Abstract
Objective
To examine change and individual trajectories for balance, upper extremity motor capacity, and mobility in people post-stroke during the time they received outpatient therapies.
Design
Retrospective analyses of an observational cohort using hierarchical linear modeling.
Setting
Outpatient rehabilitation
Participants
366 persons post stroke
Interventions
Usual outpatient physical and occupational therapy
Main Outcomes
Berg Balance Scale (BBS), Action Research Arm Test (ARAT), and walking speed were used to assess the 3 domains. Initial scores at the start of outpatient therapy (intercepts), rate of change during outpatient therapy (slopes), and the covariance between slopes and intercepts were modeled as random-effects. Additional variables modeled as fixed effects were: Duration (months of outpatient therapy), Time (days post-stroke), Age (years), and Inpatient status (if the patient went to an inpatient rehabilitation facility (IRF)).
Results
A patient with average Age and Time started at 37 points on the BBS with a change of +1.8 points per month, at 35 points on the ARAT with a change of +2.0 points per month, and with a walking speed of 0.59 m/s with a change of +0.09 m/s per month. When controlling for other variables, patients started with lower scores on the BBS and ARAT or had slower walking speeds at admission if they started outpatient therapy later than average or went to an IRF.
Conclusions
Patients generally improved over the course of outpatient therapy, but there was considerable variability in individual trajectories. Average rates of change across all three domains were small.
Keywords: stroke, outpatient rehabilitation, standardized assessment
Although stroke has now dropped to the fifth most common cause of death in the United States,1 it remains the leading cause of long-term disability and a financial burden of over $30 billion dollars.2 About 70% of patients post stroke receive post-acute care services, with about 31% receiving outpatient rehabilitation services.2 Outpatient rehabilitation is provided to people who are living at home and come to an outpatient facility. 3 Community stroke rehabilitation has been shown to be beneficial,4, 5 and evidence suggests that sub-groups of people post-stroke, perhaps those who are younger or more mildly affected, may have a greater benefit.4, 5
A challenge for clinicians referring to and providing outpatient rehabilitation services is not being able to easily predict who might benefit from outpatient services and how much change is likely to occur. Trajectories of recovery post stroke have been reasonably well-characterized,6–9 but this information does not directly speak to the challenge of determining benefit and change during outpatient therapy at an individual patient level. Persons post-stroke arrive at outpatient therapy at different times post stroke, have different clinical presentations, and receive services for various amounts of time. Knowing if an individual is likely to experience a change in function and the anticipated magnitude of that change would assist clinicians, patients, and families as they make decisions about therapy services. The purpose of this study therefore, was to examine balance, upper extremity functional capacity, and mobility changes in people post-stroke who received outpatient physical and/or occupational therapy. To enhance generalizability to clinical practice, a heterogeneous sample of people with stroke undergoing routine outpatient services was studied.
METHODS
Sample & Measures
This study utilized a convenience sample of 366 records stored in the Brain Recovery Core database for patients admitted to outpatient physical or occupational therapy from March 2010 through September 2014.10, 11 All patients in the database have a primary diagnosis of stroke (ischemic, hemorrhagic, or transient ischemic attack), and have provided informed consent to have his or her stroke rehabilitation data stored and used for research. The Washington University Human Research Protection Office approved the database and studies using de-identified data.
During outpatient therapy, patients received motor therapies from licensed therapists, as deemed appropriate by their healthcare team. Based on previous observations,12 therapeutic activities and exercises generally include: strength, transfer, gait, balance, upper limb, and/or activities of daily living training, and prescription of equipment, assistive devices and/or orthotics. Patients who were only evaluated at admission were included in our analysis to determine factors influencing scores at admission to outpatient therapy. Not all patients had multiple time points however, so only data from patients evaluated at least twice influence our estimates of change over time. Standardized evaluations are performed at admission and on a monthly basis at this facility for patients receiving outpatient therapy. The evaluations are completed by licensed clinicians who have been trained and monitored for consistency. Assessment data reported in this paper are part of the evaluations.10, 13 Three motor assessments were used for this analysis: 1) the Berg Balance Scale (BBS), a measure of static and dynamic balance;14, 15 2) the Action Research Arm Test (ARAT) on the paretic side, a measure of upper extremity functional capacity;16, 17 and 3) self-selected walking speed from the 10-m Walk Test (10m WT), a measure of mobility.18–21 Specific methods to standardize administration of each measure have been previously described.10, 13 Additional characteristics were also collected for description of the sample and for consideration in the analysis: age at time of stroke, sex, if the patient went to an inpatient rehabilitation facility (IRF), time post-stroke at admission to outpatient therapy, and duration of outpatient therapy.
