Abstract
There is growing evidence that some terrestrial avian species may play a role in the genesis of influenza viruses with pandemic potential. In the present investigation, we examined whether quail, a widespread-farmed poultry, possess the proper characteristics for serving as an intermediate host for the zoonotic transmission of influenza viruses. Using a lectin-based staining based on specific agglutinins, we found that, in addition to the presence of sialic acid α2,3-galactose (SAα2,3-gal) linked receptors, there are abundant sialic acid α2,6-galactose (SAα2,6-gal) linked receptors in quail trachea and intestine. The presence of abundant SAα2,6-gal-linked receptors explains, at least in part, the circulation of avian influenza viruses with human-like receptor specificity in quail. In quail trachea, SAα2,3-gal linked receptors are present primarily in non-ciliated cells, while SAα2,6-gal linked receptors are localized predominantly on the surface of ciliated cells. In quail intestine, both types of receptors were found on epithelial cells as well as in crypts. In a solid-phase overlay binding assay, both avian and human influenza viruses bind to plasma membranes prepared from epithelial cells of quail trachea and intestine, strongly suggesting that these receptors are functional for binding of influenza viruses from different species. Together with previous observations, these results are consistent with the notion that quail could provide an environment for the spread of reassortants between avian and human influenza viruses, thus acting as a potential intermediate host.
Keywords: Influenza virus, Sialic acid receptors, Plasma membranes, Quail
Introduction
Influenza virus infections are mediated by specific interactions between the viral hemagglutinin (HA) and cell oligosaccharides containing sialic acid (SA) residues. The majority of avian influenza viruses bind to receptors with sialic acids having an α2,3 linkage to the penultimate galactose (SAα2,3-gal), while human viruses prefer receptors that are present with an α2,6 linkage (SAα2,6-gal). Avian influenza viruses replicate poorly in humans (Beare and Webster, 1991), partially due to restrictions in receptor specificity. It is commonly accepted that, to alter their host range, avian influenza viruses need to overcome this selective binding mechanism. One possible way of doing so is through infection of animals that have both types of receptors. Pigs carry both types of receptors and are postulated to act as intermediate hosts (Ito et al., 1998; Scholtissek et al., 1998), in which avian and human viruses can reassort and consequently generate viruses with the ability to overcome the host barrier. Recent evidence suggests that other animals, particularly some terrestrial poultry, may also provide an environment similar to the one in pigs by displaying both SAα2,3-gal and SAα2,6-gal receptors. Gambaryan et al. (2002) have demonstrated the presence of both SAα2,3-gal and SAα2,6-gal receptors in chickens, adding to the notion that chicken could act as a potential intermediate host for the transmission of influenza viruses from aquatic birds to humans. Japanese quail (Coturnix coturnix) have also been suggested to be involved in the interspecies transmission of influenza viruses (Gambaryan et al., 2002; Liu et al., 2003; Perez et al., 2003a; Webster et al., 2002).
