Abstract
Background
Liver resection is the most effective treatment for intrahepatic cholangiocarcinoma (IHCC). Recurrent disease is frequent, however, recurrence patterns are ill-defined, and prognostic models are lacking.
Study Design
A primary cohort of 189 patients who underwent resection for IHCC was used for recurrence patterns analysis within and after 24 months. Based on independent factors for disease free survival (DFS) identified in Cox regression analysis, preoperative and postoperative models were developed using a recursive partitioning method. Models were externally validated using a multicenter cohort of 522 resected patients (Association Française de Chirurgie-IHCC study group).
Results
Recurrence within 24 months most often involved the liver (82.7%) while most recurrences after 24 months were strictly extrahepatic (61.1%). In multivariable analysis of the primary cohort, independent preoperative factors for DFS were tumor size and multifocality (based on imaging), while tumor size, multifocality, vascular invasion and lymph node metastases (based on pathology) were independent postoperative factors. The preoperative model allowed patient classification into low risk and high risk groups for recurrence. In the validation cohort (n=522), high risk patients had a greater likelihood of recurrence (HR=2.17, 95% CI 1.74–2.72; p<0.001). Postoperative model included tumor size, vascular invasion and positive nodal disease on pathology and classified patients in low, intermediate and high risk groups in the primary cohort. As compared to low risk patients in the validation cohort, intermediate and high risk patients were more likely to experience recurrence (HR=1.9, 95% CI 1.41–2.47; p<0.001 and HR=2.99, 95% CI 2.08–4.31; p<0.001, respectively).
Conclusions
Recurrence patterns are time dependent. Both models as developed and validated in this study classified patients in distinct recurrence risk groups, which may guide treatment recommendations.
INTRODUCTION
Intrahepatic cholangiocarcinoma (IHCC) incidence has risen over the last 3 decades(1, 2). To date, the only potentially curative treatment is complete resection, which offers a 5-year overall survival (OS) ranging from 21 to 35% and a median OS up to 39 months(3–6). According to the National Comprehensive Cancer Network guidelines, adjuvant therapy is mainly recommended in patients at risk of recurrence(7), since postoperative recurrence rates range from 53 to 79%, and most patients eventually die of disease(6–10). The most frequent site of failure is the liver, either alone (ranging from 60.9 to 62.7%) or associated with extrahepatic recurrence (18.6%), while extrahepatic only recurrence is less common (21%) (8, 9). Further understanding of recurrence patterns could help to better appraise the recurrence risk, to tailor postoperative monitoring and to guide perioperative treatment strategies, especially as locoregional therapies for IHCC are emerging(11–14). Additionally, some patients recurring early and ultimately dying shortly after resection likely do not benefit from surgery alone, and identification of these patients at presentation could optimize their management.
While evidence supporting the use of perioperative chemotherapy versus surgery alone for resectable IHCC is lacking, several studies reported promising results in initially unresectable patients who experienced significant tumor reduction and conversion to resection after preoperative systemic or hepatic intraarterial chemotherapy(15–17). Based on these data, high risk resectable patients might benefit from a multimodal approach involving systemic and/or liver directed therapy.
The current study sought to identify patients at greatest risk for early recurrence by exploring the predictive factors associated with recurrence patterns and disease free survival and by developing a recurrence risk model.
METHODS
Patients and Study Design
A retrospective study was conducted on a cohort of patients who underwent curative-intent hepatectomy from January 1993 to May 2013 for IHCC at Memorial Sloan Kettering Cancer Center (MSKCC). Data were collected from a prospectively maintained liver resection database. Patients were deemed resectable, according to the following criteria: (a) R0 resection potentially achievable, (b) adequate future liver remnant function and volume (minimum of 2 contiguous liver segments), with adequate perfusion, and venous and biliary drainage, (c) general health conditions suitable with liver surgery. The authors’ approach to intraoperative and perioperative management has been published previously (8, 18). Exclusion criteria included a diagnosis of mixed cholangiocarcinoma-hepatocellular carcinoma and a palliative-intent resection such as R2 resection. Additionally, patients deceased within 90 days after surgery were excluded from the outcome analyses (19). The Institutional Review Board approved this study.
A distinct cohort of patients who underwent curative-intent partial hepatectomy for IHCC was retrospectively analyzed and formed the validation cohort of this study. Briefly, data from all consecutive patients submitted to curative-intent resection for IHCC from January 1989 to March 2009 at 24 tertiary hepatobiliary centers were collected from a dedicated multi-institutional database related to previous published studies from the AFC-IHCC study group (4, 20). Authorization from the Association Française de Chirurgie (AFC) was obtained for using these data. Inclusion and exclusion criteria to the present study were those aforementioned.
Data Collection
Clinical preoperative variables included demographics and preoperative tumor markers (CA 19-9). Preoperative tumor features based on imaging including CT, MRI, ultrasonography (US) and PET scan were documented. Operative data were also collected. Liver resection of three or more segments was defined as major resection. In both cohorts, resections were extended to extrahepatic structures when required to achieve a macroscopically complete resection. Lymphadenectomy was performed at the discretion of the surgeon, either as a formal peripancreatic and portocaval lymph node dissection or as a targeted excision according to preoperative imaging and intraoperative findings.
Pathology Data
Pathologic variables included size and number of tumors, differentiation grade, resection margin status, vascular invasion, perineural invasion, nodal status, and histology of the non-tumoral liver parenchyma. Extrahepatic invasion (EHI) was defined as direct invasion of any extrahepatic organs excluding the gallbladder (pT3). Morphological subtype was defined as mass-forming (MF), periductal infiltrating (PI), intraductal growth (IG) and mixed (21, 22). Tumor staging was determined using the 7th edition of the American Joint Committee on Cancer Staging System (23).
