Abstract
The brain transforms physical sensory stimuli into meaningful perceptions. In animals making choices about sensory stimuli, neuronal activity in successive cortical stages reflects a progression from sensation to decision. Feedforward and feedback pathways connecting cortical areas are critical for this transformation. However, the computational roles of these pathways are poorly understood because pathway-specific activity has rarely been monitored during a perceptual task. Using cellular-resolution, pathway-specific imaging, we measured neuronal activity across primary (S1) and secondary (S2) somatosensory cortices of mice performing a tactile detection task. S1 encoded the stimulus better than S2, while S2 activity more strongly reflected perceptual choice. S1 neurons projecting to S2 fed forward activity that predicted choice. Activity encoding touch and choice propagated in an S1–S2 loop along feedforward and feedback axons. Our results suggest that sensory inputs converge into a perceptual outcome as feedforward computations are reinforced in a feedback loop.
INTRODUCTION
Perceptual decisions involve propagation and transformations of sensory signals across multiple hierarchically organized cortical areas1. Feedback projections are a ubiquitous feature of cortical organization2, and are implicated in numerous functions, such as contextual modulation of perception, attention, sensory expectation, and perceptual learning3,4. Feedback to primary sensory cortical areas is even hypothesized as essential for sensory awareness5. Sensory cortex feedforward and feedback connections form recurrent neural networks. Recurrent networks can exhibit complex dynamics and perform sophisticated computations, such as by forming content-addressable memory networks for pattern completion6, amplification of input signals7–9, and the maintenance of neural activity over timescales longer than permitted by the biophysics of individual neurons10.
How feedforward and feedback cortical dynamics mediate the transformation from raw sensory input to actionable interpretations of the sensory world (i.e., to perceptual decisions) is not understood. This is largely due to the difficulty of using traditional methods to measure neural activity within defined synaptic pathways during behavior. Progress requires theoretical work on recurrent networks to be embodied in specific circuitry11. In vivo two-photon imaging, combined with strategies to mark axons or neurons by their projection patterns, allows the activity of specific cortico-cortical pathways to be monitored12 during behavior13–18. Here, we used a combination of pathway-specific imaging and optogenetics to investigate the perception-related dynamics of a recurrent network between primary and secondary somatosensory cortex.
RESULTS
We trained mice to perform a head-fixed tactile detection task in which they reported by licking or withholding licking whether a single whisker received a brief sinusoidal deflection (20 Hz, 0.5 s, ~800 degrees s−1 peak speed; Fig. 1). Trial outcomes comprised a mixture of successful detections (“Hits”) and failed detections (“Misses”) following stimulus delivery, as well as correct (“Correct Rejection”) and incorrect (“False Alarms”) responses in the absence of the stimulus (Fig. 1c; Supplementary Fig. 1). The relationship between touch perception and responses across large-scale populations of cortical neurons remains poorly understood, even for S1. Anatomy19,20 and physiology in anesthetized/narcotized rodents21–23 suggest that mouse S2 is a higher, or more integrative, cortical area compared with S1. However, responses of rodent S2 neurons during tactile behavior are nearly entirely unexplored (but see: 24,25). We thus began by mapping responses to stimulation of a single whisker across whisker representation areas of S1 and S2, in separate mice, as they performed the detection task.
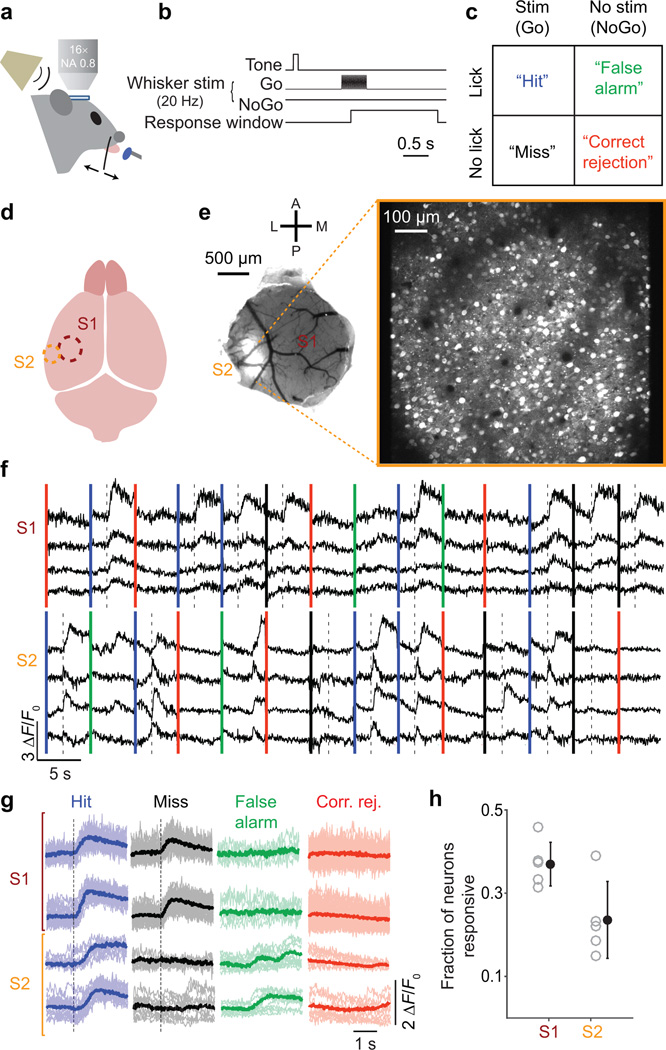
Figure 1.
Cellular resolution imaging of population activity in S1 and S2 during tactile detection. (a) Schematic of experimental setup. Mice were trained to report detection of single whisker deflections by licking a lick-port for a liquid reward. Activity of L2/3 neurons in the whisker area of S1 or S2 was monitored by two-photon calcium imaging. (b) Trials began with an auditory cue. On 50% of trials (“Go” trials), a single whisker was deflected with a sinusoidal waveform (0.5 s, 20 Hz). The whisker was not deflected on the other 50% of trials (“NoGo” trials). Trial outcome was determined by lick responses occurring during a response window. (c) Four possible trial outcomes based on the stimulus condition (present vs absent) and the animal’s response (lick vs no-lick). (d) Schematic of brain areas monitored by two-photon imaging. S1 (brown) or S2 (orange) were identified using intrinsic signal optical imaging. (e) Left, View through a cranial imaging window implanted over S1 and S2. GCaMP6 expression (white) is evident in S2. Right, Example field of view for two-photon calcium imaging (max projection through time for a trial). (f) Example GCaMP6 fluorescence traces concatenated across trials from S1 and S2 imaging sessions. Vertical colored lines indicate start of each trial (blue: Hit; black: Miss; green: False Alarm; red: Correct Rejection). Dashed lines: onset of whisker stimulus. (g) Example fluorescence traces (thin lines) and means (thick) showing two S1 and two S2 neurons (one neuron per row). Traces are grouped and colored by trial type. Dashed lines: onset of whisker stimulus. (h) Fraction of neurons that were task-responsive in S1 or S2, for individual mice (gray circles) and means (black; ± SEM).
Mapping activity in S1 and S2 during tactile detection
We used in vivo two-photon imaging of GCaMP6 genetically encoded calcium indicators26 to measure spiking-related fluorescence signals from the cell bodies of layer 2/3 (L2/3) neurons. For our single-whisker stimuli, we found that 37.0 ± 2.3% (mean ± SEM across mice) of neurons in S1 and 23.6 ± 4.1% of neurons in S2 gave task-related responses (“responsive” neurons; Online Methods; Fig. 1h). Thus, behaviorally relevant whisker stimulation is represented robustly in whisker regions of both S1 and S2. We limited subsequent analyses to responsive neurons, except where noted, and used responses occurring in a time window preceding the typical reaction times (0.25 s after stimulus onset; blue shaded rectangle in Fig. 2a,b; the end of this window precedes 96.5% of reaction times; Supplementary Fig. 1).
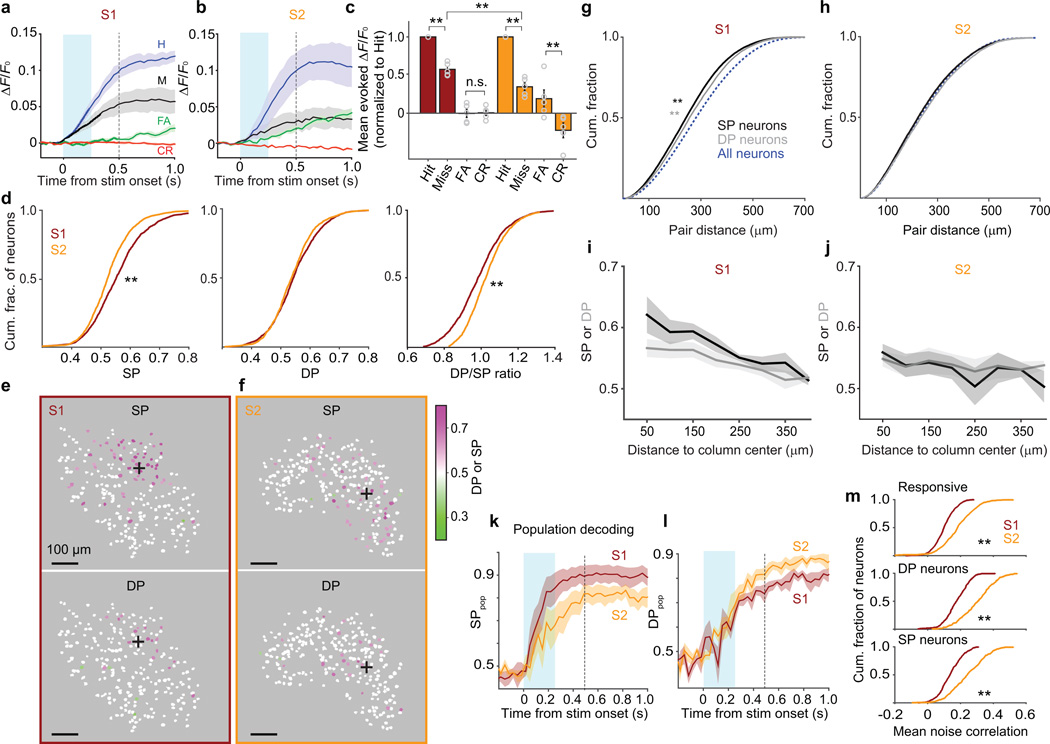
Figure 2.
