Abstract
Objective
Psychostimulant medications are the gold standard of treatment for attention-deficit/hyperactivity disorder (ADHD); however, a significant minority (~30%) of individuals with ADHD fail to respond favorably. Noradrenergic agents are increasingly used as ADHD monotherapies or adjuncts for suboptimal stimulant response, yet knowledge of their cortical effects is limited. This study is the first to examine comparative effects of guanfacine (an alpha adrenergic 2A agonist), psychostimulant, and their combination on resting state cortical activity in ADHD.
Method
The sample comprised 179 participants aged 7 to 14 years old with ADHD (113 boys, 55 girls). Participants were randomized to one of three blinded conditions: guanfacine (GUAN), d-methylphenidate (DMPH), or a combination (COMB). Electroencephalography (EEG) was performed pre-, mid-, and post-medication titration, with concomitant assessment of behavioral and cognitive functioning.
Results
Analyses of spectral power measures during resting EEG suggested that each medication condition displayed a distinct profile of effects on cortical activity. Significant time effects suggested that GUAN decreased global alpha-band (8–12 hertz [Hz]) power, DMPH and COMB increased centro-parietal beta-band (13–21 Hz) power, and COMB resulted in decreased theta-band (4–7 Hz) power. Relative to other medication groups, COMB was associated with significantly lower theta power and DMPH with higher beta-band power compared to the GUAN group. Medication-related changes in theta power were correlated with improvements in behavioral and cognitive functioning.
Conclusion
These data revealed distinct underlying medication-related effects on neural mechanisms. The COMB condition uniquely exhibited an EEG profile that was associated with improved behavioral and cognitive functioning.
Clinical trial registration information
Single Versus Combination Medication Treatment for Children With Attention Deficit Hyperactivity Disorder; http://clinicaltrials.gov/; NCT00429273.
Keywords: electroencephalography, children, medication, treatment, stimulants
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders in children, affecting approximately 5–10% of youths1. The core symptoms of this neurodevelopmental disorder include inattention, hyperactivity, and impulsivity and result in significant academic, psychological, and social impairment. While considerable progress has been made in elucidating genetic, neurobiological, and cognitive correlates of ADHD, a complete mechanistic model of ADHD has been elusive. The same can be said regarding our understanding of the mechanisms of successful treatment. A host of brain imaging methodologies has been applied to the study of ADHD and its treatment, revealing a number of abnormalities at the level of brain structure, function, connectivity, and neurochemistry. Among brain imaging modalities, electroencephalography (EEG) has long been used to assess the neural correlates of ADHD. EEG is especially well-suited for these studies, as it is more tolerant of ADHD-related movement artifacts, is much less expensive and more easily administered than other brain imaging modalities such as functional magnetic resonance imaging (fMRI), and has been used extensively to identify resting state differences in cortical activity among children with ADHD.
One of the most consistent neurophysiologic correlates of ADHD relative to typically developing children is higher frontocentral slow-wave power in the theta frequency band (4–7 Hertz [hz])2. In meta-analytic studies of EEG that include over 1,400 participants affected by ADHD, resting theta band power is significantly increased by 32% on average versus controls, with an effect size of 1.31 (95% CI, 1.14 – 1.48)3. In addition to higher frontocentral theta band power, lower global alpha and beta band activity have also been associated with ADHD. However, findings in these frequency bands are more variable and may differ according to age, ADHD subtype, and psychiatric comorbidities4. Although EEG measures such as theta power as well as the ratio of theta power to beta power, i.e. theta/beta ratio (TBR) have been suggested as having clinical utility in ADHD, recent studies suggest that neither has the sensitivity/specificity needed to function as stand-alone diagnostic markers5.
Psychostimulant medications (e.g. methylphenidate, amphetamine) have been first-line ADHD treatments for over 70 years; however, a substantial number (25–35%) of children do not respond satisfactorily to initial stimulant trials6. Noradrenergic alpha-2 adrenoceptor agonists (α agonists), such as guanfacine and clonidine, have been increasingly used both as alternative monotherapies or adjunctive treatments in cases of inadequate stimulant response7,8. The α2A mechanism of action of these agonists has been supported as treatment for disorders associated with prefrontal cortical (PFC) dysfunction based on preclinical studies that demonstrated guanfacine increased firing in PFC neurons during task-related activities and strengthened functional network connectivity between the PFC and other cortical regions9. Furthermore, a recent study using an animal model for ADHD, the spontaneously hypertensive rat (SHR), reported that guanfacine achieves its effects through stimulation of the postsynaptic adrenergic α2A receptors in the prefrontal cortex as opposed to α2B or α2C receptors or through presynaptic α2A receptor-regulated noradrenaline release10. Results of randomized clinical trials indicate that guanfacine, a selective α2A noradrenergic agonist, is effective in reducing the core symptoms of ADHD as a monotherapy11,12 and as an adjunctive treatment for those individuals with suboptimal responses to psychostimulants alone8. Further work on the mechanistic actions of α2A agonists, however, is needed to better understand the cognitive and neural effects of these medications as monotherapies and in combination with stimulants in human participants with ADHD.
