Abstract
Multimorbidity, defined as the presence of 2 or more chronic conditions, is common among older adults with cardiovascular disease. These individuals are at increased risk for poor health outcomes and account for a large proportion of healthcare utilization. Clinicians are challenged with the heterogeneity of this population, the complexity of the treatment regimen, limited high-quality evidence, and fragmented healthcare systems. Each treatment recommended by a clinical practice guideline for a single cardiovascular disease may be rational, but the combination of all evidence-based recommendations can be impractical or even harmful to individuals with multimorbidity. These challenges can be overcome with a patient-centered approach that incorporates the individual’s preferences, relevant evidence, the overall and condition-specific prognosis, clinical feasibility of treatments, and interactions with other treatments and coexisting chronic conditions. The ultimate goal is to maximize benefits and minimize harms by optimizing adherence to the most essential treatments, while acknowledging trade-offs between treatments for different health conditions. It may be necessary to discontinue therapies that are not essential or potentially harmful to decrease the risk of drug-drug and drug-disease interactions from polypharmacy. A decision to initiate, withhold, or stop a treatment should be based on the time horizon to benefits vs. the individual’s prognosis. In this review, we illustrate how cardiologists and general practitioners can adopt a patient-centered approach to focus on the aspects of cardiovascular and non-cardiovascular health that have the greatest impact on functioning and quality of life in older adults with cardiovascular disease and multimorbidity.
Multimorbidity, the presence of ≥2 chronic conditions, affects more than two thirds of the older population.1–3 Older adults with multimorbidity are at increased risk for mortality, disability, institutionalization, and healthcare utilization.4–6 The annual risk of hospital admission rises exponentially from 4% for those with 0 or 1 condition to 63% for those with ≥6 conditions; the latter group accounts for over 50% of total hospital and post-acute care costs and 70% of readmissions.1 Furthermore, almost half of readmissions after heart failure or myocardial infarction are due to non-cardiovascular conditions.7 While cardiovascular diseases (CVD) are common components of multimorbidity, the presence of multimorbidity affects management of CVD. As such, optimal management of CVD cannot be accomplished without consideration of multimorbidity. In this review, we provide guidance to cardiologists and general practitioners about evaluation and management of older adults with CVD and multimorbidity.
COMMON PATTERNS OF CVD AND MULTIMORBIDITY
In several population-based studies, CVD or a metabolic condition in conjunction with osteoarthritis was the most common multimorbidity pattern.8 In the United States, the dyad of hypertension and hyperlipidemia was most frequently observed, followed by ischemic heart disease, arthritis, and diabetes.9 In addition, over 50% of patients with heart failure or atrial fibrillation had ≥5 chronic conditions;1 common conditions were arthritis (prevalence: 41–46%), anemia (39–51%), cataract (22–23%), chronic lung disease (21–31%), and dementia (26%).9 A treatment for CVD may impact coexisting conditions, and vice versa (i.e., drug-disease interaction). Bidirectional drug-disease interactions occur when a drug used to treat CVD worsens another chronic condition and a drug for that condition worsens CVD; this is called therapeutic competition.10 The risk of adverse events may also increase with certain drug combinations (i.e., drug-drug interaction).
CHALLENGES IN MANGEMENT OF CVD AND MULTIMORBIDITY
Multimorbidity presents several challenges to clinicians. High-quality evidence from randomized controlled trials (RCTs) is lacking.9 Evidence-based management of CVD often requires therapeutic polypharmacy, yet CVD medications account for 25% of preventable drug-related adverse events.11 Clinical practice guidelines focus on disease-specific benefits of individual medications, but the incremental benefit of a medication on functioning and quality of life when added to an already-complex regimen is difficult to estimate.12 In the absence of strong evidence or clear direction from guidelines, clinicians struggle to identify patients who will benefit from novel drugs (e.g., new oral anticoagulants) and procedures (e.g., transcatheter aortic valve replacement).
PRINCIPLES OF MANAGING OLDER ADULTS WITH MULTIMORBIDITY
To promote a patient-centered approach in clinical management of older adults with multimorbidity, the American Geriatrics Society Expert Panel developed 5 guiding principles (Table 1) and an algorithm (Figure 1).13 The Expert Panel reports and recommendations are available at www.americangeriatrics.org and as a mobile application. Below we briefly introduce these principles.
Table 1.
Summary of Guiding Principles of Managing Older Adults with Multimorbidity*
|
1. Patient preferences: Elicit and incorporate patient preferences into medical decision-making.
|
|
|
|
|
2. Interpreting the evidence: Interpret and apply the medical literature, recognizing the limitations of the evidence base.
|
|
|
|
|
3. Prognosis: Frame clinical management decisions within the context of risks, burdens, benefits, and prognosis.
|
|
|
|
|
4. Clinical feasibility: Consider treatment complexity and feasibility in making clinical management decisions.
|
|
|
|
|
5. Optimizing therapies and care plan: Choose therapies that optimize benefit, minimize harm, and enhance quality of life.
|
|
Abbreviation: STOPP/START, the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions and Screening Tool to Alert to Right Treatment Criteria.30
Summarized from: Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60(10):e1–e25.
