Abstract
Purpose
To examine stress reactivity in a sample of prenatally drug exposed (PDE) adolescents by examining the consequences of PDE on stress-related adrenocortical reactivity, behavioral problems and drug experimentation during adolescence.
Methods
Participants (76 PDE, 61 non-drug exposed [NE]; 99% African-American; 50% male; Mage=14.17 years, SD=1.17) provided a urine sample, completed a drug use questionnaire, and provided saliva samples (later assayed for cortisol) before and after a mild laboratory stress task. Caregivers completed the Behavior Assessment System for Children II and reported their relationship to the adolescent.
Results
The NE group was more likely to exhibit task-related cortisol reactivity, compared to the PDE group. Overall behavior problems and drug experimentation were comparable across groups with no differences between PDE and NE groups. In unadjusted mediation analyses, cortisol reactivity mediated the association between PDE and BASC II aggression scores (95% bootstrap CI: 0.04-4.28), externalizing problems scores (95% bootstrap CI: 0.03-4.50) and drug experimentation (95% bootstrap CI: 0.001-0.54). The associations remain with the inclusion of gender as a covariate but not when age is included.
Conclusions
Findings support and expand current research in cortisol reactivity and PDE by demonstrating that cortisol reactivity attenuates the association between PDE and behavioral problems (aggression) and drug experimentation. If replicated, prenatal drug exposure may have long lasting effects on stress-sensitive physiological mechanisms associated with behavioral problems (aggression) and drug experimentation in adolescence.
Approximately 5.4% of pregnant women ages 15 to 44 use illegal drugs (e.g., cocaine/heroin), illustrating that prenatal drug exposure (PDE) is a public health issue.1 According to the current research on stress, prenatal stressors (such as PDE) are thought to impact long-term health and well-being by influencing fetal brain development and the Hypothalamic-Pituitary-Adrenal axis (HPA).2 Stress (e.g., poverty, psychopathology, drug use, etc.) during pregnancy increases activation of the fetal HPA axis, with increasing exposure to glucocorticoids and disruption to brain regions involved in the development and regulation of the HPA axis.2
PDE may act as an intrauterine stressor that leads to behavioral, emotional, and health problems later in life by altering gene expression,3 or disrupting the HPA axis.4,5 Postnatal stressors can continue to disrupt brain development and regulation of the HPA axis.6 Chronic stress (e.g., poverty) often accompanies PDE which can lead to prolonged, repeated elevations in glucocorticoids, resulting in the hyporeactivity of the HPA axis response.7 The correlates and concomitants include developmental trajectories characterized by problems with behavioral and emotional regulation.2,3
Current theory links physiological, cognitive, and affective tendencies with altered central dopaminergic activity leading to changes in brain function that may foster impulsive and risky behaviors.8 Disruption of the HPA axis can have negative effects on brain regions that are associated with behavior development, such as the amygdala and hippocampus.2 The amygdala is responsible for activating the stress response to cope with emotions such as fear and anxiety, while the hippocampus and pre-frontal cortex (PFC) antagonize the amygdala to suppress the stress response.2 Disruptions in the PFC, operationalized by impairments in decision-making and impulse control, may also disrupt emotional development.2 Several studies have demonstrated an association between lower or flattened cortisol levels and problem behaviors in children and adolescents both concurrently and longitudinally.9-11 In addition to lower cortisol levels, several studies examined the association between cortisol reactivity (in reaction to experimental stressors) and externalizing behavioral problems, finding a link between lower levels of HPA reactivity and higher levels of externalizing behavioral problems.10,12
Studies examining cortisol levels in adolescents with PDE have produced mixed results, though most show some alteration in the HPA axis.13 Adolescents with PDE have higher basal levels of cortisol than those not exposed,13 and cortisol levels in response to a stressor have been both flattened but also more reactive.4,13,14 Differences in the postnatal environment may be a reason for these mixed findings, as caregiving instability is associated with cortisol response in children with PDE.15 Given the evidence linking low cortisol levels and cortisol reactivity with externalizing problems and the well-characterized effects of PDE on the function of the amygdala and hippocampus, cortisol reactivity may explain the association between PDE and externalizing problems.16 Additionally, living with an alternative caregiver, rather than the biological mother, may add additional stress.