Inpatient status (if the patient went to an IRF) was used as a proxy for categorizing initial functional status as more or less disabled for several reasons. First, not all patients included in the outpatient sample went to our acute care hospital; therefore multiple acute measures were not available. Second, the ARAT is not administered at the acute care time point precluding us from using this measure as a covariate. Third, patients who go to an IRF immediately post stroke typically have more complex rehabilitation needs than people who can be at home and receive outpatient services.22 Within the current data set, for example, we examined assessment scores from the acute hospital admission for both the BBS and 10m WT which were statistically worse for participants who were known to have received inpatient services prior to starting outpatient therapy (ps < 0.001, t-tests correcting for unequal variances). Inpatient status was determined by discharge destination from the acute hospital, presence within the IRF (as part of the Brain Recovery Core), or by report at admission to outpatient therapy. If Inpatient status was missing, it was conservatively coded as no inpatient services, given that we could not find the patients in other electronic inpatient records/systems. The validity of this assumption was confirmed by comparing analyses including vs. excluding these individuals; both analyses yielded the same statistical conclusions.
Statistical Analyses
In order to quantify changes in balance (BBS), upper limb functional capacity (ARAT), and mobility (walking speed), a series of hierarchical linear models were constructed. For these models, Duration of therapy (measured in months of outpatient therapy) was nested within patients. The mixed-effect regressions treated Duration, Time (days post-stroke at outpatient admission), Age (years), and Inpatient status (−1 = did not go to IRF or status uncertain, +1 = did go to IRF) as fixed-effects. Initial assessment scores at the start of outpatient therapy (intercepts), variation in the rate of change during outpatient therapy (individual slopes), and the covariance between intercepts and slopes were modeled as random-effects. A multi-level modeling approach was chosen because it is similar to conventional regression analyses but allows us to model the entire trajectory of outpatient therapy.23, 24 The multi-level modeling approach is more complete and more precise for analyzing longitudinal data than alternative approaches (e.g., repeated measures ANOVA) because it uses all available data from a larger number of patients, whereas repeated-measures ANOVA or regressions based on change scores would require data to be omitted and/or would not allow data collected at different time points for different individuals.25
All statistical analyses were conducted using R and R Studio26 using the “lme4”27 and “dplyr”28 packages. To model changes in the BBS, ARAT, and walking speed as a function of Duration, a “step-up” procedure was used in which variables were added to successive models. Models were compared based on the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and Wald Tests for the change in deviance with α = 0.05.25 The BIC is included for completeness, but the AIC is the best estimate of the long-run predictive deviance given our sample size. Thus, weight is placed on the AIC and the p-value of the Wald Tests in selecting models. As shown in Table 1, all models started with assessment scores at admission to outpatient rehabilitation (random intercepts, model 0) and rates of change (random slopes, model 1) for each assessment (BBS, ARAT, walking speed). Given that initial functional status is a strong predictor of rehabilitation outcome post stroke,3, 5 we reasoned that patients who went to IRF (Inpatient status) would have lower initial assessment scores (intercepts) at the beginning of outpatient therapy. We also reasoned that Age29 and Time30–32 (days post-stroke at the start of outpatient therapy) would have negative effects on rate of change (i.e. slopes) during outpatient therapy. For these reasons, we incrementally added fixed-effects (Age, Time, and Inpatient status) that would interact with the intercept and the slope (models 2–4) before adding potential interactions of between-subjects factors with each other (model 5). Parameter values in a given model control for all other parameters in that model.
Table 1.
Step-up procedure for testing regression models.
| Model | Statistical Model | Description |
|---|---|---|
| 0 | y = 1 + (1|Patient) | Random intercepts model. |
| 1 | y = 1 + Duration + (1 + Duration| Patient) | Random slopes and intercepts model. |
| 2 | y = 1 + Duration*lnTime.c + (1 + Duration| Patient) | Effects of Time on slopes and intercepts. |
| 3 | y = 1 + Duration*lnTime.c + Duration*Age.c + (1 + Duration| Patient) | Adding the effects of Age on slopes and intercepts, controlling for Time. |
| 4 | y = 1 + Duration*lnTime.c + Duration*Age.c + Duration*Inpatient + (1 + Duration| Patient) | Adding the effects of Inpatient status on slopes and intercepts controlling for Age and Time. |
| 5 | y = 1 + Duration*lnTime.c + Duration*Age.c + Duration*Inpatient + lnTime.c*Age.c*Inpatient + (1 + Duration| Patient) | Adding the interaction of the between-subject factors, controlling for previous factors. |
Note. All models used maximum likelihood estimation.
Duration: months of outpatient therapy
lnTime.c: natural log of Time (days post stroke at the start of outpatient therapy) centered around the mean
Age.c: Age (years) centered around the mean.
