Abstract
Purpose/Objectives
Radiation injury to parahippocampal cingulum white matter is associated with cognitive decline. Diffusion tensor imaging (DTI) detects micropathologic changes in white matter. Increased radial diffusion (RD) and decreased axial diffusion (AD) correspond to demyelination and axonal degeneration/gliosis respectively. We aimed to develop a predictive model for radiation-induced cognitive changes based upon DTI changes.
Materials/Methods
Twenty-seven adults with benign or low-grade tumors received partial brain radiation therapy (RT) to a median dose of 54 Gy. Patients underwent DTI before RT, during RT, and at the end of RT. Cognitive testing was performed before RT, and 6 and 18 months after RT. Parahippocampal cingulum white matter was contoured to obtain mean values of AD and RD.
Results
By univariate analysis, decreasing AD and increasing RD during RT predicted declines in verbal memory and verbal fluency. By multivariate analysis, baseline neurocognitive score was the only clinical variable predicting verbal memory change; no clinical variables predicted verbal fluency change. In a multivariate model, increased RD at the end of RT significantly predicted decline in verbal fluency 18 months after RT.
Conclusions
Imaging biomarkers of white matter injury contributed to predictive models of cognitive function change after RT.
Keywords: magnetic resonance imaging, cognitive function, late effects, glioma
Introduction
Changes in cognitive function have been observed following brain radiation therapy (RT) in adults [1,2], however mechanisms are poorly understood and predictive models are limited. Cognitive decline may be due in part to white matter injury caused by radiation damage to vascular and glial progenitor cells as well as chronic inflammation [3]. Previous studies have established that radiation to the hippocampus and associated structures increases risk of cognitive decline [4,5]. However, improvements in cognitive performance after partial brain irradiation have also been seen, possibly due to tumor control or test practice effects [6,7].
In this study we examined the parahippocampal cingulum, a medial temporal lobe white matter structure that is an afferent connection to the hippocampus [8]. We used diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI) technique that is more sensitive to white matter microstructural changes than standard T1- and T2-weighted MRI [9]. Two measurements derived from diffusion tensor eigenvalues are radial diffusion (RD) and axial diffusion (AD). Increased RD is associated with histologic evidence of demyelination, and decreased AD is associated with axonal degeneration and inflammatory gliosis [10,11]. We have previously found that the parahippocampal cingulum shows greater diffusion changes after radiation than other white matter exposed to the same dose [12,13], and that late-delayed cognitive function changes are associated with concurrent diffusion changes in the parahippocampal cingulum [14].
In the present study, we sought to identify a predictive imaging biomarker of cognitive function after RT by conducting a prospective assessment of the cognitive abilities of adults with benign or low-grade brain tumors treated with partial brain RT. Patients were followed 18 months after RT to study both early-delayed (6 months) and late-delayed (18 months) effects. We hypothesized that diffusion changes in the parahippocampal cingulum consistent with white matter injury during and immediately after RT would be independent predictors of later cognitive function.
Methods
Study Design
Adults with benign or low-grade intracranial tumors were enrolled in a prospective, institutional review board approved study. All patients received a standard 6 or 7-week course of daily-fractionated RT. Functional status was assessed before RT using Karnofsky Performance Status (KPS), Folstein Mini-Mental State Examination (MMSE), and Radiation Therapy Oncology Group neurological function class. All enrolled patients had a KPS score ≥ 80, MMSE score ≥ 27, and neurological function class ≤ 2, indicating no major functional impairments. Patients included in the current analysis had at least two time points of imaging data and no tumor progression or radiation necrosis during follow-up. Surgical resection and any complications such as hydrocephalus or hemorrhage occurred before study enrollment.
Treatment Planning and Dosimetry
3D-conformal or intensity-modulated radiation therapy planning was performed on computed tomography images acquired using a Brilliance 16-slice system (Philips Healthcare, Best, Netherlands). Dose values were corrected to 2 Gy per fraction equivalents using the linear-quadratic model with α/β = 2.5 Gy [15]. The contribution of radiation dose to the risk of cognitive function impairment was estimated using generalized uniform equivalent dose (gEUD) calculated from the whole brain volume excluding gross target volume [16]. Our model used a = 14, indicating sensitivity to low-volume, high-dose areas. This parameter was determined from a maximum likelihood analysis of the Lyman normal tissue complication probability model [17] for cognitive function impairment from a dataset of 32 patients [18].
