Abstract
Background
Exposure to air pollution has been associated with cardiorespiratory morbidity and mortality. However, the chemical constituents and pollution sources underlying these associations remain unclear.
Method
We conducted a cohort panel study involving 97 elderly subjects living in the Los Angeles metropolitan area. Airway and circulating biomarkers of oxidative stress and inflammation were measured weekly over 12 weeks and included, exhaled breath condensate malondialdehyde (EBC MDA), fractional exhaled nitric oxide (FeNO), plasma oxidized low-density lipoprotein (oxLDL), and plasma interleukin-6 (IL-6). Exposures included 7-day personal nitrogen oxides (NOX), daily criteria-pollutant data, five-day average particulate matter (PM) measured in three size-fractions and characterized by chemical components including transition metals, and in vitro PM oxidative potential (dithiothreitol and macrophage reactive oxygen species). Associations between biomarkers and pollutants were assessed using linear mixed effects regression models.
Results
We found significant positive associations of airway oxidative stress and inflammation with traffic-related air pollutants, ultrafine particles and transition metals. Positive but nonsignificant associations were observed with PM oxidative potential. The strongest associations were observed among PM variables in the ultrafine range (PM <0.18 μm). It was estimated that an interquartile increase in 5-day average ultrafine polycyclic aromatic hydrocarbons was associated with a 6.3% (95% CI: 1.1%, 11.6%) increase in EBC MDA and 6.7% (95% CI: 3.4%, 10.2%) increase in FeNO. In addition, positive but nonsignificant associations were observed between oxLDL and traffic-related pollutants, ultrafine particles and transition metals while plasma IL-6 was positively associated with 1-day average traffic-related pollutants.
Conclusion
Our results suggest that exposure to pollutants with high oxidative potential (traffic-related pollutants, ultrafine particles, and transition metals) may lead to increased airway oxidative stress and inflammation in elderly adults. This observation was less clear with circulating biomarkers.
Keywords: Air pollution, Inflammation, Oxidative stress, Particulate matter components, Biomarkers
1. Introduction
Epidemiological studies have shown positive associations between short-term exposures to air pollutants and cardiopulmonary morbidity and mortality as reviewed by Franklin et al. (Franklin et al. 2015). The particular mechanisms linking air pollution to acute respiratory and cardiovascular events are not completely understood. Particulate matter (PM) air pollution consists of discrete particles that range from coarse-sized particles 2.5–10 μm (PM2.5–10), to accumulation mode particles 0.1–2.5 μm (PM0.1–2.5), and finally to ultrafine particles < 0.1 μm in diameter (PM0.1). Particle size is an important determinant of deposition in the respiratory tract, and an indicator of chemical composition and source (Delfino et al. 2005; Sioutas et al. 2005). Smaller particles have a higher pulmonary deposition fraction and penetrate deeper in the lung (Lippmann 1977). The large surface area of ultrafine particles also carries high concentrations of pro-oxidant chemical components, such as polycyclic aromatic hydrocarbons (PAHs) and transition metals, each of which has been shown to induce oxidative stress responses (Li et al. 2003) and can translocate from pulmonary sites to the circulatory system (Elder et al. 2006). Experimental studies have provided evidence that ultrafine particles can induce the greatest amount of oxidative stress and inflammation per unit of PM mass (Li et al. 2002; Cho et al. 2005). This may in-turn impact physiologic responses that ultimately increase the risk of acute cardiorespiratory morbidity (Weichenthal 2012). In epidemiological studies, some have shown that ultrafine particulate matter air pollution is more strongly associated with adverse health effects when compared to larger PM diameters (Delfino et al. 2009; Franck et al. 2011) while other studies have shown PM2.5 to have stronger or as strong associations as ultrafine PM (Ruckerl et al. 2014; Lanzinger et al. 2016). The inconsistent epidemiological evidence may be due to the fact that the sources and components of pollutants vary from study to study. In urban cities, such as Los Angeles, traffic-related pollutants are the main source for PM mass concentrations (Pant et al. 2013). Those traffic-related pollutants have been shown to contain redox active chemicals that are able to generate reactive oxygen species (ROS) responsible for increases in oxidative stress (Ayres et al. 2008). By quantifying the inherent capacity of PM to oxidize target molecules, oxidative potential is proposed to be a more attractive and biologically more relevant exposure metric than PM mass (Borm et al. 2007). Therefore, the direct measurement of PM oxidative potential may show stronger or more precise associations than either ultrafine PM or traffic-related air pollutants.
Most previous epidemiologic studies have used ambient air monitoring data from central air monitoring stations, and focused primarily on the U.S. Environmental Protection Agency (EPA)’s criteria air pollutants, namely, PM2.5, PM10, ozone (O3), nitrogen dioxide (NO2) and carbon monoxide (CO). Several research groups including ours have been investigating the associations between biomarkers of effect and size-fractionated and/or chemically-characterized particulate air pollutants (Delfino et al. 2008; Delfino et al. 2009; Delfino et al. 2010a; Chen et al. 2015; Wu et al. 2015). However, there is still a need to systematically explore oxidative stress and inflammatory biomarkers in both pulmonary and circulatory systems in relation to chemically-characterized PM.
To better understand the underlying mechanism of the association between cardiorespiratory morbidity and short-term exposure to air pollution, we conducted a panel study to investigate the potential roles of air pollutant components and pollution source tracers on both airway and systemic biomarkers of oxidative stress and inflammation in an elderly cohort. The focus on an elderly cohort is motivated by findings that reveal that older subjects tend to be more susceptible to air pollution-induced health effects, and hence represent a high risk population (Brook et al. 2010). We hypothesized that chemicals from fossil fuel combustion, related ultrafine particles, and PM oxidative potential would have stronger adverse health effects than other air pollutant variables. To our knowledge, this is the first study to relate biomarkers of airway and systemic oxidative stress responses to exposure markers of PM oxidative potential in an elderly population.
2. Methods
2.1. Study design
To investigate short-term health effects of exposures to air pollution, we conducted a cohort panel study of repeated measures of outcomes and exposures for 97 elderly non-smoking adults (age ≥65) living in two Los Angeles California metropolitan areas (downtown Los Angeles and Anaheim, CA) between 2012 and 2014. The repeated measures design can effectively allow for the control of between and within-subject variability in regression models. A study design flowchart can be found in Appendix, Fig. A.1. Briefly, two groups of subjects in each area were followed alternatively for up to 12 weeks in two discrete 6-week periods in order to incorporate seasonal differences in air pollution levels in the Los Angeles metropolitan area (Daher et al. 2013; Hasheminassab et al. 2014b). Specifically, one six-week period took place during the warm season (July–October) and the other took place the cool season (November-February). Weekly clinical visits for study participants were scheduled on the same day of week and at the same time of day in order to minimize potential biases induced by weekly and circadian variation (94% of the subjects arrived to clinic with a variability of ± 2 hours).
Subjects were excluded from study participation if they lived or were employed outside of the monitored community (18 km radius), smoked within the last 12 months, abused drugs or alcohol, or reported exposure to environmental tobacco smoke at home or on a regular basis at other locations. Additional health criteria for exclusion included the presence of psychiatric disorders or dementia that would prevent the subject from full participation, dialysis treatment or renal failure, daily oral corticosteroids, and active cancer. Observations following the previous 7 days when subjects reported any acute infection (7.62%) were excluded a priori given the known major impact of infections on systemic and respiratory inflammation.
2.2. Baseline questionnaires
Background questionnaires collected at the beginning of the study included information on socioeconomic status, medical history, current medication use, history of smoking, and environmental exposure profile. In addition, a baseline fasting blood sample was taken to obtain plasma lipid profiles and glucose levels.
2.3. Markers of airway oxidative stress and inflammation
Exhaled breath condensate (EBC) sampling and malondialdehyde (MDA) analysis
We collected EBC samples during normal breathing with the RTube™ Collection System (Respiratory Research, Inc., Austin, TX) using standard procedures recommended by the American Thoracic Society and European Respiratory Society (Holvoet et al. 2003). Room air was inhaled through a one-way valve and the exhaled air was directed into a collection chamber (solid aluminum tube pre-chilled on dry ice) where vapors, aerosols and moisture in the breath condense. EBC was then collected from the walls of the condenser. The samples were transported to our laboratory on dry ice and then frozen at −80°C until analysis. We analyzed EBC samples for MDA using HPLC analysis by modifying the Laerstad et al. 2002 protocol (Larstad et al. 2002) (details are given in the online supplement). The estimated limit of quantification (LOQ) for MDA in EBC samples is 3 nM. All values < LOQ were set to 1.5 nmol. We excluded MDA results if the concentration was greater than the upper limit of quantification (12.5 nM) and coefficient of variation (CV) > 25% (occurring in 1.16% of samples).
Fractional concentration of exhaled nitric oxide (FeNO) measurement
We used the NIOX MINO (Aerocrine Inc, New Providence, NJ) to noninvasively measure FeNO. Based on previous research (ATS/ERS 2005), a questionnaire was administered to ascertain the following information prior to the FeNO measurement: (1) did the subject have a meal (breakfast if in the morning, lunch if in the afternoon) or exercise within an hour before the test; (2) was the subject’s previous meal high in fat or sugar. An affirmative answer to any of these questions was tested as independent variables for their influence on FeNO and on air pollutant regression parameters. An NO scrubbing filter was used at the air intake to control for indoor NO.