The overall effect of psychostimulants has been shown to improve, but not normalize, many features of ADHD-related abnormal EEG activity. Specifically, MPH tends to decrease theta band and increase beta band power13–15, particularly when associated with medication-related improvements in cognition16,17. The effects of non-stimulant medications on cortical activity in ADHD have not been well studied. One study examined the effects of atomoxetine, a selective norepinephrine reuptake inhibitor, on EEG18. Atomoxetine appears to have some similarities to psychostimulants, such as its ability to significantly reduce posterior theta band power and increase absolute beta band power with acute administration; however, these changes were modest, and differences versus reported stimulant effects were also seen in the increase in slow-wave delta power17. Thus, some shared effects associated with partial normalization of the EEG profile apparent in ADHD may be associated with both psychostimulants and non-stimulant medications used to treat ADHD. To date, the cortical effects of α2A agonists compared to and in combination with psychostimulants in children with ADHD have not been reported.
The current study takes a step in that direction by testing the effects of the single enantiomer stimulant d-methylphenidate (DMPH), the α2A agonist guanfacine (GUAN), and the combination of DMPH and GUAN (COMB) on resting state EEG measures in youth with ADHD. Goals of this study are to contrast cortical activity effects under each medication condition during resting state and test whether acute changes in cortical activity during rest are predictive of cognitive functioning. Given that the two medication classes, psychostimulants and α2A agonists, have different proposed neural mechanisms that underlie their clinical benefits, we hypothesized that these mechanistic differences would be evident in differential EEG effects on resting state spectral power. The COMB condition was predicted to have additive effects of the monotherapies, and hence more beneficial effects on the spectral power profile. We hypothesized that significant medication-related EEG changes will be associated with improvements in behavioral and cognitive functioning.
METHOD
Sample
The sample consisted of 179 participants (113 boys, 55 girls), aged 7 to 14 years old who were diagnosed with ADHD. All participants were enrolled in the Translational Research to Enhance Cognitive Control ADHD (TRECC; McCracken et al., under review) project. Participants were recruited from clinic referrals, radio and newspaper advertisements, community organizations (CHADD; www.chadd.org), local schools, and primary care physicians. After receiving verbal and written explanations of study requirements, and prior to any study procedures, all parents/participants provided written informed permission/assent as approved by the University of California, Los Angeles (UCLA) Institutional Review Board. The study was also overseen by a local data safety and monitoring board (DSMB), which provided a tri-annual review.
Procedure
As part of their participation in the TRECC study, participants underwent phenotypic assessment, including diagnostic interviews and EEG recording. For a detailed description of diagnostic and cognitive assessment, please see McCracken et al, submitted; Bilder et al, submitted. Briefly, individuals were evaluated based on a semi-structured diagnostic interview with the primary caretaker (usually mother) and a direct interview if 8 years of age or older using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS-PL)19. Teacher reports were solicited and used to supplement clinical interview data. Psychiatric disorders were considered present if the participant currently met full DSM-IV diagnostic criteria. All interviews were conducted by clinical psychologists or other highly trained interviewers with extensive experience in psychiatric diagnoses and training in using the KSADS. Best estimate diagnoses were determined after individual review of diagnoses, symptoms, and impairment level by senior clinicians (J.J.M., J.P.). Participants were included if they had a current diagnosis of ADHD, any subtype, and excluded if positive for any neurological disorder, head injury resulting in concussion, diagnoses of autism, chronic tic disorder, current major depression, panic disorder, lifetime bipolar disorder or psychosis, or estimated Full Scale IQ < 80. Participants were off medication for baseline assessments.
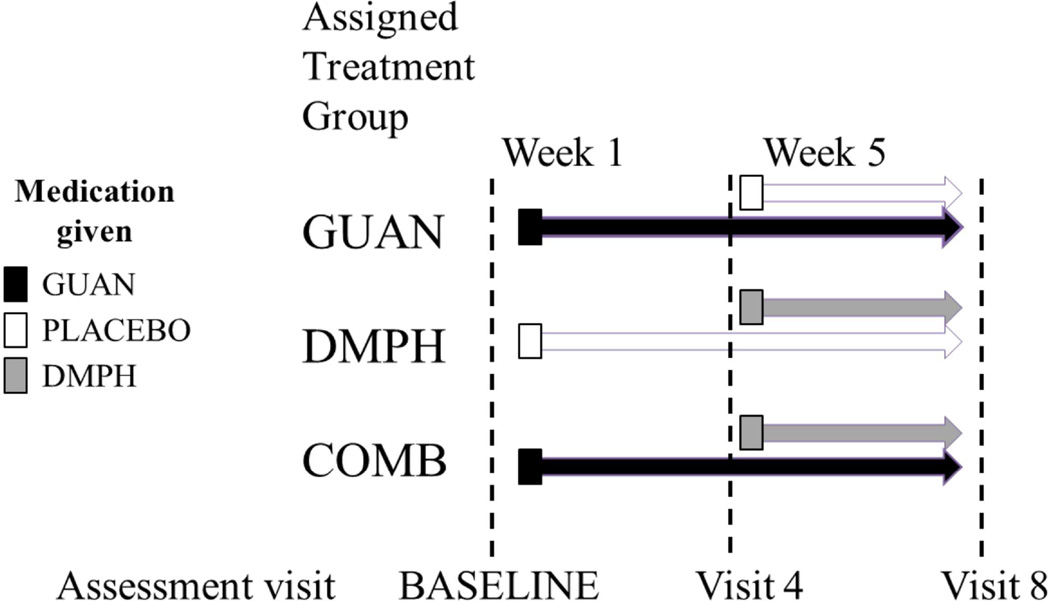
After confirmation of eligibility, participants were enrolled in a double-blind, comparative clinical trial and randomly assigned to one of three medication conditions: immediate release guanfacine (GUAN) administered twice daily, d-methylphenidate extended release (DMPH) once daily, or the combination of GUAN and DMPH (COMB) (see Figure 1). Treatments were applied sequentially: the first 4 weeks, participants received GUAN or placebo, beginning at 0.5 mg administered twice daily in week 1 and increased as tolerated to 0.5 – 1.5 mg twice daily doses in subsequent weeks. Participants remained on the optimal GUAN dose (determined by Clinical Global Impression [CGI]-Improvement ratings, ADHD Rating Scale-IV [ADHD-RS-IV] scores, and side effects for Week 4) for the remainder of the study. Beginning in Week 5, participants initially randomized to GUAN continued taking GUAN with added DMPH or placebo, while participants initially randomized to placebo received added DMPH. Low, medium, and high stimulant doses were assessed weekly for behavioral response and tolerability to determine the “optimal” DMPH dose at Week 7, according to the identical process described above. Participants remained on optimal GUAN and/or DMPH doses for a final study week prior to end-of-study Week 8 assessments. For consort chart summarizing participant completion rates for each medication group as well as adverse side effects reported within each medication group, please see McCracken et al. (under review). EEG was administered at the following time points and medication conditions: at baseline with no medication, at Week 4, which compared GUAN and placebo, and at Week 8, which compared one week of optimized treatment with GUAN, DMPH, or COMB.