Study Image Acquisition
Patients underwent MRI at three time points: 1–2 weeks before RT (pre-RT), 3 weeks after starting RT (mid-RT), and within 1 week of completing RT (end-RT). At each time point, DTI, T1- and T2-weighted MR images were acquired in a single session. Due to technology upgrades, three different MRI systems were used in the study, but each patient completed imaging on a single system. Diffusion imaging parameters by system: 1.5T Signa (GE Healthcare, Milwaukee, USA), matrix 128 × 128, voxels 2.5 × 2.5 × 4 mm, 9 diffusion directions, 2 averaged diffusion image sets, b = 1000 s/mm2. 3T Achieva (Philips Healthcare, Best, Netherlands), matrix 128 × 128, voxels 1.75 × 1.75 × 2 mm, 15 diffusion directions, 2 averaged diffusion image sets, b = 800 s/mm2. 3T Skyra (Siemens Healthcare, Erlangen, Germany), matrix 220 × 220, voxels 1.72 × 1.72 × 3.9 mm, 20 diffusion directions, 3 averaged diffusion image sets, b = 1000 s/mm2.
Image Pre-processing and Masking
MRI pre-processing was performed using the FMRIB Software Library (FSL) (FMIRB Analysis Group, Oxford, UK) [19]. Diffusion tensor eigenvalues were calculated at each voxel, from which three parameter maps were generated: axial diffusion (AD), radial diffusion (RD), and fractional anisotropy (FA). All images were interpolated to 1 mm3 voxels. On all image sets, abnormal tissue masks were contoured using post-contrast T1- and T2-weighted images. Volumes of tumor mass, edema, and visibly affected areas were manually contoured and excluded from registration and statistical analysis.
Within-Patient Longitudinal MR Image Registration
To improve contouring uniformity, for each patient FA images from multiple time points were co-registered to derive a within-patient template using an iterative registration method [20]. Final registration parameters were then applied to the tumor mask, AD image, and RD image from each time point, co-registering all images to the within-patient template. Non-linear registrations were performed by the FSL registration algorithm FNIRT [19,21].
Structure Contouring
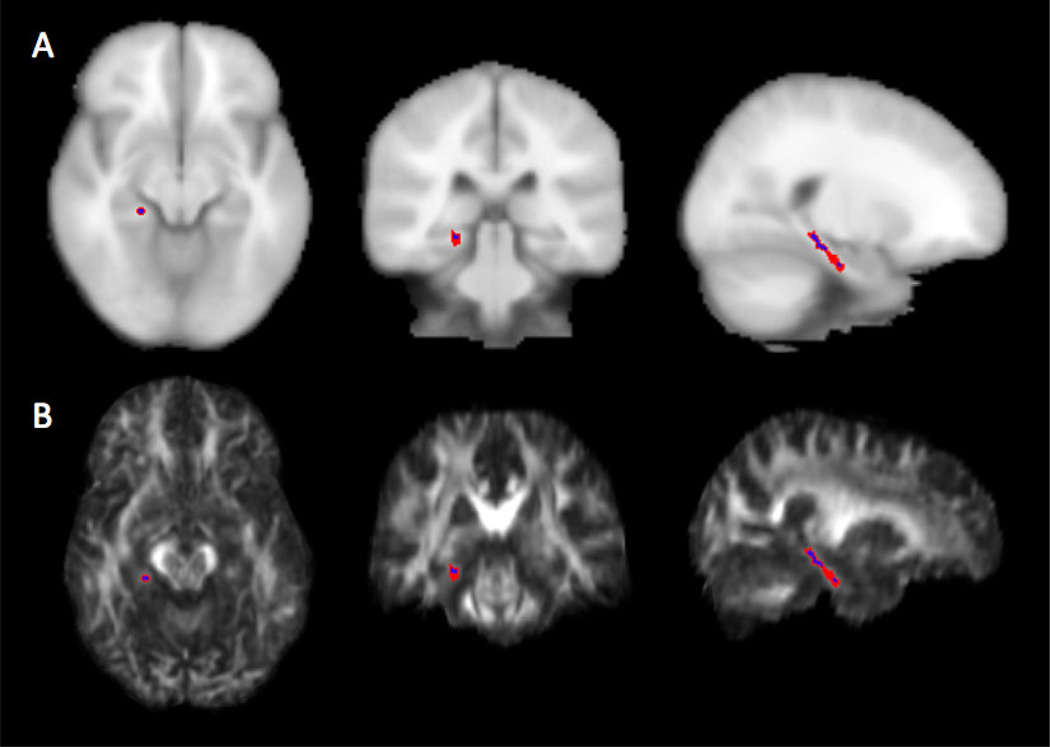
For each patient, the parahippocampal cingula were manually contoured on the within-patient FA template image. The structure was defined as the temporal portion of the cingulum white matter inferior to the corpus callosum. A 1-voxel erosion operation was performed on manual contours to reduce averaging error from edges (Figure 1). The mean values of AD and RD were then calculated from contour volumes excluding the abnormal tissue masks. FA values were not used for statistical analysis to avoid biases introduced by using FA images for registration and contouring.
Figure 1.
Example right parahippocampal cingulum contour: (A) T1-weighted reference image and (B) patient FA image with contour before (red) and after (blue) 1-voxel 3D erosion operation.
Cognitive Testing
