Abstract
Background
Adults with irritable bowel syndrome (IBS) frequently identify foods as exacerbating their gastrointestinal (GI) symptoms. In children with IBS the prevalence of perceived food intolerances and their impact are unknown.
Objectives
To determine the prevalence of self-perceived food intolerances and the relationship of these intolerances to abdominal pain, psychosocial distress, and quality of life in children with IBS.
Design
Cross-sectional; questionnaire and prospective diary data were collected from 2008–2014 by trained research coordinators.
Participants/Setting
Children 7–18 years (pediatric Rome III IBS, n=154; age-sex matched healthy children (HC), n=32) in Houston, Texas.
Measures
Perceived food intolerances and avoided foods were captured using the Childhood Food and Symptom Association Questionnaire. IBS severity was assessed by a ≥7-day Pain Diary and validated psychosocial questionnaires assessing quality of life, somatization, functional disability, depression, and anxiety.
Statistical Analyses Performed
Descriptive, Spearman bivariate correlation, chi-square, Poisson log-linear generalized model with Wald chi-square statistics.
Results
A greater proportion of children with IBS (143/154, 92.9%) vs HC (20/32, 62.5%) identified at least one self-perceived food intolerance (χ2=22.5, P<0.001). Children with IBS identified a greater number (median [25=75%]=4[2–6]) of perceived symptom-inducing foods than HC (median=2[0–4]; χ2=28.6,P<0.001). Children with IBS avoided more foods (median=2[1–4]) than HC (median=0 [0–2.75]; χ2=20.8, P<0.001). The number of self-perceived food intolerances was weakly associated (r-value range of −0.17 to 0.21) with pain frequency, pain severity, somatization, anxiety, functional disability, and decreased quality of life.
Conclusions
Children with IBS have a high prevalence of self-perceived food intolerances; the number of these intolerances is weakly associated with measures of IBS severity.
Keywords: Pediatric, Children, Diet, IBS, FODMAP
INTRODUCTION
Irritable bowel syndrome (IBS) is a prevalent functional gastrointestinal (GI) disorder affecting up to 20% of school aged children.1 Factors which play a role in the clinical phenotype expressed include: visceral hyperalgesia, the gut microbiome, psychosocial distress (e.g., anxiety), and diet.2–4 Up to 84% of adults with IBS identify foods within their diet that exacerbate GI symptoms.5, 6 This is higher than the self-perceived food intolerance prevalence of up to 34% in the general population.7, 8
The number of perceived food intolerances in adults with IBS is associated with more severe GI symptoms.6, 9 Self-reported food intolerances in adults also are associated with psychosocial distress such as somatization, anxiety, and decreased quality of life, though these associations have not been consistently identified.6, 10 Sex may also play a role, as women with IBS are more likely to identify food intolerances as compared to men.6, 10
In children with IBS there is a dearth of information regarding self-perceived food intolerances and their clinical impact. A small mixed methods study in children with several functional GI disorders found that self-perceived food intolerances qualitatively negatively impacted several areas of quality of life.11 Another study in Finnish 10 and 11-year-olds with functional GI disorders found that a majority of parents identified their child as having a food-induced GI symptom.12 Neither study evaluated the relationship of intolerances to child-reported psychosocial distress and/or GI symptoms. Given the possibility that self-perceived food intolerances may have a large influence on the clinical severity of childhood IBS, further elucidating the prevalence and role of self-perceived food intolerances is clinically important.
Given this unexplored but clinically important area, the primary objective of this study was to determine the prevalence of self-perceived food intolerances in children with IBS. In addition, the study secondarily sought to determine whether there was a relationship between the number of self-perceived food intolerances and the severity of GI symptoms (e.g., abdominal pain), psychosocial distress (e.g., somatization), and quality of life.