Figure 1. Medication titration schedule.
Note: This was an 8-week randomized 1:1:1, comparative parallel-group fixed-flexible dosing study of three treatments. COMB=combination; DMPH= d-methylphenidate; GUAN=guanfacine.
Symptom Assessment
The ADHD-RS-IV20 was used as a measure of ADHD symptom severity. A clinician blind to medication status completed this measure at baseline, Week 4, and at Week 8 or last visit based on parent, teacher, and other available data.
Cognitive Assessment
As part of the larger cognitive battery, estimated intelligence (IQ) was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI). Other cognitive variables are factor analytic scores derived from the full cognitive battery that represent four primary cognitive processes of interest: working memory (WM), response inhibition (RI), reaction time (RT), and reaction time variability (RTV). The individual tests used in the construction of the cognitive factors are described in the accompanying article by Bilder et al (under review) and are available as supplemental material.
Electrophysiologic Methods
EEG recording was carried out using 40 Ag/AgCl surface electrodes that were embedded in an electrode cap arrayed in an extended International 10/20 configuration (ElectroCap, Eaton, OH) and was referenced to linked ears. Impedance was below 10 kOhms, and EEG signal was recorded using MANSCAN (Sam Technology, San Francisco, CA) hardware and software. EEG data were sampled at a rate of 256 samples per second. Eye movements were monitored by electrodes placed on the outer canthus of each eye for horizontal movements and by electrodes above the eye for vertical eye movements. EEG recording for all participants consisted of a baseline condition lasting 5 minutes during which participants sat quietly with their eyes closed.
Continuous EEG data were reviewed off-line (i.e., after the recording was complete and the participant had left) by a technician experienced in EEG and all segments containing eye or head movement or muscle artifact were removed from further analysis. In order to be included in the following EEG analyses, at least 30 seconds of artifact-free EEG were required. Using a Fast Fourier Transform, average spectral power (µV2) was computed for the following frequency bands: theta (4–8 Hz), alpha (8–12 Hz), beta1 or sensorimotor rhythm (12–16 Hz), and beta2 (16–21 Hz). Baseline absolute power for all three medication groups is presented in Figure S1 (available online). Relative power for each frequency band was calculated (using total power from 1–21 Hz for each electrode as the denominator) and natural log transformed to assume a normal distribution. To reduce the number of comparisons, spectral power was averaged by region as follows: frontal (F3, F4, Fz), central (C3, C4, Cz), and parietal (P3, P4, Pz). EEG technicians were blind to medication group status.
Data Analytic Strategy
Analyses were conducted using IBM SPSS statistics version 21. Because age has significant effects on EEG power, it was used as a covariate in all analyses. In order to control for Type 1 error, two procedures were used. First, we performed analyses on regional spectral power estimates from proximal electrodes to reduce the number of contrasts from 36 individual electrodes to 3 regions. Second, we used the false discovery rate (FDR21) to maintain the family-wise experimental error at p<.05. According to the FDR analysis, p-values < .01 are significant; raw p-values between <.01 and .05 will be presented here as trend-level findings that may provoke further testing.
To assess the medication effects on EEG spectral power measures, generalized linear mixed model analyses were used to compare EEG spectral power by region and frequency band at baseline (B), week 4 (W4), and week 8 (W8) for each medication group. Primary effects of interest were: 1) TIME: the main effect of time, which tests within-medication group changes in spectral power at three time points, 2) MEDICATION: the main effect of medication is a between-subjects effect that indicates whether any of the medication groups were significantly different from others across all time points, and 3) MEDICATION × TIME: interaction effect of medication group by time, which tests if spectral power changes between medication groups were significantly different over the course of the medication trial. Significant omnibus effects (p<.01) and trends (p<.05) were followed by individual contrasts to determine specific time point (B, W4, W8) or medication group at Week 8 (GUAN, DMPH, COMB) differences.
Pearson partial correlations (with age as a covariate) between EEG variables and behavioral measures/cognitive factor scores were conducted to assess the association of medication-related changes in EEG power. Behavioral variables consisted of the ADHD-RS-IV Inattentive and Hyperactive-Impulsive scores were used to reflect ADHD symptom severity at Week 8. In addition, the degree of treatment-related change in ADHD symptom severity was used in the correlation analysis. To calculate treatment change, baseline ADHD symptom severity was subtracted from Week 8 ADHD symptom severity under the assumption that there would be a reduction of ADHD symptoms through the course of treatment, thus a larger number for the treatment change variables suggests a bigger reduction in symptom severity from baseline to end of trial. The treatment change and Week 8 ADHD symptom severity variables provide different information regarding the relationship of EEG to ADHD symptomatology and consequently show different patterns of association.