Coding of stimulus and choice in S1 and S2. (a) Activity (mean ± SEM ΔF/F0 across mice) averaged across Hit (blue), Miss (black), False Alarm (green) and Correct Rejection (red) trials from S1 imaging sessions (12 sessions, 5 mice, 274 ± 52 neurons per mouse; mean ± SEM). Evoked ΔF/F0 responses on Hits were larger than on Misses (t(1,369) = 16.79, P = 1.17 × 10−57; 1,370 neurons). Cyan shading: first 0.25 s after stimulus onset, which preceded 96.5% of first licks. Dashed line: last time point before median first-lick time. (b) Same as a for S2 (11 sessions, 5 mice,103 ± 26 neurons per mouse). Evoked ΔF/F0 responses on Hits were larger than on Misses (t(606) = 20.32, P = 2.22 × 10−70; 607 neurons; paired t-tests). In S2, evoked ΔF/F0 responses on False Alarms were larger than on Correct Rejections (t(606) = 4.07, P = 5.33 × 10−5; paired t-tests). (c) Mean evoked ΔF/F0 responses normalized to Hits across individual neurons in S1 and S2 (mean ± SEM across mice; circles show individual mice). For both S1 and S2 neurons, responses on Misses were smaller than on Hits (Miss/Hit ratio: S1: 0.57 ± 0.04; z = 22.3, **P = 1.73 × 10−110; 1,370 neurons; S2: 0.32 ± 0.05; z = 18.84, **P = 3.76 × 10−79; 607 neurons; Wilcoxon sign rank tests), with stronger modulation for S2 compared with S1 (z = 7.46, **P = 8.38 × 10−14; Wilcoxon rank sum test). (d) Cumulative histograms (means across mice) of “stimulus probability” (SP, left), “detect probability” (DP, middle) and DP/SP ratio (right) for all (responsive and non-responsive) neurons in S1 (brown) and S2 (orange). DP/SP ratio was higher in S2 (medians: 1.01 vs 0.97; D = 0.093, **P = 1.96 × 10−7; 2,490 S1 and 1,471 S2 neurons; Kolmogorov-Smirnov test); SP was higher in S1 compared with S2 (medians: 0.55 vs 0.52; D = 0.063, **P = 0.0013; Kolmogorov-Smirnov test) while DP was similar (D = 0.044, P = 0.056; Kolmogorov-Smirnov test). S1: 12 sessions, 5 mice, 498 ± 74 neurons per mouse; S2: 11 sessions, 5 mice, 294 ± 47 neurons per mouse). (e) Maps from one mouse showing distributions of stimulus- and decision-encoding neurons in S1. White neurons are those whose SP or DP 95% confidence intervals included 0.5. Responsive and non-responsive neurons are included. Black “+” marks: center of the somatotopic column of the stimulated whisker. (f) Same as e for S2. (g) Cumulative histograms of pairwise distances among SP neurons (black), DP neurons (gray), and all neurons (dashed blue; responsive and non-responsive) in S1. Both SP and DP neurons had smaller pairwise distances among themselves compared with all neurons (both **P < 5 × 10−5; 26,898 SP, 12,387 DP, and 431,962 all-neuron pairs; permutation tests). (h) Same as g for S2. (i) Mean SP (black) and DP (gray) values averaged across all neurons (8 bins of 50 µm; ± SEM) as a function of distance from the center of the somatotopic column representing the stimulated whisker in S1. SP and DP both decreased with distance from the column center (SP: −0.028 per 100 µm; DP: −0.017 per 100 µm; test of zero slope: F(1,74) = 54.84, P = 1.711 × 10−10; difference in slopes for SP and DP: F(1,74) = 3.58, P = 0.062; 78 binned values total from 5 mice; ANCOVA). (j) Same as i for S2 (test of zero slope: F(1,54) = 3.11, P = 0.083; difference in slopes for SP and DP: F(1,54) = 0.92, P = 0.342; 58 values from 5 mice). (k) Performance of a classifier (mean ± SEM across mice) in decoding the stimulus condition from population activity at each time point reached higher levels for S1 (84 ± 7% correct by 0.25 s after stimulus onset, and 89 ± 5% by the median reaction time of 0.52 s) compared with S2 (68 ± 8% correct by 0.25 s, and 82 ± 4% by 0.52 s; performance diverged by 0.32 s: U = 76, P = 0.045; 7 S1 and 9 S2 sessions; one-tailed Wilcoxon rank sum test). Cyan shading and vertical dashed line as in a. (l) Same as in k for decoding choice. Performance was higher for S2 compared with S1 (79 ± 3% vs 72 ± 1% at 0.35 s; U = 40, P = 0.021; one-tailed Wilcoxon rank sum test). (m) Mean pairwise noise correlations between each responsive neuron and other responsive neurons (top), each DP neuron and other DP neurons (middle), or each SP neuron and other SP neurons (bottom). Noise correlations were higher in S2 (all **P < 5 × 10−5; S1: 1,370 responsive, 536 SP and 338 DP neurons; S2: 607 responsive, 287 SP and 259 DP neurons; permutation tests).
Overall, responses of individual neurons to the whisker stimulus tended to be larger in S1 compared with S2 (S1: Hit: 0.036 ± 0.006 ΔF/F0; Miss: 0.024 ± 0.006 ΔF/F0; S2: Hit: 0.029 ± 0.016 ΔF/F0; Miss: 0.015 ± 0.008 ΔF/F0; mean ± SEM across mice; z = 3.19, P = 0.0014, for comparison of Hits; z = 7.46, P = 8.38 × 10−14 for comparison of Misses; 1,370 neurons in S1; 607 neurons in S2; Wilcoxon rank sum tests; Fig. 2a,b), suggesting a more robust representation of the tactile stimulus in S1.
Touch-evoked activity in both S1 and S2 predicted the subsequent perceptual choice of the mouse. Responses on Hit trials were larger than on Miss trials in both S1 and S2 (Hit − Miss mean ± [95% confidence interval, CI] for evoked ΔF/F0: S1: 0.013 ± [0.008, 0.019] ΔF/F0; S2: 0.012 ± [0.010, 0.023] ΔF/F0; Fig. 2a,b). Thus, activity in each area predicted whether the mouse would succeed or fail to detect an identical stimulus. In the absence of a whisker stimulus, activity was higher on False Alarm trials compared with Correct Rejections in S2 (False Alarm − Correct Rejection mean ± [95% CI]: S1: 0.000 ± [−0.006, 0.005] ΔF/F0; S2: 0.006 ± [0.009, 0.011] ΔF/F0; Fig 2a,b). To quantify modulation of neuronal activity by behavioral choice, we normalized responses of individual neurons to the mean Hit response, in order to account for overall differences in evoked activity between the two areas. We observed a smaller normalized Miss response in S2 (Fig. 2c). Thus, individual neurons were more modulated by choice in S2 compared with S1.
Trial-by-trial coding by single neurons and populations
Ideal observer analysis is frequently used to correlate the trial-by-trial activity of single sensory cortex neurons with stimuli and perceptual choices27,28. To investigate stimulus- and choice-encoding by single neurons, we first calculated "stimulus probability” (SP). SP gives the probability with which an ideal observer could correctly categorize the stimulus condition (present vs absent) of a trial based on the response of a single neuron (Online Methods). We also calculated “detect probability” (DP, which is mathematically identical to “choice probability”27 but often renamed in the context of detection tasks29). DP is the probability with which an ideal observer could correctly categorize the behavioral choice of the mouse on a single trial (in our case, lick vs no-lick) based on the response of a neuron27. We observed robust (above chance level, > 0.5) SP and DP in S2 as well as S1 (Fig. 2d). S1 trended toward having a higher fraction of neurons with significant SP (“SP neurons,” defined as neurons whose 95% confidence interval for SP did not include 0.5; 49.7 ± 3.1% of responsive neurons in S1 vs 32.2 ± 3.4% of responsive neurons in S2; mean ± SEM across mice; P = 0.24, permutation test; Supplementary Fig. 2). The distribution of SP showed larger values overall in S1 compared with S2 (Fig. 2d). The distribution of DP was similar in the two cortical areas, despite the weaker stimulus representation in S2 (Fig. 2d). The ratio of DP to SP for neurons in S2 was higher compared with neurons in S1 (Fig. 2d), consistent with a greater choice-related modulation of S2 responses.
