Abstract
The potential therapeutic effects of Costa Rican guava (Psidium friedrichsthalianum) extracts for chronic obstructive pulmonary disease were examined. The ethyl acetate fraction displayed the highest antioxidant activity, as compared to the hexane, chloroform, and n-butanol fractions, as well as the crude extract. This fraction was evaluated for its anti-inflammatory activity response relationship against interleukin-8 (IL-8) and inhibition of matrix metalloproteinase-1 (MMP-1) expression before and after treatment with cigarette smoke. The ethyl acetate fraction exhibited inhibitory activity against IL-8 production and MMP-1 expression, showing the most potent inhibitory activities in both assays at 100 μg/mL, and nine compounds (1–9) were found. Phenolic compounds 1-O-trans-cinnamoyl-β-D-glucopyranose (2), ellagic acid (3), myricetin (4), quercitrin (7), and quercetin (9) were identified using standard compounds or literature reports from related species. Compounds 1, 5, 6, and 8 were tentatively identified as 1,5-dimethyl citrate (1), sinapic aldehyde 4-O-β-D-glucopyranose (5), 3,3′,4-tri-O-methylellagic acid-4′-O-D-glucopyranoside (6), and 1,3-O-diferuloylglycerol (8), All nine compounds are reported for the first time in Costa Rican guava.
Keywords: Costa Rican guava, Psidium friedrichsthalianum, Antioxidants, Interleukin-8 (IL-8), Matrix metalloproteinase-1 (MMP-1), Chronic obstructive pulmonary disease (COPD)
1. Introduction
The genus Psidium consists of approximately 150 species, but only about twenty produce commonly eaten fruits. The most widely cultivated species is the common guava (Psidium guajava L.), and other cultivated species include strawberry guava (Psidium cattleianum Sabine), the Brazilian guava (Psidium guineense Sw.), and Costa Rican guava (Psidium friedrichsthalianum Ndz.) (Mani, Mishra, & Thomas, 2011).
The Costa Rican guava is a small tree with small and sour fruits native to the seasonally flooded forest of Central America, from south Mexico to northern South America (Bailey, 1941; Dinesh & Iyer, 2005). The fruits are consumed as juices and also made into sweets and jellies; they are often described as being more aromatic than the common guava (Pino, Marbot, & Vazquez, 2002). A large number of terpenes and terpenic derivatives have been identified in Costa Rican guava (Pino et al., 2002), as well as in a variety of other guava species (Pino, Marbot, & Vazquez, 2001; Pino, Ortega, & Rosado, 1999).
Common guavas (P. guajava) are often included among super-fruits (Sanda, Grema, Geidman, & Bukar-Kolo, 2011). A review focused on guavas reported that the leaves and fruits of this plant showed anti-oxidant, anti-inflammatory, antimicrobial, antispasmodic, hepatoprotective, anti-allergy, antigenotoxic, antiplasmodial, antidiabetic, cardioactive, anti-cough, and anticancer effects (Gutierrez, Mitchell, & Solis, 2008). We hypothesise that as a closely related edible Psidium, Costa Rican guava shares some of these useful pharmacological properties. However, no reports have examined the biological properties and the composition of this guava species.
Chronic obstructive pulmonary disease (COPD), a major disease which causes death and disability, is expected to be the third leading cause of death worldwide by 2020 (Murray & Lopez, 1997). Cigarette smoke is the main aetiological factor associated with the development of COPD, for which there is no cure. Currently, little progress has been made toward developing effective therapies for COPD, and although treatments can improve symptoms, their effects are limited and they have not reduced disease progression (Calverley et al., 2007; Vestbo et al., 1999).
The inflammatory reaction to cigarette smoke is crucial in the pathogenic mechanisms of COPD (Repine, Bast, & Lankhorst, 1997). The high concentration of oxidant molecules in cigarette smoke, in addition to the oxidants endogenously formed by inflammatory cells (macrophages and neutrophils), overcome the capacity of the antioxidant protective physiological mechanisms and induce oxidative stress (Cross, Van der Vliet, O’Neill, Louie, & Halliwell, 1994; Frei, Forte, Ames, & Cross, 1991). This oxidative stress may result in direct damage to structural cells, amplification of inflammation, and promotion of proteolytic degradation of tissues by inhibiting antiprotease systems. In vitro studies of human alveolar macrophages have shown that oxidative stress caused by acute cigarette smoke extract (CSE) exposure increases the release of interleukin-8 (IL-8) (Walters et al., 2005). Matrix metalloproteinases (MMPs), a family of zinc endo-peptidases, may also play an important role in COPD pathology (Babusyte et al., 2007; Haq et al., 2010). Particularly it has been described that MMP-1 is expressed in the lung of human patients with emphysema but not in normal control subjects (Imai et al., 2001). Kim et al. (2004) demonstrated that cigarette smoke stimulates MMP-1 production by human lung fibroblasts through the extracellular-signal-regulated kinases (ERK)1/2 pathway.
Therefore, it is anticipated that drugs that inhibit MMP-1 expression, reduce pulmonary inflammation and decrease oxidative stress in the lungs of patients with COPD will provide effective disease therapies.
