Summary
Waterfowl species are known to harbor the greatest diversity of low pathogenicity influenza A virus (LPAIV) subtypes and are recognized as their main natural reservoir. In Guatemala there is evidence of circulation of LPAIV in wild ducks, however the bird species contributing to viral diversity during the winter migration in Central America are unknown. In this study, samples obtained from 1,250 hunter-killed birds from 22 different species were collected on the Pacific coast of Guatemala during three winter migration seasons between 2010 and 2013. Prevalence of LPAIV detected by real-time reverse-transcriptase polymerase chain reaction was 38.2%, 23.5% and 24.7% in the 2010-11, 2011-12, and 2012-13 seasons respectively. The highest virus prevalence was detected in the northern shoveler (Anas clypeata), followed by the blue-winged teal (Anas discors). The majority of positive samples and viral isolates were obtained from the blue-winged teal. Analysis of LPAIV prevalence over time in this species indicated a decreasing trend in monthly prevalence within a migration season. Sixty-eight viruses were isolated and 9 HA and 7 NA subtypes were identified in 19 subtype combinations. In 2012-13, the most prevalent subtype was H14, a subtype identified for the first time in the western hemisphere in 2010. The results from this study represent the most detailed description available to date of LPAIV circulation in Central America.
Keywords: Avian influenza, Guatemala, Central America, surveillance, wild birds, prevalence, subtype diversity
Resumen
Las aves acuáticas albergan la mayor diversidad de los virus de Influenza Aviar (IA) en la naturaleza, siendo reconocidas como su principal reservorio. En Guatemala, se ha documentado que los virus de IA circulan en aves acuáticas silvestres, sin embargo poco se sabe de cuáles son las especies que contribuyen a la diversidad de los virus durante la época migratoria. En este estudio se colectaron 1.250 muestras de 22 especies diferentes de aves cazadas en la costa del Pacífico de Guatemala durante tres estaciones migratorias, entre 2010 y 2013. La prevalencia de IA detectada por transcripción-inversa y reacción en cadena de la polimerasa en tiempo real fue 38.2%, 23.5% y 24.7% durante las estaciones de 2010-11, 2011-12 y 2012-13, respectivamente. La mayor prevalencia viral se observó en el pato cuchara (Anas clypeata), seguido del pato aliazul (Anas discors). Sin embargo, la mayoría de muestras positivas y aislados virales fueron obtenidos del pato aliazul. A través del análisis temporal de la prevalencia en esta especie, se observó que la prevalencia de IA disminuye a lo largo de la migración. Se aislaron sesenta y ocho virus de 9 subtipos de HA y 7 subtipos de NA en 19 combinaciones diferentes. Durante la estación migratoria de 2012-13 el subtipo más detectado fue el H14, un subtipo identificado por primera vez en el hemisferio occidental en 2010. A la fecha, los resultados de este estudio representan la única descripción a detalle de la circulación IA de baja patogenicidad reportados para Centro América.
Introduction
Despite the increased awareness of the role of wild birds in the spread of avian influenza and the need to expand global efforts for surveillance of influenza A viruses (IAV), there are still significant knowledge gaps in the ecology of IAV. This is particularly the case with respect to the viruses that circulate in Central and South America (13). In 2012, an IAV outbreak caused by a highly pathogenic avian influenza virus (HPAIV) H7N3 strain of wild bird origin in Mexico resulted in significant economic losses for one of the biggest egg and poultry producers of Latin America (6, 23). Most recently the introduction of highly pathogenic H5 viruses of Eurasian origin (22, 26) raises concerns about further virus spreading across the region, with potentially devastating consequences for the developing countries in the Americas.
In North America, mallards (Anas platyrhynchos) and northern shovelers (Anas clypeata) are two of the duck species with the highest prevalence of influenza A virus (IAV) (11, 19, 20, 43). In addition, the blue-winged teal (Anas discors) has been observed to harbor high diversity of virus subtypes in wintering grounds, in particular in locations across the Mississippi and Central migration flyways (7, 8, 16, 37, 43). Studies in wintering grounds in North America and Europe suggest that viral prevalence in waterfowl species tend to be low in comparison to the levels generally observed at the breeding grounds prior to the onset of autumn migration. The number of virus infections decreases over time in the population during an annual migration cycle that concludes with the spring migration, as birds return from the wintering grounds (15, 19, 30, 31).
In Guatemala, we have previously reported evidence of circulation of IAV from the North American lineage in wild ducks (12); however the main bird species that contribute to viral diversity during and between migration seasons remains poorly understood. Convergence of multiple flyways into a reduced geographical area, distinguishes the wintering grounds of Central America from those in North America (32). In Europe, IAV surveillance in wild birds in a location where multiple flyways overlap, provided evidence of increased gene flow between host populations from different geographical regions, resulting in high diversity of locally circulating viruses (28). Similarly, congregation of bird populations from multiple migration flyways into a geographical bottleneck in Central America may provide unique conditions for virus reassortment during the winter migration.