Follow-Up and Recurrence data
Clinical and radiographic monitoring was performed every 4–6 months. Adjuvant therapy was offered at the discretion of the multidisciplinary team, primarily to patients considered high risk for recurrence. Recurrence was defined as any sign of recurrent cholangiocarcinoma, either biopsy-proven or suspected on cross sectional imaging (with documented progression on serial imaging) with or without elevated CA19- 9 level. In the primary cohort, initial recurrence site was categorized as hepatic only or extrahepatic or synchronous hepatic and distant recurrence. Recurrence treatment initiation date and treatment modalities were documented. Multimodal therapy was defined as recurrence management involving systemic chemotherapy associated with liver-directed therapy.
Due to missing data, recurrence site and management was not fully documented in the validation cohort. Consequently, recurrence patterns could be assessed in the primary cohort only.
Study Objectives
The first aim of this study was to develop and validate prognostic models of recurrence based on independent prognostic factors for disease-free survival (DFS). Although OS remains the standard endpoint in survival analysis, DFS stands as a relevant endpoint in the setting of IHCC. Recurrence after curative-intent hepatectomy is frequently observed and patients eventually die of their recurrent disease. However, early and multimodal management of the recurrence is reported as associated with prolonged survival. Thus, recurrence-specific prognostic models might be helpful for identifying patients at high risk of recurrence, helping for perioperative decision-making and improving early recurrence detection and management.
The second objective was to define recurrence patterns. Although recurrence may be observed long after resection, Spolverato et al. recently reported that recurrences are generally observed within 5 years, with the highest risk being within the 24 months after surgery (24). Additionally, median DFS does not exceed 24 months (range from 20 to 26 months) in the current literature (8, 9, 24, 25). Therefore, patterns of recurrence were assessed based on its occurence within or after 24 months of resection.
Statistical analysis
Categorical variables were summarized using percentages and continuous variables were summarized using mean and standard deviation (SD) or median (range), as appropriate. Characteristics of patients were compared using the chi-square test for categorical variables and the t-test or the Mann-Whitney U test for continuous variables, as appropriate. OS and DFS were estimated using the Kaplan-Meier method and corresponded to the interval between primary resection date and the date of last follow-up or the recurrence date, respectively. Patients who were dead or with recurrence at last follow-up were considered as event whereas patients who were alive and disease-free at last follow-up were censored for DFS analysis. In turn, patients who were dead at last follow-up were considered as event whereas patients who were alive at last follow-up were censored for OS analysis. Differences in terms of DFS between groups were compared using the log-rank test. Variables in the univariate analysis with p<0.1 were included in a Cox proportional hazard model in order to identify independent significant prognostic factors. Backward selection was used with a 0.1 cut-off for entry into the model. The first model included only preoperative data and the second included postoperative histopathologic data derived from the resected specimen.
Further, based on the independent predictors for DFS in either preoperative and postoperative model, patients were classified into preoperative and postoperative risk groups of recurrence, using a recursive partitioning method (26, 27). Briefly, a recursive partitioning consists in creating a decision tree that strives to correctly classify members of the population based on several dichotomous independent variables. Performance of both preoperative and postoperative models was validated using the validation cohort in terms of stratification of recurrence rate and DFS. All p values were based on two-tailed statistical analysis and a p value <0.05 was considered to indicate statistical significance. All analyses were performed with SPSS software, version 22.0 for Windows (SPSS Inc., Chicago, IL) and R software, version 3.1.1.
RESULTS
Perioperative Data in Primary and Validation Cohorts
During the study period, 200 consecutive patients underwent liver resection for IHCC at MSKCC. Patients with mixed-type primary liver tumours (n=5), distant metastatic disease at the time of resection (n=1) or postoperative death within 90 days after surgery (n=5) were excluded. The remaining 189 patients were included in the analysis, as the primary cohort. For the validation cohort, 522 patients with curative-intent resection were included. Preoperative, operative and pathologic characteristics in the primary and validation cohorts are listed in Table 1. There were significant differences in terms of gender, total bilirubin and CA19-9 levels, extent of resection and tumor features such as extrahepatic invasion rate, morphological subtypes and resection margin status between the primary and the validation cohorts.
Table 1.
Clinicopathologic Features in the Primary (Memorial Sloan Kettering Cancer Center) and Validation (Association Française de Chirurgie) cohorts of Patients Resected for Intrahepatic Cholangiocarcinoma
| MSKCC cohort (n=189) | AFC cohort (n=522) | p Value | |
|---|---|---|---|
| Preoperative | |||
| Age at surgery, y (SD) | 65.4 (11.8) | 64 (11.7) | 0.35 |
| Female, n (%) | 114 (60.3) | 268 (51.3) | 0.04 |
| Hepatitis, n (%) | 18 (9.5) | 32 (6.1) | 0.14 |
| HBV | 9 (4.8) | NA | |
| HCV | 9 (4.8) | NA | |
| PSC/IBD, n (%) | 7 (3.7) | NA | |
| Imaging modality, n (%) | |||
| CT | 170 (89.9) | NA | |
| MRI | 114 (60.3) | NA | |
| US | 70 (37) | NA | |
| PET | 59 (31.2) | NA | |
| Preoperative tumor size, cm (SD) | 6.5 (3.6) | 6.8 (3.8) | 0.16 |
| Preoperative multiple tumor | 33 (17.5) | 79 (15.1) | 0.49 |
| Preoperative enlarged lymph node | 16 (8.5) | NA | |
| Total bilirubin, mg/L (SD) | 1.2 (3.1) | 1.55 (3.4) | <0.001 |
| CA19-9, U/mL (SD) | 1847.7 (5354.1) | 1547 (7101) | 0.001 |
| Neoadjuvant therapy, n (%) | 10 (5.3) | 34 (6.5) | 0.6 |
| Postoperative | |||
| Major resection, n (%) | 124 (65.6) | 401 (76.8) | 0.004 |
| Tumor size, cm (SD) | 6.9 (3.9) | 7.1 (4) | 0.9 |
| Multiple lesions, n (%) | 54 (28.6) | 187 (35.8) | 0.08 |
| Underlying liver, n (%) | 0.053 | ||
| Steatosis | 69 (36.5) | 142 (27.2) | |
| Cirrhosis | 9 (4.8) | 25 (4.8) | |
| Vascular invasion, n (%) | 0.6 | ||
| Absent | 121 (64) | 321 (61.5) | |
| Present | 68 (36) | 201 (38.5) | |
| Microvascular | 46 (24.3) | NA | |
| Macrovascular | 22 (11.6) | NA | |
| Perineural invasion, n (%) | 54 (28.6) | 124 (23.8) | 0.21 |
| Extrahepatic invasion, n (%)* | 22 (11.6) | 34 (6.5) | 0.012 |
| Morphologic subtype, n (%) | <0.001 | ||
| Mass-forming | 176 (93.1) | 367 (70.3) | |
| Periductal invasion | 13 (6.9) | 9 (1.7) | |
| Intraductal growth | - | 6 (1.1) | |
| Mixed subtype | - | 58 (11.1) | |
| Unknown | - | 82 (15.7) | |
| Margin status, n (%) | 0.006 | ||
| Negative | 152 (80.4) | 365 (69.9) | |
| Positive | 37 (19.6) | 157 (30.1) | |
| pN stage, n (%) | 0.22 | ||
| pNx | 97 (51.3) | 246 (47.1) | |
| pN0 | 71 (37.6) | 191 (36.6) | |
| pN1 | 21 (11.1) | 85 (16.3) | |
| Adjuvant therapy, n (%) | 51 (27) | 178 (34.1) | 0.084 |
Gallbladder excluded.
