Abstract
Why do animals and humans do anything at all? Arousal is the most powerful and essential function of the brain, a continuous function that accounts for the ability of animals and humans to respond to stimuli in the environment by producing muscular responses. Following decades of psychological, neurophysiological and molecular investigations, generalized CNS arousal can now be analyzed using approaches usually applied to physical systems. The concept of “criticality” is a state that illustrates an advantage for arousal systems poised near a phase transition. This property provides speed and sensitivity and facilitates the transition of the system into different brain states, especially as the brain crosses a phase transition from less aroused to more aroused states. In summary, concepts derived from applied mathematics of physical systems will now find their application in this area of neuroscience, the neurobiology of CNS arousal.
1. Introduction
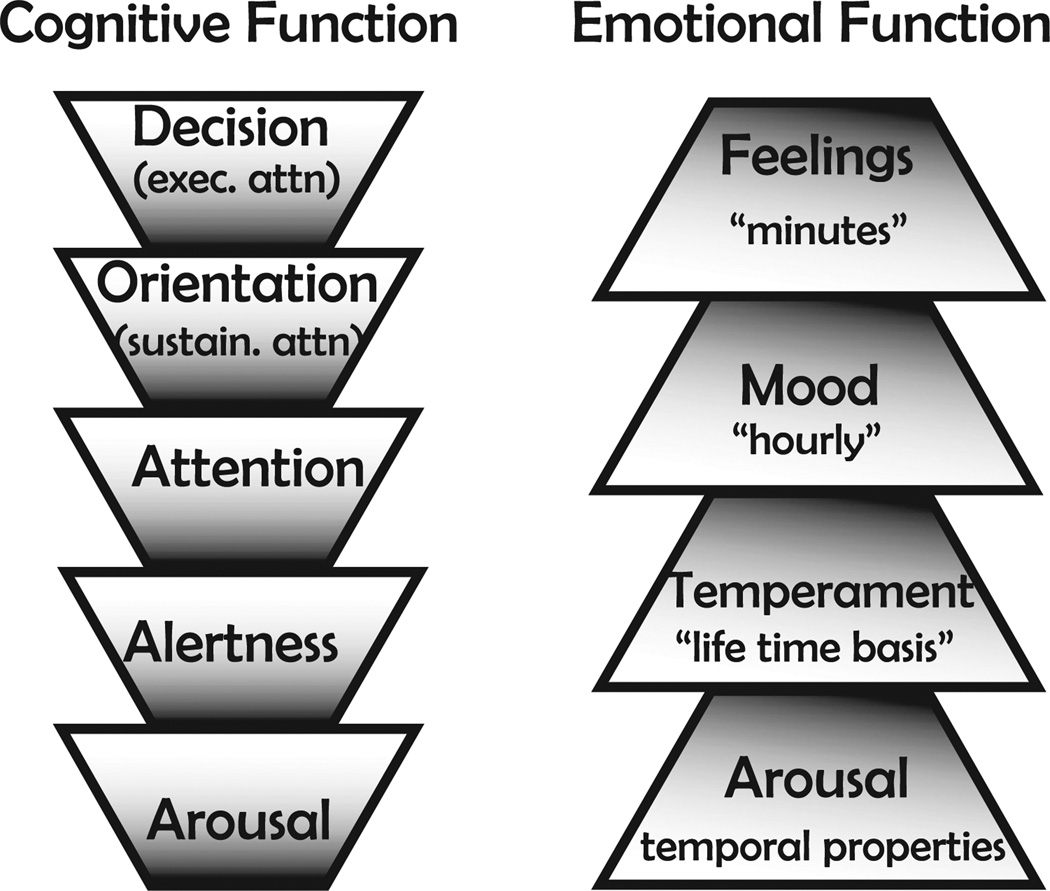
Underlying all motivated and emotional behaviors there is a primitive and elementary non-specific neuronal ‘force’ that activates a set of ascending and descending systems, and facilitates the initiation of any behavior, that we define as GA. Fig. 1 illustrates, in cartoon form, a logical perspective of GA, a fundamental property of the CNS, as it lies beneath and supports higher cognitive and emotional faculties (depicted, e.g., as a hierarchical relation between GA and decision, among cognitive functions, and GA and feelings, among emotional functions).The relationship of GAto specific motivational states (e.g. thirst, fear, hunger) and consequent motivated behaviors may be expressed as a differential equation of the following form:
Fig. 1.
Generalized arousal. Generalized CNS arousal is fundamental to all cognitive functions and to all emotional expressions. E.G., you can be aroused without being alert but cannot be alert without being aroused, and so forth, up toward more complex cognitive functions such as sustained attention (sustain attn.) and executive attention (exec attn.). In this stack of trapezoids more fundamental functions are placed at the bottom and higher-level complex functions are placed at the top. Likewise for emotional expressions, whether your emotional expressions are viewed on a lifetime basis (temperament), hourly basis (moods) or minute-by-minute basis (feelings), the strength of expression depends on CNS arousal. If emotional behavior were to be pictured as a vector, the angle of the vector would be a function of the nature of the feeling, but the magnitude of the vector would depend on CNS arousal.
That is, CNS arousal can be expressed as some sort of linear equation in which each component contributes to CNS arousal. As expected, each component has a weight represented in the equation by a coefficients kij, where Aj is a type of arousal and i,j represent GA (g), and for example, hunger(h),thirst (th), fear(f), anger (a) and sources presently obscure and unnamed (Ax…n). Coefficients (kij) are expected to be estimated using data. Notice that GA(g) is a dominant component. We note that quantitative relations among components are yet to be discovered.
Most evident are the extreme levels of GA: in a comatose patient, GA is lost. At the other extreme, sexual excitement and panic, for example, are fueled by high levels of GA. The utility of GA as a concept lies in its usefulness in explaining how all motivated behaviors can be initiated and allows us to propose an operational definition of a GA system as well as its operational requirements.
1.1. Operational definition
A more highly aroused animal or human being – – an individual with high generalized CNS arousal (GA) – – is (i.) more highly active motorically; and (ii.) more sensitive to sensory stimuli; and (iii.) more reactive, emotionally (Pfaff, 2006). The absence of these elements contributes to define disorders of consciousness (Giacino et al, 2014; Laureys and Schiff, 2012). Similarly, the exaggerated presence of these aspects results in anxiety disorders (Ressler and Mayberg, 2007). Clearly, the motoric, sensory and emotional elements of GA do not always covary. This review of GA mechanisms applies to the wide variety of situations in which they do covary.
1.2. Operational requirements for a generalized arousal system
(1) Speed, lability, ‘hair triggered’; (2) Sensitivity; (3) Convergence of all sensory stimuli onto the GA system; (4) Divergence from the GA system to affect cortical arousal and autonomic arousal; and (5) Robustness - - the GA system must not be allowed to fail Pfaff et al, 2012 . Requirement (1) for speed of GA response, particularly in threatening situations, signals the need for “criticality” (state in which natural systems self-tune to be in the vicinity of a critical point) in the GA system. A critical regime optimizes information processing and transmission (Shew et al., 2011). Likewise, critical dynamics makes the system exquisitely sensitive to perturbations (Solovey et al., 2015). Thus, criticality contributes to another operational requirement, sensitivity.
Criticality is a common state identified during recording from vertebrate neural systems including humans, nonhuman primates and rodents (Shew et al, 2015; Solovey et al., 2012; Tkacik et al., 2015; Tagliazucchi et al., 2012). A cerebral network that represents this condition in the cortex is the “default mode network” (DMN). This is a network that was discovered by Marcus Raichle and his colleagues (Raichle et al., 2001) and is characterized by higher levels of activity in areas such as the medial prefrontal cortex, the precuneus and posterior cingulate cortex in the absence of an immediate task to be performed, a form of activity characterized as ‘self-referential (Raichle, 2015). Recent papers strongly suggest that DMNs spend most of the time near criticality (Tagliazucchi et al, 2012; Shew et al, 2015; Haimovici et al., 2013). In fact, the absence of the lability seen in the stabilization of cortical activity is associated with loss of consciousness (Solovey et al., 2015). Interestingly, computational neuroscientific results, from network control theory, suggest that cortical areas within the default mode network innervate the rest of the cortex with high density connections (hubs). These “hubs” have the ability to steer the system into many easily reachable states (GU et al, 2014) suggesting that the “default mode network” may carry the effects of GA system.