Cognitive testing was performed at three time points: pre-RT, 6 months after completing RT, and 18 months after completing RT. Testing included the Hopkins Verbal Learning Test (revised edition) Total and Percent Retained components of short-term and delayed verbal memory (HVLT-T and HVLT-PR), the Benton Controlled Oral Word Association Test of verbal fluency (COWAT), and Trail Making Test B of attention and task-switching (TMT-B; preceded by the simpler version Trail Making Test A). Testing was performed under the supervision of a clinical neuropsychologist (HAB). Published data were used to convert raw scores to normalized Z-scores based on age, sex, and years of education [22–24].
Statistics
Diffusion change was calculated as a percentage change from pre-RT. Thresholds for significant changes in individual DTI measurements (AD: ±3.9%, RD: ±2.9%) were determined from previously derived repeatability coefficients [25]. Cognitive test score changes were calculated as difference in Z-score from pre-RT value. Thresholds for significant changes in individual cognitive score changes were determined using the reliable change index [26]. Student’s t-tests were used to assess group changes in diffusion and cognitive scores. Simple linear regression and Student’s t-tests were used to determine whether changes in diffusion were significantly related to clinical variables or gEUD. Univariate and multivariate analysis was used to determine if changes in cognitive scores after radiation therapy were related to clinical variables, gEUD, baseline cognitive scores, or changes in diffusion. Clinical variables assessed included patient age, patient sex, invasive tumor (glioma), and frontal or temporal lobe location (“frontotemporal”). Univariate analysis was performed using simple linear regression or two-sample Student’s t-test. Multivariate analysis was performed using linear regression models in two stages. First stage models included all predictor variables except for diffusion changes. Second stage models excluded variables from stage one with parameter significance p > 0.10, then added diffusion as a predictor variable. Intercepts were unconstrained in all models. All tests of significance were two-tailed with significance threshold p ≤ 0.05. Correction for multiple comparisons was performed on the second stage multivariate models with Bonferroni correction. All models for one cognitive score constituted a single hypothesis family with 8 hypothesis tests (2 diffusion indices × 2 imaging time points × 2 cognitive test time points). Significance threshold for each second stage hypothesis test was p ≤ 0.00625.
Results
Patient Characteristics
Twenty-seven patients met study inclusion criteria (Table 1). Twenty-three of the patients had pituitary adenomas, low-grade gliomas, or meningiomas. The remaining four tumors were two craniopharyngiomas, one hemangioblastoma, and one adenoid cystic carcinoma (included due to intracranial extension). One patient missed mid-RT imaging and one patient missed end-RT imaging. One patient missed 6 month cognitive testing and two patients missed 18 month cognitive testing. One patient did not complete TMT-B at pre-RT, therefore post-RT changes in this score were not calculable. All patients had either stability or decrease in tumor size on follow-up imaging.
