Abstract
Kaiso, a bi-modal transcription factor, regulates gene expression, and is elevated in breast, prostate, and colon cancers. Depletion of Kaiso in other cancer types leads to a reduction in markers for the epithelial–mesenchymal transition (EMT) (Jones et al., 2014), however its clinical implications in pancreatic ductal adenocarcinoma (PDCA) have not been widely explored. PDCA is rarely detected at an early stage but is characterized by rapid progression and invasiveness. We now report the significance of the subcellular localization of Kaiso in PDCAs from African Americans. Kaiso expression is higher in the cytoplasm of invasive and metastatic pancreatic cancers. In males, cytoplasmic expression of Kaiso correlates with cancer grade and lymph node positivity. In male and female patients, cytoplasmic Kaiso expression correlates with invasiveness. Also, nuclear expression of Kaiso increases with increased invasiveness and lymph node positivity. Further, analysis of the largest PDCA dataset available on ONCOMINE shows that as Kaiso increases, there is an overall increase in Zeb1, which is the inverse for E-cadherin. Hence, these findings suggest a role for Kaiso in the progression of PDCAs, involving the EMT markers, E-cadherin and Zeb1.
Keywords: Kaiso, Pancreatic ductal carcinoma, EMT markers
Introduction
In the United States, pancreatic ductal adenocarcinoma (PDCA) is the fourth leading cause of cancer death. Worldwide, more than 200,000 people are diagnosed each year; only about 4% live for 5 years after diagnosis [1]. The incidence of PDCA in African Americans is 50%–90% higher than in other racial groups [2]. African American patients also have the poorest prognosis of any racial group. These devastating statistics are likely due to the fact that, at diagnosis, ~85% of patients have advanced, unresectable disease [3]. Although chemotherapeutics such as FOLFIRINOX (5-fluorouracil, leucovorin, oxaliplatin, and irinotecan) and Abraxane® (protein-bound paclitaxel) have been approved as mono- and multimodality therapies for late-stage disease, PDCA remains largely unresponsive to most chemotherapeutic agents [4,5]. This is particularly evident for African Americans, who demonstrate worse outcomes compared to Caucasians [2]. Thus, there is a need to understand, particularly for African Americans, the biological mechanisms that contribute to development and progression of PDCAs and to develop early indicators of aggressive disease.
Kaiso, a bimodal transcription factor, belonging to the BTB/POZ (Broad Complex, Tramtrak, Bric à brac/Pox virus and Zinc finger) subfamily of zinc-finger proteins, binds both methylated DNA and consensus Kaiso binding sites (KBS) on DNA to regulate gene expression [6–8]. A function of Kaiso in tumorigenesis is methylation-dependent silencing of various tumor suppressor genes. For example, in prostate and breast cancers, Kaiso regulates genes that relate to de-differentiation, including those associated with the epithelial–mesenchymal transition (EMT), such as E-cadherin [8,9], Wnt 11 [10], and matrilysin [11]. Additionally, in MCF-7 breast cancer cells, Kaiso regulates the cyclin D1 promoter via binding to methylated CpG-dinucleotides and KBS [12]. Progeny of KaisoTg/+ (Kaiso-overexpressing) mice crossed with ApcMin/+ mice (used for studies on colon carcinogenesis) demonstrates elevated expression of Wnt-related genes, increased inflammation, and increased tumorigenesis [13].
Based on its subcellular localization, elevated expression of Kaiso has shown promise as a prognostic biomarker for various tumor types [14,15]. In chronic leukemia and in non-small cell lung cancers, elevated levels of Kaiso are present predominantly in the cytoplasm [16,17]. However, in colorectal, prostate, and breast cancers, Kaiso is present in both the cytoplasm and the nucleus. In more aggressive tumors, Kaiso shows a trend of increased nuclear expression [12,18]. In prostate cancer cells, nuclear Kaiso silences E-cadherin and induces these cells to undergo an EMT [8,9]. Also, depletion of Kaiso in breast cancer cell lines causes a reduction in the EMT markers, N-cadherin and vimentin [8]. Nuclear Kaiso is elevated in prostate and breast tumors of African American patients, who typically have highly aggressive tumors of these types [8,9]. To date, however, there are no studies examining the expression of Kaiso in PDCA.
Herein we present findings that Kaiso expression is elevated in African American patients with PDCA. Tumors of males have a higher cytoplasmic expression that is associated with cancer grade and total lymph node positivity. Also, there is a correlation between cytoplasmic Kaiso expression and cancer aggressiveness in males and females. Nuclear Kaiso expression increases with increased invasiveness and total positive lymph nodes. There is also a correlation between the expression of Kaiso and Zeb1 with E-cadherin, an EMT marker. Furthermore, as determined with the largest PDAC patient data set available on ONCOMINE, there is elevated expression of Kaiso and ZEB1, which was inverse for expression of E-cadherin. Hence, these results show that Kaiso has an oncogenic role in the metastasis of PDCA.
Materials and methods
Patient population
PDCA tissue microarrays (TMAs) were obtained from Emory University (Atlanta, GA). All specimens were originally collected utilizing Institutional Review Board-approved protocols. Additionally, the Institutional Review Boards of Tuskegee University and Troy University approved the use of these tissues for this study. For each patient, the following clinicopathological characteristics were assessed: gender, histological grade, pre-invasive tumor size, size and extent of the invasive tumor, lymph node positive tissues, and extent of regional lymph node metastases.
Immunohistochemistry (IHC)
IHC was performed with the anti-Kaiso clone 6F (Upstate Biotechnology, MA), and TMAs were stained for evaluation by IHC [19]. Briefly, cells were scored blindly, and individual specimens stained for Kaiso were scored separately for cytoplasmic and nuclear staining. Immunostaining was evaluated by determining the percentage of malignant cells in four random fields that demonstrated staining on a scale of 0–3. Scores of 0 (no staining), 1 (10–30% staining), 2 (40–70% staining), and 3 (71–100% staining) were assigned as previously described [8,9,20].