Choice-related activity is thought to depend on correlations among neuronal responses, which in turn reflect cortical topography29,30. However, while detect (or choice) probability is frequently quantified, its cellular-resolution organization within cortex is unknown. We therefore mapped SP and DP across S1 and S2 in separate mice (Fig. 2e–j). First, we tested whether SP neurons and “DP neurons” (defined as a neuron with 95% confidence interval for DP not including 0.5) were clustered. In S1, pairwise distances among SP neurons were smaller than pairwise distances among all neurons (median distances: 243 µm vs 281 µm; Fig. 2g). Clustering of SP neurons is expected due to the somatotopy of barrel cortex. Remarkably, DP neurons were also clustered in S1, with smaller pairwise distances than expected based on the distribution for all neurons (median distances 256 µm vs 281 µm; Fig. 2g). In S2, neither SP nor DP neurons formed obvious clusters (Fig. 2h).
We next examined how SP and DP were distributed as a function of distance from the center of the somatotopic representation of the stimulated whisker. In S1, this somatotopic representation corresponds to the stimulated whisker’s barrel column. We estimated the center of the barrel column using intrinsic signal imaging (ISI; Online Methods). In S2, we also used the ISI response to estimate the center of the somatotopic representation of the stimulated whisker. In S1, both mean SP and mean DP (averaged across responsive and non-responsive neurons) decreased with distance from the center of the barrel column (Fig. 2i). In S2, SP and DP both declined at most modestly with distances up to ~400 µm from the center of the somatotopic representation (Fig. 2j). Thus, choice-related activity was clustered across somatosensory cortex, but clustering depended on cortical area.
Sensory and motor variables can be encoded not just by single neurons but also by activity patterns across neural populations. We quantified how well large populations of neurons in S1 and S2 encoded the whisker stimulus and the perceptual choice of the mouse using a machine learning classifier (Random Forests31,32; Online Methods). The classifier attempted to decode the stimulus condition (present vs absent) and the behavioral choice (lick vs no-lick) from the simultaneous activity of all responsive neurons, at each time point within a trial. Performance of the classifier in decoding the stimulus condition (“SPpop”) rose following stimulus onset for both S1 and S2, and reached higher levels in S1 compared with S2 (Fig. 2k). Classifier performance in decoding the choice of the mouse (“DPpop”) rose following stimulus onset in both S1 and S2, but reached somewhat higher levels in S2 (Fig. 2l). Thus, decoding of tactile stimuli from population responses was better in S1, but decoding of choice was slightly better in S2.
Choice-related activity is thought to depend on trial-to-trial correlations in the responses of pairs of neurons29,30 (“noise correlations”33; Online Methods). Consistent with stronger encoding of choice, S2 showed higher average noise correlations compared with S1 (0.19 ± 0.05 vs 0.09 ± 0.01; mean ± SEM across mice; Fig. 2m). Stronger noise correlations in S2 were especially prominent in pairs of DP neurons (i.e. pairs of neurons that both encoded choice; Fig. 2m).
To ensure that the differences in touch-related dynamics we observed between S1 and S2 were not due to differences among animals, we monitored S1 and S2 simultaneously in individual mice (11 sessions total from 4 mice; Supplementary Fig. 3; Online Methods). Responses on Hits were larger than on Misses in both S1 and S2 (Supplementary Fig. 3e). The Miss/Hit response ratio was smaller in S2 compared to S1 (Supplementary Fig. 3f). The ratio of DP to SP for neurons in S2 was higher compared with neurons in S1 (Supplementary Fig. 3i). At the population level, decoding of choice was slightly better in S2 than in S1 (Supplementary Fig. 3k). These results are consistent with S2 responses depending to a greater degree on choice.
Two results from simultaneous S1–S2 imaging experiments suggest that perceptual detection may be associated with coordination of activity between S1 and S2. First, pairs of simultaneously recorded S1 and S2 neurons showed slightly higher noise correlations on Hit compared to Miss trials (Supplementary Fig. 3g,h). Second, population decoding of choice was superior when using the pooled sets of S1 and S2 neurons, compared with decoding from either set alone (Supplementary Fig. 3k), implying that choice was encoded in at least a partly non-redundant manner across S1 and S2.
Feedforward propagation of task-related activity
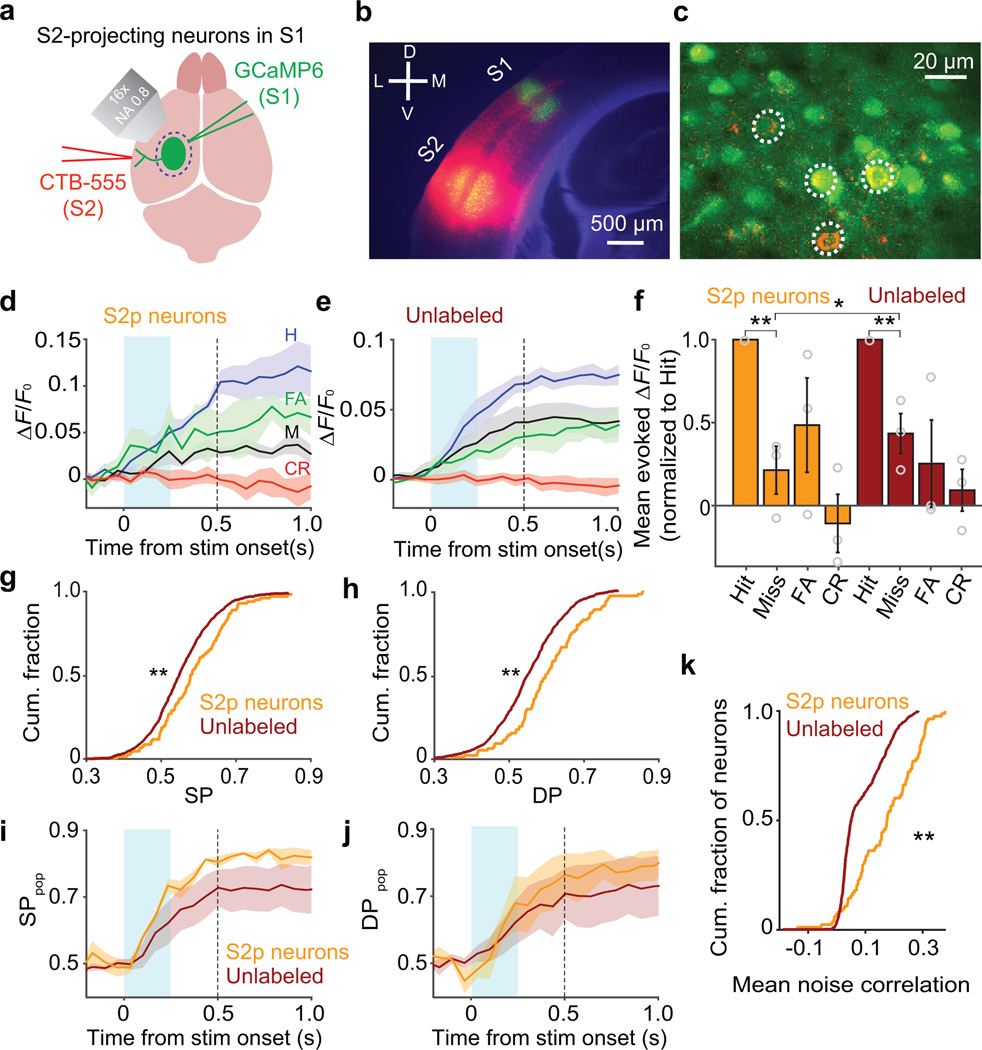
To what degree can the strong encoding of choice in S2 be attributed to feedforward inputs from S1? To quantify feedforward propagation of activity from S1 to S2, we labeled S2-projecting (S2p) neurons in S1 using injections into S2 of cholera toxin subunit B conjugated to fluorescent dye, a retrograde tracer14–17,34 (Fig. 3a,b). We then identified these S2p neurons among the larger set of GCaMP6s expressing neurons using in vivo imaging (8 sessions total from 3 mice; Fig. 3c). Unlabeled neurons presumably comprised both neurons that did not project to S2 and false-negative neurons that did project to S2 but were not labeled. Responses on Hit trials were larger than on Miss trials for both S2p neurons (Hit − Miss mean ± [95% CI]: 0.019 ± [0.005, 0.040] ΔF/F0; Fig. 3d,f) and unlabeled neurons (Hit − Miss mean ± [95% CI]: 0.010 ± [0.002, 0.017] ΔF/F0; Fig. 3e,f). However, S2p neurons showed slightly larger choice-related modulations (Fig. 3f; see also: 17).
Figure 3.
