Abstract
We report genome-wide ancient DNA from 44 ancient Near Easterners ranging in time between ~12,000-1,400 BCE, from Natufian hunter-gatherers to Bronze Age farmers. We show that the earliest populations of the Near East derived around half their ancestry from a ‘Basal Eurasian’ lineage that had little if any Neanderthal admixture and that separated from other non-African lineages prior to their separation from each other. The first farmers of the southern Levant (Israel and Jordan) and Zagros Mountains (Iran) were strongly genetically differentiated, and each descended from local hunter-gatherers. By the time of the Bronze Age, these two populations and Anatolian-related farmers had mixed with each other and with the hunter-gatherers of Europe to drastically reduce genetic differentiation. The impact of the Near Eastern farmers extended beyond the Near East: farmers related to those of Anatolia spread westward into Europe; farmers related to those of the Levant spread southward into East Africa; farmers related to those from Iran spread northward into the Eurasian steppe; and people related to both the early farmers of Iran and to the pastoralists of the Eurasian steppe spread eastward into South Asia.
Between 10,000-9,000 BCE, humans began practicing agriculture in the Near East1. In the ensuing five millennia, plants and animals domesticated in the Near East spread throughout West Eurasia (a vast region that also includes Europe) and beyond. The relative homogeneity of present-day West Eurasians in a world context2 suggests the possibility of extensive migration and admixture that homogenized geographically and genetically disparate sources of ancestry. The spread of the world's first farmers from the Near East would have been a mechanism for such homogenization. To date, however, due to the poor preservation of DNA in warm climates, it has been impossible to study the population structure and history of the first farmers and to trace their contribution to later populations.
In order to overcome the obstacle of poor DNA preservation, we took advantage of two methodological developments. First, we sampled from the inner ear region of the petrous bone3,4 that can yield up to ~100 times more endogenous DNA than other skeletal elements4. Second, we used in-solution hybridization5 to enrich extracted DNA for about 1.2 million single nucleotide polymorphism (SNP) targets6,7, making efficient sequencing practical by filtering out microbial and non-informative human DNA. We merged all sequences extracted from each individual, and randomly sampled a single sequence with minimum mapping and sequence quality to represent each SNP, restricting to individuals with at least 9,000 SNPs covered at least once (Methods). We obtained genome-wide data passing quality control for 45 individuals on whom we had a median coverage of 172,819 SNPs. We assembled radiocarbon dates for 26 individuals (22 new generated for this study) (Supplementary Data Table 1).
The newly reported ancient individuals date to ~12,000-1,400 BCE and come from the southern Caucasus (Armenia), northwestern Anatolia (Turkey), Iran, and the southern Levant (Israel and Jordan) (Supplementary Data Table 1, Fig. 1a). (One individual had a radiocarbon date that was not in agreement with the date of its archaeological context and was also a genetic outlier.) The samples include Epipaleolithic Natufian hunter-gatherers from Raqefet Cave in the Levant (12,000-9,800 BCE); a likely Mesolithic individual from Hotu Cave in the Alborz mountains of Iran (probable date of 9,100-8,600 BCE); Pre-Pottery Neolithic farmers from ‘Ain Ghazal and Motza in the southern Levant (8,300-6,700 BCE); and early farmers from Ganj Dareh in the Zagros mountains of western Iran (8,200-7,600 BCE). The samples also include later Neolithic, Chalcolithic (~4,800-3,700 BCE), and Bronze Age (~3,350-1,400 BCE) individuals (Supplementary Information, section 1). We combined our data with previously published ancient data7,8,9,10,8,10-15 to form a dataset of 281 ancient individuals. We then further merged with 2,583 present-day people genotyped on the Affymetrix Human Origins array13,16 (238 new) (Supplementary Data Table 2; Supplementary Information, section 2). We grouped the ancient individuals based on archaeological culture and chronology (Fig. 1a; Supplementary Data Table 1). We refined the grouping based on patterns evident in Principal Components Analysis (PCA)17 (Fig. 1b; Extended Data Fig. 1), ADMIXTURE model-based clustering18 (Extended Data Fig. 2a), and ‘outgroup’ f3-analysis (Extended Data Fig. 3). We used f4-statistics to identify outlier individuals and to cluster phylogenetically indistinguishable groups into ‘Analysis Labels’ (Supplementary Information, section 3).
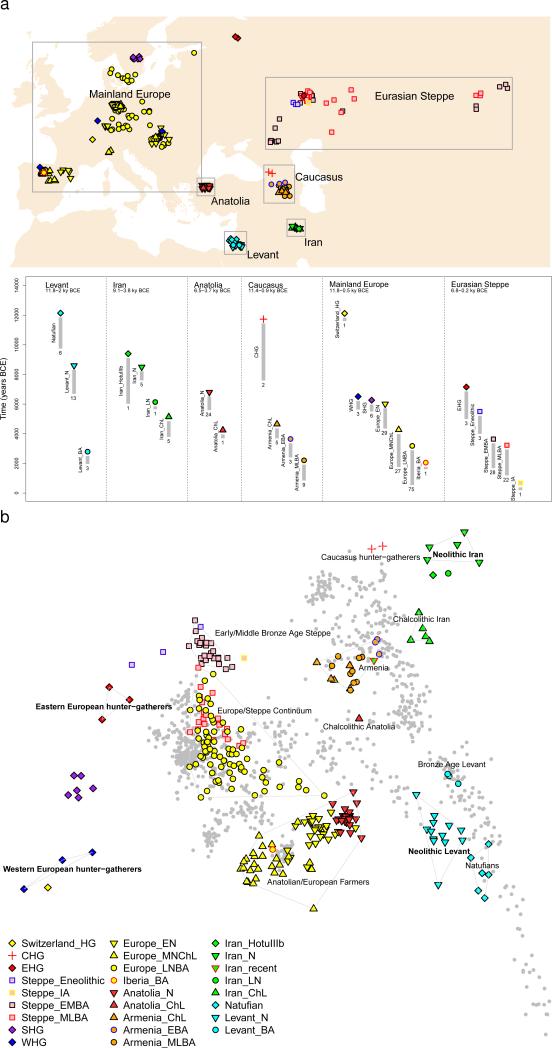
Figure 1. Genetic structure of ancient West Eurasia.
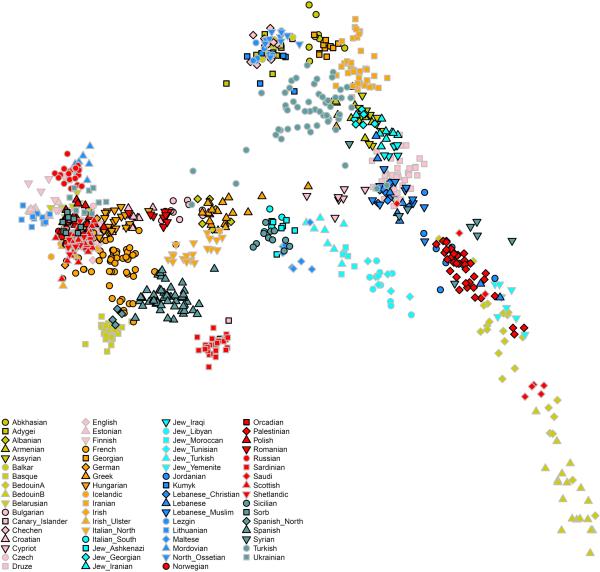
(a) Sampling locations and times in six regions. Sample sizes for each population are given below each bar. Abbreviations used: E: Early, M: Middle, L: Late, HG: Hunter-Gatherer, N: Neolithic, ChL: Chalcolithic, BA: Bronze Age, IA: Iron Age. (b) Principal components analysis of 991 present-day West Eurasians (grey points) with 278 projected ancient samples (excluding the Upper Paleolithic Ust’-Ishim, Kostenki14, and MA1). To avoid visual clutter, population labels of present-day individuals are shown in Extended Data Fig. 1.
We analyzed these data to address six questions. (1) Previous work has shown that the first European farmers harboured ancestry from a Basal Eurasian lineage that diverged from the ancestors of north Eurasian hunter-gatherers and East Asians before they separated from each other13. What was the distribution of Basal Eurasian ancestry in the ancient Near East? (2) Were the first farmers of the Near East part of a single homogeneous population, or were they regionally differentiated? (3) Was there continuity between late pre-agricultural hunter-gatherers and early farming populations, or were the hunter-gatherers largely displaced by a single expansive population as in early Neolithic Europe?8 (4) What is the genetic contribution of these early Near Eastern farmers to later populations of the Near East? (5) What is the genetic contribution of the early Near Eastern farmers to later populations of mainland Europe, the Eurasian steppe, and to populations outside West Eurasia? (6) Do our data provide broader insights about population transformations in West Eurasia?