Inpatient: contrast-coded variable denoting if a patient did/did not attend an inpatient rehabilitation facility
Individual patient trajectories were plotted to evaluate how Duration of therapy should be modeled. Visual inspection indicated that a linear effect of Duration was most appropriate. Thus, modeling for the three assessments (BBS, ARAT, and walking speed) started with estimating the starting score (intercept) at admission to outpatient rehabilitation for each patient, adding a linear effect of Duration, and then adding interactions with other variables (Age, Time, Inpatient status). Subsequent exploratory analyses also considered stroke type (ischemic vs. hemorrhagic) and number of strokes (first vs. multiple) in the model. Stroke type was removed when it had no influence on slopes or intercepts. Number of strokes was correlated with age and shared some of the same predictive value. Since age information is generally more accessible in routine service delivery, age and not number of strokes was retained. Contrast codes and centering were employed to ease the interpretation of regression models. In a mean-centered model, the coefficient of one variable (e.g., Duration of therapy) is being evaluated at average values of the other variables. Thus, we present the variable Age.c, which is the Age variable centered on the mean age of 58.2 years, and lnTime.c, which is the natural log of the Time variable centered on the mean lnTime of 4.37 (79 days). This centering allows the regression coefficients to be interpreted like main effects and interactions in a conventional regression. The variable Time was log-transformed to achieve a normal distribution of residuals. All other assumptions of the regression were met, and multicolinearity was low (maximum variance inflation factor across all models was 1.6).
RESULTS
Descriptive statistics for the sample and the variables in the models are provided in Table 2.
Table 2.
Descriptive statistics for data included in the regression models.
| Demographic Variables | Mean (SD) | [Min, Max] |
|---|---|---|
| Age (yrs) | 58.2 (12.7) | [20, 93] |
| Time (Days from stroke to beginning of outpatient therapy) | Median = 59.0 | [4, 6451.0] |
| 254.2 (611.6) | ||
| ln(Time) | 4.37 (1.34) | [1.39, 8.77] |
| Duration (months of outpatient therapy) | 4.02 (3.02) | [1, 12] |
|
| ||
| Counts (%) | Notes | |
|
| ||
| Females | 166 (45%) | |
| Type of Stroke | ||
| Ischemic | 189 (52%) | |
| Hemorrhagic | 40 (11%) | |
| TIA* | 1 (<1%) | |
| Missing | 136 (37%) | |
| First stroke | 214 (58%) | |
| Received inpatient therapy | 221 (60%) | Coded as “1” in regression. |
| No inpatient therapy | 41 (12%) | Coded as “−1” in regression. |
| Inpatient therapy unlikely | 104 (28%) | Coded as “−1” in regression. |
|
| ||
| Outcome Variables | Group Mean (SD) | Individual Patient [Min, Max] |
|
| ||
| BBS (minimum) | 37.0 (16.3) | [0, 56] |
| ΔBBS (max-min) | 6.1 (7.2) | [0, 36] |
| ARAT (minimum) | 33.7 (22.4) | [0, 57] |
| ΔARAT (max-min) | 4.3 (9.0) | [0, 57] |
| 10mWT (minimum) | 0.57 (0.48) | [0.00, 1.90] |
| Δ10mWT (max-min) | 0.25 (0.33) | [0.00, 2.35] |
Note. The group mean for the outcome variable is the average of the lowest scores for each patient. The minimum and maximum values for the outcome variables reflect individual patients. Thus, the Delta; values represent the change score for each subject over the course of therapy (e.g., for the BBS, some patients showed no change, some changed by 36 points, and on average patients changed by 6.1 points).
While TIA is usually defined by resolution of symptoms, the fact that this person received outpatient rehabilitation suggests lingering stroke-induced deficits.