Several epidemiological studies have established a beneficial link between phenolic compounds intake and reduced risk of disease, which were attributed to both their antioxidant and anti-inflammatory properties (Arts & Hollman, 2005). A significant inverse correlation between phenolic compounds intake and the incidence of COPD has been reported in a study with over 13,000 subjects (Tabak, Arts, Smit, Heederik, & Kromhout, 2001). It was reported that increased phenolic compounds, such as catechin, flavonol and flavone, intake can improve symptoms such as phlegm production, cough, and breathlessness; lung function also can improved by measured by forced expiratory volume in 1 s (Tabak et al., 2001).
Other studies have demonstrated a direct impact of specific phenolic compounds on inflammation in vitro and in vivo. For example, the flavanoid resveratrol inhibits inflammatory cytokine release from macrophages isolated from COPD patients (Culpitt et al., 2003). Meja et al. (2008) have observed that curcumin can inhibit inflammation and restore glucocorticoid efficacy in response to oxidative stress.
In our ongoing study of phenolic compounds with therapeutic effects for COPD from tropical fruits (Dastmalchi, Flores, Petrova, Pedraza-Penalosa, & Kennelly, 2011; Flores et al., 2012a; Floreset al., 2012b; Reynertson et al., 2006), the Costa Rican guava was investigated. The focus of this study is to evaluate the phenolic constituents of Costa Rican guava for their antioxidant and anti-inflammatory activities. Our ultimate goal is to identify natural products as new therapeutics for the treatment of COPD.
2. Materials and methods
2.1. General experimental procedures
Solvents for chromatography, HPLC-grade MeOH, formic acid and acetonitrile were obtained from J.T. Baker (Phillipsburg, NJ). GR-grade MeOH, hexane, chloroform, ethyl acetate, and n-butanol were supplied by VWR Inc. (Bridgeport, PA). Ultrapure water was prepared using a Millipore Milli-RO 12 plus system (Millipore Corp., Bedford, MA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), Trolox, and potassium peroxosulfate were purchased from Sigma–Aldrich (St. Louis, MO). 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonate) diammonium salt (ABTS) was obtained from TCI-Ace (Tokyo, Japan). Ellagic acid, myricetin, quercitrin, and quercetin were supplied by Extrasynthèse (Genay, France).
2.2. Plant material
Fruits of Costa Rican guava were collected at the Fruit and Spice Park (Homestead, FL). Fruits were frozen and shipped by overnight courier on dry ice to the laboratory, where they were kept in cold (−20 °C) dark storage until processed.
2.3. Extraction
Fruits of Costa Rican guava (900 g) were freeze–dried to obtain 90 g of fruit. The freeze–dried edible pulp (10 g) was extracted three times with 200 mL of MeOH/H2O (70:30) at room temperature with a blender for 5 min per extraction. The combined extract was dried in vacuo at temperatures not exceeding 40 °C.
The extract was suspended in water and sequentially partitioned three times with hexane (60 mL × 3), chloroform (60 mL × 3), ethyl acetate (60 mL × 3), and n-butanol (60 mL × 3). The four fractions were concentrated in vacuo.
2.4. ABTS assay
Determination of the ABTS·+ scavenging assay effect of the Costa Rican guava crude extract and of the hexane, chloroform, ethyl acetate, and n-butanol fractions was performed according to the method of Re et al. (1999). Initially, an ABTS·+ stock solution was prepared by reacting ABTS aqueous solution (7 mM) with K2S2O8 (2.45 mM, final concentration) at ambient temperature in the dark for 12–16 h. Absorbance of the reactant was later adjusted to 0.700 ± 0.020 at ambient temperature at a wavelength of 734 nm. A Molecular Devices Versamax microplate reader (Sunnyvale, CA) was used. In a final volume of 200 μL, the reaction mixture was compromised of 198 μL of ABTS·+ solution and 2 μL of the sample at different concentrations (50–750 μg/mL). Absorbances at 734 nm were measured at 5 min intervals for 40 min. The reduction in absorbance of the ABTS·+ solution in the presence of different concentrations of Trolox (0.5–10 μg/mL) was also determined. The ABTS·+ percentage inhibition of the extracts, the fractions and Trolox was calculated from Eq. (1). Results were expressed as μmol Trolox equivalent antioxidant capacity (TEAC) per gram of sample:
| (1) |
2.5. DPPH assay
The DPPH assay was performed according to the method developed by Smith, Reeves, Dage, and Schnettler (1987) slightly modified. To a 50 μL aliquot of the sample 150 μL of DPPH (400 μM) were added. Decrease of absorbance was monitored at 517 nm after 30 min of incubation at 37 °C on a Molecular Devices Versamax microplate reader (Sunnyvale, CA). The percentage inhibition of the DPPH by each dilution of samples was calculated considering the percentage of the steady DPPH in solution after reaction (Eq. (1)). A plot of percentage inhibition versus concentration was made for the reference standard Trolox. On the basis of this plot, the TEAC values for different samples were calculated.