2.4. Markers of systemic oxidative stress and inflammation
Oxidized low-density lipoprotein (oxLDL) and Interleukin-6 (IL-6)
At each follow-up visit, venous peripheral blood samples were drawn by a trained phlebotomist using anti-coagulant PPT BD Vacutainer® tubes and immediately centrifuged to separate plasma and transported at −20 ºC before storage in our laboratory at −80 ºC until analysis. The blood samples on one week (n=21) were lost due to improper storage procedures. The systemic oxidative stress biomarker oxLDL was measured in plasma by standardized ELISA using monoclonal antibodies directed against a neo-epitope in the aldehyde-substituted apoB-100 moiety of LDL (oxLDL-4E6 assay, Mercodia AB, Sweden). The lower limit of detection of oxLDL was 28.8 U/L and the results were considered invalid if the estimated CV was > 25% (occurring in 0.34% of samples). Systemic inflammation biomarker IL-6 was measured in plasma using 96-well immunoassay kits (Quantikine High Sensitivity, Minneapolis, MN). The lower and upper limit of detection of IL-6 were 0.156 pg/ml and 10 pg/ml, respectively. IL-6 values were considered invalid if the estimated CV was > 25%. This occurred in 15.09% of samples.
2.5. Air pollution and meteorology
Targeted study areas included an approximate 18 km radius around the central air monitoring stations. Ambient hourly concentrations of PM2.5, CO, nitrogen oxides (NOx, NO+ NO2) and O3 were ascertained from the South Coast Air Quality Management District (SCAQMD) monitoring stations (Appendix, Fig. A.2.). Measurements were based on Federal Reference Methods of the US EPA, including hourly PM2.5 from Beta attenuation monitors (BAM PM2.5). Hourly PM2.5 black carbon (BC, Aethelometer model AE22, Magee Scientific, Berkeley, CA) were measured at the University of Southern California (USC) monitoring sites near downtown Los Angeles and in Anaheim. Daily exposure data were calculated from the hourly data at the central air monitoring stations when ≥75% of daily data were available. Missing rates for daily PM2.5, CO, NOx, O3, and BC were 7.38%, 6.83%, 25.96%, 9.02%, and 3.28%, respectively. We used coefficients derived from regression modeling where measured exposures from the missing station were predicted by the exposure data from a neighboring station within the study area. All imputation linear models had R2 > 0.8. If there were no other available data from nearby stations in the study area, then linear interpolation was used to impute missing data. We had a maximum of three continuous days of missing (except for BC in Los Angeles had one 8-day period that was missing). Less than 4% of the exposure data were linearly interpolated (Detailed information was presented in Appendix, Table A.1.). Ambient air pollutant concentrations for 1-day, 3-day, 5-day and 7-day averages preceding clinic follow-ups were calculated from the daily data. We preselected these averaging times to represent effect estimation for up to 7 days of exposure while avoiding an excessive presentation of data using all seven daily averaging times.
We also collected 5-day integrated concentrations of PM0.18, PM0.18–2.5 and PM2.5–10 (MOUDI, model 100-1, MSP, Inc., Minneapolis, MN) at the University of Southern California (USC) monitoring sites near downtown Los Angeles and in Anaheim. The three particle size ranges represent the ultrafine mode (PM0.18), the accumulation mode (PM0.18–2.5), and the coarse mode (PM2.5–10). Although the typical size range for ultrafine particles have been stated in studies to be < 0.1 μm, the reported upper size cut point for particles that dominate the particle number concentration (which may be considered in the ultrafine mode) has varied temporally and spatially from 0.1 to 0.2 μm (Sioutas et al. 2005). We used five days of continuous particle collection because it was necessary to obtain a sufficient amount of PM mass loading for the chemical and oxidative potential assays described below and this duration had been previously demonstrated to be associated with cardiovascular and biomarker outcomes (such as IL-6) in our previous studies (Delfino et al. 2008; Delfino et al. 2010b; Delfino et al. 2010c). The USC monitoring site for Los Angeles was approximately 3 km southwest of the SCAQMD central air monitoring station where criteria air pollutants were measured, and was at the same location as the SCAQMD station in Anaheim (Appendix, Fig. A.2.). Carbonaceous species were measured in the ultrafine and accumulation mode fractions, and we included as exposure variables the sum of PAHs, the sum of hopanes (Appendix, Table A.2.), and elemental carbon (EC) as markers of various fossil fuel combustion products. Organic carbon (OC) was measured and is representative of both primary and secondary organic carbon sources. We also used the sum of organic (n-alkanoic) acids as a tracer of secondary organic aerosols (Rogge et al. 1993). For elemental species, we included the sum of available transition metals (V, Cr, Mn, Ni, Cu, and Fe) in all three size-fractions because they are known to participate in catalyzing Fenton’s reaction that could generate oxidative stress (Ghio et al. 2012). Particle extracts were analyzed for oxidative potential [alveolar macrophage reactive oxygen species (ROS) assay and dithiothreitol (DTT) activity] as described below.
Following gravimetric measurements using a precision microbalance (Mettler Toledo Inc., Columbus, OH, USA) (± 0.001 mg), filters were sectioned and particles extracted for chemical characterization. EC and OC were quantified from a 1.5 cm2 punch taken from the quartz/aluminum filter according to National Institute for Occupational Safety and Health Thermal Optical Transmission method (Schauer et al. 2003). PAHs and hopanes were analyzed using gas chromatography mass spectrometry (Stone et al. 2008). Total elemental composition of three size fractions was measured by digestion of a section of the Teflon filter-collected PM and subsequently analyzed by high-resolution sector-field inductively-coupled plasma mass spectrometry (SF-ICPMS).
The Ogawa passive badge sampler (Ogawa & Co. USA, Inc. Pompano Beach, FL) was used to collect seven-day average personal exposures to NOX. Subjects were instructed to wear the sampler clipped outside of clothing and placed near the bedside at night. Personal NOX was collected on cellulose fiber filters and concentrations were determined by a spectrophotometer at a wavelength of 545 nm following the manufacturer’s instructions (Ogawa & Company 2006).
Meteorological data including temperature and relative humidity were obtained from central monitorning stations operated by SCAQMD. We calculated hourly heat index, the combined effects of temperature and relative humidity, using methods developed in a previous study (Anderson et al. 2013). Heat index was chosen because it is a better predictor of skin temperature, which is the main trigger of the body’s cooling and warming mechanisms and thus is considered to be more closely related to health effects than temperature and/or relative humidity.
2.6. Measures of PM oxidative potential
Alveolar macrophage ROS assay represents the biotic oxidative potential of particle mixtures. Details of these methods are described elsewhere (Landreman et al. 2008). Briefly, biotic ROS production was quantified by extracting the 5-day composites of the three different size-fractionated PM filters with 1.00 ml of Milli-Q water. Rat alveolar macrophage cells (NR8383, American Type Culture Collection) in a 96-well plate were then exposed to PM extracts of both unfiltered (total ROS) and filtered (water-soluble ROS, 0.22 μm polypropylene syringe filter). The fluorescent probe DCFH-DA (2′,7′-dichloroluorescein diacetate) was used and the fluorescence intensity was measured using a plate reader, thus representing the cell-based oxidative generating capacity of PM. A model of microbial particles, un-opsonized Zymosan (a β-1,3-polysachharide of D-glucose) served as a positive ROS control as it binds to Toll-like receptor-2 on macrophage cells and then activates a strong respiratory burst and ROS production. ROS results are reported in μg Zymosan equivalent units per m3 air.
DTT activity represents the capacity of the PM extract to generate abiotic chemically-produced ROS (electron transfer from DTT to oxygen). DTT activity was quantified using a well-established method (Cho et al. 2005) on extracts of 5-day composites of the three different size-fractionated PM from quartz filters.
2.7. Statistical Analysis
Data were analyzed using a linear mixed effect model to account for the clustering of longitudinal repeated measurements taken on each subject. All models include a random subject intercept to account for the correlation of repeated measures. We selected an autoregressive moving average (ARMA) (1,1) covariance structure based on Akaike’s information criterion (AIC) for the covariance matrix to model the correlation between repeated measures in each subject. The magnitude of the effect was expressed relative to an interquartile range (25th to 75th percentile) difference in each pollutant concentration to compare associations across different pollutants.
Covariates
Time-invariant subject characteristics were controlled by the study design and the specified repeated measures model, and hence were not included as adjustment covariates. However, oxLDL levels were found to be correlated to LDL level. In order to test for the independent effect of oxLDL, we controlled for LDL level in the model of oxLDL. An a priori adjustment variable was heat index with the same lag average as the pollutant and was included in the model as a covariate to adjust for the potential confounding effect of weather. Time-dependent potential confounders were tested in the models, and were included in the model if they improved model fit. Days of gas stove use per week were significantly associated with increased personal NOX and were adjusted in the models of personal NOx a priori because the exposure of interest was outdoor fossil fuel sources of NOX. To take into account seasonal variations in blood markers, long-term temporal trend (counts of the days during the study period) was tested by including cubic splines using differing numbers and locations of knots. However, this did not significantly change the parameter estimates of association or improve the model fit and so was not included in our final models. In exploratory models, exercise, food intake, sugar and fat intake prior to the clinic visit did not change the estimations or improve the model fit for FeNO and thus were not included in any models. All biomarker outcome variables were log-transformed before the analyses to fulfill the model assumption of residual normality. The results of biomarkers are given as the percent change in geometric mean.