Current Study
This study examines how the HPA axis (measured by cortisol reactivity to a mild stressor) relates to behavioral problems (including drug experimentation) among adolescents who vary in PDE status and current caregiving status (with biological mother or alternative caregiver). In keeping with findings that both PDE and low cortisol reactivity are associated with multiple behavioral problems,10,12 we hypothesize that the association between PDE and behavioral problems is mediated by cortisol reactivity. Current evidence further suggests that PDE is associated with other pre and postnatal stressors, including prenatal alcohol and tobacco exposure and caregiving instability, and that multiple stressors may alter the cortisol response.15 Therefore, we include two exploratory hypotheses related to multiple pre and postnatal stressors, including PDE, prenatal alcohol and tobacco exposure, and living with an alternative caregiver (e.g., not the biological mother). We hypothesize that multiple pre and postnatal stressors increase the likelihood for flattened stress reactivity and increased behavioral problems. We also hypothesize that the association between multiple pre and postnatal stressors and behavioral problems is attenuated by cortisol reactivity.
Method
Participants and Procedures
Participants in the current study are 137 adolescents (M=14.17 years, SD=1.17, range 11.93 to 16.64), 50% male and predominantly (99%) African American (Table 1). More than half (54%) of the PDE group was exposed to both cocaine and heroin. The PDE group was significantly more likely than the NE group to have been exposed to alcohol and tobacco prenatally (Table 1). Over 60% of the PDE group, compared to 100% of the NE group, was in the care of their biological mother during the adolescent phase of the study (Table 1).
Table 1.
Sample description overall and by prenatal drug exposure (n=137)
| Overall | Prenatally Drug Exposed (n=76) | Non-Exposed (n=61) | p | |
|---|---|---|---|---|
|
| ||||
| Mean Age (SD) | 14.17 (1.17) | 14.26 (1.13) | 14.05 (1.21) | ns |
| % Male | 50.4 | 50.0 | 50.8 | ns |
| % African American | 99.3 | 98.7 | 100 | ns |
| % Care with Biological Mother | 79.6 | 63.2 | 100 | <.01 |
| Prenatal Drug Exposures | ||||
| % heroin (no cocaine) | -- | 13.16 | -- | -- |
| % cocaine (no heroin) | -- | 32.89 | -- | -- |
| % cocaine and heroin | -- | 53.95 | -- | -- |
| % alcohola | 38 | 53.9 | 18.0 | <.01 |
| % tobaccoa | 53.3 | 78.9 | 21.3 | <.01 |
Alcohol and tobacco exposures overlap with one another and with cocaine and heroin exposures;
ns=not significant
The original study was a randomized, controlled trial of a home-based intervention for substance abusing women and their children who were prenatally exposed to cocaine and/or heroin. Mothers and their infants were recruited shortly after delivery (1991-1993) at an urban university hospital and tracked through adolescence; 265 completed the baseline evaluation 2 weeks post-delivery. Eligibility for infants included prenatal cocaine and/or heroin exposure validated by positive maternal or infant urine toxicology or maternal self-report of use, gestational age >32 weeks, birth weight >1,750g, and no serious birth complications or admission to the neonatal intensive care unit. The study was conducted during a time when toxicology screens were conducted routinely during delivery.17
Two groups of non-exposed (NE) children were recruited, one at age 5 (n=70 mother-child dyads) and the other in early adolescence (n=24 mother-child dyads),17 to serve as a community comparison. Participants in the NE group resided in the same communities as participants in the PDE group and were matched for socioeconomic status (e.g., maternal education), maternal age at first pregnancy, and child age, gender, and race. Recruitment took place at a primary care clinic serving the same university hospital where the PDE participants were recruited. NE participants were identified by reviewing medical records of children delivered at the university hospital who were born during the same time period as children from the PDE group and who had negative toxicology screens and no evidence of maternal substance use. All NE participants were in the care of their biological mother at the time of recruitment.17
During the adolescent phase, participants and their caregivers were contacted and invited to participate in a laboratory-based assessment. Caregiver-adolescent dyads completed several measures of behavior functioning and three assessments of cortisol (collected over a 4.5-6 hour period). Since cortisol can be influenced by glucose levels, participants fasted for three hours prior to their appointment and were scheduled for morning appointments with 85% arriving at 10:30 am or earlier.4 Upon arrival, research assistants established rapport, and discussed consent/assent forms. After approximately 30 minutes, the first cortisol sample was collected (pre task; M=9:41am, SD=.85 hrs).4 Participants then were presented with computer-administered mild stressors, followed by a questionnaire. The second cortisol sample was collected (post task; M=10:42 am, SD=.86 hrs) approximately 30 minutes after the mild stressors. 99% of pre and 97% of post task collections were completed before noon.4 After the completion of the pre and post task cortisol collections, adolescents completed structured tasks and assessments and were offered breakfast and lunch. A third cortisol collection occurred at the end of the visit, but only the pre and post task collections are considered in the analyses because they directly assess the response to mild stressors.4 This study was approved by the Institutional Review Board of the urban university, and all participants provided written consent (caregivers) or assent (adolescents).