Figure 1. An Algorithm to Evaluate and Manage Older Adults with Multimorbidity.
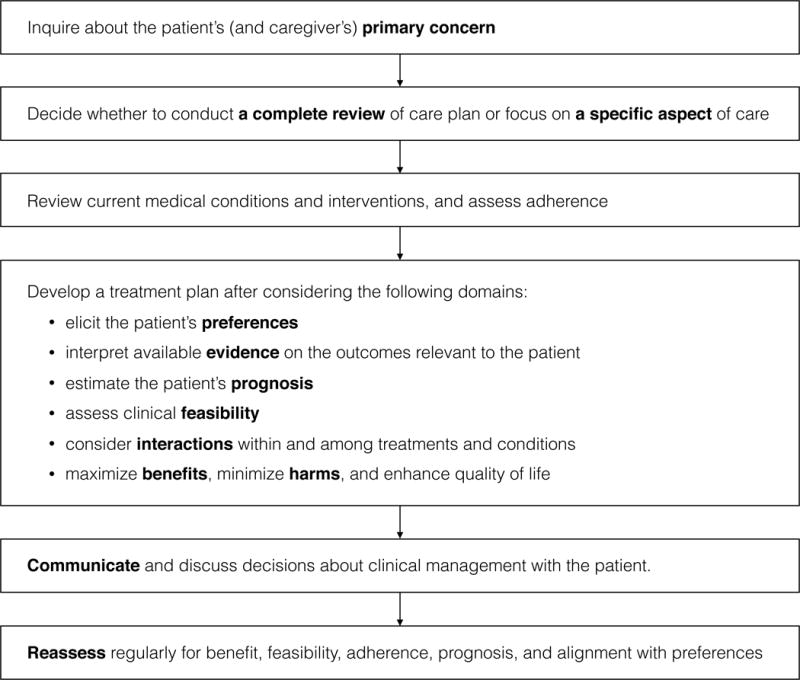
* Modified from: Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60(10):e1–e25.
1. Patient preferences
Clinicians should recognize when the individual is facing a preference-sensitive decision (i.e., more than one treatment option). Preferences should be elicited after adequately informing the patient about expected benefits and harms of available treatment options. It is recommended that clinicians present numerical likelihood and absolute risk reduction rather than descriptive terms (e.g., “rarely” or “frequently”) and assess the patient’s understanding using a “teach back” method. As preferences may change over time, it is important to reassess them periodically, especially when there is a change in health status. Cognitively impaired patients should be asked about their preferences; for those who do not understand the benefits and harms of treatment, surrogate decision-makers should be involved. This principle does not imply that patients and surrogates can demand treatments when there is no reasonable expectation of benefit.
2. Interpreting the evidence
To determine whether findings from the medical literature are applicable to older adults with multimorbidity, clinicians should evaluate how closely the patient resembles the research population by reviewing selection criteria and population characteristics, study quality (e.g., RCT or registry), relevance of outcomes to older patients, and information on harms and treatment burden that may negatively affect adherence. One should examine absolute risk reduction, as relative risk reduction can be misleading when the baseline risk is low. It is important to consider the time horizon to benefit in relation to the individual’s prognosis, because some treatments have a long time horizon to benefit (e.g., tight glycemic control or primary prevention of CVD) and may pose a greater immediate risk of harm.
3. Prognosis
Clinicians should consider the overall prognosis (e.g., life expectancy, functional status, and quality of life) as well as condition-specific prognosis (e.g., risk of cardiovascular events, stroke, and gastrointestinal bleeding). Prognosis determination can help prioritize the most pressing health issues for the patient and inform clinical decision-making about prevention, treatment (e.g., device or procedure), or type of health services to use (e.g., intensive care).14,15 Validated tools are available to predict mortality in various settings based on demographic characteristics, diagnoses, and functional status (eprognosis.ucsf.edu).14,16 Frailty assessment tools are also useful to predict mortality and institutionalization. Validated instruments include the cumulative deficit frailty index (count of health deficits, including diagnoses and severity, functional limitations, and abnormalities on physical examination),17 Clinical Frailty Scale (7 stages from very fit to severely frail; Figure 2),17 and frailty phenotype (having ≥3 of weight loss, exhaustion, inactivity, slow gait, and low grip strength).18 A brief mobility assessment, such as the Timed Up-and-Go (TUG) test (cut-point 12 sec: sensitivity 0.72, specificity 0.86) or gait speed (cut-point 0.8 m/sec: sensitivity 0.99, specificity 0.64), provides a good screening test for frailty.19,20
Figure 2. Clinical Frailty Scale and Estimation of Prognosis.