In this study, samples from hunter-killed waterfowl were collected on the Pacific coast of Guatemala during three consecutive winter migrations between 2010 and 2013. We estimated prevalence values for different bird species and compared subtype diversity among different seasons. In addition, we analyzed the patterns of IAV prevalence during the winter migration for blue-winged teal, the most abundantly sampled bird species, in order to characterize the dynamics of IAV circulation in wintering grounds in Central America.
Methods
Sample collection
Samples were collected from hunter-killed ducks during the winter migration season from 2010 to 2013 in the villages of El Pumpo in the department of Santa Rosa, Pasaco in the department of Jutiapa and in La Gomera in the department of Escuintla. Sampling sites and tracheal and cloacal swab collection methods from birds has been as previously described (12). Permits for sampling different bird species at the different sampling sites were obtained from the Center for Conservation Studies (CECON) and the National Council of Protected Areas (CONAP). Sampling of hunter-killed birds was exempt from animal use and care regulations from the Institutional Animal Use and Care Committees of the University of Maryland and the Universidad del Valle de Guatemala.
Virus detection
All samples were tested for the presence of IAV RNA by real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) (42). The details of the methods for RNA extraction, molecular testing and virus isolation have been described elsewhere (12). Only IAV positive samples by rRT-PCR were tested for virus isolation. The subtype of all viral isolates was identified by partial sequencing of the HA and NA genes with universal primers (21, 35).
Prevalence of IAV was estimated for each bird species as the total number of rRT-PCR positives divided by the total number of individuals from the same species tested by rRT-PCR. Confidence intervals were computed for prevalence values at the 95% confidence level when n>30. Differences in prevalence among duck species, and seasons (2010-11, 2011-12 and 2012-13) were analyzed with χ2 test at the 95% confidence level; only species with n>5 per sampling point (i.e. each collection date) were included in this analysis (blue-winged teal and northern shoveler). The calculations were done in GraphPad Prism v.6.0 (La Jolla, CA, www.graphpad.com). The variable location was excluded from the analysis as not all locations were uniformly sampled during all seasons. In addition, differences in monthly prevalence were analyzed for blue-winged teals with χ2 test at the 95% confidence level. The number of samples for the northern shoveler was not enough to do this analysis. For all analyses, a two-sided alternative hypothesis was assumed with p-values <0.05 considered significant.
In order to estimate viral diversity, the Simpson diversity index was calculated based on the number of different subtype combinations (40). This analysis was done for all the virus subtypes identified, regardless of the bird species and localities. Confidence intervals were estimated according to Grundmann et al, 2001 (14). Viral diversity was compared between seasons (2011-12 vs 2012-13) using the Sørensen-Dice coefficient (5, 41), given by CC=n1,1/(n1,0+n0,1), where n1,1 is the number of subtypes present in both years, n1,0 and n0,1 are the number of unique subtypes observed in the first and the second season respectively. A CC = 1 describes identical communities, with an increased level of differences as CC approaches to zero.
Results
Paired cloacal and tracheal swab samples were collected from 1,250 birds on three localities between October 2010 and February 2011, and between November and January in 2011-12 and in 2012-13. Eight species were sampled in 2010-11, 16 in 2011-12, and 10 in 2012-13. The number of samples birds were n=102 for the 2010-11 season, n=550 for the 2011-12 season and n=598 for the 2012-13 season. Most of the sampled birds were duck species, in particular blue-winged teals, followed by northern shovelers and green-winged teals. A small number of samples were obtained from other bird families including doves, coots, and shorebirds (Table 1).