Statistical analyses
For all experiments, statistical calculations were performed with Microsoft Excel or GraphPad prism software; version 6. Independent Student’s t-tests were utilized to determine statistical differences between means of experimental and control values. Tissue correlations were made using chi-square and Fisher’s exact test. Any tissues that were damaged or lacked su3cient information were removed from the overall cohort. All p values were considered statistically significant at <0.05.
Results
Kaiso expression in human PDCA tissue
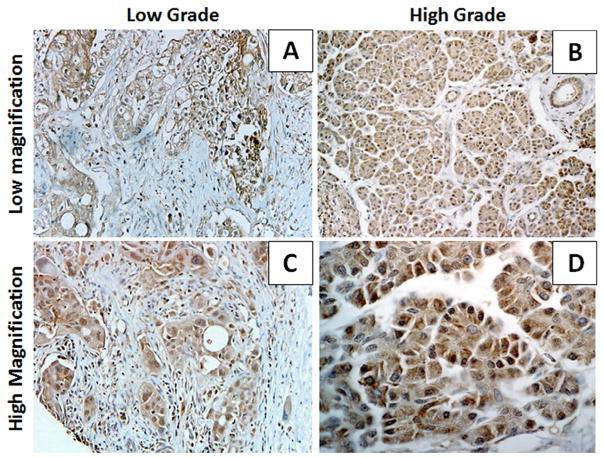
Previously, we reported that Kaiso expression is higher in aggressive breast and prostate cancers and in tumors of African Americans [21]. However, there have been few reports of Kaiso expression in PDCAs. To evaluate Kaiso expression and localization in PDCA progression, IHC was used to evaluate samples of tumors from 31 African American patients, 17 females and 14 males (Table 1). For low-grade malignant tissues, there was high expression of Kaiso in the cytoplasm and low levels in the nuclei (Fig. 1A and C, Table 2). In high-grade tumors, there was staining for Kaiso in both cytoplasm and nuclei (Fig. 1B and D, Table 2). As determined by immunofluorescence staining for Kaiso, there was a similar pattern of expression in low-grade and high-grade tumors, with low to absent expression of Kaiso in normal and adjacent normal tissue (Supplemental Fig. S1). Thus, Kaiso is robustly expressed in PDCAs.
Table 1.
Clinical and pathological characteristics of patient cohort.
| Number of patients | ||
|---|---|---|
| Characteristics | 31* | |
| Gender | ||
| Female | 17 | 54.80% |
| Male | 14 | 45.16% |
| Race | ||
| AA | 31 | 100% |
| Grade** | ||
| High | 10 | 32.26% |
| Low | 20 | 64.52% |
| Tumor size** | ||
| ≥2 | 6 | 19.35% |
| ≤2 | 24 | 77.42% |
| LN positive** | ||
| ≥2 | 20 | 64.52% |
| ≤2 | 10 | 32.26% |
| Stage T** | ||
| ≥2 (T1–T2) | 7 | 22.58% |
| ≤2 (T3–T4) | 21 | 67.74% |
| Stage N** | ||
| N0 | 10 | 32.26% |
| N1–N3 | 18 | 58.06% |
NOTE:
Patients missing due to the lack of clinical characteristics or tissue damage was excluded from the analysis.
Data are missing for few patients.
Fig. 1.
Subcellular localization of Kaiso in PDCAs. 6F8 Kaiso antibody was used to perform IHC on a pancreatic TMA. (A) Low-magnification images demonstrated that Kaiso is expressed in the cytoplasm of grade 1 tumors, but with weak to absent nuclear expression. (B) Low-magnification images of grade 1 tumors demonstrated intense Kaiso expression in the cytoplasm and the nucleus. (C) High-magnification images showing intense Kaiso expression in the cytoplasm and weak expression in the nucleus. (D) High-magnification images showing that Kaiso is expressed at high levels in both the cytoplasm and nucleus. The quality of each specimen was determined based on hematoxylin and eosin staining (data not shown). Low magnification ×100; high magnification ×400.
Table 2.
Correlation of sub-cellular Kaiso expression with patient and tumor characteristics.
| Characteristics | Patients | Cytoplasmic expression
|
p* | Nuclear expression
|
p* | ||
|---|---|---|---|---|---|---|---|
| <1.5 | ≥1.5 | <1.5 | ≥1.5 | ||||
| Gender | |||||||
| Male | 42 | 9 | 21 | 0.9186 | 18 | 12 | 0.1524 |
| Female | 51 | 14 | 31 | 34 | 11 | ||
| Stage T (male) | |||||||
| Non-invasive | 6 | 4 | 1 | 0.0101 | 5 | 0 | 0.0608 |
| Invasive | 30 | 4 | 16 | 11 | 9 | ||
| Stage T (female) | |||||||
| Non-invasive | 15 | 6 | 5 | 0.0418 | 7 | 4 | 0.3421 |
| Invasive | 33 | 7 | 25 | 25 | 7 | ||
| LN positive (male) | |||||||
| No | 27 | 9 | 11 | 0.0112 | 15 | 5 | 0.0177 |
| Yes | 15 | 0 | 10 | 3 | 7 | ||
| LN positive (female) | |||||||
| No | 33 | 10 | 19 | 0.3824 | 22 | 7 | 0.7549 |
| Yes | 15 | 3 | 11 | 10 | 4 | ||
| Grade (male) | |||||||
| Low | 21 | 7 | 7 | 0.0701 | 10 | 4 | 0.232 |
| High | 18 | 3 | 13 | 8 | 8 | ||
| Grade (female) | |||||||
| Low | 39 | 12 | 24 | 0.5196 | 26 | 10 | 0.2981 |
| High | 12 | 2 | 7 | 8 | 1 | ||
p value considered significant at ≤0.05.
Chi square test was employed for determining correlation.