Measures
Behavioral problems
The Behavior Assessment System for Children, Second Edition (BASC II) measures adolescent behavioral problems as reported by caregivers.18 Constructs included are the hyperactivity, aggression, and conduct problems scales as well as the externalizing problems composite (composite of hyperactivity, aggression, and conduct problems scales). Cronbach’s alpha is adequate for all 3 scales and the composite in the current sample (hyperactivity=0.75, aggression=0.87, conduct problems=0.80, externalizing problems=0.92). Both the composite and individual scales are included in the analyses to identify areas of problem behaviors. Scores from 60 to 69 are considered at-risk and scores 70 or higher are in the clinical range.
Biological mother
Caregivers who attended the laboratory session were asked to report their relationship to the participant. Responses were dichotomized into biological mother versus alternative caregiver (e.g., grandmother, aunt). NE adolescents were significantly more likely to be cared for by their biological mother than adolescents with PDE (100% vs. 64%, p<.01).
Drug experimentation
Adolescents completed the Adolescent Health Behavior Survey (AHB), adapted from the YRBS with questions about the use of tobacco, alcohol, marijuana, glue, inhalants, steroids, prescription drugs, cocaine, heroin, “club drugs,” amphetamines, and injection drugs.19 Adolescents provided a urine sample which was tested using the Fischer Scientific Triage Drugs of Abuse Panel and included testing for amphetamines/methamphetamines, barbiturates, benzodiazepines, cocaine, marijuana, methadone, opiates, PCP, propoxyphene, and tricyclic antidepressants. Using procedures that we have previously reported, “abstainers” were defined as adolescents who denied drug use on the AHB and provided a negative urine test. “Experimenters” were defined by having a positive result for any drug on the urine test and/or indicating any drug use on the AHB. A drug use variable indicating any vs. no drug use was created. Two subcategories of drug experimenters were created: (1) experimentation with tobacco and/or alcohol only, but no marijuana or other illegal drugs versus no drug use and (2) experimentation with marijuana and/or other illegal drugs regardless of tobacco/alcohol use versus no drug use.19
Mild stressors
Two computer-administered tasks, the Balloon Analogue Risk Task-Youth (BART-Y) and the Behavioral Indicator of Resiliency to Distress (BIRD), were administered to impose mild stress and to elicit an individual cortisol reactivity score. Both tasks are impossible to complete at times. The BART-Y20 was designed to measure risk-taking propensity from a cognitive decision making perspective by requiring respondents to inflate a computerized balloon over multiple trials. The goal is to make the balloon as large as possible without popping; the larger the balloon, the larger the prize. Accumulated points are lost if the balloon explodes, and the balloon can explode at any time, making a loud popping noise. The BIRD21 was created based on the adult computerized distress tolerance task. On the computer screen, ten numbered boxes are presented, and respondents use a mouse to click on a box when a green dot appears above it in order to earn a prize. Respondents must click on the indicated box before the green dot moves to another box. The green dot frequently changes speeds and sometime moves very quickly, seemingly at random, between the boxes. Participants always received at least one small prize after completing the computerized tasks.