2.6. IL-8 immunoassay
Human small airway epithelial (SAE) cells were cultured according to supplier instructions (Lonza, Walkersville, MD) and maintained in a controlled atmosphere of air/5% CO2 at 37 °C. 80% confluent SAE cells at passages 2–5 were used for experiments. CSE was prepared using a modified protocol (Laurent, Janoff, & Kagan, 1983). Briefly, a Barnet vacuum pump operating at constant flow was used to draw the smoke of one 3R4F research grade cigarette (University of Kentucky) through 25 mL of Dulbecco’s phosphate-buffered saline. This solution (100% CSE) was adjusted to pH 7.4, filtered, diluted with small airway growth medium to a final concentration of 5%, and added to the cells immediately.
Cells were treated with 5% CSE or pure compounds or pre-treated with pure compounds 1 h prior to 5% CSE exposure. After 24 h, measurement of human IL-8 in cell culture supernatants was performed by ELISA (R&D Systems Inc., Minneapolis, MN).
2.7. MMP-1 mRNA expression
The cells were cultured as described above. After 24 h of treatment, total RNA from the human SAE cells was isolated (RNeasy kit, Qiagen, Valencia, CA) and converted into cDNA (high capacity cDNA kit, Applied Biosystems, Carlsbad, CA). Relative expression of MMP-1 was measured using real-time quantitative PCR and Taqman probes with GAPDH as an endogenous control (Applied Biosystems).
2.8. HPLC–PDA
The analytical HPLC system (Waters Corp., Milford, MA) consisted of a Waters 2695 Separation Module equipped with a 2996 photodiode-array detector (PDA) and coupled to the Waters Empower (version 5.0) for data acquisition and processing. Separation was carried out using a 250 × 4.6 mm, 4 μm Synergi Hydro-RP 80A column (Phenomenex, Torrance, CA). The mobile phase consisted of solvents A (1% aqueous formic acid solution) and B (acetonitrile) as follows: 80–70% A over 5 min; 70–60% A over 5–10 min; 60–50% from 10 to 35 min at 1 mL/min, and these conditions were kept isocratic for 10 min. The composition was then changed to initial conditions in 5 min, and maintained for 10 min prior to the next injection. Stock solution of ellagic acid, myricetin, quercitrin, and quercetin were prepared in 70% (v/v) methanol to final concentration of 1 mg/mL. Each stock solution was further diluted to obtain six concentrations of the standard (ranging from 10 to 750 g/mL) for HPLC–PDA quantification and they were injected in triplicate. To determine the concentration of 3,3′,4-tri-O-methylellagic acid-4′-O-D-glucopyranoside, ellagic acid was used as standard. The fruit extracts were also reconstituted in 70% (v/v) methanol and injected in triplicate at a concentration of 10 mg/mL. Peak areas for the extracts and standards were integrated from HPLC–PDA chromatograms by use of Waters Empower2 software at 360 nm.
2.9. LC–MS analyses
An LCT Premier XE TOF mass spectrometer (Waters Corp.) equipped with an ESI interface and controlled by MassLynx V4.1 software was used to perform the LC–MS analysis. Mass spectra were acquired in both positive and negative modes over the mass range m/z 100–1000. MS parameters were: capillary voltage, 3000 V (positive mode) and 2800 V (negative mode); cone voltage, 20 V; desolvation and cone gas flow rates, 600 and 20 L/h; desolvation temperature, 400 °C; and the source temperature, 120 °C. The analytical column used was a 250 × 4.6 mm, 4 μm Synergi Hydro-RP 80A column (Phenomenex), and the same elution solvent and method as the one described above for HPLC–PDA were applied.
2.10. Statistical analysis
Data are expressed as means values ±95% confidence interval. One-way analysis of variance (ANOVA) was performed with significant differences between means determined by the Student’s t-test. JMP Statistics software package version 8 was used for statistical analyses (SAS Institute Inc., Cary, NC).
3. Results and discussion
The potential effects of Costa Rican guava extract and fractions were examined for the treatment of COPD. Oxidative stress and inflammation are two causes for COPD; therefore polyphenols may be a useful treatment for COPD (Rahman, 2012). The ABTS·+ and DPPH· scavenging activities of the crude extract and the hexane, chloroform, ethyl acetate, and n-butanol fractions of Costa Rican guava were evaluated. Ethyl acetate fraction demonstrated higher antioxidant activity in the ABTS and DPPH assay, and therefore the inhibitory effect of IL-8 and MMP-1 expression in cells treated with CSE was investigated. Further, a compositional analysis of this fraction was performed using LC–PDA and TOF LC–MS and nine compounds (1–9) were found.
3.1. Extraction
The extraction yield for the crude extract was 476.2 (mg/g of dry fruit). The highest extraction yield of the four fractions was obtained for n-butanol (30.23% w/w) followed by ethyl acetate (4.12% w/w) then chloroform (0.75% w/w). The lowest yield was obtained for hexane (0.25% w/w).