Cytoplasmic and nuclear Kaiso expression is associated with higher tumor grade and size
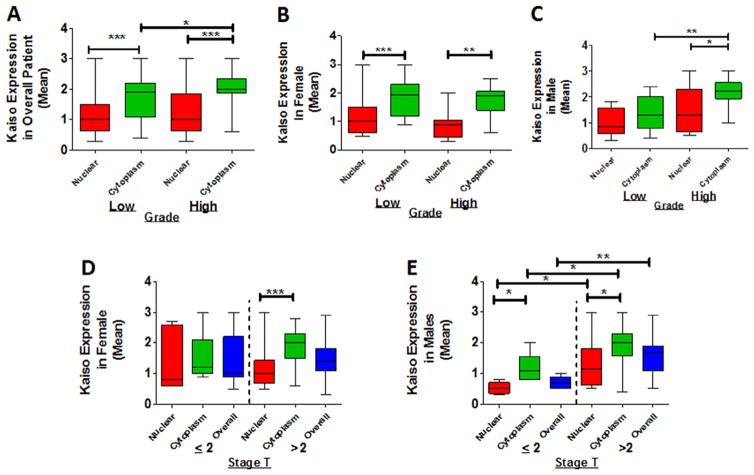
To determine the clinical relevance of Kaiso in PDCA, the association of Kaiso expression with the clinical grade of tumors, which is typically used as a prognostic factor, was analyzed. The tissues were analyzed and scored (0–3) blindly and subsequently analyzed quantitatively. In the overall patient population, expression of cytoplasmic Kaiso was higher in grade 2 tumors compared to grade 1 tumors (P = 0.0435) (Fig. 2A). For nuclear expression, there was no significant difference between tumor grades (Fig. 2A). When the population was separated according to gender, there were also no differences between nuclear expression and cytoplasmic expression in relation to tumor grade (Fig. 2B). Females, however, showed elevated expression of cytoplasmic Kaiso in both grade 1 and grade 2 tumors, compared to nuclear expression (P = 0.0001 and P = 0.0028, respectively). Grade 2 tumors from males displayed elevated expression of cytoplasmic Kaiso (P = 0.0013) (Fig. 2C). Although not statistically significant, there was elevated expression of nuclear Kaiso in grade 2 tumors of males (Fig. 2C). Furthermore, females with grade 1 tumors and males with grade 2 tumors displayed similar cytoplasmic Kaiso expression pattern.
Fig. 2.
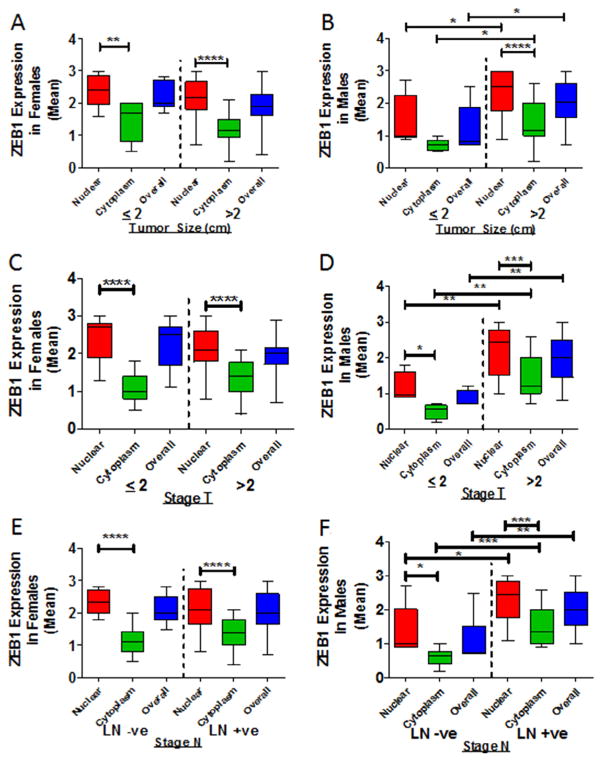
Elevated cytoplasmic in high-grade PDCAs. Scoring of Kaiso expression in the cytoplasm and nucleus was compared for grade 1 and grade 2 tumors. (A) Overall, there was elevated expression of cytoplasmic Kaiso (P = 0.0435). (B) For female patients, there was no increase in expression of cytoplasmic or nuclear Kaiso. (C) For males, there was an increase in cytoplasmic Kaiso (P = 0.0013). (D) For stage T2 tumors of females, there was no appreciable difference in cytoplasmic expression. (E) For males, there was an increase in cytoplasmic Kaiso (P = 0.0441) and nuclear Kaiso (P = 0.0236) in tumors >2 cm in size. *P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant.
Tumor size is a prognostic factor used to predict the resectability of PDCAs. To determine if there is an association between Kaiso expression and tumor size, tumor samples were analyzed based on size classification of ≤2 cm or >2 cm. Tumors of females exhibited high levels of cytoplasmic Kaiso expression in both smaller and larger tumors, but there was no significant difference (Fig. 2D). However, in tumors of males, overall Kaiso (P = 0.0369) and cytoplasmic Kaiso (P = 0.0441) were associated with tumors >2 cm (Fig. 2E). These findings suggest that, in tumors of men with PDAC, there is a cytoplasmic to nuclear shift of Kaiso expression.