Influential observations
The impact of in uential observations was assessed using the Cook’s D statistic and standardized residual diagnostics, at both the individual observations level and clustered subjects level (Cook 1977). One influential subject (out of 88 subjects) was identified for EBC MDA and after excluding that subject the positive associations became stronger and more significant for BC, NOx, CO, and ultrafine mass and components (Appendix, Table A.3). Three influential observations out of 719 observations for IL-6 were detected by restricted likelihood distance >1 or Cook’s D value> 4/n (n=number of observations) (Cook et al. 1982; Fox et al. 1990). The estimated of regression coefficients for covariates of interest were generally unchanged but the confidence interval (CI) became smaller after excluding these three influential observations (Appendix, Table A.4).
Effect modification
In exploratory analyses we considered whether the following variables may be potential effect modifiers of the associations between biomarkers and air pollution: Age (≤75 years, > 75 years), sex (male, female), obesity (body mass index: BMI ≥ 30 kg/m2), history of hypertension (yes, no), intake of anti-hypertensive medication (yes, no), diabetes mellitus (yes, no), hypercholesterolemia by history (yes, no), total cholesterol/HDL ratio (≥ 3.5, < 3.5) cardiovascular risk score (Goff et al. 2014) (≥ 0.2, < 0.2), intake of statin (yes, no), study area (Los Angeles, Anaheim), and former smokers (yes, no). Additionally, for airway biomarkers, we assessed effect modification by asthma and/or chronic obstructive pulmonary disease (COPD). Evidence of a significant interaction was considered at a nominal product term p-value<0.1 to avoid increased type II errors in these hypothesis-generating analyses.
Sensitivity analysis
Several sensitivity analyses were conducted. First, to investigate potential exposure error, we restricted the analysis to the subjects who lived within the 90th percentile of subjects’ residential distance to the stations (11.3 km for the SCAQMD monitoring stations and 13.1 km for the USC monitoring sites). Second, we limited the analysis to measured ambient exposures, rather than exposures including imputed data from the next nearest air monitoring station or interpolated from the adjacent day’s data. Third, we excluded days with extreme heat index (< 52.5 and > 75.04, 10th percentile and 90th percentile of the heat index during the study period, respectively) because the decreased ventilation at subjects’ residential buildings during those days may lead to increased exposure error for the representation of personal exposures by ambient measurements. Lastly, in order to test for the independent effects of primary and photochemically-produced air pollutants, we applied two-pollutant models by including a primary air pollutant (BC, NOx and PAHs) and O3, which showed unexpected inverse associations with some biomarkers. Note that this two-pollutant analysis is exploratory since O3 and the primary pollutants were inversely correlated (Spearman R ≥ 0.6). Interactions are also of interest because the highly oxidizing capability of O3 give it the potential to react with primary pollutant components to produce other pro-oxidant components that can adversely affect the airways. In order to address this in an exploratory analysis we tested interactions between the same primary air pollutants and O3 on the airway biomarkers (FeNO and EBC MDA).
All statistical analyses were performed using R version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria) or SAS 9.3 software (SAS, Cary, NC).
3. Results
3.1. Descriptive data
One hundred and ninety-one subjects (191) were recruited and 87 subjects dropped out or became ineligible (cancer, smoker, or moved out of the study area) before or soon after the start of follow-up. We additionally required a priori that subjects have at least 4 repeated outcome measures to provide sufficient within-subject exposure-response data. We did a sensitivity analysis by including subjects with at least 2 repeated measurements and no fundamental changes in associations were observed. The median number of repeated measures for FeNO, EBC MDA, IL-6, and oxLDL are 11, 8, 8, and 10, respectively. Those subjects excluded from regression analyses due to lack of sufficient repeated outcomes were 16 subjects for EBC MDA, 8 subjects for FeNO, 11 subjects for IL-6, and 11 subjects for oxLDL. Among these listed above, 7 subjects were completely excluded for all outcomes leaving 97 with at least some outcome measurements. Among 97 subjects, 48 were from the Los Angeles area and 49 were from the Anaheim area. Descriptive characteristics of 97 subjects and descriptive data for the biomarker measurements are shown in Table 1 and the descriptive data by region are shown in Appendix Table A.5. The subjects were between 65 and 96 years old (mean, 74.8 years), with two-thirds of the subjects being female. Among the 97 subjects, there are 59 non-Hispanic Whites, 9 Hispanics, 11 African Americans, 9 Asians and 5 other race/ethnicities. Depending on the biomarker outcomes, the numbers of observations without any report of infection in the previous week ranged from 643 to 901 (Table 1). Spearman correlations showed no correlations (−0.04 to 0.11) among these 4 biomarkers. Additionally, we assessed the relationships among 4 biomarker outcomes in linear mixed models. No significant associations were observed (data not shown).
Table 1.
Characteristics of 97 subjects (Max n= 946)
| Characteristic | Mean ± SD or N (%) |
|---|---|
| Age (years) ± SD | 74.8 ± 7.5 |
| BMI (kg/m2) ± SD | 28.1 ± 5.6 |
| Overweight (25–29.9) | 34 (35.1) |
| Obesity (≥30) | 31 (32.0) |
| Male | 25 (25.8) |
| Former smoker | 42 (43.3) |
| Cardiovascular Disease | 16 (16.5) |
| Hypertension | 66 (68.0) |
| Hypercholesterolemia (by history) | 52 (53.6) |
| Lipid Profile | |
| Total cholesterol | 188.7 ± 44.7 |
| > 200 mg/dL | 47 (48.5) |
| LDL-C | 116.4 ± 36.7 |
| > 130 mg/dL | 30 (30.9) |
| HDL-C | 55.2 ± 18.5 |
| Female <50 mg/dL, male <40 mg/dL | 32 (33.0) |
| Adult-onset Diabetes Mellitus | 22 (22.7) |
| COPD | 8 (8.3) |
| Asthma | 12 (12.4) |
| Medications | |
| Anti-hypertensive medication | 63 (65.0) |
| HMG-CoA reductase inhibitors (statins) | 45 (46.4) |
| Biomarkers | |
| IL-6 (pg/mL; n=716) | 2.4 ± 1.7 |
| oxLDL (U/L; n=841) | 59.2 ± 20.2 |
| FeNO (ppb; n=901) | 26.6 ± 13.4 |
| EBC MDA (nmol; n=643) | 8.4 ± 5.1 |
Abbreviations: BMI, body mass index; COPD: chronic obstructive pulmonary disease; EBC: exhaled breath condensate; MDA: malondialdehyde.
Descriptive statistics of the exposures are shown in Table 2 and Appendix Table A.6 (by region). Data for specific transition metals are presented in the Appendix Table A.7. For 24-hour ambient pollutants, except for NOX (missing rate = 28%), all pollutants had less than 10% of data that were missing. The observations of personal NOX had 13% missing due to subject noncompliance, incorrect sampling times, or measurement errors where NOX concentration was less than NO2. For size-fractionated PM components, up to 4 of the planned 48 weeks were missing due to equipment failure or power outages at the Anaheim site. Ninety-seven percent of the study period had average 24-hour PM2.5 below the National Air Quality Standard recommended upper limit of 35 μg/m3. The concentration of total PAHs, hopanes, OC/EC, DTT and ROS were highest in PM0.18–2.5. For total transition metals (the sum of V, Cr, Mn, Cu and Fe), the highest mass concentrations were observed in PM2.5–10.
Table 2.
Descriptive statistics of air pollutant measurements.
