Abstract
The antisaccade task has been widely used to investigate cognitive action control. While the general network for saccadic eye movements is well defined, the exact location of eye fields within the frontal cortex strongly varies between studies. It is unknown whether this inconsistency reflects spatial uncertainty or is the result of different involvement of subregions for specific aspects of eye movement control. The aim of the present study was to examine functional differentiations within the frontal cortex by integrating results from neuroimaging studies analyzing pro- and antisaccade behavior using meta-analyses. The results provide evidence for a differential functional specialization of neighboring oculomotor frontal regions, with lateral frontal eye fields (FEF) and supplementary eye field (SEF) more often involved in prosaccades while medial FEF and anterior midcingulate cortex (aMCC) revealed consistent stronger involvement for antisaccades. This dissociation was furthermore mirrored by functional connectivity analyses showing that the lateral FEF and SEF are embedded in a motor output network, while medial FEF and aMCC are integrated in a multiple demand network.
Keywords: eye movements, fMRI, medial FEF, lateral FEF, SEF, aMCC, meta-analysis, functional connectivity (FC), meta-analytic connectivity modeling (MACM), resting state
1. Introduction
Visual exploration of our environment depends on different kinds of saccadic eye movements requiring distinct levels of control processes. Visually guided saccades are reflexive rapid eye movements directed towards a particular stimulus location while volitional saccades are initiated endogenously and modulated by higher order control processes (cf. McDowell et al., 2008). A classical paradigm to investigate different aspects of control demands during oculomotor behavior in a well-controlled experimental setup is the antisaccade task (Hallett, 1978). In its most common implementation, the participant is instructed to fixate a central location and is then presented with a lateralized visual stimulus. In (previously cued) prosaccade trials participants are required to make a saccade towards the stimulus, while in antisaccade trials participants are required to perform a saccade to the mirror symmetrical position. While a prosaccade is a direct sensorimotor transformation, it has been suggested that correct performance in antisaccade trials requires additional higher cognitive processes compared to the (more reflexive) prosaccade trials (Herweg et al., 2014; McDowell et al., 2008; Munoz and Everling, 2004). During antisaccade trials participants first have to inhibit the reflexive response to look towards the visual target and then transform the stimulus location into an endogenous volitional response to the contralateral side (Munoz and Everling, 2004). Comparing performance in antisaccade trials with those in prosaccade trials usually reveals increased error rates and an increase in reaction times for antisaccades (for a review see Everling and Fischer, 1998).
An extensive literature covering electrophysiological and neuroimaging findings has attempted to delineate the underlying neural substrates of saccadic eye movements in humans and non-human primates (e.g. Brown et al., 2006; Chikazoe et al., 2007; Ettinger et al., 2008; Schlag-Rey et al., 1997; Wegener et al., 2008; for a review see Munoz and Everling, 2004). The fundamental neural circuitry underlying the transformation of visual input into motor output does not seem to differ substantially between visually driven prosaccades and more complex saccades, such as antisaccades (Leigh and Zee, 1999). Visual information enters the brain through the retina and is sent via the optic tract to the lateral geniculate nucleus and from there to the primary visual cortex (for a review see McDowell et al., 2008). Then, information is sent to the extrastriate visual areas and further to the parietal cortex, particularly the superior parietal lobe and parietal eye fields. From the parietal cortex information is forwarded to the frontal eye fields (FEF) and supplementary eye field (SEF) to which it exerts reciprocal connections (Barbas and Mesulam, 1981; Ferraina et al., 2002; Leichnetz, 2001). These cortical regions have direct connections to the subcortical saccade circuit such as the thalamus, basal ganglia and superior colliculus which modulate information via feedback loops (cf. Lynch and Tian, 2006). When the context requires additional higher cognitive processes, such as when performing an antisaccade, changed activity levels in the basic saccade circuitry as well as activity increases in additional brain areas (specifically in the prefrontal cortex (PFC)) have been observed (cf. McDowell et al., 2008).