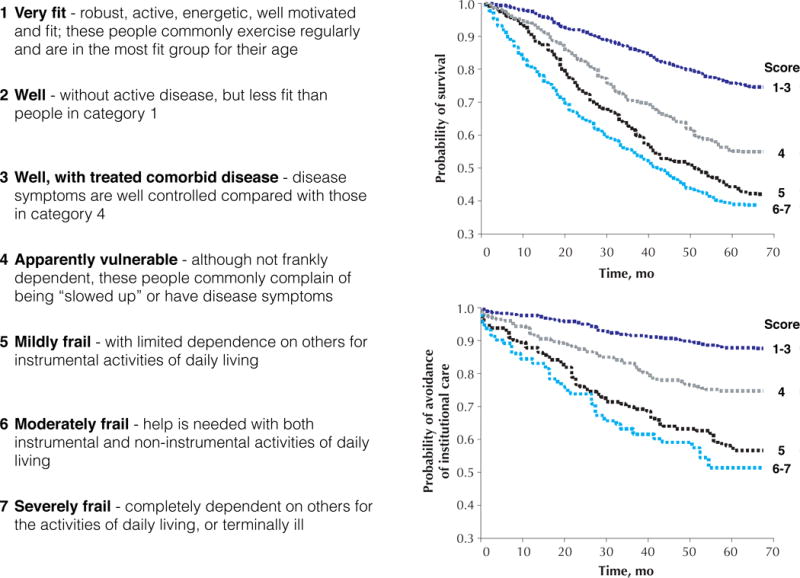
* Adopted from: Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173(5):489–95.
4. Clinical feasibility
A complex regimen is associated with non-adherence,21 adverse drug events,22 economic burden,23 and caregiver stress.24 These risks can worsen with impairments in cognitive and physical function. Thus, it is recommended to periodically evaluate the patient’s capability to manage medications. Several tools are available, but there is no simple instrument appropriate for primary care use.25 Nonetheless, clinicians should discuss patients’ treatment preferences, support system (e.g., family and caregivers), and medication management support. Simplifying the regimen and providing monitoring with feedback can improve adherence.26
5. Optimizing therapies and care plan
Clinicians should aim to minimize harms and maximize benefits by optimizing adherence to essential treatment. It may be necessary to discontinue treatments that are not essential or potentially harmful.27 As the risk of drug-drug interactions rises with polypharmacy, efforts to reduce the medication burden are critical.28 Expert consensus criteria, such as the Beers list29 (Supplemental Table 1) or STOPP/START criteria30 (Supplemental Table 2) can be useful in guiding medication choices.31,32 Decision to not initiate or stop a treatment should be made based on the likelihood of benefits vs. harms, considering the time horizon to benefit and the individual’s prognosis. A time-limited withdrawal trial may be necessary to determine whether a drug is needed.
APPLYING THE PRINCIPLES IN MANAGEMENT OF CVD AND MULTIMORBIDITY
Optimal care of older adults with multimorbidity is best achieved by a collaborative effort that involves patients, family members, and health care providers. Below, we illustrate how cardiologists can work with general practitioners to apply these guiding principles to evaluate and manage older adults with CVD and multimorbidity. Patients with more complex health and psychosocial issues may benefit from comprehensive evaluation by geriatricians, psychiatrists, social workers, and home care providers, as well as integrated care management programs.
Case 1: an 85-year-old man with heart failure, atrial fibrillation, and multimorbidity
Case presentation
You are the cardiologist treating an 85-year-old man with the following CVDs:
Heart failure with preserved ejection fraction (HFpEF), including 3 admissions within the last year
Non-obstructive coronary artery disease, asymptomatic
Persistent atrial fibrillation and history of transient ischemic attack on warfarin
Peripheral arterial disease, treated with femoral-popliteal bypass surgery
Hypertension
Dyslipidemia
The patient reports fatigue and inability to walk more than a block for the past 2 weeks. He has been eating take-out meals frequently because he cannot afford an aide to assist with grocery shopping, cooking, cleaning, and driving. He is a retired engineer and lives at home on a fixed income with his wife who also has multiple health problems. His children live in another city. He performs basic activities of daily living (ADLs) without help but needs assistance with instrumental ADLs that involve physical activity, such as shopping, cooking, housework, and transportation. He manages his medications by himself. He wishes to remain at home as long as possible and does not want to become a burden to his children. Physical examination reveals blood pressure 131/53 mmHg, pulse 93/min, oxygen saturation 94% on ambient air, irregularly irregular rhythm, jugular venous pressure 14 cmH2O, crackles in both lung bases, modest pitting edema at both ankles, and 27 of 30 on the Mini-Mental State Examination. He walks steadily without an assistive device. Electrocardiography shows atrial fibrillation with no ischemic changes. You diagnose a heart failure exacerbation.