Table 1.
Percentage prevalence for waterfowl species sampled during the wintering migration seasons in Guatemala, 2010-2013
| 2010-2011 | 2011-2012 | 2012-2013 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Family | Species (Common name) | Positive (%) |
Total | Positive (%) |
Total | Positive (%) |
Total | Positive (%) |
Total |
| Anatidae | Anas acuta (Northern Pintail) | 0(0) | 2 | 0(0) | 3 | 0(0) | 5 | ||
| Anas americana (American Wigeon) | 2(28.6) | 7 | 2(28.6) | 7 | |||||
| Anas clypeata (Northern Shoveler) | 1(25) | 4 | 28(37.8) | 74 | 8(29.6) | 27 | 37(35.2) | 105 | |
| Anas crecca (Green-winged Teal) | 0(0) | 1 | 1(6.3) | 16 | 1(100) | 1 | 2(11.1) | 18 | |
| Anas discors (Blue-winged Teal) | 29(35.4) | 82 | 99(22.8) | 434 | 135(24.5) | 550 | 263(24.7) | 1066 | |
| Anas sp. (Dabbling duck) | 4(57.1) | 7 | (0) | 4(57.1) | 7 | ||||
| Aythya affinis (Lesser Scaup) | 0(0) | 1 | 0(0) | 1 | 0(0) | 2 | |||
| Cairina moschata (Muscovy Duck) | 0(0) | 2 | 0(0) | 2 | |||||
|
Dendrocygna autumnalis (Black-bellied Whistling- Duck) |
1(50) | 2 | (0) | 1(50) | 2 | ||||
| Dendrocygna bicolor (Fulvous Whistling-Duck) | 2(100) | 2 | (0) | 2(100) | 2 | ||||
| Oxyura jamaicensis (Ruddy Duck) | 0(0) | 1 | 0(0) | 1 | 0(0) | 2 | |||
| Ciconiidae | Mycteria americana (Wood Stork) | 0(0) | 1 | 0(0) | 1 | ||||
| Columbidae | Columba flavirostris (Red-billed Pigeon) | 0(0) | 1 | 0(0) | 1 | ||||
| Streptopelia decaocta (Eurasian Collared-Dove) | 0(0) | 2 | 0(0) | 2 | |||||
| Zenaida asiatica (White-winged Dove) | 0(0) | 8 | 2(40) | 5 | 2(15.4) | 13 | |||
| Zenaida macroura (Mourning Dove) | 0(0) | 2 | 0(0) | 2 | |||||
| Pelecanidae | Pelecanus erythrorhynchos (American White Pelican) | 0(0) | 1 | 0(0) | 1 | ||||
| Phalacrocoracidae | Phalacrocorax brasilianus (Neotropic Cormorant) | 0(0) | 1 | 0(0) | 1 | ||||
| Rallidae | Fulica americana (American Coot) | 1(25) | 4 | 1(25) | 4 | ||||
| Scolopacidae | Catoptrophorus semipalmatus (Willet) | 1(50) | 2 | 1(50) | 2 | ||||
| Limnodromus scolopaceus (Long-billed Dowitcher) | (0) | 1 | 0(0) | 1 | |||||
| Threskiornithidae | Eudocimus albus (White Ibis) | 1(50) | 2 | (0) | 1 | 0(0) | 1 | 1(25) | 4 |
| Total | 39(38.2) | 102 | 129(23.5) | 550 | 148(24.7) | 598 | 316(25.3) | 1250 | |
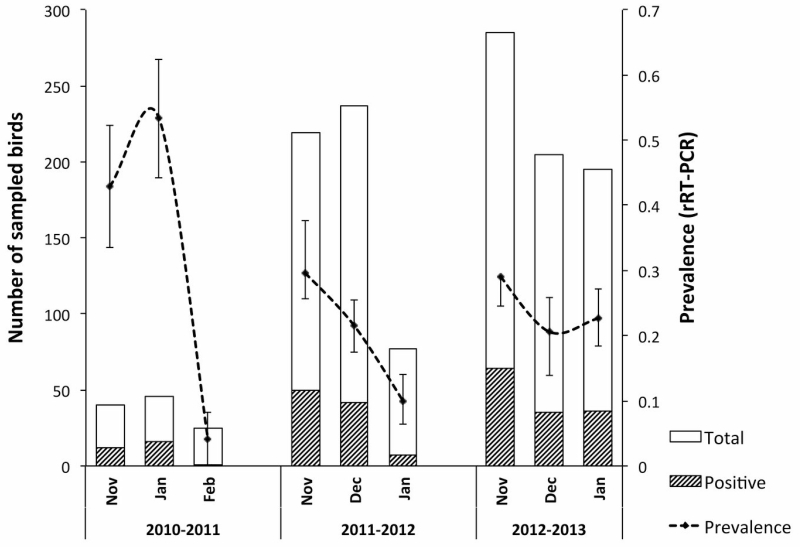
IAV was detected by rRT-PCR in 39 [38.2%(95% CI: 28.8-47.7%)] birds in 2010-11, in 129 [23.5%(95% CI: 19.9-27.0%)] birds in 2011-12 and 148 [24.7%(95% CI: 21.3-28.2%)] birds in 2012-13. Among duck species, with more than 10 sampled individuals, the higher prevalence was observed in northern shovelers [35.2%(95% CI: 26.1-44.3%), n=105], followed by blue-winged teals [24.7%(22.1-27.3%), n=1066] and green-winged teals (11.1%, n=18) (p=0.0168, χ2=8.173, df=2). Two white-winged doves tested positive for IAV (n=13) (Table 1). For the northern shoveler, a small number of samples was obtained during the 2010-11 season, and prevalence was compared only between the 2011-12 and the 2012-13 season. For this species, prevalence was not significantly different between seasons. For the blue-winged teal, prevalence was compared among all seasons and a higher prevalence was observed in 2010-11 (p=0.0117, χ2=8.901, df=2), the prevalence was similar between 2011-12 [22.8%(95%CI: 18.9-26.8%)] and 2012-13[24.5%(95%CI: 20.9-28.1%)] seasons. In the 2011-12 season, the prevalence of IAV in northern shovelers [37.8%(95%CI: 26.8-48.9)] was significantly higher (p=0.0089, χ2=6.833) than in blue-winged teals [22.8%(95%CI: 18.9-26.8%)]. Similarly, in the subsequent season (2012-13) a higher prevalence in northern shovelers was observed [29.6%(12.4-46.9) vs 24.5%(20.9-28.1)], but this difference was not significant. Differences in monthly prevalence were observed each season for blue-winged teals. For the first two seasons, a decrease in monthly IAV prevalence towards the end of the migration season was observed (Figure 1). Differences in monthly prevalence were significant for the 2011-12 season (p=1.95×10-6, χ2=22.6). In 2012-13 the differences in monthly prevalence were not significant.