Table 1.
Patient and treatment characteristics
| Age | ||
| Median | 48 years | |
| Range | 26 – 71 years | |
| Sex | ||
| Male | 15 (56%) | |
| Female | 12 (44%) | |
| Education | ||
| Median | 15 years | |
| Range | 8 – 18 years | |
| Tumor Pathology | ||
| Pituitary Adenoma | 9 (33%) | |
| Low-Grade Glioma | 8 (30%) | |
| Meningioma | 6 (22%) | |
| Other | 4 (15%) | |
| Tumor Laterality | ||
| Midline | 13 (48%) | |
| Right | 9 (33%) | |
| Right | 5 (19%) | |
| Tumor Location | ||
| Pituitary | 11 (41%) | |
| Frontal Lobe | 6 (22%) | |
| Temporal Lobe | 5 (19%) | |
| Parietal Lobe | 3 (11%) | |
| Occipital Lobe | 1 (4%) | |
| Cerebellum | 1 (4%) | |
| Treatment Planning | ||
| 3D-CRT | 19 (70%) | |
| IMRT | 8 (30%) | |
| Prescription Dose | ||
| Median | 54 Gy | |
| Range | 50.4 – 70 Gy | |
| Whole-brain gEUD | ||
| Median | 40.46 Gy | |
| Range | 32.57 – 52.82 Gy | |
| Pre-RT Surgery | ||
| Full or Partial Resection | 23 (85%) | |
| None | 3 (11%) | |
| Biopsy Only | 1 (4%) | |
| Concurrent Chemotherapy | ||
| None | 23 (85%) | |
| Temozolomide | 4 (15%) | |
| Imaging System | ||
| Philips Achieva 3T | 15 (56%) | |
| GE Signa 1.5T | 9 (33%) | |
| Siemens Skyra 3T | 3 (11%) | |
Diffusion Value Changes
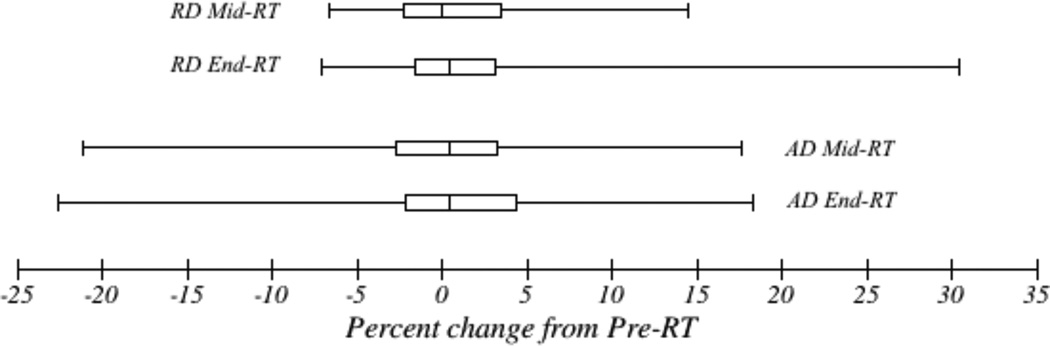
Percentage changes in parahippocampal cingulum diffusion values from pre-RT are summarized in Figure 2 and Table S1. There was heterogeneity of individual diffusion changes from baseline, with changes in AD at end-RT ranging from −22.6% to +18.3%, and changes in RD at end-RT ranging from −7.1% to +30.4%. However, individual patient changes at mid-RT and end-RT were similar, with no significant difference by pairwise t-test. There were no significant linear correlations between gEUD or patient age and diffusion changes, and there were no significant differences by patient sex, tumor location, or tumor histology (test statistics not shown).
Figure 2.
Diffusion changes from pre-treatment. Box represents median and interquartile range. Whiskers represent minimum and maximum values.