Quail are raised worldwide, especially in East Asian countries, and are often sold in live-bird markets. The first reported case of influenza A respiratory disease in quail occurred in Italy during 1966 to 1968 (Nardelli et al., 1970). Influenza viruses have since been isolated from quail in sporadic surveillance studies in North America, Europe and Asia (Guan et al., 1999; Saito et al., 1993; Suarez et al., 1999), and it appears that certain subtypes (H6, H9) of influenza viruses have established stable lineages in this species in Asia (Chin et al., 2002; Guan et al., 1999, 2000, 2002, 2004). Interestingly, quail infected with the highly pathogenic virus Turkey/Ontario/7732/66 (H5N9) showed no signs of disease but could transmit the virus to chickens and cause death (Tashiro et al., 1987). More recently, quail have been shown to carry avian influenza viruses whose genes were similar to the H5N1 and H9N2 viruses associated with infections in humans (Guan et al., 1999; Guo et al., 2000a; Lin et al., 2000). These observations highlight the need for a better understanding of the role of quail as an intermediate host of influenza A viruses. To further evaluate the molecular basis for the role of quail as a potential intermediate host, we examined the types and distribution of receptors for influenza viruses in quail. We also assayed the relative binding of both avian and human viruses to plasma membranes prepared from quail trachea and intestine and compared them to plasma membranes from other animal species including duck, pig and chicken.
Results and discussion
Quail carry both SAα2,3-gal and SAα2,6-gal linked receptors
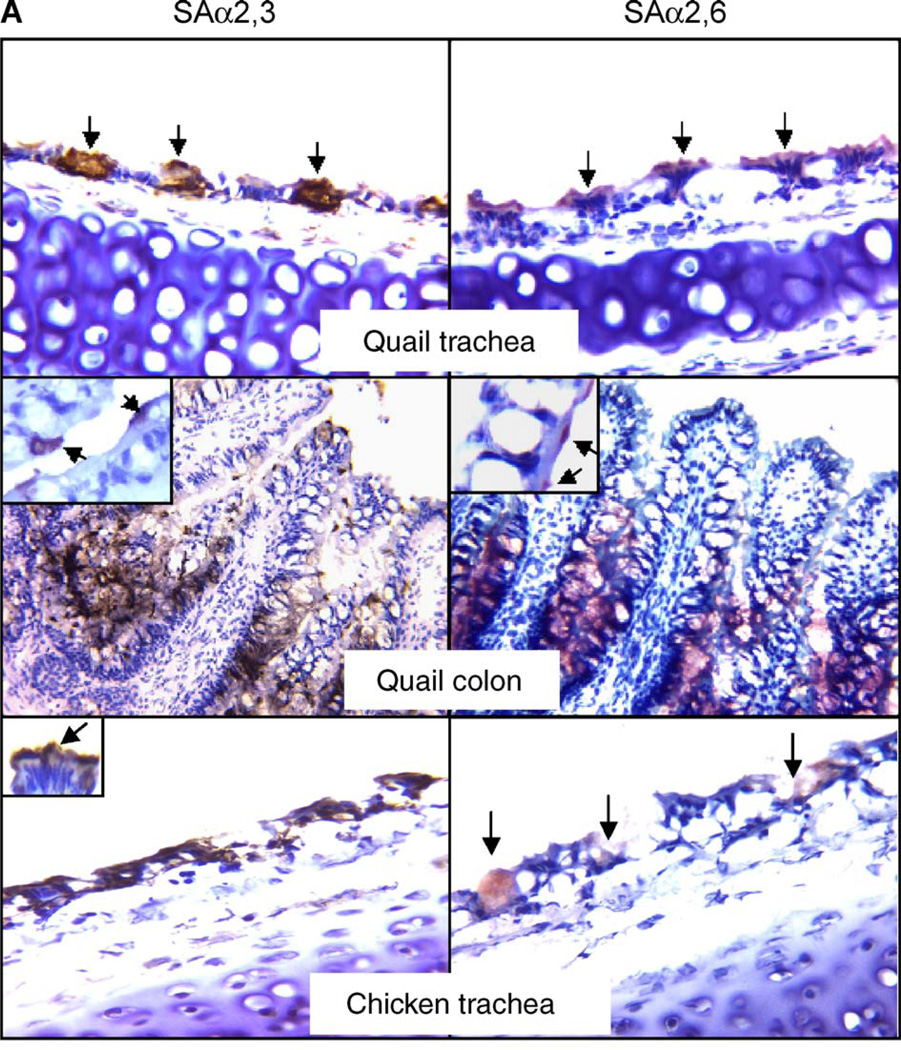
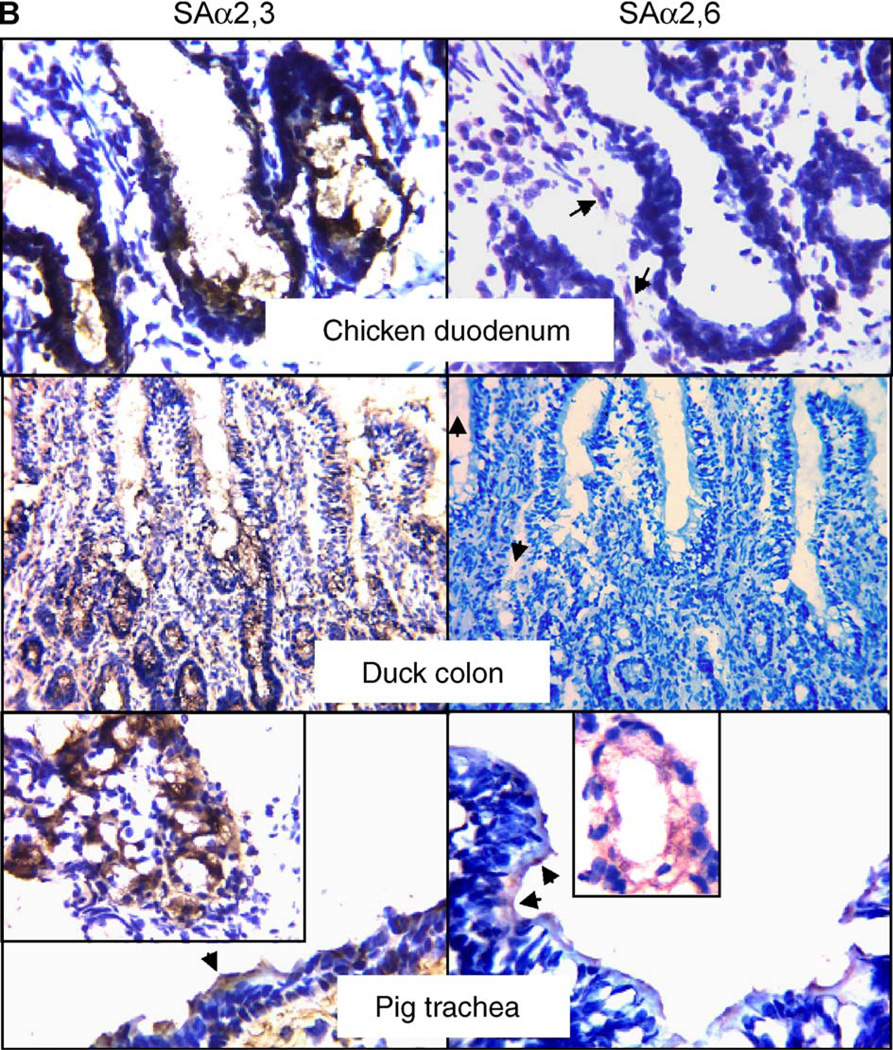
By performing a lectin-based staining, we found that quail trachea and intestine show strong reaction with Maackia amurensis agglutinin (MAA) specific for SAα2,3-gal and Sambucus nigra agglutinin (SNA) specific for SAα2,6-gal. This result, shown in Fig. 1, suggests that both SAα2,3-gal and SAα2,6-gal linked receptors are present in quail. The positive reaction of SNA with quail tissues was readily blocked by transferrin in a competitive assay for SAα2,6-gal receptors (Fig. 2), supporting the specificity of our assay and suggesting the presence of SAα2,6-gal in quail tissues. In quail trachea, the majority of epithelial cells (62%) are positive for SAα2,6-gal staining, with 30% of epithelial cells positive for SAα2,3-gal staining. In quail colon, approximately 75% of the epithelial cells are positive for SAα2,3-gal staining, while 36% are positive for SAα2,6-gal staining. By comparing similar sections stained for either SAα2,3-gal receptors, SAα2,6-gal receptors or β-tubulin, which acts as a marker for ciliated cells, we found that, in quail trachea, the SAα2,3-gal receptors are present primarily in non-ciliated cells, while the SAα2,6-gal residues are located predominantly on ciliated cells (Fig. 3). In the quail colon, SAα2,3-gal linked receptors were found on the surface of microvillus epithelial cells, as well as in crypts. The SAα2,6-gal moieties were mainly observed in cryptal cells, although some microvillus epithelial cells showed positive staining (see Fig. 1). As a comparison, we utilized a similar methodology to determine the presence of sialic acid receptors in chickens. Chicken duodenum was used instead of colon, due to some difficulties in obtaining colon sections with adequate morphology. We observed that both chicken trachea and intestine contain large amounts of cells positive for SAα2,3-gal linked receptors, with those in the intestine (duodenum) located predominantly in crypts. Approximately 85% of the epithelial cells in chicken trachea are positive for SAα2,3-gal staining, while only approximately 10% of cells were observed to be positive for SAα2,6-gal receptor staining. Chicken intestine epithelial cells were negative for SAα2,6-gal linked receptor staining except for a weak positive staining in the connective tissue of microvillus. We included duck colon and pig trachea as experimental controls for the lectin-binding assays. Duck colon displayed only SAα2,3-gal linked receptors. Pig trachea was found to carry both types of receptors, with the most intensive staining being in the seromucous gland. Our results are consistent with previous observations and suggest that our findings in quail and chicken tissues are unlikely to be the result of spurious or nonspecific staining.