Feedforward propagation of activity from S1 to S2 predicts choice. (a) Schematic of retrograde labeling of S2-projecting (S2p) neurons in S1. (b) Coronal section showing CTB-Alexa555 (red) at the S2 injection site and GCaMP6 (green) in S1. (c) In vivo identification of S2p neurons. Dashed circles indicate GCaMP6-expressing neurons labeled with CTB-Alexa555. (d) Activity (mean ± SEM ΔF/F0 across 3 mice, 8 sessions total) of S2p neurons averaged across trial types. Responses on Hits were larger than on Misses (0.040 ± 0.003 ΔF/F0 vs 0.020 ± 0.006 ΔF/F0; t(87) = 5.28, P = 9.24 × 10−7; 88 neurons; paired t-test). Conventions as in Fig. 2a. (e) Same as d for unlabeled neurons (Hit: 0.027 ± 0.003 ΔF/F0; Miss: 0.019 ± 0.007 ΔF/F0; t(647) = 13.19, P = 2.43 × 10−35; 648 neurons; paired t-test). (f) Mean evoked ΔF/F0 responses normalized to Hits across individual neurons (mean ± SEM across mice; circles show individual mice). For both S2p and unlabeled neurons, responses on Misses were smaller than on Hits (Miss/Hit ratio: S2p: 0.21 ± 0.14; z = 6.51, **P = 7.43 × 10−11; 88 neurons; unlabeled: 0.43 ± 0.12; z = 13.81, **P = 2.11 × 10−43; 648 neurons; Wilcoxon sign rank tests), with slightly stronger modulation for S2p compared with unlabeled neurons (*P = 0.031; permutation test). (g) S2p neurons showed higher SP compared with unlabeled neurons (medians: 0.57 vs 0.54; **P < 5 × 10−5; 133 S2p and 1,134 unlabeled neurons; permutation test). Includes responsive and non-responsive neurons. (h) S2p neurons showed higher DP compared with unlabeled neurons (medians: 0.58 vs 0.54; **P < 5 × 10−5; permutation test). (i) Performance of a classifier (mean ± SEM across mice) in decoding the stimulus condition from population activity reached a higher level for S2p compared with unlabeled neurons (73 ± 2% vs 63 ± 5% at 0.24 s after stimulus onset; R = 34, P = 0.012; 8 sessions; one-tailed Wilcoxon sign rank test). Conventions as in Fig. 2k. (j) Same as i for decoding choice (68 ± 7% vs 62 ± 5% at 0.24 s; R = 30, P = 0.055; one-tailed Wilcoxon sign rank test). (k) Cumulative histograms of mean pairwise noise correlations between each S2p neuron and other S2p neurons (orange), or each unlabeled neuron and other unlabeled neurons (brown). Noise correlations were higher among S2p neurons (**P < 5 × 10−5; 88 S2p and 648 unlabeled neurons; permutation test).
Ideal observer analysis showed higher values of both SP (Fig. 3g) and DP (Fig. 3h) among S2p neurons compared with unlabeled neurons. At the population-coding level, classifier performance in decoding the stimulus condition from S2p neurons reached a higher level compared with unlabeled neurons (Fig. 3i). Classifier performance in decoding choice trended toward a higher level with S2p neurons compared with unlabeled neurons (Fig. 3j). Moreover, noise correlations were stronger among pairs of S2p neurons than among pairs of unlabeled neurons (0.14 ± 0.04 vs 0.10 ± 0.02; mean ± SEM across sessions; Fig. 3k). Thus, S2p neurons showed a more coordinated response and stronger association with perceptual choice.
A cortico-cortical loop for task-related activity
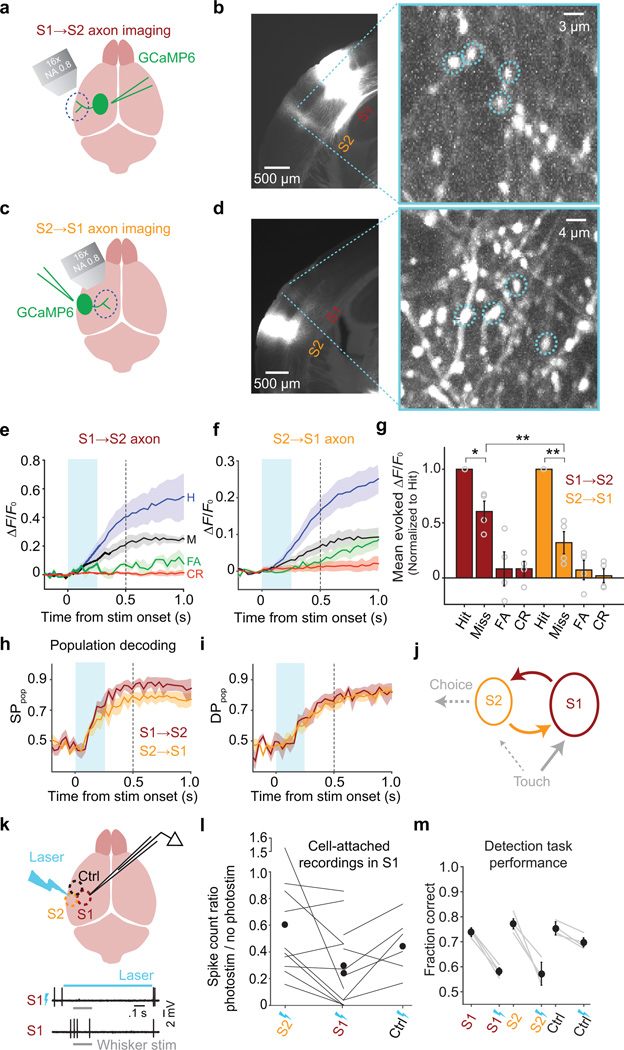
S1 and S2 are strongly and bidirectionally interconnected by long-range axonal projections35–37. Recurrent loops in cortical circuits can serve many computational roles, including amplifying and prolonging stimulus evoked activity8,10. To determine whether S1 and S2 could act in concert as a feedback loop during sensory decision making, we quantified stimulus- and choice-related activity propagating along feedforward (S1→S2) and feedback (S2→S1) axonal pathways (4 of 10 sessions of the S2→S1 data are from previously published experiments38 and are reanalyzed here in greater detail). We expressed GCaMP6s in axons using adeno-associated virus (AAV) injections into either S1 (to monitor S1→S2 axons; Fig. 4a,b) or S2 (for S2→S1 axons38; Fig. 4c,d). We then imaged axonal activity during behavior13 in S2 (S1→S2 axons, imaged in L2/3; Fig. 4b), or in S1 (S2→S1 axons38, imaged in L1, a site of major top-down input to the dendrites of L2/3 neurons; Fig. 4d; Supplementary Fig. 4).
Figure 4.
Activity in a feedback loop between S2 and S1. (a) Schematic of S1→S2 axon imaging experiment. AAV-GCaMP6 was injected in S1. A glass window was placed over S2. (b) Left, Image of a coronal section showing GCaMP6 fluorescence in the injection (S1) and imaging (S2) areas. Right, Example field of view from L2/3 of S2. Dashed circles indicate example regions of interest (ROIs). (c) Schematic of S2→S1 axon imaging experiment. AAV-GCaMP6 was injected in S2. A glass window was placed over S1. (d) Left, Image of a coronal section showing GCaMP6 fluorescence in the injection (S2) and imaging (S1) areas. Right, Example field of view and ROIs from L1 of S1. (e) Activity (mean ± SEM ΔF/F0 across 4 mice, 7 sessions total, 160 axons total) of S1→S2 axons for each trial type. Responses on Hits were larger than on Misses (0.11 ± 0.027 ΔF/F0 vs 0.079 ± 0.029 ΔF/F0; t(159) = 3.25, P = 0.001; 160 axons; paired t-test). Conventions as in Fig. 2a. (f) Same as e for S2→S1 axons (10 sessions, 4 mice, 440 axons) of S2→S1 axons. Responses on Hits were larger than on Misses (0.031 ± 0.004 ΔF/F0 vs 0.016 ± 0.002 ΔF/F0; t(439) = 3.13, P = 0.002; paired t-test). A subset of S2→S1 data in panels f, g–i are reanalyzed from ref. 38. (g) Mean evoked ΔF/F0 responses normalized to Hits across individual axons (mean ± SEM across mice; circles show individual mice). For both S1→S2 and S2→S1 axons, responses on Misses were smaller than on Hits (Miss/Hit ratio: S1→S2: 0.61 ± 0.10; S = 99, *P = 0.003; 160 axons; S2→S1: 0.29 ± 0.11; S = 313, **P = 1.15 × 10−18; 440 axons; sign test), with stronger modulation for S2→S1 axons (z = 3.82, **P = 1.33 × 10−4; Wilcoxon rank sum test). (h) Performance of a classifier (mean ± SEM across mice) in decoding the stimulus condition from population activity reached a higher level for S1→S2 compared with S2→S1 axons (82 ± 2% vs 66 ± 6% at 0.28 s after stimulus onset; z = 2.05, P = 0.044; 7 S1→S2 and 10 S2→S1 sessions; Wilcoxon rank sum test). Conventions as in Fig. 2k. (i) Same as h for decoding choice. (j) Schematic of feedforward and feedback propagation of task-related activity (dashed: hypothetical functional pathways). (k) Top, schematic of optogenetic silencing experiment. In mice expressing channelrhodopsin-2 in GABAergic neurons, a 473 nm laser was directed over the C2 column (in S1), whisker S2 or a control area (within whisker S1 but ~1 mm from the C2 column). Photostimulation was randomly delivered on 30–40% of all behavioral trials. Bottom, example electrophysiology traces showing responses of an S1 neuron to whisker stimulation with and without laser illumination. (l) Cell-attached electrophysiology recordings were targeted to the C2 column in awake mice (16 neurons in 2 mice). The C2 whisker was stimulated in the presence or absence of laser illumination directed to the recording site (centered over the C2 column in S1), S2 or the control area. Silencing was quantified as the ratio of whisker-evoked spike count in the presence vs absence of illumination. S1 (C2 area) illumination produced stronger silencing of whisker-evoked responses compared with illumination of either S2 or the control area. Silencing was similar for S2 and the control area. Vertical axis is broken to accommodate one outlier. (m) Task performance (mean ± SEM across 4 mice) was reduced by illuminating either S1 (from 74 ± 1.7% to 58 ± 1.5% correct) or S2 (from 77 ± 2.0% to 57 ± 4.6% correct), to a similar degree (S1 vs S2 reductions: z = −0.81, P = 0.421; 12 S1 and 15 S2 sessions; Wilcoxon rank sum test). Illuminating the control area caused a much smaller drop in performance (from 75 ± 2.6% to 70 ± 1.8% correct; S2 vs control area reductions: z = −3.27, P = 0.001; 12 control area sessions; Wilcoxon rank sum test). Gray lines: performance of individual mice averaged across sessions for interleaved trials with (bolts) and without (no bolts) illumination. Black symbols: mean ± SEM across mice.