3.2. Antioxidant activity
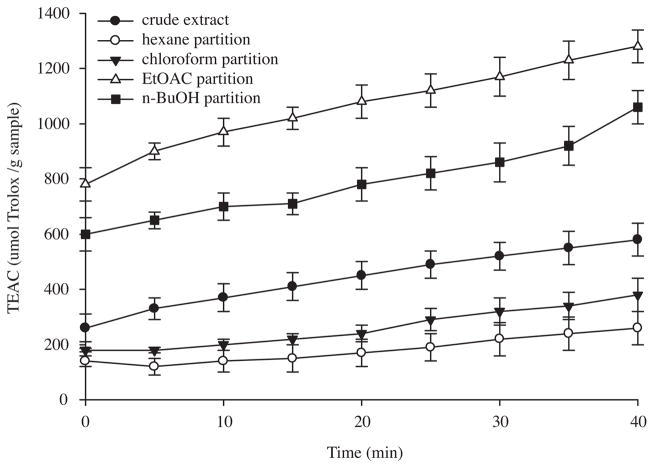
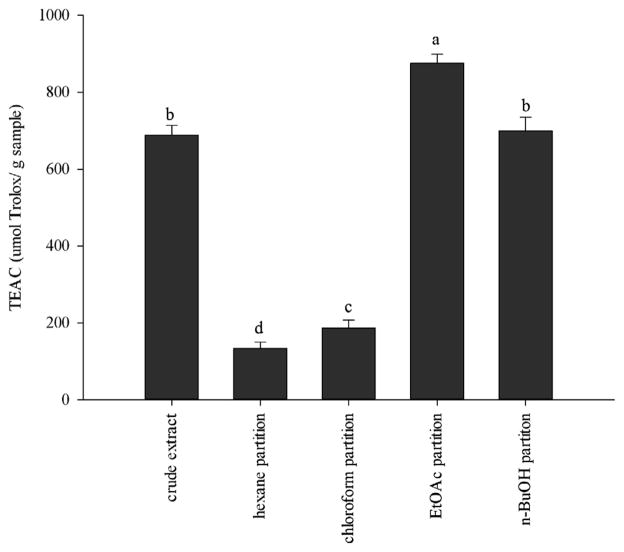
In order to measure the antioxidant activities of the Costa Rican guava extracts, ABTS·+ and DPPH· scavenging assays were used. The ABTS·+ scavenging activity was monitored over time, allowing the slow-acting antioxidants to have enough time to exert their effects. Due to their contribution to the scavenging activity, the order of activity among the samples changed during the assay, reaching their highest activity at 40 min (Fig. 1). All the extracts demonstrated a wide range of ABTS·+ scavenging activities. They exerted an increase in their activity over time; and the order of activity remained the same from 0 to 40 min. The ethyl acetate fraction was the most potent, followed by the crude extract, and the n-butanol, chloroform, and hexane fractions. The order of DPPH· scavenging activity of the Costa Rican guava extracts was ethyl acetate fraction > n-BuOH fraction and crude extract (not significantly different, p > 0.05) > chloroform fraction > hexane fraction (Fig. 2).
Fig. 1.
ABTS·+ scavenging activity of Costa Rican guava crude extract, and hexane, chloroform, ethyl acetate, and n-butanol fractions. Values are expressed as means ±95% confidence intervals (n = 8) of Trolox equivalent antioxidant capacity (TEAC) (μmol of Trolox per gram of dry extract).
Fig. 2.
DPPH scavenging activity of Costa Rican guava crude extract, and hexane, chloroform, ethyl acetate, and n-butanol fractions. Values are expressed as means ±95% confidence intervals (n = 8) of Trolox equivalent antioxidant capacity (TEAC) (μmol of Trolox per gram of dry extract). Bars with different letters (a–e) are significantly (p < 0.05) different.
3.3. IL-8 inhibition
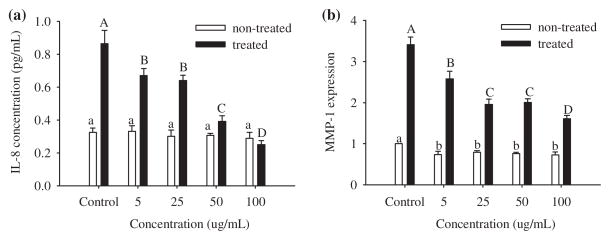
The ethyl acetate fraction, due to its high antioxidant activity in the ABTS and DPPH assays, was evaluated for its efficacy in the inhibition of IL-8 in small airway epithelial (SAE) cells untreated and treated with CSE. Concentrations of 5, 25, 50, and 100 μg/mL were used. When control cells were exposed to CSE the amount of IL-8 increased threefold (Fig. 3a). A dose of 5 μg/mL of the ethyl acetate fraction was enough to decrease the concentration of IL-8. SAE cells were more susceptible to IL-8 inhibition when 50 and 100 μg/mL were added to the medium and 100 μg/mL showed the highest decrease on the IL-8 production. In untreated cells the ethyl acetate fraction did not decrease the basal production of IL-8 at all of the concentrations tested.
Fig. 3.