Fig. 1.
Immunologic detection of SAα2,3-gal and SAα2,6-gal linked receptors in quail, chicken, duck and pig. Sections were exposed to specific agglutinins and corresponding antibody for immunostaining and were counterstained with hematoxylin. The brown color (developed from DAB) indicates the presence of SAα2,3-gal. The red color (developed from AEC) highlights the presence of SAα2,6-gal. The original magnification of quail colon and duck colon is 400×, while those of the remaining sections are at 1000×. Insets are magnified views of SAα2,3-gal or SAα2,6-gal present in epithelial cells of corresponding tissues, while those in pig photos show the staining of receptors in seromucous glands. Arrows emphasize the positively stained cells. Note that, in photos of quail trachea, the staining for SAα2,3-gal is located mainly in mucin-producing cells, whereas the staining for SAα2,6-gal is seen mainly in ciliated cells. The arrows in the photo of chicken duodenum show slight, nonspecific staining in the connective tissue. Likewise, weak positive for SAα2,6-gal staining is detected in the connective tissue of duck intestine.
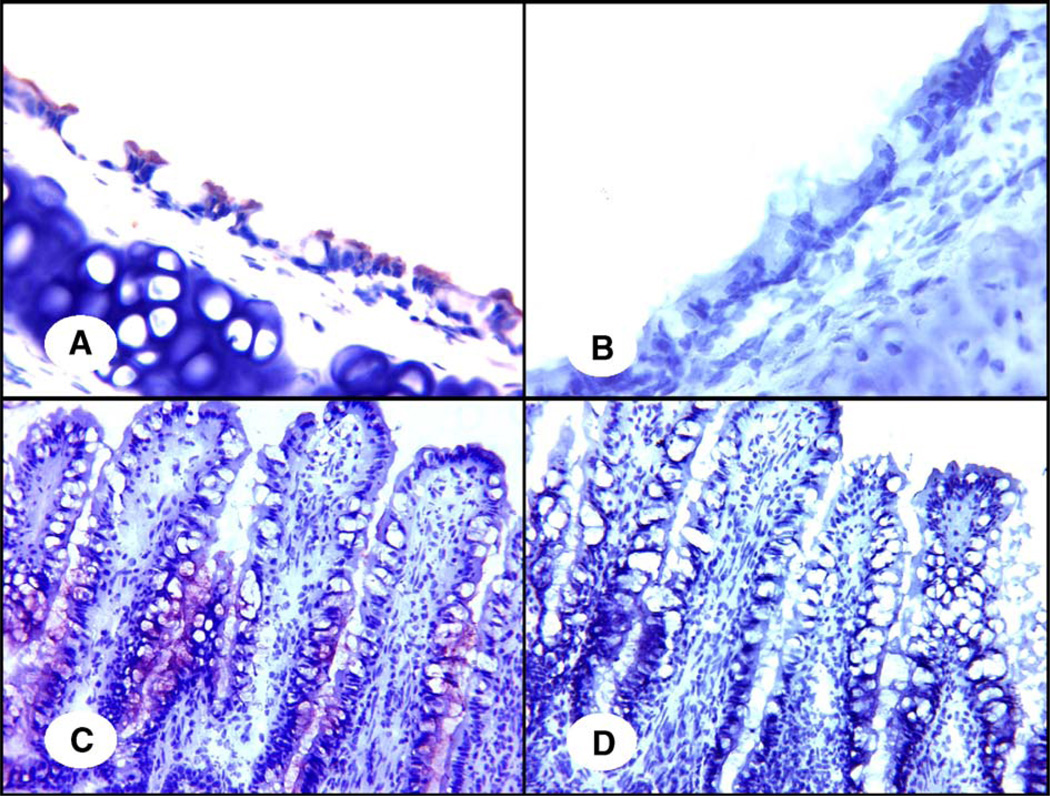
Fig. 2.
Specificity of the reaction between SNA and quail tissues. In panels A and C, the SAα2,6-gal-specific SNA (1 µg/ml) was applied directly in the lectin staining as described under Materials and methods. In panels B and D, the SAα2,6-gal specific SNA was incubated with 20 µg/ml transferrin before staining. Panels A and B indicate the trachea; panels C and D indicate the colon. Original magnification is 400×. Red staining indicates the presence of SAα2,6-gal receptors.
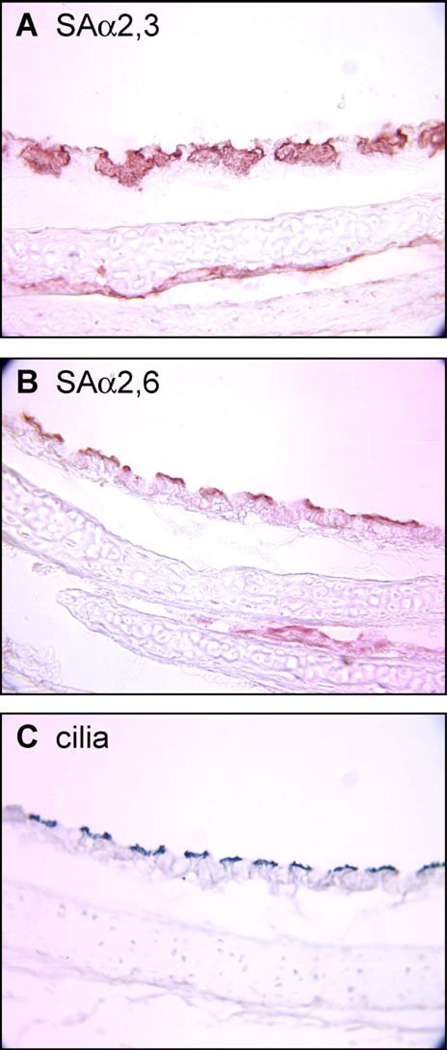
Fig. 3.