RESULTS
Demographics
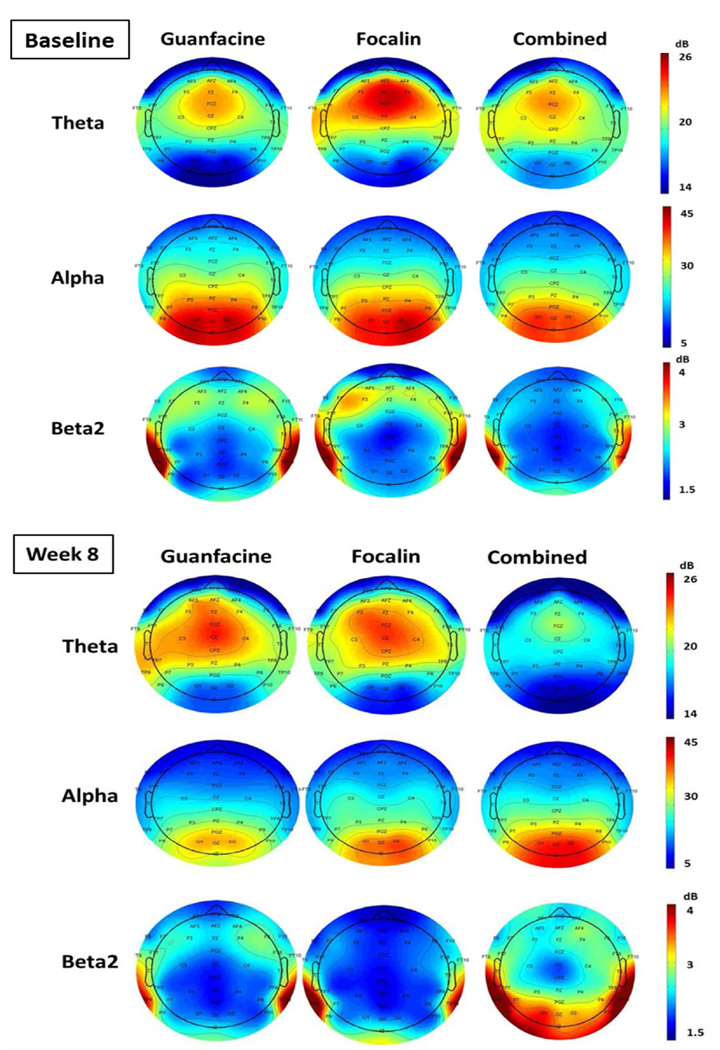
Of the 212 randomized participants, 182 completed the clinical trial (Week 8) and were included in the current study. Three participants were excluded based on the requirement to have at least 30 seconds of artifact EEG, leaving a final sample of 179 (GUAN n=59; DMPH n=60; COMB n=60). The 179 participants were 67% male, an average age of 10.1 years (SD=2.1), predominantly Caucasian (75%), and mean IQ of 103 (SD=14). At baseline, participants had, on average, ADHD-Inattentive symptoms= 7.9 (SD=1.3) and ADHD-Hyperactive/Impulsive symptoms=5.1 (SD=2.6). None of these variables differed significantly across medication groups. Average EEG spectral power for each medication group within each frequency is presented in Figure 2 for baseline and Week 8 time points. There were no significant differences in mean relative spectral power in any band or cluster across the groups at baseline (all p’s >.2).
Figure 2. Resting-state electroencephalography spectral power topographs.
Note: Relative power for each medication group by frequency band and study visit is shown. There were no significant differences in mean relative spectral power in any frequency band or region across the groups at baseline (all p’s >.2).