Review current medical conditions and interventions, and assess adherence
In addition to the CVDs listed above, the patient has the following non-cardiovascular conditions:
Type 2 diabetes, hemoglobin A1c 10%
Chronic kidney disease, serum creatinine 141 μmol/L (1.6 mg/dL), estimated glomerular filtration rate 35 ml/min/1.73m2, serum potassium 4.5 mmol/L
Gastroesophageal reflux disease
Insomnia
Current therapy includes 11 medications and 4 non-pharmacological interventions.
aspirin (81mg daily)
atorvastatin (40mg daily)
carvedilol (6.25mg twice daily)
furosemide (40mg twice daily)
lisinopril (20mg daily)
insulin glargine (20 units daily) and lispro (three times daily based on meals)
omeprazole (20mg daily)
docusate (100mg twice daily)
warfarin (2–3mg daily according to INR)
trazodone (50mg daily at bedtime as needed for insomnia)
check glucose level four times daily
check INR every 2 weeks
walk daily for 30 minutes
low-sodium diabetic diet
The patient complains of taking too many medications. He admits to difficulty checking glucose and injecting insulin frequently and adhering to a low-sodium diabetic diet. He wants to avoid furosemide in the evening due to frequent trips to the bathroom at night. Because of worsening dyspnea, he has not been walking for the past 2 weeks.
Develop a treatment plan after considering preferences, evidence, prognosis, feasibility, interactions, and benefits vs. harms of treatment
1) Preferences
The patient’s primary goal is to remain independently at home as long as possible. After each hospitalization, he stayed at a rehabilitation facility for 2–3 weeks to regain his strength; therefore, preventing hospitalization is consistent with his goal. He also wants to simplify his medication schedule by reducing the total number of pills and the frequency of glucose checks and insulin injections.
2) Evidence
You review the evidence on interventions to reduce admissions in HFpEF.
Candesartan (absolute risk reduction 2.4%, relative risk reduction 16%)33 and spironolactone (absolute risk reduction 2.2%, relative risk reduction 17%)34 reduced hospitalizations over 3 years, with increased risks of hyperkalemia and worsening renal function.
A small RCT showed that a 12-week home-based exercise program improved functional capacity and quality of life in women with HFpEF.35
It is noted that a majority of RCT participants were <70 years of age.33–35
A disease management program led by a heart failure nurse specialist and involving close telephone follow-up and home visits may reduce heart failure and all-cause admissions and mortality.36 Older patients with mild-to-moderate frailty may benefit more from such a program than non-frail or severely frail patients.37
The rate control strategy in atrial fibrillation was associated with fewer adverse events and admissions than the rhythm control strategy, without difference in mortality, cardiovascular events, or quality of life.38,39
3) Prognosis
It is important to consider overall prognosis and condition-specific prognosis. According to a validated prediction model,40 the patient’s estimated risk of 5-year mortality is 69%. According to the Clinical Frailty Scale, his 5-year risk for morality is around 55% (Figure 2).17 The CHA2DS2-VASc score41 is 8 and HAS-BLED score42 is 3, which correspond to annual risks of 11% for stroke and 4% for major bleeding.
4) Clinical feasibility
To reduce treatment burden and improve adherence, it is preferable to change medications to once-daily dosing, if possible. Once fluid overload has been treated, furosemide can be given once in the morning or after lunch to reduce nocturia43 and improve sleep quality. Carvedilol can be switched to the extended release formulation (although this would increase cost) or another long-acting agent (e.g., bisoprolol or metoprolol succinate) taken once daily. Digoxin is not as effective for rate control as beta-blockers. His adherence to the dietary regimen may improve with reducing take-out meals; a social worker can help arrange appropriate services for grocery shopping and cooking. Decreasing the frequency of glucose monitoring and insulin injections may improve his adherence to the diabetes regimen. Treatment of nocturnal hyperglycemia may reduce nocturia.
5) Interactions with medications and medical conditions
Increasing furosemide or adding spironolactone can potentially cause worsening renal function and electrolyte abnormalities. There is no evidence of benefit when aspirin is added to warfarin.44
6) Maximize benefits and minimize harms
As his cardiologist, you formulate an individualized management plan for his CVD based on the above factors.
Heart failure: To treat fluid overload, you increase furosemide and request a visiting nurse to monitor fluid status and check basic metabolic panel in 1 week. Candesartan or spironolactone may reduce heart failure admissions, but the risk of worsening renal function and hyperkalemia may outweigh the modest benefit, especially when added to lisinopril therapy. However, switching lisinopril to candesartan could be considered, since candesartan may reduce heart failure admissions in HFpEF,33 offers similar renoprotective effects,45,46 and is generally better tolerated than lisinopril.47 Furosemide and carvedilol are changed to once-daily dosing. In addition, he should be referred to a disease management program or a heart failure clinic.
Atrial fibrillation: The rate control strategy is preferred to the rhythm control strategy to reduce admissions. Because his annual risk of stroke is greater than that of major bleeding (11% vs. 4%), therapeutic anticoagulation is appropriate. Switching warfarin to a new oral anticoagulant (with dose adjustment for kidney function) may eliminate the need for INR monitoring, reduce risk for drug-drug interactions, and limit dietary restrictions, if cost is not a limiting factor. Aspirin is discontinued due to lack of benefit coupled with increased risk of bleeding when combined with warfarin.
Hypertension: With the increased dose of furosemide, the patient should be educated about and monitored for orthostatic hypotension.