AFC, Association Française de Chirurgie; CA19-9, carcinogen antigen 19-9; CT, computed tomography; HBV, hepatitis B virus; HCV, hepatitis C virus; IBD, inflammatory bowel disease; MRI, magnetic resonance imaging; MSKCC, Memorial Sloan Kettering Cancer Center; PET, positron emission tomography; PSC, primary sclerosing cholangitis; US, ultrasonography.
Survival Data, Recurrence Patterns and Management
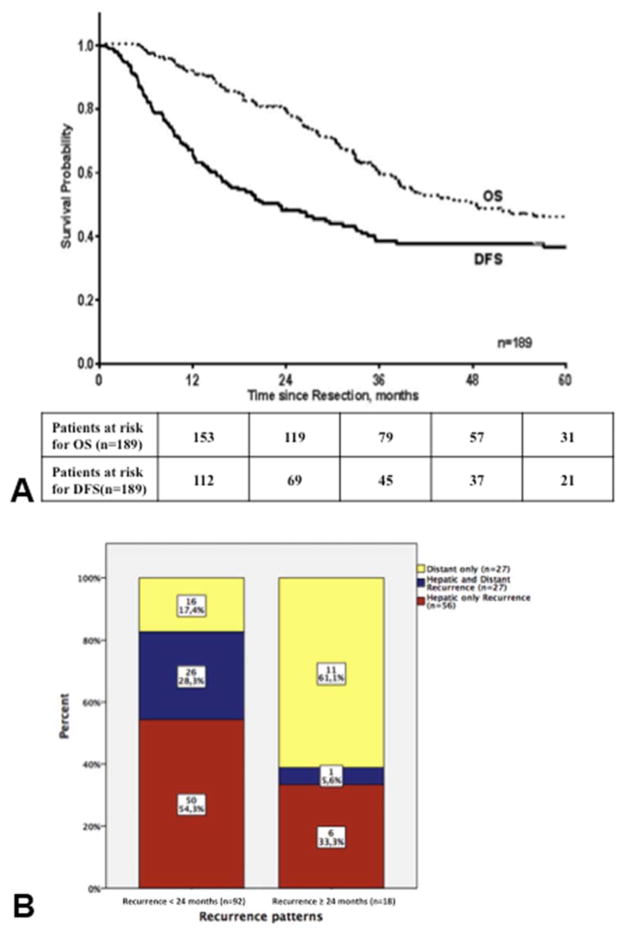
In the primary cohort, median OS was 47.8 months (95% CI, 30.3–65.4 months) (Figure 1A). After primary resection, median DFS was 23.1 months (95% CI: 14.6–31.6 months). After a median follow up of 42.5 months (range, 5–192), recurrence was documented in 110 patients (58.2%). Fifty six patients (50.9%) experienced recurrence confined to the liver. Extrahepatic recurrences were strictly extrahepatic in 27 patients and simultaneously involving the liver in 27 patients. Recurrence rate within 24 months was 83.6% (n=92) and 18 patients eventually recurred after 24 months, at a median follow-up time of 64.3 months (range, 26–192). Recurrence patterns were significantly different between the 2 groups (p<0.001) (Figure 1B). Hepatic recurrence, whether confined to the liver or associated with distant recurrence (n=83), overwhelmingly occurred in patients who recurred within 24 months (n=76; 91.6%). In this group, the liver was involved in 82.7% of patients, compared to 38.9% in patients who recurred after 24 months. In patients who failed after 24 months (n=18), 11 (61.1%) recurred distantly (lung, n=6; retroperitoneal nodes, n=2; bone, n=2; ovarian, n=1). Recurrence rate and patterns did not differ over time.
Figure 1.
Kaplan-Meier survival curves for (A) all patients included (n=189) and (B) recurrence patterns for patients categorized by their disease-free survival. Fifty two patients have not recurred at last follow-up. Dotted line, overall survival (OS) curve; black line, disease-free survival (DFS) curve. (In each group, the proportion of patients experiencing each recurrence patterns is labeled on each corresponding bar).