Basal Eurasian ancestry was pervasive in the ancient Near East and associated with reduced Neanderthal ancestry
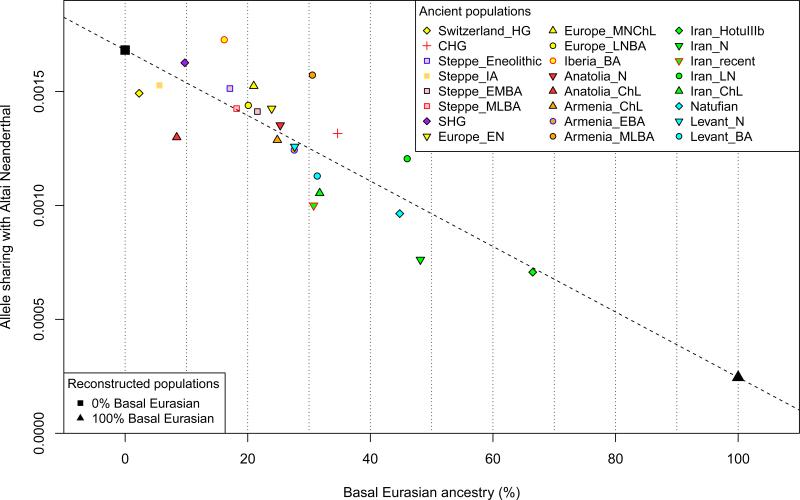
The ‘Basal Eurasians’ are a lineage hypothesized13 to have split off prior to the differentiation of all other Eurasian lineages, including both eastern non-African populations like the Han Chinese, and even the early diverged lineage represented by the genome sequence of the ~45,000 year old Upper Paleolithic Siberian from Ust’-Ishim11. To test for Basal Eurasian ancestry, we computed the statistic f4(Test, Han; Ust’-Ishim, Chimp) (Supplementary Information, section 4), which measures the excess of allele sharing of Ust’-Ishim with a variety of Test populations compared to Han as a baseline. This statistic is significantly negative (Z<−3.7) for all ancient Near Easterners as well as Neolithic and later Europeans, consistent with their having ancestry from a deeply divergent Eurasian lineage that separated from the ancestors of most Eurasians prior to the separation of Han and Ust’-Ishim. We used qpAdm7 to estimate Basal Eurasian ancestry in each Test population. We obtain the highest estimates in the earliest populations from both Iran (66±13% in the likely Mesolithic sample, 48±6% in Neolithic samples), and the Levant (44±8% in Epipaleolithic Natufians) (Fig. 2), showing that Basal Eurasian ancestry was widespread across the ancient Near East.
Figure 2. Basal Eurasian ancestry explains the reduced Neanderthal admixture in West Eurasians.
Basal Eurasian ancestry estimates are negatively correlated to a statistic measuring Neanderthal ancestry f4(Test, Mbuti; Altai, Denisovan).
West Eurasians harbour significantly less Neanderthal ancestry than East Asians19-21, which could be explained if West Eurasians (but not East Asians) have partial ancestry from a source diluting their Neanderthal inheritance22. Supporting this theory, we observe a negative correlation between Basal Eurasian ancestry and the rate of shared alleles with Neanderthals19 (Supplementary Information, section 5; Fig. 2). By extrapolation, we infer that the Basal Eurasian population had lower Neanderthal ancestry than non-Basal Eurasian populations and possibly none (ninety-five percent confidence interval truncated at zero of 0-60%; Fig. 2; Methods). The finding of little if any Neanderthal ancestry in Basal Eurasians could be explained if the Neanderthal admixture into modern humans 50,000-60,000 years ago11 largely occurred after the splitting of the Basal Eurasians from other non-Africans.
It is striking that the highest estimates of Basal Eurasian ancestry are from the Near East, given the hypothesis that it was there that most admixture between Neanderthals and modern humans occurred19,23. This could be explained if Basal Eurasians thoroughly admixed into the Near East before the time of the samples we analyzed but after the Neanderthal admixture. Alternatively, the ancestors of Basal Eurasians may have always lived in the Near East, but the lineage of which they were a part did not participate in the Neanderthal admixture.
A population without Neanderthal admixture, basal to other Eurasians, may have plausibly lived in Africa. Craniometric analyses have suggested an affinity between the Natufians and populations of north or sub-Saharan Africa24,25, a result that finds some support from Y chromosome analysis which shows that the Natufians and successor Levantine Neolithic populations carried haplogroup E, of likely ultimate African origin, which has not been detected in other ancient males from West Eurasia (Supplementary Information, section 6) 7,8. However, no affinity of Natufians to sub-Saharan Africans is evident in our genome-wide analysis, as present-day sub-Saharan Africans do not share more alleles with Natufians than with other ancient Eurasians (Extended Data Table 1). (We could not test for a link to present-day North Africans, who owe most of their ancestry to back-migration from Eurasia26,27.) The idea of Natufians as a vector for the movement of Basal Eurasian ancestry into the Near East is also not supported by our data, as the Basal Eurasian ancestry in the Natufians (44±8%) is consistent with stemming from the same population as that in the Neolithic and Mesolithic populations of Iran, and is not greater than in those populations (Supplementary Information, section 4). Further insight into the origins and legacy of the Natufians could come from comparison to Natufians from additional sites, and to ancient DNA from north Africa.
Extreme regional differentiation in the ancient Near East
PCA on present-day West Eurasian populations (Methods) (Extended Data Fig. 1) on which we projected the ancient individuals (Fig. 1b) replicates previous findings of a Europe-Near East contrast along the horizontal Principal Component 1 (PC1) and parallel clines (PC2) in both Europe and the Near East (Extended Data Fig. 1)7,8,13. Ancient samples from the Levant project at one end of the Near Eastern cline, and ancient samples from Iran at the other. The two Caucasus Hunter Gatherers (CHG)9 are less extreme along PC1 than the Mesolithic and Neolithic individuals from Iran, while individuals from Chalcolithic Anatolia, Iran, and Armenia, and Bronze Age Armenia occupy intermediate positions. Qualitatively, the PCA has the appearance of a quadrangle whose four corners are some of the oldest samples: bottom-left: Western Hunter Gatherers (WHG), top-left: Eastern Hunter Gatherers (EHG), bottom-right: Neolithic Levant and Natufians, top-right: Neolithic Iran. This suggests the hypothesis that diverse ancient West Eurasians can be modelled as mixtures of as few as four streams of ancestry related to these populations, which we confirmed using qpWave7 (Supplementary Information, section 7).
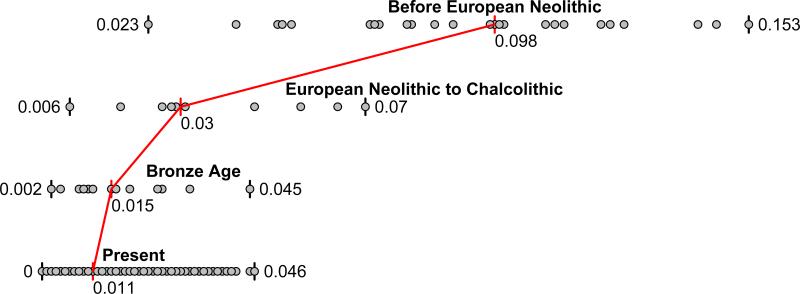
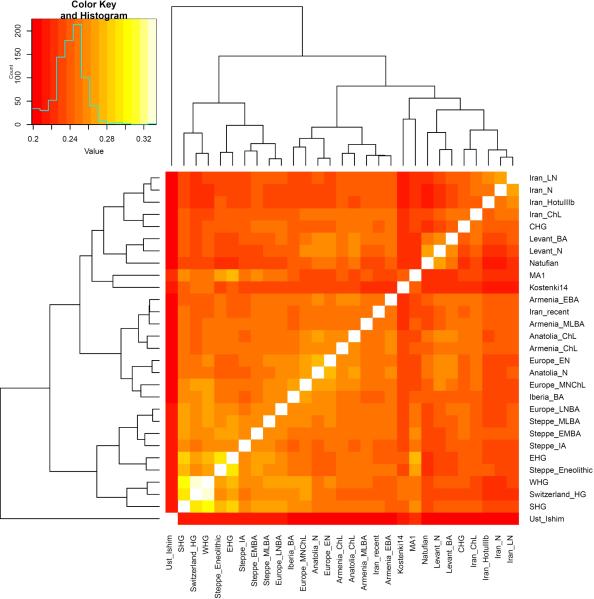
We computed squared allele frequency differentiation between all pairs of ancient West Eurasians28 (Methods; Fig. 3; Extended Data Fig. 2b; Extended Data Fig. 4), and found that the populations at the four corners of the quadrangle had differentiation of FST=0.08-0.15, comparable to the value of 0.09-0.13 seen between present-day West Eurasians and East Asians (Han) (Supplementary Data Table 3). In contrast, by the Bronze Age, genetic differentiation between pairs of West Eurasian populations had reached its present-day low levels (Fig. 3): today, FST is ≤0.025 for 95% of the pairs of West Eurasian populations and ≤0.046 for all pairs (Fig. 3). These results point to a demographic process that established high differentiation across West Eurasia and then reduced this differentiation over time.
Figure 3. Genetic differentiation and its dramatic decrease over time in West Eurasia.
Pairwise FST distribution among populations belonging to four successive time slices in West Eurasia; the median (red) and range of FST is shown.
Continuity between pre-farming hunter-gatherers and early farmers of the Near East
Our data document continuity across the hunter-gatherer / farming transition, separately in the southern Levant and in the southern Caucasus-Iran highlands. The qualitative evidence for this is that PCA, ADMIXTURE, and outgroup f3 analysis cluster Levantine hunter-gatherers (Natufians) with Levantine farmers, and Iranian and Caucasus Hunter Gatherers with Iranian farmers (Fig. 1b; Extended Data Fig. 1; Extended Data Fig. 3). We confirm this in the Levant by showing that its early farmers share significantly more alleles with Natufians than with the early farmers of Iran: the statistic f4(Levant_N, Chimp; Natufian, Iran_N) is significantly positive (Z=13.6). The early farmers of the Caucasus-Iran highlands similarly share significantly more alleles with the hunter-gatherers of this region than with the early farmers from the Levant: the statistic f4(Iran_N, Chimp; Caucasus or Iran highland hunter-gatherers, Levant_N) is significantly positive (Z>6).