As GA is a theoretical concept bearing a need for proof of its existence, this review first offers evidence of it using different perspectives: genetic, mechanistic and statistical. Second, we review some of the most recently discovered mechanisms that regulate GA, including neuroanatomical pathways, electrophysiological features and molecular underpinnings. Third, we offer a theory of the dynamics that describe the transition from a not-aroused CNS state to an aroused CNS state, including mathematical and statistical evidence for power law behavior generated by intrinsic CNS mechanisms and an argument for how such mechanisms evolved, tuned to any animal’s (or human’s) environment. Finally, we offer perspectives on increased current attention to global low brain activity states of medical importance, such as disorders of consciousness; the opportunity to bring this interesting area of neuroscience to a level of precision that can be treated with the techniques of applied mathematics; and the possibility that a default mode network may maintain a basal arousal state in the absence of specific tasks.
2. Evidence for the existence of GA
In the last century, experimental psychologists, explained a large body of human behavioral data in terms of personality characteristics by incorporating arousal constructs obviously reflected in Central Nervous System arousal (Morgan, 1943; Thompson, 1967; Harlow, 1958). For instance, Hebb (1955) used ‘generalized activation’ as part of his psychological theory of motivation to account for the vigor of motivated behavior. One concrete example is the ‘circumplex’ theory of personality: regarding motivation and emotion. It features arousal as a central variable(Russell and Barrett, 1999; Remmington et al., 2000; Russell, 1980). Likewise, ethologist Konrad Lorenz (Lorenz, 1971)] wanted to account for “variations in intensity in the performance of instinctive responses”, and relied upon a global arousal force to do that. In general, ethological approaches to the biology of behavior, emphasizing the study of animal behavior in natural settings rather than the laboratory, have shown arousal as being fundamental to the initiation of instinctive behaviors. For example, in a variety of species they observed “a general motor restlessness and the expression of a specific readiness to act” (Eibl-Eibesfeldt, 1970). In their view, a wide variety of motivated behaviors have needs and drives supported by the existence of a concept of GA. It is straightforward to cite further evidence for the existence of GA that may synergize with specific forms of CNS arousal such as hunger, thirst, sex, etc.
2.1. Mechanistic evidence of GA
In parallel with the psychological observations described above, scientists like, Dempsey and Morison (1941) and Moruzzi and Magoun (1949) revealed, using electrophysiological approaches, a first line of evidence for the existence of GA. They showed the capacity of systems in the reticular core of the brainstem, midbrain and thalamus to activate a wide range of functions: cortical, autonomic and behavioral. These findings showed for the first time the ability of the brain to actively evoke awakening in response to sensory stimulation and generate and maintain arousal. The arousal systems comprise ascending networks projecting to the cerebral cortex, which produce cortical activation; and descending networks, projecting to the spinal cord, that results in motor and sensory activation (Jones, 2003).
More recently, imaging studies have provided the opportunity to examine more complex cognitive tasks that require GA. For instance, fMRI studies determined the relevance of GA as a basic precondition and modulator of a sustained cognitive performance: vigilant attention (Langner and Eickhoff, 2013) and to states of sleep that feature reduced GA (Picchioni et al., 2013).
Knowing that GA is considered the net result of several neuromodulatory brain systems, especially glutamatergic neurons, Hyder et al., (2013) used electroencephalography and positron emission tomography (PET) measurements of oxygen and glucose utilization to analyze the oxidative demand imposed by GA defined here as “gray-matter signaling” Over several conditions oxidative demand for signaling was four times greater than ‘non-signaling events’. This indicates that the most of the metabolic activity is used to support neuromodulatory brain systems, GA.
2.2. Genetic evidence
To examine whether genetic factors directly influence generalized arousal, thus helping further to make it a tangible concept, one would ask if external manipulations producing changes in behavior (high or low GA) would result in effects on gene expression and therefore, in circuitry, anatomy and function. Certainly, there are many examples in the literature (see below) that support this concept. Note that in these examples the definition of GA varies according with the study and considers some, but not all the factors that contribute to GA.
In a laboratory breeding study involving rats (Albert et al, 2008), animals were bred for low GA (here defined as tameness and tolerance to human presence and interaction). Derived from wild rats, tame ones were selected for 32 years (Belyaev, 1969). This group expressed less aggression, fear or defensive behaviors compared to controls (not tamed) in eleven out of thirteen components scored in the behavior of rats towards a human hand. They were also more exploratory and exhibited fewer anxiety-like behaviors. Interestingly, these behaviors were associated with smaller adrenal glands, lower levels of corticosterone and serotonin in serum. In addition, cross-fostering experiments showed that these behavioral results were not related to maternal care suggesting that the behavioral differences observed between the lines have a genetic basis. Albert FW et al. mapped the locations of DNA sequence differences (quantitative trait loci; QTL) that contributed to differences between the tame and aggressive rat lines (Albert et al., 2009). More recently, they also identified using mRNA sequencing and a QTL mapping method multiple genes influenced by the genetic variants from the tame and aggressive lines (Heyne et al., 2014). Candidate genes that may play a causal role in these behaviors have been implicated in anxiety and stress.
Further evidence is derived from the breeding of dogs from wolves. The domestication of dogs started approximately 30,000 years ago (Thalmann et al., 2013), and the very existence of domesticated dogs (which emerged about 15,000 years ago in East Asia) (Thalmann et al, 2013) indicates that genetic factors contribute to arousal-dependent behaviors and thus are implicated in GA. Domestication involved animals being selected for specific behavioral traits, and this has resulted in many different dog strains with very different behavioral profiles. For example, the cocker spaniel and Shetland sheepdog have much lower behavioral reactivity to startling stimuli than the beagle, basenji, or wire-haired fox terrier (Spady and Ostrander, 2008; Scott, 1965)
Similarly, horses can be bred for specific behavioral characteristics (Oki et al., 2007; Scott, 1965); for example, the rapid responsivity of the thorough bred race horse (high) GA compared to the calm and unresponsiveness of the horse to which it is tethered (low GA) for behavioral management in loud, unpredictable environments.
Experiments exposing animals to absolutely constant environmental conditions and rigorous behavioral assessment according to the operational definition of GA obtained similar results. Starting with a strain of mice (Lindzey and Thiessen, 1970) resulted from an extensive intercross of more than eight out bred strains (Het-8), we were successful in establishing high and low GA lines, using a high throughput 24 h/7 day assay that quantitatively measures motor activity, sensory response to external stimuli and emotional reactivity (Weil et al, 2010). In each generation large populations of mice were ranked according to the three components of the operational definition of GA, and highest males mated to highest females, lowest to lowest. Animals defined as high-arousal animals by the assay exhibited greater levels of anxiety-like behavior, reduced exploratory behavior and were more sexually excitable (Weil et al., 2010) in comparison with those defined as low arousal. The divergence of behavior after the seventh generation obtained with these experiments offers independent evidence for the existence of GA, as one cannot breed for a function that is not real.
Lastly, we generated evidence of specific genetic changes that influence GA. In one study it was determined that mice with disruption of the gene coding for the classical estrogen receptor (ER-α) displayed greatly reduced GA. In contrast, the disruption of a very similar hormone-activated transcription factor like ER-β did not (Garey et al., 2003). ER- α modifying GA adds to the evidence for the existence of GA.
2.3. Statistical evidence
The impact of GA on behavior was revealed by using principal component analysis (PCA) in a set of five populations of ovariectomized mice described in detailed in Garey et al. (2001), Morgan and Pfaff (2001), Frohlich et al. (2001) and Frohlich et al. (2002) that were subjected to a high throughput assay that quantitatively measured: (1) motor activity: distance traveled, total movement duration and voluntary movement in a running wheel; (2)sensory responses to external stimuli: auditory, vestibular, tactile and olfactory stimuli; and (3) emotional reactivity: motor activity and freezing behavior in a conditioned fear paradigm (Garey et al., 2003; Weil et al, 2010). These variables were weighted by their contribution to the explanation of the variance. Despite multiple experimental variables such as experimental design and mice populations, the covariance analysis of various arousal-related response measures reported that GA accounted for between 29 and 45% of the variance in behavioral results across the studies. GA accounts for significantly more real data than in any random-number control. Likewise, nothing similar to the GA factor appeared in control data sets in which data entries were scrambled.