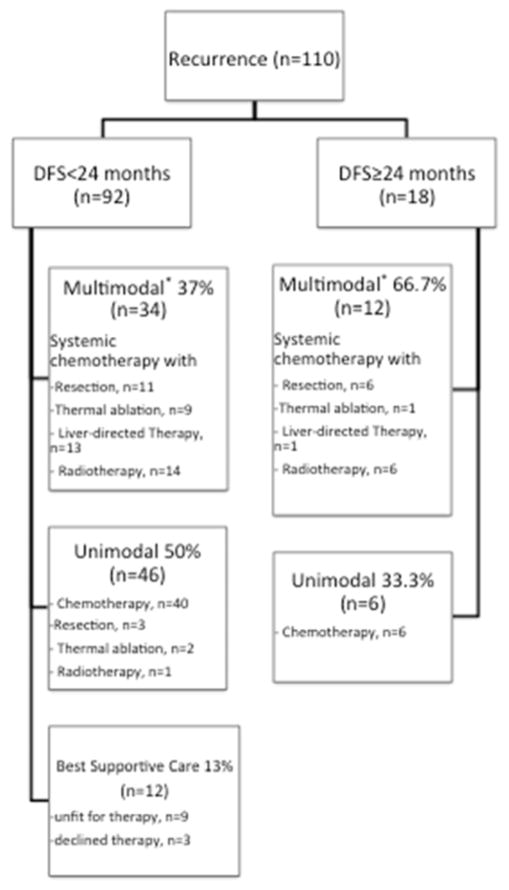
Of note, among patients treated with neoadjuvant therapy (n=10), eight patients (80%) experienced recurrence, all of which were within 24 months after resection and were extrahepatic only in four cases. As shown in Figure 2, recurrence treatment modalities were different across the DFS groups (p=0.033). Two thirds of patients who recurred ≥24months received multimodal therapy. Surgical resection was performed in 20 patients (liver, n=10; lung, n=6; bone, n=3, ovary, n=1). Metastasis ablation was exclusively performed for recurrent disease isolated to the liver (n=11; radiofrequency ablation, n=9; microwave ablation, n=2) and was combined with liver directed therapy in five patients (HAI-FUDR, n=3; hepatic artery embolization, n=2). Overall, systemic chemotherapy was used in 92 patients and consisted of gemcitabine-based regimen in 60 patients (65.2%). Median OS after recurrence treatment initiation was 19 months (95% CI 14.1–23.9) and was significantly prolonged in patients managed with multimodal therapy (p<0.001).
Figure 2.
Recurrence management according to the recurrence patterns. *Patients may have undergone more than 2 different treatment modalities as multimodal therapy.
In the validation cohort, median OS was 49 months (95% CI, 41–56.9 months). After primary resection, median DFS was 18 months (95% CI: 16.6–19.4 months). After a median follow up of 35 months (range, 3–211), recurrence was documented in 248 patients (47.5%). Recurrence rate within 24 months was 89.9% (n=223) and 25 patients eventually recurred after 24 months, at a median follow-up time of 35 months (range, 25–101).
Prognostic Factors for Disease Free Survival in the Primary Cohort
The full cohort (n=189) was included in DFS analyses. Univariable and multivariable analysis for DFS are shown in Table 2. Preoperative tumor size (HR=1.09, 95%CI 1.04–1.14; p<0.001) and multifocality on imaging (HR=1.73, 95% CI 1.12–2.70; p=0.013) were independently associated with a shorter DFS. Regarding postoperative factors, tumor size (HR=1.10, 95% CI 1.05–1.15; p<0.001), multifocality (HR=1.82, 95% CI 1.22–2.71; p=0.003), vascular invasion and positive nodal disease (HR= 2.77, 95% CI 1.52–5.03; p<0.001) on pathology were independent factors of shorter DFS.
Table 2.
Univariable Analysis and Cox Proportional Hazards Regression Model of Preoperative and Postoperative Features Associated with Disease-Free Survival in the Primary Cohort (Memorial Sloan Kettering Cancer Center, n=189 Patients)
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Median DFS, mo | HR (95% CI) | p Value | HR (95%CI) | p Value | |
| Preoperative | |||||
| Age | 0.98 (0.97–0.99) | 0.047 | 0.98 (0.97–1.01) | 0.13 | |
| Sex | |||||
| Female | 23.4 | 0.93 | |||
| Male | 19.6 | ||||
| Hepatitis | |||||
| Yes | 16.6 | 0.11 | |||
| No | 20 | ||||
| PSC/IBD | |||||
| Yes | 28.1 | 0.45 | |||
| No | 17.8 | ||||
| Preoperative tumor size | 1.10 (1.05–1.15) | <0.001 | 1.09 (1.04–1.14) | <0.001* | |
| Preoperative multiple tumor | |||||
| Yes | 12 | 0.002 | 1.73 (1.12–2.70) | 0.013* | |
| No | 23.4 | ||||
| Preoperative enlarged lymph node | |||||
| Yes | 16.9 | 0.66 | |||
| No | 19.7 | ||||
| Total bilirubin, mg/L | 1.03 (0.98–1.09) | 0.224 | |||
| CA19-9, U/mL | 1 (1-1) | 0.004 | 1 (1-1) | 0.3 | |
| Neoadjuvant therapy | |||||
| Yes | 15.6 | 0.32 | |||
| No | 20 | ||||
| Postoperative | |||||
| Tumor size, cm | 1.11 (1.06–1.15) | <0.001 | 1.10 (1.05–1.15) | <0.001* | |
| Multiple lesions | |||||
| Yes | 13.2 | <0.001 | 1.82 (1.22–2.71) | 0.003* | |
| No | 26.9 | ||||
| Underlying liver | |||||
| Normal | 16.9 | 0.66 | |||
| Steatosis | 21 | ||||
| Cirrhosis | 23.7 | ||||
| Tumor differentiation | 0.037 | 0.79 | |||
| Vascular invasion | |||||
| Absent | 32 | <0.001 | Reference† | 0.022* | |
| Micro | 12.4 | 1.65 (1.05–2.58) | 0.028* | ||
| Macro | 9.6 | 1.93 (1.13–3.31) | 0.016* | ||
| Perineural invasion | |||||
| Yes | 15 | 0.008 | 1.26 (0.80–1.98) | 0.32 | |
| No | 20 | ||||
| Extrahepatic invasion‡ | |||||
| Yes | 13.2 | 0.054 | 0.83 | ||
| No | 20.5 | ||||
| Morphological type | |||||
| Mass-forming | 19.7 | 0.78 | |||
| Periductal invasion | 17.8 | ||||
| Margin status | |||||
| Negative | 20 | 0.54 | |||
| Positive | 19.5 | ||||
| pN stage | |||||
| pN0 | 26.9 | <0.001 | Reference§ | <0.001* | |
| pN1 | 8.2 | 2.77 (1.52–5.03) | <0.001* | ||
| pNx | 20 | 1.03 (0.69–1.53) | 0.89 | ||
| Adjuvant therapy | |||||
| Yes | 15 | 0.021 | 0.95 (0.58–1.56) | 0.84 | |
| No | 26.4 | ||||
All variables with p>0.1 in univariable analysis were included in the Cox proportional hazards regression model.
