Abstract
Background
The incidence of opioid-related death in women has increased five-fold over the past decade. For many women, their initial opioid exposure will occur in the setting of routine medical care. Approximately 1 in 3 deliveries in the U.S. is by Cesarean and opioids are commonly prescribed for post-surgical pain management.
Objective
The objective of this study was to determine the risk that opioid naïve women prescribed opioids after Cesarean delivery will subsequently become consistent prescription opioid users in the year following delivery, and to identify predictors for this behavior.
Study Design
We identified women in a database of commercial insurance beneficiaries who underwent Cesarean delivery and who were opioid-naïve in the year prior to delivery. To identify persistent users of opioids, we used trajectory models, which group together patients with similar patterns of medication filling during follow-up, based on patterns of opioid dispensing in the year following Cesarean delivery. We then constructed a multivariable logistic regression model to identify independent risk factors for membership in the persistent user group.
Results
285 of 80,127 (0.36%, 95% confidence interval 0.32 to 0.40), opioid-naïve women became persistent opioid users (identified using trajectory models based on monthly patterns of opioid dispensing) following Cesarean delivery. Demographics and baseline comorbidity predicted such use with moderate discrimination (c statistic = 0.73). Significant predictors included a history of cocaine abuse (risk 7.41%; adjusted odds ratio 6.11, 95% confidence interval 1.03 to 36.31) and other illicit substance abuse (2.36%; adjusted odds ratio 2.78, 95% confidence interval 1.12 to 6.91), tobacco use (1.45%; adjusted odds ratio 3.04, 95% confidence interval 2.03 to 4.55), back pain (0.69%; adjusted odds ratio 1.74, 95% confidence interval 1.33 to 2.29), migraines (0.91%; adjusted odds ratio 2.14, 95% confidence interval 1.58 to 2.90), antidepressant use (1.34%; adjusted odds ratio 3.19, 95% confidence interval 2.41 to 4.23) and benzodiazepine use (1.99%; adjusted odds ratio 3.72, 95% confidence interval 2.64 to 5.26) in the year prior to Cesarean delivery.
Conclusions
A very small proportion of opioid-naïve women (approximately 1 in 300) become persistent prescription opioid users following Cesarean delivery. Pre-existing psychiatric comorbidity, certain pain conditions, and substance use/abuse conditions identifiable at the time of initial opioid prescribing were predictors of persistent use.
Keywords: opioids, Cesarean delivery, pregnancy, pain, cohort studies
INTRODUCTION
The rapidly escalating increase in the incidence of opioid medication overdose among women in the United States has received much recent attention from the U.S. Centers for Disease Control and Prevention (CDC) and other leading health care organizations.(1, 2) From 1999 to 2010, the rate of death from this cause increased five-fold among women. Opioid-related emergency room visits also increased substantially over this time period. These risks were particularly high among women of reproductive age.
While for some women the initial exposure to opioids comes from illicit purchase and use, for many others it occurs in the course of routine medical care. A common medical event in such patients is Cesarean delivery. Approximately 1.3 million women undergo Cesarean delivery each year in the U.S.,(3) making it the most common inpatient surgical procedure(4), as it is in many other developed countries. Women who undergo this procedure typically have postoperative pain(5) and are frequently discharged with prescriptions for opioid analgesics. Such prescriptions therefore represent a common source of exposure to opioids in young women, many of whom may have never used these drugs previously.
Given concerns about prescription opioid use and abuse in women of reproductive age, we sought to determine the risk that opioid-naïve women prescribed these medications after Cesarean delivery will subsequently become persistent users of prescription opioid medications and to identify potentially actionable predictors of this outcome.
METHODS
Data source
Study data were derived from the Clinformatics Data Mart, a database of health care utilization drawn from the transactions of the nationwide commercial U.S. health insurer UnitedHealthcare, for the years 2003 to 2011. The database contains transactional data on reimbursement for outpatient pharmacy dispensings, inpatient and outpatient services, and procedures and associated diagnoses (recorded using ICD-9 CM codes). Only those beneficiaries with both medical and prescription drug coverage are included in the database. Claims submitted for payment by providers and pharmacies are verified, adjudicated, adjusted, and de-identified prior to inclusion in the database. The use of this de-identified database for research was approved by the Institutional Review Board of the Partners Healthcare System (Boston, MA, United States of America).