Subcellular expression of Kaiso is associated with the size and extent of invasive tumor and lymph node metastases
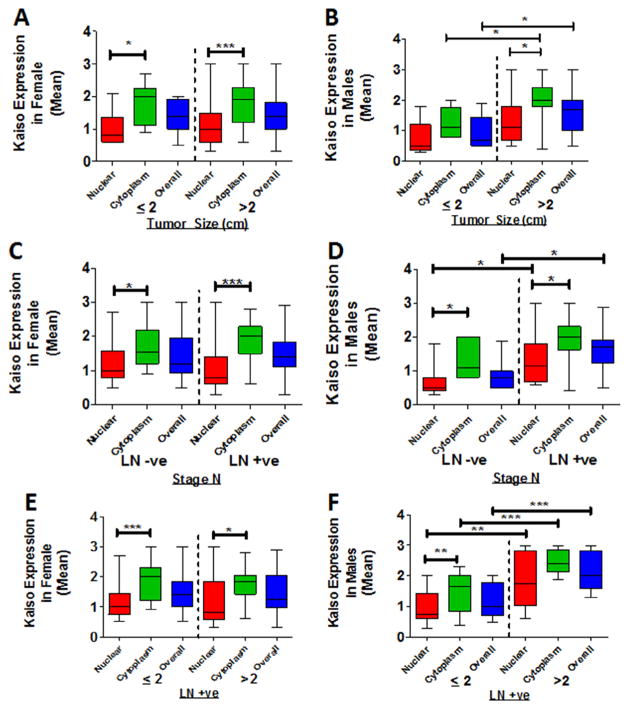
Early stage PDCA is slow growing and is often undetectable, preventing early diagnosis; however, patients often experience rapid and robust metastases after diagnosis [22]. An elevated cytoplasmic expression of Kaiso in larger tumors (>2 cm) implies that there is an association of Kaiso expression with the size and extent of the invasive or metastatic tumor (stage T) of PDCA. Stage T2 tumors have not yet spread outside the pancreas, whereas stage T3 and T4 tumors are large and have extended outside the pancreas. Therefore, stage T2 was utilized as the cut-off stage to analyze samples from the patient population. Cytoplasmic Kaiso expression was predominant in specimens from female patients with Stage T3 and above (n = 48; P < 0.0001) (Fig. 3A). Tumors of males displayed elevated Kaiso expression in both cytoplasmic and nuclear compartments as Stage T advanced (n = 36; P = 0.0384 and P = 0.0236, respectively) (Fig. 3B).
Fig. 3.
Cytoplasmic and nuclear expression of Kaiso in male and female PDAC patients. Cytoplasmic and nuclear expression of Kaiso was compared in tumors ≤2 or ≥2 cm in size. (A) There were no significant differences in cytoplasmic or nuclear Kaiso expression in tumors of females. (B) Tumors of males ≥2 cm in size demonstrated higher levels of cytoplasmic (P = 0.0384) and nuclear Kaiso (P = 0.0236). (C) Tumors of females did not demonstrate a significant difference with positive or negative lymph nodes (Stage N). (D) For lymph node-positive patients compared to lymph node-negative patients, tumors of males showed elevated Kaiso in the nucleus (P = 0.0413) and overall (P = 0.0141). (E) For females, there was no significant difference in tumors ≥2 or ≤2 cm in size that had positive or negative lymph nodes. (F) Tumors of males ≥2 cm in size with positive lymph nodes showed elevated expression of Kaiso in the cytoplasm (P = 0.0001), in the nucleus (P = 0.0012), and overall (P = 0.0002). LN+ve, lymph node positive; LN−ve, lymph node negative. *P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant.
Since lymph node metastasis is a prognostic factor for surgical extirpation of the primary tumor and metastatic tissues [23], the expression of Kaiso in tumors of patients with lymph node metastases was analyzed (Stage N). Tissue specimens of female patients with lymph node metastases (N11–N3), showed higher cytoplasmic Kaiso (n = 30; P < 0.0001) than those with no lymph node metastasis (N0) (n-18) (Fig. 3C). However, cancer tissues of male patients with lymph node metastases (N1–N3) showed higher levels of nuclear and overall Kaiso (n = 24; P = 0.0413 and P = 0.0141 respectively) (Fig. 3D). For tumors of females with lymph node positivity, there were no appreciable effects of tumor stage (Fig. 3E). For males, however, there was elevated, nuclear, cytoplasmic, and overall expression of Kaiso in those with advanced lymph node positivity (P = 0.0012, P = 0.0001 and P = 0.0002 respectively) (Fig. 3F). These findings suggest, for PDCAs, an association of sub-cellular Kaiso expression and lymph node metastasis and tumor size.
Kaiso expression correlates with clinicopathological characteristics of PDCA patients
To determine the correlation of Kaiso expression with clinicopathological characteristics, chi-square analyses were performed (Table 2). In the tumors of males, expression of cytoplasmic and nuclear Kaiso correlated with lymph node metastasis (P = 0.0112 and P = 0.0177, respectively). For tumors of male and female patients, expression of cytoplasmic Kaiso correlated with tumor invasion (Stages T3–T4) (P = 0.0101 and P = 0.0418, respectively). Although there was no statistical correlation of tumor grade with Kaiso expression, there was a trend toward higher cytoplasmic expression in high-grade tumors of males (P = 0.0013). These analyses indicate that subcellular Kaiso expression during the metastasis of PDCAs correlates with clinicopathological features used to diagnose metastatic stages.
