Abstract
Background
Rice ingestion is an important dietary exposure pathway for methylmercury. There are few studies concerning prenatal methylmercury exposure through rice ingestion, yet the health risks are greatest to the developing fetus, and thus should be investigated.
Objectives
Our main objective was to quantify dietary methylmercury intake through rice and fish/shellfish ingestion among pregnant mothers living in southern China, where rice was a staple food and mercury contamination was considered minimal.
Methods
A total of 398 mothers were recruited at parturition, who donated scalp hair and blood samples. Total mercury and/or methylmercury concentrations were measured in biomarkers, in rice samples from each participant’s home, and in fish tissue purchased from local markets. Additional fish/shellfish mercury concentrations were obtained from a literature search. Dietary methylmercury intake during the third trimester was equivalent to the ingestion rate for rice (or fish/shellfish) × the respective methylmercury concentration.
Results
Dietary methylmercury intake from both rice and fish/shellfish ingestion averaged 1.2 ± 1.8 μg/day (median=0.79 μg/day, range=0–22 μg/day), including on average 71% from rice ingestion (median: 87%, range: 0–100%), and 29% from fish/shellfish consumption (median 13%, range: 0–100%). Median concentrations of hair total mercury, hair methylmercury, and blood total mercury were 0.40 μg/g (range: 0.08–1.7 μg/g), 0.28 μg/g (range: 0.01–1.4 μg/g), and 1.2 μg/L (range: 0.29–8.6 μg/L), respectively, and all three biomarkers were positively correlated with dietary methylmercury intake through rice ingestion (Spearman’s rho=0.18–0.21, p ≤ 0.0005), although the correlations were weak. In contrast, biomarkers were not correlated with fish/shellfish methylmercury intake (Spearman’s rho=0.04–0.08, p=0.11–0.46).
Conclusions
Among pregnant mothers living in rural inland China, rice ingestion contributed to prenatal methylmercury exposure, more so than fish/shellfish ingestion.
Keywords: Mercury, Rice, Fish, China, Prenatal exposure
1. Introduction
Fish consumption is the main dietary source for methylmercury (MeHg), a potent neurotoxin (National Research Council (NRC), 2000); however MeHg exposure also occurs through rice ingestion (Rothenberg et al., 2014). Most rice is cultivated under 5–10 cm of standing water (unlike other terrestrial crops), providing optimal conditions for microbial mercury (Hg) methylation (Qiu et al., 2008; Rothenberg et al., 2014; Windham-Myers et al., 2014). In submerged rice paddy fields, anaerobic microorganisms convert less toxic inorganic Hg to MeHg (Rothenberg and Feng, 2012), which is efficiently translocated from paddy soil to the rice endosperm (i.e., polished rice grain) (Rothenberg et al., 2011a, 2014).
The prenatal period is the most vulnerable exposure window for MeHg (Clarkson and Magos, 2006), yet there are few studies reporting dietary MeHg intake through rice consumption among pregnant mothers. We searched Thomas Reuters (ISI) Web of Science using the phrase “rice and mercury” combined with “hair”, “blood”, or “urine”, which yielded a total of 101 studies. Hair Hg is a biomarker for dietary MeHg intake, blood Hg reflects both MeHg and inorganic Hg exposure, and urine Hg mostly represents elemental and inorganic Hg (reviewed by Clarkson and Magos (2006)). Overlapping studies were excluded, as well as those not reporting data for human health or for rice ingestion and/or rice Hg, resulting in 17 studies (Table A1).
In summary, rice consumption was positively correlated with blood Hg in a U.S. population (n=16,236) (Davis et al., 2014). In a Hg-contaminated area of China, hair and blood Hg were positively correlated with rice MeHg (n=17) (Rothenberg et al., 2013), and one or both biomarkers were correlated with MeHg intake through rice ingestion (μg/kg bw/day) (n=98, Feng et al., 2008; n=168, Li et al., 2015). Only two studies reported data for pregnant mothers (Maramba et al., 2006; Rothenberg et al., 2013); both had small sample sizes (n=35 and n=17, respectively), and pregnant mothers were recruited from Hg-contaminated sites.
In 2013, we initiated a birth and child cohort study in rural China, where rice is a staple food. In this report, our main objective was to quantify dietary MeHg intake through rice and fish/shellfish ingestion, and correlate with biomarkers for prenatal MeHg exposure. We also queried mothers concerning other potential sources of inorganic Hg, including proximity to a mine or working in a mine, whether mothers had dental fillings, and the use of skin whiteners during pregnancy; the latter often contain Hg and are routinely used in Asia (Murphy et al., 2009). We did not specify the kind of mine because Hg often co-occurs with other minerals, which are simultaneously emitted into the environment (Feng et al., 2006). We chose a background site for this investigation rather than a Hg-contaminated site because findings would be relevant to a wider range of rice-eating communities, and to minimize confounding from other Hg sources (e.g., atmospheric Hg). In a companion report, we assessed the impacts of prenatal MeHg exposure on offspring neurodevelopment at 12 months, including maternal hair total Hg (THg), hair MeHg, and blood THg also reported in the present study (Rothenberg et al., submitted for publication).
Rice is a staple food for half the global population (Food and Agriculture Association (FAO), 2015). Compared to fish, rice does not contain the same beneficial micronutrients (Rothenberg et al., 2011b). Thus maternal rice ingestion may represent a potentially important exposure pathway for MeHg.
2. Methods
2.1. Recruitment and data collection
The study site was located inland, in Daxin county, Guangxi Zhuang Autonomous Region, near the China-Vietnam border. Daxin county is 2742 km2 with a population of 359,800 (Guangxi Daxin County Government, 2011), including ~50,000 residents (14%) living in the town of Daxin. There were no significant sources of Hg nearby (e.g., coal-fired power plants). Rice THg from the surrounding villages was low, averaging 1.7 ng/g (range: 0.30–3.7 ng/g, n=15, unpublished data), which was 4.8 times lower compared to other non-contaminated sites (average rice THg: 8.2 ng/g, reviewed by Rothenberg et al. (2014)), suggesting the area was relatively non-contaminated for Hg.
Between May 2013 and March 2014, adult women were recruited at parturition at the Maternal and Child Health Hospital in Daxin county. Eligible mothers were in good general health, resided in Daxin county during the three previous months, and planned to remain for the next year. Protocols were reviewed and approved by the Institutional Review Boards at the University of South Carolina (USA) and XinHua Hospital (China). Mothers provided written informed consent prior to enrollment in the study.
Upon enrollment, a hair sample was collected form the occipital region using stainless steel scissors, the proximal end was tied with dental floss, and the hair sample was stored in a plastic bag at room temperature. A blood sample was collected by venipuncture (6 mL) into two vials, including one with lithium heparin anticoagulant, and a second vial for separation of serum by centrifugation (Yu et al., 2011). Whole blood and aliquots of serum were stored frozen at −26 °C for up to 10 months, then stored at −80 °C until analysis. A family member brought a ~100 g polished rice sample from home (all participants donated a rice sample), which was stored at −26 °C, and then archived at −80 °C until analysis.