Dyslipidemia: Statins provide cardiovascular benefits in individuals with CVD, but the benefit of high-intensity therapy vs. moderate-intensity therapy is not well established in stable CVD, especially in older adults.48 Thus, reducing atorvastatin to 20mg is reasonable. Stopping statin therapy near the end of life is safe and may improve quality of life.49 This should be discussed with the patient.
Fatigue and worsening exercise tolerance: Although the evidence on exercise for HFpEF is weak, low-to-moderate intensity exercise can be recommended to improve functional status and quality of life without a significant safety concern.
Importantly, the CVD management strategy should be part of the general practitioner’s overall management plan that also addresses the patient’s non-cardiovascular conditions.
Type 2 diabetes: Reducing the frequency of glucose monitoring and insulin injection to twice daily can improve adherence. His hemoglobin A1c goal should be 8–9%, with emphasis on preventing an immediate risk of hypoglycemia.50
Chronic kidney disease: To prevent progression of diabetic nephropathy, he should receive a treatment that inhibits the renin-angiotensin system (i.e., continue lisinopril or switch to candesartan, but not both agents due to increased adverse events46,51). The 1-year mortality of octogenarians initiating hemodialysis is 46%52; he is unlikely to benefit meaningfully from hemodialysis.
Medication management: Certain medications can be discontinued to improve adherence and reduce the risk of polypharmacy. Docusate is no more effective than placebo.53 A time-limited withdrawal of omeprazole can be attempted to assess its ongoing need. With once-daily dosing of furosemide, his sleep may be less interrupted; discontinuation of trazodone can be considered.
Psychosocial conditions: The possibilities of depression, caregiver stress (from caring for his sick wife), and executive dysfunction should be evaluated, as these might have contributed to his health decline and recurrent hospitalizations. The Mini-Mental State Examination score 27 of 30 should not be considered normal; he should undergo further evaluation with a more sensitive test (e.g., the Montreal Cognitive Assessment) to detect executive dysfunction.54
Communicate and discuss decisions about clinical management with the patient
In discussing the management plan with the patient, it is important to ensure that the plan is consistent with his preferences and that he agrees with the recommendations. A written summary can facilitate clear communication of the rationale for and details of the management plan with his family and other members of his healthcare team.
Reassess at selected intervals for benefit, feasibility, adherence, prognosis, and alignment with preferences
Once the heart failure exacerbation has been stabilized and the management plan has been implemented, you and his general practitioner should coordinate follow-up care to monitor treatment outcomes, adherence, and prognosis. Since his overall prognosis is poor, advance care planning should be discussed in the near future.
Case 2. an 82-year-old woman with hypertension and multimorbidity
Case presentation
You are seeing an 82-year-old woman referred to you for management of hypertension. She is accompanied by her daughter who provides the following information on her CVDs:
Hypertension, home blood pressure ranging from 130–180/50–100 mmHg
Moderate aortic stenosis based on echocardiography performed 1 year ago
Hypercholesterolemia
The patient complains of lack of energy. Her daughter is worried about her widely fluctuating blood pressure. Although the patient has mild memory loss and macular degeneration, she lives alone in her apartment. Her daughter, who lives nearby, visits her daily to ensure her safety; she also helps with grocery shopping, financial management, medication management, and transportation. She performs basic ADLs independently and goes out for a 30-minute walk daily without getting lost. Despite having regular meals, she gradually lost 10 lbs over the past year. Her goal is to live at home and avoid nursing home admission. Physical examination reveals blood pressure 170/90 mmHg in sitting position, 155/80 mmHg in standing position, pulse 63/min, oxygen saturation 95% on ambient air, regular rhythm, grade 2/6 systolic ejection murmur at right upper sternal border, clear lungs, no edema in legs, and 23 of 30 on the Mini-Mental State Examination. She completed the TUG test in 19 sec (cut-point 12 sec) with evidence of difficulty standing from the chair and slow pace. Electrocardiography shows sinus rhythm with left ventricular hypertrophy.
Review current medical conditions and interventions, and assess adherence
In addition to the CVDs listed above, the patient has the following non-cardiovascular conditions:
Mild cognitive impairment
Osteoarthritis of the knee
History of fall, once within the past year
Osteopenia
Vision loss due to macular degeneration
Seasonal allergy
Current treatments include 9 medications and 1 non-pharmacological intervention.
aspirin (81mg daily)
calcium carbonate (600mg twice daily)
cetirizine (10mg daily)
simvastatin (40mg daily)
hydrochlorothiazide (12.5mg daily)
ibuprofen (400mg twice daily)
losartan (100mg daily)
vitamin D (1000 international units daily)
eye vitamins (vitamin C, E, zinc, copper, omega-3 fatty acid, lutein, zeaxanthin)
Walk daily for 30 minutes
With help from her daughter, she has been compliant with the treatment regimen.