Kaiso expression in pancreatic tissue correlates with EMT markers Zeb1 and E-cadherin
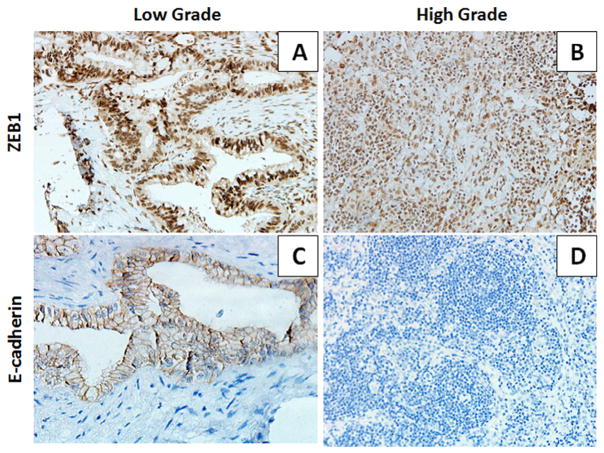
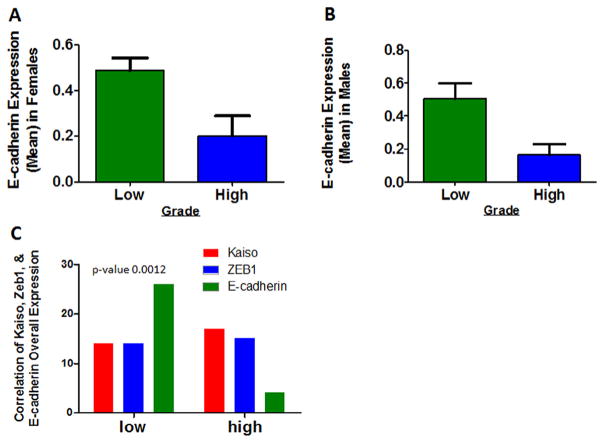
ZEB1 is a transcriptional repressor involved in the induction and regulation of the EMT and, in various tumor types, including PNCAs, in invasion and metastasis through regulation of E-cadherin expression [24]. Moreover, metastasis is related to loss of E-cadherin [8,9]. Kaiso regulates various genes, including E-cadherin [8,9]. To explore this relationship in PDCAs, tissue samples of grade 1 and grade 2 PDCAs were stained for ZEB1 and E-cadherin and scored as described above (Fig. 4). There were no appreciable increases in ZEB1 expression in grade 1 and grade 2 tumors from female patients (Fig. 5A). There were, however, increases of cytoplasmic, nuclear, and overall ZEB1 in tumors of male patients (Fig. 5B). This trend continued for other clinical features; tumors from males, but not females, exhibited elevation of cytoplasmic, nuclear and overall ZEB1 expression in tumors of Stage T > 2 (Fig. 5C and D) and in tumors of patients with positive lymph nodes (Fig. 5E and F). When data for tumors from male and female patients were combined, there were no significant changes in expression of Kaiso, ZEB1, or E-cadherin (data not shown). High-grade tumors showed elevated expression of Kaiso (P < 0.0004) (Fig. 2A) as compared to expression of E-cadherin (Fig. 6A and B). However, there was a correlation between the expression of Kaiso and ZEB1 with E-cadherin (P < 0.0238), as determined by chi-square analysis (Fig. 6C). These results suggest that Kaiso, together with ZEB1, has an influence on the expression of E-cadherin.
Fig. 4.
Expression and subcellular localization of ZEB1 and E-cadherin in PDACs. Anti-ZEB1 and anti-E-cadherin antibodies were used for IHC analysis of grade 1 and grade 2 tumors. Images are representative of ZEB1 and E-cadherin expression. (A) Low-magnification image of ZEB1 expression in grade 1 tumors. (B) Low-magnification image of ZEB1 expression in grade 2 tumors. (C) Low magnification image of E-cadherin expression in grade 1 tumors. (D) Low magnification image of E-cadherin expression in grade 2 tumors. Low magnification ×100.
Fig. 5.
Subcellular localization of ZEB1 in PDACs. Expression of cytoplasmic and nuclear ZEB1 was assessed in tumors ≤2 or ≥2 cm in size. (A) For tumors of females, there was no appreciable elevation in expression of cytoplasmic or nuclear ZEB1. (B) For males, high-grade tumors >2 cm in size showed high nuclear (P = 0.0298), cytoplasmic (P = 0.0441) and overall (P = 0.0369) expressions of ZEB1. (C) Stage T tumors of females did not demonstrate a significant difference in expression of ZEB1. (D) For males, Stage T tumors ≥2 cm in size showed elevated expression of ZEB1 in the cytoplasm (P = 0.05), in the nucleus (P = 0.041), and overall (P = 0.014). (E) In lymph node-positive relative to lymph node-negative patients, tumors of females did not demonstrate a significant difference in expression of ZEB1. (F) Lymph node-positive compared to lymph node-negative tumors of males demonstrated elevated expression of ZEB1 in the cytoplasm (P = 0.0001), in the nucleus (P = 0.0012), and overall (P = 0.0002). LN+ve, lymph node positive; LN−ve, lymph node negative. *P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant.
Fig. 6.
ZEB1 and E-cadherin expression correlates with Kaiso expression. Scoring of E-cadherin expression in grade 1 and grade 2 tumors. (A) Grade 2 tumors of females demonstrated lower expression of E-cadherin (P = 0.0182). (B) Grade 2 tumors of males also demonstrated lower E-cadherin expression (P = 0.0044). (C) As determined by chi-square analysis, there was a correlation between the expressions of Kaiso and ZEB1 with E-cadherin (P < 0.0238).
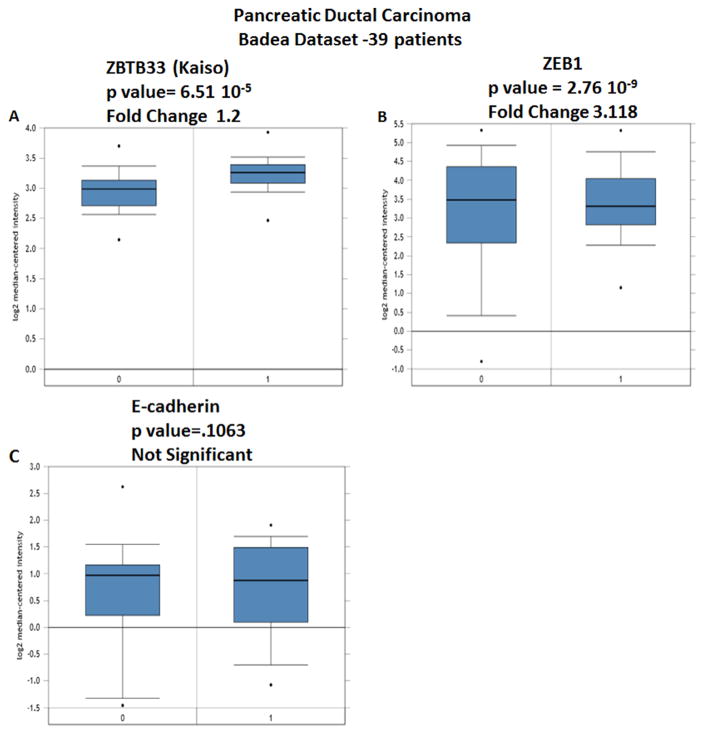
To validate the expression of Kaiso, ZEB1, and E-cadherin in PDCAs, levels of mRNA expression of all three genes were analyzed using the Badea et al. data set, available on Oncomine, which contains expression values for 39 patients [25]. Expressions of Kaiso p = 6.51 × 10−5 (Fig. 7A) and ZEB1 2.76 × 10−9 (Fig. 7B) are elevated in PDCAs compared to tissues from normal patients. There was no significant difference in E-cadherin expression in PDCAs compared to normal tissue (Fig. 7C). Thus, in PDCAs, Kaiso is elevated at both the mRNA and protein levels.