Cohort
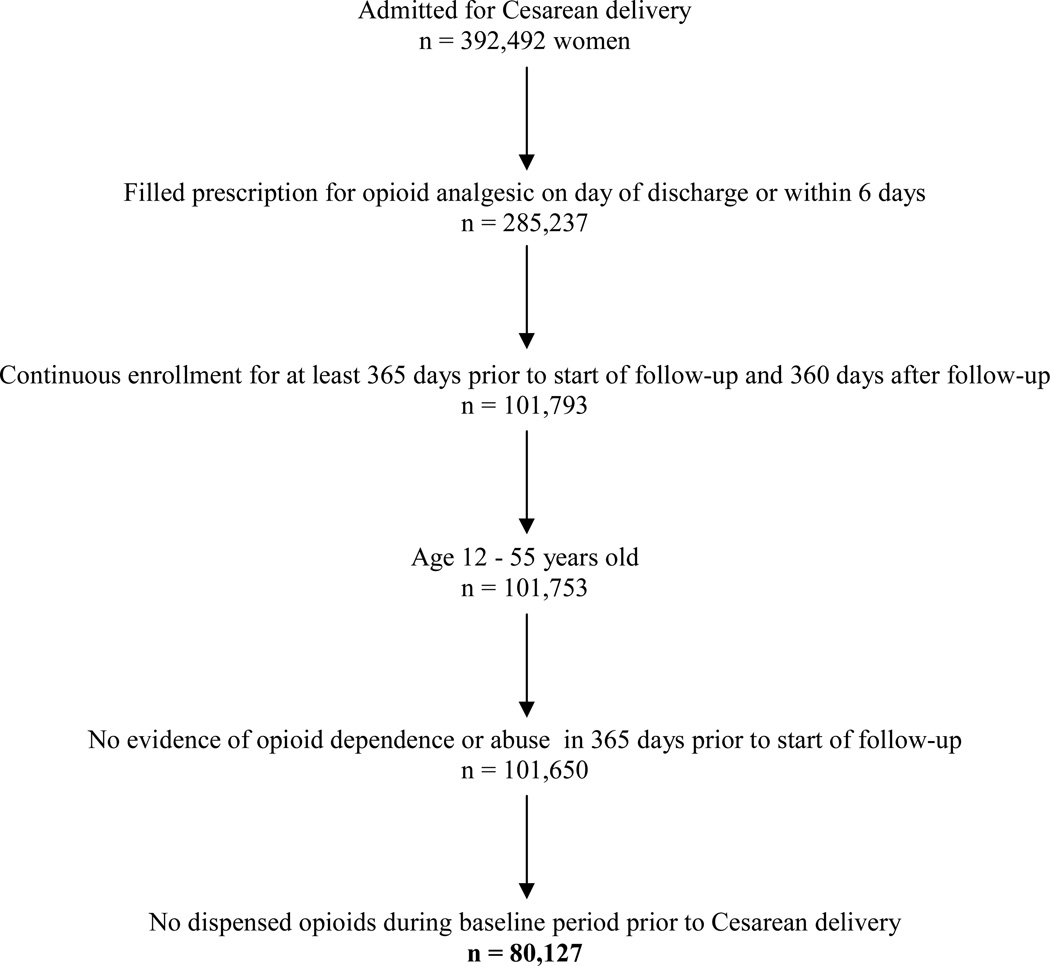
We included all women who underwent a Cesarean delivery identified by the procedure codes 74.0, 74.1, 74.2, 74.4, and 74.99 from the ICD-9 (International Classification of Diseases, 9th edition) and who filled a prescription for an opioid medication on the day of hospital discharge or within six days thereafter. This was done based on the assumption that a filled prescription during this window would likely be to treat acute postoperative pain related to the Cesarean delivery. Opioids considered in the analysis included hydrocodone, oxycodone, codeine, meperidine, hydromorphone, morphine, fentanyl, methadone, and oxymorphone. Follow-up began on the seventh day following discharge from the delivery hospitalization. To allow for adequate ascertainment of baseline characteristics and assessment of persistent post-delivery opioid use, the analysis was restricted to patients with continuous enrollment with the insurer for at least 365 days prior to and 360 days after the beginning of follow-up. We further restricted our cohort to women age 12 to 55 years at the time of delivery. As the focus of the study was on the risk of persistent opioid use after initial exposure to opioids following Cesarean delivery, we excluded women who had filled outpatient prescriptions for opioids during the baseline period prior to delivery or who had diagnoses in the baseline period indicating opioid abuse or dependence (although it is possible that the woman may have used opioids prior to the baseline period). If a woman met the inclusion criteria for more than one delivery, only the first delivery was considered in the analysis. The final cohort consisted of 80,127 women (Figure 1).
Figure 1.
Cohort selection.
In order to identify persistent users of opioids after Cesarean delivery, we used trajectory models, which group together patients with similar patterns of medication filling during follow-up. The trajectory model approach has previously been applied to the evaluation of medication adherence.(6)
To define the trajectory groups for opioid use in our cohort, we first determined whether a woman filled a prescription for an opioid medication during each of 12 consecutive 30-day periods of follow-up. Based on this definition, we then fit a group-based trajectory model to define five distinct groups of opioid use(7, 8) In this model, the log odds of filling an opioid during each 30-day period was estimated as a third-order polynomial of time separately within each group, as in prior evaluations of 12-month trajectories.(6) The model also estimated the probability of group membership for each patient; patients were assigned to the group with the highest membership probability.
Based on this model, we defined the group of patients with the highest probability of filling over time as “persistent users.” The other four trajectory groups were considered “non-persistent users”. The model was estimated using “Proc Traj,” in SAS (SAS, Version 9.2, Cary, NC). A five-group model was chosen based on a combination of the Bayesian Information Criterion and clinical interpretability of resulting trajectory groups; models with other numbers of groups were also considered.
Trajectory models make it possible to use the observed data to define distinct filling patterns of opioids in our cohort during the year after delivery, and classify patients into groups with similar medication use histories. It is one approach to identifying distinct patterns of opioid use over time that does not rely on a priori and potentially arbitrary definitions of what constitutes high levels of opioid use. The trajectory model curves that are generated show the estimated proportion of each group that filled an opioid prescription during each month during the 12-month follow-up period.