MATERIALS AND METHODS
Participants
Participants included children ages 7–18 years meeting the Rome III diagnostic criteria for pediatric IBS1 who were part of ongoing dietary intervention studies from 2008 – 2014. All children with IBS had completed a physician visit within the past year of enrollment and had not been identified with an organic etiology for their symptoms. Only baseline (prior to any intervention) information was used for this current report. Children with IBS and healthy children (HC) were recruited from the Texas Children’s Pediatric Associates general pediatrics practices and additional children with IBS from the Texas Children’s Hospital pediatric gastroenterology program as previously described.13 The screening process included investigator review of the subject’s medical record to ensure investigations of symptoms (e.g., lab testing) were negative.13
Healthy children were recruited based on absence of chronic illness and GI complaints and matched by age and sex in a 5:1 ratio to those with IBS. Though the differences between healthy children and those with IBS was unknown, the 5:1 ratio was initially chosen based on the much higher anticipated number of food intolerances in those with IBS vs. HC.
Demographic information was captured for all participants. Baylor College of Medicine Institutional Review Board approved the study protocol. Parents provided written informed consent, and children gave their assent.
Procedure
Study procedures were completed during a home visit or hospital visit for all children with IBS. Children completed all questionnaires in a room independent from their parent. For children who were 7–10 years of age, questionnaires were read by a research coordinator using a standardized protocol to ensure reading difficulties did not affect responses. Healthy children were interviewed over the phone in the same manner as would have occurred during a visit. Healthy children only completed the Childhood Food and Symptom Association Questionnaire. The comprehensive severity of IBS was evaluated through the use of the prospective Pain Diary as well as through the use of the questionnaires.
Determination of Food Intolerances and Avoided Foods
Participants completed the Childhood Food and Symptom Association Questionnaire assessing whether any of 97 foods (e.g., oranges) or food types (e.g., barbeque food) exacerbated their GI symptoms.11 The food symptom association questionnaire was originally created and initially validated to assess symptoms in children with functional gastrointestinal disorders.11 This questionnaire was read to the child by a coordinator outside the presence of a caregiver. If a food or food type was identified, the child was asked if he/she avoided eating the identified food. The questionnaire further assessed what type of symptom was elicited. Foods and food types were subsequently categorized into one of 8 groups for this study: Fruits, Vegetables, Dairy, Condiments, High Protein, Grains, Beverages, and Sweets (Table 1).
Table 1.
Food and Food Type Assignments into Food Categories from Items in the Childhood Food and Symptom Association Questionnaire11
| Food Category | Food and/or Food Type |
|---|---|
| Fruits | Apples, Pears, Mangoes, Watermelon, Melons, Oranges, Grapefruit, Lemons, Peaches, Plums, Cherries, Nectarines, Bananas, Apricots, Grapes, Raisins, Berries, Fruit juices, Dried fruits, Other fruits |
| Vegetables | Onions, Broccoli, Cauliflower, Asparagus, Cabbage, Beans, Peas, Tomatoes, Corn, Potatoes, Brussels sprout, Green or Red peppers, Cooked vegetables, Raw vegetables, Other vegetables |
| Dairy | Milk (cow’s), Soy milk, Ice cream, Cheese, Creams, Yogurt, Butter, Margarine, Coconut milk/cream |
| Condiments | BBQ sauce, Ketchup, Mayonnaise, Salad dressing, Mustard, Pancake syrup, Honey, Jellies/Jam, Peanut butter, Sweet and sour sauce, Gravy, Salsa |
| Meats/Protein | Pork, Sausage, Ham, Beef, Turkey, Hot dogs, Chicken, Fish, Eggs, Peanuts, Other nuts |
| Grains | Pasta, Pasta sauce, Wheat bread, Rye bread, White bread, Bagels, Sourdough bread, Cold breakfast cereal, Cookies, Crackers, Cakes, Pizza, Rice |
| Beverages/Other | Pickles, Sodas, Diet sodas, Sports drinks, Coffee, Tea, Alcohol, Smoked foods, Deep fried foods, Fried foods, Spicy foods, Fast food |
| Sweets | Pastries, Chocolate, Non-chocolate candy, Sugar-free gum, Sugar gum |
Pain Diary
Children with IBS were instructed on the completion of a daily Pain Diary for ≥. 7 days. Children rated abdominal pain for 3 intervals each day (morning, midday/afternoon, and evening/nighttime) using a numeric rating scale of 0 to 10, anchored with the phrases “no pain at all” and “the worst pain you can imagine”.14 This method of pain assessment has been validated in children and successfully used in children with IBS.15, 16 Parents were asked to remind children to complete the diaries daily and to allow children to independently rate abdominal pain without influencing their responses.17 Responses for each day were called in to a telephone data entry system. Research coordinators ensured compliance with completion of the Pain Diary by monitoring the phone call data entry and contacting families (at least once) when needed to ensure completion.