Significant.
Patients with microvascular invasion and macrovascular invasion were respectively compared to patients without vascular invasion on tumor specimen.
Gallbladder excluded.
pN1 and pNx patients were respectively compared to pN0 patients.
CA19-9, carcinogen antigen 19-9; PSC/IBD, primary sclerosing cholangitis/inflammatory bowel disease.
Development of Recurrence Risk Models on the Primary Cohort
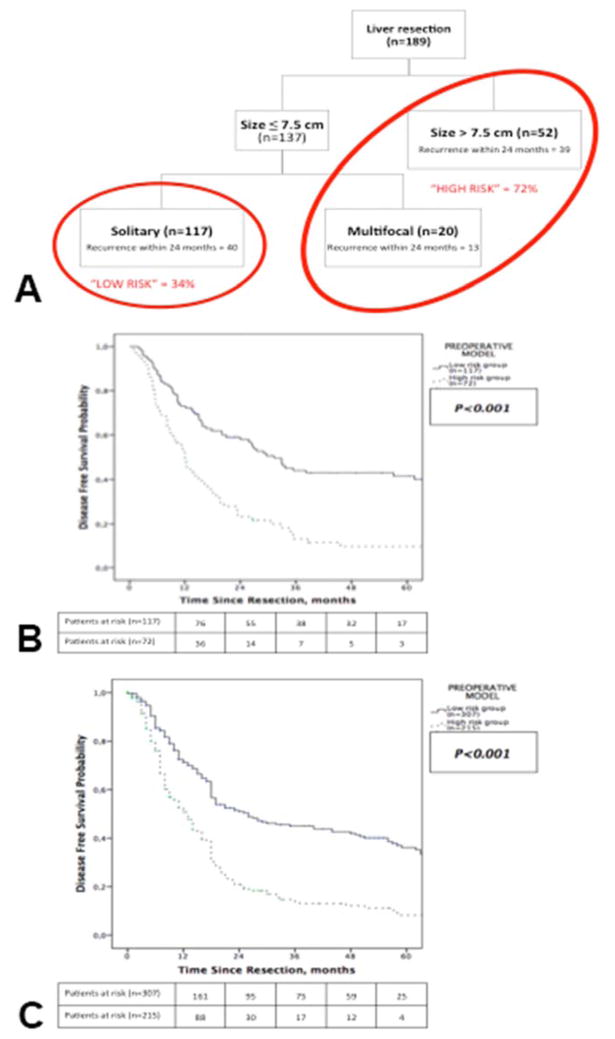
Using a recursive partitioning method, preoperative and postoperative independent factors for DFS, as cited above, were used for developing preoperative and postoperative recurrence risk models, respectively. Patient subsets with low and high recurrence risk were then identified using the preoperative model (Classification tree, Figure 3A). Tumor size was the most important variable and multifocal disease helped to further separate patients in low and high risk groups into the preoperative model. Patients preoperatively classified as low risk had a significantly longer DFS than patients classified as high risk of recurrence (median DFS = 31.3 months vs. 12 months; p<0.001, Figure 3B). Recurrence patterns observed in the full primary cohort remained comparable between the two groups with recurrence mostly involving the liver within 24 months while later recurrences were mostly isolated to an extrahepatic site (Supplemental Table 1, online only).
Figure 3.
Preoperative model classifying patients into (A) recurrence risk groups, and Kaplan-Meier estimates of disease-free survival for patients stratified by groups in the (B) primary cohort and (C) validation cohort.