Changes in balance (BBS) with outpatient therapy
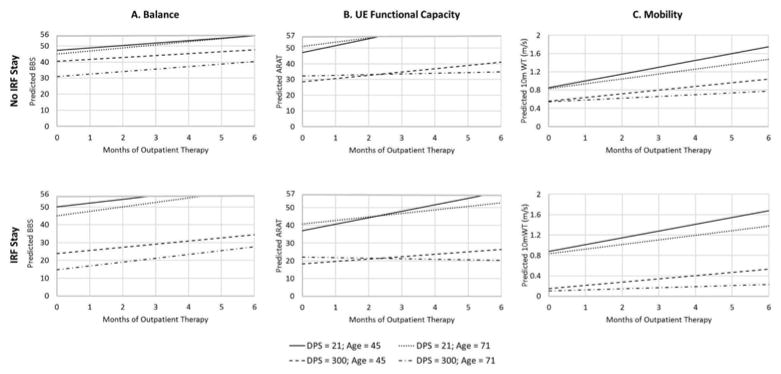
Comparing across different regression models for BBS scores, the most complex model, B5, provided the lowest AIC and a statistically significant decrease in deviance beyond model B4, see Supplemental Table I. As shown in Table 3, a patient of average Age and Time started at 37 points on the BBS (intercept estimate) and improved by approximately 1.8 points per month on average (slope estimate), controlling for other factors in the model. Increasing Age, increasing Time, and Inpatient status were all associated with lower starting assessment scores. For every one year increase in Age, the estimated BBS score at the start of outpatient therapy was 0.25 points lower. Similarly, for every one point increase in lnTime (e.g. starting therapy at 55 days where ln=4 instead of 21 days where ln=3), the BBS score at the start of outpatient therapy was 7.25 points lower. Having gone to an IRF also led to a lower assessment score at the start of outpatient therapy. For the average Age and Time, a patient who went to an IRF had an initial BBS score 7.5 points lower than a patient who did not. In addition, the effects of Time and Inpatient status interacted such that the effect of Time was greater for patients who went to an IRF than those who did not, shown in Figure 1 (A panels). This indicates that patients who took longer to start outpatient therapy and who went to inpatient rehabilitation had a lower initial BBS score at the start of outpatient therapy, compared to those who did not go to an IRF. Furthermore, the correlation between random-effects was r = −0.17, suggesting that patients’ rate of change (i.e. slopes) were relatively independent of their starting assessment scores (i.e. intercepts).
Table 3.
Details of the best fitting regression model (B5) for the Berg Balance Scale.
| Random-Effects | |||
|---|---|---|---|
|
| |||
| Groups | Name | Variance | Correlation |
| Subject | Intercept | 171.54 | -- |
| Duration | 1.74 | −0.16 | |
| Residual | 14.51 | ||
|
| |||
| # of Observations | 998 | ||
| # of Participants | 328 | ||
| Fixed-Effects | |||
|---|---|---|---|
|
| |||
| Estimate | Standard Error | 95% MOE | |
| Intercept | 37.20 | 0.85 | ± 1.67* |
| lnTime.c | −7.25 | 0.73 | ± 1.43* |
| Age.c | −0.25 | 0.07 | ± 0.14* |
| Inpatient | −3.77 | 0.85 | ± 1.67* |
| lnTime.c X Age.c | −0.09 | 0.05 | ± 0.10 |
| lnTime.c X Inpatient | −3.36 | 0.68 | ± 1.37* |
| Age X Inpatient | −0.02 | 0.65 | ± 1.27 |
| lnTime.c X Age.c X Inpatient | 0.02 | 0.05 | ± 0.10 |
| Duration | 1.83 | 0.16 | ± 0.31* |
| Duration X lnTime.c | −0.13 | 0.13 | ± 0.25 |
| Duration X Age.c | 0.01 | 0.01 | ± 0.02 |
| Duration X Inpatient | 0.30 | 0.16 | ± 0.31 |
Note. lnTime.c is centered on a mean value of 4.37 (or ~79 days). Age.c is centered on a mean age of 58.16 years. Inpatient status was contrast coded as {−1 = no inpatient therapy/status uncertain; +1 = received inpatient therapy}.
denotes a 95% confidence interval that does not contain zero (i.e., p < 0.05). MOE = margin of error; Time = the number of days from the stroke to the beginning of outpatient therapy. For generating individual patient profiles, a non-centered version of this equation is also presented in the supplemental information.
Figure 1.
Predictions of the regression model B5 for the Berg Balance Scale (BBS, left), model A4 for the Action Research Arm Test (ARAT, center), model T5 for the 10m Walk Test (10m WT, right). Predicted scores are shown as a function of duration of outpatient therapy, patient age, time (as days post-stroke; DPS), and Inpatient status (the top shows predictions for patients who did not attend an inpatient rehab facility/had uncertain status, the bottom row shows predictions for patients who did attend an inpatient rehab facility). Predictions were generated for ±1 standard deviation in lnTime, which translates to approximately 21 and 300 days post-stroke, and ±1 standard deviation in Age, which translates to approximately 45 and 71 years old. UE = upper extremity.
Changes in upper limb functional capacity (ARAT) with outpatient therapy
Comparing the different models for ARAT scores, model A4 provided the lowest AIC and a statistically significant decrease in deviance beyond model A3, see Supplemental Table I. As shown in Table 4, a patient of average Age and Time started at 34.6 points on the ARAT and improved by approximately 2 points per month on average, controlling for other factors in the model. Time and Inpatient status, but not Age, were associated with lower scores on the ARAT at admission to outpatient therapy. For every one point increase in lnTime (i.e. a later start to outpatient therapy), the initial ARAT score was 7.2 points lower. Similarly, Inpatient status also showed a negative effect on the initial ARAT scores. For the average Age and Time, a patient who received inpatient therapy began outpatient therapy 10.1 points lower than a patient who did not.