While in the hospital mothers filled out a questionnaire, including maternal health, maternal/paternal education, occupation, and household income, and neonatal statistics (e.g., gender, length, and weight). Mothers also filled out a modified 102-item semi-quantitative food frequency questionnaire (FFQ) (Cheng et al., 2009) about their food intake during the third trimester, including rice, fish/shellfish, pork, other meat, eggs, fruits and vegetables. The FFQ asked mothers to chose from eight options ranging from “never or rarely” to “ ≥ twice/day”, and frequencies were converted to servings per day as follows: 0=never or rarely, 1/30.5=monthly, 2.5/30.5=two to three times/month, 1/7=once per week, 2.5/7=two to three times/week, 5/7=four to six times/week, 1=once per day, and 2.5=at least twice per day. For rice, mothers were asked to select portion size (grams per serving) from one of three bowls using a picture and/or actual bowls. The daily rice ingestion rate (grams per day) was calculated by multiplying frequency (servings per day) × the portion size (grams per serving). Mothers also reported the consumption frequency for seven categories of fish/shellfish (including freshwater fish, ocean fish, shrimp, eel, shrimp, crab, and other shellfish) using the same eight options (from “never or rarely” to “ ≥ twice/day”) and frequencies were converted to servings per day as described above. To calculate fish/shellfish ingestion (grams per day), we assumed 170 g/serving for ocean fish and freshwater fish [170 g=6 oz, the recommended serving size from the U.S. Food and Drug Administration (USFDA) (2001)], and 100 g/serving for other categories (Cheng et al., 2009).
Dietary MeHg intake from rice and fish/shellfish consumption (micrograms per day) was quantified by multiplying the ingestion rate (grams per day) × the concentration of rice MeHg (micrograms per gram) or fish/shellfish THg (micrograms per gram). Rice MeHg was measured in each participant’s rice sample brought from home. We measured rice MeHg (not THg) because average rice %MeHg (of THg) ranged from 17 to 75% (reviewed by Rothenberg et al., 2014), while fish THg was >90% MeHg (NRC, 2000). THg concentrations for freshwater fish were analyzed in seven commonly consumed varieties purchased in Daxin markets in 2014 (n=13); however other fish/shellfish varieties were not available for purchase. Therefore a comprehensive literature search was conducted using Thomas Reuters (ISI) Web of Science and the phrase “mercury and China”, which was combined with “seafood”, “fish”, “eel”, “shrimp”, “crab”, “mollusk”, “shellfish”, “snail”, “scallop”, “oyster”, “lobster”, “spiral shell”, or “bivalve”, resulting in 209 studies. Data were included if most or all fish/shellfish samples were collected after 1 January 2011, studies were conducted in non-contaminated sites in China, and THg (or MeHg) concentrations were reported in wet weight (n=11 studies). For “eel”, just one study for China was published in 2006 and was included, resulting in a total of 12 studies (Table 1 and Table A2). For fish/shellfish varieties from the literature search, summary statistics were calculated if possible (mean, median and standard deviation); for studies reporting the sample size, summary statistics were calculated using the sample size as the analytical weight. Daily MeHg intake through fish/shellfish (micrograms per day) was quantified by multiplying the ingestion rate (grams per day) × the average THg concentration (micrograms per gram). The distribution for fish/shellfish THg was highly skewed; therefore the daily MeHg intake was also calculated using the median fish/shellfish THg concentration.
Table 1.
Concentrations of rice methylmercury and fish/shellfish total mercury (see Table A2).
| N | Average ± SD (ng/g) | Median (Range) (ng/g) | References | |
|---|---|---|---|---|
| Rice | 398 | 2.7 ± 2.1 | 2.2 (0.32–15) | This study |
| Freshwater fish | 13 | 31 ± 31 | 23 (2, 98) | This study |
| Marine fisha | 1164 | 56 ± 30 | 32 (0, 760) | Chen et al. (2013), Gao et al. (2014), Geng et al. (2015), Liu et al. (2014a, 2014b), Pan et al. (2014), and Wu et al. (2016) |
| Eela | 21 | 50 ± 10 | NA | Yamashita et al. (2006) |
| Shrimpa | 10 | 10 | NA | Tong et al. (2015) |
| Craba | 10 | 74 | NA | Tong et al. (2015) |
| Snailsa | 10 | 31 | NA | Tong et al. (2015) |
| Other shellfisha | 755 | 68 ± 110 | 24 (7, 450) | Li and Gao (2014), Li et al. (2013), and Zhang and Zhang (2015) |
For marine fish and other shellfish, sampling statistics (average, SD and median) were weighted based on sample size. The average ± SD was provided for eel, while the SD was not provided for shrimp, crab, and snails.
Total dietary MeHg intake (micrograms per day) was equivalent to the daily rice MeHg intake+the daily fish/shellfish MeHg intake; proportional contributions from rice and fish/shellfish ingestion were also determined.
2.2. Hg analyses
Prior to Hg analysis for hair and rice, all labware was acid-washed for >24 h using 1.2 N hydrochloric acid, then triple-rinsed with deionized-water (DDI-H2O), and dried in a Class II biosafety Hood (Baker Company, Sanford, USA) to prevent further Hg contamination, then double-bagged. Blood THg was analyzed directly, and no preparatory steps were needed.
Hair samples corresponding to trimester 3 (34 mm) were analyzed, assuming a growth rate of 0.41 mm/day for Asian women (Loussouarn et al., 2005), and 83 days for trimester 3, reflecting a 10-day lag between ingestion and incorporation of MeHg into the hair shaft (Cernichiari et al., 1995). Before hair THg and MeHg analyses began, two hair-washing methods were compared, including Formula 409™ and 2-mercaptoethanol. Formula 409™ is considered as effective than acetone (the International Atomic Energy Agency hair washing method) (Rothenberg et al., 2013), while 2-mercaptoethanol removes high concentrations of inorganic Hg from hair (Li et al., 2011) and plant material (North and Nobel, 2000). Hair samples were weighed into acid-washed porcelain dishes, 25 mL of 1% (v/v) Formula 409™ or 0.1% (v/v) 2-mercaptoethanol were added, samples were gently shaken for 1 h, then triple-rinsed with DDI-H2O, and samples were air-dried in a biosafety cabinet (Baker Company, Sanford, USA). Two-mercaptoethanol removed on average 19% more exogenous Hg compared to Formula 409™ (range: 14–26%, n=6 samples, 1–3 replicates/sample) (Table A3), and was used for all hair samples.
THg concentrations for hair, blood and fish tissue were measured by thermal decomposition, amalgamation and atomic absorption spectrophotometry following EPA Method 7473 (U.S. Environmental Protection Agency (USEPA), 2007) using a Lumex for hair and fish tissue (Model RA-915+/PYRO-915+, St. Petersburg, Russia) and a DMA-80 for blood (Milestone, Inc., Shelton, CT, USA).
