase in FMO catalytic activity measured with methimazole. Methimazole was reported to be metabolized by FMO1 and FMO3 but not FMO5 when mouse FMOs are expressed as fusion proteins in Escherichia coli (Zhang et al., 2007). However, selectivity of substrates for different forms of FMO in various animal species in vivo remains poorly defined (Yeung and Rettie, 2006). The limited information on selectivity creates difficulties in the FMO field because, in contrast to P450 enzymes, there is a general lack of “probe” substrates that are reliable indicators of catalytic function for specific forms of FMO.
It is possible that the abundant FMO mRNAs in TCDD-treated mice are not able to be effectively translated into their respective proteins. Numerous microRNAs are predicted to target mouse FMO2 and mouse FMO3 (Supplemental Table 2). We previously tested the effect of TCDD on expression of microRNAs in rodent livers (Moffat et al., 2007a). Of the list of microRNAs that are predicted to target mouse FMO3 mRNA, we found that TCDD treatment caused an approximately 1.2-fold increase in levels of hsa-miR-519 in livers of C57BL/J mice. In addition, mmu-miR203 and mmu-miR-148b each are increased approximately 1.5-fold by TCDD in an AHR-dependent manner (Moffat et al., 2007a); these latter two miRNAs are closely related in sequence to miRNAs (mmu-miR-203* and mmu-148a*) that are predicted to target mouse FMO3 mRNA. It is conceivable that the concerted increase in levels of these three microRNAs in TCDD-treated mice interferes with translation of FMO3 mRNA into protein. Our previous microRNA study (Moffat et al., 2007a) also showed a 1.2-fold increase in hepatic levels of mmu-miR-203 in TCDD-treated mice; miR-203 is predicted to target mouse FMO2 mRNA, again providing a potential explanation for the relatively low increase in FMO catalytic activity compared with the large increase in FMO2 mRNA in TCDD-treated mice.
Mouse and rat are the two most extensively studied laboratory species in biochemistry, pharmacology, and toxicology. In regard to FMOs, there are notable differences in regulation between mouse and rat. As mentioned earlier, our initial expression array studies (Tijet et al., 2006) and the results of the current study show that FMO2 and FMO3 mRNAs are strongly up-regulated by TCDD in mouse liver, but this is not the case in rat liver (I. D. Moffat, P. C. Boutros, R. Pohjanvirta, and A. B. Okey, manuscript in preparation).
Moreover, for FMO1 the effect of TCDD is opposite in the two rodent species: FMO1 is significantly up-regulated in mouse liver (this study; Boutros et al., 2008) and significantly down-regulated in rat liver (Boutros et al., 2008). Our experiments in mice that express transgenic wild-type AHR show that the rat AHR is fully competent to drive induction of FMO2 and FMO3 in mice, indicating that the lack of FMO induction in rat resides in differences in non-AHR regulatory factors between rat and mouse, perhaps in the FMO gene structures themselves. Our current ChIP studies in mice revealed binding of AHR/ARNT to an AHRE in an upstream enhancer region of the mouse Fmo3 gene. Although neither the rat nor the mouse Fmo3 gene contain full-length AHRE-I motifs in its up-stream regulatory regions, both contain extended AHRE-I motifs, as well AHRE-II and antioxidant response element motifs (Supplemental Figs. 1–4). Very recently Klick et al. (2008) showed that structural differences in responsive elements in the promoter region can account for some differences among mammalian species in hepatic constitutive FMO3 expression. Although structural differences in aryl hydrocarbon response elements potentially could explain the fact that that FMO3 mRNA levels are highly up-regulated by TCDD in mouse but not in rat, our analysis of AHREs in the two species indicates that the difference in FMO inducibility cannot be explained solely by differences in regulatory motifs between their FMO3 genes (Supplemental Figs. 1–4). Instead, epigenetic factors such as DNA methylation patterns, nucleosome placements, histone modifications, or the presence/absence of interfering or obligate partner proteins may be involved.
It is not known whether TCDD can induce hepatic FMO3 in humans. We aligned the mouse AHR-associated region with human and rat genomes by a BLAT analysis (Kent, 2002) (Supplemental Fig. 5). The mouse AHR-associated region showed minimal overlap with genomic sequences in human (26 of 78 base pairs aligning) or in rat (20 of 78 base pairs aligning). Furthermore, the aligned regions are on separate chromosomes from the FMO3 gene in each species. Taken together these results suggest that the AHR-binding element is part of a mouse-specific regulatory module and, at present, it cannot be inferred from promoter analysis in silico whether human FMO3 is likely to be inducible by AHR agonists.