Figure 1.
Temporal distribution of IAV prevalence in blue-winged teals in Guatemala, 2010-2013
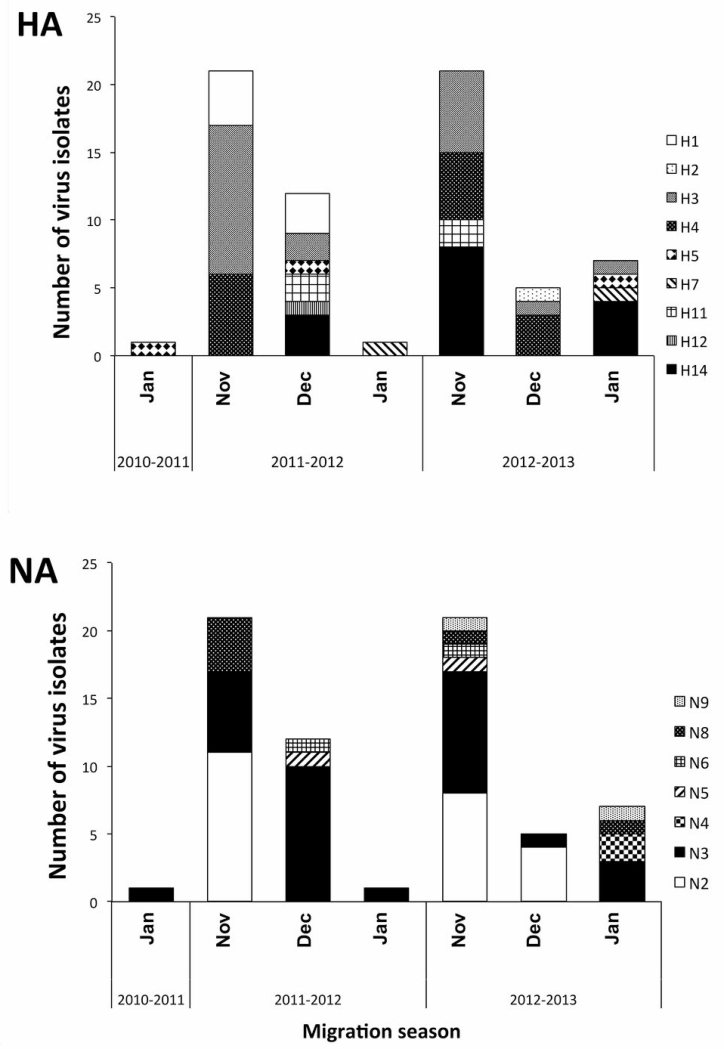
Sixty-eight viruses were isolated from 316 rRT-PCR positive samples with an isolation rate of 21.5%. The majority of isolates were obtained from blue-winged teals (n=61), and the remaining isolates were obtained from the northern shoveler (n=5), the green winged teal (n=1) and the American wigeon (n=1) (Table 2). The percentage of IAV isolation per species was 5.7% for blue-winged teals, 4.7% for northern shovelers, 5.5% for green-winged teals and 14.3% for American wigeons. A total of 19 subtype combinations were found, from 9 different HA subtypes and 7 different NA subtypes. Only one virus isolate, of the H5N3 subtype was obtained in the 2010-11 season. In the 2011-12 season, 34 isolates were obtained and the most prevalent subtype combinations were H3N2 (n=10, 29%) and H1N3 (n=7, 20%). In the season of 2012-13, 33 isolates were obtained and the most prevalent subtype was H14N3 (n=9, 27%), followed by H4N2 (n=7, 21%) and H3N2 (n=5, 15%). From the HA subtypes found, in 2011-12 the most prevalent were the H3 (nH3=14, 41%), the H1 (nH1=7, 20%) and the H4 (nH4=5, 15%), and in 2012-13, the H14 (nH14=12, 36%), H3 and H4 (nH3,4=8, 23% both). The most frequent NA subtypes were N3 (nN3=17, 50%) and N2 (nN2=12, 35%) in 2011-12; both subtypes were also the most frequently isolated during 2012-13 (nN3=13, 39%, and nN2=12, 36%). H5 virus isolates were obtained during all three seasons (one isolate per season) and two H7 isolates were obtained in the second and third seasons respectively. All H5 and H7 viruses were determined to be of low pathogenicity, by analysis of the monobasic amino acid sequence of the HA cleavage site (44). For the H5, the translated amino acid sequence of the cleavage site was PQRETRG and for the two H7 isolates PENPTRG. H1 and H12 subtypes were only detected during 2011-12, and the H2 subtype was only detected in 2012-13, with the remaining six HA subtypes identified both seasons. The N9 and N4 subtypes were only found during the 2012-13 season; the remaining 5 NA subtypes (N2, N3, N5, N6, N8) were found during both seasons. From the 19 subtype combinations found, only seven were observed during both seasons. The estimated indexes of diversity were 0.860(95%CI: 0.794-0.925) and 0.843(95%CI: 0.776,0.910%) for 2011-12 and 2012-13, respectively. The diversity index indicated high diversity of subtypes during both seasons; based on the subtype combinations, the composition of the virus populations between the two seasons was different as indicated by the Sørensen-Dice coefficient (CC = 0.269).