Fig. 7.
Kaiso, ZEB1, and E-cadherin gene analysis in PDCA (Oncomine database). Box plots derived from gene expression data in Oncomine comparing expression of each gene in normal (left plot) and PDCA tissue (right plot). (A) ZBTB33 (Kaiso); (B) ZEB1; and (C) E-cadherin. Oncomine box plots were retrieved from Badea et al. [25].
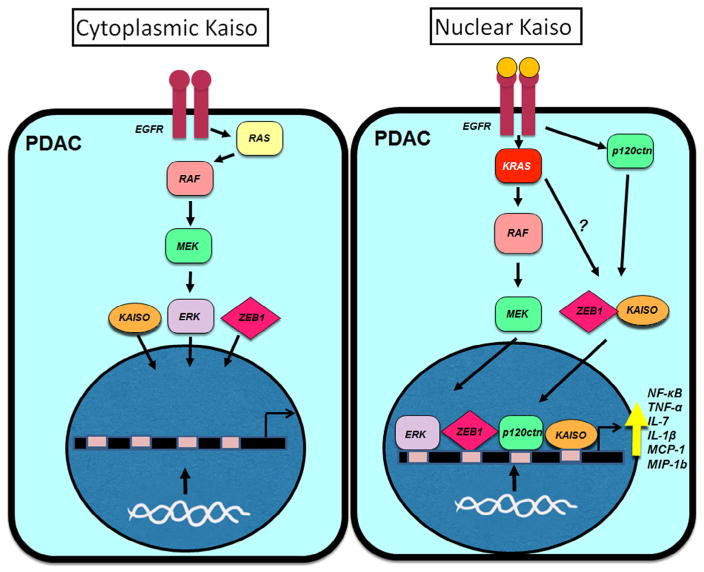
There is a growing literature on the role of Kaiso in cancer, therefore a proposed model for the role of Kaiso in male and female PDAC patients is included, where upregulation of EGFR or constitutively active mutant KRAS is responsible for nuclear localization of Kaiso (Fig. 8).
Fig. 8.
Proposed model of Kaiso subcellular localization in male and female patients with PDAC. Upregulation of EGFR or constitutively active mutant KRAS is responsible for nuclear localization of Kaiso, ZEB1 and ERK. Nuclear localization of ERK could be responsible for increasing Kaiso expression, and nuclear Kaiso could inhibit the anti-inflammatory effects of nuclear p120ctn and increase the expression of pro-inflammatory cytokines IL-7, IL-3, MCP1, MIP1β, GM-CSF and G-CSF.
Discussion
There is a 50–90% higher incidence of PDCA for African Americans compared to the general population [26,27]. Furthermore, PDACs are more prevalent in African American male patients as compared to females [8,39]. Cigarette smoking, which causes about 25% of PDCAs [1], is more common among African American males [2] and may explain, in part, why PDCA is more prevalent in this group. Other risk factors for PDCA more common in African Americans include diabetes mellitus, pancreatitis, and being overweight. Also, late detection and rapid progression of PDCA may contribute to their low survival rates [27]. Socioeconomic status is not the major factor contributing to this disparity, however, as African Americans present a pro-tumorigenic gene profile [28,29], thus, there is a need to elucidate the factors that contribute to tumorigenesis.
Kaiso, which is involved in silencing of various tumor suppressor genes, is a promising prognostic biomarker and therapeutic target for multiple tumor types [8,9,15,16]. Kaiso expression and localization varies in different types of tumors. For example, cytoplasmic localization of Kaiso is associated with poor prognosis of chronic myeloid leukemia and non-small cell lung cancer [16,17], while nuclear Kaiso is a poor prognostic marker in colon, breast, and prostate tumors [8,9,13]. However in all tumor types, evaluated expression, particularly in African American cancer patients compared to non-malignant tissue [8,9,13]. Interestingly, loss of Kaiso results in the re-activation of the tumor suppressor genes silenced by hypermethylation, indicating that Kaiso can be targeted therapeutically [14]. Additionally, however, there are no reports regarding Kaiso expression in PDCAs, and thus its role and association with clinicopathological characteristics of PDCA need to be elucidated.
In the present study, a TMA-based survey of tumors from African American patients was used to analyze the expression pattern of Kaiso in PDCA tissues. Tumors from both male and female patients displayed high levels of cytoplasmic Kaiso; however, only male patients demonstrated increases in grade 2 tumors compared to grade 1 tumors. In tumors of males, there were elevated levels of both cytoplasmic and nuclear Kaiso, and this correlated with other factors that determine tumor aggressiveness, such as tumors <2 cm in size and positivity for lymph node metastases. Although there were no appreciable differences in Kaiso levels in tumors of females, grade 1 tumors from females had similar Kaiso expression levels as males with aggressive disease. These results suggest that Kaiso has a gender-specific role in promoting aggressiveness in patients with PDCA.
The cytoplasmic-to-nuclear shift of Kaiso in males with lymph node-positive tumors is similar to our findings with lymph node-positive breast and prostate cancers, in which tumors have undergone an EMT [8,9]. Similar to results for primary tumors, males with regional lymph node metastases displayed a higher expression of both nuclear and cytoplasmic Kaiso that was not observed in tumors from females. To verify that these changes are associated with tumors undergoing EMT, the same patient samples were probed for the EMT markers, ZEB1 and E-cadherin, which are prognostic factors for PDAC [30]. Similar to our finding with Kaiso, both cytoplasmic and nuclear expressions of ZEB1 correlate with tumor stage and positive lymph node status. Additionally, high levels of Kaiso and ZEB1 are associated with decreased expression of E-cadherin [24], which was not observed in a comparison of E-cadherin in grade 1 and grade 2 tumors. Furthermore, there were elevated expressions of Kaiso and ZEB1 as determined with Oncomine data from the Badea et al. study [25]. Thus, given the widespread association of ZEB1 and E-cadherin as prognostic markers for multiple tumor types, including PDAC, the present data suggest that Kaiso could be added to these markers.