Table 4.
Details of the best fitting regression model (A4) for the Action Research Arm Test.
| Random-Effects | |||
|---|---|---|---|
|
| |||
| Groups | Name | Variance | Correlation |
| Subject | Intercept | 398.2 | -- |
| Duration | 7.29 | -0.21 | |
| Residual | 15.85 | ||
|
| |||
| # of Observations | 528 | ||
| # of Participants | 242 | ||
| Fixed-Effects | |||
|---|---|---|---|
|
| |||
| Estimate | Standard Error | 95% MOE | |
| Intercept | 34.65 | 1.40 | ± 2.74* |
| lnTime.c | −7.20 | 1.10 | ± 2.16* |
| Age.c | 0.16 | 0.11 | ± 0.22 |
| Inpatient | −5.07 | 1.46 | ± 2.86* |
| Duration | 2.04 | 0.35 | ± 0.68* |
| Duration X lnTime.c | −0.88 | 0.28 | ± 0.55* |
| Duration X Age.c | −0.07 | 0.02 | ± 0.04* |
| Duration X Inpatient | −0.37 | 0.37 | ± 0.73 |
Note. lnTime.c is centered on a mean value of 4.37 (or ~79 days). Age.c is centered on a mean age of 58.16 years. Inpatient status was contrast coded as {−1 = no inpatient therapy/status uncertain; +1 = received inpatient therapy}.
denotes a 95% confidence interval that does not contain zero (i.e., p < 0.05). MOE = margin of error; Time = the number of days from the stroke to the beginning of outpatient therapy. For generating individual patient profiles, a non-centered version of this equation is also presented in the supplemental information.
Although the between-subject effects did not seem to interact with each other, both Time and Age interacted with Duration of therapy. The interaction of Duration with Time showed that for every one point below the mean lnTime a patient was (i.e. an earlier start in outpatient therapy), the improvement from outpatient therapy (slope) increased by 0.88 points/month. Similarly, for every one year below the mean age a patient was, the effect of outpatient therapy increased by 0.07 points/month. Thus, younger patients who start therapy earlier show a faster rate of improvement in outpatient therapy, shown in Figure 1 (B panels). Furthermore, when fixed-effects are controlled (Time, Age, Inpatient status), the correlation between random-effects was r = −0.21, suggesting that patient’s rate of change (i.e. slopes) were relatively independent of their starting point (i.e. intercepts).
Changes in mobility (10m WT speed) with outpatient therapy
Comparing the different models for walking speed, model T5 provided the lowest AIC and a statistically significant decrease in deviance beyond model T4, see Supplemental Table I. As shown in Table 5, a patient of average Age and Time began outpatient therapy with a walking speed of approximately 0.59 m/s and improved by approximately 0.09 m/s per month on average, controlling for other factors in the model. Time, Age, and Inpatient status all had negative effects on the intercept, meaning that the later a patient started therapy, the older a patient was, or if the patient had gone to an IRF, walking speed at admission to outpatient therapy was slower. For every one point increase in lnTime (i.e. a later start in outpatient therapy), the intercept was 0.196 m/s slower. For every one year increase in age the intercept decreased by 0.006 m/s. For a patient of average Age and Time that went to an IRF, this led to an intercept 0.21 m/s slower than a patient who did not go to an IRF. These effects were complicated by an interaction of lnTime and Inpatient status, such that the negative effects of Time were greater for patients that went to an IRF, shown in Figure 1 (C panels).
Table 5.
Details of the best fitting regression model (T5) for the 10 m Walk Test.
| Random-Effects | |||
|---|---|---|---|
|
| |||
| Groups | Name | Variance | Correlation |
| Subject | Intercept | 0.152 | -- |
| Duration | 0.004 | 0.490 | |
| Residual | 0.028 | ||
|
| |||
| # of Observations | 987 | ||
| # of Participants | 318 | ||
| Fixed-Effects | |||
|---|---|---|---|
|
| |||
| Estimate | Standard Error | 95% MOE | |
| Intercept | 0.594 | 0.027 | ± 0.053* |
| lnTime.c | −0.196 | 0.022 | ± 0.043* |
| Age.c | −0.006 | 0.002 | ± 0.004* |
| Inpatient | −0.103 | 0.027 | ± 0.053* |
| lnTime.c X Age.c | 0.0001 | 0.00018 | ± 0.004 |
| lnTime.c X Inpatient | −0.085 | 0.022 | ± 0.043* |
| Age X Inpatient | −0.0007 | 0.0021 | ± 0.004 |
| lnTime.c X Age.c X Inpatient | −0.0000 | 0.0020 | ± 0.004 |
| Duration | 0.085 | 0.007 | ± 0.0.014* |
| Duration X lnTime.c | −0.027 | 0.006 | ± 0.012* |
| Duration X Age.c | −0.0018 | 0.0005 | ± 0.001* |
| Duration X Inpatient | −0.008 | 0.007 | ± 0.014 |
Note. lnTime.c is centered around a mean value of 4.37 (or ~79 days). Age.c is centered around a mean age of 58.16 years. Inpatient status was contrast coded as {−1 = no inpatient therapy/status uncertain; +1 = received inpatient therapy}.