Presence of SAα2,6-gal in ciliated cells of the respiratory tract of quail. Three similar sections of quail trachea were stained to detect the presence of either SAα2,3-gal (A), SAα2,6-gal (B) or β-tubulin, a marker for ciliated cells (C) as described in Materials and methods. The staining shows the abundant presence of SAα2,3-gal receptors in mucin-producing, nonciliated cells. The SAα2,6-gal receptor expression matches the expression pattern of β-tubulin in ciliated cells.
A previous investigation (Couceiro et al., 1993) established that in human trachea SAα2,6-gal residues were found on the surface of ciliated tracheal epithelium, whereas SAα2,3-gal residues were mostly localized to intracellular mucin droplets. This information is in sharp contrast to the distribution pattern of receptors reported by Matrosovich et al. (2004) using a human tracheal/bronchial epithelial cell system. Residues from SAα2,6-gal were observed on the surface of non-ciliated cells in the human tracheal/bronchial system, with the SAα2,3-gal present on the surface of ciliated cells. Whether this contrasting difference is the result of the complexities associated with modulation of glycosylation patterns in human cells in vivo versus human cells artificially differentiated in vitro remains unknown. Our results suggest that the distribution model of receptors in quail fits the pattern observed in human trachea by Couceiro et al. Our studies also showed a less defined pattern of receptor distribution in chickens. SAα2,6-gal residues in chicken trachea were observed only in a small number of non-ciliated epithelial cells, whereas strong reaction to SAα2,3-gal moieties staining was found in both ciliated and non-ciliated cells. As the ciliated cells and non-ciliated cells differ in both physiology and function, it may be of importance to examine their role in supporting the replication and spread of influenza viruses in avian species. This question, however, is beyond the scope of this report.
The presence of abundant SAα2,6-gal residues provides a molecular platform for the potential infection of quail with human influenza viruses. Indeed, a human-like H1N1 virus has been isolated from quail during a surveillance study in Nanchang, China (Liu et al., 2003). The virus is antigenically and molecularly similar to A/New Caledonia/20/99, the prototype of H1N1 viruses currently circulating in humans worldwide. Experimental studies show that some quail can be infected with A/New Caledonia/20/99 virus, causing a respiratory infection and virus shedding for up to 4 days (Liu et al., 2003). This suggests the possibility that human influenza viruses could replicate, albeit inefficiently, in this species. On the other hand, some H9N2 viruses isolated from quail exhibited human-virus-like receptor specificity, preferentially binding to SAα2,6-gal receptor analogs but not to that of SAα2,3-gal residues (Matrosovich et al., 2001). This indicates that viruses with human-virus-like receptor specificity can replicate and transmit in quail. Furthermore, the H9N2 viruses that caused human infection in 1999 (Guo et al., 2000b; Peiris et al., 1999) were genetically (99% or greater homology) and antigenically very closely related to the viruses from quail (Lin et al., 2000), suggesting epidemic links between the quail H9N2 viruses and the human infection. Taken together, these results highlight the possibility that quail can be one of the sources for influenza viruses with potential risk for humans.
Both avian and human influenza viruses can bind to plasma membranes from quail
To further refine our investigation of the functionality and distribution of receptors for influenza virus present in quail, we performed a solid-phase overlay binding assay to test the ability of influenza viruses from different species to bind to the plasma membranes of epithelial cells derived from quail trachea and intestine. Plasma membranes from chicken, pig and duck were included for comparison purposes.
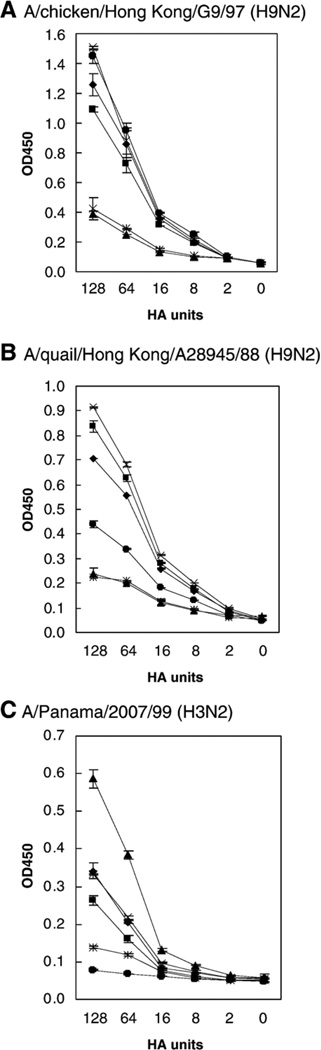
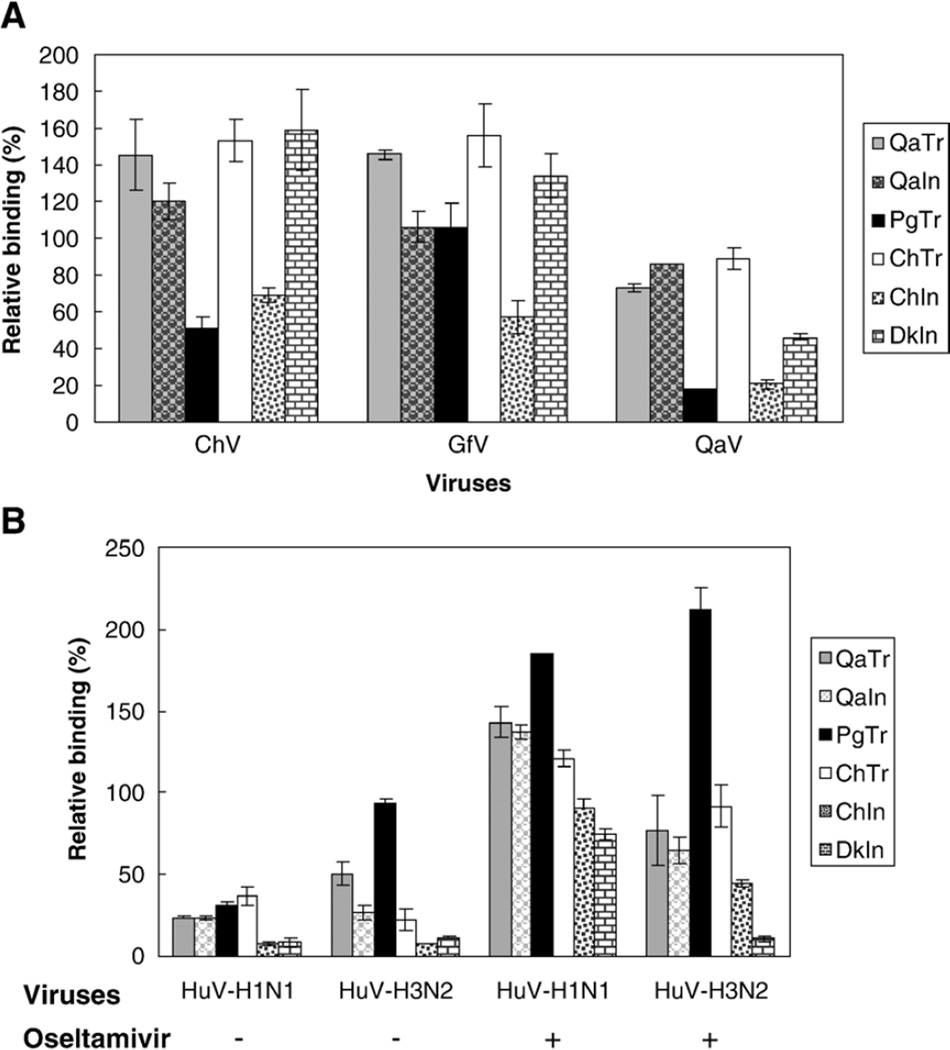