Dose–response relationship of Costa Rican guava ethyl acetate fraction at 5, 25, 50, and 100 mg/mL on the expression of (a) IL-8 (b) and MMP-1 mRNA in SAE cells untreated (open bars) and treated (bold bars) with cigarette smoke extract (CSE). Data are presented as mean values ±95% confidence limits (n = 3). Open bars with the same lower case letters (a) and bold bars with the same upper case letters (A–C) are not significantly (p > 0.05) different.
3.4. MMP-1
Before treatment with the guava ethyl acetate fraction, SAE cells demonstrated MMP-1 expression, which increased threefold after 24 h of CSE exposure (Fig. 3b). The addition of 5 μg/mL of the ethyl acetate fraction reduced the production of MMP-1. This reduction was higher when 25 and 50 μg/mL were added to the cells. The highest inhibitory activity was observed when 100 μg/mL of the extract were added to the cells. In untreated cells the expression of MMP-1 decreased with all the concentrations evaluated in this study to the same level.
3.5. Characterisation and quantification of the ethyl acetate fraction components by LC–PDA and LC–TOF
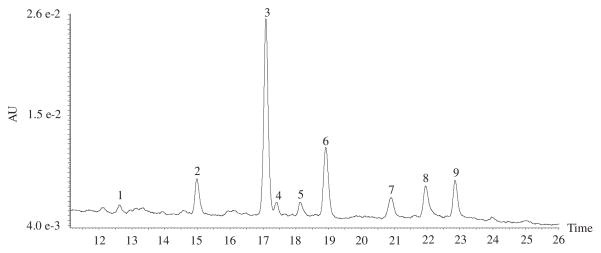
The ethyl acetate fraction of Costa Rican guava was analysed by LC–PDA and LC–TOF, to identify the components with significant biological activity. The nine major peaks detected in the ethyl acetate fraction by HPLC–PDA at 254 nm (Fig. 4), were identified by their elution order, UV/Vis and MS characteristics were compared with reported data in the literature, and, when possible, by co-injection of standards. In this study negative and positive modes of ESI mass detection were employed. TOF LC–MS (negative and positive modes) with ESI mass detection was conducted, and fragmentation data, retention time, spectrum information, and quantitative data are displayed in Table 1.
Fig. 4.
HPLC chromatogram of Costa Rican guava ethyl acetate fraction at 254 nm.
Table 1.
Chemical profile of the identified compounds in the Costa Rican guava ethyl acetate fraction.
| Compound numbera | Retention time (min) | Concentration (mg/g of dry extract) | UV data (λmax) | Marker ion exact mass (ppm) | Selected fragmental ion exact masses [adduct molecular ions-neutral molecules or radicals]+,− (molecular formula, ppm) | Identification |
|---|---|---|---|---|---|---|
| 1 | 12.7 | 205 | 221.0649 [M+H]+ (C8H13O7, −5.4) | 243.0492 [M+Na]+ (C8H12O7Na, 4.5); 463.1057 [2M+Na]+ (C16H24O14Na, −1.5) 219.0480 [M−H]− (C8H11O7, −11.4); 265.0540 [M−H+HCOOH]− (C9H13O9, −7.5); 439.1057 [2 M−H] − (C16H23O14, −7.1) |
1,5-Dimethyl citrate | |
| 2 | 15.0 | 224, 300 | 333.0946 [M+Na]+ (C15H18O7Na, −1.2) | 643.2001 [2M+Na]+ (C30H36O14Na, −0.3) 355.1011 [M−H+HCOOH]− (C16H19O9, −5.1) |
1-O-trans-Cinnamoyl-β-D-glucopyranose | |
| 3 | 17.1 | 10.5 | 254, 364 | 300.0073 [M−H] − (C14H5O8, −3.7) | 603.0048 [M−H+HCOOH]− (C28H11O16, 0.2) | Ellagic acid |
| 4 | 17.4 | 1.1 | 254, 370 | 317.0280 [M−H] − (C15H9O8, −5.4) | 319.0465 [M+H]+ (C15H11O8, 3.4); 635.0687 [2 M−H]− (C30H19O16, 0.5) | Myricetin |
| 5 | 18.2 | 265, 365 | 415.1219 [M−H+HCOOH]− (C18H23O11, −5.1) | 393.1161 [M+Na]+ (C17H21O9Na, −0.3) | Sinapic aldehyde 4-O-β-D-glucopyranoside | |
| 6 | 18.9 | 5.8 | 250, 365 | 551.1033 [M−H+HCOOH] − (C24H23O15, −0.7) | 507.1158 [M+H]+ (C23H23O13, 3.7); 529.0938 [M+Na]+ (C23H22O13Na, −3.8); 345.0554 [M+H-glucosyl group]+ (C17H13O8, −16.2) 343.0470 [M−H–glucosyl group]− (C17H11O8, 4.7) |
3,3′,4-Tri-O-methylellagic acid-4′-O-D-glucopyranoside |
| 7 | 20.9 | 6.0 | 257, 353 | 449.1099 [M+H]+ (C21H21O11, 3.3) | 493.0970 [M−H+HCOOH] − (C22H21O13, −2.4) | Quercitrin |
| 8 | 22.0 | 250, 365 | 445.1501 [M+H]+ (C23H25O9, 0.4) | 443.1326 [M−H] − (C23H23O9, −3.6) | 1,3-O-Diferuloylglycerol | |
| 9 | 22.9 | 9.2 | 254, 367 | 301.0366 [M−H]− (C15H9O7, 6.0) | 303.0534 [M−H]+ (C15H11O7, 9.6) | Quercetin |
Compounds numbers and retention times refers to the numbers given in Fig. 4.