| Pollutant | N (Missing) | Mean (SD) | IQR | Min | Max |
|---|---|---|---|---|---|
| Personal Exposures (7-day average) | |||||
| NOx (ppb) | 820 (126) | 29.3 (21.8) | 21.3 | 2.2 | 160.1 |
| Ambient Exposures (24-hr Averages) | |||||
| Black Carbon (μg/m3) | 320 (16) | 1.3 (0.9) | 1.1 | 0.2 | 5.2 |
| PM2.5 (μg/m3) | 309 (27) | 17.6 (7.7) | 9.6 | 3.8 | 49.2 |
| CO (ppm) | 311 (25) | 0.5 (0.2) | 0.3 | 0.1 | 1.6 |
| Ozone (ppb) | 303 (33) | 23.2 (9.3) | 12.7 | 1.3 | 48.5 |
| NOx (ppb) | 241 (95) | 35.4 (27.7) | 31.0 | 3.6 | 175.6 |
| Heat Index (F°) | 366 (0) | 66.1 (8.4) | 12.6 | 42.6 | 84.3 |
| Size-fractionated PM (5-day average) | |||||
| Mass (μg/m3) | |||||
| PM0.18 | 45 (3) | 2.4 (0.9) | 1.1 | 1.2 | 4.8 |
| PM0.18–2.5 | 45 (3) | 8.6 (3.2) | 4.0 | 4.4 | 19.4 |
| PM2.5–10 | 44 (4) | 14.9 (6.8) | 7.8 | 4.5 | 35.4 |
| Total PAH (ng/m3) | |||||
| PM0.18 | 45 (3) | 0.3 (0.2) | 0.3 | 0.0 | 0.8 |
| PM0.18–2.5 | 45 (3) | 0.5 (0.5) | 0.5 | 0 | 2.0 |
| Hopanes (ng/m3) | |||||
| PM0.18 | 45 (3) | 0.2 (0.1) | 0.2 | 0.0 | 0.4 |
| PM0.18–2.5 | 45 (3) | 0.2 (0.2) | 0.2 | 0 | 0.8 |
| Organic acid (μg/m3) | |||||
| PM0.18 | 45 (3) | 26.2 (10.8) | 14.5 | 9.6 | 58.0 |
| PM0.18–2.5 | 45 (3) | 18.1 (14.0) | 16.8 | 0.6 | 61.4 |
| OC (μg/m3) | |||||
| PM0.18 | 45 (3) | 1.2 (0.4) | 0.4 | 0.5 | 2.4 |
| PM0.18–2.5 | 45 (3) | 1.5 (0.8) | 1.1 | 0.5 | 3.5 |
| PM2.5–10 | 45 (3) | 0.7 (0.2) | 0.3 | 0.3 | 1.1 |
| EC (μg/m3) | |||||
| PM0.18 | 45 (3) | 0.3 (0.1) | 0.2 | 0.1 | 0.6 |
| PM0.18–2.5 | 45 (3) | 0.2 (0.1) | 0.2 | 0.0 | 0.5 |
| PM2.5–10 | 45 (3) | 0.0 (0.0) | 0.1 | 0 | 0.09 |
| Total ROS (μg Zym/m3)a | |||||
| PM0.18 | 45 (3) | 19.8 (12.2) | 16.6 | 2.1 | 53.2 |
| PM0.18–2.5 | 45 (3) | 136.7 (94.6) | 125.9 | 26.2 | 394.0 |
| PM2.5–10 | 44 (4) | 60.2 (49.7) | 76.5 | 8.7 | 181.0 |
| Dithiothreitol (nmol/min/m3) | |||||
| PM0.18 | 45 (3) | 0.1 (0.1) | 0.1 | 0.0 | 0.2 |
| PM0.18–2.5 | 45 (3) | 0.3 (0.1) | 0.1 | 0.1 | 0.5 |
| PM2.5–10 | 44 (4) | 0.2 (0.1) | 0.1 | 0.1 | 0.5 |
| GPx-10.18 (% activity/100 μg) | 45 (3) | 30.5 (13.5) | 8.8 | 10.9 | 100.0 |
| Transition metalsb (ng/m3) | |||||
| PM0.18 | 45 (3) | 49.2 (40.4) | 56.2 | 7.2 | 153.4 |
| PM0.18–2.5 | 45 (3) | 72.0 (52.0) | 46.0 | 12.9 | 237.1 |
| PM2.5–10 | 44 (4) | 396.6 (171.2) | 220.0 | 112.0 | 914.6 |
Abbreviations: IQR: interquartile range; CO: carbon monoxide; GPx: glutathione peroxidase; PM: particulate matter; PAHs: polycyclic aromatic hydrocarbons; OC: organic carbon; EC: elemental carbon; ROS: Reactive oxygen species;
Zym: μg Zymosan equivalent units;
Total sum of transition metals include V, Cr, Mn, Ni, Cu and Fe.
Correlations among traffic-related particulate air pollutants (BC, total PAHs in PM0.18 and PM0.18–2.5, EC in PM0.18) and between the traffic-related pollutants and total transition metals in PM0.18 and PM0.18–2.5 were strongly positive (R ≥ 0.64, Table 3). These pollutants were also positively correlated with OC, which represents a mixture of primary and secondary organic aerosols. OC was positively correlated with organic acids (R = 0.52 and 0.70 for PM0.18 and PM0.18–2.5, respectively), which are tracers of secondary organic aerosols (Saffari et al. 2015). Traffic-related air pollutants were positively correlated with DTT, especially in PM0.18, but not with ROS, and negatively correlated with O3 and heat index (except EC0.18–2.5).
Table 3.
Spearman correlation matrix of selected pollutants.a
| PM2.5 | BC | T PAHs0.18 | T PAHs0.18–2.5 | OC0.18 | OC0.18–2.5 | EC0.18 | EC0.18–2.5 | T ROS0.18 | T ROS0.18–2.5 | DTT0.18 | DTT0.18–2.5 | GPx0.18 | T metals0.18 | T metal0.18–2.5 | heat index | Personal NOXa | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O3 | 0.06 | −0.71 | −0.84 | −0.81 | −0.57 | −0.71 | −0.60 | −0.13 | 0.12 | −0.07 | −0.55 | −0.38 | −0.30 | −0.61 | −0.52 | 0.67 | −0.43 |
| PM2.5 | 0.02 | −0.12 | −0.07 | −0.17 | 0.15 | 0.08 | 0.19 | 0.05 | 0.45 | −0.17 | 0.20 | −0.18 | −0.27 | −0.03 | 0.17 | 0.16 | |
| BC | 0.92 | 0.92 | 0.84 | 0.89 | 0.82 | 0.47 | 0.18 | 0.24 | 0.79 | 0.62 | 0.18 | 0.81 | 0.72 | −0.55 | 0.47 | ||
| T PAHs0.18 | 0.94 | 0.80 | 0.87 | 0.75 | 0.37 | 0.10 | 0.18 | 0.76 | 0.52 | 0.25 | 0.81 | 0.68 | −0.70 | - | |||
| T PAHs0.18–2.5 | 0.73 | 0.85 | 0.64 | 0.32 | −0.02 | 0.09 | 0.68 | 0.49 | 0.29 | 0.76 | 0.63 | −0.68 | - | ||||
| OC0.18 | 0.76 | 0.83 | 0.46 | 0.45 | 0.26 | 0.86 | 0.61 | 0.19 | 0.80 | 0.70 | −0.43 | - | |||||
| OC0.18–2.5 | 0.76 | 0.47 | 0.12 | 0.31 | 0.63 | 0.62 | 0.28 | 0.64 | 0.63 | −0.53 | - | ||||||
| EC0.18 | 0.58 | 0.42 | 0.39 | 0.77 | 0.57 | 0.02 | 0.68 | 0.66 | −0.27 | - | |||||||
| EC0.18–2.5 | 0.34 | 0.41 | 0.50 | 0.25 | −0.21 | 0.32 | 0.27 | 0.09 | - | ||||||||
| T ROS0.18 | 0.30 | 0.41 | 0.17 | −0.08 | 0.25 | 0.18 | 0.14 | - | |||||||||
| T ROS0.18–2.5 | 0.14 | 0.37 | −0.07 | 0.13 | 0.30 | 0.12 | - | ||||||||||
| DTT0.18 | 0.44 | 0.10 | 0.81 | 0.68 | −0.39 | - | |||||||||||
| DTT0.18–2.5 | 0.20 | 0.48 | 0.57 | −0.39 | - | ||||||||||||
| GPx0.18 | 0.24 | 0.18 | −0.44 | - | |||||||||||||
| T metalsb0.18 | 0.80 | −0.54 | - | ||||||||||||||
| T metals0.18–2.5 | −0.37 | - | |||||||||||||||
| heat index | −0.25 |
Abbreviations: DTT: dithiothreitol; EC: elemental carbon; OC: organic carbon; PM: particulate matter; ROS: reactive oxygen species; T: total;
Bold numbers indicate correlation values ≥ 0.60 and P < 0.05;
Correlations for personal NOx were calculated with 7-day average of ambient pollutants and heat index;
Total sum of transition metals include V, Cr, Mn, Ni, Cu and Fe.
3.2. Airway biomarkers and air pollutant associations
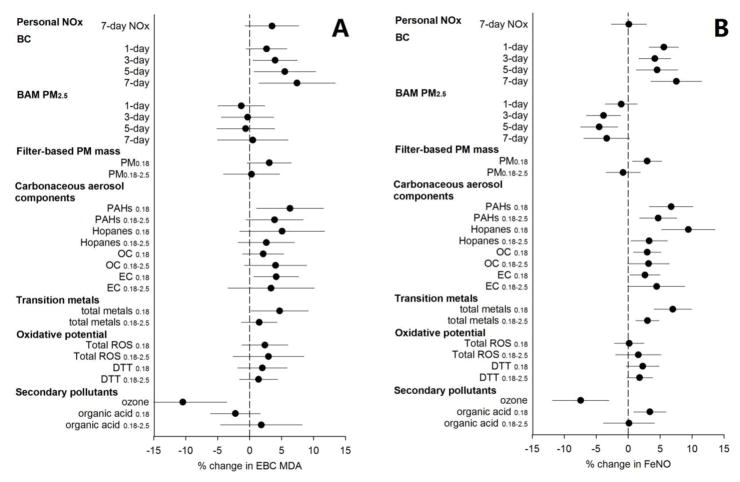
Associations between airway biomarkers and air pollutants are shown in Figure 1. Since Daily ambient traffic-related pollutants (BC, CO, and NOx) were strongly correlated and showed similar results, we only display results of BC. PM within the ultrafine and accumulation mode ranges was of primary interest. Therefore, we only present PM components with these two size modes. For detailed information, see Appendix Table A.3 and Appendix Table A.8. The biomarker of airway oxidative stress, EBC MDA, was positively associated with daily ambient traffic-related air pollutants and 7-day personal NOx, but not with BAM PM2.5 (Figure 1A). The strongest positive associations were observed for 7-day averages. For example, MDA had an estimated 7.4% increase (95% CI: 1.7%,13.4%) per interquartile increase of 7-day average BC. For 5-day PM components, MDA was positively associated with total PM0.18 mass, carbonaceous aerosol components, and total transition metals (more strongly so with the PM0.18 fraction). We observed small but statistically nonsignificant positive associations of MDA with measurements of in vitro oxidative potential (ROS and DTT). The estimates of association for water-soluble ROS and total ROS were similar for all biomarkers, therefore, we do not present water-soluble ROS results. No associations were observed for organic acid while the associations of MDA with O3 were negative.