Table 2.
Subtypes of IAV isolated during the wintering season in Guatemala, 2010-2013
| Species | November |
December |
January |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2010 | 2011 | 2012 | 2012 | 2013 | ||
| Anas americana (American wigeon) | - | - | - | - | - | H4N2 | - | - | 1 |
| Anas clypeata (Northern shoveler) | - | H1N3 | - | - | H11N3 | H4N2 | H7N3 | H3N8 | 5 |
| Anas crecca (Green-winged teal) | - | - | - | - | - | - | - | H7N9 | 1 |
| Anas discors (Blue-winged teal) | H5N3 | H1N3 (3) | H3N2 (4) | - | H14N6 | H2N3 | - | H14N3 (2) | 61 |
| H3N2 (10) | H3N3 | H1N3 (3) | H3N2 | H14N4 (2) | |||||
| H3N8 (2) | H3N8 | H3N3 (2) | H4N2 | H5N3 | |||||
| H4N2 (2) | H4N2 (4) | H5N3 | |||||||
| H4N3 (2) | H4N6 | H11N3 | |||||||
| H4N8 | H11N3 | H12N5 | |||||||
| H11N9 | H14N3 (2) | ||||||||
| H14N3 (7) | |||||||||
| H14N5 | |||||||||
| Total | 1 | 21 | 21 | - | 12 | 5 | 1 | 7 | 68 |
Variation of subtype diversity over time is shown in Figure 2. The most prevalent subtypes H3 and H4, N3 and N2 were found in at least two months of each season. The N3 subtype was the only NA subtype detected in 2010 and observed during all months in the following seasons. The highest diversity of virus subtypes was detected in the months during peak prevalence as detected by rRT-PCR. All other subtypes, with the exception of the H14 (see below), were found sporadically or only during one month within a season. H5 viruses were isolated in November in 2010, December in 2012, and in January in 2013, whereas the H7 viruses were only detected in January, in the 2011-12 and 2012-13 seasons. Three H14 viruses were recovered during the 2011-2012 season (37), and during the 2012-13 season the H14 was the most prevalent subtype, detected in November (8 of 21 isolates) and January (4 of 7 isolates).
Figure 2.
Virus subtypes (HA and NA) by month obtained from wintering waterfowl in Guatemala between 2010 and 2013.
Discussion
Sampling of hunter-harvested waterfowl in Guatemala revealed relatively high detection rates of IAV in wintering grounds. Prevalence estimates based on rRT-PCR for northern shovelers and blue-winged teals are higher than reported in wintering populations in other geographical regions (7, 19). Overall prevalence estimates based on virus isolation for blue-winged teals (5.7%) and northern shovelers (4.7%) are comparable with studies from wintering ducks in California and Louisiana (19, 37, 43). A higher prevalence based on rRT-PCR is not surprising, as only rRT-PCR positive samples were tested for virus isolation. Low virus isolation rates when rRT-PCR CT-values >30 have been reported (27, 29). In this study, CT-values for all rRT-PCR positive samples ranged from 19 to 41 with a median of 34 (data not shown). For samples positive for virus isolation the CT-values ranged between 19 and 38 with a median of 31. In addition, selection of the strains that are able to replicate in embryonating chicken eggs (ECE) during virus isolation may also explain the differences in prevalence estimates obtained with each method. Higher prevalence estimates based on rRT-PCR data when compared to virus isolation are in agreement with other similar studies (27, 39). An increase in rRT-PCR virus prevalence compared to previous years was observed previously during the 2009-10 (12). The limited sample size may have influenced the unexpectedly high prevalence estimates obtained in 2010 (12) and during the first season of the present study. During the subsequent seasons (2011-12 and 2012-13) the number of samples was larger (n>500), however the estimated prevalence was still high in comparison to estimates reported for blue-winged teals in North America (31). The high diversity of co-circulating subtypes and subtype combinations detected may help explain this observation. High diversity of AIV subtypes have been observed in wintering grounds in California and Texas (7, 8, 19), with comparable virus isolation rates.