How diverse first farmers of the Near East mixed to form the region's later populations
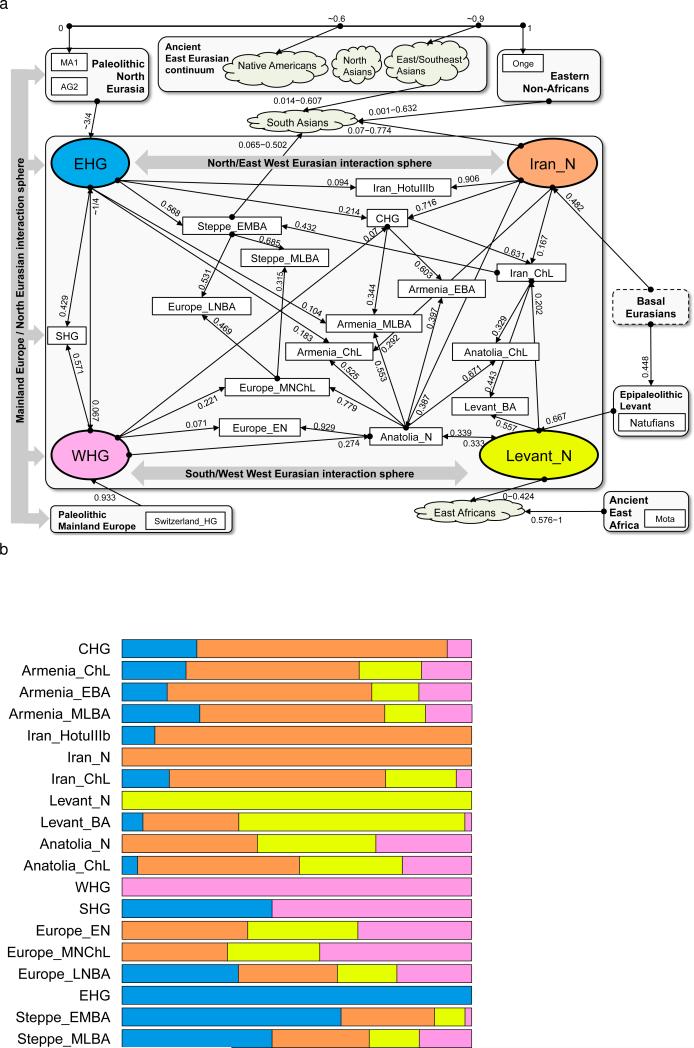
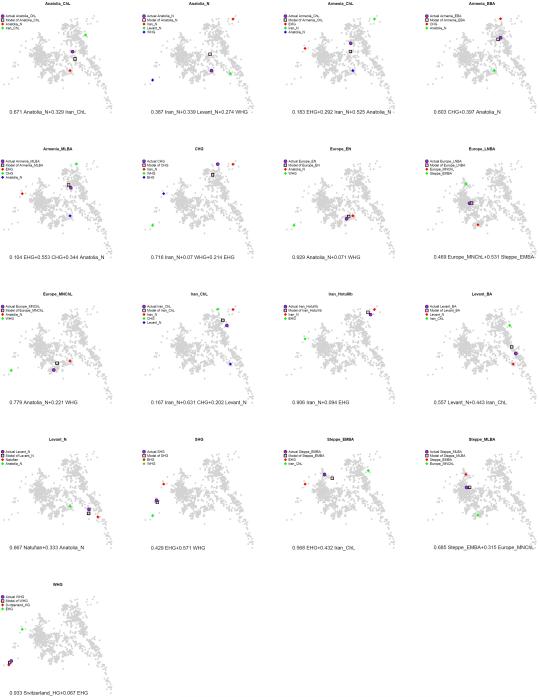
Almost all ancient and present-day West Eurasians have evidence of significant admixture between two or more ancestral populations, as documented by statistics of the form f3(Test; Reference1, Reference2) which if negative, show that a Test population's allele frequencies tend to be intermediate between two Reference populations16 (Extended Data Table 2). To better understand the admixture history beyond these patterns, we used qpAdm7, which can evaluate whether a particular Test population is consistent with being derived from a set of proposed source populations, and if so, infer mixture proportions (Methods). We used this approach to carry out a systematic survey of ancient West Eurasian populations to explore their possible sources of admixture (Fig. 4; Supplementary Information, section 7).
Figure 4. Modelling ancient West Eurasians, East Africans, East Eurasians and South Asians.
(a) All the ancient populations can be modelled as mixtures of two or three other populations and up to four proximate sources (marked in colour). Mixture proportions inferred by qpAdm are indicated by the incoming arrows to each population. Clouds represent sets of more than one population. Multiple admixture solutions are consistent with the data for some populations, and while only one solution is shown here, Supplementary Information, section 7 presents the others. (b) A flat representation of the graph showing mixture proportions from the four proximate sources.
Among first farmers, those of the Levant trace ~2/3 of their ancestry to people related to Natufian hunter-gatherers and ~1/3 to people related to Anatolian farmers (Supplementary Information, section 7). Western Iranian first farmers cluster with the likely Mesolithic HotuIIIb individual and more remotely with hunter-gatherers from the southern Caucasus (Fig. 1b), and share alleles at an equal rate with Anatolian and Levantine early farmers (Supplementary Information, section 7), highlighting the long-term isolation of western Iran.
During subsequent millennia, the early farmer populations of the Near East expanded in all directions and mixed, as we can only model populations of the Chalcolithic and subsequent Bronze Age as having ancestry from two or more sources. The Chalcolithic people of western Iran can be modelled as a mixture of the Neolithic people of western Iran, the Levant, and Caucasus Hunter Gatherers (CHG), consistent with their position in the PCA (Fig. 1b). Admixture from populations related to the Chalcolithic people of western Iran had a wide impact, consistent with contributing ~44% of the ancestry of Levantine Bronze Age populations in the south and ~33% of the ancestry of the Chalcolithic northwest Anatolians in the west. Our analysis show that the ancient populations of the Chalcolithic Iran, Chalcolithic Armenia, Bronze Age Armenia and Chalcolithic Anatolia were all composed of the same ancestral components, albeit in slightly different proportions (Fig. 4b; Supplementary Information, section 7).
The Near Eastern contribution to Europeans, East Africans and South Asians
Admixture did not only occur within the Near East but extended towards Europe. To the north, a population related to people of the Iran Chalcolithic contributed ~43% of the ancestry of early Bronze Age populations of the steppe. The spread of Near Eastern ancestry into the Eurasian steppe was previously inferred7 without access to ancient samples, by hypothesizing a population related to present-day Armenians as a source7,8. To the west, the early farmers of mainland Europe were descended from a population related to Neolithic northwestern Anatolians8. This is consistent with an Anatolian origin of farming in Europe, but does not reject other sources, since the spatial distribution of the Anatolian/European-like farmer populations is unknown. We can rule out the hypothesis that European farmers stem directly from a population related to the ancient farmers of the southern Levant29,30, however, since they share more allele with Anatolian Neolithic farmers than with Levantine farmers as attested by the positive statistic f4(Europe_EN, Chimp; Anatolia_N, Levant_N) (Z=15).
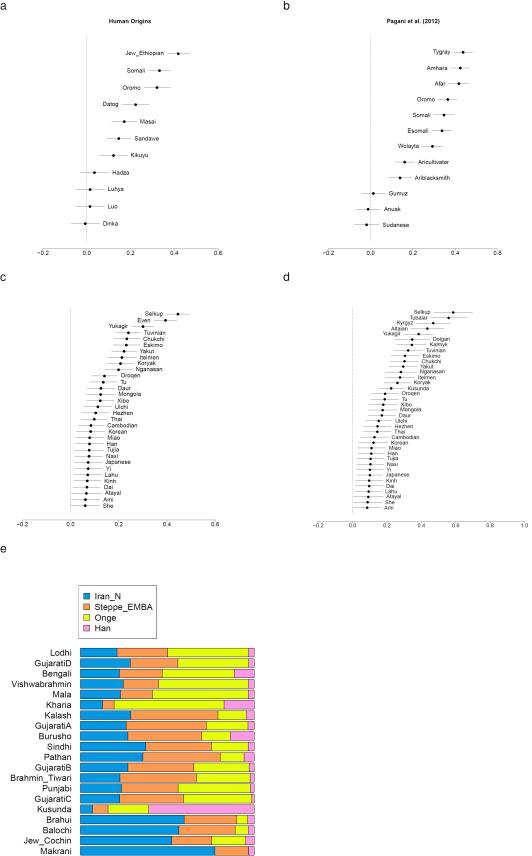
Migrations from the Near East also occurred towards the southwest into East African populations which experienced West Eurasian admixture ~1,000 BCE31,32. Previously, the West Eurasian population known to be the best proxy for this ancestry was present-day Sardinians32, who resemble Neolithic Europeans genetically13,33. However, our analysis shows that East African ancestry is significantly better modelled by Levantine early farmers than by Anatolian or early European farmers, implying that the spread of this ancestry to East Africa was not from the same group that spread Near Eastern ancestry into Europe (Extended Data Fig. 5; Supplementary Information, section 8).