For hair MeHg analysis, samples were weighed into 50 mL Teflon tubes (Savillex, MN, USA) and 5 mL of 25% (w/v) sodium hydroxide-DDI H2O were added, and samples were digested for 3 h at 75ºC, then DDI-H2O was added to a total volume of 50 mL (personal communication, Lian Liang, CEBAM). Digests were analyzed following EPA Method 1630 (USEPA, 2001a), including ethylation with sodium tetraethylborate, purge and trap onto Tenax traps, and quantification using gas chromatography-cold vapor atomic fluorescence spectrometry (Brooks Rand Model III, Seattle, WA, USA).
Rice samples were ground into a powder using a coffee grinder, which was cleaned with ethanol after each sample to prevent carry-over of Hg. Rice MeHg was extracted following Liang et al. (1996), including solvent extraction and back extraction into water. Briefly, ~0.5 g rice was digested in 2 mL of 25% (w/v) potassium hydroxide-methanol for 3 h at 75 °C, then 6 mL of dichloromethane and 1.5 mL hydrochloric acid were added, samples were shaken for 30 min, centrifuged (4000 rpm=3000 × g, 30 min), and the phases were separated, DDI-H2O was added, and samples were heated for 1.5 h in a water bath at 60–70 °C to expel dichloromethane. Extracts were measured using EPA Method 1630 as described above for hair MeHg (USEPA, 2001a).
Quality assurance and quality control for Hg analyses are summarized in Table A4. For hair, rice and fish tissue, average recovery of standard reference material ranged from 78 to 96%, matrix spikes averaged 96–98%, and the average relative percent difference between sample replicates ranged from 4.2 to 8.4%. For blood, recovery of standard reference material ranged from 85 to 115%, and the relative percent difference between sample replicates was < 20%. The limits of detection were instrument- and matrix-specific, including hair THg (0.0095 μg/g), hair MeHg (0.0001 μg/g), blood THg (0.14 μg/L), fish THg (0.001 μg/g), and rice MeHg (0.002 ng/g). All observations were above the limits of detection.
Rice and hair THg and/or MeHg analyses were completed at the University of South Carolina, USA, fish tissue THg was analyzed at Beijing Lumex Analytical Co. Ltd., China, and blood THg was analyzed at the Shanghai State Key Lab for Children’s Environmental Health, China.
2.3. Statistical analyses
Data were assessed using univariate and bivariate analyses. Correlations were determined using Spearman’s correlation (for non-symmetrical variables) or Pearson’s correlation (for symmetrical variables). For Pearson’s correlation, a log10-transformation was applied to variables with a right-skewed distribution. Prior to the log10-transformation, a value of 0.01 was added to all observations for fish/shellfish MeHg intake (micrograms per day) because more >40% of mothers rarely or never ingested fish/shellfish. We defined an alpha-level of < 0.05 as a guide for significance, where appropriate. Analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) or the R-platform.
For the rice ingestion rate, 5.5% of mothers did not choose a frequency (servings per day) or quantity (grams per serving). Missing observations were imputed based on the multivariate normal distribution (Schafer, 1997), conditional on biomarker concentrations and maternal, paternal and offspring characteristics. For fish/shellfish consumption, 4.3% did not choose a frequency for one or more fish/shellfish categories, and a value of 0 was imputed for these observations, which was appropriate for this inland region in rural China. We assumed mothers skipped some categories for fish/shellfish because these varieties were often unavailable. Data analyses were checked with and without imputed observations, and results did not differ.
3. Results
3.1. Recruitment
During the 10-month recruitment window, a total of 1261 mothers gave birth at the Maternal and Child Health Hospital, including 408 (32%) eligible mothers who provided informed consent. Ten mothers were subsequently excluded because they did not give birth in the hospital (n=1), gave birth to twins (n=1), did not live in Daxin during the previous three months (n=3), or data collection was incomplete (n=5), resulting in a final cohort of 398 mothers. The average maternal age at parturition was 28 ± 5.7 years, a majority of mothers were Zhuang ethnicity (85%), 13% were Han (the majority in China), and 2% of mothers were other ethnicities, 6.8% lived near a mine, one or both parents worked as a miner in 2.9% of households, 11% of mothers had dental fillings, and 5.2% used whitening cosmetics during pregnancy. Most mothers were farmers (77%), while 8.1% were workers (including civil servants, white-collar workers, skilled/unskilled workers or shopkeepers), 11% were unemployed, and 13% selected “other” for occupation.
3.2. Rice and fish/shellfish consumption
On average mothers ingested 1.8 servings/day of rice (median: 2.5 servings/day, range: 0–2.5 servings/day). Most mothers ingested rice daily (86%), and only three mothers (< 1%) rarely or never ingested rice. Rice consumption averaged 231 g/day (median: 210 g/day; range: 0–650 g/day), which was similar to the rate of the general Chinese population (214 g/day) (FAO, 2015), and was similar to the rice ingestion rate among 17 pregnant mothers in rural Guizhou province, China (200 g/day, from Rothenberg et al. (2013)).
On average mothers ingested 0.13 servings/day of fish/shellfish (median: 0.03 servings/day, range: 0–2.9 servings/day), i.e., 14 times lower compared to servings per day of rice. A total of 172 mothers (43%) rarely or never consumed fish/shellfish, while 45 mothers (11%) consumed fish/shellfish ≥ twice/weekly. Fish/shellfish consumption averaged 18 g/day (median: 5.6 g/day, range: 0–470 g/day), which was lower than the average rate in China (95 g/day) (FAO, 2015). Of the seven categories of fish/shellfish, the average consumption for freshwater fish was highest (12 g/day), followed by shrimp (2.3 g/day), marine fish: (1.6 g/day), and eel (1.1 g/day), and other categories each averaged < 1 g/day (snails, other shellfish, and crabs) (median=0 g/day for all categories).
Rice and fish/shellfish ingestion (servings per day) were weakly positively correlated (Spearman’s rho=0.12, p=0.02).
3.3. Rice and fish/shellfish Hg concentrations
Concentrations of rice MeHg and fish/shellfish THg were considered low-level (Table 1). Among Daxin participants, 92% (n=367) of rice MeHg concentrations were < 5.8 ng/g, i.e., within the range previously reported for background sites (Rothenberg et al., 2014). THg concentrations for freshwater fish purchased in Daxin markets averaged 0.031 ± 0.031 μg/g (n=13), which was 16 times lower than the Chinese dietary guideline (0.5 μg/g) (U.S. Department of Agriculture (USDA), 2014) and 9.7 times lower than the U.S. EPA human health criterion (0.3 μg/g) (USEPA, 2001b). Average THg concentrations for fish/shellfish obtained through a literature search were also up to 30 times lower than the U.S. EPA criterion (USEPA, 2001b).