Within-group changes in EEG spectral power by medication group
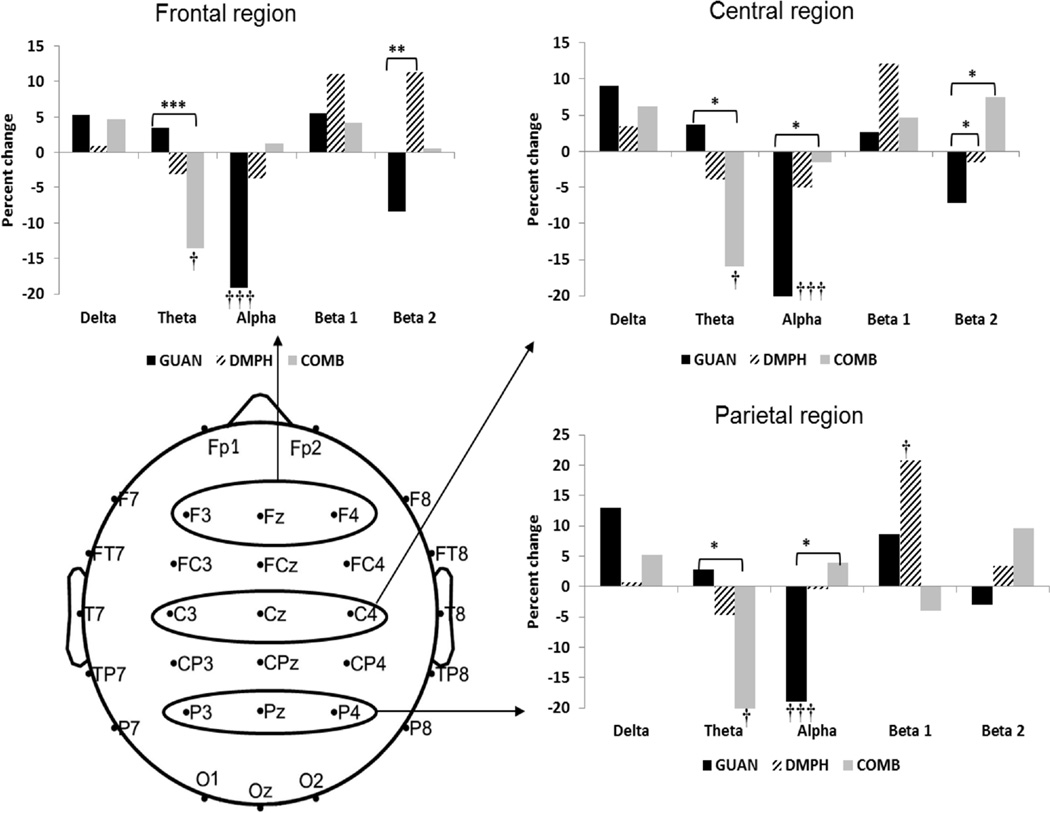
Significant time effects informed whether a significant effect of medication was observed on EEG power among participants within each medication group. Results of the linear mixed models suggested significant time effects on EEG spectral power for each medication condition; this is depicted by percent change in EEG power for each medication group within each frequency band by region in Figure 3. Each medication group was associated with significant changes in a different frequency band. The primary effect of COMB medications was in central and parietal theta-band power (central: F[2, 238.3]=3.0, p=.049, and parietal F[2, 238.3]=3.4, p=.034), which was reduced at Week 8 compared to baseline and Week 4. In contrast, the DMPH group exhibited increased parietal beta-band power (F[2, 238.3]=5.0, p<.01) at Week 8 relative to baseline levels. Finally, administration of GUAN resulted in decreased global alpha-band (8–12 Hz) spectral power at Weeks 4 and 8, where significant effects of time emerged in all regions (frontal: F[2, 238.3]=10.0, p<.001, central: F[2, 238.3]=14.7, p<.001, parietal: F[2, 238.3]=4.8, p=.008).
Figure 3. Change in electroencephalography spectral power from baseline by medication group.
Note: Each figure depicts regional change in relative power in each frequency band from baseline to Week 8 for each medication group. Significant medication group differences are represented by * p<.05, ** p<.01, and significant time effects are represented by † p<.05, ††† p<.01, ††† p<.001. COMB=combination; DMPH= d-methylphenidate; GUAN=guanfacine.
Between-group changes in EEG spectral power
Significant medication and medication × time interaction effects informed whether a significant medication effect was observed on EEG power relative to other medication conditions. These effects are also depicted in Figure 2. In the beta2 band (17–21 Hz), a significant medication effect emerged in the frontal and central regions for the theta and beta2 frequency bands (central theta: F[2, 172.5]=3.0, p=.050; frontal beta2: F[2, 172.5]=3.9, p=.022; central beta2: F[2, 172.5]=3.5, p=.033). In the central region, the COMB group exhibited lower theta band power relative to the GUAN group (p<.05), although this effect became a trend-level finding after FDR correction. DMPH also had significantly higher spectral beta band power compared to the GUAN group in frontal regions (p’s <.01), and both DMPH and COMB trended towards higher beta2 power relative to GUAN in the central region (p<.05).
Significant medication × time interaction effects emerged in the theta-, alpha-, and beta2 frequency bands. In frontal and central regions (frontal F[4, 238.3]=2.9, p=.024, central: F[4, 238.3]=4.0, p=.004; parietal F[4, 238.3]=3.1, p<.016), theta power was significantly lower in the COMB group compared to GUAN (p <.005) across all regions and trended in the same direction relative to the DMPH group in central and parietal regions (p’s < .05). Significant interaction effects for alpha power in central (F[4, 238.3]=4.2, p=.002) and parietal (F[4, 238s.3]=4.8, p=.001) region emerged. The GUAN group exhibited lower alpha power relative to both the COMB and DMPH group in the central region (p<.05) and the COMB condition in the parietal region (p<.05) only. The COMB group alone exhibited an EEG profile of reduced theta-band and higher alpha and beta band power after medication administration.
Correlations between EEG and behavioral and cognitive variables
Partial Pearson correlations (controlling for age) were used to assess the relationship between resting state spectral power and behavioral and cognitive functioning at the Week 8 time point. Significant between-group medication condition differences were observed in global theta, central, and parietal alpha and frontal and central beta band power; therefore, these EEG variables were tested for association with behavioral and cognitive measures. As seen in Table 1, severity of ADHD inattentive or hyperactive-impulsive symptoms at Week 8 was not associated with EEG spectral power beyond two trend-level correlations between theta-band power and hyperactive-impulsive symptoms.
Table 1.