Predictors of persistent opioid use
We sought to define predictors of persistent use that could be identified at the time of the initial opioid prescription based upon diagnosis/procedure codes or medication use during the baseline period, defined as in the 365 days prior to initiation of follow-up. Potential predictors included maternal age, year of delivery, delivery type, illicit substance use/abuse and tobacco use, pain conditions, psychotropic medication use, and characteristics of the initial opioid prescription. Maternal age at delivery was categorized as <20, 20–29, 30–39, and ≥40 years. Delivery type was classified as primary or repeat Cesarean. Substance abuse or dependence was assessed for marijuana, cocaine, other or unspecified drugs/medications (including anxiolytic, stimulant, or hallucinogenic drugs), alcohol, and tobacco based on recorded ICD-9 CM codes. Pain conditions assessed included back pain, fibromyalgia, and migraine or other headache syndromes, again using ICD-9 CM codes. Filled prescriptions in the baseline period were identified for benzodiazepines, antidepressants, antipsychotics, and central nervous system stimulants. We used prescription information rather than diagnostic codes to identify psychiatric conditions on the assumption that this approach is likely to define the presence of significant psychiatric comorbidity with greater specificity.
Finally, we assessed characteristics of the initial filled outpatient opioid prescription (which occurred following discharge from the Cesarean delivery and prior to the start of follow-up) including the class of opioid (divided into oxycodone, hydrocodone, or other), number of days supply, and oral morphine equivalent daily dose (mg). Days supply was divided into tertiles of <4 days, 4–5 days, and ≥6 days. Morphine equivalent daily dose for the initial prescription was also determined, based on potency(9) and the assumption that the medication was taken regularly based on the days supply and quantity dispensed. Daily dose was divided into tertiles for the analysis (corresponding to thresholds of <81 mg, 81 mg to 112.5 mg and >112.5 mg of oral morphine equivalent).
Univariate odds ratios (OR) and 95% confidence intervals (CI) describing the association of these predictors with membership in the persistent-user group were determined. We then constructed a multivariable logistic regression model predicting membership in the persistent user group. All potential predictors were forced into the model without selection and adjusted OR and 95% CI determined. The c-statistic, representing the model’s discrimination, was calculated. We then used 10 bootstrap samples to measure prediction accuracy as applied to a new set of patients not used for estimation.(10)
We also determined the frequency of potential indications for chronic opioid use in the persistent opioid-user group based on inpatient and outpatient diagnosis codes during the follow-up period.
Alternative approaches to defining the amount of opioid initially dispensed
We examined two alternative ways of assessing characteristics of the initial prescription that might be associated with persistent use: the total number of opioid pills dispensed and the total number of oral morphine milligram equivalents dispensed. The total number of pills dispensed was divided into groups of <30, 30 to 39, and ≥40. The total number of oral morphine equivalents initially dispensed was divided into tertiles of <330 mg, 330 to 400 mg, and >400 mg morphine equivalents. Each of these characteristics was separately included in a model predicting membership in the persistent user group along with all of the covariates included in the primary analysis (excluding the characteristics of the initial prescription as defined in the primary analysis).
Comparative frequency of onset of persistent opioid use
To understand how Cesarean delivery and post-delivery opioid exposure contributed to the risk of persistent opioid use beyond the background rate at which similar women become persistent opioid users, we defined a comparator group of women who delivered vaginally who met the same eligibility criteria as patients in our Cesarean cohort (i.e, opioid-naive for the year prior to delivery, 12 months of follow-up) and who did not fill an opioid within seven days following discharge from the delivery. This comparator group was designed to capture the baseline risk of chronic opioid use during the follow-up period in a population with characteristics similar to our Cesarean delivery cohort (i.e. women who have recently given birth). Unlike Cesarean delivery in which opioids are frequently dispensed to treat post-surgical pain, opioids are not routinely prescribed following vaginal delivery, which is typically not associated with severe pain in the post-delivery period. We compared the frequency of three different outcomes in women with Cesarean delivery who were dispensed an opioid and women with vaginal delivery who were not dispensed an opioid, (1) filling an opioid in ≥4 months of follow-up, (2) filling an opioid in ≥6 months of follow-up, and (3) filling an opioid in ≥8 months of follow-up. These endpoints were selected as it is not possible to directly compare the frequency of “persistent use” defined by the trajectory model, since the trajectory is dependent on the data. We calculated unadjusted ORs and 95% CI for the association between Cesarean delivery with an opioid dispensing and each of these outcomes versus those who delivered vaginally and were not dispensed an opioid. We then used logistic regression models to determine the association with each of the outcomes adjusted for age, prior Cesarean delivery, substance use and abuse conditions, pain conditions, and psychiatric medications (as shown in Table 1).
Table 1.
Distribution of patient characteristics and initial opioid prescription characteristics in persistent users and non-persistent users of opioids following Cesarean delivery. Shown as N (%).