denotes a 95% confidence interval that does not contain zero (i.e., p < 0.05). MOE = margin of error; Time = the number of days from the stroke to the beginning of outpatient therapy. For generating individual patient profiles, a non-centered version of this equation is also presented in the supplemental information.
Furthermore, there were interactions of both Age and Time with Duration, suggesting that younger patients who started outpatient therapy sooner had faster rates of improvement during outpatient therapy. The interaction of Duration with Time showed that for every one point below the mean lnTime (i.e. an earlier start in outpatient therapy), the rate of improvement increased by 0.027 m/s per month. Similarly, for every one year below the mean age, a patient’s rate of improvement increased by 0.002 m/s per month. In contrast to the models for the BBS and the ARAT, there was also a moderate positive correlation between the random-effects, r = 0.49, suggesting that patients with higher intercepts also tended to have steeper slopes, controlling for the fixed-effects. Thus, if there were two participants with the same Age, Time, and Inpatient status, the participant with the higher starting assessment score (i.e. higher intercept) is likely to have a faster rate of change.
For all three motor assessments, equations for estimating scores for individual patients are provided in the Supplemental Information. These supplemental equations are statistically equivalent to the models presented above (with respect to significance and explained variance), but are based on uncentered predictors. Although centering simplifies the interpretation of coefficients, centering also makes it more difficult to use the regression equations to make novel predictions.
DISCUSSION
Patients generally improved over the course of outpatient therapy across the measured activity domains, but there was considerable variability in individual trajectories. Patients who started outpatient therapy later or went to an inpatient rehabilitation facility had lower initial scores at admission to outpatient therapy. People who were older also had slightly worse scores at admission to outpatient therapy for balance and mobility, but the magnitude of these effects was quite small. Across all three domains, the average rate of change was positive. For upper-extremity functional capacity and mobility, but not balance, the rate of change was faster for patients who started outpatient therapy sooner and/or were younger. While there are large amounts of data regarding the time course of recovery from early to later after stroke,6–9, 33 the current results provide the first quantitative profiles of change during outpatient therapy at various times post stroke and across the domains of balance, upper-extremity functional capacity, and mobility.
Standardized functional outcomes, such as the Functional Independence Measure,20, 34 have been a part of inpatient services for a long time.3 It is only recently, with new requirements for functional outcome reporting, that outcomes are more routinely collected during outpatient therapy. Although much variability exists in the specific measures used to track outcomes, balance, upper extremity functional capacity and mobility are three functional domains that are highly relevant to people with stroke.3, 21, 35–37 Outpatient therapy interventions delivered at our facility for people post-stroke are similar to services provided across the United States and Canada.12 Our data describe the changes that may be expected to occur during usual and customary outpatient rehabilitation services in these domains, but causal attribution of the changes cannot be determined. The prediction equations generated here (see Supplemental Information) can be used to estimate how much a person might change during the time outpatient rehabilitation services are provided, given his/her initial clinical presentation. Since these data were collected at a single site, it will be important to collect similar data from other facilities to understand the extent to which these data can be generalized.
There was tremendous variability in the time post-stroke that people arrived to outpatient therapy. The majority arrived within the first two months, but about 25% of the people arrived six months or more post stroke. This is part of the complexity of outpatient service delivery. Information presented here will allow clinicians and facilities to better manage referrals and clinical services, as well as assist in clinical decision making related to prognosis and plan of care. Interestingly, for mobility and upper extremity functional capacity, the people who were younger and arrived to outpatient therapy sooner had faster rates of improvement. The faster rates of improvement for mobility and upper extremity functional capacity earlier may be because of the additive benefit of natural recovery early after stroke. Another possibility is that people who are arriving earlier were more responsive to the services because the presence of endogenous recovery mechanisms might augment the effectiveness of therapy.38, 39 Why there were faster rates of improvement for two domains but not the balance domain is unknown.