The other contributions to arousal- - -or example due to thirst, hunger, fear, anger and so forth - - would all be expected to account for some of the variance and are reflected in the partial differential equation written above. Thus, in quantitative terms, if behavioral arousal were viewed as a differential equation with many different types of arousal represented by many independent terms, the GA term would be large and significant, but would account for less than half of the variance, compared with the sum of the other terms (for example, arousal due to hunger, thirst or fear). Thus, the statistics of principal components analysis support the conclusion that GA exists, but also indicates the importance of other, specific forms of arousal.
3. Molecular regulation of GA
Clearly, large numbers of genes and their products contribute to the modification of GA and therefore to its existence. A more molecular approach shows that each neurotransmitter or neuropeptide that contributes to activity changes in GA pathways depends on at least four classes of genes for its proper regulation: genes for synthetic enzymes, genes for receptors, transporter genes and genes encoding catabolic enzymes. At minimum, genes encoding for norepinephrine, glutamate, acetylcholine, dopamine and histamine are involved as all these neurotransmitters contribute to GA, in addition to the more recently discovered hypocretin/orexin gene (Pfaff, 2006).
For brevity, we review the newest studies on the hypocretin and noradrenergic system, in which new optogenetic and pharmacogenetics techniques have been employed to demonstrate that specific neuronal subpopulations genetically identified regulate GA.
3.1. Ascending neuromodulatory pathways
The gene encoding hypocretin peptides is especially interesting to GA because of the broad range of neuroanatomical projections from hypocretin-expressing neurons and because of the diverse and wide range of inputs - metabolic, circadian and limbic - that are integrated by these neurons (de Lecea and Huerta, 2014). The hypothesis has been offered, and is being confirmed by optogenetics, that hypocretin effectively lowers the threshold for GA to occur (Sutcliffe and de Lecea, 2002). Optogenetic activation of hypocretin neurons at frequencies greater than 5 Hz woke up mice from sleep (Adamantidis et al., 2007), whereas optogenetic silencing of these neurons could induce sleep during the light phase of the daily light cycle (Tsunematsu et al., 2011). As mentioned, two studies (Carter et al., 2010; Sears et al., 2013) have shown that hypocretin neurons have important behavioral effects by working through the LC. de Lecea and Huerta (2014) and Sears et al. (2013) show the roles of hypocretin neurons in “coordinating arousal centers” rather than acting as “master switches” for GA, thus re-emphasizing the multigenic nature of GA regulation.
The classical literature on the anatomy, physiology and chemistry of locus coeruleus (LC) as it contributes to arousal and attention has been reviewed comprehensively (Foote et al., 1983). Adrenergic projections ascending from locus coeruleus (LC) have a role in modulating GA, as reflected in cortical arousal (increased power of high frequencies observed in the electroencephalogram) especially, and transitions between sleep and waking states. Effects of LC neuronal activation have been tested under states of isoflurane anesthesia (Vazey and Aston-Jones, 2014) using DREADDS (designer receptors exclusively activated by designer drugs) or RASSLs (receptors activated solely by synthetic ligands). Activation of LC neurons induced a shift in cortical EEG patterns from delta to theta waves (marking cortical arousal) during anesthesia. These effects of LC were blocked by adrenergic receptor antagonists, indicating that noradrenergic neurons were effective in changing the EEG from slow waves to an activated state. By contrast, as predicted, treatment with adrenergic receptor antagonists prolonged the duration of anesthesia. These results indicate that noradrenergic neurons in the LC modulate cortical arousal. Apart from the well-studied roles of the LC in alertness, attention and perceptual shifts, this study reveals its role in modulating shifts from anesthetized to active states (Vazey and Aston-Jones, 2014). These experiments reflect direct manipulations of generalized CNS arousal via specific activity modulation of adrenergic neurons.
Interestingly, two modes of activity have been observed in LC neuronal activity (Aston-Jones and Cohen, 2005): LC engages in phasic firing that is associated with the processes of decision-making, attention and vigilance towards salient stimuli; and a phasic-to-tonic transition of firing pattern was observed during disengagement from the current task and explorative behavior in non-human primates. As the salience of sensory stimuli wanes (either because of satiety or removal of reward), the firing pattern of LC shifts from phasic to tonic. The Yerkes-Dodson law states that maximum performance of a task is achieved with intermediate levels of GA that varies with different tasks. As shown by Aston-Jones and Cohen (2005), the balance between intermediate and high levels of GA is mediated by firing patterns of LC neurons.
Carter et al. (2012) used optogenetic and pharmacological tools to provide evidence that blocking LC neuronal excitability during hypocretin neuronal excitation blocked the sleep-to-wake transition usually observed during hypocretin activation (Carter et al., 2010). In addition, Sears, de Lecea and Ledoux (Sears et al., 2013) used a combination of electrophysiological, pharmacological and optogenetic approaches to demonstrate that fear conditioning, a classic example of emotional GA is due to increased hypocretin activity in the amygdala mediated by the LC. Thus, even for hypocretin, the GA-peptide par excellence, the noradrenergic LC cell group is essential.
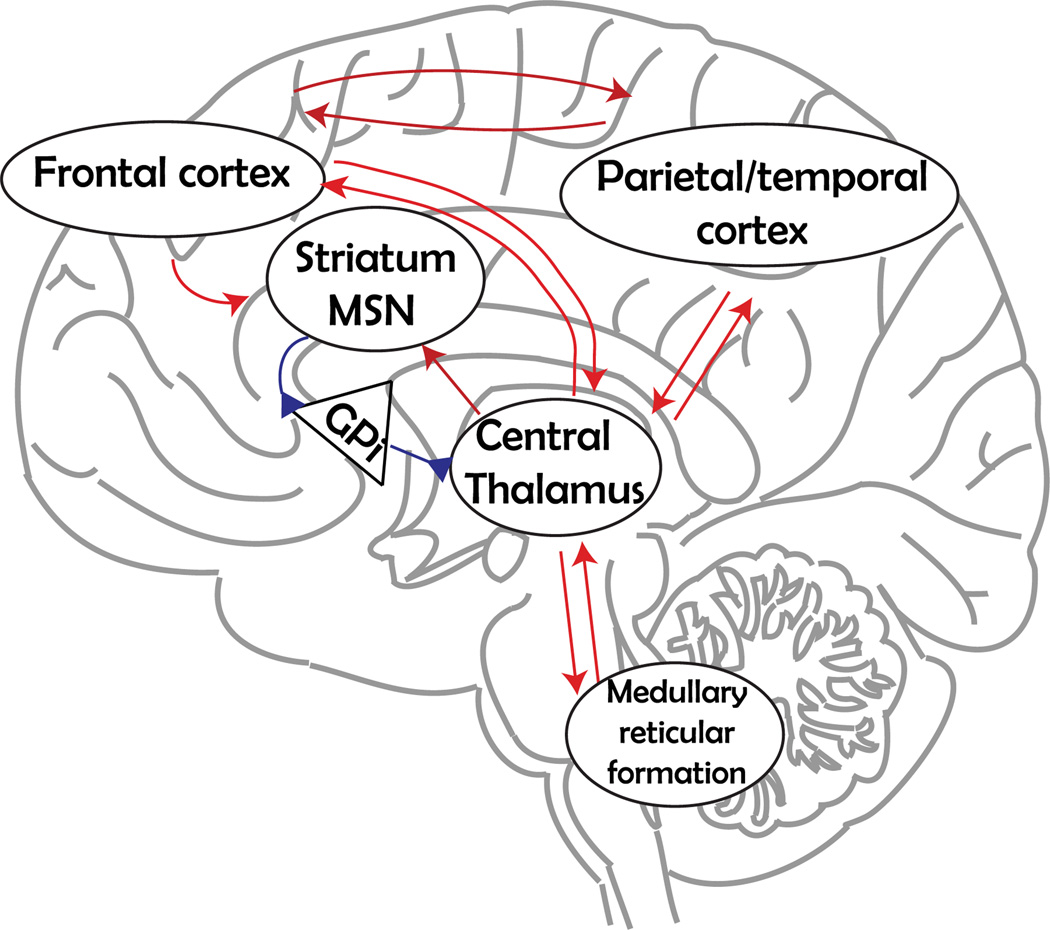
A clinically important source of evidence for the role of noradrenergic neurons in GA comes from work with brain-damaged human patients who suffer from disorders of consciousness (Laureys and Schiff, 2012; Giacino et al., 2014; Boly and Seth, 2012). Noradrenergic signaling ascending from the LC synergizes with glutamatergic and cholinergic signaling from the pedunculopontine nucleus to provide a drive to the intralaminar thalamic nuclei (Schiff, 2008) - notably the centralis lateralis - to activate a ‘mesocircuit’ that is necessary for forebrain activation and that reflects GA as patients significantly recover behavioral responsiveness (Fig. 2). This precise thinking of how ascending GA pathways contribute to consciousness allows it, for the first time, to be treated with the quantitative methods of physical science (see below).