| Persistent users (n = 285) |
Non-persistent users (n = 79,842) |
|
|---|---|---|
| Patient characteristics | ||
| Age at delivery (years) | ||
| <20 | 6 (2.1) | 1352 (1.7) |
| 20–29 | 113 (39.7) | 25545 (32) |
| 30–39 | 147 (51.6) | 45819 (57.4) |
| >=40 | 19 (6.7) | 7126 (8.9) |
| Repeat Cesarean delivery | 126 (44.2) | 33288 (41.7) |
| Substance use and abuse | ||
| Marijuana | 1 (0.4) | 72 (0.1) |
| Cocaine | 2 (0.7) | 25 (0) |
| Other substance abuse* | 6 (2.1) | 248 (0.3) |
| Tobacco use | 29 (10.2) | 1976 (2.5) |
| Alcohol abuse | 0 (0) | 150 (0.2) |
| Pain conditions | ||
| Fibromyalgia | 13 (4.6) | 1730 (2.2) |
| Migraines/other headache sydromes | 56 (19.7) | 6089 (7.6) |
| Back pain | 83 (29.1) | 12025 (15.1) |
| Psychiatric medications | ||
| Benzodiazepines | 49 (17.2) | 2408 (3) |
| Antidepressants | 85 (29.8) | 6275 (7.9) |
| Antipsychotics | 5 (1.8) | 161 (0.2) |
| Stimulants | 5 (1.8) | 410 (0.5) |
| Initial opioid prescription characteristics | ||
| Days supply (days) | ||
| ≤3 | 98 (34.4) | 28492 (35.7) |
| 4–5 | 126 (44.2) | 37500 (47.0) |
| ≥6 | 61 (21.4) | 13850 (17.4) |
| Daily dose tertile (milligrams of oral morphine equivalent) | ||
| <81 | 134 (47) | 36309 (45.5) |
| 81–112.5 | 53 (18.6) | 16791 (21.0) |
| >112.5 | 98 (34.4) | 26742 (33.5) |
| Type of opioid | ||
| Oxycodone | 181 (63.5) | 50066 (62.7) |
| Hydrocodone | 80 (28.1) | 24714 (31) |
| Other | 24 (8.4) | 5062 (6.3) |
Includes anxiolytic, stimulant, hallucinogenic drugs, or abuse of unspecified drugs/medications
RESULTS
Cohort
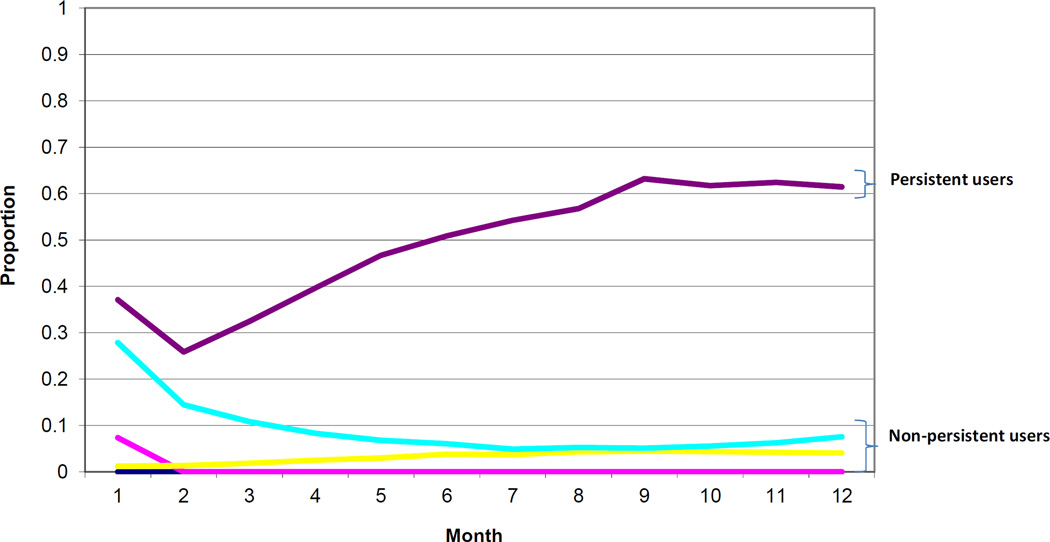
Our analytic cohort consisted of 80,127 women who underwent Cesarean delivery from 2003 to 2011 and who were opioid-naïve in the year prior to delivery (Figure 1). Patterns of opioid dispensing for each group estimated by the trajectory model in twelve 30-day periods of follow up are presented in Figure 2. For groups 1 to 3 (n = 76,557, 95.5% (95% confidence interval (CI) 95.4% to 95.7%) of cohort), fewer than 10% of the group members filled an opioid prescription in each month of the follow-up period, following the index prescription. For members of group 4 (n = 3285, 4.1% (95% CI 4.0 to 4.2) of cohort), the estimated proportion filling an opioid prescription was 28% in the first month of follow-up, but then decreased for each subsequent month such that by month 4 and thereafter the proportion dispensed an opioid was less than 10%. In Group 5 (n = 285, 0.36% (95% CI 0.32 to 0.40) of cohort), the persistent-user group, the estimated proportion filling an opioid prescription was 37% in the first month of follow-up and increased steadily during the follow-up such that at month 12, the proportion filling an opioid prescription was 61%. On average, members of the persistent user group were dispensed opioids in 6.1 (standard deviation 2.1) of the 12 follow-up months. All 285 patients filled an opioid during at least four of the follow-up months, 218 (76%) during at least five months, 146 (51%) during at least six months, and 59 (21%) during at least eight months; 229 (80.3%) filled an opioid during the final two months of follow-up. The filling patterns for patients in the persistent user group during each month of follow-up is shown in Supplementary Table 1 and the class of opioid medication filled one or more times by patients in the persistent user group during each month of follow-up is shown in Supplementary Table 2.
Figure 2.
Estimated average proportion in each trajectory group dispensed an opioid during each follow-up month.