The estimated daily MeHg intake from rice and fish/shellfish was calculated using mother’s responses recorded in the FFQ and the MeHg concentrations for rice and THg concentrations for fish/shellfish (Table 2). Using the average THg fish/shellfish concentration, for mothers ingesting rice and/or fish/shellfish (n=396), %MeHg intake from rice averaged 71% (median: 87%, range: 0–100%), while %MeHg intake from fish/shellfish averaged 29% (median 13%, range: 0–100%). Using the median THg fish/shellfish concentration, results did not change; %MeHg intake from rice averaged 73% (median: 89%, range: 0–100%), while %MeHg intake from fish/shellfish averaged 27% (median 11%, range: 0–100%). Results indicated maternal rice ingestion was a significant source of dietary MeHg intake in this non-contaminated site in rural China.
Table 2.
Food intake, dietary methylmercury intake, and biomarker mercury concentration (n=398 mothers).
| Mean | Median (Range) | ||
|---|---|---|---|
| Foods (servings/day) | Rice | 1.8 | 2.5 (0, 2.5) |
| Fish/shellfish | 0.13 | 0.033 (0, 2.9) | |
| Rice+Fish/Shellfish | 0.99 | 1.0 (0, 3.9) | |
| Porka, b | 1.9 | 1.3 (0, 9.6) | |
| Eggsb | 0.13 | 0.08 (0, 1) | |
| Chickenb | 0.14 | 0.08 (0, 1) | |
| Beef and Sheepb | 0.02 | 0 (0, 1) | |
| Dietary MeHg intake (μg/day) | Rice | 0.63 | 0.44 (0, 5.0) |
| Fish/Shellfish | 0.60 | 0.15 (0, 20) | |
| Rice+Fish/Shellfish | 1.2 | 0.79 (0, 22) | |
| Hg Biomarkers | Hair THg (μg/g) | 0.48 | 0.40 (0.077, 1.7) |
| Hair MeHg (μg/g) | 0.32 | 0.28 (0.011, 1.4) | |
| Hair %MeHg (of THg) | 67 | 67 (14, 108) | |
| Blood THg (μg/L) | 1.5 | 1.2 (0.29, 8.6) |
Hg (mercury), MeHg (methylmercury), THg (total mercury).
Pork includes 6 options (pork chops, pork spare ribs, pork feet, pork fat, lean pork, and pork meat).
Missing data for pork (n=18), eggs (n=25), chicken (n=21), and beef and sheep (n=23), n=398 otherwise.
3.4. Hg biomarkers
Hair and blood THg concentrations for Daxin mothers (Table 2) were compared with hair and blood THg concentrations corresponding to the EPA reference dose (i.e., 0.1 μg/kg bw/day, USEPA, 1997). A total of eight mothers (2.0%) had hair THg concentrations >1.2 μg/g, which is the National Research Council hair THg level corresponding to the reference dose (NRC, 2000), an additional four mothers (1.0%) had hair THg concentrations >1.1 μg/g, which is the EPA hair THg level corresponding to the reference dose (USEPA, 1997), and four mothers (1%) had blood Hg concentrations exceeding 5.8 μg/L, which is the National Research Council cord blood THg level corresponding to the reference dose (NRC, 2000). Just one mother had blood and hair THg concentrations exceeding both the NRC and EPA recommended levels.
Hair %MeHg (of THg) averaged 67% (median: 67%, range: 14–108%) (Table 2), which was lower than the expected value of 90% (NRC, 2000). This result was not surprising given the wide range of values previously reported in the literature (13–100%, reviewed by Rothenberg et al. (2013)), suggesting more research is needed to understand potential demethylation of MeHg after incorporation into the hair shaft.
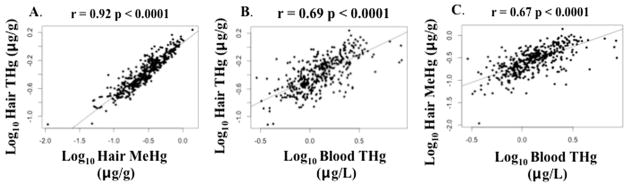
Hair THg and MeHg concentrations were highly correlated (Spearman’s rho=0.92, p < 0.0001), and both were positively correlated with blood THg concentrations (for both: Spearman’s rho=0.69, p < 0.0001). Using log10-transformed variables, hair THg and MeHg were strongly correlated (Pearson’s rho=0.92, p < 0.0001), and both variables were strongly correlated with blood THg (for both: Pearson’s rho=0.67–0.69, p < 0.0001) (Fig. 1).
Fig. 1.
Bivariate scatterplots relating biomarker concentrations of total mercury (THg) and methylmercury (MeHg) for A) hair THg versus hair MeHg, B) hair THg versus blood THg, and C) hair MeHg versus blood THg (all variables log10-transformed), including p-value for Pearson’s correlation (n =397–398 mothers).
3.5. Hg biomarkers and rice/fish ingestion
Associations between Hg biomarkers and rice and fish/shellfish ingestion (servings per day) were determined. Trends were positive (but non-significant) between Hg biomarkers and servings per day of rice (Spearman’s rho: 0.04–0.09, p=0.07–0.42) and servings per day of fish/shellfish (Spearman’s rho: 0.03–0.08, p=0.14–0.54). Using rice+fish/shellfish servings per day, trends were weakly positive and significant for hair THg (Spearman’s rho=0.10, p=0.04) and non-significant for hair MeHg and blood THg (Spearman’s rho=0.05–0.06, p=0.23–0.30).
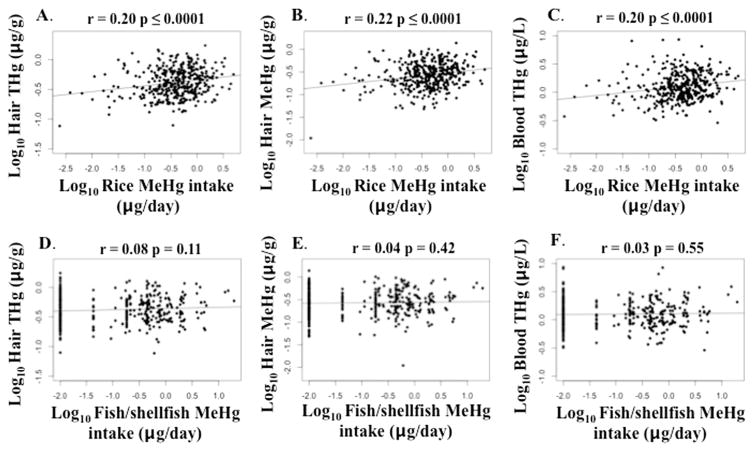
Associations between Hg biomarkers and dietary MeHg exposure were strengthened when using dietary MeHg intake for rice+fish/shellfish (micrograms MeHg per day), although associations remained weak (Spearman’s rho=0.16–0.18, p ≤ 0.002). The contributions of rice and fish/shellfish to dietary MeHg intake were considered separately. Dietary MeHg intake through rice ingestion was weakly positively correlated with all three biomarkers (hair THg, hair MeHg and blood THg) (Spearman’s rho=0.17–0.21, p ≤ 0.0005), while the trends for Hg biomarkers and dietary MeHg intake through fish/shellfish ingestion were non-significant (Spearman’s rho=0.04–0.08, p=0.11–0.46). Results were similar after applying the log10-transformation (Fig. 2): all three biomarkers were weakly positively correlated with MeHg intake through rice ingestion (Pearson’s rho=0.20–0.22, p ≤ 0.0001), while MeHg intake through fish/shellfish ingestion was not strongly correlated with Hg biomarkers (Pearson’s rho=0.03–0.08, p=0.11–0.55).