Axonal activity increased following stimulus onset, to higher levels on Hits compared with Misses in both S1→S2 axons (Hit − Miss mean ± [95% CI]: 0.029 ± [0.007, 0.054] ΔF/F0; Fig. 4e) and S2→S1 axons (Hit − Miss mean ± [95% CI]: 0.014 ± [0.005, 0.022] ΔF/F0; Fig. 4f). S2→S1 axons showed larger choice-related modulation compared with S1→S2 axons (Fig. 4g).
Performance of a classifier in decoding the stimulus condition from populations of axons rose following stimulus onset for both S1→S2 and S2→S1 axons, to a higher level for S1→S2 axons (Fig. 4h). Classifier performance in decoding choice also rose following stimulus onset, to similar levels based on S1→S2 axons or S2→S1 axons (Fig. 4i). Thus, activity encoding both the sensory stimulus and the upcoming perceptual choice propagated along feedforward and feedback cortico-cortical axons between S1 and S2.
Our results are consistent with a model in which touch-evoked activity propagates in a feedback loop between S1 and S2 during formation of a perceptual choice, with “readout” by downstream circuits occurring at least in part from S2 neurons (Fig. 4j). This model predicts that silencing S2 should impair task performance. However, S1 and S2 comprise densely interconnected networks35,36, and the spatial resolution of cortical silencing is inherently too coarse to silence the two areas independently (for a description of the spatial resolution of cortical silencing, see: 39). Therefore, to test for a causal role of S2 in task performance, we devised a strategy based on comparing the behavioral impact of partially silencing S2 and S1 to different degrees.
We directed a laser to S1 (centered over the cortical column representing the C2 whisker, which was used to solve the task), to S2, or to a nearby “control” area (at stereotactic coordinates within the S1 barrel field but ~1 mm away from the C2 column) to optogenetically silence regions of cortex by stimulation of GABAergic neurons39,40 (Fig. 4k). First, we recorded whisker stimulus-evoked spikes from S1 neurons using loose-seal cell-attached recordings while directing the laser beam to S1, S2, or the control area. Silencing S2 or the control area led to decreases in S1 stimulus responses to 61% and 46% of baseline responses measured without photostimulation, respectively (Fig. 4l). Direct silencing of S1 by illumination of the recording site reduced responses further to 29% of baseline (Fig. 4l). Thus, direct silencing of somatosensory cortex yielded a 1.5-fold to 2-fold greater reduction in spiking than indirect silencing (via illumination of a nearby area).
Although silencing S2 or the control area reduced activity to similar levels in S1 (Fig. 4l), the behavioral impact of silencing S2 was dramatically greater (Fig. 4m; Supplementary Fig. 5). In other words, task performance was more effectively disrupted by silencing S2 compared with silencing a region of barrel cortex representing whiskers not used in the task, despite similar indirect effects on S1. Moreover, direct silencing of either S2 or S1 decreased performance to similar levels (Fig. 4m). We conclude that the behavioral impact of reducing activity in either S1 or S2 was similar, indicating causal roles for both areas in tactile detection.
DISCUSSION
Our results show that as mice attempted to detect a faint whisker stimulus, larger responses within a cortico-cortical loop predicted perceptual choice (successful detection) on a trial-by-trial basis. Interestingly, “late” stimulus responses in S1 have been linked to perceptual detection in both rodents17,40 and primates5. These late responses occur at delays longer than feedforward activity can be sustained cell-autonomously by individual cortical neurons. We show here that touch-related activity propagates in specific pathways that form a direct loop between S1 and S2 (activity may also propagate between S1 and S2 indirectly41). Propagation in a loop can create circuit dynamics that act to amplify and sustain sensory activity8, such as those of a “Hebbian assembly.” Although future work with electrophysiology will be required to define temporal dynamics, our work is consistent with the hypothesis that perception-related5,40 late sensory responses in S1 reflect reverberation of activity10 among cortical areas42.
Overall, patterns of activity in S1 and S2 had obvious similarities and subtle but intriguing contrasts. In both S1 and S2, activity encoded the stimulus and the perceptual choice. Neurons in both S1 and S2 propagated stimulus- and choice-related activity to the other area. However, neurons in each area also showed key differences in their response properties (see also:24,25). S2 activity was more associated with perceptual outcome compared with S1, whereas S1 activity better encoded the stimulus. While sensory stimuli are usually analog in nature, perception is often all-or-none. Neural representations of sensory stimuli must therefore converge into states representing discrete perceptions, perhaps via attractor dynamics. We found that trial-to-trial noise correlations (coordinated variability not explained by the stimulus) among pairs of S2 neurons were higher than among pairs of S1 neurons, consistent with a model in which S2 neurons encoded the binary perceptual outcome of each trial. Recent computational modeling has shown that top-down feedback of choice-related activity (similar to what we observed between S2 and S1) may be critical for the development of neuronal responses that reflect perceptual outcome43.
Recently, a small number of studies have examined in vivo responses of S1 neurons that project to S214–17,25,34,44, and shown that these neurons have distinct intrinsic and task-related response properties. Here, we found that L2/3 neurons that project from S1 to S2 tended to show higher choice-related activity15,17 and stronger noise correlations compared with other L2/3 neurons. These characteristics were similar to those of L2/3 neurons located in S2, suggesting a partly feedforward inheritance of response properties45,46 and even choice-related activity (cf. 47).
A limitation of our work is that we focused analysis of L2/3 neurons on the relatively small fractions in S1 (~37%) and S2 (~24%) that were task-responsive (Fig. 1h). Studies across a wide variety of experimental contexts have revealed sparse activity patterns among cortical L2/3 neurons, but the nature of this sparseness remains poorly understood (reviewed in: 48). While we made no attempt to optimize our stimulus for the studied neurons, even systematic exploration of subthreshold whisker receptive fields has shown that activity is sparse among L2/3 neurons in S1 over the course of an experimental session49. The degree to which responses of L2/3 neurons change during learning of tactile tasks remains an active area of research15,17,50. Intriguingly, a recent study using a single-whisker tactile detection task found that detection-related activity emerged with learning specifically in S1 neurons that project to S217.
We examined cortico-cortical dynamics during detection of passive touch in head-fixed animals, but S1–S2 interactions could depend on motor-sensory context. A recent study24 used electrophysiology to quantify S1 and S2 spiking as rodents moved freely to interact with and identify textured surfaces. This study found that stimuli and choice were encoded via both the rate and timing of spikes across the S1–S2 network24. A second recent study25 imaged activity in S1 and S2, including from neurons that project in each direction between these areas, and found behavior-dependent coordination of S1 and S2 activity during texture discrimination. Jointly with our data, these studies suggest that S1–S2 dynamics are critical to touch perception across multiple behavioral contexts.
Together, our results suggest that the transformation from raw sensory input to perception occurs via feedforward computations that are reinforced through feedback in a cortico-cortical loop.
ONLINE METHODS
All procedures were in accordance with protocols approved by the Johns Hopkins University Animal Care and Use Committee.
Mice
We report calcium imaging experiments from 16 male C57BL/6NHsd (Harlan) mice, 2 male and 2 female C57BL/6J Thy1-GCaMP6s GP4.351 (Jackson Labs) mice, with ages ranging from 9–16 weeks. We report optogenetic silencing experiments from 4 females and 1 male obtained by crossing PV-IRES-Cre52(Jackson Labs: 008069; B6;129P2-Pvalbtm1(cre)Arbr/J) with Ai3253(Jackson Labs: 012569; B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J) mice on a mixed background, with ages ranging from 10–12 weeks. Mice were housed in a vivarium with reverse light-dark cycle (12 hours each phase). Experiments occurred during the dark phase. Mice were house in groups of up to 5 prior to the start of water restriction, after which mice were housed singly. Assignment of mice to experimental conditions and analyses are detailed in Supplementary Fig. 6.
Intrinsic signal imaging
After recovery from headpost surgery (> 24 hours) or headpost surgery plus GCaMP6 injection and cranial window implantation (7–9 days), mice were anesthetized with light isoflurane (0.5–1%) and chlorprothixene (0.02 ml of 0.36 mg ml−1, intramuscular). Intrinsic signal imaging (ISI) was performed as described54. In all virus-injected mice, the target whisker was right C2. ISI was performed through the cranial window or a clear skull cap39. Whisker S2 could be identified as a region of decreased reflectance clearly delineated from whisker S1. Sound from the piezo stimulator is a potential source of response during ISI mapping of regions in the vicinity S2. As a control experiment to distinguish auditory responses from tactile responses, ISI was occasionally performed without threading the target whisker into the stimulator, which otherwise remained in a nearly identical position. Areas responsive under this condition were considered auditory areas and were distinct from whisker S2. In Thy1-GCaMP6s mice used for simultaneous S1 and S2 imaging, responses to several different whiskers (right B2, B3, C2, C3) were mapped through the cranial window after recovery from surgery. We selected a target whisker for behavioral training based on the criterion that the target whisker’s responsive regions in both S1 and S2 could be covered by the same two-photon field of view. As a result, the B3 whisker was used in 2 mice, B2 in 1 mouse, and C3 in 1 mouse. We further validated S1 and S2 identification using wide-field fluorescence imaging under the same field of view used for ISI. Taking advantage of homogeneous expression of GCaMP6s across cortex, we identified regions showing evoked fluorescence in response to the same whisker stimulation protocol used for ISI. In all cases, wide-field imaging yielded two clearly separated regions (corresponding to S1 and S2) that matched those identified by ISI.