Compounds 3, 4, 7, and 9 were identified as ellagic acid, myricetin, quercitrin, and quercetin, respectively. Their identification was confirmed by co-injection of standards. Mahattanatawee et al. (2006) identified ellagic acid in the leaf and roots of P. guajava. Quercitin, quercitrin, and myrcetin have been previously reported in the leaf and fruits of P. guajava (Kubola, Siriamornpun, & Meeso, 2011; Lozoya et al., 1994).
Compound 1 showed in the positive mode m/z 243.0492 corresponding to [M+Na]+ (C8H12O7Na), and the molecular ion [M+H]+ at 221.0649 (C8H13O7) (Table 1). In the negative mode a fragmental ion [M−H+HCOOH]− at 265.0540 (C9H13O9), and the molecular ion [M−H]− at 219.0480 (C8H11O7) were found (Table 1). The maximum UV absorbance was registered at 205 nm. This compound was tentatively associated with 1,5-dimethyl citrate.
The parent ion of compound 2 was obtained at m/z 333.0946 [M+Na]+ (C15H18O7Na). It showed a deprotonated molecular ion in the negative mode at m/z 355.1011 (Table 1) corresponding, as already observed (Lunkenbein et al., 2006) to the formate adduct [M−H+COOH]− and thus, a molecular weight of 310.1048. The UV spectra showed absorption maxima at 300, 224 (sh). This compound was determined to be 1-trans-cinnamoyl-β-D-glucopyranoside, which was previously identified in P. guajava by Latza et al. (1996).
Based on their fragmentation pattern, molecular formula and UV profile (Table 1), compounds 5 and 8 were tentatively identified as sinapic aldehyde-4-O-β-D-glucopyranoside and 1,3-O-diferuloylglycerol, respectively.
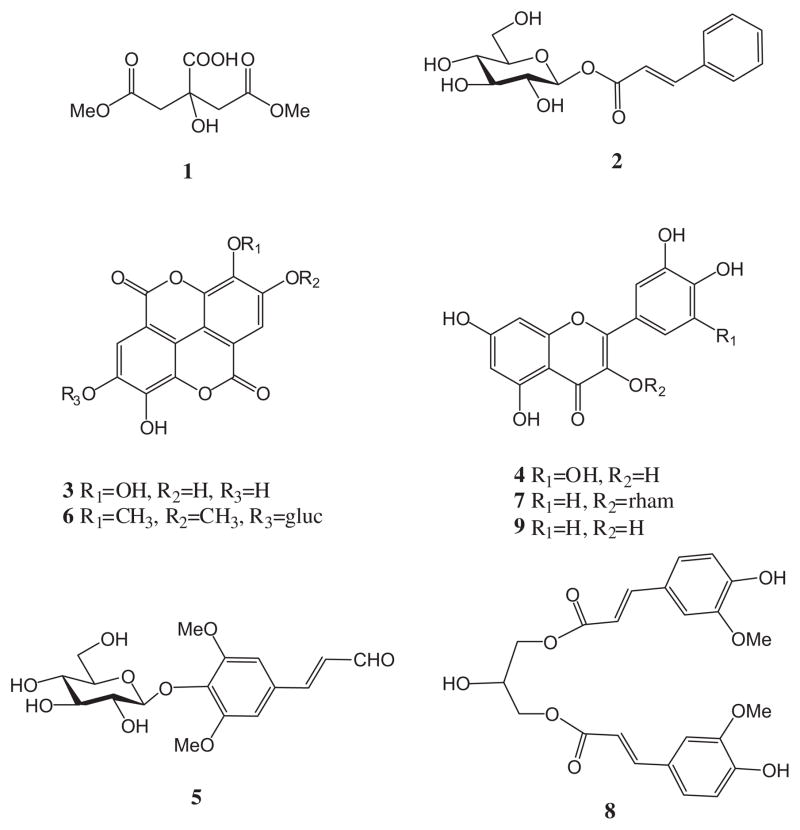
Compound 6 had a similar UV absorbance profile to 3. It showed a molecular ion [M+H]+ at 507.1158 in the positive mode. In the negative mode a fragment at 551.1033 corresponding to the formate adduct [M−H+HCOOH]− was found. The mass spectrum showed a fragment corresponding to the presence of glucose in the structure at m/z 345.0554 [M+H–glucosyl group]+ (C17H13O8) in the positive mode and at m/z 343.0470 [M−H–glucosyl group]− (C17H11O8) in the negative mode (Table 1). This compound was tentatively identified as 3,3′,4-tri-O-methylellagic acid-4′-O-D-glucopyranoside. Fig. 5 shows the structures of compounds 1–9.
Fig. 5.