The pain severity scores were calculated as the mean pain rating over the course of the diary excluding ratings of “no pain” (i.e., zero). Pain frequency was calculated as the number of ratings ≥1 over the course of the diary.
Measures of Psychosocial Distress
The Children’s Somatization Inventory (CSI) includes 35 symptoms (e.g., ”headache”) in which the child rates on a 5-point Likert scale (0=”not at all” to 4=”a whole lot”) how much the child is “bothered by each symptom” over the preceding two weeks.18 The CSI has been successfully used and validated in children with functional gastrointestinal disorders such as IBS.19, 20 Total scores for the CSI range between 0 and 140 with increasing scores indicating greater levels of somatization.
The Behavioral Assessment System for Children, Second Edition (BASC-2) is a questionnaire assessing a wide array of behavior and emotional dimensions of children, and has extensive support for reliability, content and construct validity (including in children with functional gastrointestinal disorders).21–23 The child self-report questionnaires are available for children ages 6 to 21 years. The Anxiety and Depression T scores were used for this study. The child self-report scales are derived from established norms with higher scorers having more anxiety or depression characteristics.21
The Functional Disability Index is a 15-item questionnaire which measures the degree to which children have difficulty in physical and psychosocial functioning.24 It has been successfully used and validated in children with functional gastrointestinal disorders.24, 25 The total scores range from 0–60 with higher scores reflecting higher levels of perceived disability.24, 26
The Pediatric Health-Related Quality of Life 4.0 Generic Core Scales is a 23-item, extensively validated questionnaire assessing health-related quality of life in healthy children and in those with medical conditions (including IBS).27, 28 Pediatric Health-Related Quality of Life total scores range from 0–100 with higher scores reflecting better quality of life.27
Statistical Analyses
All statistical analyses were conducted using IBS® SPSS® Statistics (version 22, 2013, IBM Corporation). Chi square (for proportions) or a generalized linear model with Poisson log-linear distribution (for data using counts) was used to compare data between groups. A binary Spearman’s rho correlation coefficient was calculated between the number of self-perceived food intolerances and pain-related variables, psychosocial questionnaire scores, and quality of life scores. A generalized linear model with Poisson log-linear distribution using factors found to significantly correlate with the total number of perceived foods was used to evaluate the main effects and interactions of these factors on the total number of self-perceived food intolerances. All factors (main effects and their potential interactions) were initially included; thereafter, statistically redundant interactions were removed one at a time until all remaining factors and interactions in the final model were statistically significant.
Unless otherwise indicated, values are presented as mean ± standard deviation or median [25% quartile, 75% quartile]. Two tailed P values <0.05 were considered statistically significant.
RESULTS
Comparison of Frequency and Type of Self-Perceived Food Intolerances in Children with IBS versus Healthy Children
A total of 154 children with IBS [89 (57.8%) girls] and 32 HC [19 (59.4%) girls] were enrolled in the study. Children in both groups were of similar age (12.5 ± 2.9 (SD) vs. 12.4 ± 3.6 years, IBS vs. HC, respectively). Children with IBS differed from HC with respect to race/ethnicity composition [97 (62.9%) vs. 10 (31.3%) White, 25 (16.2%) vs. 9 (28.1%) Hispanic, 22 (14.3%) vs. 9 (28.1%) Black, 5 (3.2%) vs. 4 (12.5%) Asian, 1 (0.6%) vs. 0 American Indian, respectively, χ2=15.5, P<0.01].