Fig. 2.
Anterior forebrain mesocircuit. The schematic shows important cortical and subcortical components of the anterior forebrain mesocircuit, based on the work and thinking of Nicholas Schiff (Schiff, 2013). Projections from the central thalamus innervate the prefrontal and frontal cortex, particularly supplementary motor area and anterior cingulate. These medial frontal regions in turn provide broad, feedforward projections to the prefrontal and frontal cortices. In addition, the same central thalamic neurons provide thalamo-striatal projections to medial spinal neurons (MSN) in the striatum, which send inhibitory projections to the globus pallidus interna (GPi) (blue synapses). This inhibition prevents potential central thalamus inhibition and therefore a shutdown of the anterior forebrain. Spontaneous recovery and pharmacological manipulations known to be effective in some severely brain-injured patients strongly modulate activity across the forebrain mesocircuit (Schiff, 2013).
3.2. Descending neuromodulator pathways
Since the operating definition of increased GA includes the measurement of increased motor activity, the relevance of descending pathways to GA is clear. Medullary and pontine reticulospinal pathways have wide-ranging projections. For example, reticulospinal neurons project to all levels of the spinal cord bilaterally, and respond to a wide variety of inputs (Peterson et al., 1975; Peterson, 1979). Moreover, the activity of reticulospinal neurons that express the homeobox genes Lhx3 and/or Chx10 is required for the initiation of movements (Bretzner and Brownstone, 2013) - an essential characteristic of arousal-related behaviors −. Electrophysiological results indicate that reticulospinal inputs to interneuronal networks in the spinal cord could be essential for a wide range of movements in the aroused animal (Vetrivelan et al, 2009; Jankowska, 2008).
Muscle tone is at its highest during alert waking, reduced in the drowsy state and at its least during stages III and IV of deep sleep (Kryger et al., 2011). The primary drivers of muscle tone appear to be glutamatergic neurons that elevate motor neuron excitability (Vetrivelan et al., 2009). Motor neurons receive glutamatergic input from descending axons that originate in rhythmogenic nuclei in the brainstem, which heighten GA (Vetrivelan et al., 2009).
Autonomic systems play a role in elevating GA (Pfaff, 2006). However, whether they are causal to changes in GA or the result of changes in GA, it has not been determined. Autonomic activation has been thought to elevate GA and thus disrupt sleep (Gilmartin and Thomas, 2004), but many occurrences of autonomic arousal during sleep are not associated with changes to high-frequency activity in the cortical EEG.
3.3. Medullary reticular neurons in nucleus gigantocellularis
Nucleus gigantocellularis (NGC) neurons can be considered crucial to elevations in GA, and their power to convey arousal owes in part to their broad connectivity (Jones and Yang, 1985; Jones, 2003). Studies using Golgi staining (Scheibel, 1958; Valverde, 1961,1962; Leontovich and Zhukova, 1963) and retrograde neuroanatomical methodologies (Martin et al., 2011; Jones and Yang, 1985) have reported that some NGC neurons contribute axons to both ascending and descending arousal pathways. Both anatomical and electrophysiological evidence that large NGC neurons contribute importantly to GA has been reviewed (Pfaff et al., 2012). Briefly, the wide range of axonal projections mentioned above provides the neuroanatomical substrate. NGC neurons can respond to stimuli in all sensory modalities (Martin et al., 2010). Importantly, elevated firing rates precede movement indicating that NGC provides the basis for sensory responsivity. Lastly, recent studies (Bretzner and Brownstone, 2013; Bouvier et al, 2015) showed that medullary reticular glutamatergic neurons are centrally involved in the activation of movement. Thus, these functional and anatomical features suggest that NGC is a strategic location for promoting arousal.
4. Mathematical analysis of CNS transition states
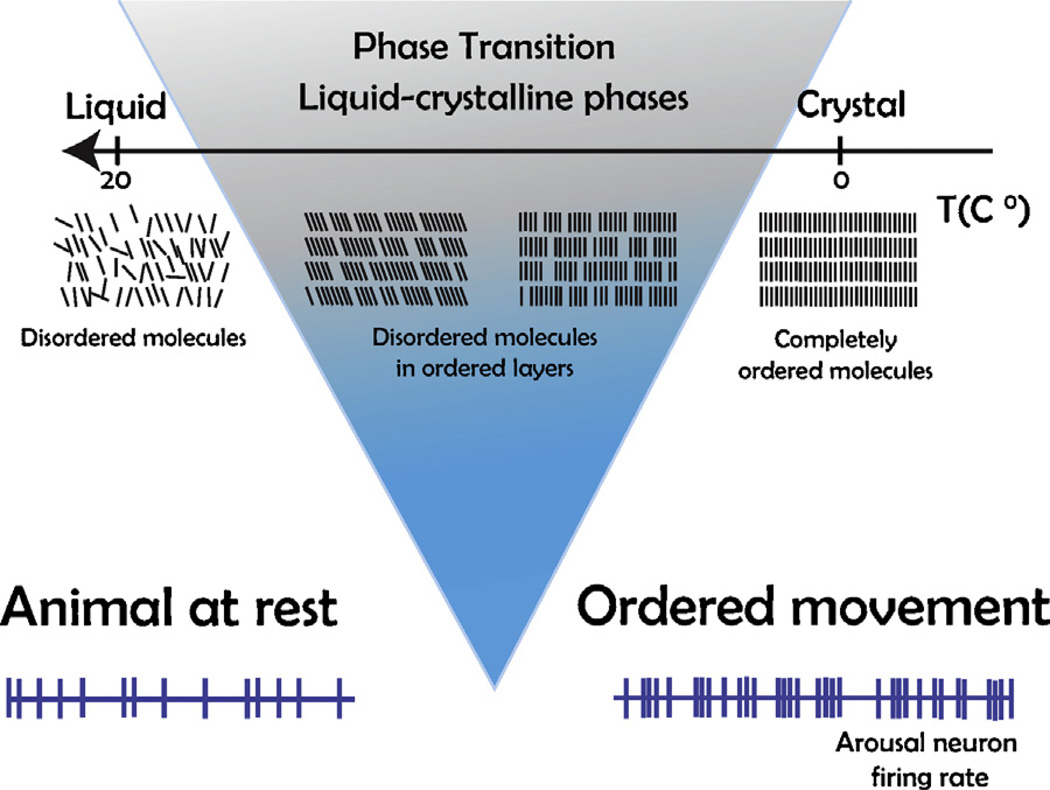
Here, we review the mathematical statistics describing the transition from low to high GA; that is, from quietude to the brain of the individual ready to activate a behavioral response (e.g. Hudson et al., 2014). This transition generates behaviors described by power laws (Proekt et al., 2012), and results in ‘dynamic criticality’ (Magnasco et al, 2009; Alonso et al., 2014) of the CNS. A theoretical framework sketched in Fig. 3 predicts the behavioral data summarized in Fig. 4. Our theoretical framework is intended to explain how the initiation of heightened CNS arousal happens as fast as would be required by sudden changes in environmental circumstances.