Develop a treatment plan after considering preferences, evidence, prognosis, feasibility, interactions, and benefits vs. harms of treatment
1) Preferences
The patient wishes to live in her apartment and avoid nursing home admission. Her daughter is in agreement and wants to slow down the rate of cognitive decline. Given her memory loss and vision impairment, keeping her treatment regimen simple is important. Additionally, the daughter is concerned about the risk of major cardiovascular events and stroke from hypertension, as well as the risk of falls.
2) Evidence
You review the evidence on blood pressure lowering in preventing major cardiovascular events, stroke, and cognitive decline in older adults.
Cardiovascular benefit: High-quality evidence suggests that lowering blood pressure to <150/90 mmHg prevents cardiovascular morbidity and mortality.55–58 In the Hypertension in the Very Elderly Trial (HYVET), which enrolled patients ≥80 years of age (mean age 84 years, 60% female), the actively treated group had a lower rate of stroke than the placebo group (absolute risk reduction, 5.3%; relative risk reduction, 30%) over 2 years, with no increase in adverse events.58 In the Systolic Blood Pressure Intervention Trial (SPRINT), lowering systolic blood pressure to 120 mmHg reduced cardiovascular morbidity and mortality vs. lowering to 140 mmHg (absolute risk reduction, 3.2%; relative risk reduction, 33%) over 3 years in adults aged ≥75 years, with significant increases in several adverse events: hypotension (absolute risk increase, 0.9%), syncope (absolute risk increase, 0.7%), acute kidney injury (absolute risk increase, 1.6%), and electrolyte abnormality (absolute risk increase, 1.3%).59 However, individuals taking numerous medications were excluded from SPRINT, which limits applicability of the results to multimorbid older adults.
Cognitive function: A meta-analysis of RCTs suggests a modest protection against dementia with treatment of stage 2 hypertension.60 In the HYVET, the rate of dementia was modestly lower in the treated group than the placebo group (absolute risk reduction, 0.5%, and relative risk reduction, 14%) over 2 years.60
3) Prognosis
The estimated 5-year mortality risk is 43% based on one validated model,40 while the risk estimate from the Clinical Frailty Scale is 55% (Figure 2). Thus, life expectancy for people with similar health status is approximately 5 years. Although the American College of Cardiology/American Heart Association pooled cohort equation does not calculate the risk of CVD events in adults aged ≥80 years,61 her 10-year CVD risk approaches 50% (the risk calculated for a 79-year-old, non-smoking, non-diabetic, white woman with total cholesterol 3.88 mmol/L [150 mg/dL], high-density lipoprotein cholesterol 1.55 mmol/L [60 mg/dL], and systolic blood pressure 170 mmHg on treatment). The rate of progression from mild cognitive impairment to dementia ranges from 11–33% over 2 years.62 Given her fall history and slow TUG performance, she is at moderate risk for falling according to the Centers for Disease Control and Prevention algorithm (http://www.cdc.gov/STEADI/). Since she is already showing symptoms and signs of frailty (i.e., weight loss, lack of energy, and poor mobility), her ability to tolerate a stressful event (e.g., treatment-related adverse events or hospitalizations) is likely to be limited. As a result, her risk of worsening disability, institutionalization, hospitalization, and mortality is high.
4) Clinical feasibility
Intensifying the antihypertensive regimen is needed to achieve better blood pressure control. Given her memory loss and visual impairment, once-daily dosing is preferred. With more aggressive blood pressure control, the burden of clinic visits and blood tests to monitor adverse events should be considered.
5) Interactions with medications and medical conditions
Adding or increasing the dose of an antihypertensive medication can potentially increase the risk of orthostatic hypotension and falls. Ibuprofen, which she takes for arthritis, can oppose the antihypertensive effects of losartan and hydrochlorothiazide, thereby contributing to uncontrolled hypertension.
6) Maximize benefits and minimize harms
As her cardiologist, you formulate an individualized management plan for her hypertension and other CVDs.
Hypertension: A blood pressure target of 150/90 mmHg is reasonable to prevent cardiovascular events and stroke. The increased treatment burden and risk of more aggressive blood pressure lowering likely outweigh the modest additional benefit. After explaining the risk of gastrointestinal bleeding and worsening hypertension associated with long-term ibuprofen use, you recommend stopping ibuprofen and contacting her general practitioner about an alternative pain regimen (see below). If blood pressure remains elevated despite discontinuation of ibuprofen, you will consider adding a new antihypertensive medication. The patient and her daughter need to be educated about the risk of orthostatic hypotension and risk of falling with initiation of a new antihypertensive medication.
Moderate aortic stenosis: There is no preventive therapy to slow the progression of aortic stenosis.63 With availability of transcatheter aortic valve replacement, the patient may benefit from this treatment in the future. However, frail patients are more likely to have limited benefit and to experience functional decline or death than less frail patients after invasive procedures, including transcatheter aortic valve replacement.64,65 Serial echocardiography every 1–2 years is reasonable given her life expectancy of 5 years. When she becomes symptomatic from progression of aortic stenosis, the overall benefit vs. risk of transcatheter aortic valve replacement should be evaluated considering the degree of cognitive impairment, frailty, and her goals of care. The procedure should be offered only when the expected benefit in achieving health status consistent with the patient’s goals outweighs the risk of harms.