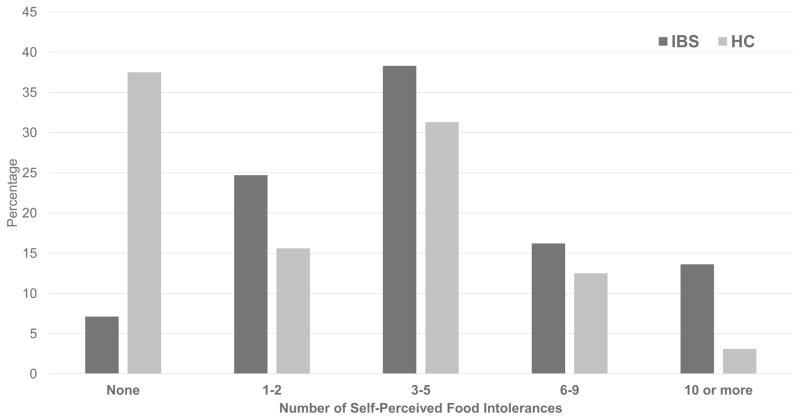
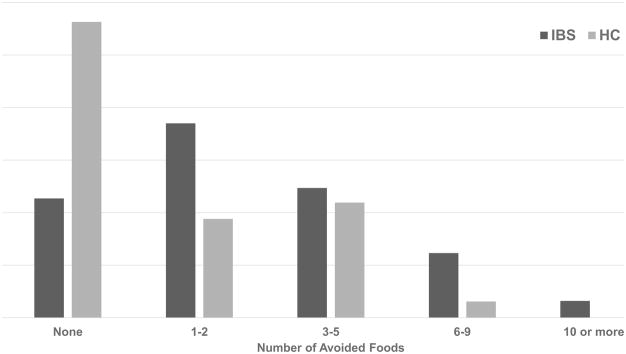
A greater proportion of children with IBS (143/154, 92.9%) vs. HC (20/32, 62.5%) identified at least one food as being able to exacerbate a GI symptom (χ2=22.5, P<0.001). Children with IBS identified a greater number (median = 4 [2–6]; Range=35) of symptom-inducing foods than HC (median = 2 [0–4]; Range=11; χ2=28.6, P<0.001). In parallel, children with IBS avoided a greater median number (2 [1–4]; Range=16) of foods than HC (median=0 [0–2.75]; χ2=20.8, Range=9; P<0.001). The number of self-perceived food intolerances and avoided foods correlated in both those with IBS (r=0.73; P<0.0001) and HC (r=0.58; P=0.001). Children with IBS vs. HC identified a wide range of self-perceived food intolerances (Figure 1) and avoided foods (Figure 2).
Figure 1.
Number of self-perceived food intolerances in children with irritable bowel syndrome (n=154) versus healthy children (n=32). IBS = children with irritable bowel syndrome; HC = healthy children
Figure 2.
Number of avoided foods in children with irritable bowel syndrome (n=154) versus healthy health controls (n=32). IBS = children with irritable bowel syndrome; HC = healthy children
Children with IBS vs. HC were more likely to have GI symptom inducing foods within the Dairy, Beverages, and Grains groups but not foods in the other assessed categories (Table 2). The ten most commonly identified GI symptom inducing foods in children with IBS in descending order of frequency were cow’s milk, fast food, cheese, ice cream, spicy food, beans, pizza, sodas, chocolate, and fried foods (Table 3).
Table 2.
Comparison of Self-Perceived Food Intolerances by Food Category in Children with Irritable Bowel Syndrome versus Healthy Children using the Food and Symptom Association Questionnaire11
| Food categorya | Children with Irritable Bowel Syndrome (n=154) | Healthy Children (n=32) | P-value |
|---|---|---|---|
| Fruit | 61 (39.6%) | 11 (34.4%) | N.S.b |
| Vegetables | 59 (38.3%) | 10 (31.3%) | N.S. |
| Dairy | 82 (53.2%) | 8 (25%) | <0.005 |
| Condiments | 44 (28.6%) | 6 (18.8%) | N.S. |
| Meat/High Protein | 39 (25.3%) | 3 (9.4%) | N.S. |
| Grains | 48 (31.2%) | 4 (12.5%) | <0.05 |
| Beverages/Other | 84 (54.5%) | 8 (25%) | <0.005 |
| Sweets | 29 (18.8%) | 3 (9.4%) | N.S. |
| Item From Any Food Category | 143 (92.9%) | 20 (62.5%) | <0.001 |
Please see Table 1 for foods and food types included in each category.