Chemical structures of compounds identified in Costa Rican guava ethyl acetate fraction. 1,5-Dimethyl citrate (1), 1-O-trans-cinnamoyl-β-D-glucopyranoside (2), ellagic acid (3), myricetin (4), sinapic aldehyde 4-O-β-D-glucopyranoside (5), 3,3′,4-tri-O-methylellagic acid-4′-O-D-glucopyranoside (6), quercitrin (7), 1,3-O-diferuloylglycerol (8), and quercetin (9).
Although the therapeutical effect of Costa Rican guava ethyl acetate fraction on COPD remains to be investigated further, the antioxidant, IL-8, and MMP-1 inhibitory activities of this fraction are likely to be due to the phytochemicals mentioned above.
The antioxidant activity of compounds 3, 4, 7, and 9 has been well documented (Boots, Haenen, & Bast, 2008; Tabart, Kevers, Pincemail, Defraigne, & Dommes, 2009). Luo, Li, and Kong (2011) studied the antioxidant activity of compound 8 using ABTS and DPPH assays. Several researchers have reported the anti-inflammatory activity of these polyphenols. Mueller, Hobiger, and Jungbauer (2010) reported that compounds 4 and 9 were notably more effective on the reduction of IL-6 and TNF-α secretion compared to cortisol. Previous studies carried out in our laboratory revealed that compound 3, the major phenolic compound, demonstrated IL-8 and MMP-1 inhibitory activity in cells treated and untreated with CSE (Dastmalchi et al., 2012). Reactive oxygen species can initiate transcription factors as well as signal-transduction pathways (Rahman & Adcock, 2006). The activation of these pro-inflammatory mediators enhances transcription of downstream inflammatory chemokines (Calixto, Campos, Otuki, & Santos, 2004). By reducing the oxidative attack, these compounds may reduce the amount of pro-inflammatory mediators, which are directly tied to IL-8 production.
Despite the many promising in vitro effects of phenolic compounds for COPD and other diseases, it is important to note that many phenolic compounds either have limited bioavailability in vivo and/or are biotransformed in the gastrointestinal tract into compounds that may have less therapeutic efficacy (Spencer, Schroeter, Rechner, & Rice-Evans, 2001). Future in vivo studies using these phenolic compounds should be designed keeping in view the preceding observations.
For the treatment of COPD, it has been proposed that the development of improved inhaled delivery techniques would allow clinically relevant concentrations of antioxidants to be deposited in the lung while avoiding the first pass metabolism that occurs during systemic absorption (Zhu, Chen, & Li, 2000).
4. Conclusion
The antioxidant activity of the Costa Rican guava ethyl acetate fraction coupled with its ability to reduce inflammation caused by secondary smoke exposure shows the potential that the constituents within this fraction may have for further drug development. Based on these results, further studies of these chemical constituents are currently being carried out in our laboratory.
Acknowledgments
Support for this study was provided by NIH-NHLBI grant 5SC1HL096016, NIH-NCCAM grant F31AT00801, and by the Spanish Ministry of Science and Innovation postdoctoral fellowship (G.F.). The authors thank the staff of the Fruit and Spice Park (Homestead, FL). The authors also thank Dr. Chunhui Ma, Lehman College (CUNY) for his help in the LC–MS analysis and Sturlainny Paulino for her help in the extraction of the guava plant material.
References
- Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. American Journal of Clinical Nutrition. 2005;81(Suppl 1):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Babusyte A, Stravinskaite K, Jeroch J, Lotvall J, Sakalauskas R, Sitkauskiene B. Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respiratory Research. 2007;8(81):1–9. doi: 10.1186/1465-9921-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LH. Hortus Second. New York: The Mac Millan Co; 1941. A concise dictionary of gardening and general horticulture and cultivated plants in North America; p. 604. [Google Scholar]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: From antioxidant to nutraceutical. European Journal of Pharmacology. 2008;585(2–3):325–327. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Medica. 2004;70:93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Cross CE, van der Vliet A, O’Neill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environmental Health Perspectives. 1994;102(Suppl 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58(11):942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastmalchi K, Flores G, Ma C, Dabo AJ, Whalen K, Reynertson KA, et al. Edible Myrciaria vexator fruits: Bioactive phenolics for potential COPD therapy. Bioorganic & Medicinal Chemistry. 2012;20(14):4549–4555. doi: 10.1016/j.bmc.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastmalchi K, Flores G, Petrova V, Pedraza-Penalosa P, Kennelly EJ. Edible neotropical blueberries: Antioxidant and compositional fingerprint analysis. Journal of Agricultural and Food Chemistry. 2011;59(7):3020–3026. doi: 10.1021/jf200367j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh MR, Iyer CPA. Significant research achievements in Guava-improvement and future needs. 1. IGS; India: 2005. pp. 7–16. [Google Scholar]