Behavioral task
Mice were trained to perform a tactile detection task while head-fixed. Behavioral apparatus was controlled by BControl software (C. Brody, Princeton University). For 7–10 days prior to training, mice received 1 ml d−1 of water. On training days, mice were weighed before and after each training session to determine water consumed. Mice were allowed to perform the task until sated. Additional water was given if mice consumed < 0.3 ml. In the first 1–2 sessions, mice received a drop of water (~6 µl) each time the tongue contacted a “lickport” tube placed near their snouts. In subsequent sessions, mice were operantly conditioned to lick at the lickport in response to a passive whisker deflection. All whiskers except the target whisker were trimmed to near the base. The target whisker was threaded into a glass pipette attached to a piezo actuator (Piezo Systems), with ~3–5 mm at the base exposed. For training, on Go trials, the whisker was deflected for 0.5 s with a 20 Hz sinusoidal deflection (rostral to caudal, peak angular speed ~800 deg s−1). A “Hit” trial occurred when mice licked the lickport within a response window, and a drop of water was delivered (~6 µl). The response window was defined from 0.2–2 s after onset of whisker stimulation. The initial 0.2 s after stimulus onset was a “grace period” in which licks had no consequence. On Go trials, if mice did not lick within the 1.8 s response window, it was scored as a “Miss” trial, and no reward or punishment was delivered. Go trials were randomly mixed with NoGo trials, in which the whisker was not deflected. No more than 3 consecutive trials of the same type were allowed. On NoGo trials, if mice licked within the response window, it was scored as a “False Alarm”, and mice were punished with a 3–5 s timeout. If mice licked during the timeout, an additional timeout was triggered. A “Correct Rejection” occurred when mice withheld licking during the response window. Correct Rejections were not rewarded. After performance reached > 65% correct, a 0.1 s auditory cue (8 kHz tone, ~80 dB SPL) was introduced starting 1 s preceding stimulus onset. During all sessions, ambient white noise (cut off at 40 kHz, ~80 dB SPL) was played through a separate speaker to mask any other potential auditory cues associated with movement of the piezo stimulator. Mice were considered trained when performance reached > 70% correct for at least two consecutive days. Typically, mice were trained one session per day for 7–10 days to reach this criterion. Trials with “premature” licks occurring close to stimulus onset (−0.51 s to +0.12 s) were excluded from subsequent analysis. Only 2% of trials with reaction times prior to the end of the grace period were not thus excluded. There was a modest trend toward higher pre-stimulus ΔF/F0 on Miss trials in S1 (Supplementary Fig. 7), which could be due to occasional pre-stimulus whisker motion. We considered the possibility that self-generated whisker or tongue movements early in the trial could partly account for the different responses we observed in Hit vs Miss trials. We used high-speed video to monitor whisker and tongue motion, and thereby to obtain a set of trials in which we could confirm negligible motion (Supplementary Fig. 8). We observed a robust Hit vs Miss difference in evoked ΔF/F0 for this subset of trials (Supplementary Fig. 8).
Two-photon calcium imaging of layer 2/3 somata
A circular craniotomy was made over the left barrel cortex (2.5 mm diameter; center relative to bregma: lateral, 3.5 mm; posterior, 1.3 mm) of P40–50 mice. The dura was left intact. GCaMP6s or GCaMP6f (one animal in S1 cell body calcium imaging)26 was expressed under the human synapsin-1 promoter following infection with recombinant adeno-associated virus (serotype 2/1, Syn.GCaMP6s.WPRE.SV40, or Syn.GCaMP6f.WPRE.SV40, University of Pennsylvania Gene Therapy Program Vector Core). Injections were made at 4–6 sites within the craniotomy (30–50 nl per site; depth, 250–300 µm; rate, ~1 nl s−1) using a glass pipette (30–50 µm diameter). After virus injection, the craniotomy was covered with an imaging window made by gluing together two pieces of microscope cover glass32. The smaller piece (Fisher; number 2 thickness) was fitted into the craniotomy and the larger piece (number 1.5 thickness) was glued to the bone surrounding the craniotomy32. Intrinsic signal imaging was used to localize a barrel column or whisker S2 within the area of the cranial window (7–9 days after window implantation). All whiskers on the right side of the snout except the relevant one (a row C whisker, except as noted below for simultaneous S1–S2 imaging) were trimmed after the intrinsic signal imaging. Mice were then water restricted for 2 weeks prior to training. Imaging was started 3–5 weeks after surgery.
For simultaneous imaging of S1 and S2, Thy1-GCaMP6s mice were prepared using the same method as described above except that a row B whisker (B2 or B3) was used for 3 of 4 mice and C3 whisker for one mouse. S1 and S2 were identified using intrinsic signal imaging and wide-field imaging of evoked GCaMP6s fluorescence as described above.
Images were acquired on a custom two-photon microscope (http://openwiki.janelia.org/wiki/display/shareddesigns/MIMMS) equipped with a resonant scanning module (Thorlabs), GaAsP photomultiplier tubes (Hamamatsu) and a 16× 0.8 NA microscope objective (Nikon). GCaMP6 was excited at 1,000 nm (40–60 mW at specimen) with a Ti:Sapphire laser (Chameleon Ultra II, Coherent). Imaging fields were restricted to areas where GCaMP6 expression overlapped with the desired barrel columns or the identified whisker area of S2. For S2 imaging sessions, we rotated mice ≤ 5 degrees from the sagittal plane to enable access of the microscope objective to the cranial window. Mice exhibited no signs of discomfort. We did not rotate mice for simultaneous imaging of S1 and S2 sessions because the imaged whisker representation in S2 was located relatively medially. The field of view ranged from 760 µm × 790 µm to 440 µm × 485 µm (440 × 512 pixels; pixel size, 1.72 µm × 1.55 µm to 1.0 µm × 0.94 µm). Images were acquired continuously at 30 Hz using ScanImage55 4.2 (www.scanimage.org). A movie, corresponding to a single trial, consisted of 140 image frames. In 2 sessions from 1 mouse, images were acquired at 15 Hz and movies comprised 65 frames.
Retrograde labeling of S2-projecting neurons in S1
A subset of mice that were used for S1 somata imaging experiments were injected post hoc with a retrograde tracer, CTB-Alexa555 (5 µg µl−1 in PBS, Invitrogen) in S2 localized by intrinsic signal imaging. The post hoc injection occurred shortly after conclusion of behavioral experiments. A small hole was drilled through the glass cranial window. One-hundred nanoliters of CTB-Alexa555 was injected (depth, 400–500 µm; rate, ~1 nl s−1) through the hole via a glass pipette (30–50 µm). Injection site was sealed with dental cement. Seven to ten days after the injection, the labeled cells were examined under the two-photon microscope. To localize co-labeling, GCaMP6-labeled cells and CTB-Alexa555-labeled cells were excited at 940 nm or 1,020 nm, respectively (40–60 mW at specimen), respectively. Fluorescence emission was separated using a 568 nm dichroic (FF568-Di01-35.5x50.2, Semrock), passed through green (ET525/70m-2p, Chroma) and red (FF01-625/90-30-D, Semrock) channel filters before detection with two GaAsP photomultiplier tubes (Hamamatsu).
Two-photon calcium imaging of axons
A subset of data for S2→S1 axons (4 of 10 sessions) comes from published experiments38 and are analyzed here in greater detail. Adeno-associated virus (serotype 2/1, Syn.GCaMP6s.WPRE.SV40) was injected into a C2 barrel column (identified by ISI) for S1→S2 axon imaging or whisker S2 area (relative to bregma: lateral, 4.3 mm; posterior, 1 mm) for S2→S1 axon imaging at 2 depths (250 µm and 350 µm; 30–40 nl each; ~1 nl s−1), and covered with a cranial window. For S2→S1 axon imaging, intrinsic signal imaging was performed through the window. GCaMP6s expression was examined under a wide-field fluorescence microscope, and mice showing excessive cell body fluorescence outside the ISI-localized S2 region were excluded. Imaging planes were from layer 2/3 of S2 (150–250 µm from pial surface) for S1→S2 axon imaging or layer 1 of S1 (70–100 µm from pial surface) for S2→S1 axon imaging. The field of view was 100 µm × 108 µm (440 × 512 pixels; pixel size, 0.23 µm × 0.21 µm). Images were acquired continuously at 30 Hz using ScanImage 4.2. A movie, corresponding to a single trial, consisted of 140 image frames.