Cortisol reactivity
Using pre-established guidelines, whole saliva samples were collected through passive drool then frozen at -20° C until transported on dry ice via overnight delivery to Salimetrics Laboratories (State College, PA). A commercially available immunoassay specifically designed for use with saliva without modification to the manufacturers recommended protocol was used to assay samples in duplicate. Test volume was 25 ul and sensitivity ranged 0.007 to 3.0 μg/dL. On average, intra- and inter-assay coefficients of variation were less than 5% and 15% respectively. The duplicate assays were averaged, and the average of the two was used in all analyses. 22
Stress index
A stress index was calculated using the entire sample. Participants were assigned a score of “1” for each of the following endorsements: PDE, prenatal exposure to alcohol, prenatal exposure to tobacco, and living with an alternative caregiver. The four variables were summed to create a stress index ranging from 0 (no stressors present) to 4 (all stressors present).
Analytic Strategy
Covariates were considered based on theoretical and statistical importance. There was a wide range of ages in this sample. Adolescents with PDE were significantly more likely than NE adolescents to have been prenatally exposed to alcohol and tobacco (see Participants and Procedures; Table 1). The time of the first cortisol sample collection varied between the PDE/NE groups, and males were significantly more reactive to the stressor than females [25% vs. 12%, χ2(df=1, N=137)=3.80, p=.05)].4 Covariates for the primary hypothesis were age, gender, prenatal tobacco exposure, prenatal alcohol exposure, alternative caregiver and sampling time of day of first saliva collection (pre task). Covariates for the exploratory hypotheses were age, gender, and sampling time of day of first saliva collection (pre task).
To assess the mediation effect of cortisol reactivity on the association between PDE and behavioral problems or drug experimentation, path analyses were conducted to assess the significance of an indirect effect (mediation effect). Mediation is not dependent on direct exposure-outcome effects. 23,24 The significance of the product of the regression coefficient for each outcome on cortisol reactivity after adjusting for PDE, and the regression coefficient for cortisol reactivity on PDE from zero was assessed using the bootstrap confidence interval, which has greater power than the Sobel test.23 The analyses were repeated to examine the exploratory hypothesis that cortisol reactivity mediates the association between multiple stressors and behavioral problems or drug experimentation. Mediation analyses were examined in unadjusted and adjusted models. Mplus statistical software was used in the mediation analyses (Mplus manual).23,25 Statistical significance was accepted at p<.05.
Results
Preliminary Analyses
Salivary cortisol values were skewed and kurtotic; pre and post task values were subjected to ln transformation, which eliminated the skewed and kurtotic values. Using procedures previously reported, two cortisol reactivity variables were calculated, a continuous change score and a dichotomous reactivity score.4 “Reactive” participants were required to meet both of the following conditions: 1) a 10% difference between pre and post task cortisol levels (twice the intra-assay coefficient of variation which is the error inherent in the assay when comparing results from the same samples assayed twice)4 and 2) an absolute difference of at least 0.02 μg/dL between pre task and post task cortisol collections (i.e., the lower limit of salivary cortisol assay sensitivity).4 Participants who did not meet both requirements were coded “less reactive.” Following data transformation, pre task cortisol was subtracted from post task cortisol levels to obtain a continuous cortisol reactivity score.4 Overall, 19% of participants were reactive to the laboratory stressor.4 In the NE group, 26% exhibited cortisol reactivity, compared to 12% in the PDE group (x2=4.49, p=.03).4
Approximately 50% of participants reported experimentation with any drug;19 27.8% ever used tobacco, 31.6% ever used alcohol, 23.3% ever used marijuana, and 11.3% ever used other drugs (Table 1). Although overall drug experimentation did not differ by PDE status, 15%of the NE group, compared to 36% of the PDE group, experimented with tobacco/alcohol use (x2=4.47, p=.04; Table 2).
Table 2.