During the 2010-11 and 2011-12 seasons a decreasing trend in prevalence was observed towards the end of the migration, supporting observations from previous years in Guatemala and similar to patterns of prevalence observed in wintering grounds in the south of the US (7, 8, 12, 19, 31). This decrease is likely explained by the accumulation of population immunity (as the number of seroconverted birds increases) resulting in a reduced number of susceptible individuals, not only to circulating subtypes but to other viruses from related genetic clades (25). During 2012-13 the pattern of IAV detection over time was different and the prevalence of IAV in January two-times higher in comparison to previous years (Figure 1). A plausible explanation could be the introduction and a potential outbreak of H14, the most prevalent HA subtype detected during that season. There is no evidence of circulation of H14 viruses in the western hemisphere prior to 2010 (4, 10). In January 2013, 4 of the 5 virus isolates obtained from blue-winged teals that month were from the H14 subtype. Current reports indicate that isolation of H14 viruses has been sporadic in North America (9, 10). It is not clear if the H14 virus persisted in wintering grounds in Guatemala after its first detection in 2011, or if it was re-introduced during the following season. In addition to the H14, other prevalent subtypes found in this study include the H3 and the H4, both of which are commonly isolated in North America (24). However, these subtypes were found in non-commonly isolated combinations such as H1N3 (the second most prevalent subtype in 2011-12), H3N3 and the H4N3. The H6 subtype, also prevalent in North America (2, 19), was not found in this study. Maintenance of virus diversity by resident duck populations has been observed in other studies (17, 18), and this diversity may be amplified upon arrival of migrants resulting in epizootic events (45). Either of these possibilities is supported by isolation of other rare subtypes (including the H14) and subtype combinations at the wintering grounds in Guatemala.
Among other species that tested positive for IAV were two white-winged doves. The number of samples collected from doves in this study was limited and no isolates were obtained from the rRT-PCR positive samples. There is natural and experimental evidence that doves and pigeons are susceptible to infection with IAV, including the H7N9 subtype from Asia (1). Although their role in IAV transmission remains unclear, association with habitats where waterfowl species are abundant, such as wetlands, may increase the probability of exposure to IAV in terrestrial birds. Nonetheless, the number of natural infections observed has been limited, suggesting that these species may solely act as incidental hosts.
In summary, we detected a wide diversity of virus subtypes in ducks during the wintering season in Guatemala. The diversity of circulating viruses seems to vary between years and overall virus prevalence seems to decrease at the end of the migration season at the studied locations. Our findings are supported by previous observations at the wintering grounds in Guatemala and other locations. The emergence of the H14 subtype in blue-winged teals (4, 10, 33, 36), and its high levels of detection during the 2012-13 season, provides further evidence that the wintering grounds in Central America may serve as places where virus variants with limited circulation may be amplified upon arrival of yearly migrants. Although additional bird species need to be investigated, the high relative abundance of the blue-winged teals in comparison to other duck species in Central America, the particular behavior of this long-distance migrant (3, 34, 38), and the diversity of viruses found in these ducks makes them a candidate species for targeted IAV surveillance in the Neotropics. We recognize that surveillance in other species, characterization of viruses that circulate in resident bird populations (during and in between migrations), and incorporation of more systematic sampling methodologies, (including the use of geo-transmitters), are needed to better understand the ecology of IAV in this region; however, as we cannot longer ignore that Eurasian HPAI H5 viruses have been introduced to the western hemisphere, we think that sampling of hunted birds is a cost effective strategy that could be replicated in neighboring countries from the region that, like Guatemala, have limited resources to establish long term disease surveillance.
Acknowledgements
The authors want to thank national regulatory agencies (CONAP and CECON) and licensed sport-hunters for allowing sample collection from birds in Guatemala. To Jorge Paniagua, Silvia Ramirez, Silvia Sosa, Carmen Yoc, Oscar de Leon, Adan Real, Johanna Lavigne, Diego Lopez for their assistance in sample collection and processing, data management, and administrative support. To Cheryl Nichols for English editing of this manuscript. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) Center for Research on Influenza Pathogenesis (CRIP) contracts No. HHSN266200700010C and HHSN272201400008C.