In South Asia, our dataset provides insight into the sources of Ancestral North Indians (ANI), a West Eurasian related population that no longer exists in unmixed form but contributes a variable amount of the ancestry of South Asians34,35 (Supplementary Information, section 9) (Extended Data Fig. 5). We show that it is impossible to model the ANI as being derived from any single ancient population in our dataset. However, it can be modelled as a mix of ancestry related to both early farmers of western Iran and to people of the Bronze Age Eurasian steppe; all sampled South Asian groups are inferred to have significant amounts of both ancestral types. The demographic impact of steppe related populations on South Asia was substantial, as the Mala, a south Indian population with minimal ANI along the ‘Indian Cline’ of such ancestry34,35 is inferred to have ~18% steppe-related ancestry, while the Kalash of Pakistan are inferred to have ~50%, similar to present-day northern Europeans7.
Broader insights into population transformations across West Eurasia and beyond
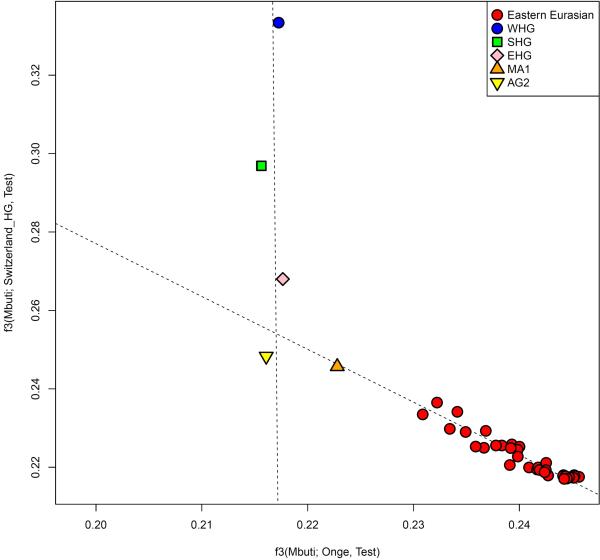
We were concerned that our conclusions might be biased by the particular populations we happened to sample, and that we would have obtained qualitatively different conclusions without data from some key populations. We tested our conclusions by plotting the inferred position of admixed populations in PCA against a weighted combination of their inferred source populations and obtained qualitatively consistent results (Extended Data Fig. 6).
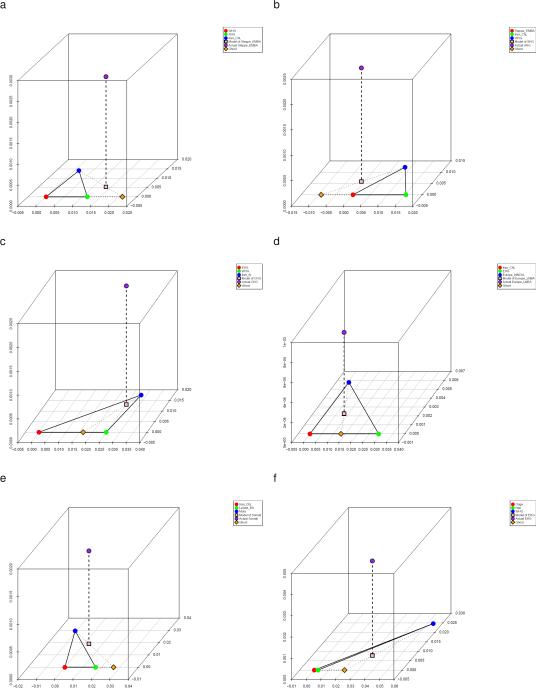
To further assess the robustness of our inferences, we developed a method to infer the existence and genetic affinities of ancient populations from unobserved ‘ghost’ populations (Supplementary Information, section 10; Extended Data Fig. 7). This method takes advantage of the insight that if an unsampled ghost population admixes with differentiated ‘substratum’ populations, it is possible to extrapolate its identity by intersecting clines of populations with variable proportions of ‘ghost’ and ‘substratum’ ancestry. Applying this while withholding major populations, we validated some of our key inferences, successfully inferring mixture proportions consistent with those obtained when the populations are included in the analysis. Application of this methods highlights the impact of Ancient North Eurasian (ANE) ancestry related to the ~22,000 BCE Mal'ta 1 and ~15,000 BCE Afontova Gora 215 on populations living in Europe, the Americas, and Eastern Eurasia. Eastern Eurasians can be modelled as arrayed along a cline with different proportions of ANE ancestry (Supplementary Information, section 11; Extended Data Fig. 8), ranging from ~40% ANE in Native Americans matching previous findings13,15, to no less than ~5-10% ANE in diverse East Asian groups including Han Chinese (Extended Data Fig. 5; Extended Data Fig. 7f). We also document a cline of ANE ancestry across the east-west extent of Eurasia. Eastern Hunter Gatherers (EHG) derive ~3/4 of their ancestry from the ANE (Supplementary Information, section 11); Scandinavian hunter-gatherers7,8,13 (SHG) are a mix of EHG and WHG; and WHG are a mix of EHG and the Upper Paleolithic Bichon from Switzerland (Supplementary Information, section 7). Northwest Anatolians—with ancestry from a population related to European hunter-gatherers (Supplementary Information, section 7)—are better modelled if this ancestry is taken as more extreme than Bichon (Supplementary Information, section 10).
The population structure of the ancient Near East was not independent of that of Europe (Supplementary Information, section 4), as evidenced by the highly significant (Z=−8.9) statistic f4(Iran_N, Natufian;WHG, EHG) which suggests gene flow in ‘northeastern’ (Neolithic Iran/EHG) and ‘southwestern’ (Levant/WHG) interaction spheres (Fig. 4d). This interdependence of the ancestry of Europe and the Near East may have been mediated by unsampled geographically intermediate populations36 that contribute ancestry to both regions.
Conclusions
By analysing genome-wide ancient DNA data from ancient individuals from the Levant, Anatolia, the southern Caucasus and Iran, we have provided a first glimpse of the demographic structure of the human populations that transitioned to farming. We reject the hypothesis that the spread of agriculture in the Near East was achieved by the dispersal of a single farming population displacing the hunter-gatherers they encountered. Instead, the spread of ideas and farming technology moved faster than the spread of people, as we can determine from the fact that the population structure of the Near East was maintained throughout the transition to agriculture. A priority for future ancient DNA studies should be to obtain data from older periods, which would reveal the deeper origins of the population structure in the Near East. It will also be important to obtain data from the ancient civilizations of the Near East to bridge the gap between the region's prehistoric inhabitants and those of the present.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Ancient DNA data
In a dedicated ancient DNA laboratory at University College Dublin, we prepared powder from 132 ancient Near Eastern samples, either by dissecting the inner ear region of the petrous bone using a sandblaster (Renfert), or by drilling using a Dremel tool and single-use drill bits and selecting the best preserved bone fragments based on anatomical criteria. These fragments were then powdered using a mixer mill (Retsch Mixer Mill 400)4.
We performed all subsequent processing steps in a dedicated ancient DNA laboratory at Harvard Medical School, where we extracted DNA from the powder (usually 75 mg, range 14-81 mg) using an optimized ancient DNA extraction protocol37, but replaced the assembly of Qiagen MinElute columns and extension reservoirs from Zymo Research with a High Pure Extender Assembly from the High Pure Viral Nucleic Acid Large Volume Kit (Roche Applied Science). We built a total of 170 barcoded double-stranded Illumina sequencing libraries for these samples38, of which we treated 167 with Uracil-DNA glycosylase (UDG) to remove the characteristic C-to-T errors of ancient DNA39. The UDG treatment strategy is (by-design) inefficient at removing terminal uracils, allowing the mismatch rate to the human genome at the terminal nucleotide to be used for authentication38. We updated this library preparation protocol in two ways compared to the original publication: first, we used 16U Bst2.0 Polymerase, Large Fragment (NEB) and 1x Isothermal Amplification buffer (NEB) in a final volume of 25μL fill-in reaction, and second, we used the entire inactivated 25μL fill-in reaction in a total volume of 100μL PCR mix with 1 μM of each primer40. We included extraction negative controls (where no sample powder was used) and library negative controls (where extract was supplemented by water) in every batch of samples processed and carried them through the entire wet lab processing to test for reagent contamination.
We screened the libraries by hybridizing them in solution to a set of oligonucleotide probes tiling the mitochondrial genome41, using the protocol described previously7. We sequenced the enriched libraries using an Illumina NextSeq 500 instrument using 2×76bp reads, trimmed identifying sequences (seven base pair molecular barcodes at either end) and any trailing adapters, merged read pairs that overlapped by at least 15 base pairs, and mapped the merged sequences to the RSRS mitochondrial DNA reference genome42, using the Burrows Wheeler Aligner43 (bwa) and the command samse (v0.6.1).
We enriched promising libraries for a targeted set of ~1.2 million SNPs8 as in ref. 5, and adjusted the blocking oligonucleotide and primers to be appropriate for our libraries. The specific probe sequences are given in Supplementary Data 2 of ref. 7 (http://www.nature.com/nature/journal/v522/n7555/abs/nature14317.html#supplementary-information) and Supplementary Data 1 of ref. 6. (http://www.nature.com/nature/journal/v524/n7564/full/nature14558.html#supplementary-information). We sequenced the libraries on an Illumina NextSeq 500 using 2×76bp reads. We trimmed identifying sequences (molecular barcodes) and any trailing adapters, merged pairs that overlapped by at least 15 base pairs (allowing up to one mismatch), and mapped the merged sequences to hg19 using the single-ended aligner samse in bwa (v0.6.1). We removed duplicated sequences by identifying sets of sequences with the same orientation and start and end positions after alignment to hg19; we picked the highest quality sequence to represent each set. For each sample, we represented each SNP position by a randomly chosen sequence, restricting to sequences with a minimum mapping quality (MAPQ≥10), sites with a minimum sequencing quality (≥20), and removing 2 bases at the ends of reads. We sequenced the enriched products up to the point that we estimated that generating a hundred new sequences was expected to add data on less than about one new SNP8.