Primary prevention of CVD: There is insufficient evidence on aspirin and statin therapy for primary prevention in adults aged ≥80 years. Since her estimated overall mortality risk is greater than her CVD risk, possible discontinuation of these preventive medications should be discussed with the patient.
In conjunction with the CVD management, the general practitioner should develop a plan to prevent disability from non-cardiovascular conditions.
Frailty: Although frailty is not a formal diagnosis, recognizing frailty is critical in managing older adults with multimorbidity. Due to frailty, she is at imminent risk for loss of independence and institutionalization whenever she experiences a stressful event, including infections, metabolic derangements, drug-related adverse events, environmental hazards, or hospitalizations. Other than regular exercise,66 there is no proven effective therapy for frailty.67 Nonetheless, efforts can be made, such as a home assessment, to avoid preventable harms and mitigate the consequences of frailty.
Mild cognitive impairment: Given her Mini-Mental State Examination score, weight loss, and dependence in instrumental ADLs, she may have mild dementia. Thus, formal cognitive testing and serial monitoring are needed. There is little evidence that cholinesterase inhibitors slow the progression to dementia over 2 years, but there is significant potential for adverse events, including diarrhea (absolute risk increase, 6.6%), nausea (absolute risk increase, 18.8%), syncope or dizziness (absolute risk increase, 5.3%).68 Moderate-intensity physical activity (e.g., walking 150 min/week) may improve cognitive function modestly.69 Her daily walking should be continued.
Osteoarthritis of the knee: Ibuprofen should be discontinued and an alternative pain management regimen, such as scheduled acetaminophen, topical agents, intraarticular steroid injection, physical therapy, or their combination, should be sought.
History of fall, slow gait, and osteopenia: Her fall risk can be decreased with physical therapy targeting gait, balance, and muscle strength; a home safety evaluation would also be appropriate. Discontinuation of cetirizine, which often causes sedation, may reduce the risk of falling, although her allergies could be exacerbated. Her fracture risk should be assessed using the FRAX® tool (http://www.shef.ac.uk/FRAX/) to guide appropriate preventive therapy.
Communicate and discuss decisions about clinical management with the patient
It is important to communicate the trade-offs between treatment benefits and harms (e.g., more vs. less aggressive blood pressure lowering) and between treatments for different health conditions (e.g., hypertension control vs. osteoarthritis pain control).
Reassess at selected intervals for benefit, feasibility, adherence, prognosis, and alignment with preferences
A periodic assessment of blood pressure, cognitive function, and functional status is necessary to monitor for treatment-related adverse events and progression to dementia. The management plan should be revised according to the patient’s prognosis, functional status, and personal preferences. Discussion of advance care planning should take place before her cognitive impairment progresses.
CONCLUSIONS
Cardiologists should work closely with general practitioners to adopt a patient-centered approach to manage CVD and non-cardiovascular health that will have the greatest impact on functioning and quality of life in older adults with CVD and multimorbidity. Before routinely applying recommendations from practice guidelines, clinicians should evaluate the adequacy of the evidence and the feasibility of treatment in the context of the patient’s preferences and prognosis. Polypharmacy should be minimized to avoid drug-drug and drug-disease interactions. Ultimately, clinicians’ efforts should be supported by high-quality evidence relevant to older patients, healthcare systems that facilitate multidisciplinary care, and reimbursement structures that reward the quality of care as measured by effectiveness in achieving patient-centered outcomes.13
Supplementary Material
SUMMARY.
The care of older adults with cardiovascular disease and multimorbidity is challenged by complexity and heterogeneity, lack of high-quality evidence, and fragmented healthcare systems. In this review, we illustrate how cardiologists and general practitioners can adopt a patient-centered approach to maximize benefits and minimize harms for these patients by incorporating the individual’s preferences, relevant evidence, overall and condition-specific prognosis, feasibility of treatments, and drug-drug and drug-disease interactions.
Acknowledgments
Funding support:
Dr. Kim is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies. He is consultant to the Alosa Foundation, a nonprofit educational organization with no relationship to any drug or device manufacturers.
Footnotes
Disclosures:
Dr. Rich has no relationships to disclose.