We first plotted the binding curves with OD450 values corresponding to different doses of virus. As shown in Fig. 4, the OD450 values correlated well with the virus doses used. Particularly, when the HA units were higher than 16, the values increased significantly and varied clearly among different plasma membranes. According to this result and those from a different group (Gambaryan and Matrosovich, 1992; Gambaryan et al., 1998), we used 64 HA units (~106 TCID50 in MDCK cells) for each virus in the following relative binding assays. As shown in Fig. 5A, we found that the two prototype H9N2 avian influenza viruses used in these initial assays bound readily to the membranes derived from avian species, corroborating the presence of the SAα2,3-gal sequences. Interestingly, although the chicken H9 subtype virus A/chicken/Hong Kong/G9/97 (H9N2) has been reported elsewhere (Matrosovich et al., 2001) to show human-virus-like receptor specificity, our studies suggest that it still retains substantial preference for avian-virus-like receptors, evidenced by the strong binding to membrane preparations from duck intestine. Similar results were found when we used another avian H9 virus A/guinea fowl/Hong Kong/WF10/99 (H9N2) (Fig. 5A). These two most recent H9 viruses bound to plasma membranes much more strongly than other H9 viruses tested isolated prior 1997 (only data for A/quail/Hong Kong/A28945/88 (H9N2) are shown). With respect to the human viruses, the binding of an H3N2 and an H1N1 virus was tested in our plasma membrane binding assays. Our initial OD values for the binding of human influenza viruses to plasma membranes were significantly lower than those obtained with the avian influenza viruses. To find out if blocking of the neuraminidase activity would result in better binding of the human influenza viruses to the membrane preparations, we applied 1 µM Oseltamivir in the reaction buffer for diluting the human viruses as described in Materials and methods. Efficient binding of A/Panama/2007/99 (H3N2) to plasma membranes was observed with quail trachea, quail intestine, pig trachea and chicken trachea, whereas little binding was noticed to duck intestine (Fig. 4C and Fig. 5B). Since the A/Panama/2007/99 (H3N2) virus grows exclusively in MDCK tissue culture cells and not in chicken eggs, it is tempting to speculate that the virus retains strict SAα2,6-gal linked receptor specificity. Taken together, these results suggest that SAα2,6-gal terminated receptors are present in quail trachea, quail intestine and chicken trachea and in pig trachea, corroborating our lectin staining studies. Interestingly, A/Panama/2007/99 (H3N2) also bound to plasma membranes from chicken intestine. We speculate that it is perhaps binding to SAα2,6-gal-like receptors present in the connective tissues, which showed positive reaction with SNA in the lectin staining assay. This last observation would explain binding data of human viruses to chicken membranes as described in other studies (Gambaryan et al., 2002). The other human virus tested, A/New Caledonia/20/99 (H1N1), bound to all plasma membranes including those from duck intestine. In a different assay (data not shown), the human H1N1 virus also bound effectively to plasma membranes prepared from horse trachea, which exclusively contains SAα2,3-gal receptors (Suzuki, 2005). Thus, A/New Caledonia/20/99 (H1N1) virus appears to maintain dual receptor specificity similar to other human H1N1 viruses (Rogers and D’Souza, 1989).
Fig. 4.
Dose–response curves of binding of influenza viruses to plasma membranes from quail, pigs, chickens and ducks. Binding assays were performed using two-fold serial dilution of influenza viruses A/chicken/Hong Kong/G9/97 (H9N2) (A), A/quail/Hong Kong/A28945/88 (H9N2) (B) or A/Panama/2007/99 (H3N2) (C). Data show OD450 values of the mean ± SD of two independent assays run in duplicates: quail trachea (QaTr ♦), quail intestine (QaIn■), pig trachea (PgTr▲), chicken trachea (ChTr×), chicken intestine (ChInҖ) and duck intestine (DkIn●). The results show good correlation values between HA units and OD450 values. A concentration of virus giving 64 HA units was used as the standard concentration for subsequent relative binding assays for these and other viruses, presented in Fig. 5.
Fig. 5.
Relative binding of influenza viruses to plasma membranes from quail, pigs, chickens and ducks. (A) The viruses used are represented in the x axis (from left to right) as A/Chicken/Hong Kong/G9/97 (H9N2) (ChV), A/Guinea fowl/Hong Kong/WF10/99 (H9N2) (GfV), A/Quail/Hong Kong/A28945/88 (H9N2) (QaV). (B) The x axis represents A/New Caledonia/20/99 (H1N1), (HuV-H1N1) and A/Panama/2007/99 (H3N2), (HuV–H3N2). Plasma membranes of epithelial cells from different animal species and tissues were used including quail trachea (QaTr), quail intestine (QaIn), pig trachea (PgTr), chicken trachea (ChTr), chicken intestine (ChIn) and duck intestine (DkIn). The y axis represents percentages of OD450 values normalized to that of nonspecific adsorption, with the value of negative controls being subtracted for each tissue, respectively. Each bar is a mean of two assays with each sample run in duplicates. In panel B, the first two sets on the left represent the data of relative binding without Oseltamivir, while the third and fourth sets represent data from the assays in which 1 µM Oseltamivir was applied in the reaction buffer for diluting viruses.