- Flores G, Dastmalchi K, Dabo AJ, Whalen K, Pedraza-Peñalosa P, Foronjy RF, et al. Antioxidants of therapeutic relevance in COPD from the neotropical blueberry Anthopterus wardii. Food Chemistry. 2012a;131(7):119–125. doi: 10.1016/j.foodchem.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Dastmalchi K, Paulino S, Whalen K, Dabo AJ, Reynertson KA, et al. Anthocyanins from Eugenia brasiliensis edible fruits as potential therapeutics for COPD treatment. Food Chemistry. 2012b;134(3):1256–1262. doi: 10.1016/j.foodchem.2012.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochemical Journal. 1991;277:133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez RM, Mitchell S, Solis RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2008;117(1):1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Haq I, Chappell S, Johnson SR, Lotya J, Daly L, Morgan K, et al. Association of MMP-2 polymorphisms with severe and very severe COPD: A case control study of MMPs-1, 9 and 12 in a European population. BMC Medical Genetics. 2010;11(7):1–11. doi: 10.1186/1471-2350-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. American Journal of Respiratory Critical Care Medicine. 2001;163(3):786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Kim H, Liu X, Kohyama T, Kobayashi T, Conner H, Abe S, et al. Cigarette smoke stimulates MMP-1 production by human lung fibroblasts through the ERK1/2 pathway. COPD. 2004;1(1):13–23. doi: 10.1081/COPD-120030164. [DOI] [PubMed] [Google Scholar]
- Kubola J, Siriamornpun S, Meeso N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chemistry. 2011;3(1):972–981. [Google Scholar]
- Latza S, Ganber D, Berger RG. Carbohydrate esters of cinnamic acid from fruits of Physalis peruviana, Psidium guajava and Vaccinium vitis-idaea. Phytochemistry. 1996;43(2):481–485. [Google Scholar]
- Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. American Review of Respiratory Disease. 1983;127(2):189–192. doi: 10.1164/arrd.1983.127.2.189. [DOI] [PubMed] [Google Scholar]
- Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Arnason JT. Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Archives of Medical Research. 1994;25(1):11–15. [PubMed] [Google Scholar]
- Lunkenbein S, Bellido M, Aharoni A, Salentijn EM, Kaldenhoff R, Coiner HA, et al. Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose: Cinnamate glucosyltransferase from strawberry. Plant Physiology. 2006;140(3):1047–1058. doi: 10.1104/pp.105.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Li L, Kong L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chemistry. 2011;131(3):1056–1062. [Google Scholar]
- Mahattanatawee K, Manthey JA, Luzio G, Talcott ST, Goodner K, Baldwin EA. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. Journal of Agricultural and Food Chemistry. 2006;54(19):7355–7363. doi: 10.1021/jf060566s. [DOI] [PubMed] [Google Scholar]
- Mani A, Mishra R, Thomas G. Elucidation of diversity among Psidium species using morphological and SPAR methods. Journal of Phytology. 2011;3(8):53–61. [Google Scholar]
- Meja KK, Rajendrasozhan S, Adenuga D, Biswas SK, Sundar IK, Spooner G, et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. American Journal of Respiratory Cell and Molecular Biology. 2008;39(3):312–323. doi: 10.1165/rcmb.2008-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chemistry. 2010;122(4):987–996. [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Pino JA, Marbot R, Vazquez C. Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) fruit. Journal of Agricultural and Food Chemistry. 2001;49(12):5883–5887. doi: 10.1021/jf010414r. [DOI] [PubMed] [Google Scholar]
- Pino JA, Marbot R, Vazquez C. Characterization of volatiles in Costa Rican guava [Psidium friedrichsthalianum (Berg) Niedenzu] fruit. Journal of Agricultural and Food Chemistry. 2002;50(21):6023–6026. doi: 10.1021/jf011456i. [DOI] [PubMed] [Google Scholar]
- Pino J, Ortega A, Rosado A. Volatile constituents of guava (Psidium guajava L.) fruits from Cuba. Journal of Essential Oil Research. 1999;11(6):623–628. [Google Scholar]
- Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochimica et Biophysica Acta. 2012;1822(5):714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. European Respiratory Journal. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biological Medicine. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. American Journal of Respiratory Critical Care Medicine. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, et al. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora) Journal of Natural Products. 2006;69(8):1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- Sanda KA, Grema HA, Geidman YA, Bukar-Kolo YM. Pharmacological aspects of Psidium guajava: An update. International Journal of Pharmacology. 2011;7(3):316–324. [Google Scholar]
- Smith RC, Reeves JC, Dage RC, Schnettler RA. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochemical Pharmacology. 1987;36:1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: Gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxidant Redox Signaling. 2001;3(6):1023–1039. doi: 10.1089/152308601317203558. [DOI] [PubMed] [Google Scholar]
- Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN Study. American Journal of Respiratory Critical Care Medicine. 2001;164(1):61–64. doi: 10.1164/ajrccm.164.1.2010025. [DOI] [PubMed] [Google Scholar]
- Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chemistry. 2009;113(4):1226–1233. [Google Scholar]
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 1999;353(9167):1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: Implications for steroid resistance. Molecular Pharmacology. 2005;68(5):1343–1353. doi: 10.1124/mol.105.012591. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen Y, Li RC. Oral absorption and bioavailability of tea catechins. Planta Medica. 2000;66(5):444–447. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]