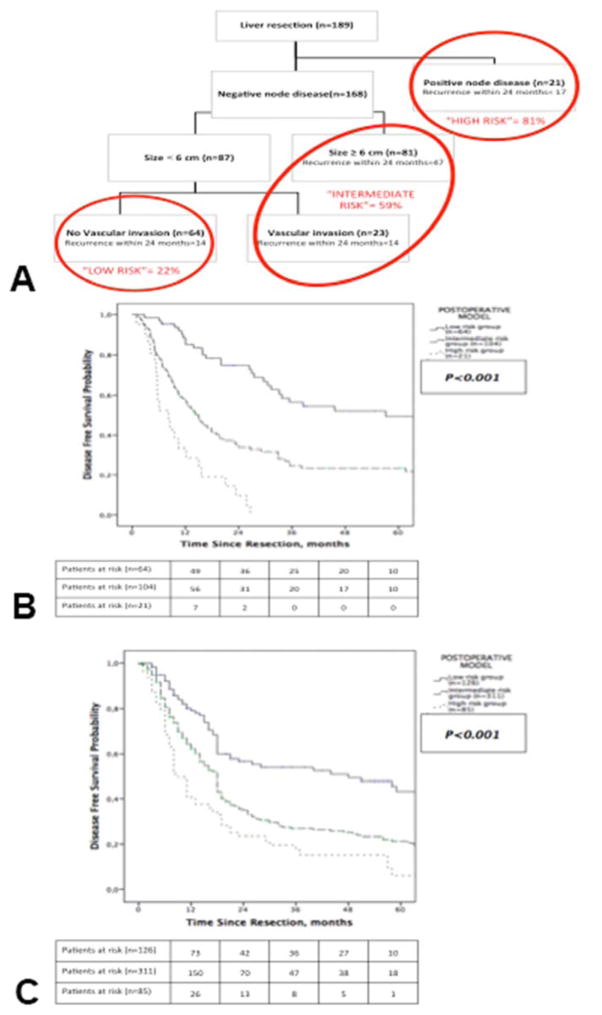
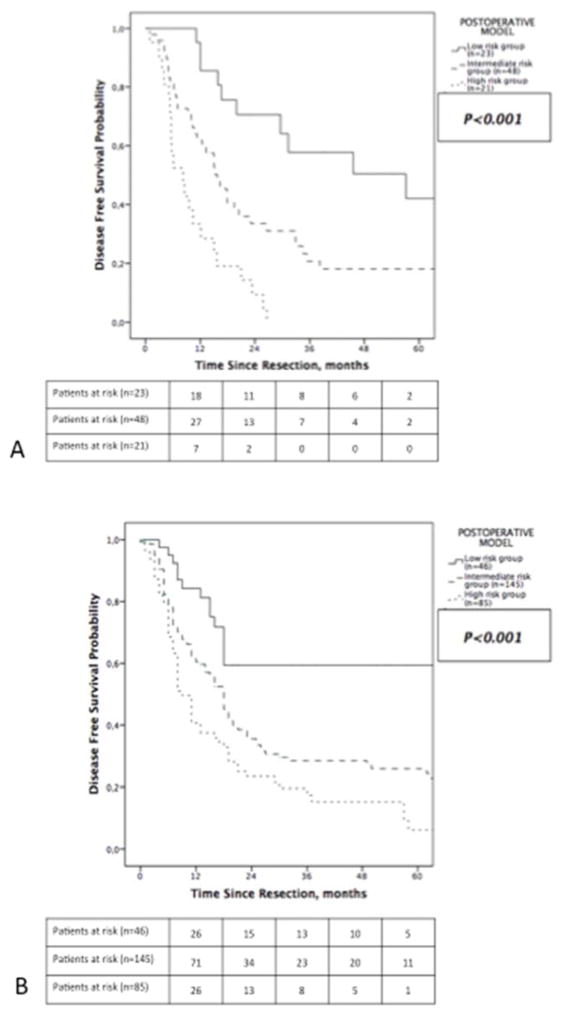
In contrast, three risk subsets were identified in the postoperative model (Figure 4A; low, intermediate and high). Nodal status was the most important variable whereas multifocal disease was replaced in the postoperative model by vascular invasion for further stratifying patients with node negative tumor smaller than 6 cm. In the full primary cohort (n=189), patients with pNx status (n=97) were considered as pN0. Median DFS differed significantly between risk groups (low risk= 48 months, intermediate risk= 18 months, high risk= 9 months; p<0.001, Figure 4B). Similarly, the time dependence of recurrence patterns was again observed across these three groups (Supplemental Table 1, online only). When restricted to the subset of patients who underwent portal lymph node dissection (n=92), the postoperative model performed similarly with significantly different median DFS across the different risk groups (low risk= 57.1 months, intermediate risk= 16 months, high risk= 8.2 months; p<0.001, Figure 5A).
Figure 4.
Postoperative model classifying patients into (A) recurrence risk groups, and Kaplan-Meier estimates of disease-free survival for patients stratified by groups in the (B) primary cohort and (C) validation cohort.
Figure 5.
Kaplan-Meier estimates of disease-free survival for patients who underwent portal lymphadenectomy classified using the postoperative model in (A) the primary cohort and (B) the validation cohort.
External Validation
The preoperative model allowed stratification in two risk groups significantly different in term of median DFS (low risk = 26 months vs. high risk= 13 months; p<0.001, Figure 3C). As compared to low risk patients, patients in the high risk group had a 117% greater likelihood of recurrence (HR=2.17, 95% CI 1.74–2.72; p<0.001).
In turn, the postoperative model stratified the full cohort (n=522) into three distinct risk groups in term of median DFS (low risk= 48 months, intermediate risk= 18 months, high risk= 9 months; p<0.001, Figure 4D). As compared to low risk patients, patients in the intermediate risk group had a 90% greater likelihood of recurrence (HR=1.9, 95% CI 1.41–2.46; p<0.001). Further, patients classified into the high risk group had a 199% greater likelihood of recurrence (HR=2.99, 95% CI 2.08–4.31; p<0.001). When strictly applied to patients who underwent portal lymphadenectomy (n=276), the postoperative model provided a similar stratification (median DFS in low risk group= 45 months, intermediate risk group= 18 months and high risk group= 9 months; p<0.001, Figure 5B).
These distinct recurrence risk groups were also significantly different in term of OS, as shown in the validation cohort (Supplemental Figure 1, online only).
DISCUSSION
The findings of the current study are important for a number of reasons. First, preoperative and postoperative prognostic models for patients with IHCC after curative-intent hepatectomy were developed and validated in a large external cohort. These models, easy to apply in clinical practice, allowed clear-cut classification of patients in groups of distinct outcomes both before and after resection. Second, distinct patterns of recurrences were identified. Recurrence within 24 months of resection overwhelmingly involved the liver (82.7%) while recurrence after 24 months were mostly isolated to an extrahepatic site.