Optogenetic silencing
PV-IRES-Cre;Ai32 mice were implanted with a clear skull cap39, or a cover-glass over the left barrel cortex following a circular craniotomy. Light from a 473 nm laser (MBL-III-473-100, Ultralasers) was passed through an acousto-optic modulator (MTS110-A3-VIS, QuantaTech), focused into a multimode optical fiber, recollimated and directed onto the targeted cortical area. The beam at the skull or cranial window had an approximately Gaussian profile with FWHM of 600 µm. Photostimulation was randomly delivered on 30–40% of all trials. Photostimulation comprised a train of 5 ms pulses at 100 Hz delivered from −300 ms to +2,200 ms relative to the time of whisker stimulus onset for Go trials. The same time window was used for NoGo trials. Average power at the brain surface was ~3 mW for glass-implanted animals (n = 2) or ~7 mW for those with a clear skull39 cap (n = 2). A visual masking flash (2 ms pulses at 10 Hz) was delivered for the duration of every trial via a 470 nm LED (7007-PB000-D, LEDdynamics) placed near the eyes. For behavioral experiments, the laser was steered over the C2 column in S1 (localized with ISI), whisker S2, or a control area in separate sessions within the same animal. The control area was separated from the C2 column and S2 illumination sites by ~1 mm, but at stereotactic coordinates still within whisker S1 (4 mm lateral, 0.3 mm caudal to bregma). Cell-attached recordings for electrophysiological calibration were performed as described previously56. One of the two mice used for recordings was previously used for the behavioral silencing experiments. The illumination site for S1 electrophysiological recordings (either S1 vs S2, or S1 vs control area) was varied every ~50 sweeps with the starting site varied across neurons. The C2 area of S1 was accessed by the recording pipette through a hole drilled in a previously implanted cranial window. Loose-seal cell-attached recordings were made in awake mice while C2 whisker was stimulated in the presence or absence of 473 nm laser pulses to the recording site (S1, C2 area), S2 or the control area (S1, non-C2).
Two-photon calcium imaging of layer 2/3 somata: data analysis
A line-by-line correction algorithm was used to correct for brain motion32,50. For each behavioral trial, we used five consecutive frames with a minimum of luminance changes to generate an average reference image. Each line was registered to the reference image by maximizing the line-by-line Pearson correlation. Regions of interest (ROIs) corresponding to individual neurons were manually selected with the help of maximum intensity and standard deviation projections across movie frames. For each ROI, the time series of raw fluorescence was estimated by averaging all pixels within the ROI. Neuropil signal surrounding each ROI was estimated by averaging all pixels, excluding those from neighboring ROIs, within a 2 pixel-wide ring that starts at 2 pixels away from the border of the ROI. This neuropil signal was subtracted from the raw fluorescence time series to yield the corrected fluorescence time series9,35: F(t) = Fraw(t) − r × Fneuropil(t), with r = 0.326,57. ΔF/F0 was calculated as (F−F0)/F0, where F0 was the mean F over 8 baseline frames immediately preceding the stimulus onset time for each trial. Evoked ΔF/F0 was calculated as the mean ΔF/F0 over 5 frames following the stimulus onset time and before the answer lick (frames 56–60 with stimulus onset between frames 52 and 53; results were similar for later 5-frame windows; Supplementary Fig. 9). For one mouse imaged at 15 Hz, F0 was the mean F over 4 baseline frames immediately preceding the stimulus onset time, and evoked ΔF/F0 was the mean ΔF/F0 over 3 frames following the stimulus onset time (frames 28–30 with stimulus onset between frames 26 and 27).
To assign each neuron as “responsive” or “non-responsive”, evoked ΔF/F0 was calculated as above, except using the 20 frames (10 frames for 15 Hz imaging) immediately following stimulus onset for the post-stimulus window. For each neuron, a Wilcoxon signed rank test (for samples with absolute value skew < 0.6) or a sign test (absolute value skew > 0.6) was performed on the evoked ΔF/F0 values. If the resulting p-value was < 0.01, the neuron was considered “responsive;” otherwise it was “non-responsive.”
Two-photon calcium imaging of axons: data analysis
Analysis procedures were as described above except that neuropil subtraction was not implemented. To distinguish ROIs that belong to the same axon from those that belong to different axons, we used a correlation-based method (adapted from: 13) to build clusters of highly correlated ROIs (Supplementary Fig. 4). Briefly, varicosities that were clearly part of the same axon were identified by visual inspection (8 sessions). Correlation coefficients of these varicosities were calculated from ΔF/F0 concatenated from trials over an entire session, and compared to all pairwise correlation coefficients. ΔF/F0 was calculated as (F−F0)/F0, where F0 was the 15th percentile of F. Correlation coefficients of varicosities from the same axon were clearly distinct from the rest and showed values larger than 0.7 on average. We selected all sets of two varicosities with correlation coefficient > 0.7. These N pairs of varicosities were randomly ordered into a N-tuple, P = (P1,P2,…,Pi,…PN). We began an iterative clustering process by initializing N clusters, C = (C1,C2,…,Ci,…CN), where the i-th cluster Ci contained the two varicosities from Pi. Next, P2 was tested for inclusion in C1. If P2 ∩ C1 ≠ {}, then the elements of P2 were added to C1. P3 was then tested for inclusion in C1 and C2, and so on. That is, for all j < i, the elements of Pi were added to Cj if Pi ∩ Cj ≠ {}. At the end of this process, C contained overlapping clusters. Finally, we reduced each cluster to its unique elements and selected for subsequent analysis only those clusters having no overlap with previous clusters. That is, Ci was selected if and only if Ci ∩ Cj = {} for all j < i. For subsequent analysis, ΔF/F0 was calculated as (F−F0)/F0, where F0 was the mean F over 8 baseline frames immediately preceding the time of possible stimulus onset on each trial. The ΔF/F0 for each putative “axon” was calculated as the mean ΔF/F0 from all responsive varicosities within a cluster.
Single neuron ideal observer analysis
We used receiver operating characteristic (ROC) analysis to calculate “detect probability” (identical to “choice probability”27) and “stimulus probability.” A decision variable (DV) was assigned for each trial based on the neural response. DV was the evoked ΔF/F0 as defined above. Trials were grouped by the mouse’s choice (lick vs no-lick, for detect probability) or by stimulus condition (present vs absent, for stimulus probability). An ROC curve was obtained by systematically varying the criterion value across the full range of DV (using MATLAB “perfcurve”). The area under the ROC curve (AUC) represents the performance of an ideal observer in categorizing trials based on the DV. Detect probability was the AUC for discriminating choice. Stimulus probability was the AUC for discriminating the stimulus condition. A 95% confidence interval for each AUC was obtained by bootstrap (MATLAB “perfcurve”).
Noise correlation analysis
Noise correlations33 were quantified as Pearson’s correlation coefficient between evoked ΔF/F0 values for a pair of neurons across all Go trials. The values were averaged across all pairs to obtain the mean noise correlation for a neuron.
Population decoding analysis
We used Random Forests ensemble classifiers31 (via MATLAB “TreeBagger” class with 300 trees and minimum leaf size of 1) to discriminate the stimulus condition (Go trials vs NoGo trials) or choice (Hits and False Alarms vs Misses and Correct Rejections) based on the vector of ΔF/F0 for all responsive neurons at a given time point (image frame) during the trial. A separate classifier was trained on each time point. Classifier performance was quantified as the area under the receiver-operating characteristic curve for classification of out-of-bag observations. We denote the performance in decoding the stimulus condition based on population responses as “SPpop,” by analogy to our ideal observer “stimulus probability” quantity for single neurons. Similarly, we denote performance on decoding choice as “DPpop.” In Fig. 3, data from 1 mouse were acquired at 15 Hz whereas data from 2 mice were acquired at 30 Hz. We accommodated the slower imaging speed for this 1 mouse as follows. For display in Fig. 3d,e, we combined 15 Hz and 30 Hz movies by excluding every other frame of the 30 Hz movies. Random Forest classification was performed using all frames (65 frames for 15 Hz, 140 frames for 30 Hz). For Fig. 3i,j, decoding performance curves were combined across different imaging speeds by excluding every other frame of the 30 Hz movies. The image frame at which decoder performance diverged was defined as the first frame at which a one-tailed Wilcoxon rank sum test (Figs. 2k,l, 4h) or a one-tailed Wilcoxon sign rank test (Fig. 3i,j, Supplementary Fig. 3j,k) reached a p-value < 0.05. Statistical tests for population decoders were one-tailed and performed across sessions rather than mice because (a) each analysis began with a hypothesis for the sign of any difference, and (b) each behavioral session yielded one decoder performance curve.
Data analysis: pooling across sessions and mice
Our convention in the text and figures is to report variability across mice. Unless otherwise noted, we used two strategies to combine data from multiple behavioral sessions for an individual mouse. For cumulative histograms in Fig. 2d, we pooled ROIs across sessions to obtain histograms for each mouse, and then averaged these histograms. For mean ΔF/F0 time series in Figs. 2a,b, 3d,e and 4e,f, bargraphs and scatterplots in Figs. 1h, 2c, 3f and 4g, plots of SP or DP vs distance in Fig. 2i,j, and population decoder time series in Figs. 2k,l, 3i,j and 4h,i, we averaged values across sessions for each mouse. Cumulative histograms in Figs. 2g,h,m, 3g,h,k were obtained by pooling all data across mice and sessions. The number of monitored or responsive neurons could vary from session to session for a given mouse. For brevity in the text and figure legends, therefore, we report the number of neurons obtained on average across sessions for each mouse. We also report the total number of neurons or observations used for each statistical test.
