Abstract
An adolescent with plastic bronchitis due to congenital heart disease had altered mental status after an interventional lymphatic procedure in which lipiodol contrast was used. Neuroimaging revealed cerebral lipiodol embolization due to direct shunting between lymphatic channels and pulmonary veins. Cerebral lipiodol embolization is a potential neurologic morbidity associated with interventional lymphatic procedures.
Plastic bronchitis is a rare complication of congenital heart disease palliation that is caused by the formation of proteinaceous casts in the tracheobronchial tree. Clinical management is challenging, and these gelatinous casts can result in airway obstruction and death from asphyxiation.1 Lymphatic abnormalities have been shown to play a major role in the pathophysiology of plastic bronchitis.2,3 A new therapeutic option for these patients is an interventional procedure to embolize the abnormally dilated lymphatic ducts.3,4
These procedures require the use of lipiodol, an iodinated poppy seed oil, which is used as a radio-opaque contrast agent in lymphangiography and for chemoembolization procedures. Data on safety and efficacy of this procedure and contrast agent are limited to a small number of case reports. Lipiodol frequently is used in the transcatheter arterial chemoembolization (TACE) of liver tumors. Cerebral lipiodol embolism (CLE) is a known complication of this procedure, although the mechanism of its neurotoxicity is uncertain.5,6 In patients with single-ventricle physiology, cerebral embolization due to right-to-left shunting is of significant concern.
We describe a child with congenital heart disease and refractory plastic bronchitis who developed CLE after embolization of peribronchial lymphatic networks. This case highlights that CLE is a potential neurologic morbidity associated with these interventional lymphatic procedures and can be diagnosed by clinical history and its unique neuroimaging pattern.
Case Description
A 15-year-old boy with pulmonary atresia with intact ventricular septum developed plastic bronchitis from worsening right heart dysfunction and elevated central venous pressure. His treatment included prednisone, inhaled tissue plasminogen activator, and sildenafil with limited success. He underwent lymphatic duct embolization with resolution of symptoms, but these recurred after surgery for pulmonary valve replacement. Consequently, he presented again for lymphatic embolization.
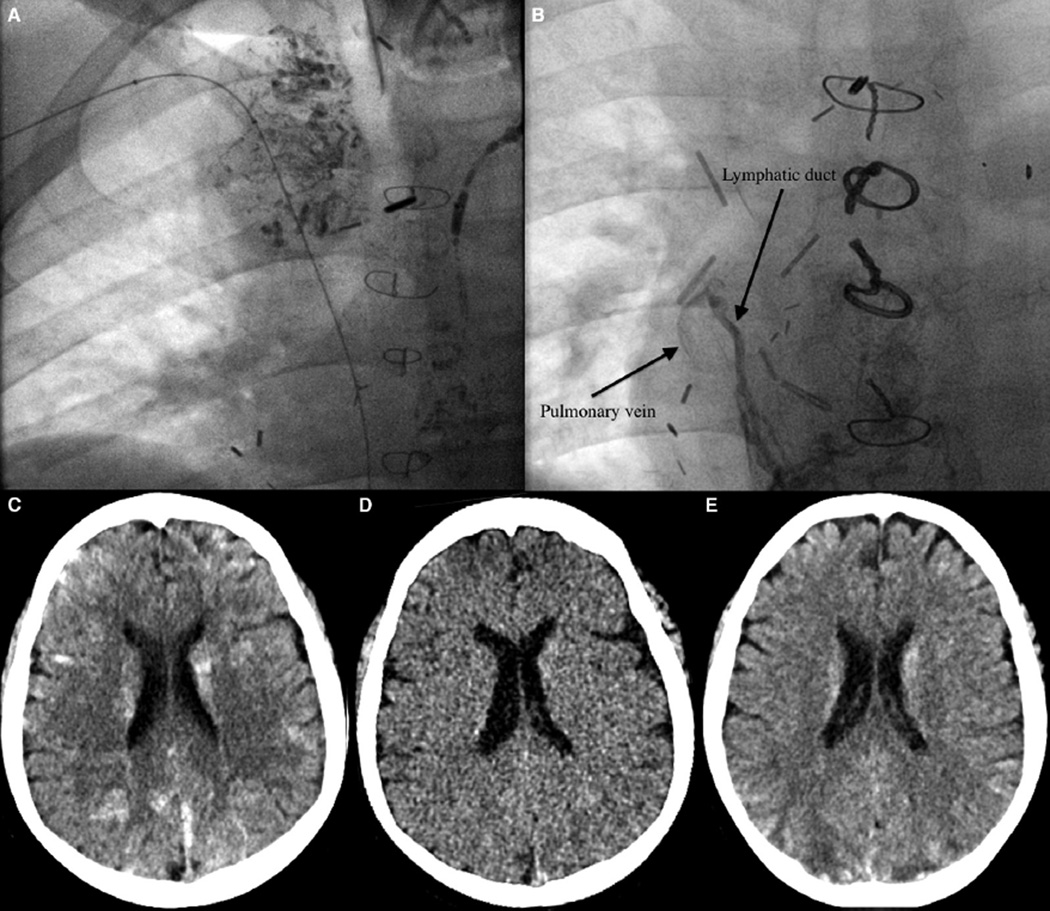