Both preoperative and postoperative models allowed patients classification in groups with distinct recurrence rates and different DFS. Preoperative model was based on simple variables obtained on imaging. This model allowed classification in low risk and high risk groups (Figure 3). Patients deemed at high risk of recurrence had a 117% greater likelihood of recurrence with a significantly shorter median DFS (12 months) as compared to 31.1 months in the high risk group (p<0.001). In the validation cohort, preoperative model performed similarly. Postoperative model including tumor features on pathology stratified patients into three risk groups (low, intermediate and high) and performed consistently in both the primary and validation cohorts (Figure 4).
To date, five staging systems have been successively used for IHCC and several prognostic models and nomograms have been recently published and externally validated (6, 21, 23, 25, 28–31). All are focused on OS estimation that remains the most relevant endpoint in clinical practice. Still, prognosis after resection of IHCC remains poor mainly due to the high recurrence rate(6, 8, 9). Hyder et al. have previously published a clinical risk score for recurrence including three items such as tumor size greater or equal to 5 cm, major vascular invasion and positive nodal disease (9). They reported that an increasing risk score was associated with an incrementally worse DFS. However, this clinical score assigned equal strength (1 point) to each risk factor. In the current study, the risk of recurrence overtime varied as different independent prognostic factors were considered. For instance, based on our Cox regression analysis (Table 2), the probability of recurrence was 82% greater in case of multifocal disease on specimen (p=0.003). This risk was 167% greater in case of positive nodal disease (p<0.001). Using a recursive partitioning method, positive nodal disease was the most important variable in our postoperative model. Tumor size and vascular invasion helped to further classified patients without positive nodal disease. One can hypothesize that this method allowed respecting the different prognostic strength of each variable in our models.
Multifocal disease and tumor size, whether on imaging or pathology, were independent prognostic factors of shorter DFS. In the current study, tumor size estimation on preoperative imaging was found to be reliable with a median difference between imaging and pathology (pathologic size – radiologic size) of + 0.41 cm. Regarding multifocality, accordingly to Okabayashi et al. (21), discrepancy between preoperative imaging and pathologic examination was observed in one third of patients but this discrepancy rate significantly decreased overtime. Of these two features, solely multifocal disease is part of the current AJCC staging system (23). In the postoperative model, tumor features such as vascular invasion and positive nodal disease replaced multifocal disease. Vascular invasion was previously reported as an independent predictor of recurrence (9, 24). As aforementioned, positive nodal disease was the strongest independent predictor of short DFS. Its prognostic value has already been extensively reported and routine portal lymphadenectomy is now widely recommended in recent guidelines (3, 32, 33). In the primary cohort, nodal disease was suspected on the preoperative work-up of 15 patients only (9.3%) and was not associated with DFS on univariable analysis (p=0.78).
Resection remains the backbone of IHCC management, providing prolonged survival. Still, patients recurring after resection such as those classified in the high risk group experienced median DFS ranging from 9 to 13 months (Figure 3 and 4) and likely do not benefit from resection. Based on results from clinical trials in the palliative setting, current practice guidelines recommend adjuvant therapy in case of adverse tumor features (positive resection margin, presence of vascular invasion, positive nodal disease, multifocal disease). In the primary and validation cohorts, adjuvant chemotherapy was delivered to 43 patients (26.5%) and 178 patients (34.1%) respectively. Among them, 32 patients (62.7%) and 92 (51.7%) experienced recurrence within 24 months, respectively. Furthermore, adjuvant therapy was not independently associated with DFS. Taken altogether, these findings are not surprising but underscore that the main determinants of DFS are tumor characteristics and question the impact of adjuvant chemotherapy on recurrence. One clinical trial (NCT01313377) is currently interrogating the impact of systemic therapy in the adjuvant setting (34). However, given that recurrence often involves the liver, especially when occurring within 24 months after resection, targeted liver therapy might represent a credible option to increase disease control in the liver. Indeed, data from published clinical trials evaluating the impact of HAI-FUDR in unresectable ICC reported a response rate of 48%, a hepatic progression-free survival reaching 12 months and a median OS of 29 months(11, 12). Based on these compelling results, a phase II trial combining HAI-FUDR with systemic therapy (NCT01938729) in the adjuvant setting is currently accruing (35). The validated preoperative and postoperative models may help for patient selection and inclusion in future clinical trials.
Although recurrence patterns are generally defined from anatomic sites, time to recurrence might represent a more relevant surrogate for tumor behavior. Most hepatic recurrence (91.5%) was seen in patients recurring within 24 months of resection. In contrast, most patients who were free of disease at 24 months had not recurred at time of last follow-up (73.5%) and recurrences were mainly observed at a solitary extrahepatic site (61.1%). This time dependence of recurrence patterns was also found in different patient subsets classified by recurrence risks. In other words, whatever the likelihood of recurrence for one patient, recurrence will be more likely to involve the liver or a distant organ when occurring within or after 24 months, respectively. In the primary cohort, recurrence management was generally more aggressive using a multimodal approach in patients who recurred after 24 months (n=12/18; 66.7%) than in those recurring earlier (n=34/92; 33.3%; p=0.033). This finding may be due to the significantly different recurrence patterns between both groups. Indeed, recurrent disease within 24 months was simultaneously intrahepatic and extrahepatic (n=26/92; 28.3%) precluding a multimodal management while recurrences after 24 months were mostly isolated to a single organ (n=17/18; 94.5%) thereby allowing an aggressive approach with combined local and systemic therapies. The timing of recurrence may also have played some role in deciding the type of therapy, with a more aggressive approach favored in patients with a longer disease free interval. Similarly to previous studies, a multimodal approach involving liver-directed therapies in selected patients was associated with a prolonged survival in previous series (36–39).