Given the results presented in this report, it would appear that, like pigs, quail could provide a more ideal milieu than chickens and ducks for replication and reassortment of avian and human viruses. While there is no convincing evidence at present that human viruses can replicate efficiently in quail, a human–avian recombinant virus containing the surface glycoprotein genes of a quail virus and the internal genes of human virus has been shown to replicate and transmit in quail (Makarova et al., 2003). As A/quail/Hong Kong/G1/97 (H9N2)-like viruses with human-virus-like receptor specificity continue to circulate in quail (Cameron et al., 2000; Cheng et al., 2002; Saito et al., 2001; Shaw et al., 2002; Subbarao and Katz, 2000), the possibility exists that another reassortment event could occur in this host and result in viruses with a similar pandemic potential to humans as the H5N1 viruses. Of particular interest is that both the lectin-based staining and the solid-phase overlay binding assay in the present study show that SAα2,6-gal moieties are abundant in quail trachea. Several investigations (Liu et al., 2003; Makarova et al., 2003; Perez et al., 2003a, 2003b; Webster et al., 2002) have established that, in comparison to chickens, quail are more sensitive to infection of influenza viruses. Quail are also more permissive for replication of influenza viruses in the respiratory tract than in the intestine. Surveillance studies in the 1970s identified H9 viruses in domestic ducks (Markwell and Shortridge, 1982; Shortridge, 1992). The isolation of H9N2 viruses in 1988 seems to be the first evidence of H9 viruses in land-based poultry. Since the mid-1990s, H9 viruses have become adapted to land-based poultry and have sporadically crossed to pigs and humans, causing mild respiratory disease (Cameron et al., 2000; Guan et al., 2000; Lin et al., 2000; Peiris et al., 1999; Subbarao and Katz, 2000). Some of these H9 viruses have acquired SAα2,6-gal-terminated receptor specificity (Matrosovich et al., 2001). In this respect, quail may have acted as an amplifier for the H9 viruses to spread from waterfowls to other land-based poultry, or as a “species modulator”, in which some viruses altered their receptor preference and tissue tropism, acquiring the ability to replicate and cause disease in humans. Additionally, quail were shown to be susceptible to most subtypes (at least 14 out of the 15 known to date) of avian influenza viruses (Makarova et al., 2003), which further supports the possibility that quail could act as “amplifiers” of avian reassortant viruses. In agreement with this notion, quail virus A/quail/Hong Kong/G1/97 (H9N2) is postulated to be the donor of the internal genes of the lethal Hong Kong H5N1 viruses (Guan et al., 1999, 2000).
In conclusion, the present study shows that, besides SAα2,3-gal linked receptors, there are abundant SAα2,6-gal receptors in quail. The SAα2,6-gal receptors provide quail epithelial cells with the ability to bind human influenza viruses in vitro. This could provide the necessary basis for quail to play a substantial role in interspecies transmission, or to serve as an intermediate host for generation of reassortant viruses with pandemic potential for human beings. Additional studies are needed to fully understand the role of quail in the zoonotic transmission of influenza viruses.
Materials and methods
Animal tissues
Four-week-old Japanese quail were obtained from the Department of Animal and Avian Sciences, University of Maryland, College Park. Four-week-old white leghorn SPF chickens were purchased from Charles River Laboratories (Wilmington, MA). One-day-old mallard ducks were purchased from McMurray Hatchery (Webster City, IA) and were maintained under BSL2 conditions in the Animal Facility in the Department of Veterinary Medicine until 4 weeks old. Pig tracheae were obtained from a slaughterhouse, Mount Airy Locker Company (Mt. Airy, MD).
Viruses
The influenza viruses used in this study were from the influenza repository at St. Jude’s Children’s Research Hospital. The avian influenza viruses, A/Chicken/Hong Kong/G9/97 (H9N2), A/Guinea fowl/Hong Kong/WF10/99 (H9N2) and A/Quail/Hong Kong/A28945/88 (H9N2), were propagated in 10-day-old embryonated SPF chicken eggs. Human influenza viruses A/New Caledonia/20/99 (H1N1) and A/Panama/2007/99 (H3N2) were grown on MDCK cells maintained in Opti-Mem I media. Freshly collected allantoic fluids or culture supernatants were clarified by low-speed centrifugation. The viruses were then pelleted by ultra-speed centrifugation at 25,000 rpm for 1.5 h. The pellets were resuspended in PBS containing 50% glycerol then aliquoted and stored at −20 °C for future use.
Detection of SAα2,3-gal and SAα2,6-gal linked receptors
Animals were humanely sacrificed. Trachea and intestine (small intestine or colon) were freshly collected, washed with TBS and cut into small pieces. The tissue samples were embedded in OCT compound (Sakura Finetek USA Inc., Torrance, CA) and cut into 5-µm-thick sections. After air-drying overnight, the sections were rinsed with tap water for 1 h followed by incubating with 3% H2O2 in methanol for 20 min and fixing with cold acetone for 15 min. Subsequently, the sections were blocked with 1% BSA in TBS for 1 h. For detection of receptors, the sections were incubated with digoxigenin (DIG)-labeled M. amurensis agglutinin (MAA, specific for SAα2,3-gal, Boehringer Mannheim Biochemicals, Germany) or S. nigra agglutinin (SNA, specific for SAα2,6-gal, Boehringer Mannheim Biochemicals, Germany) in buffer I (TBS containing Mg2+, Mn2+, Ca2+ and 1% BSA) for 1 h. After washing with TBS, the sections were incubated with peroxidase-conjugated anti-DIG Fab fragments (Boehringer Mannheim Biochemicals, Germany) in TBS containing 1% BSA for 1 h. After washing with 0.05 M Tris, pH 7.6, the sections were developed in a solution of diaminobenzidine (DAB, for SAα2,3-gal) or aminoethylcarbazole (AEC, for SAα2,6-gal) containing H2O2 for 10 min, counterstained with hematoxylin, mounted and observed with a light microscope. All incubation steps were done at room temperature unless otherwise described. Photos were taken using SPORT ADVANCED software. Duck colon, which has been known to have only SAα2,3-gal linked receptors, and pig trachea, which has been shown to present both SAα2,3-gal and SAα2,6-gal linked receptors, were used to serve as positive controls. Sections stained with buffer I containing no MAA or SNA were set up as negative controls. To count the epithelial cells positive for SAα2,3-gal or SAα2,6-gal staining, two similar sections from each tissue were stained and observed under light microscope at 1000× magnification. Five different fields in each section were counted, and the results were averaged. To further verify the specificity of the reaction between SNA and quail tissues, SNA (1 µg/ml) was incubated with transferrin (Boehringer Mannheim Biochemicals, Germany, 20 µg/ml) at room temperature for 2 h before being used in the staining with sections of quail trachea and colon.