Statistics
We report data as mean ± standard error of the mean unless otherwise noted. Statistical tests were two-tailed except where noted. We made no adjustments for multiple comparisons. We chose statistical tests in the following order of decreasing preference: (1) parametric tests when appropriate (paired and unpaired t-tests); (2) rank- and sign-based nonparametric tests (Wilcoxon signed rank, Wilcoxon rank sum, sign test); Kolmogorov-Smirnov tests; (3) randomization tests (permutation and bootstrap). Prior to using t-tests, we assessed normality using quantile-quantile plots. Prior to using Wilcoxon signed rank tests, we confirmed symmetry of the tested sample about its median (defined as absolute value skewness < 0.6); otherwise we used a sign test.
To test whether the median pairwise distance (Fig. 2g) among SP neurons, DSP, was the same as the median distance among all neurons, Dall, we used a bootstrap method. The test statistic was the observed difference in median distances, Y = Dall − DSP. A bootstrap replicate, Y*, was obtained from each of 20,000 bootstrap samples. Each replicate was calculated as the difference between Dall and the median of NSP distances sampled with replacement from the set of distances among all neurons, where NSP was the number of distances among SP neurons. The p-value was calculated as the fraction of Y* values more extreme than Y. We used the same procedure for DP neurons.
Unless otherwise noted, we calculated confidence intervals using a nonparametric multi-stage bootstrap58 method that simulates the data generation process and incorporates variability both among neurons for a given mouse, and variability among mice. We calculated the confidence interval for statistic Y (e.g. a mean Hit − Miss difference across mice) as follows, where Y was calculated using data from N mice, and where Nk is the number of neurons obtained from the k-th mouse (we first pooled neurons collected in separate sessions for a mouse). First, mice were sampled randomly with replacement to obtain a set of N primary sampling units (PSUs). Next, Nk neurons were sampled with replacement for each PSU. A bootstrap replicate, Y*, was calculated for each of 50,000 such bootstrap samples. The 95% confidence interval for Y was calculated as the 2.5 and 97.5 percentile values of Y*.
We assigned mice of appropriate genotypes to experimental groups arbitrarily, without randomization or blinding. We did not use statistical methods to predetermine sample sizes. Sample sizes are similar to what have been reported in the field.
Supplementary Material
Acknowledgments
We thank V. Jayaraman, R. Kerr, D. Kim, L. Looger, K. Svoboda and the HHMI Janelia Farm GENIE Project for GcaMP6. We thank S. Peron for MATLAB software; T. Shelley for instrument fabrication; K. Severson and E. Finkel for mouse husbandry. We thank E. Finkel, D. Xu, K. Severson, B. Bari, M. Chevee, K. Svoboda, S. Brown, J. Cohen and S. Mysore for comments on the manuscript. This work was supported by the Whitehall Foundation, Klingenstein Fund, the Johns Hopkins Science of Learning Institute, and NIH grants R01NS089652 (D.H.O.) and core grant P30NS050274. G.M. was supported by a JSPS Postdoctoral Fellowship for Research Abroad.
Footnotes
Author Contributions
S.E.K. and D.H.O. planned the project. S.E.K. performed imaging, behavioral, and optogenetics experiments. H.Y. performed electrophysiology and optogenetics experiments. S.E.K. and D.H.O. analyzed data. G.M. established S2 targeting methods. S.E.K. and D.H.O. wrote the paper with comments from H.Y. and G.M.
Competing Financial Interests
The authors declare no competing financial interests.
Data availability. The data that support the findings of this study are available from the corresponding author upon request.
Code availability. Data analyses were conducted in MATLAB using scripts available from the corresponding author upon request.
References
- 1.de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci U S A. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14:350–363. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastos AM, et al. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauller L. Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav Brain Res. 1995;71:163–170. doi: 10.1016/0166-4328(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 6.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 8.Seung HS. The Handbook of Brain Theory and Neural Networks. MIT Press; 2003. pp. 94–97. [Google Scholar]
- 9.Murphy BK, Miller KD. Balanced amplification: a new mechanism of selective amplification of neural activity patterns. Neuron. 2009;61:635–648. doi: 10.1016/j.neuron.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 11.Douglas RJ, Martin KA. Opening the grey box. Trends Neurosci. 1991;14:286–293. doi: 10.1016/0166-2236(91)90139-l. [DOI] [PubMed] [Google Scholar]
- 12.Glickfeld LL, Andermann ML, Bonin V, Reid RC. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat Neurosci. 2013;16:219–226. doi: 10.1038/nn.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petreanu L, et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature. 2012;489:299–303. doi: 10.1038/nature11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature. 2013;499:336–340. doi: 10.1038/nature12236. [DOI] [PubMed] [Google Scholar]
- 15.Chen JL, et al. Pathway-specific reorganization of projection neurons in somatosensory cortex during learning. Nat Neurosci. 2015 doi: 10.1038/nn.4046. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T, et al. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–1490. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Petersen C. Target-specific membrane potential dynamics of neocortical projection neurons during goal-directed behavior. Elife. 2016;5 doi: 10.7554/eLife.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino H, Komiyama T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci. 2015;18:1116–1122. doi: 10.1038/nn.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suter BA, Shepherd GM. Reciprocal interareal connections to corticospinal neurons in mouse M1 and S2. J Neurosci. 2015;35:2959–2974. doi: 10.1523/JNEUROSCI.4287-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvell GE, Simons DJ. Thalamic and corticocortical connections of the second somatic sensory area of the mouse. J Comp Neurol. 1987;265:409–427. doi: 10.1002/cne.902650309. [DOI] [PubMed] [Google Scholar]
- 21.Carvell GE, Simons DJ. Somatotopic organization of the second somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens Res. 1986;3:213–237. doi: 10.3109/07367228609144585. [DOI] [PubMed] [Google Scholar]
- 22.Kleinfeld D, Delaney KR. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J Comp Neurol. 1996;375:89–108. doi: 10.1002/(SICI)1096-9861(19961104)375:1<89::AID-CNE6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Kwegyir-Afful EE, Keller A. Response properties of whisker-related neurons in rat second somatosensory cortex. J Neurophysiol. 2004;92:2083–2092. doi: 10.1152/jn.00262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Y, et al. Complementary contributions of spike timing and spike rate to perceptual decisions in rat S1 and S2 cortex. Curr Biol. 2015;25:357–363. doi: 10.1016/j.cub.2014.11.065. [DOI] [PubMed] [Google Scholar]
- 25.Chen JL, Voigt FF, Javadzadeh M, Krueppel R, Helmchen F. Long-range population dynamics of anatomically defined neocortical networks. Elife. 2016;5 doi: 10.7554/eLife.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 28.Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 29.Nienborg H, Cohen MR, Cumming BG. Decision-related activity in sensory neurons: correlations among neurons and with behavior. Annu Rev Neurosci. 2012;35:463–483. doi: 10.1146/annurev-neuro-062111-150403. [DOI] [PubMed] [Google Scholar]
- 30.Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2nd. Springer; 2009. [Google Scholar]
- 32.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy KB, Schnepel P, Rao AT, Feldman DE. Structure of a single whisker representation in layer 2 of mouse somatosensory cortex. J Neurosci. 2015;35:3946–3958. doi: 10.1523/JNEUROSCI.3887-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronoff R, et al. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. doi: 10.1111/j.1460-9568.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- 36.Cauller LJ, Clancy B, Connors BW. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol. 1998;390:297–310. [PubMed] [Google Scholar]
- 37.Mao T, et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Kwon SE, Severson KS, O'Connor DH. Origins of choice-related activity in mouse somatosensory cortex. Nat Neurosci. 2016;19:127–134. doi: 10.1038/nn.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo ZV, et al. Flow of Cortical Activity Underlying a Tactile Decision in Mice. Neuron. 2013 doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CC. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci. 2013;16:1671–1677. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- 41.Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manita S, et al. A Top-Down Cortical Circuit for Accurate Sensory Perception. Neuron. 2015;86:1304–1316. doi: 10.1016/j.neuron.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Engel TA, Chaisangmongkon W, Freedman DJ, Wang XJ. Choice-correlated activity fluctuations underlie learning of neuronal category representation. Nat Commun. 2015;6:6454. doi: 10.1038/ncomms7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato TR, Svoboda K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J Neurosci. 2010;30:4256–4260. doi: 10.1523/JNEUROSCI.3774-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Shamayleh Y, Kumbhani RD, Dhruv NT, Movshon JA. Visual response properties of V1 neurons projecting to V2 in macaque. J Neurosci. 2013;33:16594–16605. doi: 10.1523/JNEUROSCI.2753-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smolyanskaya A, Haefner RM, Lomber SG, Born RT. A Modality-Specific Feedforward Component of Choice-Related Activity in MT. Neuron. 2015;87:208–219. doi: 10.1016/j.neuron.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez A, et al. Spatiotemporal receptive fields of barrel cortex revealed by reverse correlation of synaptic input. Nat Neurosci. 2014;17:866–875. doi: 10.1038/nn.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peron SP, Freeman J, Iyer V, Guo C, Svoboda K. A Cellular Resolution Map of Barrel Cortex Activity during Tactile Behavior. Neuron. 2015;86:783–799. doi: 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
References
- 51.Dana H, et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One. 2014;9:e108697. doi: 10.1371/journal.pone.0108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Connor DH, et al. Neural coding during active somatosensation revealed using illusory touch. Nat Neurosci. 2013;16:958–965. doi: 10.1038/nn.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge University Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.