Fig. 2.
Bivariate scatterplots relating biomarker concentrations of total mercury (THg) and methylmercury (MeHg) to dietary MeHg intake through rice ingestion (A–C) and fish/shellfish consumption (D–F), including A) hair THg versus rice MeHg intake, B) hair MeHg versus rice MeHg intake, C) blood THg versus rice MeHg intake, D) hair THg versus fish/shellfish MeHg intake, E) hair MeHg versus fish/shellfish MeHg intake, and F) blood THg versus fish/shellfish MeHg intake (all variables log10-transformed), including p-values for Pearson’s correlation (n=397–398 mothers).
We compared Hg sources between mothers with low hair THg ( ≤ 1.1 μg/g, n=386) and with high hair THg (>1.1 μg/g, n=12), using the EPA hair THg level corresponding to the reference dose (USEPA, 1997) (Table 3). Median fish/shellfish ingestion and MeHg intake through fish/shellfish ingestion were 1.5 and 1.3 times higher, respectively, for mothers with hair THg > 1.1 μg/g compared to mothers with lower hair Hg. Rice ingestion (median servings per day) did not differ between both groups (2.5 servings/day); however median MeHg intake through rice was 1.2 times higher for mothers with lower hair THg. A greater proportion of mothers with higher hair THg lived near a mine (25%) compared to mothers with lower hair THg (6.2%); however, 0% of mothers with higher hair THg (and fathers) did not work in the mines. Lower hair THg was also associated with higher use of skin whiteners and dental fillings. Skin whiteners contain inorganic Hg and dental fillings contain elemental Hg, which are less likely to accumulate in hair (Clarkson and Magos, 2006).
Table 3.
Comparison of mercury sources for mothers with high hair total mercury (>1.1 μg/g) (n=386 mothers) and low hair total mercury ( ≤ 1.1 μg/g) (n=12 mothers).
| Hair THg ≤ 1.1 μg/g Median or % | Hair THg>1.1 μg/g Median or % | Ratio between high/low hair THg | |
|---|---|---|---|
| Rice (servings/day) | 2.5 | 2.5 | 1 |
| Fish/Shellfish (servings/day) | 0.033 | 0.049 | 1.5 |
| Rice+Fish/Shellfish (servings/day) | 0.99 | 1.1 | 1.1 |
| MeHg Intake Through Rice (μg/day) | 0.45 | 0.37 | 0.82 |
| MeHg Intake Through Fish/Shellfish (μg/day) | 0.14 | 0.18 | 1.3 |
| MeHg Intake Through Rice+Fish/Shellfish (μg/day) | 0.79 | 0.74 | 0.94 |
| Mine Near Home | 6.2% | 25% | 4.0 |
| Use of Skin Whiteners During Pregnancya | 5.4% | 0% | 0 |
| Dental Fillingsa | 11% | 8.3% | 0.75 |
| Mothers Who Were Minersa | 1.3% | 0% | 0 |
| Fathers Who Were Minersa | 2.1% | 0% | 0 |
MeHg (methylmercury), THg (total mercury).
Missing data for skin whiteners (n=15), dental fillings (n=18), mothers mining (n=16), and fathers mining (n=13); n=398 otherwise.
3.6. Comparison with fish-eaters
We compared hair THg and blood THg from the present study to values for pregnant mothers with low-level MeHg exposure mainly through fish ingestion (Table 4). Low-level was defined as hair THg < 4 μg/g and blood THg < 12 μg/L (Karagas et al., 2012), and cohorts included at least 100 participants (there were no low-level Hg studies reporting hair MeHg). Estimated fish/shellfish consumption from other studies was up to 5.3 times higher, while mothers in the present study ingested up to 21 times more rice. For other studies, median (or geometric mean or average) hair THg ranged from 0.23 to 0.60 μg/g, while blood THg ranged from 0.38 to 3.9 μg/L. Despite differences in dietary MeHg intake (rice versus fish), hair THg and blood THg reported for the present study were comparable to other cohorts of pregnant women with low-level MeHg exposure.
Table 4.
Low-level hair and blood mercury levels in pregnant women.
| Study Site | Average rice consumption (g/capita/day)a | Average fish/shellfish consumption (g/capita/day)a | Median Hair THg (μg/g) (n) | Median Blood THg (μg/L) (n) | Reference |
|---|---|---|---|---|---|
| Guangxi, China | 231 | 18 | 0.40 (398) | 1.2 (397) | This study |
| Alaska, USA | 20 | 59 | 0.47 (150) | Arnold et al. (2005) | |
| Mexico | 15 | 25–27b | 0.39 (371) | 2.88–2.79 (217–248) | Basu et al. (2014) |
| Sweden | 17 | 25b | 0.35 (127) | Ask Björnberg et al. (2003) | |
| Norway | 13 | 42b | 1.67 (119) | Brantsaeter et al. (2010) | |
| South Africa | 47 | 16 | 0.60 (349) | Channa et al. (2013) | |
| France | 16 | 95 | 0.52 (645) | Drouillet-Pinard et al. (2010) | |
| Spain | 26 | 76 | 3.9 (140) | Garcia-Esquinas et al. (2013) | |
| Avon, UK | 19 | 52 | 1.86 (4134) | Golding et al. (2013) | |
| Slovenia | 11 | 25c | 0.297 (574) | Miklavcic et al. (2011) | |
| Massachusetts, USA | 20 | 29d | 0.45e (135) | Oken et al. (2005) | |
| Massachusetts, USA | 20 | 59 | 0.6f (393) | Orenstein et al. (2014) | |
| USA | 20 | 59 | 0.7e (1183) | Razzaghi et al. (2014) | |
| North Carolina, USA | 20 | 59 | 0.45e (210) | Sanders et al. (2012) | |
| New Jersey, USA | 20 | 39g | 0.53f (189) | 0.99f (149) | Stern et al. (2001) |
| Istanbul, Turkey | 22 | 20 | 0.38e (143) | Unuvar et al. (2007) | |
| Michigan, USA | 20 | 18h | 0.23 (1024) | Xue et al. (2007) |
n (sample size), THg (total mercury).
Aside from this study and otherwise indicated in footnotes b–f, average per capita consumption of rice and fish/shellfish were obtained from FAO (2015).
Mean fish/seafood consumption reported in manuscripts.
25 g/day from Miklavcic et al. (2011).
29 g/capita/day=(1.2 fish meals/week × 170 g/meal)/7 days (Average of 1.2 fish meals/week from Oken et al. (2005)).
Geometric mean.
Average.
39 g/capita/day=(83 fish meals/year × 170 g/meal)/365 days (Average of 83 fish meals/year from Stern et al. (2001).