N.S. = Not Significant
Table 3.
Top Ten Most Commonly Identified Foods or Food Typesa Causing Self-Perceived Food Intolerance in Children with Irritable Bowel Syndrome vs. Healthy Children Using the Food and Symptom Association Questionnaire11
| Food or Food Typea | Children with Irritiable Bowel Syndrome (n=154) | Healthy Children (n=32) |
|---|---|---|
| Cow’s milk | 51 (33.1%) | 3 (9.4%) |
| Fast food | 37 (24%) | 3 (9.4%) |
| Cheese | 35 (22.7%) | 2 (6.3%) |
| Ice cream | 34 (22.1%) | 3 (9.4%) |
| Spicy Food | 32 (20.8%) | 4 (12.5%) |
| Beans | 23 (14.9%) | 3 (9.4%) |
| Pizza | 23 (14.9%) | 1 (3.1%) |
| Sodas | 21 (13.6%) | 1 (3.1%) |
| Chocolate | 20 (13%) | 3 (9.4%) |
| Fried foods | 18 (11.7%) | 2 (6.3%) |
The food or food type is from the Food and Symptom Association Questionnaire11
Association of Self-Perceived Food Intolerances with Sex, Race/Ethnicity, and Age
In children with IBS, girls identified a greater number (median = 4 [2–7]; Range=35] of self-perceived food intolerances than boys (median = 3 [1.5–5]; Range=15; χ2=1337.8, P<0.001]. In addition, girls avoided a higher number (median = 2 [1–5; range: 0–16] of foods than boys (median = 1[1–3]; Range=10; χ2=378.8, P<0.001) within the IBS cohort. No differences were found in HC between boys and girls in the total number of food intolerances or avoided foods (data not shown). We did not identify an association between either the total number food intolerances or avoided foods with race/ethnicity (data not shown). In children with IBS there is a weak correlation between age and the number of self-perceived food intolerances (r=0.16, P=0.05). In contrast, there is a weak negative correlation in HC between age and the number of self-perceived food intolerances (r=−0.38, P<0.05).
Association of Self-Perceived Food Intolerances with Abdominal Pain Characteristics, Psychosocial Distress, and Quality of Life in Children with IBS
In children with IBS, the number of self-perceived food intolerances was weakly related (r-value range, −0.17 to 0.21; Table 4) with abdominal pain frequency, pain severity, somatization, anxiety, functional disability, and lower quality of life scores. Depression was not associated with the number of identified food intolerances.
Table 4.
In children with Irritable Bowel Syndrome (n=154), the Number of Self-perceived Food Intolerances Correlates with Somatization, Anxiety, Functional Disability, Number of Abdominal Pain Episodes and Median Pain Severity. The Number of Food Intolerances Negatively Correlates with Quality of Life but is not Correlated to Depression.
| Characteristic | Median Value [25–75% quartile] | Spearman Correlation r-value | P value |
|---|---|---|---|
| Number of daily pain episodesa | 1 [0.5–1.6] | 0.17 | <0.05 |
| Median pain severitya | 3.25 [2.3–4.0] | 0.2 | <0.05 |
| Somatizationb | 24 [15–35.7] | 0.22 | <0.01 |
| Anxiety (BASC-2 T-score)c | 51 [43–62] | 0.21 | 0.01 |
| Functional disabilityd | 9 [4–19] | 0.16 | <0.05 |
| Quality of lifee | 81.8 [70.7–89.1] | − 0.17 | <0.05 |
| Depression (BASC-2 T- score)c | 45 [41–51] | 0.1 | 0.2 |
DISCUSSION
To date the self-perceived role of diet in IBS symptom generation in children has not been comprehensively assessed. This study suggests that children with IBS, particularly girls, frequently perceive diet as playing an important role in exacerbating their IBS symptoms. Presumably due to these self-perceived food intolerances, children with IBS avoid a high number of foods. The number of food intolerances was weakly associated with more frequent and severe abdominal pain, as well as increased functional disability, increased somatization and anxiety, and decreased quality of life. Overall, these findings in children with IBS suggest a high prevalence of self-perceived food intolerances in children but a weak correlation with clinical phenotype.