Correlations Between Week 8 Electroencephalogram (EEG) Spectral Power and Behavioral Characteristics and Cognitive Performance
| Theta power | Alpha power | Beta power | |||||
|---|---|---|---|---|---|---|---|
| Front | Cen | Par | Cen | Par | Front | Cen | |
| INsxs | 0.08 | 0.06 | 0.08 | −0.08 | −0.03 | 0.04 | 0.03 |
| HIsxs | 0.21* | 0.14 | 0.17 | −0.10 | 0.11 | 0.02 | 0.01 |
| Tx chg IN | −0.26** | −0.29*** | −0.23** | 0.14 | 0.12 | −0.02 | −0.07 |
| Tx chg HI | −0.22* | −0.25** | −0.23** | 0.06 | 0.06 | −0.08 | −0.11 |
| WM factor ⇧ | −0.14 | −0.17 | −0.20* | 0.06 | 0.04 | 0.02 | 0.11 |
| IN factor ⇧ | −0.02 | 0.06 | 0.13 | −0.21* | −0.22* | 0.01 | −0.13 |
| RT factor ⇧ | 0.11 | 0.19* | 0.24** | −0.32*** | −0.30*** | −0.16 | −0.14 |
| RTV factor ⇧ | 0.27*** | 0.29*** | 0.30*** | −0.31*** | −0.29*** | −0.07 | −0.11 |
Note: Arrows next to cognitive factors show the direction of score indicating better performance (i.e., higher scores on working memory [WM] and inhibition [IN] indicate better performance; lower scores on reaction time [RT] and reaction time variability [RTV] suggest quicker reaction time and lower reaction time variability. Significant correlations are in bold; trend level findings are in italics.
HI = hyperactivity/impulsivity; HIsxs=Number of hyperactive-impulsive symptoms at week 8; INsxs=Number of inattentive symptoms at week 8; Tx chg=treatment change (Baseline-Week 8).
p≤.05,
p≤.01,
p≤.005.
In contrast, theta band spectral power was significantly negatively associated with treatment-related change in ADHD symptoms such that lower theta band power was correlated with a larger reduction in inattentive symptoms (r’s range −0.23 to −0.29, p< .01). In central and parietal regions, reduced theta power was also associated with larger treatment change in hyperactive-impulsive symptoms (r’s range 0.-23 to −0.25, p< .01). Spectral power in alpha and beta bands was not associated with ADHD symptom severity at Week 8 or treatment-related change in ADHD symptomatology. The primary effect of the COMB medications was lower theta power, which was significantly associated with medication-related improvement in ADHD symptom severity.
Within the cognitive domain, the relationship between the same EEG variables and the factor scores for broad domains of cognitive functioning were tested (see Table 1). Although the correlations were modest, significant correlations emerged between resting-state EEG power and cognitive performance, both of which were measured at Week 8. Theta band power in the parietal region was negatively correlated at a trend level with the working memory factor (r=0.20, p=.033) and significantly correlated with reaction time (r =0.24, p=.010) and reaction time variability (r=0.30, p=.002). These correlations suggest a relationship between improved cognitive performance (better working memory performance and lower reaction time variability) with lower spectral power in the theta frequency band during resting state. Coupled with the behavioral findings above, these data further strengthen the idea that reduced theta band power, which is evident in the COMB medication condition, is associated with enhanced functioning in ADHD. Alpha band power, on the other hand, was significantly negatively correlated with reaction time (central r = 0.32, p=.001; parietal r = 0.30, p=.001) and reaction time variability (central r =0.31, p=.001; parietal r = .29, p=.002) and trended toward significance with the inhibition factor score (central r = −0.21, p≤.031; parietal r = −0.22, p<.019). Thus, the primary effect of GUAN, which was lower alpha band power, was associated with a slower and more variable cognitive task performance and slightly poorer performance on inhibition tasks. Beta band activity was not significantly associated with any of the behavioral or cognitive variables, suggesting that resting state cortical activity in this frequency band may be a poor predictor of later behavior or cognitive performance.
DISCUSSION
The current study is the first to report the comparative medication effects of α2A agonist, guanfacine, d-MPH, and their combination on resting state EEG spectral power among a large sample of youth with ADHD. Analyses of spectral power measures during resting EEG suggest that each medication is associated with unique effects on cortical activity: GUAN with decreased alpha band power, DMPH with increased frontal and central beta power, and COMB with decreased theta band power and focal increases in beta power. Correlations between spectral power and behavior/cognitive variables, although modest, suggest that lower theta and higher alpha at posttreatment are associated with improved behavioral and cognitive functioning. Thus, these data suggest that each medication has a distinct neural signature, which is associated with behavioral and cognitive functioning.
The GUAN-related decrease in alpha band power is especially interesting to consider due to a seemingly paradoxical effect. Behaviorally, GUAN leads to improvement in ADHD symptoms8,11,12; however, neurophysiologically, guanfacine appeared to lower alpha power, which was subsequently associated with slower reaction time, higher reaction time variability, and a trend towards lower performance on tasks measuring inhibition. This result is similar to the only previous study examining EEG correlates of guanfacine among humans22. Among 10 healthy adults, guanfacine resulted in alpha power decrease with concomitant participant report of significantly reduced alertness, both of which were interpreted as being consistent with a central nervous system depressant effect. Resting alpha power has been negatively associated with vigilance23 and arousal regulation24, potentially arising from idling of thalamocortical circuits25. Recent studies suggest that resting state EEG alpha power is negatively correlated with functional connectivity (as measured by resting state functional magnetic resonance [rs-fMRI]) across a broad range of regions26, particularly within the visual network.27 In addition, researchers have also found an association between alpha power and the degree of anti-correlation between the default network and task-positive attention networks28, which was also found to be aberrant in ADHD.29 Finally, alpha power has been associated with affective dysregulation,30 and recent studies suggest that guanfacine may modulate the influence of emotional cues on cognitive control by altering fronto-limbic connectivity31. These associations suggest that guanfacine’s neural effect may be to decrease thalamo-cortical arousal or affective modulation of cognitive processing, which leads to slower and more variable reaction time. Thus, guanfacine may be most effective for those individuals with ADHD who have high arousal levels or affective dysregulation, perhaps in line with the reported secondary effect of decreasing emotional lability and irritability42.