Abbreviations
- AIV
Avian influenza virus
- HA (H)
hemagglutinin
- NA (N)
neuraminidase
- LPAIV
Low pathogenicity avian influenza virus
- HPAIV
Highly pathogenic avian influenza virus
- rRT-PCR
real-time reverse-transcriptase polymerase chain reaction
References
- 1.Abolnik C. A current review of avian influenza in pigeons and doves (Columbidae) Veterinary Microbiology. 2014;170:181–196. doi: 10.1016/j.vetmic.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Bahl J, Vijaykrishna D, Holmes EC, Smith GJ, Guan Y. Gene flow and competitive exclusion of avian influenza A virus in natural reservoir hosts. Virology. 2009;390:289–297. doi: 10.1016/j.virol.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botero JE, Rusch DH. Recoveries of North American waterfowl in the neotropics. Waterfowl in winter. University of Minnesota Press; Minneapolis, MN: 1988. pp. 469–482. [Google Scholar]
- 4.Boyce WM, Schobel S, Dugan VG, Halpin R, Lin X, Wentworth DE, Lindsay LL, Mertens E, Plancarte M. Complete genome sequence of a reassortant H14N2 avian influenza virus from California. Genome announcements. 2013;1:e00543–00513. doi: 10.1128/genomeA.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945;26:297–302. [Google Scholar]
- 6.FAO, F. a. A. O. o. t. U. N Highly Pathogenic Avian Influenza in Mexico (H7N3) - A significant threat to poultry production not to be underestimated. EMPRES Watch. 2012;26:9. [Google Scholar]
- 7.Ferro PJ, Budke CM, Peterson MJ, Cox D, Roltsch E, Merendino T, Nelson M, Lupiani B. Multiyear surveillance for avian influenza virus in waterfowl from wintering grounds, Texas coast, USA. Emerg Infect Dis. 2010;16:1224–1230. doi: 10.3201/eid1608.091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferro PJ, Khan O, Peterson MJ, Batchuluun D, Reddy SM, Lupiani B. Avian influenza virus surveillance in hunter-harvested waterfowl, Texas coast, September 2009-January 2010. Avian Dis. 2012;56:1006–1009. doi: 10.1637/10194-041012-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 9.Fries AC, Nolting JM, Bowman AS, Killian ML, Wentworth DE, Slemons RD. Genomic analyses detect Eurasian-lineage H10 and additional H14 influenza A viruses recovered from waterfowl in the Central United States. Influenza and other respiratory viruses. 2014;8:493–498. doi: 10.1111/irv.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries AC, Nolting JM, Danner A, Webster RG, Bowman AS, Krauss S, Slemons RD. Evidence for the circulation and inter-hemispheric movement of the H14 subtype influenza A virus. PLoS One. 2013;8:e59216. doi: 10.1371/journal.pone.0059216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goekjian VH, Smith JT, Howell DL, Senne DA, Swayne DE, Stallknecht DE. Avian influenza viruses and avian paramyxoviruses in wintering and breeding waterfowl populations in North Carolina, USA. J Wildl Dis. 2011;47:240–245. doi: 10.7589/0090-3558-47.1.240. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Reiche AS, Morales-Betoulle ME, Alvarez D, Betoulle JL, Muller ML, Sosa SM, Perez DR. Influenza a viruses from wild birds in guatemala belong to the north american lineage. PLoS ONE. 2012;7:e32873. doi: 10.1371/journal.pone.0032873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Reiche AS, Perez DR. Where do avian influenza viruses meet in the Americas? Avian Dis. 2012;56:1025–1033. doi: 10.1637/10203-041412-Reg.1. [DOI] [PubMed] [Google Scholar]
- 14.Grundmann H, Hori S, Tanner G. Determining Confidence Intervals When Measuring Genetic Diversity and the Discriminatory Abilities of Typing Methods for Microorganisms. Journal of Clinical Microbiology. 2001;39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson BA, Stallknecht DE, Swayne DE, Lewis LA, Senne DA. Avian Influenza Viruses in Minnesota Ducks during 1998-2000. Avian Diseases. 2003;47:867–871. doi: 10.1637/0005-2086-47.s3.867. [DOI] [PubMed] [Google Scholar]
- 16.Hanson BA, Swayne DE, Senne DA, Lobpries DS, Hurst J, Stallknecht DE. Avian Influenza Viruses and Paramyxoviruses in Wintering and Resident Ducks in Texas. Journal of wildlife diseases. 2005;41:624–628. doi: 10.7589/0090-3558-41.3.624. [DOI] [PubMed] [Google Scholar]
- 17.Hénaux V, Samuel MD, Dusek RJ, Fleskes JP, Ip HS. Presence of Avian Influenza Viruses in Waterfowl and Wetlands during Summer 2010 in California: Are Resident Birds a Potential Reservoir? PLoS ONE. 2012;7:e31471. doi: 10.1371/journal.pone.0031471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill NJ, Takekawa JY, Ackerman JT, Hobson KA, Herring G, Cardona CJ, Runstadler JA, Boyce WM. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos) Mol Ecol. 2012;21:5986–5999. doi: 10.1111/j.1365-294X.2012.05735.x. [DOI] [PubMed] [Google Scholar]