Unadjusted differences in cortisol reactivity, drug experimentation, behavioral problems, and weight status by prenatal drug exposure status (n=137)
| Prenatally Drug Exposed (n=76) | Non-exposed (n=61) | ||
|---|---|---|---|
|
| |||
| %/M(SD) | %/M(SD) | p | |
|
| |||
| Cortisol reactivity | 12 | 26 | .03 |
| Tobacco/alcohol use only | 36 | 15 | .04 |
| Marijuana/other drug use | 27 | 37 | ns |
| Drug Experimentation (any drug) | 53 | 47 | ns |
| BASC Aggression | 50.73 (7.46) | 51.26 (11.32) | ns |
| BASC Hyperactivity | 52.38 (9.33) | 51.62 (11.51) | ns |
| BASC Conduct problems | 53.24 (9.32) | 52.79 (12.59) | ns |
| BASC Externalizing problems | 52.31 (8.15) | 52.00 (12.04) | ns |
BASC=Behavior Assessment Scale for Children II (caregiver-report)
None of the BASC II scales differed by PDE status (Table 2). Additionally, we examined whether the percentage in the at-risk/clinical range on the BASC II for each outcome varied by PDE status, and the differences were not statistically significant (hyperactivity: PDE 22.97% vs. NE 14.75%; conduct problems: PDE 21.62% vs NE 21.31%; aggression: PDE 12.16% vs NE 16.39%; externalizing problems: PDE 16.22% vs NE 16.39%).
Regarding the stress index, 31% of all adolescents received a score of 0, indicating no stressors (70% in NE group, 0% in the PDE group,), 14% received a score of 1 (20% in NE group, 9% in PDE group), 21% a score of 2 (10% in NE group, 30% in PDE group), 23% a score of 3 (0 in NE group, 42% in PDE group), 10% a score of 4 (0% in NE group, 18% in PDE group).
Hypothesis Testing
PDE is significantly related to a lower likelihood of cortisol reactivity (OR=0.38, 95% CI=0.15-0.93, p=0.03). Cortisol reactivity is significantly related to a lower likelihood of drug experimentation (OR=0.31, 95% CI: 0.12-0.81, p=0.02) and lower scores on the BASC externalizing problem composite score (b=-4.53, 95% CI:-8.88, -0.18, p=0.04), aggression scores (b=-4.63, 95% CI: -8.67, -0.58, p=0.03), and marginally related to lower hyperactivity scores (b=-4.12, 95% CI: -8.61, 0.37, p=0.07), but is not associated with BASC conduct problem scores (ps>0.10). There is no direct association between PDE status and any behavioral problems, including drug experimentation.
Unadjusted mediation analyses show that cortisol reactivity significantly mediates the association between PDE and BASC II aggression scores (95% bootstrap CI: 0.04-4.28; Figure 1A), BASC II externalizing problem composite scores (95% bootstrap CI: 0.03-4.50; Figure 1B), and drug experimentation (95% bootstrap CI: 0.001-0.54; Figure 1C). When the covariates are added to the model, the mediation becomes non-significant. Further investigation of the covariates reveals that, when gender is included as a covariate, the mediation effect remains (BASC II aggression scores: 95% bootstrap CI: 0.02-4.89; drug experimentation: 95% bootstrap CI: 0.002-0.57; BASC II externalizing problems scores: 95% bootstrap CI: 0.05-5.38). When age is added, the mediation becomes non-significant. When any other covariate is added, the mediation becomes non-significant.
Figure 1. Cortisol reactivity mediates the association between PDE status and behavioral problems.