These data provide new information to inform clinical decision making at a variety of levels. At the present time, people are referred to and participate in outpatient therapy services with the assumption that therapy services will help them ‘get better’. The prognostic data presented here estimates, based on individual characteristics, how much change someone might anticipate for a given duration of therapy services. The average rates of change over time (slopes) observed in each of the three domains were smaller than anticipated, with monthly changes smaller than estimates of minimal detectable change40, 41 or most estimates of minimally clinically important difference.42–45 These rates of change are likely to be smaller than many patient and family perceptions of ‘getting better’. As an example of how these data might be used, patients are often referred for balance deficits with a pre-specified amount (or duration) of allowable services. Using the prognostic data here, one could estimate the anticipated change from those services. These anticipated changes could be shared with the patient and family, and if the anticipated changes are not enough to make a meaningful difference in balance or safety, then other approaches (e.g. home modifications, fall alert device, training with an assistive device) might be implemented right away. Alternatively, more aggressive therapies and/or a longer commitment to participating in services could be considered. While no prognostic data can perfectly predict how an individual will change over time, repeated (e.g. monthly) assessments will determine if the patient is progressing as expected and can be used to make adjustments to the plan of care. Given the basic information of age and time since stroke, a clinician now has a more robust mechanism for predicting changes in balance, upper extremity functional capacity, and mobility.
Study Limitations
There are several limitations that should be considered when interpreting these data. First, the data are from a clinical database. Thus, the analyses include only patients who agreed to have their data included in the database. We cannot make any statements about the people who do not consent nor know if they are in some way systematically different. Similarly, description of the study population and the conclusions drawn are limited to only the variables available. Additional descriptive variables, such as co-morbidities and clinical imaging test results, were limited. Given the heterogeneity of the sample, it is possible that individual trajectories may have been more or less influenced by missing descriptive variables. It is noteworthy however, that in many outpatient rehabilitation environments, access to these variables is often limited as well. A second limitation is that data from all patients who were seen for the admission time point in outpatient therapy were included in the model. Patients with a single time-point influence only the estimated intercepts, whereas patients who were evaluated at least two times will influence the estimated slopes. The inclusion of all patients (including those with only an admission time point for the intercept) could have led to an over-fitting of the model, but this is unlikely as people with only one time point are a minority of the patients in each analysis and when we repeated the analyses after removing those patients we obtained equivalent results for all three dependent measures. Third, although our model predicts changes in outpatient therapy for durations up to twelve months, the bulk of the patients had therapy durations between 1–6 months, starting at various time points post stroke. Thus, the models’ estimates will be most accurate for those durations. Finally, the exact intervention delivered to each patient and the frequency of that intervention is not known. It is possible that patient change across the measures of balance, upper limb functional capacity, and mobility could have been (positively or negatively) influenced by a specific therapist or intervention. With our sample size however, these influences are likely minimal.
Conclusions
Patients generally improved over the course of outpatient therapy across the measured activity domains of balance, upper extremity functional capacity, and mobility. Demographics of patients coming to outpatient therapy were variable, with initial admission scores heavily dependent on time post-stoke and whether or not the patient went to an inpatient rehabilitation facility. The average rates of change across all three domains for the duration of outpatient therapy were small, but younger patients who started outpatient therapy sooner had greater rates of improvement. The simplest conclusion overall may be that, for the average patient, a single month of outpatient therapy is going to yield relatively little benefit. Most patients may require multiple months of outpatient therapy in order to achieve clinically meaningful benefits with any degree of certainty. The quantitative profiles generated here may be used to predict patient change during outpatient therapy across these different activity domains.
Supplementary Material
Acknowledgments
Funding: Funding was provided by the Barnes Jewish Hospital Foundation and the Washington University McDonnell Center for Systems Neuroscience. Salary support (CEL) was provided by NIH R01HD068290.
We thank the staff, administrators, and data entry team at Barnes Jewish Hospital, The Rehabilitation Institute of Saint Louis, and Washington University for their enthusiasm, support, and efforts on this project.