Testing for contamination and quality control
For each ancient DNA library, we evaluated authenticity in several ways. First, we estimated the rate of matching to the consensus sequence for mitochondrial genomes sequenced to a coverage of at least 10-fold from the initial screening data. Of the 76 libraries that contributed to our dataset (coming from 45 samples), 70 had an estimated rate of sequencing matching to the consensus of >95% according to contamMix5 (the remaining libraries had estimated match rates of 75-92%, but gave no sign of being outliers in principal component analysis or X chromosome contamination analysis so we retained them for analysis) (Supplementary Data Table 1). We quantified the rate of C-to-T substitution in the final nucleotide of the sequences analyzed, relative to the human reference genome sequence, and found that all the libraries analyzed had rates of at least 3%38, consistent with genuine ancient DNA. For the nuclear data from males, we used the ANGSD software44 to estimate a conservative X chromosome estimate of contamination. We determined that all libraries passing our quality control and for which we had sufficient X chromosome data to make an assessment had contamination rates of 0-1.5%. Finally, we merged data for samples for which we had multiple libraries to produce an analysis dataset.
Affymetrix Human Origins genotyping data
We genotyped 238 present-day individuals from 17 diverse West Eurasian populations on the Affymetrix Human Origins array16, and applied quality control analyses as previously described13 (Supplementary Data Table 2). We merged the newly generated data with data from 2,345 individuals previously genotyped on the same array13. All individuals that were genotyped provided individual informed consent consistent with studies of population history, following protocols approved by the ethical review committees of the institutions of the researchers who collected the samples; the ethical reviews applying to each of these individual sample collections are specified in Supplementary Data Table 2. The collection and analysis of genome-wide on anonymized samples at Harvard Medical School for the purpose of studying population history was approved by the Harvard Human Research Protection Program, protocol 11681, re-reviewed on July 12 2016. Anonymized aliquots of DNA from all individuals were sent to the core facility of the Center for Applied Genomics at the Children's Hospital of Philadelphia for genotyping and data processing. For 127 of the individuals with newly reported data, the informed consent was consistent with public distribution of data, and the data can be downloaded at http://genetics.med.harvard.edu/reich/Reich_Lab/Datasets.html. To access data for the remaining 111 samples, researchers should send a signed letter to D.R. containing the following text: “(a) I will not distribute the data outside my collaboration; (b) I will not post the data publicly, (c) I will make no attempt to connect the genetic data to personal identifiers for the samples, (d) I will use the data only for studies of population history, (e) I will not use the data for any selection studies, (f) I will not use the data for medical or disease-related analyses, (g) I will not use the data for commercial purposes.” Supplementary Data Table 2 specifies which samples are consistent with which type of data distribution.
Datasets
We carried out population genetic analysis on two datasets: (i) HO includes 2,583 present-day humans genotyped on the Human Origins array13,16 including 238 newly reported (Supplementary Data Table 2; Supplementary Information, section 2), and 281 ancient individuals on a total of 592,146 autosomal SNPs. (ii) HOIll includes the 281 ancient individuals on a total of 1,055,186 autosomal SNPs, including those present in both the Human Origins and Illumina genotyping platforms, but excluding SNPs on the sex chromosomes or additional SNPs of the 1240k capture array that were included because of their potential functional importance8. We used HO for analyses that involve both ancient and present-day individuals, and HOIll for analysis on ancient individuals alone. We also use 235 individuals from Pagani et al.31 on 418,700 autosomal SNPs to study admixture in East Africans (Supplementary Information, section 8). Ancient individuals are represented in ‘pseudo-haploid’ form by randomly choosing one allele for each position of the array.
Principal Components Analysis
We carried out principal components analysis in the smartpca program of EIGENSOFT17, using default parameters and the lsqproject: YES13 and numoutlieriter: 0 options. We carried out PCA of the HO dataset on 991 present-day West Eurasians (Extended Data Fig. 1), and projected the 278 ancient individuals (Fig. 1b).
ADMIXTURE Analysis
We carried out ADMIXTURE analysis18 of the HO dataset after pruning for linkage disequilibrium in PLINK45,46 with parameters --indep-pairwise 200 25 0.4 which retained 296,309 SNPs. We performed analysis in 20 replicates with different random seeds, and retained the highest likelihood replicate for each value of K. We show the K=11 results for the 281 ancient samples in Extended Data Fig. 2a (this is the lowest K for which components maximized in European hunter-gatherers, ancient Levant, and ancient Iran appear).
f-statistics
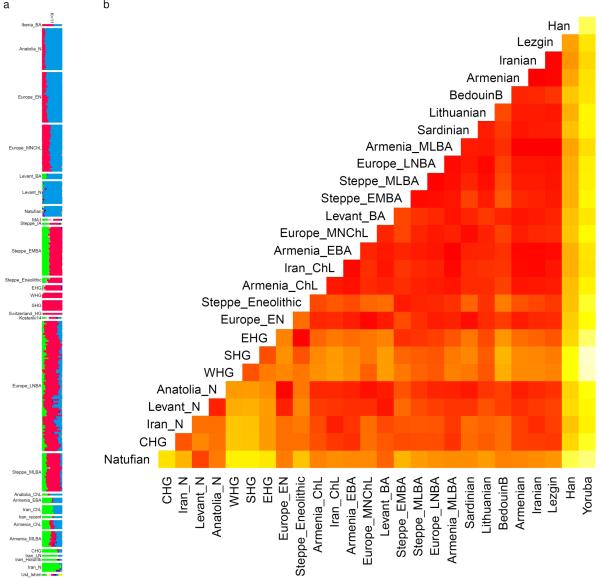
We carried out analysis of f3-statistics, f4-ratio, and f4-statistics statistics using the ADMIXTOOLS16 programs qp3Pop, qpF4ratio with default parameters, and qpDstat with f4mode: YES, and computed standard errors with a block jackknife47. For computing f3-statistics with an ancient population as a target, we set the inbreed:YES parameter. We computed f-statistics on the HOIll dataset when no present-day humans were involved and on the HO dataset when they were. We computed the statistic f4(Test, Mbuti; Altai, Denisovan) in Fig. 2 on the HOIll dataset after merging with whole genome data on 3 Mbuti individuals from Panel C of the Simons Genome Diversity Project48. We computed the dendrogram of Extended Data Fig. 3 showing hierarchical clustering of populations with outgroup f3-statistics using the open source heatmap.2 function of the gplots package in R.
Negative correlation of Basal Eurasian ancestry with Neanderthal ancestry
We used the lm function of R to fit a linear regression of the rate of allele sharing of a Test population with the Altai Neanderthal as measured by f4(Test, Mbuti; Altai, Denisovan) as the dependent variable, and the proportion of Basal Eurasian ancestry (Supplementary Information, section 4) as the predictor variable,. Extrapolating from the fitted line, we obtain the value of the statistic expected if Test is a population of 0% or 100% Basal Eurasian ancestry. We then compute the ratio of the Neanderthal ancestry estimate in Basal Eurasians relative to non-Basal Eurasians as f4(100% Basal Eurasian, Mbuti; Altai, Denisovan)/ f4(0% Basal Eurasian, Mbuti; Altai, Denisovan). We use a block jackknife47, dropping one of 100 contiguous blocks of the genome at a time, to estimate the value and standard error of this quantity (9±26%). We compute a 95% confidence interval based on the point estimate ±1.96-times the standard error: −42 to 60%. We truncated to 0-60% on the assumption that Basal Eurasians had no less Neanderthal admixture than Mbuti from sub-Saharan Africa.
Estimation of FST coefficients
We estimated FST in smartpca17 with default parameters, inbreed: YES, and fstonly: YES.
Admixture Graph modeling
We carried out Admixture Graph modeling with the qpGraph software16 using Mbuti as an outgroup unless otherwise specified.
Testing for the number of streams of ancestry
We used the qpWave34,49 software, described in Supplementary Information, section 10 of ref.7, to test whether a set of ‘Left’ populations is consistent with being related via as few as N streams of ancestry to a set of ‘Right’ populations by studying statistics of the form X(u, v) = F4(u0, u; v0, v) where u0, v0 are basis populations chosen from the ‘Left’ and ‘Right’ sets and u, v are other populations from these sets. We use a Hotelling's T2 test49 to evaluate whether the matrix of size (L-1)*(R-1), where L, R are the sizes of the ‘Left’ and ‘Right’ sets has rank m. If this is the case, we can conclude that the ‘Left’ set is related via at least N=m+1 streams of ancestry related differently to the ‘Right’ set.
Inferring mixture proportions without an explicit phylogeny
We used the qpAdm methodology described in Supplementary Information, section 10 of ref. 7 to estimate the proportions of ancestry in a Test population deriving from a mixture of N ‘reference’ populations by exploiting (but not explicitly modeling) shared genetic drift with a set of ‘Outgroup’ populations (Supplementary Information, section 7). We set the details: YES parameter, which reports a normally distributed Z-score estimated with a block jackknife for the difference between the statistics f4(u0, Test; v0, v) and f4(u0, Estimated Test; v0, v) where Estimated Test is , the average of these f4-statistics weighed by the mixture proportions αi from the N reference populations.