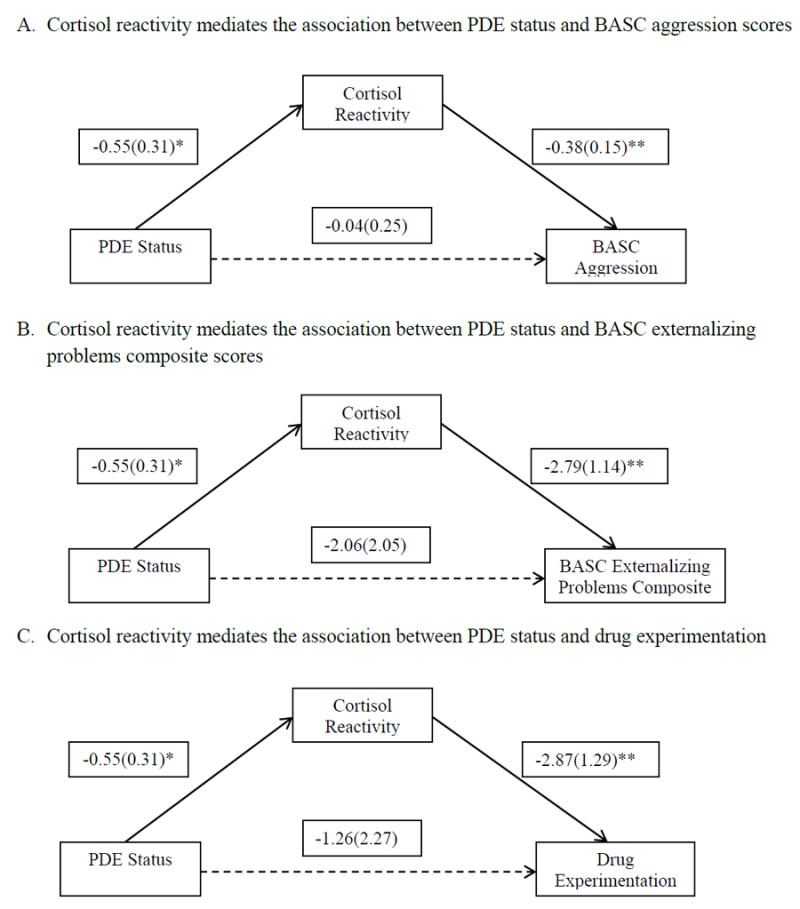
Note: Unadjusted mediation analyses without covariates; * indicates 0.05<=p<0.10, **indicates p<0.05; The 95% bootstrap confidence intervals for the indirect effect are 0.04-4.28 for aggression scores, 0.03-4.50 for externalizing problems scores and 0.001-0.54 for drug experimentation. Probit regression for the mediator (cortisol reactivity) and weighted least squares estimation were used for all the models. Probit regression was also conducted for the categorical dependent variable (drug experimentation).
The stress index was not associated with cortisol reactivity (p>0.10). In addition, it was not related to any outcome variables including behavioral problems or drug experimentation (ps>0.10). There was no mediation effect via cortisol reactivity on the relation between stress index and any outcome variable.
Discussion
Our findings suggest long-lasting developmental effects of prenatal drug exposure on acute regulation of the HPA axis. These findings suggest that PDE adversely impacts adolescent behavior through disruptions to cortisol reactivity.2 This research adds to similar findings in comparable samples of adolescents with PDE,15,26 building evidence that adolescents with PDE may have a dysregulated HPA axis.
Similar to our previous findings, the NE reactive group appears to be demonstrating an expected reaction to the laboratory-presented mild stressor. Previously we found a connection between the NE group, demonstrating an expected reaction, and better cognitive function.4 These results extend those findings to behavior and drug use. The group with the expected cortisol reactivity manifested positive behavior outcomes on the BASC (aggression and externalizing problems composite) with small effect sizes and drug experimentation (tobacco/alcohol and any drug use). Repeated activation of the HPA axis increases glucocorticoid levels which disrupt the function of the amygdala and prefrontal cortex. These brain areas are integral to emotion and impulse control by “remembering” prior experiences and interpreting the stressfulness of events.27 Emotion and impulse control are integral factors in adolescent behavioral problems and drug experimentation, suggesting that the NE reactive group is demonstrating a typically developing HPA axis. In contrast, in the PDE group, the lack of association between cortisol reactivity and behavior outcomes (externalizing, aggression, and drug experimentation) suggests dysregulation in the HPA axis and companion brain structures. These findings advance our understanding of the development of the HPA axis, particularly in adolescents with PDE.
Prevalence of drug use (50%) was high and did not differ by PDE status. The recent Monitoring the Future survey asks 16,500 eighth-graders about their drug use. The prevalence of cigarette smoking (28% vs. 18%) and marijuana use (23% vs. 16%) was higher in our sample, but the prevalence of alcohol (32% vs. 33%) and other drugs (11% vs. 10%) was similar.28 Our findings combined with a national survey support that adolescent drug experimentation is of serious national concern regardless of PDE. Also, our unadjusted mediation analysis demonstrated that adolescents with PDE had an attenuated cortisol response which was associated with increased likelihood of drug experimentation. This finding provides further evidence that attenuated cortisol reactivity is associated with negative behavioral outcomes.