Fig. 1.
Percent Change (mean and 95 % confidence intervals) in airway inflammatory biomarkers EBC MDA (A) and airway oxidative stress biomarker FeNO (B) with a one interquartile range increase of ambient and personal air pollutants. Exposures were averaged across 5 days except as specified. BC: black carbon; CO: carbon monoxide; DTT: dithiothreitol; EBC MDA: malondialdehyde in exhaled breath condensate; EC: elemental carbon; FeNO: exhaled nitric oxide; NOX: nitrogen oxides; OC: organic carbon; PAHs: polycyclic aromatic hydrocarbons; ROS: reactive oxygen species.
The biomarker of airway inflammation, FeNO, was positively associated with markers of traffic-related air pollutants (BC, CO, NOx, all carbonaceous aerosol components including OC), ultrafine PM0.18 mass, and total transition metals (Figure 1B and Appendix Table A.8.). For daily ambient measurements of traffic-related air pollutants (BC, CO, and NOx), the strongest associations were observed for 7-day averages. For example, BC at 7-day average had the strongest estimated association (7.5%, 95% CI: 3.6%,11.5%). For the 5-day average PM variables, we observed stronger estimated associations for ultrafine PM0.18 than larger size-fractions for total mass, PAHs, hopanes, transition metals and organic acid. Hopanes in ultrafine PM0.18 had the strongest association (9.4%, 95% CI: 5.2%, 13.6%). Associations between FeNO and DTT were positive, with borderline significance, while no association was observed for FeNO with ROS. Unexpectedly, FeNO was inversely associated with O3.
3.3. Systemic biomarkers and air pollutant associations
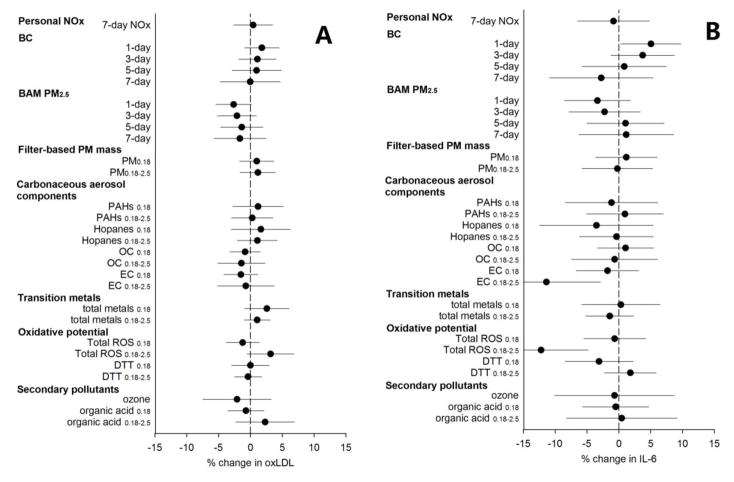
We did not observe significant associations between oxLDL and PM air pollutant components (Figure 2A and Appendix Table A.9). However, positive (expected) but nonsignificant associations of oxLDL with traffic-related pollutants (BC, PAHs, hopanes), total transition metals, ROS (especially in accumulation mode PM0.18–2.5), and organic acids in PM0.18–2.5, were observed. The systemic inflammation biomarker IL-6 was significantly and positively associated with daily ambient traffic-related air pollutants at 1-day averages and then gradually became weaker and nonsignificant at 3-day, 5-day and 7-day averages (Figure 2B and Appendix Table 4). We observed unexpected inverse associations with EC and with ROS in PM0.18–2.5. The associations of IL-6 with 7-day personal NOX and other 5-day average PM components were nonsignificant.
Fig. 2.
Percent Change (mean and 95 % confidence intervals) in systemic inflammatory biomarkers oxLDL (A) and systemic oxidative stress biomarker IL-6 (B) with a one interquartile range increase of ambient and personal air pollutants. Exposures were averaged across 5 days except as specified. BC: black carbon; CO: carbon monoxide; DTT: dithiothreitol; EC: elemental carbon; IL-6: Interleukin 6; NOX: nitrogen oxides; OC: organic carbon; oxLDL oxidized low-density lipoprotein; PAHs: polycyclic aromatic hydrocarbons; ROS: reactive oxygen species.
3.4. Effect modification and sensitivity analysis
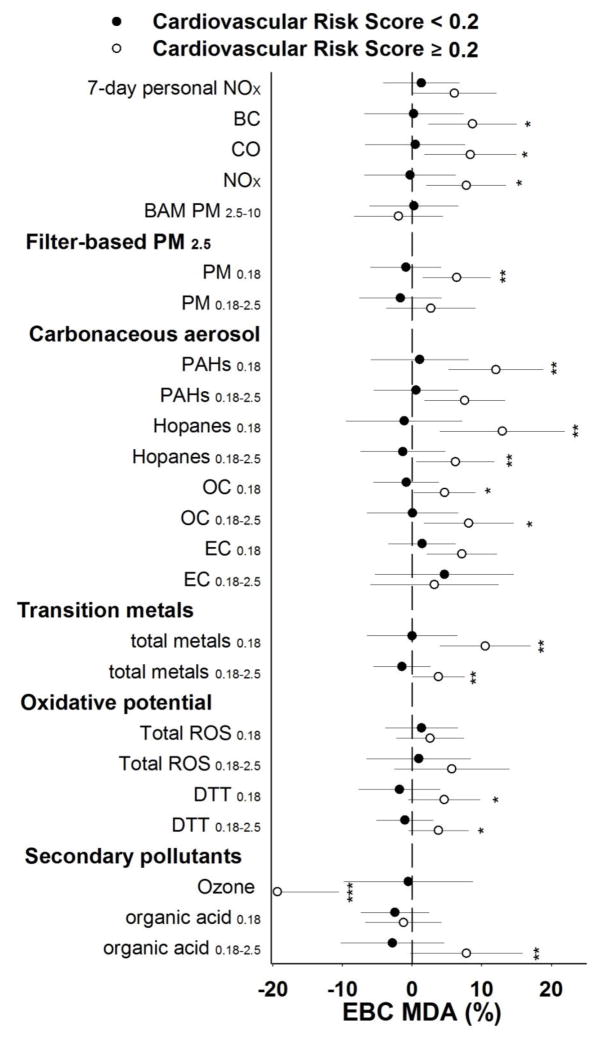
We found evidence of potential effect modification by cardiovascular risk score on the associations between EBC MDA and air pollutants. Subjects with higher cardiovascular risk score (≥ 0.2) were more likely to have positive associations (Figure 3). Consistent with this finding, the components of cardiovascular risk score: age (Appendix, Fig. A.3) and total cholesterol to HDL ratio (Appendix, Fig. A.4) also had similar modifying effects on the associations. The positive associations were pronounced in subjects who were older or had greater total cholesterol to HDL ratio. However, this effect modification by cardiovascular risk score was not observed in other outcome biomarkers.
Fig. 3.
Effect modification of relations between exhaled breath condensate malondialdehyde (EBC MDA) and air pollution by cardiovascular score. Percent Change (mean and 95 % confidence intervals) in EBC MDA with one interquartile range increase of selected air pollutant exposures averaged across 5 days (7 days for personal nitrogen oxides; NOX) preceding each subject’s clinic visits: *p < 0.1, **p < 0.05, compared with no effect modification by cardiovascular score.
We also found regional differences of the associations between IL-6 and 1-day average traffic-related air pollutants and 7-day average personal NOX (Figure 4A). Stronger positive associations were observed for traffic-related air pollutants in Los Angeles region than in Anaheim, while IL-6 was inversely associated with personal NOX in Anaheim and positively but non-significantly associated with personal NOX in Los Angeles. For O3, significant inverse associations were observed in the Los Angeles, while positive but non-significant associations were observed in Anaheim. Regional differences were also observed between EBC MDA and daily traffic-related air pollutants and personal NOx. Positive associations were stronger in Anaheim than in Los Angeles (Fig 4B). However, these differences were not observed for the 5-day PM components. Other independent variables showed no statistically significant effect modification for any outcomes (data not shown).
Fig. 4.
Effect modification of relations between interleukin-6 (IL-6) and air pollution (A), and exhaled breath condensate malondialdehyde (EBC MDA) and air pollution (B) by region. Percent Change (mean and 95 % confidence intervals) in EBC MDA with one interquartile range increase of selected air pollutant exposures averaged across 1-day, 3-day, 5-day, and 7-day (7-day for personal nitrogen oxides; NOX) preceding each subject’s clinic visits: *p < 0.1, **p < 0.05, compared with no effect modification by region.
Results from the sensitivity analyses did not qualitatively change the main findings when restricting subjects to a smaller study area radius around central air monitors, excluding extreme weather conditions, or excluding imputed ambient exposure data. Specifically, after excluding imputed BC exposure data, the estimated percent change of MDA per interquartile increase of 7-day average BC slightly decreased from 7.4% (95% CI: 1.7%, 13.4%) to 7.2% (1.1% – 13.4%). Results were similar for FeNO.
The estimates of association from two-pollutant models of O3 with a primary air pollutant (BC, NOx and PAH) became largely nonsignificant with effect estimates attenuating toward the null for airway biomarkers, except for associations between FeNO and PAHs in PM0.18 that remained significant. The results for the systemic biomarkers were similar using two-pollutant models and single-pollutant models (data not shown).