Modeling admixture from ghost populations
We model admixture from a ‘ghost’ (unobserved) population X in the specific case that X has part of its ancestry from two unobserved ancestral populations p and q. Any population X composed of the same populations p and q resides on a line defined by two observed reference populations r1 and r2 composed of the same elements p and q according to a parametric equation x = r1 + λ(r2 – r1) with real-valued parameter λ. We define and solve the optimization problem of fitting λ and obtain mixture proportions (Supplementary Information, section 10).
Code availability
Code implementing the method to model admixture from ghost populations is available on request from I.L.
Extended Data
Extended Data Figure 1. Principal components analysis of 991 present-day West Eurasians.
The PCA analysis is performed on the same set of individuals as are reported in Fig. 1b, using EIGENSOFT. Here, we color the samples by population (to highlight the present-day populations) instead of using grey points as in Fig. 1b (where the goal is to highlight ancient samples).
Extended Data Figure 2. Genetic structure in ancient West Eurasian populations across time and decline of genetic differentiation over time.
(a) ADMIXTURE model-based clustering analysis of 2,583 present-day humans and 281 ancient samples; we show the results only for ancient samples for K=11 clusters. (b) Pairwise FST between 19 Ancient West Eurasian populations (arranged in approximate chronological order), and select present-day populations.
Extended Data Figure 3. Outgroup f3(Mbuti; X, Y) for pairs of ancient populations.
The dendrogram is plotted for convenience and should not be interpreted as a phylogenetic tree. Areas of high shared genetic drift are ‘yellow’ and include from top-right to bottom-left along the diagonal: early Anatolian and European farmers; European hunter-gatherers, Steppe populations and ones admixed with steppe ancestry; populations from the Levant from the Epipaleolithic (Natufians) to the Bronze Age; populations from Iran from the Mesolithic to the Late Neolithic.
Extended Data Figure 4. Reduction of genetic differentiation in West Eurasia over time.
We measure differentiation by FST. Each column of the 5×5 matrix of plots represents a major region and each row the earliest population with at least two individuals from each major region.
Extended Data Figure 5. West Eurasian related admixture in East Africa, Eastern Eurasia and South Asia.
(a) Levantine ancestry in Eastern Africa in the Human Origins dataset, (b) Levantine ancestry in different Eastern African population in the dataset of Pagani et al. (2012); the remainder of the ancestry is a clade with Mota, a ~4,500 year old sample from Ethiopia50. (c) EHG ancestry in Eastern Eurasians, or (d) Afontova Gora (AG2) ancestry in Eastern Eurasians; the remainder of their ancestry is a clade with Onge. (e) Mixture proportions for South Asian populations showing that they can be modelled as having West Eurasian-related ancestry similar to that in populations from both the Eurasian steppe and Iran.
Extended Data Figure 6.
Inferred position of ancient populations in West Eurasian PCA according to the model of Fig. 4.
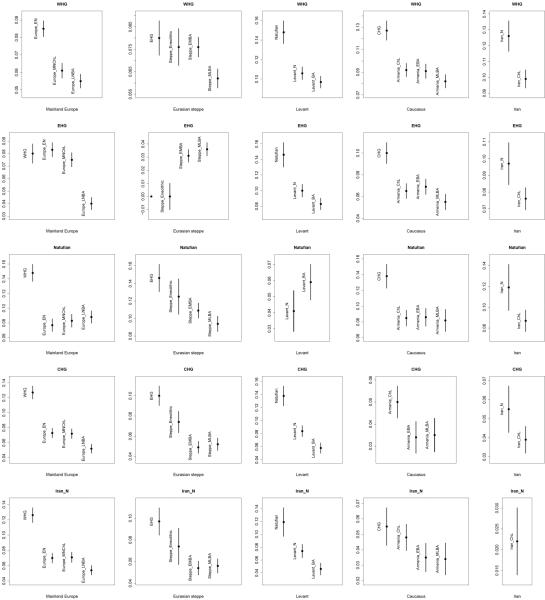
Extended Data Figure 7. Admixture from ghost populations using ‘cline intersection’.
We model each Test population (purple) in panels (a-f) as a mixture (pink) of a fixed reference population (blue) and a ghost population (orange) residing on the cline defined by two other populations (red and green) according to the visualization method of Supplementary Information, section 10. (a) Early/Middle Bronze Age steppe populations are a mixture of Iran_ChL and a population on the WHG→SHG cline. (b) Scandinavian hunter-gatherers (SHG) are a mixture of WHG and a population on the Iran_ChL→Steppe_EMBA cline. (c) Caucasus hunter-gatherers (CHG) are a mixture of Iran_N and both WHG and EHG. (d) Late Neolithic/Bronze Age Europeans are a mixture of the preceding Europe_MNChL population and a population with both EHG and Iran_ChL ancestry. (e) Somali are a mixture of Mota50 and a population on the Iran_ChL→Levant_BA cline. (f) Eastern European hunter-gatherers (EHG) are a mixture of WHG and a population on the Onge→Han cline.
Extended Data Figure 8. Admixture from a ‘ghost’ ANE population into both European and Eastern Eurasian ancestry.
EHG, and Upper Paleolithic Siberians Mal'ta 1 (MA1) and Afontova Gora 2 (AG2) are positioned near the intersection of clines formed by European hunter-gatherers (WHG, SHG, EHG) and Eastern non-Africans in the space of outgroup f3-statistics of the form f3(Mbuti; Papuan, Test) and f3(Mbuti; Switzerland_HG, Test).
Extended Data Table 1. No evidence for admixture related to sub-Saharan Africans in Natufians.
We computed the statistic f4(Natufian, Other Ancient; African, Chimp) varying African to be Mbuti, Yoruba, Ju_hoan_North, or the ancient Mota individual. Gene flow between Natufians and African populations would be expected to bias these statistics positive. However, we find most of them to be negative in sign and all of them to be non-significant (|Z|<3), providing no evidence that Natufians differ from other ancient samples with respect to African populations.
| Other Ancient | African | f4(Natufian, Other Ancient; African, Chimp) | z | Number of SNPs |
|---|---|---|---|---|
| EHG | Mbuti | −0.00044 | −1.0 | 254033 |
| EHG | Yoruba | 0.00029 | 0.7 | 254033 |
| EHG | Ju_hoan_North | −0.00015 | −0.4 | 254033 |
| EHG | Mota | −0.00022 | −0.4 | 253986 |
| WHG | Mbuti | −0.00067 | −1.7 | 261514 |
| WHG | Yoruba | −0.00045 | −1.1 | 261514 |
| WHG | Ju_hoan_North | −0.00046 | −1.2 | 261514 |
| WHG | Mota | −0.00129 | −2.3 | 261461 |
| SHG | Mbuti | −0.00076 | −2.0 | 255686 |
| SHG | Yoruba | −0.00039 | −1.0 | 255686 |
| SHG | Ju_hoan_North | −0.00052 | −1.4 | 255686 |
| SHG | Mota | −0.00091 | −1.7 | 255641 |
| Switzerland_HG | Mbuti | −0.00018 | −0.4 | 261322 |
| Switzerland_HG | Yoruba | 0.00019 | 0.4 | 261322 |
| Switzerland_HG | Ju_hoan_North | 0.00009 | 0.2 | 261322 |
| Switzerland_HG | Mota | −0.00062 | −0.9 | 261276 |
| Kostenki14 | Mbuti | 0.00034 | 0.7 | 246765 |
| Kostenki14 | Yoruba | 0.00120 | 2.3 | 246765 |
| Kostenki14 | Ju_hoan_North | 0.00069 | 1.4 | 246765 |
| Kostenki14 | Mota | 0.00036 | 0.5 | 246719 |
| MA1 | Mbuti | −0.00038 | −0.7 | 191819 |
| MA1 | Yoruba | 0.00009 | 0.2 | 191819 |
| MA1 | Ju_hoan_North | −0.00010 | −0.2 | 191819 |
| MA1 | Mota | −0.00038 | −0.5 | 191782 |
| CHG | Mbuti | −0.00051 | −1.2 | 261505 |
| CHG | Yoruba | −0.00012 | −0.3 | 261505 |
| CHG | Ju_hoan_North | −0.00013 | −0.3 | 261505 |
| CHG | Mota | −0.00042 | −0.7 | 261456 |
| Iran_N | Mbuti | −0.00018 | −0.4 | 232927 |
| Iran_N | Yoruba | 0.00036 | 0.8 | 232927 |
| Iran_N | Ju_hoan_North | 0.00041 | 0.9 | 232927 |
| Iran_N | Mota | 0.00006 | 0.1 | 232880 |
Extended Data Table 2. Admixture f3-statistics.