To assess whether patients might have had more distant histories of opioid use or misuse we identified patients with 730 days of enrollment prior the start of follow-up and no dispensed opioid or codes indicating opioid abuse/dependence during this baseline period. In this case, the cohort size decreased to 37,623 of whom, 87 (0.23% (95% CI 0.19 to 0.28)) were in the persistent user trajectory group.
Predictors of persistent opioid use
The characteristics of the persistent user group were significantly different from those of the rest of the cohort (Table 1). Persistent users were younger and more likely to be previous users of illicit substances and tobacco. They had a higher frequency of pain conditions and/or symptoms diagnosed in the baseline period including back pain, fibromyalgia, and migraines. They were also more likely to have been users of psychotropic medications.
The multivariable logistic regression model predicting membership in the persistent user group identified maternal age of 20–29 years (compared to 30–39, adjusted odds ratio (aOR) 1.40, 95% confidence interval (CI) 1.09 to 1.80), use of cocaine (aOR 6.11, 95% CI 1.03 to 36.31) or other illicit substances (aOR 2.78, 95% CI 1.12 to 6.91), tobacco use (aOR 3.04, 95% CI 2.03 to 4.55), back pain (aOR 1.74, 95% CI 1.33 to 2.29), migraines (aOR 2.14, 95% CI 1.58 to 2.90), antidepressant use (aOR 3.19, 95% CI 2.41 to 4.23), and benzodiazepine use (aOR 3.72, 95% CI 2.64 to 5.26) as independent predictive factors. None of the characteristics of the initial opioid prescription assessed, including type of opioid dispensed, days supply or daily dose in morphine equivalents, were associated with persistent use. The c-statistic for the model was 0.73, indicating moderate discrimination. The average c-statistic across 10 bootstrapped samples was 0.67.
In sensitivity analyses examining additional characteristics of the initial prescription, neither the total number of opioid pills in the initial prescription nor the total number of oral morphine equivalents was associated with persistent use after adjusting for other covariates. Compared to patients whose initial opioid prescription was for <30 pills, those dispensed either 30 to 40 pills or >40 pills had a similar risk of persistent use (aOR 0.94, 95% CI 0.70 to 1.25 and aOR 1.12, 95% CI 0.80 to 1.57, respectively). Likewise, compared to those whose initial opioid prescription was for a total of <330 mg of morphine equivalents, those with a prescription for either 330 to 400 mg, and >400 mg of morphine equivalents were at comparable risk for persistent use (aOR 0.84, 95% CI 0.61 to 1.16 and 0.90, 95% CI 0.68 to 1.20, respectively).
While most patients in the persistent use group had a pain condition diagnosed in the follow-up period, only a small fraction had chronic medical conditions based on inpatient and outpatient diagnosis codes recorded during the follow-up period that would normally be considered strong indications for chronic opioid use; only 2.8% had cancer, 2.5% had rheumatoid arthritis, and 0.7% had chronic pancreatitis (Table 3). The proportions of these diagnoses among non-persistent users were generally lower.
Table 3.
Distribution during the follow-up period of possible indications for chronic opioid use in persistent users and non-persistent users of opioids following Cesarean delivery. Shown as N (%).
| Persistent users (n = 285) | Non-persistent users (n = 79,842) | |
|---|---|---|
| Malignancy | 8 (2.8) | 933 (1.2) |
| Rheumatoid arthritis | 7 (2.5) | 378 (0.5) |
| Chronic pancreatitis | 2 (0.7) | 27 (0.03) |
| Sickle cell disease | 0 (0) | 25 (0.03) |
| Migraine/other headache sydromes | 45 (15.8) | 2191 (2.7) |
| Back pain | 155 (54.4) | 12328 (15.4) |
| Fibromylagia | 40 (14.0) | 2156 (2.7) |
The frequency of filling opioids during four, six, and eight or more months during the follow-up period was substantially higher in women who underwent a Cesarean delivery and filled an opioid in the immediate post-delivery period compared to women who delivered vaginally and did not fill an opioid in the seven days following the delivery admission. After adjusting for differences in age, prior Cesarean delivery, substance use and abuse conditions, pain conditions, and psychiatric medications measured during the baseline period, women who delivered by Cesarean and were prescribed an opioid in the seven days following the delivery admission were at higher risk for chronic opioid use during the follow up period compared to women who delivered vaginally and were not dispensed an opioid in the immediate post-delivery period. The adjusted odds ratio (aOR) was 2.21 (95% CI 1.89 to 2.58) for filling an opioid prescription in ≥4 months, 2.68 (95% CI 2.02 to 3.55 for filling in ≥6 months, and 3.47 (95% CI 2.15 to 5.50) for filling in ≥8 months.
DISCUSSION
We found that opioid-naïve women who filled a prescription for an opioid analgesic after Cesarean delivery have a small risk – approximately 1 in 300 – of becoming persistent users of prescription opioids in the year following delivery. This frequency is relatively low, which should be reassuring to physicians and patients. However, as approximately 1.3 million women undergo Cesarean delivery in the United States (and many more worldwide) annually, the absolute number of persistent opioid use after Cesarean delivery is likely to be substantial. Our findings further suggest that women at high risk for this behavior are identifiable at the time that they initiate opioid therapy, based on evident characteristics and conditions. Physicians should be aware of the potential for persistent opioid use in this population.