In the product term models testing interactions between ambient 1-day, 3-day, 5-day and 7-day averages of BC and O3 on the airway biomarkers (FeNO and EBC MDA) we found significant positive interactions between the pollutants across all averaging times in relation to FeNO, with the strongest effect at the 5-day BC average (Figure 5). Similar interactions on FeNO were also observed between O3 and PAHs in both PM0.18 and PM0.18–2.5 (data not shown). However, this interaction effect was not observed for EBC MDA.
Fig. 5.
Relation of FeNO (log value) to interaction between black carbon and O3. Changes in log FeNO per unit increase of BC, O3 and their interaction across 1 day (A), 3 days (B), 5 days (C), and 7 days (D) averaged before each subject’s outcome measurement. BC: black carbon; FeNO: exhaled nitric oxide; O3: ozone.
4. Discussion
4.1. Summary
We investigated the effects of short-term exposure to personal and ambient air pollutants, including their chemically-characterized components, on biomarkers of oxidative stress and inflammation in the airways and circulation in a susceptible elderly cohort in the Los Angeles air basin. We found evidence that pollutants with high oxidative potential, including markers of traffic-related pollutants, ultrafine particles (PM0.18), and transition metals, were associated with elevated airway oxidative stress (EBC MDA) and inflammation (FeNO). This is further evidenced by the positive, albeit non-significant, association between airway biomarkers and measurements of in vitro oxidative potential (ROS and DTT). In general, the associations for both of the airway biomarkers were stronger for traffic-related air pollutants in the ultrafine range than the larger particle size range. Moreover, we found acute positive associations between ambient traffic-related pollutants (1-day and 3-day pollutant averages of BC, NOx and CO) and the biomarker of systemic inflammation (IL-6).
4.2. Airway biomarkers and air pollutant associations
MDA is a lipid peroxidation end-product and has been recognized as a biomarker of oxidative stress (Nordenhall et al. 2000). Higher levels of MDA in EBC have been observed in subjects with diseases characterized by oxidative stress, such as COPD (Lee et al. 2014) and diabetes (Dierckx et al. 2003). Few air pollution epidemiological studies have used EBC MDA as the biomarker of airway oxidative stress (Romieu et al. 2008; Gong et al. 2013; Sarnat et al. 2014). A study during the Beijing Olympic Games showed that a significant decrease in EBC MDA concentration in healthy young adults was associated with a substantial improvement in air quality (Gong et al. 2013). In a more recent study, EBC MDA was estimated to be higher three hours after the highway commutes of subjects during morning rush hours in the metropolitan Atlanta area in both asthmatic and non-asthmatic adults (Sarnat et al. 2014). However, to our knowledge, the present study is the first epidemiologic study that has shown positive associations of EBC MDA with components of aerosols with potentially high oxidative potential (ultrafine particle mass, PAHs, and transition metals) and with direct measures of PM oxidative potential (ROS and DTT).
Nitric oxide (NO) is produced in the respiratory tract as a part of the inflammatory process. It has previously been shown that NO is increased in exhaled air with exposure to air pollution among patients with asthma (Delfino et al. 2006), COPD (Brindicci et al. 2005), other respiratory conditions (Mar et al. 2005), and coronary artery disease (Delfino et al. 2010a). Previous epidemiological studies have shown that FeNO is associated with increases in traffic-related air pollutants in children (Delfino et al. 2006) and in young adults (Huang et al. 2012). However, this association has rarely been investigated in the general elderly population. We previously reported null associations of FeNO with traffic-related air pollutants (including markers of primary organic aerosols) in subjects with coronary artery disease, but found positive associations of FeNO with markers of secondary organic aerosols (Delfino et al. 2010a). In the present study, the estimated associations between FeNO and primary organic aerosols were positive, while we also found associations of FeNO with the one marker of secondary organic aerosols (organic acids) in PM0.18, but not in PM0.18–2.5. It is important to note that our previous study (Delfino et al. 2010a) had better exposure characterization including modeled hourly secondary organic carbon as a marker of secondary organic aerosol (Polidori et al. 2007). Another study of a group of elderly subjects in Steubenville, Ohio showed that FeNO was positively associated with exposure to 1-day PM2.5 mass concentrations (not chemically characterized). However, in our current study, we did not find a positive association between FeNO and PM2.5. Instead, we found FeNO was positively associated with exposure to 5-day ultrafine PM0.18 mass, but not accumulation mode PM0.18–2.5 mass concentrations. This may be due to the different chemical components in ultrafine versus accumulation mode size fractions of PM2.5 in Los Angeles as compared with Steubenville. Consistent with our current results, controlled human exposure studies have shown that acute exposure to diesel exhaust particles can transiently increase airway inflammation (Behndig et al. 2006; Bosson et al. 2008).
4.3. Systemic biomarkers and air pollutant associations
Our findings of increased systemic inflammation with exposure to traffic-related air pollutants are generally consistent with previous studies (Rückerl et al. 2006; Rückerl et al. 2007; Delfino et al. 2009; Fang et al. 2012). However, the averaging time showing the strongest association has varied from hours to 9-days in these studies. It is unclear why different studies have shown different pollutant averaging times in association with biomarkers of systemic inflammation, but some combination of varying exposure error and population susceptibilities may be at play here.
Our previous study in elderly subjects with coronary artery disease reported significant associations of both airway and systemic inflammation with contrasting PM air pollutant characteristics (secondary organic aerosols and primary organic aerosols, respectively) (Delfino et al. 2010a). Notably, the air pollutant species that were significantly associated with airway versus systemic outcomes differed with the present study as discussed above. In contrast, our current study also revealed associations of biomarkers in the airways with markers of combustion-related air pollutants that were less clear for biomarkers of inflammation in the circulation. This may due to the fact that study subjects in the current cohort are relatively healthier than the subjects in the previous study. Healthy subjects may have stronger antioxidant defense mechanisms in their circulation that combats the effects of air pollution and chemical components that may spillover from the lungs. Furthermore, with considerably greater resources than the present study, the previous study measured air pollutants at the residential location (retirement communities) of each subject group that likely greatly reduced exposure error. Another possible explanation for why our current results for the systemic biomarkers are not as definitive as our previous studies could be due to the fact that the air pollutant levels, especially ultrafine PM, EC, OC, and tracers of mobile sources measured by our research group, have decreased over the last decade in the Los Angeles area (Shirmohammadi et al. 2016). This resulted from more stringent regulations on mobile source emissions by the United States EPA, California Air Resources Board and SCAQMD (Hasheminassab et al. 2014a).
4.4. Plausible biological mechanisms
In response to these pro-oxidant components in PM (traffic-related pollutants, ultrafine PM, and transition metals), alveolar macrophages produce nitric oxide, which can combine with superoxide anion to produce peroxynitrite, a potent oxidizing compound, to generate ROS (Laumbach et al. 2010). Subsequently, excessive ROS can induce oxidative stress and inflammation in the airways. This is partly supported by our finding of positive, albeit nonsignificant, associations of both airway outcomes with in vitro macrophage ROS and DTT from ultrafine and accumulation mode PM extracts. This finding is consistent with our report of significant positive associations of FeNO with macrophage ROS and DTT from PM2.5 extracts in children and adolescents with asthma living in the Los Angeles air basin (Delfino et al. 2013).
The associations of PM oxidative potential (ROS and/or DTT) with airway and systemic biomarkers were less strong than we expected. Nevertheless, the observed nonsignificant positive associations may be mediated by ROS-induced activation of Nrf2 which is the major regulator of over 250 antioxidant and detoxifying enzymes and related proteins (Li et al. 2009). This potential mechanism is supported by our previous study that exposure markers of combustion-related air pollutants were positively associated with expression of the Nrf2 gene (NFE2L2) and Nrf2-mediated genes (HMOX1, NQO1, and SOD2) in a cohort of elderly subjects with coronary artery disease (Wittkopp et al. 2015).
Previous epidemiological studies have shown that impaired vascular function is associated with short-term exposure to air pollutants, especially with pollutants linked to traffic sources (Ljungman et al. 2014; Provost et al. 2016). However, in our current study, we found relatively weak evidence of an elevation in systemic (plasma) oxidative stress and inflammation biomarkers with exposure to traffic-related pollutants. It is possible that these pollutants are translocated from the airways into the systemic circulation, where they may directly impact the endothelium and promote cardiovascular events without prior induction of systemic oxidative stress or inflammatory responses (Yamawaki et al. 2006; Wallenborn et al. 2007).
4.3. Effect modification
In assessing effect modification, we found suggestive evidence that subjects with a high cardiovascular risk score, as well as older subjects, and subjects with higher LDL/HDL ratio experienced stronger and more significant positive associations of EBC MDA with air pollutants compared with healthier subjects. With the current study design, it is not possible to disentangle the independent effect of cardiovascular risk score, age or LDL/HDL ratio. The stronger observed associations may simply be driven by single components of the cardiovascular risk score, such as age.
The observed regional differences for EBC MDA and IL-6 may be due to the fact that on average the baseline levels were different for the subjects in these two regions. For IL-6, the concentration in Los Angeles (1.95 pg/ml) is lower than in Anaheim (2.75 pg/ml) (Appendix Table A.5). While for EBC MDA, the average concentration in Los Angeles is 10.47 nmol, which was higher than in Anaheim (6.39 nmol). From the known medical conditions, we cannot explain different levels of these two biomarkers in Los Angeles and Anaheim, but the higher levels may be due to unmeasured factors that confound air pollution associations, which may explain the stronger associations of IL-6 and EBC MDA in regions where their plasma concentrations were lower. It is important to note that in these exploratory analyses, we tested many interactions, hence multiple testing bias is a concern and the significant effect modification may be due to chance. However, we did not adjust for multiple comparisons as it is not explicitly required in exploratory analyses (Bender et al. 2001).