Cognitive Test Results
Baseline Z-scores and post-treatment changes are summarized in Table 2. The proportion of individuals significantly impaired before radiation ranged from 11% for HVLT-PR (delayed verbal memory) to 33% for HVLT-T (short-term verbal memory). Group mean performance at pre-RT was significantly below population normal for COWAT (verbal fluency) and HVLT-T. HVLT-PR at 18 months and TMT-B (attention and task-switching) at both follow-up points showed more patients with significant individual decline than improvement, while other tests showed more patients with individual improvement. Of all tests, only COWAT showed a statistically significant change of group mean performance, with improvement at 6 months.
Table 2.
Summary of cognitive test score results
| COWAT | HVLT-T | HVLT-PR | TMT-B | |
|---|---|---|---|---|
| Pre-RT | ||||
| Group Mean ± 95% CI | −0.53 ± 0.47* | −1.09 ± 0.59* | −0.31 ± 0.64 | −0.66 ± 0.93 |
| Individual Impairment | 7/27 (26%) | 9/27 (33%) | 3/27 (11%) | 4/26 (15%) |
| 6 months | ||||
| Mean Change from Pre-RT | +0.40 ± 0.26† | +0.29 ± 0.50 | +0.14 ± 0.71 | +0.00 ± 0.59 |
| Individual Improvement | 6/26 (23%) | 5/26 (19%) | 3/26 (12%) | 6/25 (24%) |
| Individual Decline | 0/26 (0%) | 1/26 (4%) | 2/26 (8%) | 7/25 (28%) |
| 18 months | ||||
| Mean Change from Pre-RT | +0.24 ± 0.30 | +0.36 ± 0.36 | −0.32 ± 0.82 | −1.59 ± 1.83 |
| Individual Improvement | 5/25 (20%) | 2/25 (8%) | 2/25 (8%) | 7/24 (29%) |
| Individual Decline | 1/25 (4%) | 0/25 (0%) | 4/25 (16%) | 9/24 (38%) |
Group results are mean Z-scores ± 95% confidence interval.
Significant difference from population norm (p ≤ 0.05).
Significant difference from pre-RT scores (p ≤ 0.05).
COWAT: Controlled Oral Word Association Test. HVLT-T: Hopkins Verbal Learning Test, Total. HVLT-PR: Hopkins Verbal Learning Test, Percent Retained. TMT-B: Trail Making Test B.
Univariate Modeling of Cognitive Score Changes
By univariate analysis, only baseline cognitive scores and diffusion changes significantly predicted cognitive score changes at 6 and 18 months post-RT. For HVLT-T and HVLT-PR, baseline Z-score was negatively correlated to post-RT changes in test scores at 6 and 18 months (HVLT-T at 6 months, R = −0.44, p = 0.02; HVLT-T at 18 months, R = −0.47, p = 0.02; HVLT-PR at 6 months, R = −0.45, p = 0.02; HVLT-PR at 18 months, R = −0.57, p = 0.003). This suggests that for verbal recall and short-term memory, patients with poor performance at baseline improved following RT. Conversely, baseline Z-score for TMT-B was positively correlated to score change at 18 months post-RT (R = 0.42, p = 0.04). This suggests that for attention and task-switching, patients with poor performance at baseline worsened following RT.
Change in AD at mid-RT was positively correlated to change in HVLT-PR score at 6 months (R = 0.54, p = 0.005, Table 3). This signifies that patients with white matter changes related to axonal damage (decreased AD) experienced either decline or less improvement in verbal memory after RT. Change in RD at end-RT was negatively correlated to change in COWAT score at 18 months (R = −0.60, p = 0.002, Table 3). Patients with white matter changes related to demyelination (increased RD) experienced either decline or less improvement in verbal fluency after RT.
Table 3.