The prevalence of reported food intolerance in the children with IBS in this study is higher than that (65–84%) reported in adult IBS studies evaluating postprandial symptoms.5, 6, 10, 29 Whether this suggests differences in children with IBS vs. adults with IBS with respect to food responses (e.g., immunological) needs to be evaluated in future studies. Given the high prevalence of perceived food intolerances in children with IBS, future dietary studies in this population may consider taking into account the number of self-perceived food intolerances. In addition, intervention studies (both dietary and non-dietary) may need to formally address dietary regimens consumed during the intervention as they may influence GI symptom expression.
Though not the primary aim of this study, this study reports for the first time the number of food intolerances in a small population of HC. A group of HC was used in this initial assessment to provide context to the findings in those children with IBS. HC were matched for age/sex and not by race/ethnicity to those with IBS to reflect better Houston (where the study took place) demographics. The healthy children participants had a wide range and overall higher prevalence of self-perceived food intolerances as compared to primarily adult-based population studies which identified perceived food reactions in 20–34%, though some of the reported symptoms were not specifically GI related.7, 8 This may be related in part either to differences in biological factors related to food perception such as food neophobia (which is more pronounced in children vs. adults) or methodological differences through this study’s use of a more comprehensive questionnaire assessing more foods and/or food types. Young et al. only evaluated 8 foods, while Zuberbier et al. mentioned “more than 40” assessed foods and/or food additives.7, 8 Healthy children (and those with IBS) may have incorrectly associated dislike of the food due to taste or other reasons with a food intolerance. Future studies of perceived food intolerances in HC of various races/ethnicities and of different geographic locations with different cuisines are needed.
This study found that avoidance of foods that may induce symptoms was common in children with IBS as has been reported in adults with IBS.10 Avoidance of a culprit food would be an expected coping strategy that children with IBS may employ to better control their symptoms. Though this study did not address whether nutritional deficiencies were present due to food avoidance, studies in adults with IBS have found nutritionally inadequate diets in a small number of subjects.5 Families and clinicians caring for children with IBS with a large number of self-perceived food intolerances and subsequent avoidance of these foods may consider formally assessing the child’s nutrient intake.
Girls with IBS have increased visceral hypersensitivity, more frequent autonomic abnormalities, and more psychological distress (e.g., depression) as compared to boys with IBS.30, 31 The etiology for these sex differences has not been fully established but is also seen in adult women versus men.32 Paralleling these sex differences, girls with IBS in the study had a higher number of self-perceived food intolerances than boys. These differences (particularly increased visceral hypersensitivity) are believed to play a significant role in food-induced symptoms. Alternatively, increased psychological distress influencing perception may account for the sex findings. Another possibility may be differences in self-awareness between boys and girls. Given that these sex differences may extend into adulthood, future studies are needed to determine the true sex-based pathophysiologic connections with food intolerance (including differences in self-awareness).6, 10
The primary limitation of the study is that it is cross-sectional. Given the number of factors/relationships evaluated, the number of children studied was modest. Although this approach allows for insight into the evaluated relationships to food intolerance, causality will need to be determined in future studies. Another limitation in the study is that food intolerances were not documented by blind challenges, prospective means (e.g., electronic diaries) or corroboration with healthcare providers and/or parents.33 However, while the role of healthcare provider opinion is unknown, significant differences between parental perception and child complaints have been reported in children with functional GI disorders.34, 35 Another limitation (given mode of administration of a questionnaire may influence results) is having HC interviewed via phone rather than in person.36 However differences were minimized by having the same research coordinators administer the food symptom association questionnaire using a standardized protocol.