We show the lowest Z-score of the statistic f3(Test; Reference1, Refrence2) for every ancient Test population with at least 2 individuals and every pair (Reference1, Refrence2) of ancient or present-day source populations. Z-scores lower than −3 are highlighted and indicate that the Test population is admixed from sources related to (but not identical to) the reference populations. Z-scores greater than −3 are consistent with the population either being admixed or not.
| Test | Reference1 | Reference2 | f3(Test; Reference1, Refrence2) | Z-score | Number of SNPs |
|---|---|---|---|---|---|
| Anatolia_N | Iberia_BA | Levant_N | −0.00034 | −0.2 | 111632 |
| Armenia_ChL | EHG | Levant_N | −0.00249 | −1.5 | 167020 |
| Armenia_EBA | Anatolia_N | CHG | −0.01017 | −7.9 | 195596 |
| Armenia MLBA | Anatolia_N | Steppe_EMBA | −0.00809 | −7.3 | 203796 |
| CHG | Anatolia_ChL | Iran_Hotulllb | 0.02612 | 3.6 | 9884 |
| EHG | Steppe_Eneolithic | Switzerland_HG | −0.00282 | −0.9 | 67938 |
| Europe_EN | Anatolia_N | WHG | −0.00494 | −11.2 | 380684 |
| Europe_LNBA | Europe_MNChL | Steppe_EMBA | −0.00920 | −41.8 | 414782 |
| Europe_MNChL | Anatolia_N | WHG | −0.01351 | −26.8 | 363672 |
| Iran_ChL | Anatolia_N | Iran_N | −0.01285 | −10.6 | 167941 |
| Iran_N | Iran_LN | Gana | −0.00462 | −1.1 | 17804 |
| Levant_BA | Iran_N | Levant_N | −0.00853 | −4.7 | 118269 |
| Levant_N | Europe_MNChL | Natufian | −0.00671 | −3.6 | 61845 |
| Natufian | Iberia_BA | Iran_Hotulllb | 0.07613 | 3.4 | 1054 |
| SHG | Steppe_Eneolithic | Switzerland_HG | 0.00728 | 3.2 | 154825 |
| Steppe_EMBA | EHG | Abkhasian | −0.00756 | −11.2 | 349359 |
| Steppe_Eneolithic | EHG | Iran_LN | −0.01637 | −4.2 | 25100 |
| Steppe_MLBA | Europe_MNChL | Steppe_EMBA | −0.00573 | −18.0 | 378298 |
| WHG | Switzerland_HG | Saudi | −0.01562 | −7.7 | 218758 |
| Abkhasian | CHG | Sardinian | −0.00754 | −13.1 | 387956 |
| Adygei | Anatolia_N | Eskimo | −0.00699 | −14.4 | 413128 |
| Albanian | Europe_EN | Burusho | −0.00650 | −16.8 | 395851 |
| Armenian | Anatolia_N | Sindhi | −0.00603 | −19.5 | 406021 |
| Assyrian | Iran_N | Sardinian | −0.00672 | −11.8 | 309055 |
| Balkar | Anatolia_N | Chukchi | −0.00975 | −18.8 | 401928 |
| Basque | Switzerland_HG | Druze | −0.00726 | −12.6 | 416070 |
| BedouinA | Europe_EN | Yoruba | −0.01584 | −42.8 | 460762 |
| BedouinB | Iran_Hotulllb | Natufian | 0.01384 | 4.1 | 32266 |
| Belarusian | WHG | Iranian | −0.00974 | −19.8 | 392363 |
| Bulgarian | Anatolia_N | Steppe_EMBA | −0.00807 | −26.7 | 400263 |
| Canary_Islander | Europe_MNChL | Mende | −0.00829 | −5.9 | 353172 |
| Chechen | Anatolia_N | Eskimo | −0.00440 | −7.9 | 396678 |
| Croatian | WHG | Druze | −0.00871 | −18.6 | 394032 |
| Cypriot | Anatolia_N | Sindhi | −0.00562 | −16.1 | 401141 |
| Czech | SHG | Druze | −0.00919 | −21.7 | 374705 |
| Druze | Iran_N | Sardinian | −0.00269 | −5.8 | 343813 |
| English | Steppe_EMBA | Sardinian | −0.00628 | −20.6 | 402502 |
| Estonian | SHG | Druze | −0.00789 | −17.6 | 371575 |
| Finnish | SHG | Assyrian | −0.00716 | −12.6 | 355744 |
| French | Steppe_EMBA | Sardinian | −0.00669 | −37.9 | 441807 |
| Georgian | CHG | Sardinian | −0.00782 | −13.7 | 390744 |
| German | WHG | Druze | −0.01103 | −22.9 | 391302 |
| Greek | Europe_EN | Pathan | −0.00600 | −30.0 | 421984 |
| Hungarian | Steppe_EMBA | Sardinian | −0.00644 | −31.2 | 420017 |
| Icelandic | WHG | Abkhasian | −0.00974 | −17.0 | 394625 |
| Iranian | Anatolia_M | Sindhi | −0.00594 | −30.9 | 443011 |
| Irish | Steppe_EMBA | Sardinian | −0.00590 | −22.8 | 416663 |
| Irish_Ulster | SHG | Assyrian | −0.00909 | −15.6 | 350547 |
| Italian_North | Europe_EN | Steppe_EMBA | −0.00627 | −26.4 | 419169 |
| Italian_South | Iberia_BA | Iran_Hotulllb | 0.01224 | 2.6 | 17678 |
| Jew_Ashkenazi | Anatolia_N | Koryak | −0.00532 | −9.4 | 389012 |
| Jew_Georgian | Iran_N | Sardinian | −0.00306 | −4.2 | 292410 |
| Jew_Iranian | Iran_N | Sardinian | −0.00385 | −5.8 | 302446 |
| Jew_Iraqi | Iran_N | Sardinian | −0.00486 | −6.5 | 287673 |
| Jew_Libyan | Europe_EN | Yoruba | −0.00397 | −7.2 | 415797 |
| Jew_Moroccan | Europe_EN | Yoruba | −0.00649 | −10.9 | 405193 |
| Jew_Tunisian | Anatolia_N | Mende | −0.00276 | −4.1 | 399354 |
| Jew_Turkish | Anatolia_N | Burusho | −0.00571 | −16.4 | 405254 |
| Jew_Yemenite | Natufian | Kalash | −0.00341 | −3.8 | 174052 |
| Jordanian | Europe_EN | Yoruba | −0.01283 | −26.7 | 423649 |
| Kumyk | Anatolia_N | Chukchi | −0.01025 | −19.6 | 396439 |
| Lebanese | Anatolia_N | Yoruba | −0.01022 | −19.5 | 414854 |
| Lebanese_Christian | Anatolia_N | Sindhi | −0.00504 | −15.7 | 404858 |
| Lebanese_Muslim | Anatolia_N | Brahmin_Tiwari | −0.00616 | −20.4 | 415129 |
| Lezgin | Steppe_EMBA | Jew_Yemenite | −0.00481 | −13.1 | 398974 |
| Lithuanian | WHG | Abkhasian | −0.00999 | −17.7 | 386718 |
| Maltese | Anatolia_N | Brahmin_Tiwari | −0.00518 | −14.5 | 404438 |
| Mordovian | WHG | Iranian | −0.00912 | −18.4 | 395230 |
| North_Ossetian | Anatolia_N | Chukchi | −0.00894 | −17.2 | 401729 |
| Norwegian | WHG | Abkhasian | −0.00957 | −16.5 | 393546 |
| Orcadian | SHG | Druze | −0.00662 | −15.8 | 379656 |
| Palestinian | Europe_EN | Yoruba | −0.01129 | −31.3 | 464066 |
| Polish | SHG | Druze | −0.00924 | −27.8 | 394654 |
| Romanian | Europe_EN | Steppe_EMBA | −0.00549 | −16.9 | 397119 |
| Russian | SHG | Turkish | −0.00731 | −25.0 | 398393 |
| Sardinian | Anatolia_N | Switzerland_HG | −0.00587 | −9.6 | 417931 |
| Saudi | Anatolia_N | Dinka | −0.00326 | −5.1 | 404923 |
| Scottish | Steppe_EMBA | Sardinian | −0.00622 | −26.6 | 426660 |
| Shetlandic | WHG | Abkhasian | −0.00868 | −14.6 | 386562 |
| Sicilian | Anatolia_N | Brahmin_Tiwari | −0.00646 | −22.2 | 411481 |
| Sorb | SHG | Palestinian | −0.00787 | −16.8 | 366924 |
| Spanish | Steppe_EMBA | Sardinian | −0.00557 | −32.2 | 447735 |
| Spanish_North | WHG | Armenian | −0.00825 | −10.9 | 356832 |
| Syrian | Europe_EN | Dinka | −0.01002 | −17.3 | 410920 |
| Turkish | Europe_EN | Sindhi | −0.00709 | −41.1 | 448975 |
| Ukrainian | WHG | Abkhasian | −0.01183 | −21.4 | 388282 |
Supplementary Material
Acknowledgements
We thank the 238 human subjects who donated samples for genome-wide analysis, and D. Labuda and P. Zalloua for sharing samples from Poland and Lebanon. The Fig. 1a map was plotted in R using the worldHiRes map of the 'mapdata' package (using public domain data from the CIA World Data Bank II). We thank O. Bar-Yosef, M. Bonogofsky, I. Hershkowitz, M. Lipson, I. Mathieson, H. May, R. Meadow, I. Olalde, S. Paabo, P. Skoglund, and N. Nakatsuka for comments and critiques, and D. Bradley, M. Dallakyan, S. Esoyan, M. Ferry and M. Michel, and A. Yesayan, for contributions to bone preparation and ancient DNA work D.F. and M.N. were supported by Irish Research Council grants GOIPG/2013/36 and GOIPD/2013/1, respectively. S.C. was funded by the Irish Research Council for Humanities and Social Sciences (IRCHSS) ERC Support Programme. Q.F. was funded by the Bureau of International Cooperation of the Chinese Academy of Sciences, the National Natural Science Foundation of China (L1524016) and the Chinese Academy of Sciences Discipline Development Strategy Project (2015-DX-C-03). The Scottish diversity data was funded by the Chief Scientist Office of the Scottish Government Health Directorates [CZD/16/6], the Scottish Funding Council [HR03006], and a project grant from the Scottish Executive Health Department, Chief Scientist Office [CZB/4/285]. M.S., A.Tön., M.B. and P.K. were supported by the German Research Foundation (CRC 1052; B01, B03, C01). M.S.-P. was funded by a Wenner-Gren Foundation Dissertation Fieldwork Grant (#9005), and by the National Science Foundation DDRIG (BCS-1455744).P.K. was funded by the Federal Ministry of Education and Research, Germany (FKZ: 01EO1501). J.F.W acknowledges the MRC “QTL in Health and Disease” programme grant. The Romanian diversity data was supported by the EC Commission, Directorate General XII (Supplementary Agreement ERBCIPDCT 940038 to the Contract ERBCHRXCT 920032, coordinated by Prof. A. Piazza, Turin, Italy). M.R. received support from the Leverhulme Trust's Doctoral Scholarship programme. O.S. and A.Tor. were supported by the University of Pavia (MIGRAT-IN-G) and the Italian Ministry of Education, University and Research: Progetti Ricerca Interesse Nazionale 2012. The Raqefet Cave Natufian project was supported by funds from the National Geographic Society (Grant #8915-11), the Wenner-Gren Foundation (Grant #7481) and the Irene Levi-Sala CARE Foundation, while radiocarbon dating on the samples was funded by the Israel Science Foundation (Grant 475/10; E. Boaretto). R.P. was supported by ERC starting grant ADNABIOARC (263441). D.R. was supported by NIH grant GM100233, by NSF HOMINID BCS-1032255, and is a Howard Hughes Medical Institute investigator.