4.4. Pollutant interactions
The weakened associations for airway biomarkers with primary pollutants in the two-pollutant models with O3 are likely due to the high inverse correlations between primary pollutants and O3 (BC, NOx and PAHs correlations with O3, R ≥ 0.6). However, interactions may be of more interest because the highly oxidizing capability of O3 give it the potential to react with primary pollutant components (such as those indicated by BC, NOx and PAHs) leading to synergistic or additive adverse effects on human health. It was evidenced in an in vitro experimental study that PM and O3 act synergistically in generating a sustained production of oxidative stress (Valavanidis et al. 2009). Consistent with these concepts we found positive interactions between primary pollutants and O3 on FeNO. This may indicate that the underlying effect of air pollutant mixtures may not be fully explained by only one air pollutant. Based on this observation, we built a model using ROS or DTT as the dependent variable and tested for interaction effects between BC/PAHs and O3. For BC and O3, the interaction effect was significant in Los Angeles but not in Anaheim. While for PAHs and O3, the interaction effect was significant in both areas, but stronger in Los Angeles than in Anaheim. This indicates that there may be other unmeasured secondary pollutant species that are formed during periods with both high primary air pollutants, and high O3 that lead to higher oxidative potential of the particle mixtures.
4.5. Personal NOX
Our results for personal NOx were nonsignificant. The association between personal NOX and EBC MDA was borderline significant but weaker than ambient 7-day NOX (Appendix Table A.6). Meanwhile, we observed the strongest association of FeNO with ambient NOX at 7-day average, but the relation was null with 7-day personal NOx (Appendix Table A.8). The lack of association for personal NOX may be due to subject non-compliance, face velocity effects on the passive NOx badge from variations in airflow, and unadjusted indoor sources of NOX.
4.6. Strengths and limitations
The strengths of our study include the comprehensive characterization of PM components, including directly measuring PM oxidative potential using in vitro macrophage ROS and DTT, the repeated measurements of biomarkers over time, which minimize confounding from time-invariant between-subject and within-subject factors, and the evaluation of biomarkers in both the airways and the circulation.
One of the limitations is that different biomarker outcomes were collected approximately at the same time. Therefore, we cannot investigate the relationships with respect to the time-sequence of oxidative stress and subsequent inflammatory responses to air pollution. Our size-fractionated PM composition data were collected across five-day integrated periods rather than daily limiting comparisons with ambient daily exposures that were measured continuously (BC and air pollutant gases). For example, we observed significant associations for IL-6 with continuously measured air pollutants at 1-day averages. Second, time-varying subject characteristics, such as daily physical activity and diet, can change associations between biomarkers and air pollution. However, we did not have this information in any detail resulting in potential unmeasured confounding. Third, measurement errors in exposure may have attenuated true associations. Most of our exposure data were obtained from central monitoring stations, which may not account for variations in personal exposure levels or variations in indoor air quality. Given that our elderly subjects spent most of their time indoors, our ambient outdoor exposure data may have introduced exposure error. Finally, external validity of the findings is limited to elderly subjects living in the selected regions of Los Angeles and Orange Counties.
5. Conclusions
Our results suggest that in a cohort of elderly adults, airway biomarkers of oxidative stress and inflammation are positively associated with traffic-related air pollutants, ultrafine particles and transition metals. We identified that pollutants with high oxidative potential, especially PM in the ultrafine range, have stronger estimated effects than in the larger PM size fractions. Acute and transient elevations of the systemic inflammatory biomarkers were observed with exposure to ambient daily traffic-related pollutants. Our findings thus add mechanistic plausibility to the hypothesis that short-term exposure to traffic-related pollutants is associated with cardiorespiratory morbidity and mortality.
Supplementary Material
Highlights.
This study included 97 elderly adults with up to 12 repeated measures.
Air pollution exposure was more clearly associated with airway than systemic biomarkers
Biomarkers were positively associated with traffic-related pollutants, ultrafine PM and transition metals
Positive but nonsignificant associations were observed for biomarkers with PM oxidative potential.
Acknowledgments
Funding sources
This study was supported by grant number R01 ES12243 from the National Institute of Environmental Health Sciences, U.S. National Institutes of Health (NIH), and Grant UL1 TR000153 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, and by contract numbers BPG-55175, BPG-53003, and AQMD-14172 administered by the South Coast Air Quality Management District (SCAQMD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or SCAQMD.
We thank the staff and students at the University of California Irvine Department of Epidemiology, and staff at the Wisconsin State Laboratory of Hygiene for their assistance with the chemical analysis. We also acknowledge the support of University of Southern California’s Provost and Viterbi PhD fellowships.
Footnotes
Ethics Approval
This study was approved by the institutional Review Board of the University of California, Irvine. All subjects provided written informed consent before participation.
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GB, Bell ML, Peng RD. Methods to Calculate the Heat Index as an Exposure Metric in Environmental Health Research. Environ Health Persp. 2013;121(10):1111–1119. doi: 10.1289/ehp.1206273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS/ERS. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. 171/8/912 [pii] [DOI] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential - A workshop report and consensus statement. Inhalation Toxicology. 2008;20(1):75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Behndig AF, I, Mudway S, Brown JL, Stenfors N, Helleday R, Duggan ST, Wilson SJ, Boman C, Cassee FR, Frew AJ, Kelly FJ, Sandstrom T, Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27(2):359–365. doi: 10.1183/09031936.06.00136904. 27/2/359 [pii] [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing - when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/S0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Borm PJA, Kelly F, Kunzli N, Schins RPF, Donaldson K. Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric. Occupational and Environmental Medicine. 2007;64(2):73–74. doi: 10.1136/oem.2006.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosson J, Barath S, Pourazar J, Behindig AF, Sandstrom T, Blomberg A, Adelroth E. Diesel exhaust exposure enhances the ozone-induced airway inflammation in healthy humans. Eur Respir J. 2008;31(6):1234–1240. doi: 10.1183/09031936.00078407. [DOI] [PubMed] [Google Scholar]
- Brindicci C, Ito K, Resta O, Pride NB, Barnes PJ, Kharitonov SA. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J. 2005;26(1):52–59. doi: 10.1183/09031936.05.00125304. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD E American Heart Association Council on, C. o. t. K. i. C. D. Prevention, P. A. Council on Nutrition and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhao Z, Sun Q, Lin Z, Zhao A, Wang C, Xia Y, Xu X, Kan H. Size-fractionated Particulate Air Pollution and Circulating Biomarkers of Inflammation, Coagulation, and Vasoconstriction in a Panel of Young Adults. Epidemiology. 2015;26(3):328–336. doi: 10.1097/EDE.0000000000000273. [DOI] [PubMed] [Google Scholar]
- Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005;99(1):40–47. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cook RD. Detection of Influential Observation in Linear-Regression. Technometrics. 1977;19(1):15–18. doi: 10.2307/1268249. [DOI] [Google Scholar]
- Cook RD, Weisberg S. Residuals and Influence in Regression. New York, NY: Chapman & Hall; 1982. [Google Scholar]
- Daher N, Hasheminassab S, Shafer MM, Schauer JJ, Sioutas C. Seasonal and spatial variability in chemical composition and mass closure of ambient ultrafine particles in the megacity of Los Angeles. Environ Sci-Proc Imp. 2013;15(1):283–295. doi: 10.1039/c2em30615h. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Persp. 2005;113(8):934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, George SC, Kleinman MT. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Persp. 2006;114(11):1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Persp. 2008;116(7):898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Persp. 2009;117(8):1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, George SC, Shafer MM, Schauer JJ, Sioutas C. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010a;21(6):892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, Kleinman MT, Schauer JJ, Sioutas C. Association of biomarkers of systemic inflammation with organic components and source tracers in quasi-ultrafine particles. Environ Health Persp. 2010b;118(6):756–762. doi: 10.1289/ehp.0901407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, Jamner L, Sioutas C, Longhurst J. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010c;21(3):396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J Expo Sci Env Epid. 2013;23(5):466–473. doi: 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx N, Horvath G, van Gils C, Vertommen J, van de Vliet J, De Leeuw I, Manuel-y-Keenoy B. Oxidative stress status in patients with diabetes mellitus: relationship to diet. Eur J Clin Nutr. 2003;57(8):999–1008. doi: 10.1038/sj.ejcn.1601635. [DOI] [PubMed] [Google Scholar]
- Elder A, Oberdorster G. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med. 2006;5(4):785–796. doi: 10.1016/j.coem.2006.07.003. S1526-0046(06)00044-6 [pii] [DOI] [PubMed] [Google Scholar]