Areas where future studies may be better able to address self-perceived food intolerances in children with IBS include the role of self-awareness, the influence of ongoing treatments, and parental influences (e.g., parental IBS status). Double-blind placebo controlled food challenge trials (often employed in food allergy research) may help determine causality of foods and IBS symptoms.37 In addition these trials may be able to be paired with non-invasive mechanistic evaluations (such as breath testing for assessment of colonic fermentation) to understand the underlying pathophysiology.15 Information from future trials will help healthcare providers and families in determining how best to identify (e.g., self-perception vs. formal challenges) and address potential foods that induce symptoms.
There are several strengths of this study. First, all IBS children were defined as having IBS per Rome III criteria. Second, only validated and previously published measures were used, increasing the validity of the study. Third, children themselves completed the measures with the assistance of research coordinators independent of parents rather than having parents fill out the measures as surrogates. Fourth, abdominal pain characteristics were captured prospectively via using the Pain Diary which has been shown to be more reliable than relying on recall alone in children with IBS.38 Finally, children with IBS were recruited from both primary care and tertiary care settings, lending greater generalizability to the results.
CONCLUSIONS
Children with IBS have a high prevalence of self-perceived food intolerances and avoid foods due to this perception. The number of self-perceived food intolerances in children with IBS is weakly associated with a more severe IBS clinical phenotype characterized by increased GI symptoms, increased psychosocial distress, and decreased quality of life. These study findings indicate a need for clinicians to address self-perceived food intolerance in children with IBS. Future studies related to self-perceived food intolerance may lead to identification of successful strategies to address this important and common experience of children with IBS.
Abbreviations
- FODMAP
fermentable oligosaccharides disaccharides monosaccharides and polyols
- GI
gastroenterology
- IBS
irritable bowel syndrome
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Bruno Chumpitazi receives funding from the National Institutes of Health (NIH), QOL Medical Inc. and is a consultant for Mead Johnson Nutrition. Robert Shulman receives funding from the NIH, and Mead-Johnson Inc. and is a consultant for Nutrinia Inc. and Gerson Lehrman Group. The remaining authors do not conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica M. Weidler, Email: baimbrid@bcm.edu.
Diana Y. Lu, Email: diana24@yahoo.com.
Cynthia M. Tsai, Email: ct2@bcm.edu.
Robert J. Shulman, Email: rshulman@bcm.edu.
References
- 1.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McOmber ME, Shulman RJ. Recurrent abdominal pain and irritable bowel syndrome in children. Curr Opin Pediatr. 2007;19(5):581–5. doi: 10.1097/MOP.0b013e3282bf6ddc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AE, Czyzewski DI, Self MM, Shulman RJ. Are child anxiety and somatization associated with pain in pain-related functional gastrointestinal disorders? J Health Psychol. 2013;14(9):921–30. doi: 10.1177/1359105313502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39(3):312–21. doi: 10.1111/apt.12573. [DOI] [PubMed] [Google Scholar]
- 5.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60(5):667–72. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 6.Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 7.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343(8906):1127–30. doi: 10.1016/s0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 8.Zuberbier T, Edenharter G, Worm M, et al. Prevalence of adverse reactions to food in Germany - a population study. Allergy. 2004;59(3):338–45. doi: 10.1046/j.1398-9995.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37(11):1074–83. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–15. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 11.Carlson MJ, Moore CE, Tsai CM, Shulman RJ, Chumpitazi BP. Child and parent perceived food-induced gastrointestinal symptoms and quality of life in children with functional gastrointestinal disorders. J Acad Nutr Diet. 2014;114(3):403–13. doi: 10.1016/j.jand.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haapalahti M, Mykkanen H, Tikkanen S, Kokkonen J. Meal patterns and food use in 10- to 11-year-old Finnish children. Public Health Nutr. 2003;6(4):365–70. doi: 10.1079/PHN2002433. [DOI] [PubMed] [Google Scholar]