Repeat dynamic contrast lymphangiography demonstrated abnormal peribronchial lymphatic ducts on the right side originating from a network of ducts below the previously occluded thoracic duct. Intranodal lymphangiogram was performed to opacify a target central lymphatic vessel.7 Access to this vessel was then performed with a 22-gauge Chiba needle (Cook Medical Inc, Bloomington, Indiana) via a transabdominal approach.7 A V18 control guide wire (Boston Scientific, Natick, Massachusetts) was advanced into the lymphatic duct and manipulated cephalad. Over the wire, a 60-cm 2.3 F Rapid Transit microcatheter (Cordis Corp, Warren, New Jersey) was advanced further into the duct for imaging of the duct and its branches. Embolization of the lymphatic ducts was performed with 1–2 mL of lipiodol and 1 mL of n-butyl cyanoacrylate (Trufill glue; Cordis Corporation, Warren, New Jersey) (Figure 1, A). During the procedure, an abnormal connection between a lymphatic duct and the pulmonary vein was seen (Figure 1, B). There were no intraprocedural complications, and the patient had a normal neurologic examination on recovery from anesthesia.
Figure 1.
A, Chest radiograph demonstrating lipiodol in peribronchial lymphatic ducts. B, Chest radiograph demonstrating an abnormal connection between a lymphatic duct and the pulmonary vein. C, Noncontrast CT shows numerous hyperdensities scattered throughout the brain bilaterally. D, A virtual noncontrast CT image derived from dual energy CT proves that the hyperdensities were iodine containing material (lipiodol) rather than just multifocal hemorrhage. E, CT image from 5 days later shows mild interval decrease and improvement in the hyperdensities, with development of foci of hypodensity (eg, in the bilateral posterior parietal lobes).
Approximately 5 hours after the procedure, the patient became acutely unresponsive and had a seizure with rightward gaze deviation, lip smacking, and stiffness of the left lower extremity. The seizure lasted less than 3 minutes and was aborted with lorazepam. No further anticonvulsants were required. A head computed tomography (CT) scan showed innumerable hyperdensities bilaterally throughout the supra- and infratentorial brain (Figure 1, C). The hyperdensities demonstrated spectral characteristics consistent with iodine on dual energy imaging, indicating that they were iodine-based (lipiodol) rather than primarily multifocal hemorrhage (Figure 1, D). The patient’s mental status improved during the next several hours, although he had persistent difficulties with speech, impaired vision, and a right-sided hemiparesis.
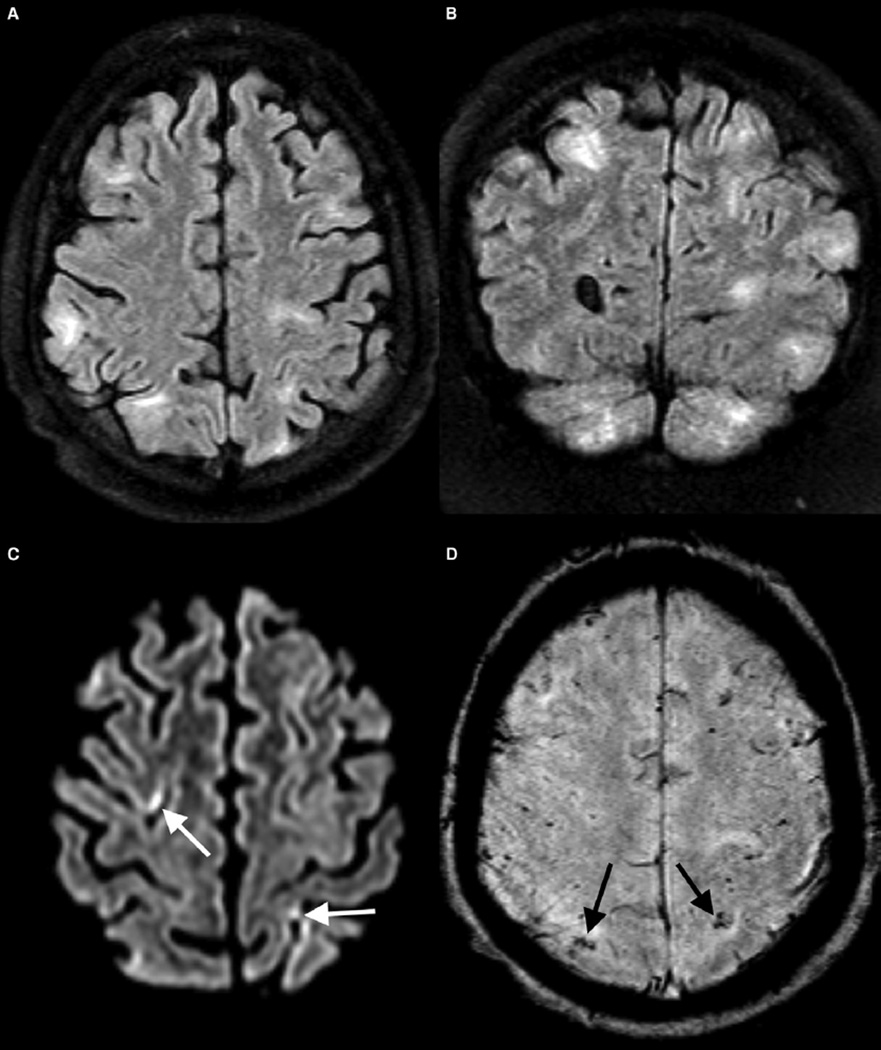
A CT scan done 5 days later showed interval improvement in the hyperdensities, with new, small foci of wedge-shaped hypodensity (Figure 1, E). A brain magnetic resonance scan at 8 days showed multiple areas of T2/fluid-attenuated inversion recovery hyperintense signal abnormality (Figure 2). There were punctate foci of susceptibility within these areas, and a few small residual foci of mild restricted diffusion (Figure 2). The child’s neurologic deficits gradually improved during the next week with rehabilitation therapies and had completely resolved by hospital discharge.