- 19.Hill NJ, Takekawa JY, Cardona CJ, Meixell BW, Ackerman JT, Runstadler JA, Boyce WM. Cross-seasonal patterns of avian influenza virus in breeding and wintering migratory birds: a flyway perspective. Vector Borne Zoonotic Dis. 2012;12:243–253. doi: 10.1089/vbz.2010.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw VS, Webster RG, Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Canadian Journal of Microbiology. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 22.Ip HS. Novel Eurasian highly pathogenic influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis. 2015 doi: 10.3201/eid2105.142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapczynski DR, Pantin-Jackwood M, Guzman SG, Ricardez Y, Spackman E, Bertran K, Suarez DL, Swayne DE. Characterization of the 2012 highly pathogenic avian influenza H7N3 virus isolated from poultry in an outbreak in Mexico: pathobiology and vaccine protection. J Virol. 2013;87:9086–9096. doi: 10.1128/JVI.00666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauss S, Walker D, Pryor SP, Niles L, Chenghong L, Hinshaw VS, Webster RG. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4:177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- 25.Latorre-Margalef N, Grosbois V, Wahlgren J, Munster VJ, Tolf C, Fouchier RA, Osterhaus AD, Olsen B, Waldenstrom J. Heterosubtypic immunity to influenza A virus infections in mallards may explain existence of multiple virus subtypes. PLoS Pathog. 2013;9:e1003443. doi: 10.1371/journal.ppat.1003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D-H, Torchetti MK, Winker K, Ip HS, Song C-S, Swayne DE. Intercontinental Spread of Asian-origin H5N8 to North America through Beringia by Migratory Birds. Journal of Virology. 2015 doi: 10.1128/JVI.00728-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis NS, Javakhishvili Z, Russell CA, Machablishvili A, Lexmond P, Verhagen JH, Vuong O, Onashvili T, Donduashvili M, Smith DJ, Fouchier RA. Avian influenza virus surveillance in wild birds in Georgia: 2009-2011. PLoS One. 2013;8:e58534. doi: 10.1371/journal.pone.0058534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis NS, Javakhishvili Z, Russell CA, Machablishvili A, Lexmond P, Verhagen JH, Vuong O, Onashvili T, Donduashvili M, Smith DJ, Fouchier RAM. Avian Influenza Virus Surveillance in Wild Birds in Georgia: 2009-2011. PLoS ONE. 2013;8:e58534. doi: 10.1371/journal.pone.0058534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munster VJ, Baas C, Lexmond P, Bestebroer TM, Guldemeester J, Beyer WE, de Wit E, Schutten M, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Practical considerations for high-throughput influenza A virus surveillance studies of wild birds by use of molecular diagnostic tests. J Clin Microbiol. 2009;47:666–673. doi: 10.1128/JCM.01625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WE, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nallar R, Papp Z, Epp T, Leighton FA, Swafford SR, DeLiberto TJ, Dusek RJ, Ip HS, Hall J, Berhane Y, Gibbs SE, Soos C. Demographic and Spatiotemporal Patterns of Avian Influenza Infection at the Continental Scale, and in Relation to Annual Life Cycle of a Migratory Host. PLoS One. 2015;10:e0130662. doi: 10.1371/journal.pone.0130662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton I. The migration ecology of birds. Academic Press; 2010. [Google Scholar]
- 33.Nolting J, Fries AC, Slemons RD, Courtney C, Hines N, Pedersen J. Recovery of H14 influenza A virus isolates from sea ducks in the Western Hemisphere. PLoS currents. 2012;4:RRN1290. doi: 10.1371/currents.RRN1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 35.Phipps LP, Essen SC, Brown IH. Genetic subtyping of influenza A viruses using RT-PCR with a single set of primers based on conserved sequences within the HA2 coding region. Journal of virological methods. 2004;122:119–122. doi: 10.1016/j.jviromet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Ramey AM, Poulson RL, Gonzalez-Reiche AS, Perez DR, Stallknecht DE, Brown JD. Genomic characterization of H14 subtype Influenza A viruses in new world waterfowl and experimental infectivity in mallards (Anas platyrhynchos) PLoS One. 2014;9:e95620. doi: 10.1371/journal.pone.0095620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramey AM, Poulson RL, Gonzalez-Reiche AS, Wilcox BR, Walther P, Link P, Carter DL, Newsome GM, Muller ML, Berghaus RD, Perez DR, Hall JS, Stallknecht DE. Evidence for Seasonal Patterns in the Relative Abundance of Avian Influenza Virus Subtypes in Blue-Winged Teal (Anas discors) J Wildl Dis. 2014;50:916–922. doi: 10.7589/2013-09-232. [DOI] [PubMed] [Google Scholar]
- 38.Ramey AM, Walther P, Link P, Poulson RL, Wilcox BR, Newsome G, Spackman E, Brown JD, Stallknecht DE. Optimizing Surveillance for South American Origin Influenza A Viruses Along the United States Gulf Coast Through Genomic Characterization of Isolates from Blue-winged Teal (Anas discors) Transbound Emerg Dis. 2014 doi: 10.1111/tbed.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runstadler JA, Happ GM, Slemons RD, Sheng ZM, Gundlach N, Petrula M, Senne D, Nolting J, Evers DL, Modrell A, Huson H, Hills S, Rothe T, Marr T, Taubenberger JK. Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks at Minto Flats State Game Refuge, Alaska, during August 2005. Arch Virol. 2007;152:1901–1910. doi: 10.1007/s00705-007-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson EH. Measurement of diversity. Nature. 1949 [Google Scholar]
- 41.Sørensen T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons. I kommission hos E. Munksgaard. 1948 [Google Scholar]
- 42.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, Kearney MT. Avian Influenza Viruses from Migratory and Resident Ducks of Coastal Louisiana. Avian Diseases. 1990;34:398–405. [PubMed] [Google Scholar]
- 44.Steinhauer DA. Role of Hemagglutinin Cleavage for the Pathogenicity of Influenza Virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 45.Verhagen JH, van Dijk JG, Vuong O, Bestebroer T, Lexmond P, Klaassen M, Fouchier RA. Migratory birds reinforce local circulation of avian influenza viruses. PLoS One. 2014;9:e112366. doi: 10.1371/journal.pone.0112366. [DOI] [PMC free article] [PubMed] [Google Scholar]