Fig. 3.
Phase transitions illustrated here produce data in physical systems and in the nervous system that fit power law curves. Top sketch shows that a small elevation in temperature (fine tuning parameter) can cause the material to go through a phase transition from rigidly ordered molecules (right) to disordered molecules in liquid phase (left). Bottom sketch shows GA relevant neurons firing at relatively low and inconsistent rates in the brain of the quiescent animal (left). According to our physical analogy, they go through a phase transition to high rates of temporally patterned electrical activity (right) in the high GA animal showing ordered motoric responses. In particular, the physical analogy sketched here predicts the kind of GA behavioral data plotted in Fig. 4.
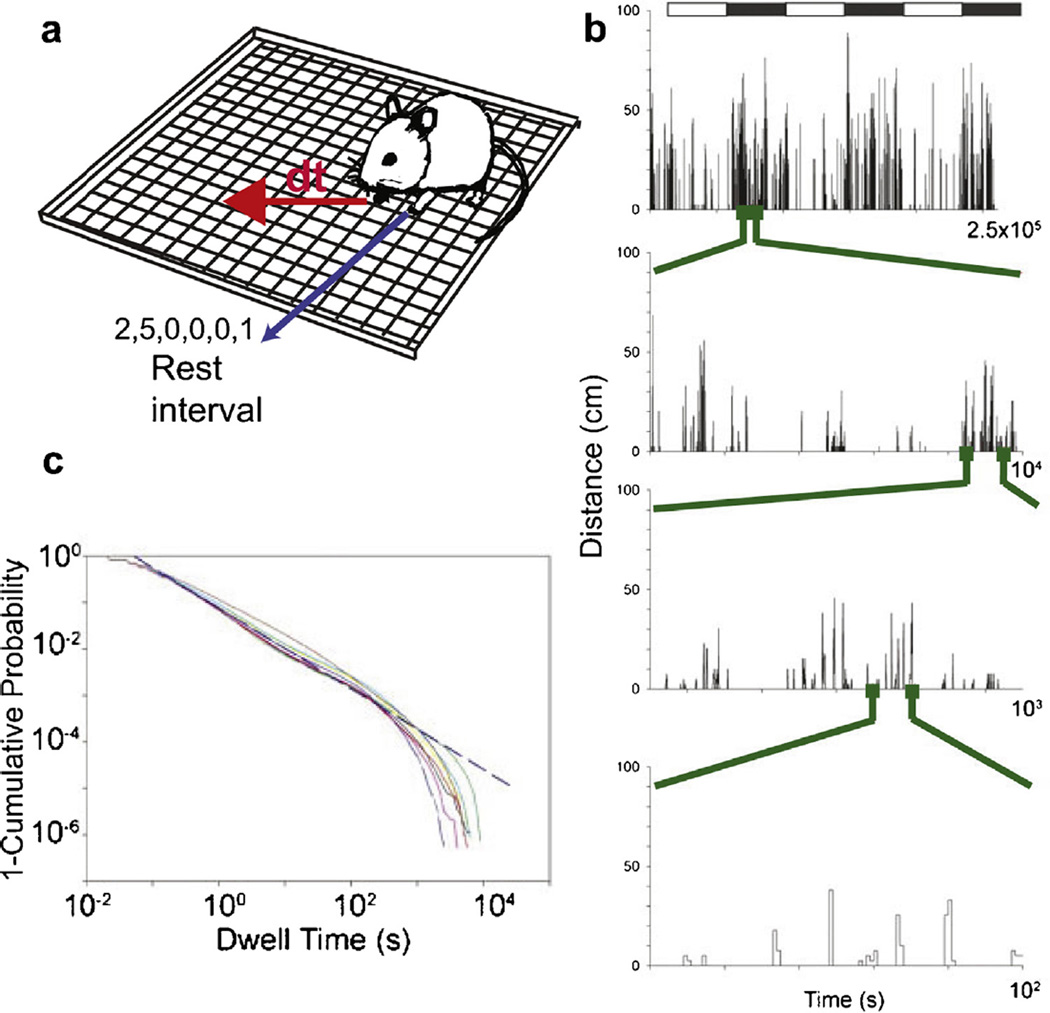
Fig. 4.
Dynamics of spontaneous fluctuations between activity and rest are scale-invariant. (a) This figure shows schematic illustrating a distance traveled (dt) by a single C57/BL6 adult male mouse in consecutive 1-s intervals (b) Plot shows distance traveled by mouse maintained in a constant measured environment. White and black rectangles show light and dark phases (12 h:12 h), respectively. Each subsequent lower trace shows progressive zooming in on the time axis in the region shown by the green dots (number at the right of the x axis shows the range plotted). Regardless of scale, the pattern of fluctuations between locomotion and rest remains unchanged, signaling scale invariance over more than three orders of magnitude. (c) Cumulative distributions of dwell times for nine male mice. Each mouse was recorded continuously for ~22 d (15,027,257 dwell times total, 1,669,695 ±432,903 dwell times per mouse ±SD). Distributions are linear on a log-log scale and have similar slopes indicating power law decay with nearly the same exponent for these mice. Data points from panel (b) and (c) were adapted from reference (Proekt et al., 2012).
GA is a continuous neurobiological function but we sometimes, below, refer to ‘high’ and ‘low’ for the purpose of simplicity of discussion.
4.1. Theoretical framework
How does the brain accomplish rapid changes of state, from low to high GA? In order to be functionally effective, the brain, in low GA, must be poised in the vicinity of the transition to higher GA; that is, at a critical point between two states (Botcharova et al., 2014; Alonso et al., 2014)
Indeed, a pioneering study (Beggs and Plenz, 2003) measured spontaneous potentials in slices of rat cortex and organotypic cultures. They discovered that neural activity occurred in bursts and that these “neuronal avalanches may be a generic property of cortical networks”. Recent studies based on simultaneous measurements of the spiking patterns of multiple retinal ganglion cells in response to natural visual stimuli as well as cortical cells in the visual cortex of cats and monkeys (Gollisch, 2013; Van Hateren et al., 2002) have shown a similar behavior. Through an elegant characterization of the correlations between the dynamical activities of the cells, Bialek and colleagues (Tkacik et al, 2014) have shown that unbiased maximum entropy distributions perform well in predicting the collective spiking pattern. Such data show the applicability of criticality in scale-free systems to actual neuronal system properties.
It is interesting to illustrate key differences between the finely tuned criticality in physical systems and the seemingly self-tuned critical brain (Table 1). Critical exponents determine the dynamics of the system near a continuous phase transition. The exponents characterizing algebraic behavior typically take on just a few sets of values in physical systems where there is considerable variation of the exponent values pertaining to brain dynamics. Life is necessarily a non-equilibrium phenomenon whereas the physical systems are often studied in equilibrium.
Table 1.
Differences and similarities between scale invariance in inanimate matter poised in the vicinity of a critical point and the ever-vigilant brain.
| Inanimate Matter | Brain |
|---|---|
| Power law behavior in time | Power law behavior in time |
| Equilibrium | Non-equilibrium |
| Fine-tuned | Self-tuned |
| Universal | Non-universal |
| Long length and time scales | Absence of relevant intermediate scales |
| Spontaneous symmetry breaking | Superior environmental response |
Consider ‘scale invariance’ in spontaneous behavior, a subject that has been studied by many groups (Proekt et al., 2012; Nakamura et al., 2008; Maye et al, 2007; Reynolds and Frye, 2007) and is suggestive of criticality. “Scale invariance” refers to self-similar dynamics which “scale”, that is, dynamics that leap from tiny metrics across exponents to huge metrics and thus afford the opportunity to change states of the system very rapidly. Spontaneous activation of behavior is the simplest case of arousal onset because it avoids the complexities added by specific behavioral tasks. In the simplest scenario, one might expect that the intervals between consecutive occurrences of a particular behavior will have a characteristic time scale around which most observations are centered. However, the timing of many diverse behaviors from human communication to animal foraging form complex self-similar temporal patterns reproduced on multiple time scales (Proekt et al., 2012; Nakamura et al, 2007).