18 g/capita/day=(19.6 fish meals/6 months × 170 g/meal)/30.5 days (Average of 19.6 fish meals/6 months from Xue et al. (2007).
4. Discussion
Rice ingestion is an important dietary source for MeHg (Rothenberg et al., 2013, 2014), and MeHg exposure is considered most harmful to the developing fetus (Clarkson and Magos, 2006). In rural Guangxi province, China, rice ingestion was a significant dietary source of prenatal MeHg exposure, contributing on average 71% of dietary MeHg intake (median: 87%, range: 0–100%), while fish/shellfish ingestion contributed on average 29% (median 13%, range: 0–100%). Daxin county was a relatively non-contaminated site for Hg, yet maternal biomarker Hg concentrations were comparable to populations of pregnant mothers where fish ingestion was the primary MeHg exposure pathway. Given the global importance of rice as a staple food (FAO, 2015), results suggest rice-eating communities may be at risk for MeHg exposure, similar to fish-eaters.
The correlation between Hg biomarkers and dietary MeHg sources (servings per day) was weaker compared to studies among pregnant mothers where fish ingestion was the primary dietary source of MeHg. As noted above, the strongest correlation was between hair THg and rice+fish ingestion (Spearman’s rho=0.10, p=0.04). In Mexico, Spearman’s correlation between maternal blood Hg (for trimesters 2 and 3) and seafood consumption was 0.246 and 0.173 (p < 0.001 for both), respectively (Basu et al., 2014). In Sweden, the correlation between hair THg and total seafood consumption (< 5 meals/month, 5–9 meals/month, >9 meals/month) was 0.41 (p < 0.01) (Ask Björnberg et al., 2003). In the U.K., ingestion of seafood explained 6.98% of the variability in blood THg (Golding et al., 2013), i.e., rho=0.26. In New Jersey, the correlation between total fish intake (log10-transformed) and hair THg was 0.18 (p=0.02) (Stern et al., 2001). Differences between the present study and others suggest potential variability in MeHg metabolism between rice and fish/shellfish. For example, rice contains more fiber compared to fish (cooked white rice: 0.6 g fiber/cup, fish: 0 g fiber/cup), which may reduce the accumulation of MeHg in tissues (Rowland et al., 1986), although the mechanism is uncertain.
In this analysis, we assumed rice and fish/shellfish ingestion represented the main dietary MeHg sources. However other foods may contain MeHg, including pork, chicken and hen eggs (Ask Björnberg et al., 2003; Cheng et al., 2013; Lindberg et al., 2004; Tang et al., 2015). In Sweden, this was attributed to feeding farm animals fish meal (Ask Björnberg et al., 2003; Lindberg et al., 2004), while in an e-waste region of China, pigs and hens were fed MeHg-contaminated rice (Tang et al., 2015). In the present study, MeHg concentrations were not measured in other foods; however we queried mothers concerning servings per day of pork, chicken, eggs, and other meat (beef and sheep) (Table 2). The direction of correlation between Hg biomarkers and pork, chicken and eggs was inverse or the correlation was=0 (Spearman’s rho= −0.08–0, p=0.13–0.96), while the correlation was non-significant between Hg biomarkers and other meat (Spearman’s rho=0.02 for all biomarkers, p=0.64–0.76). Results suggested these foods were not important dietary sources of MeHg in this population.
Although there are a number of strengths to our study, including a sample size of 398 mothers and recruitment of mothers from an area without significant Hg contamination, there are also limitations, which are worth acknowledging. The FFQ was self-completed by mothers, reflecting food intake for the previous three months. The FFQ was previously validated among pregnant mothers in a rural area in western China (Cheng et al., 2009); however, measures for food consumption were estimates, with potentially large error. Maternal biomarkers for prenatal MeHg exposure (hair THg, hair MeHg, and blood THg) were more strongly correlated with dietary MeHg intake through rice ingestion compared to fish/shellfish consumption. Differences may be due in part to greater precision in rice MeHg data because each participant brought a rice sample from home, while data for fish/shellfish were obtained by measuring fish THg, which was purchased in local markets, and through a literature search.
5. Conclusions
Although, fish/shellfish consumption is considered the primary MeHg exposure pathway (NRC, 2000), rice ingestion contributed significantly to prenatal MeHg exposure in this rural area of China. Rice MeHg concentrations were similar to rice MeHg from background sites, and >40% of mothers rarely or never ingested fish/shellfish. However biomarkers for Hg (hair THg and blood THg) were comparable to values reported for pregnant mothers who were fish-eaters. In China, rice ingestion rates average more than 10 times higher compared to fish/shellfish ingestion (FAO, 2015), compensating for the low MeHg concentrations in rice. This likely occurs in other communities, where rice is a staple food. Researchers investigating prenatal MeHg exposure should include rice ingestion as a dietary source of MeHg, even if rice MeHg levels are considered low (Dórea, 2014).
Supplementary Material
Acknowledgments
We gratefully acknowledge the comments from anonymous reviewers, which greatly improved the manuscript. This study was supported by grants to S.E. Rothenberg from the U.S. National Institute of Environmental Health Sciences (R15 ES022409) and the U.S. National Institutes of Health Loan Replacement Program (L30 ES023165). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2016.06.038.