The mediating effects of cortisol reactivity remain across genders, but not when age is included in the model, suggesting a possible confounding or moderating effect of age on the mediation. However, due to the small sample size, we could not conduct further examination on the effect of age. During adolescence, behavioral problems and drug experimentation increase with age, suggesting that associations between PDE and behavioral problems may be moderated by age, particularly among youth exposed to community aggression or drug use. Future research could examine this possibility.
There are several possible explanations for the lack of association between multiple pre- and post-natal stressors and adolescent behavioral problems and drug experimentation. One possibility is that the effects of PDE on the HPA axis did not extend to the other stressors examined. Another possibility is that PDE created a threshold effect, such that additional stressors did not further disrupt the HPA axis or increase the risk for adolescent behavioral problems. Subsequent investigations into the timing, severity, and duration of pre- and post-natal stressors are needed to understand how multiple stressors impact cortisol reactivity and adolescent behavioral problems and drug experimentation.
This study has several limitations to acknowledge. First, all measurements were taken at the same time point in adolescence. Hyporeactivity of the HPA axis may occur earlier in development, and measurement of cortisol reactivity earlier in development may further help with understanding the mechanisms linking cortisol reactivity with long-term behavior. Second, this study includes a small, racially homogeneous sample and cannot be generalized to other populations. However, the sample is from a high-risk population, and the findings contribute to understanding the mechanisms that may influence long-term development through adolescence. Third, caregiving status, measured by living with the biological mother versus an alternative caregiver, differed between groups (60% PDE, 100% NE). There may have been other unmeasured stressors associated with PDE. Fourth, cortisol studies often include two baseline cortisol samples to account for any effects that novel environments or procedures may have on cortisol reactivity. Future studies including cortisol should consider the inclusion of two baseline measures for further precision. Finally, medication use was not available and should be included in additional research. Although theoretically plausible, findings should be regarded as preliminary and should be replicated.
This study also has several strengths. First, hypotheses were driven by current developmental theories.2 Second, the use of mild to moderate stressors typically produces 20-30% of participants who have a salivary cortisol increase from time 1 to time 2 of at least 10%;22 thus, the NE group is well within the expected range. Third, multiple methods of measurement were used (direct measurement of cortisol, self-report and urine toxicology for drug use, and caregiver report of adolescent behavioral problems) with findings consistent across measurements. Finally, findings extend previous research to a high risk sample, providing additional validity that dysregulation of the HPA axis, caused by adverse childhood experiences, has negative consequences for behavior and development in adolescence.
Overall, this study finds that cortisol reactivity mediates the association between PDE status and behavioral problems, including drug experimentation and suggests that prenatal drug exposure may have long-term effects on stress-sensitive physiological mechanisms. A similar pattern was detected in this same sample regarding cognitive outcomes4 indicating a robust association of cortisol reactivity and both cognitive and behavioral outcomes assessed using multiple methods. Future research might investigate these associations in a larger sample, longitudinally because hyporeactivity of the HPA axis may occur earlier in development. Measurement of cortisol reactivity earlier in development may further help with understanding mechanisms to explain long-term behavior and metabolic outcomes. If replicated, the current findings suggest that PDE may have long-lasting effects on cortisol reactivity which may operate as a mechanism associated with behavioral problems (aggression) and drug experimentation in adolescence. There is potential for disrupting this process, as demonstrated by a recent finding that psychosocial interventions have been successful in improving cortisol regulation in children.29 Altering cortisol regulation early in life may disrupt the development of behavioral problems in adolescence.
Acknowledgments
We thank Grace Paik, BS, for her assistance with editing this manuscript.
This study was supported by the National Institute on Drug Abuse (R01-DA07432, R01-DA021059, F32DA036274) and the National Institute of Diabetes and Digestive and Kidney Diseases’ Summer Program in Obesity, Diabetes, and Nutrition Research Training (SPORT) program (T35DK095737).