Footnotes
Author Contributions R.P. and D.R. conceived the idea for the study. D.N., G.R., D.C.M., S.C., S.A.R., G.L., F.B., B.Gas., J.M.M., M.G., V.E., A.M., C.M., F.G., N.A.H. and R.P. assembled archaeological material. N.R., D.F., M.N., B.Gam., K.Si., S.C., K.St., E.H., Q.F., G.G.-F., E.R.J., R.P. and D.R. performed or supervised ancient DNA wet laboratory work. L.B, M.B., A.C., G.C., D.C., P.F., E.G., S.M.K., P.K., J.K., D.M., M.M., D.A.M., S.O., M.B.R., O.S., M.S.-P., G.S., M.S., A.Tön., A.Tor., J.F.W., L.Y. and D.R. assembled present-day samples for genotyping. I.L, N.P. and D.R. developed methods for data analysis. I.L., S.M., Q.F., N.P. and D.R. analyzed data. I.L., R.P. and D.R. wrote the manuscript and supplements.
Author Information The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB14455. Fully public subsets of the analysis datasets are at (http://genetics.med.harvard.edu/reichlab/Reich_Lab/Datasets.html). The complete dataset including present-day humans for which the informed consent is not consistent with public posting of data is available to researchers who send a signed letter to D.R. indicating that they will abide by specified usage conditions (Supplementary Information, section 2). The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Supplementary Information is available in the online version of the paper.
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
References
- 1.Barker G, Goucher C. The Cambridge World History Volume II: A world with agriculture, 12,000 BCE-500 CE. Cambridge University Press; 2015. [Google Scholar]
- 2.Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton University Press; 1994. [Google Scholar]
- 3.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat Commun. 2014;5:5257. doi: 10.1038/ncomms6257. doi:10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinhasi R, et al. Optimal Ancient DNA Yields from the Inner Ear Part of the Human Petrous Bone. PLoS ONE. 2015;10:e0129102. doi: 10.1371/journal.pone.0129102. doi:10.1371/journal.pone.0129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. USA. 2013;110:2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015:524, 216. doi: 10.1038/nature14558. doi:10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. doi:10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones ER, et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun. 2015;6 doi: 10.1038/ncomms9912. doi:10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allentoft ME, et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. doi:10.1038/nature14507 http://www.nature.com/nature/journal/v522/n7555/abs/nature14507.html - supplementary-information. [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. doi:10.1038/nature13810 http://www.nature.com/nature/journal/v514/n7523/abs/nature13810.html - supplementary- information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günther T, et al. Ancient genomes link early farmers from Atapuerca in Spain to modern-day Basques. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1509851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. doi:10.1038/nature13673 http://www.nature.com/nature/journal/v513/n7518/abs/nature13673.html - supplementary- information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olalde I, et al. A common genetic origin for early farmers from Mediterranean Cardial and Central European LBK cultures. Molecular Biology and Evolution. 2015 doi: 10.1093/molbev/msv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. doi:10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Research. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. doi:10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prufer K, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. doi:10.1038/nature12886 http://www.nature.com/nature/journal/v505/n7481/abs/nature12886.html - supplementary- information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer M, et al. A High-Coverage Genome Sequence from an Archaic Denisovan Individual. Science. 2012 doi: 10.1126/science.1224344. doi:10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall JD, et al. Higher levels of neanderthal ancestry in East Asians than in Europeans. Genetics. 2013;194:199–209. doi: 10.1534/genetics.112.148213. doi:10.1534/genetics.112.148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer M, et al. A High-Coverage Genome Sequence from an Archaic Denisovan Individual. Science. 2012;338:222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. doi:10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brace CL, et al. The questionable contribution of the Neolithic and the Bronze Age to European craniofacial form. Proc Natl Acad Sci U S A. 2006;103:242–247. doi: 10.1073/pnas.0509801102. doi:10.1073/pnas.0509801102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferembach D. Squelettes du Natoufien d'Israel., etude anthropologique. L'Anthropologie. 1961;65:46–66. [Google Scholar]
- 26.Fadhlaoui-Zid K, et al. Genome-Wide and Paternal Diversity Reveal a Recent Origin of Human Populations in North Africa. PLoS ONE. 2013;8:e80293. doi: 10.1371/journal.pone.0080293. doi:10.1371/journal.pone.0080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henn BM, et al. Genomic ancestry of North Africans supports back-to-Africa migrations. PLoS genetics. 2012;8:e1002397. doi: 10.1371/journal.pgen.1002397. doi:10.1371/journal.pgen.1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia G, Patterson N, Sankararaman S, Price AL. Estimating and interpreting F(ST): The impact of rare variants. Genome Res. 2013;23:1514–1521. doi: 10.1101/gr.154831.113. doi:10.1101/gr.154831.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez E, et al. Ancient DNA analysis of 8000 B.C. near eastern farmers supports an early neolithic pioneer maritime colonization of Mainland Europe through Cyprus and the Aegean Islands. PLoS genetics. 2014;10:e1004401. doi: 10.1371/journal.pgen.1004401. doi:10.1371/journal.pgen.1004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammerman AJ, Pinhasi R, Banffy E. Comment on “Ancient DNA from the first European farmers in 7500-year-old Neolithic sites”. Science. 2006;312:1875. doi: 10.1126/science.1123984. author reply 1875, doi:10.1126/science.1123936. [DOI] [PubMed] [Google Scholar]
- 31.Pagani L, et al. Ethiopian Genetic Diversity Reveals Linguistic Stratification and Complex Influences on the Ethiopian Gene Pool. American journal of human genetics. 2012;91:83–96. doi: 10.1016/j.ajhg.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickrell JK, et al. Ancient west Eurasian ancestry in southern and eastern Africa. Proc. Natl. Acad. Sci. USA. 2014;111:2632–2637. doi: 10.1073/pnas.1313787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller A, et al. New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698. doi: 10.1038/ncomms1701. doi: http://www.nature.com/ncomms/journal/v3/n2/suppinfo/ncomms1701_S1.html. [DOI] [PubMed] [Google Scholar]
- 34.Moorjani P, et al. Genetic evidence for recent population mixture in India. American journal of human genetics. 2013;93:422–438. doi: 10.1016/j.ajhg.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reich D, Thangaraj K, Patterson N, Price AL, Singh L. Reconstructing Indian population history. Nature. 2009;461:489–494. doi: 10.1038/nature08365. doi: http://www.nature.com/nature/journal/v461/n7263/suppinfo/nature08365_S1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu Q, et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. doi:10.1038/nature17993 http://www.nature.com/nature/journal/v534/n7606/abs/nature17993.html - supplementary- information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. doi:10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil–DNA– glycosylase treatment for screening of ancient DNA. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2014;370 doi: 10.1098/rstb.2013.0624. DOI: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs AW, et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic acids research. 2010;38:e87. doi: 10.1093/nar/gkp1163. doi:10.1093/nar/gkp1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korlevic P, et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. BioTechniques. 2015;59:87–93. doi: 10.2144/000114320. doi:10.2144/000114320. [DOI] [PubMed] [Google Scholar]
- 41.Meyer M, et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature. 2014;505:403–406. doi: 10.1038/nature12788. doi:10.1038/nature12788. [DOI] [PubMed] [Google Scholar]
- 42.Behar DM, et al. A “Copernican” Reassessment of the Human Mitochondrial DNA Tree from its Root. American journal of human genetics. 2012;90:675–684. doi: 10.1016/j.ajhg.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15:1–13. doi: 10.1186/s12859-014-0356-4. doi:10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell S, et al. PLINK: a tool set for whole-genome association and population- based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. doi:10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang C, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busing FTA, Meijer E, Leeden R. Delete-m Jackknife for Unequal m. Statistics and Computing. 1999;9:3–8. doi:10.1023/A:1008800423698. [Google Scholar]
- 48.Sudmant PH, et al. Global diversity, population stratification, and selection of human copy-number variation. Science. 2015;349 doi: 10.1126/science.aab3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reich D, et al. Reconstructing Native American population history. Nature. 2012;488:370–374. doi: 10.1038/nature11258. doi:10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llorente MG, et al. Ancient Ethiopian genome reveals extensive Eurasian admixture in Eastern Africa. Science. 2015;350:820–822. doi: 10.1126/science.aad2879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.