References
- Arnold SM, Lynn TV, Verbrugge LA, Middaugh JP. Human biomonitoring to optimize fish consumption advice: reducing uncertainty when evaluating benefits and risks. Am J Public Health. 2005;95:393–397. doi: 10.2105/AJPH.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ask Björnberg KA, Vahter M, Petersson-Grawé K, Glynn A, Cnattingius S, Darnerud PO, Atuma S, Aune M, Becker W, Berglund M. Methyl mercury and inorganic mercury in Swedish pregnant women and in cord blood: influence of fish consumption. Environ Health Perspect. 2003;111:637–641. doi: 10.1289/ehp.111-1241457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Tutino R, Zhang Z, Cantonwine DE, Goodrich JM, Somers EC, Rodriguez L, Schnaas L, Solano M, Mercado A, Peterson K, Sánchez BN, Hernández-Avila M, Hu H, Téllez-Rojo MM. Mercury levels in pregnant women, children, and seafood from Mexico City. Environ Res. 2014;135:63–69. doi: 10.1016/j.envres.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsaeter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA, Alexander J, Meltzer HM. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- Cernichiari E, Toribara TY, Liang L, Marsh DO, Berlin MW, Myers GJ, Cox C, Shamlaye CF, Choisy O, Davidson P, Clarkson TW. The biological monitoring of mercury in the Seychelles study. Neurotoxicology. 1995;16:613–627. [PubMed] [Google Scholar]
- Channa K, Odland JØ, Kootbodien T, Theodorou P, Naik I, Sandanger TM, Röllin HB. Differences in prenatal exposure to mercury in South African communities residing along the Indian Ocean. Sci Total Environ. 2013;463–464:11–19. doi: 10.1016/j.scitotenv.2013.05.055. [DOI] [PubMed] [Google Scholar]
- Chen X, Han C, Cheng H, Wang Y, Liu J, Xu Z, Hu L. Rapid speciation analysis of mercury in seawater and marine fish by cation exchange chromatography hyphenated with inductively coupled plasma mass spectrometry. J Chromatogr A. 2013;1314:86–93. doi: 10.1016/j.chroma.2013.08.104. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dibley MJ, Zhang X, Zeng L, Yan H. Assessment of dietary intake among pregnant women in a rural area of western China. BMC Public Health. 2009;9:222. doi: 10.1186/1471-2458-9-222. http://dx.doi.org/10.1186/1471-2458-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Wang HS, Du J, Sthiannopkao S, Xing GH, Kim KW, Yasin MSM, Hashim JH, Wong MH. Dietary exposure and risk assessment of mercury via total diet study in Cambodia. Chemosphere. 2013;92:143–149. doi: 10.1016/j.chemosphere.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, Frost HR. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS One. 2014;9(9):e104768. doi: 10.1371/journal.pone.0104768. http://dx.doi.org/10.1371/journal.pone.0104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dórea JG. Comments on neonatal hair-Hg and birth weight in China: mercury in rice and fish. Environ Pollut. 2014;184:654. doi: 10.1016/j.envpol.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Drouillet-Pinard P, Huel G, Slama R, Forhan A, Sahuquillo J, Goua V, Thiébaugeorges O, Foliguet B, Magnin G, Kaminski M, Cordier S, Charles MA. Prenatal mercury contamination: relationship with maternal seafood consumption during pregnancy and fetal growth in the “EDEN mother-child” cohort. Br J Nutr. 2010;104:1096–1100. doi: 10.1017/S0007114510001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Li G, Qiu G. A preliminary study on mercury contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang County, Guizhou, China: part 2. Mercury contaminations to soil and crop. Sci Total Environ. 2006;368:47–55. doi: 10.1016/j.scitotenv.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Feng X, Li P, Qiu G, Wang S, Li G, Shang L, Meng B, Jiang H, Bai W, Li Z, Fu X. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol. 2008;42:326–332. doi: 10.1021/es071948x. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) [accessed 19.05.16];Statistics Division Homepage. 2015 Available: < http://faostat3.fao.org/home/E>.
- Gao YX, Zhang H, Yu X, He JL, Shang X, Li X, Zhao Y, Wu Y. Risk and benefit assessment of potential neurodevelopmental effect resulting from consumption of marine fish from a coastal archipelago in China. J Agric Food Chem. 2014;62:5207–5213. doi: 10.1021/jf500343w. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E, Pérez-Gómez B, Fernández-Navarro P, Fernández MA, de Paz C, Pérez-Meixeira AM, Gil E, Iriso A, Sanz JC, Astray J, Cisneros M, de Santos A, Asensio Á, García-Sagredo JM, García JF, Vioque J, López-Abente G, Pollán M, González MJ, Martínez M, Aragonés N. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health. 2013;13:841. doi: 10.1186/1471-2458-13-841. http://dx.doi.org/10.1186/1471-2458-13-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Li H, Liu JP, Yang Y, Jin ZL, Zhang YN, Zhang ML, Chen LQ, Du ZY. Nutrients and contaminants in tissues of five fish species obtained from Shanghai markets: risk–benefit evaluation from human health perspectives. Sci Total Environ. 2015;536:933–945. doi: 10.1016/j.scitotenv.2015.06.057. [DOI] [PubMed] [Google Scholar]
- Golding J, Steer CD, Hibbeln JR, Emmett PM, Lowery T, Jones R. Dietary predictors of maternal prenatal blood mercury levels in the ALSPAC birth cohort study. Environ Health Perspect. 2013;121:1214–1218. doi: 10.1289/ehp.1206115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [accessed 25.05.16];Guangxi Daxin County Government. 2011 < http://www.daxin.gov.cn/>.
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huang ZY, Hu Y, Yang H. Potential risk assessment of heavy metals by consuming shellfish collected from Xiamen, China. Environ Sci Pollut Res. 2013;20:2937–2947. doi: 10.1007/s11356-012-1207-3. [DOI] [PubMed] [Google Scholar]
- Li Peimiao, Gao X. Trace elements in major marketed marine bivalves from six northern coastal cities of China: concentrations and risk assessment for human health. Ecotoxicol Environ Saf. 2014;109:1–9. doi: 10.1016/j.ecoenv.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Li Ping, Feng X, Qiu G, Wan Q. Hair can be a good biomarker of occupational exposure to mercury vapor: simultaneous experiments and field data analysis. Sci Total Environ. 2011;409:4484–4488. doi: 10.1016/j.scitotenv.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Li Ping, Feng X, Chan HM, Zhang X, Du B. Human body burden and dietary methylmercury intake: the relationship in a rice-consuming population. Environ Sci Technol. 2015;49:9682–9689. doi: 10.1021/acs.est.5b00195. [DOI] [PubMed] [Google Scholar]
- Liang L, Horvat M, Cernichiari E, Gelein B, Balogh S. Simple solvent extraction technique for elimination of matrix interferences in the determination of methylmercury in environmental and biological samples by ethylation-gas chromatography-cold vapor atomic fluorescence spectrometry. Talanta. 1996;43:1883–1888. doi: 10.1016/0039-9140(96)01964-9. [DOI] [PubMed] [Google Scholar]
- Lindberg A, Ask Björnberg K, Vahter M, Berglund M. Exposure to methylmercury in non-fish-eating people in Sweden. Environ Res. 2004;96:28–33. doi: 10.1016/j.envres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu X, Yu S, Cheng H, Hong Y, Feng X. Mercury pollution in fish from South China Sea: levels, species-specific accumulation, and possible sources. Environ Res. 2014b;131:160–164. doi: 10.1016/j.envres.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Liu JL, Xu XR, Yu S, Cheng H, Peng JX, Hong YG, Feng XB. Mercury contamination in fish and human hair from Hainan Island, South China Sea: implication for human exposure. Environ Res. 2014a;135:42–47. doi: 10.1016/j.envres.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44:6–9. doi: 10.1111/j.1365-4632.2005.02800.x. [DOI] [PubMed] [Google Scholar]