Although males develop 30% more PDCA relative to females, the molecular mechanism remains elusive. Previously, we demonstrated that stimulation of epidermal growth factor receptor (EGFR) is at least partially responsible for shuttling Kaiso to the nucleus [9]. There is overexpression of EGFR in up to 90% of PDACs [31,32], with males expressing more than female patients [33]. Nevertheless, EGFR inhibitors have only a marginal clinical benefit [34]. This is largely due to downstream mutations in KRAS, which activate various EGFR signaling pathways independent of EGFR ligand activation [34]. Since both Kaiso and ZEB1 are transcriptional repressors, it is possible that the cytoplasmic-to-nuclear shift is the result of EGFR or mutant KRAS signaling causing increased invasiveness and aggressiveness of PDCA. A second option is that nuclear localization of Kaiso inhibits the anti-inflammatory effects of p120-catenin (a Kaiso binding partner), which is responsible for IκB-α degradation [35]. This is particularly evident in studies where KaisoTg/+ (Kaiso-overexpressing) mice crossed with ApcMin/+ mice demonstrate increased tumorigenesis and significant increases in NFκB and pro-inflammatory cytokines IL-7, IL-3, CCL2-monocyte chemotactic protein 1 (MCP1), CCL4-macrophage inflammatory protein-1 (MIP1β), GM-CSF and G-CSF. Similarly, Kaiso-null mice crossed with ApcMin/+ mice displayed a delayed onset of intestinal tumors [15]. While more studies should focus on the interaction of Kaiso and p120ctn in regulating inflammation, it is well characterized that subcellular expression of p120ctn in tumors is associated with a lack of E-cadherin [36,37]. Multiple studies provide evidence that males have higher incidence of certain cancers compared to females [35,38], and PDACs represent an excellent example of inflammation driven tumors. Thus, it is plausible that Kaiso has a role in inducing both inflammation and EMT, factors that are necessary for metastases to occur. Although we have not determined the influence of other methyl binding proteins like ZBTB4 on EMT or inflammation, our observations support the multiple findings that subcellular localizations of Kaiso and ZEB1 regulate the loss of E-cadherin [8].
In summary, due to the slow progression of PDCAs in early stages, Kaiso expression and localization varies relative to other cancer types such as prostate and breast cancers. In PDCAs, Kaiso may have a dual function. It is likely that cytoplasmic Kaiso drives high-grade, larger tumors (>2 cm) toward metastases with simultaneous loss of E-cadherin. Once the tumors metastasize from its primary site, Kaiso localizes to the nucleus and, together with ZEB1 and other tumor suppressors, promotes invasiveness of PDCAs. The exact reason is that there are gender differences in Kaiso expression that are largely known; more work should be done to determine the hormonal influence on Kaiso in both males and females. Additional studies in females pre- and post-menopause would also add insight into the gender/hormonal influences on Kaiso.
Supplementary Material
Highlights.
Kaiso expression is elevated in High Grade Pancreatic Tumors.
Males’ expression more significantly increases in cytoplasmic and nuclear Kaiso.
Kaiso and ZEB1 correlate with loss of E-cadherin expression.
Acknowledgments
Funding
This work was supported by the Department of Defense Prostate Cancer Research Program (PC120913) [CY], G12 RR03059-21A1 (NIH/RCMI) [CY], (NIH/NCI) 1 R21 CA188799-01 [CY] and U54 CA118623 (NIH/NCI) [CY], a pilot project on Pancreatic SPORE 2 P50 CA101955-11 [CY]. Statistical analysis was performed by Tuskegee University Statistical Core.
We would like to thank Drs. Donald Buchsbaum at University of Alabama at Birmingham (UAB) and Ritu Anjea at Georgia State University for their comments and discussions.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2016.06.025.
Footnotes
Conflict of interest
None of the authors have any conflicts of interest to report on this project.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Khawja SN, Mohammed S, Silberfein EJ, Musher BL, Fisher WE, Van Buren G., 2nd Pancreatic cancer disparities in African Americans. Pancreas. 2015;44:522–527. doi: 10.1097/MPA.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 3.Niederhuber JE, Brennan MF, Menck HR. The national cancer data base report on pancreatic cancer. Cancer. 1995;76:1671–1677. doi: 10.1002/1097-0142(19951101)76:9<1671::aid-cncr2820760926>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21:9297–9316. doi: 10.3748/wjg.v21.i31.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon D, McFarland K, Velanovich V, Martin RC., 2nd Borderline and locally advanced pancreatic adenocarcinoma margin accentuation with intraoperative irreversible electroporation. Surgery. 2014;156:910–920. doi: 10.1016/j.surg.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 6.Dalla-Favera R, Ye BH, Lo Coco F, Chang CC, Cechova K, Zhang J, et al. BCL-6 and the molecular pathogenesis of B-cell lymphoma. Cold Spring Harb Quant Biol. 1994;59:117–123. doi: 10.1101/sqb.1994.059.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones J, Wang H, Karanam B, Theodore S, Dean-Colomb W, Welch DR, et al. Nuclear localization of Kaiso promotes the poorly differentiated phenotype and EMT in infiltrating ductal carcinomas. Clin Exp Metastasis. 2014;31:497–510. doi: 10.1007/s10585-014-9644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones J, Wang H, Zhou J, Hardy S, Turner T, Austin D, et al. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am J Pathol. 2012;181:1836–1846. doi: 10.1016/j.ajpath.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, et al. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol. 2004;6:1212–1220. doi: 10.1038/ncb1191. [DOI] [PubMed] [Google Scholar]