References
- 1.Centers for Medicare and Medicaid Services. Chronic Conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Baltimore, MD: 2012. [Google Scholar]
- 2.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport J, Jacobs P, Bell NR, Klarenbach S. Refining the measurement of the economic burden of chronic diseases in Canada. Chronic Dis Can. 2004;25(1):13–21. [PubMed] [Google Scholar]
- 4.Cornoni-Huntley JC, Foley DJ, Guralnik JM. Co-morbidity analysis: a strategy for understanding mortality, disability and use of health care facilities of older people. Int J Epidemiol. 1991;20(Suppl 1):S8–17. [PubMed] [Google Scholar]
- 5.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999;52(1):27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 6.Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15(1):83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 7.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and U.S. Department of Health and Human Services. J Am Coll Cardiol. 2014;64(17):1851–1856. doi: 10.1016/j.jacc.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum C, Johnell K. Managing therapeutic competition in patients with heart failure, lower urinary tract symptoms and incontinence. Drugs Aging. 2014;31(2):93–101. doi: 10.1007/s40266-013-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 12.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 13.Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuben DB. Medical care for the final years of life: “When you’re 83, it’s not going to be 20 years”. JAMA. 2009;302(24):2686–2694. doi: 10.1001/jama.2009.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covinsky KE, Hilton J, Lindquist K, Dudley RA. Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care. 2006;44(2):149–157. doi: 10.1097/01.mlr.0000196955.99704.64. [DOI] [PubMed] [Google Scholar]
- 17.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68(4):441–446. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- 20.Castell MV, Sanchez M, Julian R, Queipo R, Martin S, Otero A. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract. 2013;14:86. doi: 10.1186/1471-2296-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171(9):814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 22.Willson MN, Greer CL, Weeks DL. Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother. 2014;48(1):26–32. doi: 10.1177/1060028013510898. [DOI] [PubMed] [Google Scholar]
- 23.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 24.Giovannetti ER, Wolff JL, Xue QL, et al. Difficulty assisting with health care tasks among caregivers of multimorbid older adults. J Gen Intern Med. 2012;27(1):37–44. doi: 10.1007/s11606-011-1831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farris KB, Phillips BB. Instruments assessing capacity to manage medications. Ann Pharmacother. 2008;42(7):1026–1036. doi: 10.1345/aph.1G502. [DOI] [PubMed] [Google Scholar]
- 26.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Wehling M. Multimorbidity and polypharmacy: how to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: fit for the aged. J Am Geriatr Soc. 2009;57(3):560–561. doi: 10.1111/j.1532-5415.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 28.Nobili A, Pasina L, Tettamanti M, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34(4):377–386. doi: 10.1111/j.1365-2710.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 29.American Geriatrics Society. 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 30.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–854. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 32.O’Conner M, O’Sullivan D, Gallagher P, Byrne S, Eustace J, O’Mahony D. Prevention of adverse drug events in hospitalized older patients: a randomised controlled trial using STOPP/START criteria. Eur Geriatr Med. 2012;3(Supplement 1):S132. [Google Scholar]
- 33.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 35.Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210–218. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752. doi: 10.1002/14651858.CD002752.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Pulignano G, Del Sindaco D, Di Lenarda A, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med (Hagerstown) 2010;11(10):739–747. doi: 10.2459/JCM.0b013e328339d981. [DOI] [PubMed] [Google Scholar]
- 38.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 39.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 40.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379(9816):648–661. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 42.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 43.Heckman GA, Tannenbaum C, Costa AP, Harkness K, McKelvie RS. The journey of the frail older adult with heart failure: implications for management and health care systems. Rev Clin Gerontol. 2014;24(4):269–289. [Google Scholar]
- 44.Larson RJ, Fisher ES. Should aspirin be continued in patients started on warfarin? J Gen Intern Med. 2004;19(8):879–886. doi: 10.1111/j.1525-1497.2004.30419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD006257. doi: 10.1002/14651858.CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 47.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 48.Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40 000 patients. Eur Heart J. 2011;32(11):1409–1415. doi: 10.1093/eurheartj/ehr035. [DOI] [PubMed] [Google Scholar]
- 49.Kutner JS, Blatchford PJ, Taylor DH, Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA internal medicine. 2015;175(5):691–700. doi: 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Geriatrics Society Expert Panel on Care of Older Adults with Diabete M. Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 52.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 53.Fleming V, Wade WE. A review of laxative therapies for treatment of chronic constipation in older adults. Am J Geriatr Pharmacother. 2010;8(6):514–550. doi: 10.1016/S1543-5946(10)80003-0. [DOI] [PubMed] [Google Scholar]
- 54.Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. doi: 10.1016/j.ijcard.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 55.Amery A, Birkenhager W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet. 1985;1(8442):1349–1354. doi: 10.1016/s0140-6736(85)91783-0. [DOI] [PubMed] [Google Scholar]
- 56.Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338(8778):1281–1285. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 57.Perry HM, Jr, Davis BR, Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 2000;284(4):465–471. doi: 10.1001/jama.284.4.465. [DOI] [PubMed] [Google Scholar]
- 58.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 59.Group SR. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7(8):683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 61.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie K. Mild cognitive impairment: an epidemiological perspective. Dialogues Clin Neurosci. 2004;6(4):401–408. doi: 10.31887/DCNS.2004.6.4/kritchie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 64.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2013;34(9):684–692. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 65.Green P, Arnold SV, Cohen DJ, et al. Relation of Frailty to Outcomes After Transcatheter Aortic Valve Replacement (from the PARTNER Trial) Am J Cardiol. 2015;116(2):264–269. doi: 10.1016/j.amjcard.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 67.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 69.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