Repeated administration of DMPH leads to increased beta power, which is consistent with previous findings on psychostimulant effects on EEG in ADHD16,17. During eyes-closed (EC) resting, beta power is associated with deactivation of the fronto-parietal attention network as well as sensory cortices32. These associations, however, exhibited strong developmental trends and were weakest among children, perhaps explaining the lack of correlation with behavioral and cognitive measures in the current study. It is likely that measures of event-related beta band activity would show stronger association with specific aspects of cognitive performance given its association with attention and concentration in previous studies33.
Finally, the effects of COMB medication on EEG spectral power were most prominent and resulted in ~20% decrease in theta band power. In our study, lower theta band power was associated with greater treatment change in ADHD behaviors as well with improved cognitive functioning, such as fewer inattentive errors and lower reaction time variability. Elevated resting state theta power has been widely reported as a marker for ADHD; that the COMB group had the lowest theta power relative to the other medication groups suggests a selective and more robust targeted treatment effect moving towards normalization. In concurrent EEG-fMRI studies, eyes-closed theta band power has been negatively correlated with default mode network activity26,32 and has been implicated in the balance of cortical excitation/inhibition.34 Aberrent connectivity within the DMN as well as weaker anti-correlation between the DMN and other task-positive networks have been associated with greater reaction time variability in ADHD,35,36 thus supporting the relationships found here among theta band power, DMN, and reaction time variability. Thus the combination of psychostimulant and α2A agonist may have a synergistic effect of strengthening the DMN at rest, which has been reported to have weaker connectivity in ADHD.37,38
To our knowledge, this study is the first to report medication effects of GUAN, DMPH, and COMB on cortical activation among a large sample of children with ADHD. Limitations of this study include that these results represent treatment effects that do not necessarily inform what occurs with long-term therapy. In addition, the participants were a selected population of youth with ADHD who were primarily Caucasian and relatively free of psychiatric comorbidities. Caution should be used when generalizing these results to older individuals as well as non-Caucasian youth with ADHD and those with greater psychiatric comorbidity. In addition, considerable variability in oscillatory activity within all of the EEG frequency bands has been noted, particularly between arousal states (i.e., eyes closed versus eyes open). Thus, EEG spectral power should not be used for clinical purposes such as making ADHD diagnosis or determining medication type or dosages. Further study is needed to examine medication effects in other resting and cognitive activation conditions to examine whether parallel effects are observed.
In conclusion, this study is the first to examine the medication effects of GUAN, DMPH, and COMB on cortical activation among youth with ADHD. Although all three medication conditions resulted in improvement of ADHD behavioral symptoms, differential medication effects on cortical activation emerged, suggesting different underlying neural mechanisms for each medication condition. Participants in the COMB condition exhibited decreased theta band and increased beta band power, which in turn was associated with better treatment response and improved cognitive performance. Participants in the GUAN condition had reduced alpha band power, which was associated with a slower and more variable reaction time. These results are consistent with previous studies on EEG correlates of medication response13,16 and suggest distinct neural effects of the medications singly and in combination predict later cognitive functioning. Whether or not the greater effects of COMB on these previously identified ADHD-related EEG correlates are durable over time and translate to longer-term benefits on clinical functioning should compel additional research.
Supplementary Material
Note: There were no significant differences in any frequency or electrode by medication group (all p >.2). COMB = combination; DMPH= d-methylphenidate; GUAN = guanfacine.
Acknowledgments
This work is supported by National Institute of Mental Health (NIMH) grants P50MH077248, “Translational Research to Enhance Cognitive Control” (J.T.M.) and MH92829, “Brain Source Analysis of EEG in ADHD” (S.K.L.).
The authors thank all of the families that participated in this research.