Determination of the type of the cells stained with lectins
Sections of quail (Fig. 3) and chicken (not shown) trachea were processed with 3% H2O2 in methanol and cold acetone as described above. The sections were blocked with 1% BSA in PBS for 1 h and then incubated with monoclonal antibody against tubulin (Sigma, St. Louis, MO, Cat# T0198) followed by incubation with 1:200 HRP-conjugated goat-anti-mouse IgG (Sigma, St. Louis, MO, Cat# A-0168). The cilia were then demonstrated by developing the sections in Vector SG substrate (Vector laboratories, Inc., Burlingame, CA, Cat# SK-4700). Similar sections were stained with lectins as described above except that the counterstaining was omitted. The sections stained for cilia and for receptors were compared to show the type of cells stained by MAA or SNA.
Solid-phase overlay binding assay
To determine the binding ability of influenza viruses with different receptor specificities to plasma membranes of quail, we performed a solid-phase overlay binding assay as previously described (Gambaryan et al., 1998; Gambaryan and Matrosovich, 1992).
Labeling of fetuin
Bovine fetuin (Sigma, St. Louis, MO) was labeled with peroxidase, using a labeling kit (Roche Applied Science, Germany) according to the manufacturer’s protocol. The conjugate mixture was purified, using Sephacryl-200 high resolution (Amersham BioScienses, Sweden) and a PD-10 column (Amersham BioScienses, Sweden). The peroxidase–fetuin fraction was collected, stabilized, aliquoted and stored at 4 °C.
Preparation of plasma membranes
Tracheae and intestines were collected from freshly sacrificed animals and washed thoroughly with TBS. Epithelial cells were gently scratched off with a spatula, and plasma membranes were prepared as described (Gambaryan et al., 1998).
Relative binding of influenza viruses to plasma membranes
We performed the assay according to the protocols described (Gambaryan et al., 2002; Gambaryan et al., 1998) with minor modifications. Briefly, ninety six-well microplates (Costar, USA) were coated with membranes containing a protein concentration of 20 µg/ml in PBS. The plates were rinsed once with PBS. After blocking the nonspecific binding sites by incubation with 0.2% BSA in PBS, at 37 °C for 1.5 h, 50 µl per well of virus suspension in reaction buffer (PBS containing 0.02% BSA) with hemagglutination titers of 64 or other concentrations as indicated below was added and incubated at 4 °C for 1 h. After washing four times with cold 0.2× PBS containing 0.05% Tween-80, 50 µl per well of peroxidase–fetuin in PBS containing 0.02% BSA and 0.05% Tween-80 was added and incubated for 1 h at 4 °C. After washing four times with cold 0.2× PBS containing 0.05% Tween-80, 100 µl per well SureBlueTM TMB substrate (KPL, Gaithersburg, MD) was added and developed for 10 min at 37 °C. The reaction was terminated by adding 100 µl 2 N H2SO4, and OD450 was measured. For obtaining the dose–response curves, three viruses, A/Chicken/Hong Kong/G9/97, A/Quail/Hong Kong/A28945/88 and A/Panama/2007/99, were diluted with reaction buffer to get HA unit titers of 128, 64, 16, 8 and 2. Reaction buffer without virus was used as negative control for each virus. OD450 values on the y axis were plotted versus the HA units on the x axis. Results represent the average values of two independent assays performed with samples run in duplicate wells. Relative binding activities of the H3N2 and H1N1 human viruses were performed in the absence or presence of the neuraminidase inhibitor Oseltamivir (a kind gift from F. Hoffmann-La Roche Ltd.). When Oseltamivir was used, it was added to the reaction buffer to a final concentration of 1 µM. To perform the relative binding assay, all of the viruses were diluted to a final hemagglutination titer of 64. The nonspecific adsorption of virus to the plastic was set to serve as a positive control for both SAα2,3-gal and SAα2,6-gal linked receptors binding. Membrane-coated wells incubated with reaction buffer alone instead of virus suspensions were set as negative controls. Alternatively, reaction buffer containing 1 µM Oseltamivir was used as negative control. Non-coated wells incubated merely with peroxidase–fetuin were used as controls for nonspecific adsorption. We expressed the relative binding (%) as: (sample OD450 − sample control OD450) ÷ (nonspecific adsorption OD450 − nonspecific adsorption control OD450). Two separate tests were performed; duplicate wells were set in each test, and the final results are the averages of the two tests read at OD450.
Acknowledgments
The authors are grateful to Dr. Robert G. Webster and Scott Krauss for providing the influenza virus strains for this study. We would also like to thank Mt. Airy Locker Company for providing the pig tracheae used in this study. We are greatly indebted to Dr. Mary Ann Ottinger and Michael Quinn and to the Central Animal Research Facility at the University of Maryland for providing the quail used in this study. This work has been funded by grants from NIH-NIAID (RO1-AI052155) and USDA (CSREES NO 2005-35605-15388).