- 13.Shulman RJ, Eakin MN, Jarrett M, Czyzewski DI, Zeltzer LK. Characteristics of pain and stooling in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44(2):203–8. doi: 10.1097/01.mpg.0000243437.39710.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chumpitazi BP, Hollister EB, Oezguen N, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5(2):165–75. doi: 10.4161/gmic.27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42(4):418–27. doi: 10.1111/apt.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143(3):223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Self MM, Czyzewski DI, Chumpitazi BP, Weidler EM, Shulman RJ. Subtypes of irritable bowel syndrome in children and adolescents. Clin Gastroenterol Hepatol. 2014;12(9):1468–73. doi: 10.1016/j.cgh.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LS, Beck JE, Garber J, Lambert W. Children's Somatization Inventory: psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34(4):430–40. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber J, Walker LS, Zeman J. Somatization symptoms in a community sample of children and adolescents: Further validation of the Children's Somatization Inventory. Psychol Assess. 1991;3:588–95. [Google Scholar]
- 20.Williams AE, Czyzewski DI, Self MM, Shulman RJ. Are child anxiety and somatization associated with pain in pain-related functional gastrointestinal disorders? J Health Psychol. 2015;20(4):369–79. doi: 10.1177/1359105313502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. Circle Pines, MN: American Guidance Service, Inc; 2004. [Google Scholar]
- 22.Schurman JV, Danda CE, Friesen CA, Hyman PE, Simon SD, Cocjin JT. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings. 2008;15(3):241–51. doi: 10.1007/s10880-008-9120-0. [DOI] [PubMed] [Google Scholar]
- 23.Robins PM, Schoff KM, Glutting JJ, et al. Discriminative validity of the Behavioral Assessment System for Children-parent rating scales in children with recurrent abdominal pain and matched controls. Psychol Schools. 2003;40(2):145–54. [Google Scholar]
- 24.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Claar RL, Walker LS, Smith CA. Functional disability in adolescents and young adults with symptoms of irritable bowel disease: The role of academic, social, and athletic competence. J Pediatr Psychol. 1999;24:271–80. doi: 10.1093/jpepsy/24.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rippel SW, Acra S, Correa H, et al. Pediatric patients with dyspepsia have chronic symptoms, anxiety, and lower quality of life as adolescents and adults. Gastroenterology. 2012;142(4):754–61. doi: 10.1053/j.gastro.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varni JW, Lane MM, Burwinkle TM, et al. Health-related quality of life in pediatric patients with irritable bowel syndrome: a comparative analysis. J Dev Behav Pediatr. 2006;27(6):451–8. doi: 10.1097/00004703-200612000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40(1):141–62. doi: 10.1016/j.gtc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Castilloux J, Noble A, Faure C. Is visceral hypersensitivity correlated with symptom severity in children with functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr. 2008;46(3):272–8. doi: 10.1097/MPG.0b013e31814b91e7. [DOI] [PubMed] [Google Scholar]
- 31.Jarrett M, Heitkemper M, Czyzewski D, et al. Autonomic nervous system function in young children with functional abdominal pain or irritable bowel syndrome. J Pain. 2012;13(5):477–84. doi: 10.1016/j.jpain.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med. 2009;6(Suppl 2):152–67. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connelly M, Bickel J. An electronic daily diary process study of stress and health behavior triggers of primary headaches in children. J Pediatr Psychol. 2011;36(8):852–62. doi: 10.1093/jpepsy/jsr017. [DOI] [PubMed] [Google Scholar]
- 34.Czyzewski DI, Eakin MN, Lane MM, et al. Recurrent Abdominal Pain in Primary and Tertiary Care: Differences and Similarities. Child Health Care. 2007;36(2):137–53. doi: 10.1080/02739610701334970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99(12):2442–51. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 36.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27(3):281–91. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
- 37.van der Valk JP, Gerth van Wijk R, Dubois AE, et al. Multicentre Double-Blind Placebo-Controlled Food Challenge Study in Children Sensitised to Cashew Nut. PLoS One. 2016;11(3):e0151055. doi: 10.1371/journal.pone.0151055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chogle A, Sztainberg M, Bass L, et al. Accuracy of pain recall in children. J Pediatr Gastroenterol Nutr. 2012;55(3):288–91. doi: 10.1097/MPG.0b013e31824cf08a. [DOI] [PubMed] [Google Scholar]