Figure 2.
A, Axial and B, coronal/fluid-attenuated inversion recovery images from magnetic resonance imaging performed on day 8 post procedure show multifocal areas of signal abnormality and injury, predominantly in the gray matter and subcortical white matter. C, Axial diffusion image shows small foci of restricted diffusion (arrows) confirmed on apparent diffusion coefficient maps (not shown). D, Axial susceptibility-weighted imaging shows multiple punctate foci of susceptibility in keeping with foci of microhemorrhage confirmed on phase images (not shown).
Discussion
Interventional lymphatic procedures are being performed with increasing frequency in children, although data regarding complications are limited. CLE is a rare but known complication of procedures that use lipiodol, occurring in 1.02 per 1000 TACE procedures.5 The true incidence of CLE is unknown because only symptomatic patients undergo neuroimaging. In our case, CLE occurred after lipiodol lymphangiography and embolization of peribronchial lymphatic networks in an adolescent with plastic bronchitis. The neuroimaging findings resemble those described in adults with CLE after liver tumor TACE showing high-density material scattered in the brain on CT and multiple infarcts and region of T2/fluid-attenuated inversion recovery hyperintensity on magnetic resonance imaging.8
The underlying mechanism for how lipiodol reaches the cerebral circulation and causes CLE after TACE is unclear but may involve hepatopulmonary, pulmonary arteriovenous, or right-to-left intracardiac shunts. CLE after lipiodol lymphangiography and embolization likely involves migration of the lipiodol from the lymphatics into the venous system, with subsequent shunting from the venous to the arterial circulation either through arteriovenous malformations in the lungs or right to left intra-cardiac connections. In addition, lipid globules such as lipiodol can cross the pulmonary arteriolar network because of their small size, gaining access to the systemic circulation.9 In our patient, direct shunting was found between his abnormal lymphatic channels and the pulmonary venous circulation (Figure 1, B). Multiple lymphovenous connections are a common finding; however, direct connections between lymphatic ducts and the pulmonary veins have not been reported previously. Consequently, careful attention must be paid to leak of lipiodol into the systemic circulation in all cases.