With the rapid increase in opioid addiction and opioid-related deaths and other adverse outcomes observed in women in the United States,(1) there is a need to identify clinical situations in which opioid exposure commonly occurs and which can create risk for subsequent chronic use. With approximately 30 percent of all deliveries in the United States occurring by Cesarean(3) and the majority of women undergoing this operation being given opioids for acute pain, Cesarean delivery represents a common source of initial exposure to opioids in women of reproductive age. Our finding that 1 in 300 opioid-naïve women become persistent users in the year following delivery suggests that this may be a period of vulnerability, particularly for patients with certain predisposing conditions (e.g., psychiatric comorbidity, substance abuse disorders) with the potential to lead to chronic opioid use with all of its attendant risks.(11–15) In addition to the risks that are applicable to all populations, use of opioids in women of reproductive age also creates risks to the fetus when subsequent pregnancies are undertaken (including potentially increased risks for congenital malformations and neonatal abstinence syndrome).,(16)
Little has previously been known about predictors of persistent opioid use following Cesarean delivery. Our study identified younger age, certain types of illicit drug abuse, tobacco use, psychiatric comorbidity (as evidenced by filled prescriptions for psychotropic medications), and pain conditions as independent predictors of chronic opioid use in this previously opioid-naïve population. Some of these factors have been previously associated with prolonged opioid use following other surgeries(17) and/or regular prescription opioid use/abuse in other clinical contexts.(18–24) Clinicians prescribing opioids to post-Cesarean patients need to be mindful of these predictors and exercise greater caution in refilling opioid medications which may lead to persistent use, particularly in patients with characteristics associated with persistent use, such as those defined in this study (young age, history of other substance abuse, tobacco use, back pain, or headaches). Physicians and patients should cautiously evaluate whether a patient’s symptoms in the post-delivery period are best treated with opioid medications. Health systems and payers should work to eliminate barriers to the use of non-opioid approaches to the treatment of chronic pain conditions including alternative medications (e.g., triptans for migraines) and physical therapy.
While the characteristics of the initial opioid prescription filled after delivery were not predictors of persistent opioid use, there are other good reasons to limit the amount of opioid dispensed for acute pain following surgical procedures. Several recent studies have found that many patients use only a fraction of the amount of opioid prescribed after such procedures.(25, 26) Because pain following Cesarean delivery usually resolves quickly, patients may frequently have leftover medication. A recent population-based survey reported that 71% of patients with leftover opioids saved them(27); these medications can then be stolen or otherwise diverted for illicit use. Other pain medications, including non-steroidal anti-inflammatory medications and acetaminophen, lack the addictive potential of opioids and have a more favorable side-effect profile in many situations. The use of these medications could reduce the need for opioid use even more.
While the prescription of opioid medications following discharge after Cesarean delivery is common in the United States, this practice may not be routine in other countries. Therefore, future research should aim at better defining the risks and benefits associated with prescribing opioids following this common surgical procedure. Understanding the role for opioid use following vaginal delivery complicated by post-delivery pain is also needed. Research should also be directed at understanding whether the practice of rapidly discharging patients following delivery, when some patients may still be in pain, contributes to overuse of opioid medications in this population. Similarly, work is needed to understand whether the frequent prescribing of large amounts of opioids following delivery is driven by concerns about the need for readmission or use of the emergency room for uncontrolled pain.
Our study is subject to certain limitations inherent in its design. The dataset used for the study does not have information about whether patients report persistent pain associated with the Cesarean delivery or about the specific indications for the persistent opioid prescription use. It is not possible to validate the information included in this database using chart review. While a minority of patients had chronic medical conditions recorded in the follow-up period that would be considered strong indications for chronic opioid use (e.g., malignancy) we cannot definitively ascertain from these data the reasons for the prescriptions or whether the prescriptions were inappropriate. We also cannot determine the post-delivery stressors that might contribute to persistent opioid use, nor do our data capture information on socioeconomic factors that might be associated with this risk. The confidence intervals for some predictors of persistent use were wide (e.g., cocaine). It is also challenging with our data to determine the proportion of persistent use that is causally related to opioid exposure associated with Cesarean delivery. Our findings suggest that patients undergoing Cesarean delivery who are dispensed an opioid medication are more likely to use opioids heavily during the year of follow up than patients who deliver vaginally and who are not dispensed an opioid immediately after delivery (a comparator group designed to capture the baseline frequency of chronic opioid use in a similar population who were not exposed to opioids after delivery). While this association persisted even after adjusting for measured confounders, which points to exposure to opioids following caesarean as a precipitating event, we cannot exclude the possibility that this finding is driven by unmeasured confounding. It is also important to note that the absolute increase in risk is very small. A recent prospective study found that chronic pain attributable to Cesarean delivery at one-year follow-up is rare, affecting less than 1% of patients,(28) although other studies have reported higher rates;(29) some of the persistent use that we observe may be for this indication. Data from other surgical procedures suggest that most patients with chronic pain following surgery discontinue opioids, and that persistent use is more often tied to affective distress than chronic pain;(17) establishing whether this applies to opioid use after Cesarean delivery will be an important direction for future research. An additional limitation of our data source is that it does not include information on certain psychological measures such as depression or anxiety scales, or self-perceived risk of addiction, which may be important determinants of persistent use. Last, while we use trajectory models to identify persistent users, which offers several advantages, there are other methods that could have been used to summarize the amount of persistent use in the follow-up period.