Consider the dynamics of spontaneous fluctuations between activity and rest of a mouse in a generalized arousal assay, a simple unchanging environment. In one study, each mouse was recorded continuously for ~22 days. The dwell time was defined as the time during which the mouse stayed at the same location. The autocorrelation of the dwell times shows that subsequent dwell times are substantially independent of each other. However, the distribution of dwell times is approximately scale invariant - there is no single characteristic dwell time - . Quite remarkably, the resting times of a mouse, a rat, a healthy human, and a depressed human all show power law distributions but with variable exponents ranging from 1.7 to 2 (Nakamura et al, 2007). The essential ingredient for such scale-invariant behavior follows from first noting that there is a significant disparity between the time scale for the spiking of a neuron (~ms) and the characteristic macroscopic time scale T over which behavior occurs. It has been shown that the absence of any special intermediate time scale automatically yields that the waiting time distributions are fit by a power law function, as observed.
Let t = 0 represent the time at which the mouse starts resting in one of its dwell time intervals. The probability that the mouse is aroused and moves during a time interval dt after being continuously at rest during the time interval between 0 and t is equal to the arousal rate (of increase, measured in units of inverse time -recall the probability is dimensionless and has no units) times dt (which has units of time). When t is much smaller than T, there are no other relevant time scales and therefore the scale of the arousal rate is set by t itself with a dimensionless proportionality constant β: the arousal rate is given by β/t. It can be shown straightforwardly that this innocuous constant of proportionality determines the power law exponent of the dwell time distribution.
When β > 0, the probability of arousal out of the resting state ~β/t, decreases as a function of time t elapsed after the dwell time commenced. The ubiquitous scale-free dynamics is highly suggestive of critical-like behavior of the brain.
4.2. Criticality
How and why might the brain ‘tune itself to be poised in the vicinity of a critical point? What are the advantages of criticality to a GA system poised to activate behavioral responses to environmental stimuli? Self-organized criticality (SOC) is an influential idea introduced by Bak, Tang and Weisenfeld to provide a general explanation for how natural systems self-tune to be in the vicinity of a critical point (Bak et al., 1987; Bak, 1996)‥ The earliest example of SOC was a sandpile model: pebbles of sand are dropped continually on the squares of a large chessboard - the system is driven. When the number of pebbles on a square exceeds a threshold value, the pebbles topple over into the neighboring sites. Dissipation occurs at the boundary of the chessboard, when the pebbles drop out of the board. Generically, the repeated application of these dynamical rules tunes the system to a near critical state - the system has a wide variety of possible responses on adding an additional grain. This is important for a GA system that alerts the greater part of the brain to a need for action. For GA to work in a biologically adaptive fashion, neither ultra-stability nor sheer instability is ideal. The biological need is for a system poised at the boundary between stability and instability and thus in the vicinity of criticality. The dynamic of the system drives it towards the critical point.
A powerful approach, quite distinct from SOC, justifies the critical behavior of the brain in terms of its evolutionary advantage(Shew et al, 2011; Chialvo and Dante, 2006, 2010). Here the self-tuning arises because there are advantages for a functional system to be in the vicinity of a critical point and evolution drives the system to the vicinity of a critical point. An organism strives to survive and thrives in a constantly changing environment. In contrast to inanimate matter, organisms adapt, learn, and evolve through the key factors of inheritance, mutations, and selection. A key ingredient to their success is their ability to capture relevant information from the richly varying external world, synthesizing its most prominent features into manageable maps.
The brain is continually exposed to richly varying environments. The information represented in a given environment can be represented by a humongous array of bits each taking on a value of 1 or 0. As a function of time, the environmental snapshots change, often in a correlated manner and occasionally in a disconnected way. The brain needs to encapsulate the essential features of this information in an efficient and compact manner - the number of available bits associated with the brain, albeit very large, dwarfs in comparison with the bits encoding the environmental information. An efficient mapping would confer a functional advantage to an organism in being able to be responsive to new stimuli with deftness. The brain is an emergent entity of connected neurons. How is the efficient mapping maintained? If the range of environmental stimuli is narrow and monotonous, the linkages between the neurons can be adjusted just right in order to capture the environment with fidelity. And the brain does not need to be critical. On the contrary, an evolved and inherited brain can best capture an incredible range of stimuli by being tuned to be poised near criticality. This self-tuning arises as a consequence of evolution and adaptation.
In this sense, the observed criticality in the regulation of GA (see Box 1) can be understood as resulting from the need for having accurate “mappings” of the world, the need to cope with many widely diverse conditions, and the well-honed ability of the brain to react to external changes in an optimal way. Referring to the idea of accurate “mappings” we quote Hidalgo et al., “Smart cartography drives a brain to criticality” (Hidalgo et al, 2014).
Box 1: Primer on critical phenomena in physical systems and their implications for CNS.
There are key differences between criticality that arises from the fine-tuning of physical parameters and the apparent self-tuning of a brain poised in the vicinity of a critical point.
Consider, for example, a liquid-vapor system. Under normal atmospheric pressure, water in a kettle boils if you increase its temperature to 100°C. On continuing to increase the pressure, one reaches a special critical pressure at which the “boiling” occurs at a critical temperature and is characterized by vanishing density difference between the liquid and vapor phases. At this critical point, the distinction between the liquid and vapor phases has all but disappeared. One can identify local liquid-like higher density droplets and vapor-like lower density bubbles in the system at its critical point.
This phenomenon called scale invariance leads to several striking consequences in physical systems. The first is universality - even though the elementary constituents of a system can vary, criticality and scale invariance emerge as the collective behavior of a many-body system with characteristics depending only on just a few essential attributes such as the dimensionality of the system and symmetries of the problem. The underlying details such as the chemistry of the liquid are irrelevant in determining the critical behavior. In contrast, there is no evidence of universal exponents associated with the criticality of the brain.
A second consequence of scale invariance is that the dynamics of a system at criticality is characterized by a plethora of time scales - there is no single predictable time scale. Scale-free dynamics offer several fundamental advantages in the context of arousal. The ability to balance conflicting demands such as the need for rest along with the ability to readily respond to salient stimuli with efficient coding of information are all simultaneously fulfilled by scale-free dynamics.
A third important consequence is the presence of slowly decaying correlations between different parts of the system as a power law of the distance between them. (Note that the notion of distance is somewhat ill defined in the brain because of possible long range interactions between neurons.) Changes in one part of the system are felt a long distance away. This results in the system at a critical point being exquisitely sensitive to external perturbations of the right sort. In CNS, criticality leads to exquisite sensitivity to the environment, an attribute invaluable to arousal.
A prediction of this theoretical framework is that a brain exposed to or trained with relatively few kinds of stimuli ought not to be as responsive to a new kind of stimulus as a brain exposed to or trained with many more stimuli (“enriched environments”). GA will be too low. Another prediction is that a brain very high in GA, deeply enmeshed in an activity, is likely to be farther from criticality than one that is not. Thus the strength of an external stimulus needed to distract such a brain would be higher than that for an unfocussed brain, a brain not already engaged in a defined activity.
Thus, even as a careful consideration of GA and its scale-free properties leads to rigorously quantitative thinking, the quantitative approach allows prediction for neurobiological experiments.
5. Summary
The new work reviewed in this paper signifies currently increased levels of attention to global CNS states of medical importance, such as disorders of consciousness (Schiff, 2013; Laureys and Schiff, 2012; Di Perri et al., 2014). In particular, this area of work, generalized CNS arousal, benefits from the genetics (de Lecea et al., 2012) and epigenetics (Maze et al., 2014; Hunter et al., 2014; Kundakovic et al, 2014) that were not available when the early neurophysiological work on GA, cited at the top of this article, was initiated.
The review cites multiple studies that serve as independent lines of evidence to show that an elementary function like GA does in fact exist. For example, one can breed for GA, and one would not be able to breed for a function that does not exist. Likewise, one cannot have mechanisms for a function that does not exist. Thus, the fact that we can breed for GA and know many mechanisms for GA implies that GA does really exist. Notice that we have extrapolated the operational definition of GA from the cited studies in order to accomplish a unified theory.