Footnotes
In the interest of full disclosure, DAG is founder and Chief Scientific and Strategy Advisor at Salimetrics LLC and SalivaBio LLC (Carlsbad, CA) and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and Office of Research Adherence and Integrity at Arizona State University. The Institute for Interdisciplinary Salivary Bioscience Research and DAG are in the process of transitioning to the University of California where similar policies will be established.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: US Department of Health and Human Services; 2013. [Google Scholar]
- 2.Shonkoff JP, Garner AS, et al. Committee on Psychosocial Aspects of Child and Family Health. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 3.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31(1-2):23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 4.Buckingham-Howes S, Bento S, Scaletti LA, et al. Prenatal drug exposure moderates the association between stress reactivity and cognitive function in adolescence. Developmental Neuroscience. 2014;36:329–337. doi: 10.1159/000360851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckingham-Howes S, Berger SS, Scaletti LA, et al. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131:e1917–e1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sapolsky RM. Why Zebras Don’t Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping. New York, NY: St Martin’s Griffin; 2004. [Google Scholar]
- 7.Loman M, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews. 2010;34(6):867. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. International Journal of Psychophysiology. 2013;90(1):8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen FR, Raine A, Soyfer L, et al. Interaction of adrenocortical activity and autonomic arousal on children’s externalizing and internalizing behavior problems. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9900-y. [DOI] [PubMed] [Google Scholar]
- 10.Pajer K, Gardner W, Rubin RT, et al. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- 11.Snoek H, Van Goozen SH, Matthys W, et al. Stress responsivity in children with externalizing behavior disorders. Development and Psychopathology. 2004;16(2):389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- 12.Hagan MJ, Roubinov DS, Mistler AK, et al. Mental health outcomes in emerging adults exposed to childhood maltreatment: the moderating role of stress reactivity. Child Maltreatment. 2014;19:156–167. doi: 10.1177/1077559514539753. [DOI] [PubMed] [Google Scholar]
- 13.Chaplin TM, Freiburger MB, Mayes LC, et al. Prenatal cocaine exposure, gender, and adolescent stress response: a prospective longitudinal study. Neurotoxicology and teratology. 2010;32(6):595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conradt E, Abar B, Lester BM, et al. Cortisol reactivity to social stress as a mediator of early adversity on risk and adaptive outcomes. Child development. 2014;85(6):2279–2298. doi: 10.1111/cdev.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lester BM, LaGasse LL, Shankaran S, et al. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. Journal of Pediatrics. 2010;157(2):288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggins T, Cacic K, Buckingham-Howes S, et al. Memory ability and hippocampal volume in adolescents with prenatal drug exposure. Neurotoxicology and Teratology. 2012;34(4):434–441. doi: 10.1016/j.ntt.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler ME, Nair P, Black MM. Ongoing maternal drug use, parenting attitudes, and a home intervention: effects on mother-child interaction at 18 months. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):87–94. doi: 10.1097/00004703-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds C, Kamphaus W. The Behavior Assessment System for Children. Circle Pines, MN: AGS; 2004. [Google Scholar]
- 19.Wang Y, Buckingham-Howes S, Nair P, et al. Prenatal drug exposure, behavioral problems, and drug experimentation among african-american urban adolescents. Journal of Adolescent Health. 2014;55:423–431. doi: 10.1016/j.jadohealth.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lejuez CW, Aklin W, Daughters S, et al. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. Journal of Clinical Child and Adolescent Psychology. 2007;36(1):106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- 21.Lejuez CW, Daughters S, Danielson CW, et al. The Behavioral Indicator of Resiliency to Distress (BIRD) 2006 [Google Scholar]
- 22.Granger DA, Fortunato CK, Beltzer EK, et al. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. J Adolesc. 2012;35(4):1081–1095. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 23.MacKinnon DP. Introduction to statistical mediation analysis. Mahwah, NJ: Lawrence Earlbaum Associates; 2008. [Google Scholar]
- 24.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual review of psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthén LK, Muthén BO. Mplus User’s Guide, Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- 26.Bauer CR, Lambert BL, Bann CM, et al. Long-term impact of maternal substance use during pregnancy and extrauterine environmental adversity: stress hormone levels of preadolescent children. Pediatric Research. 2011;70(2):213–219. doi: 10.1203/PDR.0b013e3182291b13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future national results on adolescent drug use: Overview of key findings. The University of Michigan: Institute for Social Research; 2011. [Google Scholar]
- 29.Slopen N, McLaughlin K, Shonkoff JP. Interventions to improve cortisol regulation in children: A systematic review. Pediatrics. 2014;133(2):312–326. doi: 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]