Predictive models with significant contributions from diffusion changes
| Dependent Variable | Parameter | Estimate | Standard Error | p-value |
|---|---|---|---|---|
| Univariate Models: | ||||
| ΔZ HVLT-PR at 6 months | Intercept | −0.046 | 0.31 | 0.88 |
| %Δ AD at mid-RT | 0.14 | 0.045 | 0.0054 | |
| ΔZ COWAT at 18 months | Intercept | 0.36 | 0.14 | 0.015 |
| %Δ RD at end-RT | −0.056 | 0.016 | 0.0018 | |
| Multivariate Model: | ||||
| ΔZ COWAT at 18 months | Intercept | 0.31 | 0.17 | 0.079 |
| %Δ RD at end-RT | −0.055 | 0.02 | 0.0023 | |
| Frontotemporal | 0.15 | 0.28 | 0.60 |
Parameter estimates reflect effect on cognitive Z-score change for 1% increase in diffusion variable, or for frontotemporal versus other tumor location.
There were no significant univariate correlations between patient age or gEUD and post-RT cognitive score changes. There were no significant differences in post-RT cognitive score changes by patient sex, tumor histology (glioma or not), or frontotemporal tumor location.
Multivariate Modeling of Cognitive Score Changes
Results of first stage multivariate analysis (non-diffusion variables only) are summarized in Table S2. Baseline Z-score again had significant negative correlation to HVLT-T at 6 months, HVLT-PR at 6 months, and HVLT-PR at 18 months, indicating more improvement with worse baseline performance. A significant negative correlation was seen between gEUD and change in HVLT-T at 6 months and TMT-B at 18 months, representing worse cognitive performance change with greater effective radiation dose. Frontotemporal tumor location had a significant negative correlation with change in HVLT-PR at 6 months, representing worse performance change in verbal memory for patients with tumors in frontal or temporal lobes.
Age did not achieve statistical significance in any model, but was included in second stage models for 6 month HVLT-PR and 6 month TMT-B due to trends towards significance (p ≤ 0.10). Patient sex and tumor histology did not significantly contribute to any multivariate model (p > 0.10) and were excluded in all second stage models. There were no significant clinical predictors for 6 month COWAT and 18 month HVLT-T, and thus none were included in second stage models.
In the second stage multivariate analysis (diffusion variables added to significant clinical variables), diffusion change was an independent significant predictor in one model. Changes in RD at end-RT were negatively correlated to changes in COWAT at 18 months in a model including frontotemporal location (estimate −0.055, p = 0.0023; Table 3). This retained significance after Bonferroni correction. This indicates that diffusion imaging changes in the parahippocampal cingula associated with demyelination predicted worse performance change in verbal fluency at 18 months after RT, independent of clinical variables and with high significance.
Although significant in univariate analysis, prediction of HVLT-PR at 6 months by change in AD at mid-RT was reduced to near-significance (p = 0.062) after multivariate combination with baseline Z-score (p = 0.0066), frontotemporal tumor location (p = 0.070), and patient age (p = 0.054). No other second stage models had significant contributions from diffusion variables (test statistics not shown).
Discussion
We prospectively performed DTI and cognitive testing on patients with low-grade and benign brain tumors receiving RT. We identified early changes in parahippocampal cingulum diffusion measurements that significantly predicted changes in cognitive performance up to 18 months after completing treatment. For all significant correlations, our findings were in agreement with the hypothesis that diffusion changes in the parahippocampal cingulum associated with white matter injury, specifically increased RD and decreased AD, predicted worse cognitive performance change.