It is striking that the behavioral and physiological dynamics of GA lead naturally to thinking based in physics. Scaling phenomena referred to in the last part of the review explain how GA systems in the brain could permit the animal to quickly cross the phase transition from inactive to active.
Further, we note the opportunity to bring this interesting area of neuroscience, the science of arousal systems, to a level of precision that can be treated with the techniques of applied mathematics. Thus, we (Pfaff and Banavar, 2007) speculated that the passage of the brain from the not-aroused to aroused states would have the quantitative characteristics of a phase transition. Recently Dehaene et al. (2014), likewise, have suggested that “conscious perception may be likened to a non-linear decision that ‘ignites’ a network of distributed areas”.
Clearly, following a half-century of neuroscientific concentration on specific signaling in sensory and motor systems, the new emphasis on broader behavioral dispositions such as GA will deserve increased attention.
Key points.
Generalized CNS arousal (GA) is the most elementary function of vertebrate nervous systems. It is a non-specific neuronal ‘force’ that activates ascending and descending systems, facilitating the initiation of any behavior responding to external stimulation and emotional expression
Several components of the nervous system such as the medullary reticular formation, thalamus and cortex contribute to underlie GA. These areas have been analyzed, including neuroanatomical pathways, electrophysiological features and some of the most important genes whose expression supports GA.
Metastable activity transitions are necessary when the brain passes from an induced-unconscious state to a state of responsiveness - - the structure of these transitions has been mathematically defined by physical scientists, neurologists and anesthesiologists.
Acknowledgments
This work was supported by NIH grant: R01 NS094655-01 awarded to D.P.C
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, DE Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert FW, Shchepina O, Winter C, Rompler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, Thiery J, Morgenstern R, Plyusnina IZ, Schoneberg T, Paabo S. Phenotypic differences in behavior: physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm. Behav. 2008;53:413–421. doi: 10.1016/j.yhbeh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Albert FW, Carlborg Ö, Plyusnina I, Besnier F, Hedwig D, Lautenschläger S, Lorenz D, Mcintosh J, Neumann C, Richter H. Genetic architecture of tameness in a rat model of animal domestication. Genetics. 2009;182:541–554. doi: 10.1534/genetics.109.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso LM, Proekt A, Schwartz TH, Pryor KO, Cecchi GA, Magnasco MO. Dynamical criticality during induction of anesthesia in human ECoG recordings. Front. Neural Circuits. 2014;8:20. doi: 10.3389/fncir.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Bak P, Tang C, Wiesenfeld K. Self-organized criticality: an explanation of the 1/f noise. Phys. Rev. Lett. 1987;59:381–384. doi: 10.1103/PhysRevLett.59.381. [DOI] [PubMed] [Google Scholar]
- Bak P. How Nature Works: The Science of Self-Organised Criticality. New York, NY: Copernicus Press; 1996. [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003;23:11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev DK. Domestication of animals. Sci. J. 1969;5:47–52. [Google Scholar]
- Boly M, Seth AK. Modes and models in disorders of consciousness science. Arch. Ital. Biol. 2012;150:172–184. doi: 10.4449/aib.v150i2.1372. [DOI] [PubMed] [Google Scholar]
- Botcharova M, Farmer SF, Berthouze L. Markers of criticality in phase synchronization. Front. Syst. Neurosci. 2014;8:176. doi: 10.3389/fnsys.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O. Descending command neurons in the brainstem that halt locomotion. Cell. 2015;163:1191–1203. doi: 10.1016/j.cell.2015.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretzner F, Brownstone RM. Lhx3-Chx10 reticulospinal neurons in locomotor circuits. J. Neurosci. 2013;33:14681–14692. doi: 10.1523/JNEUROSCI.5231-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, DE Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2635–E2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chialvo Dante R. Psychophysics: Are our senses critical? Nat. Phys. 2006;2(5):301–302. [Google Scholar]
- Chialvo Dante R. Emergent complex neural dynamics. Nat. Phys. 2010;6(10):744–750. [Google Scholar]
- Dehaene S, Charles L, King JR, Marti S. Toward a computational theory of conscious processing. Curr. Opin. Neurobiol. 2014;25:76–84. doi: 10.1016/j.conb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EW, Morison RS. The production of rhythmically recurrent cortical potentials after localized thalamic stimulation. Am. J. Physiol. Legacy Content. 1941;135:293–300. [Google Scholar]
- Di Perri C, Thibaut A, Heine L, Soddu A, Demertzi A, Laureys S. Measuring consciousness in coma and related states. World J. Radiol. 2014;6:589–597. doi: 10.4329/wjr.v6.i8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl-Eibesfeldt I. Ethol. Biol. Behav. 1970 [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Morgan M, Ogawa S, Burton L, Pfaff D. Statistical analysis of measures of arousal in ovariectomized female mice. Horm. Behav. 2001;39:39–47. doi: 10.1006/hbeh.2000.1632. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Morgan M, Ogawa S, Burton L, Pfaff D. Statistical analysis of hormonal influences on arousal measures in ovariectomized female mice. Horm. Behav. 2002;42:414–423. doi: 10.1006/hbeh.2002.1832. [DOI] [PubMed] [Google Scholar]
- GU S, Pasqualetti F, Cieslak M, Grafton ST, Bassett DS. Control. Brain Networks. 2014. p. 5197. (arXiv preprint arXiv:1406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey J, Morgan MA, Frohlich J, Mcewen BS, Pfaff DW. Effects of the phytoestrogen coumestrol on locomotor and fear-related behaviors in female mice. Horm. Behav. 2001;40:65–76. doi: 10.1006/hbeh.2001.1660. [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Korach KS, Ogawa S, Pfaff DW. Genetic contributions to generalized arousal of brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nature Rev. Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Gilmartin GS, Thomas RJ. Mechanisms of arousal from sleep and their consequences. Curr. Opin. Pulm. Med. 2004;10:468–474. doi: 10.1097/01.mcp.0000143690.94442.b3. [DOI] [PubMed] [Google Scholar]
- Gollisch T. Features and functions of nonlinear spatial integration by retinal ganglion cells. J. Physiol. Paris. 2013;107:338–348. doi: 10.1016/j.jphysparis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Haimovici A, Tagliazucchi E, Balenzuela P, Chialvo DR. Brain organization into resting state networks emerges at criticality on a model of the human connectome. Phys. Rev. Lett. 2013;110:178101. doi: 10.1103/PhysRevLett.110.178101. [DOI] [PubMed] [Google Scholar]
- Harlow H. Biological and Biochemical Bases of Behavior. Harlow. Madison: University of Wisconsin press; 1958. [Google Scholar]
- Hebb DO. Drives and the C.N.S. (conceptual nervous system) Psychol. Rev. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Heyne HO, Lautenschläger S, Nelson R, Besnier F, Rotival M, Cagan A, Kozhemyakina R, Plyusnina IZ, Trut L, Carlborg Ö. Genetic influences on brain gene expression in rats selected for tameness and aggression. Genetics. 2014;198:1277–1290. doi: 10.1534/genetics.114.168948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J, Grilli J, Suweis S, Munoz MA, Banavar JR, Maritan A. Information-based fitness and the emergence of criticality in living systems. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10095–10100. doi: 10.1073/pnas.1319166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Calderon D, Pfaff D, Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Gagnidze K, Mcewen BS, Pfaff DW. Stress and the dynamic genome: steroids, epigenetics, and the transposome. Proc. Natl. Acad. Sci. 2014:201411260. doi: 10.1073/pnas.1411260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Fulbright RK, Shulman RG, Rothman DL. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J. Cereb. Blood Flow Metab. 2013;33:339–347. doi: 10.1038/jcbfm.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res. Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front. Biosci. J. Virtual Lib. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. 5th. Philadelphia, PA: Saunders/Elsevier; 2011. [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. 2014:201408355. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Leontovich TA, Zhukova GP. The specificity of the neuronal structure and topography of the reticular formation in the brain and spinal cord of carnivora. J. Comp. Neurol. 1963;121:347–379. doi: 10.1002/cne.901210305. [DOI] [PubMed] [Google Scholar]