References
- Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch. Virol. 1991;119(1–2):37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- Cameron KR, Gregory V, Banks J, Brown IH, Alexander DJ, Hay AJ, Lin YP. H9N2 subtype influenza A viruses in poultry in Pakistan are closely related to the H9N2 viruses responsible for human infection in Hong Kong. Virology. 2000;278(1):36–41. doi: 10.1006/viro.2000.0585. [DOI] [PubMed] [Google Scholar]
- Cheng X, Liu J, He J, Shan F. Virological and serological surveys for H9N2 subtype of influenza A virus in chickens and men in Shenzhen city. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2002;16(4):319–321. [PubMed] [Google Scholar]
- Chin PS, Hoffmann E, Webby R, Webster RG, Guan Y, Peiris M, Shortridge KF. Molecular evolution of H6 influenza viruses from poultry in Southeastern China: prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)-like genes in poultry. J. Virol. 2002;76(2):507–516. doi: 10.1128/JVI.76.2.507-516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29(2):155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Matrosovich MN. A solid-phase enzyme-linked assay for influenza virus receptor-binding activity. J. Virol. Methods. 1992;39(1–2):111–123. doi: 10.1016/0166-0934(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Marinina VP, Tuzikov AB, Bovin NV, Rudneva IA, Sinitsyn BV, Shilov AA, Matrosovich MN. Effects of host-dependent glycosylation of hemagglutinin on receptor-binding properties on H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology. 1998;247(2):170–177. doi: 10.1006/viro.1998.9224. [DOI] [PubMed] [Google Scholar]
- Gambaryan A, Webster R, Matrosovich M. Differences between influenza virus receptors on target cells of duck and chicken. Arch. Virol. 2002;147(6):1197–1208. doi: 10.1007/s00705-002-0796-4. [DOI] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Webster RG. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. U.S.A. 1999;96(16):9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 2000;74(20):9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Peiris JS, Lipatov AS, Ellis TM, Dyrting KC, Krauss S, Zhang LJ, Webster RG, Shortridge KF. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. U.S.A. 2002;99(13):8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Poon LL, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Sturm-Ramirez KM, Cheung CL, Leung YH, Yuen KY, Webster RG, Peiris JS. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. U.S.A. 2004;101(21):8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, Norwood M, Shortridge KF, Webster RG, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000a;267(2):279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Xie JP, Wang M, Dong J, Gou JF, Zhang H, Wu KY. A strain of influenza A H9N2 virus repeatedly isolated from human population in China. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2000b;14(3):209–212. [PubMed] [Google Scholar]
- Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998;72(9):7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. U.S.A. 2000;97(17):9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, He S, Walker D, Zhou N, Perez DR, Mo B, Li F, Huang X, Webster RG, Webby RJ. The influenza virus gene pool in a poultry market in South central china. Virology. 2003;305(2):267–275. doi: 10.1006/viro.2002.1762. [DOI] [PubMed] [Google Scholar]
- Makarova NV, Ozaki H, Kida H, Webster RG, Perez DR. Replication and transmission of influenza viruses in Japanese quail. Virology. 2003;310(1):8–15. doi: 10.1016/s0042-6822(03)00094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell DD, Shortridge KF. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl. Environ. Microbiol. 1982;43(1):110–115. doi: 10.1128/aem.43.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich MN, Krauss S, Webster RG. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001;281(2):156–162. doi: 10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 2004;101(13):4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli L, Rinaldi A, Pereira HG, Mandelli G. Influenza virus infections in Japanese quails. Arch. Exp. Vet. Med. 1970;24:231–249. [PubMed] [Google Scholar]
- Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. Human infection with influenza H9N2. Lancet. 1999;354(9182):916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- Perez DR, Lim W, Seiler JP, Yi G, Peiris M, Shortridge KF, Webster RG. Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J. Virol. 2003a;77(5):3148–3156. doi: 10.1128/JVI.77.5.3148-3156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DR, Webby RJ, Hoffmann E, Webster RG. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 2003b;47(3):1114–1117. doi: 10.1637/0005-2086-47.s3.1114. [DOI] [PubMed] [Google Scholar]
- Rogers GN, D’Souza BL. Receptor binding properties of human and animal H1 influenza virus isolates. Virology. 1989;173(1):317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Saito T, Kawaoka Y, Webster RG. Phylogenetic analysis of the N8 neuraminidase gene of influenza A viruses. Virology. 1993;193(2):868–876. doi: 10.1006/viro.1993.1196. [DOI] [PubMed] [Google Scholar]
- Saito T, Lim W, Suzuki T, Suzuki Y, Kida H, Nishimura SI, Tashiro M. Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine. 2001;20(1–2):125–133. doi: 10.1016/s0264-410x(01)00279-1. [DOI] [PubMed] [Google Scholar]
- Scholtissek C, Hinshaw VS, Olsen CW. Influenza in Pigs and their role as the intermediate host. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science Ltd; 1998. pp. 137–145. [Google Scholar]
- Shaw M, Cooper L, Xu X, Thompson W, Krauss S, Guan Y, Zhou N, Klimov A, Cox N, Webster R, Lim W, Shortridge K, Subbarao K. Molecular changes associated with the transmission of avian influenza a H5N1 and H9N2 viruses to humans. J. Med. Virol. 2002;66(1):107–114. doi: 10.1002/jmv.2118. [DOI] [PubMed] [Google Scholar]
- Shortridge KF. Pandemic influenza: a zoonosis? Semin. Respir. Infect. 1992;7(1):11–25. [PubMed] [Google Scholar]
- Suarez DL, Garcia M, Latimer J, Senne D, Perdue M. Phylogenetic analysis of H7 avian influenza viruses isolated from the live bird markets of the Northeast United States. J. Virol. 1999;73(5):3567–3573. doi: 10.1128/jvi.73.5.3567-3573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Katz J. Avian influenza viruses infecting humans. Cell. Mol. Life Sci. 2000;57(12):1770–1784. doi: 10.1007/PL00000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses (review) Biol. Pharm. Bull. 2005;28(3):399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Reinacher M, Rott R. Aggravation of pathogenicity of an avian influenza virus by adaptation to quails. Arch. Virol. 1987;93(1–2):81–95. doi: 10.1007/BF01313895. [DOI] [PubMed] [Google Scholar]
- Webster RG, Guan Y, Peiris M, Walker D, Krauss S, Zhou NN, Govorkova EA, Ellis TM, Dyrting KC, Sit T, Perez DR, Shortridge KF. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 2002;76(1):118–126. doi: 10.1128/JVI.76.1.118-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]