- Fang SC, Mehta AJ, Alexeeff SE, Gryparis A, Coull B, Vokonas P, Christiani DC, Schwartz J. Residential Black Carbon Exposure and Circulating Markers of Systemic Inflammation in Elderly Males: The Normative Aging Study. Environ Health Persp. 2012;120(5):674–680. doi: 10.1289/ehp.1103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Long JS. Modern Methods of Data-Analysis. 1990:257–291. [Google Scholar]
- Franck U, Odeh S, Wiedensohler A, Wehner B, Herbarth O. The effect of particle size on cardiovascular disorders - The smaller the worse. Sci Total Environ. 2011;409(20):4217–4221. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Arden Pope C., 3rd Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40(5):207–238. doi: 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J Toxicol Env Heal B. 2012;15(1):1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JC, Zhu T, Kipen H, Wang GF, Hu M, Ohman-Strickland P, Lu SE, Zhang L, Wang YD, Zhu P, Rich DQ, Diehl SR, Huang W, Zhang JF. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J Expo Sci Env Epid. 2013;23(3):322–327. doi: 10.1038/jes.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasheminassab S, Daher N, Ostro BD, Sioutas C. Long-term source apportionment of ambient fine particulate matter (PM2.5) in the Los Angeles Basin: A focus on emissions reduction from vehicular sources. Environ Pollut. 2014a;193:54–64. doi: 10.1016/j.envpol.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Hasheminassab S, Daher N, Shafer MM, Schauer JJ, Delfino RJ, Sioutas C. Chemical characterization and source apportionment of indoor and outdoor fine particulatematter (PM2.5) in retirement communities of the Los Angeles Basin. Sci Total Environ. 2014b;490:528–537. doi: 10.1016/j.scitotenv.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM, Simonsick EM, Colbert LH, Kritchevsky SB. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23(8):1444–1448. doi: 10.1161/01.ATV.0000080379.05071.2201.ATV.0000080379.05071.22. [pii] [DOI] [PubMed] [Google Scholar]
- Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR, Zhu P, Tong J, Gong J, Zhu T, Zhang J. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Resp Crit Care. 2012;186(11):1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreman AP, Shafer MM, Hemming JC, Hannigan MP, Schauer JJ. A macrophage-based method for the assessment of the reactive oxygen species (ROS) activity of atmospheric particulate matter (PM) and application to routine (daily-24 h) aerosol monitoring studies. Aerosol Sci Tech. 2008;42(11):946–957. doi: 10.1080/02786820802363819. [DOI] [Google Scholar]
- Lanzinger S, Schneider A, Breitner S, Stafoggia M, Erzen I, Dostal M, Pastorkova A, Bastian S, Cyrys J, Zscheppang A, Kolodnitska T, Peters A, Grp US. Associations between ultrafine and fine particles and mortality in five central European cities - Results from the UFIREG study. Environment International. 2016;88:44–52. doi: 10.1016/j.envint.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Larstad M, Ljungkvist G, Olin AC, Toren K. Determination of malondialdehyde in breath condensate by high-performance liquid chromatography with fluorescence detection. J Chromatogr B. 2002;766(1):107–114. doi: 10.1016/S0378-4347(01)00437-6. [DOI] [PubMed] [Google Scholar]
- Laumbach RJ, Kipen HM. Acute effects of motor vehicle traffic-related air pollution exposures on measures of oxidative stress in human airways. Ann Ny Acad Sci. 2010;1203:107–112. doi: 10.1111/j.1749-6632.2010.05604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Shin JH, Hwang JH, Baek JE, Choi BS. Malondialdehyde and 3-nitrotyrosine in exhaled breath condensate in retired elderly coal miners with chronic obstructive pulmonary disease. Saf Health Work. 2014;5(2):91–96. doi: 10.1016/j.shaw.2014.03.001. S2093-7911(14)00019-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Kim S, Wang M, Froines J, Sioutas C, Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal Toxicol. 2002;14(5):459–486. doi: 10.1080/089583701753678571. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Persp. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WG, Kong AN. Molecular Mechanisms of Nrf2-Mediated Antioxidant Response. Mol Carcinogen. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M. Regional deposition of particles in the human respiratory trend. In: Lee DHK, Falk HL, et al., editors. Handbook of physiology, reaction to environmental agents. Bethesda, MD: American Physiological Society; 1977. [Google Scholar]
- Ljungman PL, Wilker EH, Rice MB, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, Benjamin EJ, Mittleman MA, Hamburg NM. Short-term exposure to air pollution and digital vascular function. Am J Epidemiol. 2014;180(5):482–489. doi: 10.1093/aje/kwu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ Health Persp. 2005;113(12):1791–1794. doi: 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenhall C, Pourazar J, Blomberg A, Levin JO, Sandstrom T, Adelroth E. Airway inflammation following exposure to diesel exhaust: a study of time kinetics using induced sputum. Eur Respir J. 2000;15(6):1046–1051. doi: 10.1034/j.1399-3003.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- Ogawa & Company, U., Inc. NO, NO2, NOx and SO2 Sampling Protocol Using The Ogawa Sampler 2006 [Google Scholar]
- Pant P, Harrison RM. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmospheric environment. 2013;77:78–97. doi: 10.1016/j.atmosenv.2013.04.028. [DOI] [Google Scholar]
- Polidori A, Arhami M, Sioutas C, Delfino RJ, Allen R. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles basin. J Air Waste Manage. 2007;57(3):366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- Provost EB, Louwies T, Cox B, Op ‘t Roodt J, Solmi F, Dons E, Int Panis L, De Boever P, Nawrot TS. Short-term fluctuations in personal black carbon exposure are associated with rapid changes in carotid arterial stiffening. Environment international. 2016;88:228–234. doi: 10.1016/j.envint.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Rogge WF, Mazurek MA, Hildemann LM, Cass GR, Simoneit BRT. Quantification of Urban Organic Aerosols at a Molecular-Level - Identification, Abundance and Seasonal-Variation. Atmos Environ a-Gen. 1993;27(8):1309–1330. doi: 10.1016/0960-1686(93)90257-Y. [DOI] [Google Scholar]
- Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Almstrand AC, Diaz-Sanchez D, Sly PD, Olin AC. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immun. 2008;121(4):903–909. doi: 10.1016/j.jaci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, Dailey L, Devlin RB, Diaz-Sanchez D, Koenig W, Phipps R, Silbajoris R, Soentgen J, Soukup J, Peters A, Schneider A. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environment International. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173(4):432–441. doi: 10.1164/rccm.200507-1123OC. 200507-1123OC [pii] [DOI] [PubMed] [Google Scholar]
- Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Kuchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin-6,C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Persp. 2007;115(7):1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari A, Hasheminassab S, Wang DB, Shafer MM, Schauer JJ, Sioutas C. Impact of primary and secondary organic sources on the oxidative potential of quasi-ultrafine particles (PM0.25) at three contrasting locations in the Los Angeles Basin. Atmos Environ. 2015;120:286–296. doi: 10.1016/j.atmosenv.2015.09.022. [DOI] [Google Scholar]
- Sarnat JA, Golan R, Greenwald R, Raysoni AU, Kewada P, Winquist A, Sarnat SE, Flanders WD, Mirabelli MC, Zora JE, Bergin MH, Yip F. Exposure to traffic pollution, acute inflammation and autonomic response in a panel of car commuters. Environ Res. 2014;133:66–76. doi: 10.1016/j.envres.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer JJ, Mader BT, Deminter JT, Heidemann G, Bae MS, Seinfeld JH, Flagan RC, Cary RA, Smith D, Huebert BJ, Bertram T, Howell S, Kline JT, Quinn P, Bates T, Turpin B, Lim HJ, Yu JZ, Yang H, Keywood MD. ACE-Asia intercomparison of a thermal-optical method for the determination of particle-phase organic and elemental carbon. Environ Sci Technol. 2003;37(5):993–1001. doi: 10.1021/es020622f. [DOI] [PubMed] [Google Scholar]
- Shirmohammadi F, Hasheminassab S, Saffari A, Schauer JJ, Delfino RJ, Sioutas C. Fine and ultrafine particulate organic carbon in the Los Angeles basin: Trends in sources and composition. Sci Total Environ. 2016;541:1083–1096. doi: 10.1016/j.scitotenv.2015.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Persp. 2005;113(8):947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Snyder DC, Sheesley RJ, Sullivan AP, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmos Chem Phys. 2008;8(5):1249–1259. [Google Scholar]
- Valavanidis A, Loridas S, Vlahogianni T, Fiotakis K. Influence of ozone on traffic-related particulate matter on the generation of hydroxyl radicals through a heterogeneous synergistic effect. J Hazard Mater. 2009;162(2–3):886–892. doi: 10.1016/j.jhazmat.2008.05.124. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2007;98(1):231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- Weichenthal S. Selected physiological effects of ultrafine particles in acute cardiovascular morbidity. Environ Res. 2012;115:26–36. doi: 10.1016/j.envres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Wittkopp S, Staimer N, Tjoa T, Stinchcombe T, Daher N, Schauer JJ, Shafer MM, Sioutas C, Gillen DL, Delfino RJ. Nrf2-related gene expression and exposure to traffic-related air pollution in elderly subjects with cardiovascular disease: An exploratory panel study. J Expo Sci Environ Epidemiol. 2015 doi: 10.1038/jes.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yang D, Wei H, Wang B, Huang J, Li H, Shima M, Deng F, Guo X. Association of chemical constituents and pollution sources of ambient fine particulate air pollution and biomarkers of oxidative stress associated with atherosclerosis: A panel study among young adults in Beijing, China. Chemosphere. 2015;135:347–353. doi: 10.1016/j.chemosphere.2015.04.096. doi:10.1016/j.chemosphere.2015.04.096 S0045-6535(15)00454-3. [pii] [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circulation journal : official journal of the Japanese Circulation Society. 2006;70(1):129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.