- 11.Spring CM, Kelly KF, O’Kelly I, Graham M, Crawford HC, Daniel JM. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp Cell Res. 2005;305:253–265. doi: 10.1016/j.yexcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson NS, Pierre CC, Anstey MI, Robinson SC, Weerawardane SM, Daniel JM. Kaiso represses the cell cycle gene cyclin D1 via sequence-specific and methyl-CpG-dependent mechanisms. PLoS ONE. 2012;7:e50398. doi: 10.1371/journal.pone.0050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierre CC, Longo J, Mavor M, Milosavljevic SB, Chaudhary R, Gilbreath E, et al. Kaiso overexpression promotes intestinal inflammation and potentiates intestinal tumorigenesis in Apc(Min/+) mice. Biochim Biophys Acta. 2015;1852:1846–1855. doi: 10.1016/j.bbadis.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Lopes EC, Valls E, Figueroa ME, Mazur A, Meng FG, Chiosis G, et al. Kaiso contributes to DNA methylation-dependent silencing of tumor suppressor genes in colon cancer cell lines. Cancer Res. 2008;68:7258–7263. doi: 10.1158/0008-5472.CAN-08-0344. [DOI] [PubMed] [Google Scholar]
- 15.Prokhortchouk A, Sansom O, Selfridge J, Caballero IM, Salozhin S, Aithozhina D, et al. Kaiso-deficient mice show resistance to intestinal cancer. Mol Cell Biol. 2006;26:199–208. doi: 10.1128/MCB.26.1.199-208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cofre J, Menezes JR, Pizzatti L, Abdelhay E. Knock-down of Kaiso induces proliferation and blocks granulocytic differentiation in blast crisis of chronic myeloid leukemia. Cancer Cell Int. 2012;12:28. doi: 10.1186/1475-2867-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai SD, Wang Y, Miao Y, Zhao Y, Zhang Y, Jiang GY, et al. Cytoplasmic Kaiso is associated with poor prognosis in non-small cell lung cancer. BMC Cancer. 2009;9:178. doi: 10.1186/1471-2407-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soubry A, van Hengel J, Parthoens E, Colpaert C, Van Marck E, Waltregny D, et al. Expression and nuclear location of the transcriptional repressor Kaiso is regulated by the tumor microenvironment. Cancer Res. 2005;65:2224–2233. doi: 10.1158/0008-5472.CAN-04-2020. [DOI] [PubMed] [Google Scholar]
- 19.Manne U, Myers RB, Srivastava S, Grizzle WE. Re: loss of tumor marker-immunostaining intensity on stored para3n slides of breast cancer. J Natl Cancer Inst. 1997;89:585–586. doi: 10.1093/jnci/89.8.585. [DOI] [PubMed] [Google Scholar]
- 20.Grizzle WE, Myers RB, Manne U, Srivastava S. Immunohistochemical evaluation of biomarker expression in neoplasia. In: Walaszek MHAZ, editor. John Walker’s Methods in Molecular Medicine – Tumor Marker Protocols. Humana Press Inc; Totowa, NJ: 1998. pp. 161–179. [Google Scholar]
- 21.Abdalla MO, Karna P, Sajja HK, Mao H, Yates C, Turner T, et al. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J Control Release. 2011;149:314–322. doi: 10.1016/j.jconrel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Masuda K, Harada S, Akioka K, Sako H. Pancreatic cancer: slow progression in the early stages. Int J Surg Case Rep. 2013;4:693–696. doi: 10.1016/j.ijscr.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JL, Nguyen AH, Rochefort M, Muthusamy VR, Wainberg ZA, Dawson DW, et al. Pancreatic cancer patients with lymph node involvement by direct tumor extension have similar survival to those with node-negative disease. J Surg Oncol. 2015;112:396–402. doi: 10.1002/jso.24011. [DOI] [PubMed] [Google Scholar]
- 24.Wellner U, Brabletz T, Keck T. ZEB1 in pancreatic cancer. Cancers (Basel) 2010;2:1617–1628. doi: 10.3390/cancers2031617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. [PubMed] [Google Scholar]
- 26.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman DT, Hoover RN, Brown LM, Swanson GM, Schiffman M, Greenberg RS, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118:1334–1344. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 29.Kwabi-Addo B, Wang S, Chung W, Jelinek J, Patierno SR, Wang BD, et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin Cancer Res. 2010;16:3539–3547. doi: 10.1158/1078-0432.CCR-09-3342. [DOI] [PubMed] [Google Scholar]
- 30.Bronsert P, Kohler I, Timme S, Kiefer S, Werner M, Schilling O, et al. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery. 2014;156:97–108. doi: 10.1016/j.surg.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Pryczynicz A, Guzinska-Ustymowicz K, Kemona A, Czyzewska J. Expression of EGF and EGFR strongly correlates with metastasis of pancreatic ductal carcinoma. Anticancer Res. 2008;28:1399–1404. [PubMed] [Google Scholar]
- 32.Jimeno A, Tan AC, Coffa J, Rajeshkumar NV, Kulesza P, Rubio-Viqueira B, et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res. 2008;68:2841–2849. doi: 10.1158/0008-5472.CAN-07-5200. [DOI] [PubMed] [Google Scholar]
- 33.Perini MV, Montagnini AL, Coudry R, Patzina R, Penteado S, Abdo EE, et al. Prognostic significance of epidermal growth factor receptor overexpression in pancreas cancer and nodal metastasis. ANZ J Surg. 2015;85:174–178. doi: 10.1111/ans.12399. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S, et al. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Regul. 2015;59:65–81. doi: 10.1016/j.jbior.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Casimir GJ, Duchateau J. Gender differences in inflammatory processes could explain poorer prognosis for males. J Clin Microbiol. 2011;49:478. doi: 10.1128/JCM.02096-10. author reply 478–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayerle J, Friess H, Buchler MW, Schnekenburger J, Weiss FU, Zimmer KP, et al. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology. 2003;124:949–960. doi: 10.1053/gast.2003.50142. [DOI] [PubMed] [Google Scholar]
- 37.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 38.Soderlund S, Granath F, Brostrom O, Karlen P, Lofberg R, Ekbom A, et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology. 2010;138:1697–1703. doi: 10.1053/j.gastro.2010.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.