Our data, while accurately capturing information on filled prescriptions for opioids, does not account for diversion of prescribed opioids. This may cause us to misestimate the proportion of women who appear to become persistent opioid users. Likewise, we required 12 months without opioid use before the Cesarean delivery, but we cannot account for opioid exposure prior to that period or for unrecognized illicit opioid use; it is possible that some women who became persistent opioid users were heavy opioid users at a point in time >1 year before the period examined. When we repeated the analyses restricted to those with 2 years of baseline period without opioid use, the frequency of persistent use decreased from 0.36% to 0.23%. While this may be due to imprecision in the estimate, it may also be attributable to changes in the characteristics of the cohort when we require 2 years of enrollment without opioid exposure or may point to a small fraction of the persistent user group actually representing patients who are resuming opioid therapy after discontinuing for pregnancy. The study design required a year of enrollment with the insurer following delivery in order to observe long-term patterns of opioid use. It is possible that women who become disabled due to persistent pain and/or opioid use would differentially disenroll from this commercial insurance plan. This may lead us to underestimate the number of persistent users of opioids following Cesarean delivery. Alternatively, if patients are less likely to disenroll because they require ongoing medical attention and prescriptions for opioids, it is possible that our estimates are somewhat biased in the opposite direction. It is also possible that requiring a full year of enrollment before and after the Cesarean delivery, while necessary to conduct the study, may somewhat limit the generalizability of the cohort. We also assume that the opioid prescription filled on the day of hospital discharge or within six days thereafter is for post-Cesarean pain; while this seems very reasonable, we cannot empirically confirm this assumption. It is also very important to note that our study examines the outcome of persistent opioid use and not opioid addiction. While previous studies have suggested that a substantial fraction of patients on chronic opioid therapy exhibit patterns of aberrant medication-taking behaviors,(30) we cannot determine whether patients in our cohort meet criteria for addiction and some women may be taking the medications for appropriate indications. Finally, we studied a commercially insured population from the U.S. In Medicaid patients, opioids may be used in a different manner following Cesarean delivery and our findings may not generalize to that population; this will be an important area for future study.
Conclusion
Approximately 1 in 300 opioid-naïve women become persistent prescription opioid users following Cesarean delivery in the United States. Patients who are more likely to become persistent users can be predicted using a priori patient characteristics such as pre-existing psychiatric comorbidity and substance use/abuse. Awareness of these predictors may lead to more discerning use of these medications in this clinical setting.
Supplementary Material
Table 2.
Patient pre-operative characteristics and initial opioid prescription characteristics that predict persistent opioid use following Cesarean delivery.
| Unadjusted Odds Ratio, 95% Confidence Interval |
Adjusted Odds Ratio** 95% Confidence Interval |
|
|---|---|---|
| Patient characteristics | ||
| Age at delivery (year) | ||
| <20 | 1.38 (0.61 to 3.14) | 1.41 (0.61 to 3.24) |
| 20–29 | 1.38 (1.08 to 1.76) | 1.40 (1.09 to 1.8) |
| 30–39 | Ref | Ref |
| >=40 | 0.83 (0.52 to 1.34) | 0.76 (0.47 to 1.23) |
| Year of surgery (per year) | 1.00 (0.95 to 1.05) | 0.98 (0.93 to 1.04) |
| Repeat Cesarean delivery | 1.11 (0.88 to 1.40) | 1.21 (0.95 to 1.54) |
| Substance use and abuse | ||
| Marijuana | 3.90 (0.54 to 28.17) | 0.30 (0.02 to 3.67) |
| Cocaine | 22.56 (5.32 to 95.71) | 6.11 (1.03 to 36.31) |
| Other substance abuse* | 6.90 (3.05 to 15.65) | 2.78 (1.12 to 6.91) |
| Tobacco use | 4.47 (3.03 to 6.57) | 3.04 (2.03 to 4.55) |
| Pain conditions | ||
| Fibromyalgia | 2.16 (1.24 to 3.77) | 1.25 (0.70 to 2.24) |
| Migraines/other headache syndromes | 2.96 (2.21 to 3.97) | 2.14 (1.58 to 2.9) |
| Back pain | 2.32 (1.79 to 2.99) | 1.74 (1.33 to 2.29) |
| Psychiatric medications | ||
| Benzodiazepines | 6.68 (4.90 to 9.11) | 3.72 (2.64 to 5.26) |
| Antidepressants | 4.98 (3.86 to 6.43) | 3.19 (2.41 to 4.23) |
| Antipsychotics | 8.84 (3.60 to 21.69) | 1.36 (0.50 to 3.71) |
| Stimulants | 3.46 (1.42 to 8.42) | 1.33 (0.53 to 3.36) |
| Initial opioid prescription characteristics | ||
| Days supply (days) | ||
| ≤3 | Ref | Ref |
| 4–5 | 0.98 (0.75 to 1.27) | 0.88 (0.63 to 1.23) |
| ≥6 | 1.28 (0.93 to 1.76) | 0.99 (0.69 to 1.41) |
| Daily dose tertile (milligrams of oral morphine equivalent) | ||
| <81 | Ref | Ref |
| 81–112.5 | 0.86 (0.62 to 1.18) | 1.02 (0.74 to 1.41) |
| >112.5 | 0.99 (0.77 to 1.29) | 1.30 (0.87 to 1.93) |
| Type of opioid | ||
| Oxycodone | Ref | Ref |
| Hydrocodone | 0.90 (0.69 to 1.17) | 0.91 (0.68 to 1.2) |
| Other | 1.31 (0.86 to 2.01) | 1.30 (0.84 to 2.01) |
Includes anxiolytic, stimulant, hallucinogenic drugs, or abuse of unspecified drugs/medications.