- Maramba NPC, Reyes JP, Francisco-Rivera AT, Panganiban LCR, Dioquino C, Dando N, Timbang R, Akagi H, Castillo MT, Quitoriano C, Afuang M, Matsuyama A, Eguchi T, Fuchigami Y. Environmental and human exposure assessment monitoring of communities near an abandoned mercury mine in the Philippines: a toxic legacy. J Environ Manag. 2006;81:135–145. doi: 10.1016/j.jenvman.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Miklavcic A, Cuderman P, Mazej D, Snoj Tratnik J, Krsnik M, Planinšek P, Osredkar J, Horvat M. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ Res. 2011;111:1201–1207. doi: 10.1016/j.envres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Murphy T, Slotton DG, Irvine K, Sukontason K, Goldman CR. Mercury contamination of skin whiteners in Cambodia. Hum Ecol Risk Assess Int J. 2009;15:1286–1303. [Google Scholar]
- National Research Council (NRC) Toxicological Effects of Methylmercury. National Academy Press; Washington DC: 2000. [Google Scholar]
- North GB, Nobel PS. Heterogeneity in water availability alters cellular development and hydraulic conductivity along roots of a desert succulent. Ann Bot. 2000;85:247–255. [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein STC, Thurston SW, Bellinger DC, Schwartz JD, Amarasiriwardena CJ, Altshul LM, Korrick S. Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts. Environ Health Perspect. 2014;122:1253–1259. doi: 10.1289/ehp.1307804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K, Chan H, Tam YK, Wang WX. Low mercury levels in marine fish from estuarine and coastal environments in southern China. Environ Pollut. 2014;185:250–257. doi: 10.1016/j.envpol.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Qiu G, Feng X, Li P, Wang S, Li G, Shang L, Fu X. Methylmercury accumulation in rice (Oryza sativa L.) grown at abandoned mines in Guizhou, China. J Agric Food Chem. 2008;56:2465–2468. doi: 10.1021/jf073391a. [DOI] [PubMed] [Google Scholar]
- Razzaghi H, Tinker SC, Crider K. Blood mercury concentrations in pregnant and nonpregnant women in the United States: National Health and Nutrition Examination Survey 1999–2006. Am J Obstet Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.10.884. http://dx.doi.org/10.1016/j.ajog.2013.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SE, Feng X. Mercury cycling in a flooded rice paddy. J Geophys Res Biogeosci. 2012;117:G03003. http://dx.doi.org/10.1029/2011JG001800. [Google Scholar]
- Rothenberg SE, Feng X, Li P. Low-level maternal methylmercury exposure through rice ingestion and potential implications for offspring health. Environ Pollut. 2011a;159:1017–1022. doi: 10.1016/j.envpol.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Feng X, Dong B, Shang L, Yin R, Yuan X. Characterization of mercury species in brown and white rice (Oryza sativa L.) grown in water-saving paddies. Environ Pollut. 2011b;159:1283–1289. doi: 10.1016/j.envpol.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Yu X, Zhang Y. Prenatal methylmercury exposure through maternal rice ingestion: insights from a feasibility pilot in Guizhou Province, China. Environ Pollut. 2013;180:291–298. doi: 10.1016/j.envpol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Windham-Myers L, Creswell JE. Rice methylmercury exposure and mitigation: a comprehensive review. Environ Res. 2014;133:407–423. doi: 10.1016/j.envres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SE, Yu X, Liu J, Biasini FJ, Hong C, Jiang X, Nong Y, Cheng Y, Korrick SA. Submitted for publication. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: a prospective cohort study. doi: 10.1016/j.ijheh.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland IR, Mallett AK, Flynn J, Hargreaves RJ. The effect of various dietary fibres on tissue concentration and chemical form of mercury after methylmercury exposure in mice. Arch Toxicol. 1986;59:94–98. doi: 10.1007/BF00286730. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Flood K, Chiang S, Herring AH, Wolf L, Fry RC. Towards prenatal biomonitoring in North Carolina: assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS One. 2012;7:e31354. doi: 10.1371/journal.pone.0031354. http://dx.doi.org/10.1371/journal.pone.0031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall; London: 1997. [Google Scholar]
- Stern AH, Gochfeld M, Weisel C, Burger J. Mercury and methylmercury exposure in the New Jersey pregnant population. Arch Environ Health. 2001;56:4–10. doi: 10.1080/00039890109604048. [DOI] [PubMed] [Google Scholar]
- Tang W, Cheng J, Zhao W, Wang W. Mercury levels and estimated total daily intakes for children and adults from an electronic waste recycling area in Taizhou, China: key role of rice and fish consumption. J Environ Sci. 2015;34:107–115. doi: 10.1016/j.jes.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Tong YD, Ou LB, Chen L, Wang HH, Chen C, Wang XJ, Zhang W, Wang QG. Modeled methylmercury exposure and risk from rice consumption for vulnerable populations in a traditional fish-eating area in China. Environ Toxicol Chem. 2015;34:1161–1168. doi: 10.1002/etc.2888. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture (USDA) China’s Maximum Levels for Contaminants in Foods. 2014. Report Number: CH14058. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Mercury Study Report to Congress Volume VII: Characterization of Human Health and Wildlife Risks From Mercury Exposure in the United States. Environmental Protection Agency; Washington DC: 1997. EPA-452/R-97-009. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Method 1630, Methyl Mercury in Water by Distillation Aqueous Ethylation, Purge and Trap, and Cold Vapor Atomic Spectrometry. 2001a. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Water Quality Criterion for the Protection of Human Health Methylmercury. 2001b. EPA-823-R-01-001. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Method 7473: mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. 2007. [Google Scholar]
- U.S. Food and Drug Administration (USFDA) [accessed 19.05.16];Consumer Advisory: An Important Message for Pregnant Women of Childbearing Age Who May Become Pregnant and the Risks of Mercury in Fish. 2001 < http://www.rst2.edu/ties/mercury/university/pdfs/me35.pdf>.
- Unuvar E, Ahmadov H, Kiziler AR, Aydemir B, Toprak S, Ulker V, Ark C. Mercury levels in cord blood and meconium of healthy newborns and venous blood of their mothers: clinical, prospective cohort study. Sci Total Environ. 2007;374:60–70. doi: 10.1016/j.scitotenv.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Windham-Myers L, Fleck JA, Ackerman JT, Marvin-DiPasquale M, Stricker CA, Heim WA, Bachand PAM, Eagles-Smith CA, Gill G, Stephenson M, Alpers CN. Mercury cycling in agricultural and managed wetlands: a synthesis of methylmercury production, hydrologic export, and bioaccumulation from an integrated field study. Sci Total Environ. 2014;484:221–231. doi: 10.1016/j.scitotenv.2014.01.033. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang H, Liu G, Zhang J, Wang J, Yu Y, Lu S. Concentrations and health risk assessment of trace elements in animal-derived food in southern China. Chemosphere. 2016;144:564–570. doi: 10.1016/j.chemosphere.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect. 2007;115:42–47. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Omura Y, Okazaki E. Distinct regional profiles of trace element content in muscle of Japanese eel Anguilla japonica from Japan, Taiwan, and China. Fish Sci. 2006;72:1109–1113. [Google Scholar]
- Yu XD, Yan CH, Shen XM, Tian Y, Cao LL, Yu XG, Zhao L, Liu JX. Prenantal exposure to multiple toxic heavy metals and neonatal neurobehavioral development in Shanghai, China. Neurotoxicol Teratol. 2011;33:437–443. doi: 10.1016/j.ntt.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang L. Contribution of shellfish consumption to lower mercury health risk for residents in northern Jiaozhou Bay, China. Bioinorg Chem, Appl. 2015 doi: 10.1155/2015/159521. http://dx.doi.org/10.1155/2015/159521. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.