ABBREVIATIONS
- BBS
Berg Balance Scale
- ARAT
Action Research Arm Test
- 10m WT
Ten Meter Walk Test
- IRF
Inpatient Rehabilitation acility
- AIC
Akaike Information Criterion
- BIC
Bayesian Information Criterion
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the united states, 2013. Nchs data brief. 2014;(178) [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, et al. Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke. 2005;36:e100–143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 4.Legg L, Langhorne P Outpatient Service T. Rehabilitation therapy services for stroke patients living at home: Systematic review of randomised trials. Lancet. 2004;363:352–356. doi: 10.1016/S0140-6736(04)15434-2. [DOI] [PubMed] [Google Scholar]
- 5.Quinn TJ, Paolucci S, Sunnerhagen KS, Sivenius J, Walker MF, Toni D, et al. Evidence-based stroke r-ehabilitation: An expanded guidance document from the european stroke organisation (eso) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. J Rehabil Med. 2009;41:99–111. doi: 10.2340/16501977-0301. [DOI] [PubMed] [Google Scholar]
- 6.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: The copenhagen stroke study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 8.Tilling K, Sterne JA, Rudd AG, Glass TA, Wityk RJ, Wolfe CD. A new method for predicting recovery after stroke. Stroke. 2001;32:2867–2873. doi: 10.1161/hs1201.099413. [DOI] [PubMed] [Google Scholar]
- 9.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2015;29:614–622. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- 10.Lang CE, Bland MD, Connor LT, Fucetola R, Whitson M, Edmiaston J, et al. The brain recovery core: Building a system of organized stroke rehabilitation and outcomes assessment across the continuum of care. J Neurol Phys Ther. 2011;35:194–201. doi: 10.1097/NPT.0b013e318235dc07. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland MD, Sturmoski A, Whitson M, Harris H, Connor LT, Fucetola R, et al. Clinician adherence to a standardized assessment battery across settings and disciplines in a poststroke rehabilitation population. Arch Phys Med Rehabil. 2013;94:1048–1053. e1041. doi: 10.1016/j.apmr.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg K, Wood-Dauphinee S, Williams JI. The balance scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 15.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: Validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7–11. [PubMed] [Google Scholar]
- 16.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 18.Dean CM, Richards CL, Malouin F. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin Rehabil. 2001;15:415–421. doi: 10.1191/026921501678310216. [DOI] [PubMed] [Google Scholar]
- 19.Dobkin BH. Short-distance walking speed and timed walking distance: Redundant measures for clinical trials? Neurology. 2006;66:584–586. doi: 10.1212/01.wnl.0000198502.88147.dd. [DOI] [PubMed] [Google Scholar]
- 20.Finch EBD, Stratford PW, Mayo NE. Physical rehabilitation outcome measures. Hamilton, ON: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 21.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 22.Centers for medicare & medicaid services. medicare benefit policy manual. 2009;chapter 1:2015. section 110. [PubMed] [Google Scholar]
- 23.Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage Press; 2012. [Google Scholar]
- 24.Singer JD, Willet JB. Applied longitudinal data anlaysis: Modeling change and event occurence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 25.Long JD. Longitudinal data analysis for the behavioral sciences using r. Thousand Oaks, CA: SAGE Publications, Inc; 2012. [Google Scholar]
- 26.Team RC. R: A language and environment for statistical computing. 2015. [Google Scholar]
- 27.Bates D, Maechler M, Bolker BM, Walker S. _lme: Linear mixed-effects models using eigen and s4_. 2014. [Google Scholar]
- 28.Wickham H, Francois R. Dplyr: A grammar of data manipulation. 2015. [Google Scholar]
- 29.Veerbeek JM, Kwakkel G, van Wegen EE, Ket JC, Heymans MW. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke. 2011;42:1482–1488. doi: 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part ii: Time course of recovery. The copenhagen stroke study. Arch Phys Med Rehabil. 1995;76:406–412. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 32.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 33.Shelton FD, Volpe BT, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil Neural Repair. 2001;15:229–237. doi: 10.1177/154596830101500311. [DOI] [PubMed] [Google Scholar]
- 34.Keith RAGC, Hamilton BB, Sherwin FS. The functional independence measure: A new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 35.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2013;26:104–114. doi: 10.1016/j.jht.2012.06.005. quiz 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldie PA, Matyas TA, Evans OM. Deficit and change in gait velocity during rehabilitation after stroke. Arch Phys Med Rehabil. 1996;77:1074–1082. doi: 10.1016/s0003-9993(96)90072-6. [DOI] [PubMed] [Google Scholar]
- 37.Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19:25–30. [PubMed] [Google Scholar]
- 38.Nudo RJ. Recovery after brain injury: Mechanisms and principles. Frontiers in human neuroscience. 2013;7:887. doi: 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overman JJ, Carmichael ST. Plasticity in the injured brain: More than molecules matter. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20:15–28. doi: 10.1177/1073858413491146. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson TJ. Detecting change in patients with stroke using the berg balance scale. Aust J Physiother. 2001;47:29–38. doi: 10.1016/s0004-9514(14)60296-8. [DOI] [PubMed] [Google Scholar]
- 41.Liston RA, Brouwer BJ. Reliability and validity of measures obtained from stroke patients using the balance master. Arch Phys Med Rehabil. 1996;77:425–430. doi: 10.1016/s0003-9993(96)90028-3. [DOI] [PubMed] [Google Scholar]
- 42.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the action research arm test and the fugl-meyer assessment scale in chronic stroke patients. J Rehabil Med. 2001;33:110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 44.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.