Adjusted for all patient and initial opioid prescription characteristics.
Table 4.
Risk of filling opioids during 4, 6, and 8 or more months during follow-up in patients undergoing Cesarean delivery who were dispensed an opioid and those with a vaginal delivery who were not dispensed an opioid.
| Number of months with opioid dispensed during follow-up |
Cesarean with opioid dispensed (N=80,127) |
Vaginal delivery without opioid (N= 169,576) |
Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)* |
|---|---|---|---|---|
| ≥4 months | 468 (0.58) | 393 (0.23) | 2.53 (2.21 to 2.89) | 2.21 (1.89 to 2.58) |
| ≥6 months | 148 (0.18) | 105 (0.06) | 2.99 (2.33 to 3.84) | 2.68 (2.02 to 3.55) |
| ≥8 months | 59 (0.07) | 32 (0.02) | 3.90 (2.54 to 6.00) | 3.47 (2.15 to 5.60) |
Adjusted for age, prior Cesarean delivery, substance use and abuse conditions, pain conditions, and psychiatric medications
Acknowledgments
Thanks to Cora Allen-Coleman for her work as a research assistant on this project.
Dr. Brian Bateman has obtained written permission from all persons named in the acknowledgement. Legends:
Supported by an unrestricted research grant from CVS Caremark to Brigham and Women’s Hospital and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Award Number K08HD075831).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors report no relevant conflicts of interest.
Contributor Information
Brian T. Bateman, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Division of Obstetric Anesthesia, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Jessica M. Franklin, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
Katsiaryna Bykov, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
Jerry Avorn, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
William H. Shrank, Chief Scientific Officer and Chief Medical Officer, CVS Health, Woonsocket, RI 02895.
Troyen A. Brennan, CVS Health, Woonsocket, RI 02895.
Joan E. Landon, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
James P. Rathmell, Harvard Medical School, Chair, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women's, Boston, Massachusetts 02120.
Krista F. Huybrechts, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
Michael A. Fischer, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
Niteesh K. Choudhry, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02120.
References
- 1.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(26):537–542. [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy M. Opioid overdose deaths rose fivefold among US women in 10 years. BMJ. 2013;347:f4415. doi: 10.1136/bmj.f4415. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131(3):548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 5.Lavoie A, Toledo P. Multimodal postcesarean delivery analgesia. Clin Perinatol. 2013;40(3):443–455. doi: 10.1016/j.clp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–796. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 7.Nagin D. Group-based modeling of development. Harvard University Press; 2005. [Google Scholar]
- 8.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological methods. 1999;4(2):139. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Sinatra RS. Oral and Parenteral Opioid Analgesics for Acute Pain Management. In: Sinatra RS, de Leon-Cassasola OA, Viscusi ER, Ginsberg B, editors. Acute Pain Management. Cambridge ; New York: Cambridge University Press; 2009. pp. 188–203. [Google Scholar]
- 10.Efron B, Tibshirani R. Improvements on cross-validation: the 632+ bootstrap method. Journal of the American Statistical Association. 1997;92(438):548–560. [Google Scholar]
- 11.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9(1):28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 14.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Rhodin A, Stridsberg M, Gordh T. Opioid endocrinopathy: a clinical problem in patients with chronic pain and long-term oral opioid treatment. Clin J Pain. 2010;26(5):374–380. doi: 10.1097/AJP.0b013e3181d1059d. [DOI] [PubMed] [Google Scholar]
- 16.Meyer M. The perils of opioid prescribing during pregnancy. Obstet Gynecol Clin North Am. 2014;41(2):297–306. doi: 10.1016/j.ogc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Carroll I, Barelka P, Wang CK, Wang BM, Gillespie MJ, McCue R, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg. 2012;115(3):694–702. doi: 10.1213/ANE.0b013e31825c049f. [DOI] [PubMed] [Google Scholar]
- 18.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119(1–3):95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, regular prescription opioid use. Arch Intern Med. 2006;166(19):2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 21.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schieffer BM, Pham Q, Labus J, Baria A, Van Vort W, Davis P, et al. Pain medication beliefs and medication misuse in chronic pain. J Pain. 2005;6(9):620–629. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Krebs EE, Lurie JD, Fanciullo G, Tosteson TD, Blood EA, Carey TS, et al. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. 2010;11(1):44–52. doi: 10.1016/j.jpain.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645–650. doi: 10.1016/j.jhsa.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 26.Harris K, Curtis J, Larsen B, Calder S, Duffy K, Bowen G, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317–321. doi: 10.1001/jamadermatol.2013.1871. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Adult use of prescription opioid pain medications - Utah, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(6):153–157. [PubMed] [Google Scholar]
- 28.Eisenach JC, Pan P, Smiley RM, Lavand'homme P, Landau R, Houle TT. Resolution of pain after childbirth. Anesthesiology. 2013;118(1):143–151. doi: 10.1097/ALN.0b013e318278ccfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landau R, Bollag L, Ortner C. Chronic pain after childbirth. Int J Obstet Anesth. 2013;22(2):133–145. doi: 10.1016/j.ijoa.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Martell BA, O'Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.