In rodents there are substantial differences between males and females in FMO expression. In mice, previous studies (Falls et al., 1997; Cherrington et al., 1998; Janmohamed et al., 2004) as well as our current experiments show that constitutive FMO3 is highly expressed in liver of adult female mice but is virtually absent in livers of adult male mice. However, in juvenile animals, males express significant basal levels of FMO3 mRNA, and TCDD up-regulates FMO3 to a similar extent in both sexes (Fig. 6). In mice, testosterone suppresses hepatic FMO3 and FMO1 (Falls et al., 1997). We were surprised to find that the sex differences and response to sex steroids in rats are opposite to those in mice. In rats FMO3 expression is independent of the sex (Cherrington et al., 1998), and treatment with testosterone elevates FMO levels rather than suppressing them (Dannan et al., 1986). One way to view the effect of TCDD on FMO3 expression in mice is that TCDD overcomes the suppressive action of testosterone. Female mouse livers express high constitutive levels of FMO3 that could not be further elevated by TCDD after 2 to 4 h, whereas FMO3 mRNA levels in male livers were induced 6-fold upon exposure to TCDD for 4 h. After a 24-h exposure male liver FMO3 expression levels were increased by 6000-fold and achieved female-like levels of FMO3 mRNA. Female constitutive FMO3 expression levels were high and increased only by 8-fold upon exposure to TCDD for 24 h. The mechanism by which testosterone negatively regulates FMO3 in mice is not known. The suppression in FMO3 mRNA levels seen in adult male mouse liver was not observed in kidney and lung, indicating that FMO3 is subject to tissue-specific regulation (Janmohamed et al., 2004) in addition to apparent species-specific regulation. FMO enzyme activity was higher in female liver microsomes than in microsomes from male mice in our study, both in the constitutive state and after TCDD treatment.
In summary, our present study shows that multiple FMO mRNAs in mice can, indeed, be up-regulated by a xenobiotic chemical, TCDD. This induction is mediated by the aryl hydrocarbon receptor in a fashion similar to that for the classic AHR-regulated gene, Cyp1a1. Further analytical tools, including antibodies that are selective to individual mouse FMO isoforms and substrates that can discriminate among the isoforms will be necessary to fully reveal the downstream consequences of the profound up-regulation at the mRNA level.
Supplementary Material
Acknowledgments
This work was supported by Grant MOP-57903 from the Canadian Institutes of Health (to A.B.O.) and Grant 123345 from the Academy of Finland (to R.P.).
We thank Sharon Choi, Laura MacPherson, and Agnes Forgacs for help with harvesting tissues; Ulla Naukkarinen, Janne Korkalainen, and Arja Tamminen for technical assistance; Dr. Ivy Moffat for microRNA data; and Drs. Sharon Krueger and David Williams (Oregon State University) for advice regarding FMO enzyme assays.
ABBREVIATIONS
- FMO
flavin-containing monooxygenase
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin)
- AHR
aryl hydrocarbon receptor
- AHRE
aryl hydrocarbon response elements
- DTNB
5,5′-dithiobis(2-nitrobenzoate)
- MMI
methimazole
- DTT
dithiothreitol
- PCR
polymerase chain reaction
- ChIP
chromatin immunoprecipitation
- RT
reverse transcription
- miRNA
microRNA
- CT
threshold cycle
Footnotes
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
References
- Boutros PC, Yan R, Pohjanvirta R, Okey AB. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9:419. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46:65–100. doi: 10.1146/annurev.pharmtox.46.120604.141043. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Chen GS, Bunce NJ. Inhibition of CYP 1A2-dependent MROD activity in rat liver microsomes: an explanation of the hepatic sequestration of a limited subset of halogenated aromatic hydrocarbons. Environ Toxicol. 2003;18:115–119. doi: 10.1002/tox.10107. [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Cao Y, Cherrington JW, Rose RL, Hodgson E. Physiological factors affecting protein expression of flavin-containing monooxygenases 1, 3 and 5. Xenobiotica. 1998;28:673–682. doi: 10.1080/004982598239254. [DOI] [PubMed] [Google Scholar]
- Dannan GA, Guengerich FP, Waxman DJ. Hormonal regulation of rat liver microsomal enzymes: role of gonadal steroids in programming, maintenance, and suppression of Δ4-steroid 5α-reductase, flavin-containing monooxygenase, and sex-specific cytochromes P-450. J Biol Chem. 1986;261:10728–10735. [PubMed] [Google Scholar]
- Diliberto JJ, Burgin D, Birnbaum LS. Role of CYP1A2 in hepatic sequestration of dioxin: studies using CYP1A2 knock-out mice. Biochem Biophys Res Commun. 1997;236:431–433. doi: 10.1006/bbrc.1997.6973. [DOI] [PubMed] [Google Scholar]
- Dixit A, Roche TE. Spectrophotometric assay of the flavin-containing monooxygenase and changes in its activity in female mouse liver with nutritional and diurnal conditions. Arch Biochem Biophys. 1984;233:50–63. doi: 10.1016/0003-9861(84)90600-3. [DOI] [PubMed] [Google Scholar]
- Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- Falls JG, Ryu DY, Cao Y, Levi PE, Hodgson E. Regulation of mouse liver flavin-containing monooxygenases 1 and 3 by sex steroids. Arch Biochem Biophys. 1997;342:212–223. doi: 10.1006/abbi.1997.9965. [DOI] [PubMed] [Google Scholar]
- Fu C, Xi L, Wu Y, McCarter R, Richardson A, Hickey M, Han ES. Hepatic genes altered in expression by food restriction are not influenced by the low plasma glucose level in young male GLUT4 transgenic mice. J Nutr. 2004;134:2965–2974. doi: 10.1093/jn/134.11.2965. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Janmohamed A, Chandan P, Phillips IR, Shephard EA. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004;14:117–130. doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Hines RN. Developmental and tissue-specific expression of human flavin-containing monooxygenases 1 and 3. Expert Opin Drug Metab Toxicol. 2006;2:41–49. doi: 10.1517/17425255.2.1.41. [DOI] [PubMed] [Google Scholar]
- Janmohamed A, Hernandez D, Phillips IR, Shephard EA. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos) Biochem Pharmacol. 2004;68:73–83. doi: 10.1016/j.bcp.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Kent WJ. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klick DE, Shadley JD, Hines RN. Differential regulation of human hepatic flavin containing monooxygenase 3 (FMO3) by CCAAT/enhancer-binding protein β (C/EBPβ) liver inhibitory and liver activating proteins. Biochem Pharmacol. 2008;76:268–278. doi: 10.1016/j.bcp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Hines RN. Regulation of flavin-containing monooxygenase 1 expression by ying yang 1 and hepatic nuclear factors 1 and 4. Mol Pharmacol. 2001;60:1421–1430. doi: 10.1124/mol.60.6.1421. [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlén B, Thomsen J, Gustafsson JA. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor α to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Celius T, Lindén J, Pohjanvirta R, Okey AB. microRNAs in adult rodent liver are refractory to dioxin treatment. Toxicol Sci. 2007a;99:470–487. doi: 10.1093/toxsci/kfm189. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Roblin S, Harper PA, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor splice variants in the dioxin-resistant rat: tissue expression and transactivational activity. Mol Pharmacol. 2007b;72:956–966. doi: 10.1124/mol.107.037218. [DOI] [PubMed] [Google Scholar]
- Motika MS, Zhang J, Cashman JR. Flavin-containing monooxygenase 3 and human disease. Expert Opin Drug Metab Toxicol. 2007;3:831–845. doi: 10.1517/17425255.3.6.831. [DOI] [PubMed] [Google Scholar]
- Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci. 2007;98:5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- Patel RD, Hollingshead BD, Omiecinski CJ, Perdew GH. Aryl-hydrocarbon receptor activation regulates constitutive androstane receptor levels in murine and human liver. Hepatology. 2007;46:209–218. doi: 10.1002/hep.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips IR, Francois AA, Shephard EA. The flavin-containing monooxygenases (FMOs): genetic variation and its consequences for the metabolism of therapeutic drugs. Curr Pharmacogenomics. 2007;5:292–313. [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for General Users and for Biologist Programmers. Humana Press; Totawa, NJ: 2000. [DOI] [PubMed] [Google Scholar]
- Siddens LK, Henderson MC, Vandyke JE, Williams DE, Krueger SK. Characterization of mouse flavin-containing monooxygenase transcript levels in lung and liver, and activity of expressed isoforms. Biochem Pharmacol. 2008;75:570–579. doi: 10.1016/j.bcp.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal D, Diliberto J, Devito M, Birnbaum L. Inhibition of human and rat CYP1A2 by TCDD and dioxin-like chemicals. Toxicol Sci. 2005;84:225–231. doi: 10.1093/toxsci/kfi090. [DOI] [PubMed] [Google Scholar]
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. The aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 2006;69:140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- Treacy EP, Akerman BR, Chow LM, Youil R, Bibeau C, Lin J, Bruce AG, Knight M, Danks DM, Cashman JR, et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum Mol Genet. 1998;7:839–845. doi: 10.1093/hmg/7.5.839. [DOI] [PubMed] [Google Scholar]
- Yeung CK, Adman ET, Rettie AE. Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria. Arch Biochem Biophys. 2007;464:251–259. doi: 10.1016/j.abb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CK, Rettie AE. Prochiral sulfoxidation as a probe for flavin-containing monooxygenases. Methods Mol Biol. 2006;320:163–172. doi: 10.1385/1-59259-998-2:163. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cerny MA, Lawson M, Mosadeghi R, Cashman JR. Functional activity of the mouse flavin-containing monooxygenase forms 1, 3, and 5. J Biochem Mol Toxicol. 2007;21:206–215. doi: 10.1002/jbt.20176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