After partial brain radiation, COWAT (verbal fluency) and HVLT-T (short-term verbal memory) scores showed group trends towards improvement, while HVLT-PR (delayed verbal memory) and TMT-B (attention and task switching) showed trends toward worsening. These differences suggest that for some neurocognitive domains, tumor control had a beneficial effect. This may particularly be true for verbal memory, as patients with worse baseline performance in HVLT-T and HVLT-PR had more improvement after RT. Another consideration is the influence of practice effect, where repeated testing causes improved scores by repetition alone [27]. In concordance with previous publications, we calculated change in cognitive performance without correcting for practice effects [1,2]. In domains such as attention and task switching, global radiation effects may be more detrimental, with higher whole-brain gEUD predicting worse performance on TMT-B at 18 months after RT. The most significant and independently predictive diffusion variable was increasing RD (associated with demyelination) at the end of RT predicting worse performance on the COWAT at 18 months. The second strongest diffusion predictor was significant in univariate analysis only, which was for decreasing AD (associated with axonal degeneration) at mid-RT predicting worse performance on the HVLT-PR at 6 months. Given that both of these tests showed a general group improvement at these time points, these diffusion changes may reflect radiation injuries limiting recovery from baseline impairment. Our findings suggest there may be two opposing processes: cognitive score improvement from tumor control, reflected by improving average scores after radiation and more improvement among patients with low pre-RT performance, and cognitive score decline from normal tissue radiation effects, reflected by high gEUD and diffusion tensor correlates of normal white matter injury predicting worse later performance. Dissociation between RD and AD prediction of late-delayed and early-delayed cognitive decline may suggest also distinct mechanisms, such as injury to microvasculature and glial precursor cells (RD) versus impairment of hippocampal neurogenesis (AD) [3]. Frontotemporal location was also a significant predictor of decline in verbal memory, suggesting a relation to hippocampus radiation as previously seen [5,28].
Diffusion changes during radiation were predominantly increases in both AD and RD, with a skew towards increased RD and decreased AD, consistent with white matter radiation injury [11]. However, there was large variation between patients in diffusion index changes, with some patients showing little evidence of white matter injury after RT. Individual variation may be due to direct cingulum dose, as seen previously [14]. In the present analysis, whole-brain gEUD, patient age, patient sex, tumor location, and tumor histology were not significantly correlated with diffusion changes. However, other studies have seen greater white matter injury in older patients [29]. There may be other unmeasured factors influencing white matter radiation sensitivity such as age-related vascular disease [30]. The observed heterogeneity in white matter changes after RT may be a marker for radiation sensitivity and could inform individual treatment adaptation.
We studied white matter changes only in the parahippocampal cingulum. This structure was chosen for analysis based on previous observations that late diffusion changes in this structure are correlated to cognitive performance change [14]. Our current findings cannot determine whether injury to cingulum white matter is the causative factor or whether it is a surrogate marker for a related effect. However, diffusion changes in the cingula have previously been associated with other forms of cognitive impairment [31–33], suggesting that this is a promising structure to examine as an imaging biomarker of radiation-induced cognitive dysfunction.
A potential confounding effect in this study is that MRI was performed on three different imaging systems. Systematic differences in diffusion measurements are expected between MR imaging systems and DTI protocols [34]. Anticipating this, all patients completed imaging follow-up with the same system and protocol as their initial images, and percentage changes in diffusion values were used to minimize the impact of systematic variation. Because this study tested multiple correlations, another potential problem is the risk of type I error. To guard against this, we performed Bonferroni correction on the final hypothesis tests. Because of concerns for limited power with a small sample size, statistical modeling was performed using DTI and neurocognitive changes for all patients, not just those who were determined to have significant individual changes.
In conclusion, the results show that diffusion changes during radiation in normal appearing cingulum white matter were predictive of cognitive function change up to 18 months after RT in adults with low-grade and benign brain tumors. For one cognitive domain this correlation was significant after multivariate modeling including clinical factors. These diffusion changes have previously been shown to correlate histologically to radiation-induced white matter injury. DTI shows promise as a predictive imaging biomarker of radiation-induced cognitive decline, and further work is needed to determine its reproducibility and applicability. Development of an imaging biomarker could advance efforts to predict and prevent this complication.
Supplementary Material
Diffusion changes from pre-treatment
First stage multivariate models of clinical variables alone
Acknowledgments
Funding
This study was funded by USA National Institutes of Health grant R01 NS064973 (Cao)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
None of the authors have any real or potential conflicts of interest to disclose.