The present study had several limitations that should be addressed. First, the study is retrospective in nature, and reviewed data can be imprecise, especially regarding recurrence. Additionally, monitoring after IHCC resection is not standardized in France even though a follow-up visit every 6 months for 5 years is generally advocated. This may represent a potential bias of differential recurrence screening. Second, predictive models that have been developed are easily applicable and all included prognostic variables are routinely available in clinical practice. One methodological alternative would have been the development of a nomogram for DFS prediction. Third, portal lymph node dissection was performed in nearly half of patients. Thus, the association between nodal disease and recurrence could not be thoroughly explored in our study. However, postoperative model performed similarly when strictly applied to patients who underwent portal lymphadenectomy either in the primary or the validation cohort. Finally, these models were developed from and validated in Western cohorts. As shown in Table 1, both cohorts were different regarding baseline characteristics, extent of resection and tumor features. Such heterogeneity extends the applicability of these prediction tools to the daily clinical practice. However, further validation might be needed before applicability on Eastern cohorts.
In conclusion, recurrence patterns after resection for ICC are time dependent. Preoperative and postoperative models as developed and validated in this study distinctly classified patients at different risk of recurrence. Patients classified as high risk might benefit from perioperative therapy instead of surgery alone.
Supplementary Material
Acknowledgments
Support: Dr Doussot received research fellowship grants from the French Association of Hepatobiliary Surgery and Transplantation (ACHBT) and from Université de Bourgogne. This study was also supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant). Presented at the French Surgical Association Meeting 2015, Paris, France, October 2015.
The authors would like to thank the collaborators: Members of the AFC-IHCC study group (beside the authors): René Adam, Gérard Pascal, Denis Castaing, Daniel Cherqui (Hôpital Paul Brousse, Villejuif, France), Jacques Baulieux, Jean Yves Mabrut, Christian Ducerf (Hopital de la Croix Rousse, Lyon, France), Jacques Belghiti (Hopital Beaujon, Clichy, France), Gennaro Nuzzo, Felice Giuliante (University Catholic di Roma, Roma, Italy), Yves-Patrice Le Treut, Jean Hardwigsen (Hôpital de la Conception, Marseille, France), Patrick Pessaux, Philippe Bachellier (Hopital Hautepierre, Strasbourg, France), Francois Reńe Pruvot, Emmanuel Boleslawski (Hopital Hurriez, Lille, France), Michel Rivoire (Centre Léon Bérard, Lyon, France), Laurence Chiche (CHU Bordeaux, France).
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatol. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 4.Farges O, Fuks D, Le Treut Y-P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 5.Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Cancer Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 9.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabrizian P, Jibara G, Hechtman JF, et al. Outcomes following resection of intrahepatic cholangiocarcinoma. HPB. 2015;17:344–351. doi: 10.1111/hpb.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589–1595. doi: 10.1093/annonc/mdp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstantinidis IT, Do RKG, Gultekin DH, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol. 2014;21:2675–2683. doi: 10.1245/s10434-014-3649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: A systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213–220. doi: 10.1002/jso.23781. [DOI] [PubMed] [Google Scholar]
- 15.Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318–324. doi: 10.1245/s10434-012-2312-8. [DOI] [PubMed] [Google Scholar]
- 16.Ghiringhelli F, Lorgis V, Vincent J, et al. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59:354–360. doi: 10.1159/000362223. [DOI] [PubMed] [Google Scholar]
- 17.Rayar M, Sulpice L, Edeline J, et al. Intra-arterial Yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 18.Kingham TP, Correa-Gallego C, D’Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220:471–479. doi: 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB. 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–829. doi: 10.1097/SLA.0b013e318236c21d. discussion 830. [DOI] [PubMed] [Google Scholar]
- 21.Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374–2383. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, editor. AJCC cancer staging manual. 7. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 24.Spolverato G, Kim Y, Alexandrescu S, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016;23:235–243. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- 25.Doussot A, Groot-Koerkamp B, Wiggers JK, et al. Outcomes after resection of intrahepatic cholangiocarcinoma: external validation and comparison of prognostic models. J Am Coll Surg. 2015;221:452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breiman L. Classification and regression trees. New York, N.Y: Chapman & Hall; 1993. [Google Scholar]
- 27.Chambers JM, Hastie T, editors. Wadsworth & Brooks/Cole computer science series. Pacific Grove, Calif: Wadsworth & Brooks/Cole Advanced Books & Software; 1992. Statistical models in S. [Google Scholar]
- 28.Nathan H, Aloia TA, Vauthey J-N, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 29.Greene FL, editor. American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 30.Jiang W, Zeng Z-C, Tang Z-Y, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22:1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 31.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149:432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 32.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB. 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gemcitabine Hydrochloride and Oxaliplatin or Observation in Treating Patients With Biliary Tract Cancer That Has Been Removed by Surgery. [Accessed on line, 02/27/15]; https://clinicaltrials.gov/ct2/show/NCT01313377?term=NCT01313377&rank=1.
- 35.Hepatic Arterial Infusion With Floxuridine and Dexamethasone in Combination With Gemcitabine as Adjuvant Treatment After Resection of Intrahepatic Cholangiocarcinoma. [Accessed on line, 02/27/15]; https://clinicaltrials.gov/ct2/show/NCT01938729?term=NCT01938729&rank=1.
- 36.Zhang S-J, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20:3596–3602. doi: 10.1245/s10434-013-3035-1. [DOI] [PubMed] [Google Scholar]
- 37.Sulpice L, Rayar M, Boucher E, et al. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99:1711–1717. doi: 10.1002/bjs.8953. [DOI] [PubMed] [Google Scholar]
- 38.Kamphues C, Seehofer D, Collettini F, et al. Preliminary experience with CT-guided high-dose rate brachytherapy as an alternative treatment for hepatic recurrence of cholangiocarcinoma. HPB. 2012;14:791–797. doi: 10.1111/j.1477-2574.2012.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi Y, Ebata T, Yokoyama Y, et al. Surgery for recurrent biliary tract cancer: a single-center experience with 74 consecutive resections. Ann Surg. 2014;99:1711–1717. doi: 10.1097/SLA.0000000000000827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.