Dr. Bilder has received consulting income or honoraria from EnVivo Pharmaceuticals, Forum Pharmaceuticals, Lumos Labs, Maven Research, Neurocog Trials Inc., OMDUSA, LLC, Snapchat, Takeda-Lundbeck, and ThinkNow Inc. He has received research support from the National Institute of Mental Health, the John Templeton Foundation, and Johnson and Johnson. Dr. Piacentini has received grant or research support from the National Institute of Mental Health, Pfizer Pharmaceuticals through the Duke Clinical Research Institute CAPTN Network, Psyadon Pharmaceuticals, and the Tourette Association of America. He has received financial support from the Petit Family Foundation and the Tourette Syndrome Association Center of Excellence Gift Fund. He is a co-author of the Child OCD Impact Scale-Revised (COIS-R), the Child Anxiety Impact Scale (CAIS), the Parent Tic Questionnaire (PTQ), and the Premonitory Urge for Tics Scale (PUTS) assessment tools, all of which are in the public domain therefore no royalties are received. He has received royalties from Guilford Press and Oxford University Press. He has served on the speakers’ bureau of the Tourette Association of America, the International Obsessive Compulsive Disorder Foundation, and the Trichotillomania Learning Center. Dr. McGough has received consultant honoraria from Neurovance; research support from Purdue; material research support for investigator initiated studies from NeuroSigma and Shire; book royalties from Oxford University Press; and DSMB honoraria from Sunovion. He has provided expert testimony for Shire. Dr. McCracken has received consultant honoraria from Dart Neuroscience and Think Now, Inc. Drs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Loo, Cowen, Walshaw Welker, Levitt, Del’Homme, Mr. Cho, and Ms. Sturm report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Willcutt EG. The prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Neurotherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loo S, Makeig S. Clinical utility of EEG in Attention-Deficit/Hyperactivity Disorder: A research update. Neurotherapeutics. 2012;9:569–587. doi: 10.1007/s13311-012-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 4.Loo S, Hale T, Hanada G, et al. Familial clustering and DRD4 effects on EEG Measures in multiplex families with Attention-Deficit Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:368–377. [PMC free article] [PubMed] [Google Scholar]
- 5.Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. Journal of attention disorders. 2013;17(5):374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics. 1991;87:519–531. [PubMed] [Google Scholar]
- 7.Kollins SH, Lopez FA, Vince BD, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. Journal of child and adolescent psychopharmacology. 2011;21(2):111–120. doi: 10.1089/cap.2010.0064. [DOI] [PubMed] [Google Scholar]
- 8.Wilens TE, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten AF. Guanfacine for treatment of cognitive disorders: A century of discoveries at Yale. Yale Journal of Biology and Medicine. 2012;85:45–58. [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaura K, Karasawa J, Chaki S, Hikichi H. Stimulation of postsynapse adrenergic alpha2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2014;270:349–356. doi: 10.1016/j.bbr.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1):e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 12.Sallee FR, McGough J, Wigal T, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 13.Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2002;164(3):277–284. doi: 10.1007/s00213-002-1205-0. [DOI] [PubMed] [Google Scholar]
- 14.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG differences between good and poor responders to methylphenidate and dexamphetamine in children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2002;113(2):194–205. doi: 10.1016/s1388-2457(01)00736-2. [DOI] [PubMed] [Google Scholar]
- 15.Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR, Croft RJ. Effects of stimulant medications on the EEG of children with Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive type. Int J Psychophysiol. 2003;47(2):129–137. doi: 10.1016/s0167-8760(02)00119-8. [DOI] [PubMed] [Google Scholar]
- 16.Loo SK, Hopfer C, Teale PD, Reite ML. EEG correlates of methylphenidate response in ADHD: association with cognitive and behavioral measures. J Clin Neurophysiol. 2004;21:457–464. doi: 10.1097/01.wnp.0000150890.14421.9a. [DOI] [PubMed] [Google Scholar]
- 17.Loo SK, Teale PD, Reite ML. EEG correlates of methylphenidate response among children with ADHD: a preliminary report. Biological psychiatry. 1999;45:1657–1660. doi: 10.1016/s0006-3223(98)00250-9. [DOI] [PubMed] [Google Scholar]
- 18.Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM. Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology. 2009;57:702–707. doi: 10.1016/j.neuropharm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford Press; 1998. [Google Scholar]
- 21.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 22.Yamadera H, Ferber G, Matejcek M, Pokorny R. Electroencephalographic and psychometric assessment of the CNS effects of single doses of guanfacine hydrochloride (Estulic) and clonidine (Catapres) Neuropsychobiology. 1985;14(2):97–107. doi: 10.1159/000118212. [DOI] [PubMed] [Google Scholar]
- 23.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain research. Brain research reviews. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 24.Barry RJ, Clarke AR, Johnstone SJ, McCarthy R, Selikowitz M. Electroencephalogram theta/beta ratio and arousal in attention-deficit/hyperactivity disorder: evidence of independent processes. Biological psychiatry. 2009;66(4):398–401. doi: 10.1016/j.biopsych.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13(18):2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheeringa R, Petersson KM, Kleinschmidt A, Jensen O, Bastiaansen MC. EEG alpha power modulation of fMRI resting-state connectivity. Brain connectivity. 2012;2(5):254–264. doi: 10.1089/brain.2012.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Frontiers in human neuroscience. 2012;6:339. doi: 10.3389/fnhum.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. NeuroImage. 2013;72:227–236. doi: 10.1016/j.neuroimage.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoekzema E, Carmona S, Ramos-Quiroga JA, et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human brain mapping. 2014;35(4):1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGough JJ, McCracken JT, Cho AL, et al. A potential electroencephalography and cognitive biosignature for the child behavior checklist-dysregulation profile. J Am Acad Child Adolesc Psychiatry. 2013;52:1173–1182. doi: 10.1016/j.jaac.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz KP, Clerkin SM, Fan J, Halperin JM, Newcorn JH. Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology (Berl) 2013;226(2):261–271. doi: 10.1007/s00213-012-2893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luchinger R, Michels L, Martin E, Brandeis D. Brain state regulation during normal development: Intrinsic activity fluctuations in simultaneous EEG-fMRI. NeuroImage. 2012;60:1426–1439. doi: 10.1016/j.neuroimage.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- 34.Mayhew SD, Ostwald D, Porcaro C, Bagshaw AP. Spontaneous EEG alpha oscillation interacts with positive and negative BOLD responses in the visual-auditory cortices and default-mode network. NeuroImage. 2013;76:362–372. doi: 10.1016/j.neuroimage.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 35.Barber AD, Jacobson LA, Wexler JL, et al. Connectivity supporting attention in children with attention deficit hyperactivity disorder. Neuroimage Clin. 2015;7:68–81. doi: 10.1016/j.nicl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Fair DA, Posner J, Nagel BJ, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biological psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Cao Q, Long X, et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry research. 2012;201(2):120–127. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: There were no significant differences in any frequency or electrode by medication group (all p >.2). COMB = combination; DMPH= d-methylphenidate; GUAN = guanfacine.