Once lipiodol gains access to the cerebral circulation, its mechanism of neurotoxicity is unknown, and could be related to its vaso-occlusive, lipid, osmolar, or other chemical or physical properties. As an iodinated contrast agent, lipiodol may potentially penetrate or cause breakdown of the blood-brain barrier and extravasate into neural tissue, causing direct toxicity and eliciting local cytotoxic and vasogenic edema.10 Patients with CLE after TACE and our patient demonstrate foci of restricted diffusion, suggesting a possible component of small vessel/capillary occlusive disease from the lipiodol; however, the delay between the procedure and symptom onset argues against a hyperacute arterial ischemic stroke as the sole explanation for clinical symptoms.11 Our patient had symptom onset 5 hours after his procedure, consistent with previous reports that describe symptom onset ≤6 hours from the exposure in >85% of cases.5 The amount of contrast that reaches the brain may influence occurrence and severity of CLE. Previous studies describe a greater risk of CLE when ≥20 mL of lipiodol is used and after ≥3 exposures.5 In contrast, this was only our patient’s second lymphatic embolization, and he received <5 mL of lipiodol during each procedure.
Neurologic symptoms ultimately completely resolved in our patient, consistent with many other reported cases, although poor neurologic outcome has been reported in up to 20% of older adult patients with CLE.5 Supportive care is the mainstay of treatment for CLE. This case demonstrates that even small amounts of lipiodol administered for lymphangiography and lymphatic embolization can result in CLE. It emphasizes that although rare, CLE should be considered in the differential diagnosis for patients who develop new neurologic symptoms with this unique neuroimaging pattern after undergoing an interventional lymphatic procedure.
Acknowledgments
D.L. is supported by National Institute of Neurological Disorders and Stroke (R01NS72338, R01 NS060653), National Heart, Lung, and Blood Institute (HL090615-06), National Institute of Child Health and Human Development (UO1HD087180), and the June and Steve Wolfson Family Foundation. R.I. is supported by National Institute of Neurological Disorders and Stroke (U10NS086474) and National Heart, Lung, and Blood Institute (U01 HL094345).
Glossary
- CLE
Cerebral lipiodol embolism
- CT
Computed tomography
- TACE
Transcatheter arterial chemoembolization
Footnotes
The authors declare no conflicts of interest.
References
- 1.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;6:292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Larue M, Gossett JG, Stewart RD, Backer CL, Mavroudis C, Jacobs ML. Plastic bronchitis in patients with fontan physiology: review of the literature and preliminary experience with fontan conversion and cardiac transplantation. World J Pediatr Congenit Heart Surg. 2012;3:364–372. doi: 10.1177/2150135112438107. [DOI] [PubMed] [Google Scholar]
- 3.Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133:1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 4.Dori Y, Keller MS, Rychik J, Itkin M. Successful treatment of plastic bronchitis by selective lymphatic embolization in a Fontan patient. Pediatrics. 2014;134:590–595. doi: 10.1542/peds.2013-3723. [DOI] [PubMed] [Google Scholar]
- 5.Chu HJ, Lee CW, Yeh SJ, Tsai LK, Tang SC, Jeng JS. Cerebral lipiodol embolism in hepatocellular carcinoma patients treated with transarterial embolization/chemoembolization. PLoS One. 2015;10:e0129367. doi: 10.1371/journal.pone.0129367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo KM, Yoo BG, Kim KS, Lee SU, Han BH. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology. 2004;63:181–183. doi: 10.1212/01.wnl.0000132645.23611.2d. [DOI] [PubMed] [Google Scholar]
- 7.Nadolski G, Itkin M. Thoracic duct embolization for the management of chylothoraces. Curr Opin Pulm Med. 2013;19:380–386. doi: 10.1097/MCP.0b013e3283610df2. [DOI] [PubMed] [Google Scholar]
- 8.Lee CS, Kim SJ, Choi JW, Choi CG, Lee DH. Cerebral lipiodol embolism proven by dual- energy computed tomography: a case report. J Comput Assist Tomogr. 2010;34:105–106. doi: 10.1097/RCT.0b013e3181b382f8. [DOI] [PubMed] [Google Scholar]
- 9.Sevitt S. The significance and pathology of fat embolism. Ann Clin Res. 1977;9:173–180. [PubMed] [Google Scholar]
- 10.Utz R, Ekholm SE, Isaac L, Sands M, Fonte D. Local blood-brain barrier penetration following systemic contrast medium administration. A case report and an experimental study. Acta Radiol. 1988;29:237–242. [PubMed] [Google Scholar]
- 11.Zach V, Rapaport B, Yoo JY, Goldfeder L, Weinberger J. Multiple ischemic strokes after transcatheter arterial chemoembolization for hepatocellular carcinoma with a radiographic and pathological correlate. J Stroke Cerebrovasc Dis. 2012;21:217–224. doi: 10.1016/j.jstrokecerebrovasdis.2010.08.001. [DOI] [PubMed] [Google Scholar]