References
- 1.Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86:656–664. doi: 10.1016/j.ijrobp.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 3.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clinical Cancer Research. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiffer AM, Leyrer CM, Greene-Schloesser DM, Shing E, Kearns WT, Hinson WH, et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology. 2013;80:747–753. doi: 10.1212/WNL.0b013e318283bb0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83:e487–e493. doi: 10.1016/j.ijrobp.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres IJ, Mundt AJ, Sweeney PJ, Llanes-Macy S, Dunaway L, Castillo M, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113–1118. doi: 10.1212/01.wnl.0000055862.20003.4a. [DOI] [PubMed] [Google Scholar]
- 7.Laack NN, Brown PD, Ivnik RJ, Furth AF, Ballman KV, Hammack JE, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63:1175–1183. doi: 10.1016/j.ijrobp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19:289–298. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Assaf Y, Pasternak O. Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review. J Mol Neurosci. 2007;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 10.Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Wu EX, Qiu D, Leung LHT, Lau HF, Khong PL. Longitudinal Diffusion Tensor Magnetic Resonance Imaging Study of Radiation-Induced White Matter Damage in a Rat Model. Cancer Res. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. [DOI] [PubMed] [Google Scholar]
- 12.Chapman CH, Nazem-Zadeh M, Lee OE, Schipper MJ, Tsien CI, Lawrence TS, et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract-based spatial statistics. PLoS ONE. 2013;8:e57768. doi: 10.1371/journal.pone.0057768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazem-Zadeh M-R, Chapman CH, Lawrence TL, Tsien CI, Cao Y. Radiation therapy effects on white matter fiber tracts of the limbic circuit. Medical Physics. 2012;39:5603–5613. doi: 10.1118/1.4745560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman CH, Nagesh V, Sundgren PC, Buchtel H, Chenevert TL, Junck L, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys. 2012;82:2033–2040. doi: 10.1016/j.ijrobp.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steel GG. Basic Clinical Radiobiology. 3rd. London: Arnold; 2002. [Google Scholar]
- 16.Niemierko A. A generalized concept of equivalent uniform dose (EUD) Med Phys. 1999 doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 17.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 18.Avila R, Chapman CH, Tao Y, Schipper M, Buchtel H, Haken Ten RK, et al. Dose-Volume Effects of Partial-Brain Radiation Therapy on Late Neurocognitive Processing Speed and Executive Function. Int J Radiat Oncol Biol Phys. 2015;93:S175. [Google Scholar]
- 19.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Keihaninejad S, Zhang H, Ryan NS, Malone IB, Modat M, Cardoso MJ, et al. An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer's disease. Neuroimage. 2013;72:153–163. doi: 10.1016/j.neuroimage.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Andersson J, Jenkinson M, SMITH S. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. Non-linear registration, aka Spatial normalisation. [Google Scholar]
- 22.Benedict R, Schretlen D, Groninger L. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 23.Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: Reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 24.Tombaugh T. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 25.Nazem-Zadeh M-R, Chapman CH, Lawrence TS, Tsien CI, Cao Y. Uncertainty in assessment of radiation-induced diffusion index changes in individual patients. Phys Med Biol. 2013;58:4277–4296. doi: 10.1088/0031-9155/58/12/4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 27.Heilbronner RL, Sweet JJ, Attix DK, Krull KR, Henry GK, Hart RP. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol. 2010;24:1267–1278. doi: 10.1080/13854046.2010.526785. [DOI] [PubMed] [Google Scholar]
- 28.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welzel T, Niethammer A, Mende U, Heiland S, Wenz F, Debus J, et al. Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol. 2008;29:379–383. doi: 10.3174/ajnr.A0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopewell JW, Wright EA. The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol. 1970;43:161–167. doi: 10.1259/0007-1285-43-507-161. [DOI] [PubMed] [Google Scholar]
- 31.Chua TC, Wen W, Chen X, Kochan N, Slavin MJ, Trollor JN, et al. Diffusion Tensor Imaging of the Posterior Cingulate is a Useful Biomarker of Mild Cognitive Impairment. Am J Geriat Psychiat. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- 32.Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, et al. Evaluating the Relationship between Memory Functioning and Cingulum Bundles in Acute Mild Traumatic Brain Injury Using Diffusion Tensor Imaging. J Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, et al. White matter integrity in mild cognitive impairment: A tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 34.Vollmar C, O'Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, et al. Identical, but not the same: Intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diffusion changes from pre-treatment
First stage multivariate models of clinical variables alone