- Lindzey G, Thiessen DD. Contributions to Behavior-genetic Analysis: The Mouse as a Prototype. Ardent Media. 1970 [Google Scholar]
- Lorenz K. Studies in Animal and Human Behaviour. Cambridge MA: Harvard University Press; 1971. [Google Scholar]
- Magnasco MO, Piro O, Cecchi GA. Self-tuned critical anti-Hebbian networks. Phys. Rev. Lett. 2009;102:258102. doi: 10.1103/PhysRevLett.102.258102. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pavlides C, Pfaff D. Multimodal sensory responses of nucleus reticularis gigantocellularis and the responses’ relation to cortical and motor activation. J. Neurophysiol. 2010;103:2326–2338. doi: 10.1152/jn.01122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Devidze N, Shelley DN, Westberg L, Fontaine C, Pfaff DW. Molecular and neuroanatomical characterization of single neurons in the mouse medullary gigantocellular reticular nucleus. J. Comp. Neurol. 2011;519:2574–2593. doi: 10.1002/cne.22639. [DOI] [PubMed] [Google Scholar]
- Maye A, Hsieh CH, Sugihara G, Brembs B. Order in spontaneous behavior. PLoS One. 2007;2:e443. doi: 10.1371/journal.pone.0000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Noh KM, Soshnev AA, Allis CD. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 2014;15:259–271. doi: 10.1038/nrg3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Pfaff D. Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan CT, Stellar Eliot . Physiological Psychology. New York, NY: McGraw-Hill; 1943. [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Nakamura T, Kiyono K, Yoshiuchi K, Nakahara R, Struzik ZR, Yamamoto Y. Universal scaling law in human behavioral organization. Phys. Rev. Lett. 2007;99:138103. doi: 10.1103/PhysRevLett.99.138103. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takumi T, Takano A, Aoyagi N, Yoshiuchi K, Struzik ZR, Yamamoto Y. Of mice and men-universality and breakdown of behavioral organization. PLoS One. 2008;3:e2050. doi: 10.1371/journal.pone.0002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki H, Kusunose R, Nakaoka H, Nishiura A, Miyake T, Sasaki Y. Estimation of heritability and genetic correlation for behavioural responses by Gibbs sampling in the Thorough bred race horse. J. Anim. Breed. Genet. 2007;124:185–191. doi: 10.1111/j.1439-0388.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons: experimental brain research. Exp. Brain. Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu. Rev. Physiol. 1979;41:127–140. doi: 10.1146/annurev.ph.41.030179.001015. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Banavar JR. A theoretical framework for CNS arousal. BioEssays. 2007;29:803–810. doi: 10.1002/bies.20611. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Martin EM, Faber D. Origins of arousal: roles for medullary reticular neurons. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge Mass: Harvard University Press; 2006. [Google Scholar]
- Picchioni D, Duyn JH, Horovitz SG. Sleep and the functional connectome. Neuroimage. 2013;80:387–396. doi: 10.1016/j.neuroimage.2013.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Banavar JR, Maritan A, Pfaff DW. Scale invariance in the dynamics of spontaneous behavior. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10564–10569. doi: 10.1073/pnas.1206894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Remmington NA, Fabrigar LR, Visser PS. Reexamining the circumplex model of affect. J. Pers. Soc. Psychol. 2000;79:286–300. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AM, Frye MA. Free-flight odor tracking in Drosophila is consistent with an optimal intermittent scale-free search. PLoS One. 2007;2:e354. doi: 10.1371/journal.pone.0000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. J. Person. Soc. Psychol. 1980;39:16. [Google Scholar]
- Scheibel MSA, et al. Reticular Formation of the Brain. Boston, H. Jasper: Little, Brown and Co; 1958. [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic deep brain stimulation for support of forebrain arousal regulation in the minimally conscious state. Handb. Clin. Neurol. 2013;116:295–306. doi: 10.1016/B978-0-444-53497-2.00024-3. [DOI] [PubMed] [Google Scholar]
- Scott JPAF. Genetics and the Social Behavior of the Dog. Chicago, Ill: University of Chicago Press; 1965. [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, DE Lecea L, Ledoux JE. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20260–20265. doi: 10.1073/pnas.1320325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew WL, Yang H, Yu S, Roy R, Plenz D. Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J. Neurosci. 2011;31:55–63. doi: 10.1523/JNEUROSCI.4637-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew WL, Clawson WP, Pobst J, Karimipanah Y, Wright NC, Wessel R. Adaptation to sensory input tunes visual cortex to criticality. Nat. Phys. 2015 [Google Scholar]
- Solovey G, Miller KJ, Ojemann JG, Magnasco MO, Cecchi GA. Self-Regulated dynamical criticality in human ECoG. Front. Integr. Neurosci. 2012;6:44. doi: 10.3389/fnint.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey G, Alonso LM, Yanagawa T, Fujii N, Magnasco MO, Cecchi GA, Proekt A. Loss of consciousness is associated with stabilization of cortical activity. J. Neurosci. 2015;35:10866–10877. doi: 10.1523/JNEUROSCI.4895-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady TC, Ostrander EA. Canine behavioral genetics: pointing out the phenotypes and herding up the genes. Am. J. Hum. Genet. 2008;82:10–18. doi: 10.1016/j.ajhg.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat. Rev. Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Front. Physiol. 2012;3:15. doi: 10.3389/fphys.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann O, Shapiro B, Cui P, Schuenemann VJ, Sawyer SK, Greenfield DL, Germonpre MB, Sablin MV, Lopez-Giraldez F, Domingo-Roura X, Napierala H, Uerpmann HP, Loponte DM, Acosta AA, Giemsch L, Schmitz RW, Worthington B, Buikstra JE, Druzhkova A, Graphodatsky AS, Ovodov ND, Wahlberg N, Freedman AH, Schweizer RM, Koepfli KP, Leonard JA, Meyer M, Krause J, Paabo S, Green RE, Wayne RK. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science. 2013;342:871–874. doi: 10.1126/science.1243650. [DOI] [PubMed] [Google Scholar]
- Thompson RF. Foundations of physiological psychology. New York, NY: Harper and Row; 1967. [Google Scholar]
- Tkacik G, Mora T, Marre O, Amodei D, Palmer SE, Berry MJ, Bialek W. Thermodynamics and signatures of criticality in a network of neurons. Proc. Natl. Acad. Sci. 2015;112:11508–11513. doi: 10.1073/pnas.1514188112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacik G, Marre O, Amodei D, Schneidman E, Bialek W, 2nd, Berry MJ. Searching for collective behavior in a large network of sensory neurons. PLoS Comput. Biol. 2014;10:e1003408. doi: 10.1371/journal.pcbi.1003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. TJ. Neurosci. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Reticular formation of the pons and medulla oblongata. A Golgi study. J. Comp. Neurol. 1961;116:71–99. doi: 10.1002/cne.901160105. [DOI] [PubMed] [Google Scholar]
- Valverde F. Reticular formation of the albino rat’s brain stem cytoarchitecture and corticofugal connections. J. Comp. Neurol. 1962;119:25–53. doi: 10.1002/cne.901190105. [DOI] [PubMed] [Google Scholar]
- Van Hateren JH, Ruttiger L, Sun H, Lee BB. Processing of natural temporal stimuli by macaque retinal ganglion cells. J. Neurosci. 2002;22:9945–9960. doi: 10.1523/JNEUROSCI.22-22-09945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, Fuller PM, Tong Q, Lu J. Medullary circuitry regulating rapid eye movement sleep and motor atonia. J. Neurosci. 2009;29:9361–9369. doi: 10.1523/JNEUROSCI.0737-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Zhang Q, Hornung A, Blizard D, Pfaff DW. Impact of generalized brain arousal on sexual behavior. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2265–2270. doi: 10.1073/pnas.0914014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front. pharmacol. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Carter ME, Adamantidis A. Shining light on wakefulness and arousal. Biol. Psychiatry. 2012;71:1046–1052. doi: 10.1016/j.biopsych.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]