Three different eye fields have been identified in the non-human primate frontal lobe: the SEF and the cingulate eye field (CEF) within the posterior medial frontal cortex and the FEF lying within the dorsal premotor cortex (for a review see Amiez and Petrides, 2009). Some authors have argued for further subdivisions within these frontal oculomotor areas particularly within the FEF, which might reflect differentiable involvement in sensorimotor versus cognitive control demands during eye movement behavior (McDowell et al., 2008). This dissociation is well in line with reports of differential anatomical and functional connectivity of subregions within the FEF especially with the visual and parietal cortices as revealed by tracer and functional connectivity analyses in non-human primates (Babapoor-Farrokhran et al., 2013; Schall et al., 1995; Stanton et al., 1995). While potential homologues of (at least) the FEF and SEF have been described in the human brain using imaging techniques, localization varies between different studies making precise anatomical delineations rather difficult. The image is furthermore complicated by divergences between functional imaging results and those from electrical stimulation studies in humans (for a review see Amiez and Petrides, 2009; Sweeney et al., 2007). Possible explanations for this inconsistency might be spatial uncertainty in neuroimaging methods as well as differences in the specific paradigm settings and hence variable involvement of subregions within the dorsal premotor and posterior medial frontal cortex specifically associated with either more basal sensorimotor processes or higher volitional control demands.
One possible approach to overcome the potential bias of individual studies and look for convergent brain activation associated with specific aspects of eye movements is integrating results from different neuroimaging studies using meta-analyses. We here used coordinate-based activation likelihood estimation (ALE) meta-analysis (Eickhoff et al., 2012; Eickhoff et al., 2009; Turkeltaub et al., 2002; Turkeltaub et al., 2012) as a method to integrate results from different experiments investigating brain activity changes during the performance of pro- and antisaccades. This method allows identifying reliable region of interests robustly defined across many experiments that may in a next step be used as a priori information to investigate the specific network structure with independent datasets such as functional or structural connectivity data. In the present study we investigated functional connectivity (FC) using task-dependent and task-independent FC analyses. FC is defined as the temporal coincidence of neurophysiological events in spatially distant brain areas (cf. Aertsen et al., 1989; Friston et al., 1993). FC thus represents a general concept of describing brain connectivity based on correlative observations and may be assessed in different settings using different methods. The two most popular of those are task-dependent analyses of co-activation patterns and in particular correlations in task-free resting-state fMRI data. Methods for assessing task-dependent FC have emerged within the last years through meta-analytic connectivity modeling (MACM) (Laird et al., 2009; Eickhoff et al, 2010; Robinson et al., 2010), a method capitalizing on the large number of highly standardized neuroimaging reports as contained, e.g., in the BrainMap database (www.brainmap.org). MACM-FC analyses rely on identifying those regions that show consistent co-activation with a specific seed region across a multitude of neuroimaging experiments. Importantly, selection of experiments used for the specific analysis is solely based on the prerequisite of finding activation within the particular seed region, independent of the type of paradigm used. Then, inference is performed based on the likelihood of observing concurrent activation in any other region in the brain (cf. Jakobs et al., 2012). This data-driven approach allows to specify networks conjointly co-activating across a broad range of differing experimental tasks without any selection biases (Eickhoff and Grefkes, 2011; Cieslik et al., 2013) and hence reflects task-dependent FC.
Task-independent or resting state FC, in turn, is based on the analysis of synchronized spontaneous signal fluctuation in the absence of an external structured task (Beckmann et al., 2005; for a review see van den Heuval and Hulshoff Pol 2010). Even though resting-state (RS) connectivity cannot be mapped back to an actual task, its functional relevance has been supported by findings robustly showing that most identified resting-state networks resemble known functional networks found for basic visual processing up to higher cognitive functions, such as attention or salience detection (for a review see van den Heuvel and Holshoff Pol, 2010 and Lee et al., 2013). Moreover, a recent study could show that synchronized spontaneous neural activity across cortical regions is relevant for behavior by showing that RS correlation between two face-selective regions positively correlated with individual performance in different face processing tasks but not in non-face tasks (Zhu et al., 2011). The most common interpretation of RS connectivity is that it may help to keep systems in an active state, hence improving performance and control processes whenever functional connectivity is required (cf. van den Heuvel and Holshoff Pol, 2010).
Summarizing, as the functional role of a region is supposed to be crucially related to the neural network it is interacting with, FC analyses can provide new information about large-scale communication in the human brain and functional specialization of brain regions.
The aim of the present study was twofold: we investigated potential subdivisions within the medial (SEF/CEF) and lateral (FEF) frontal cortex reflecting differentiated functional involvement in either more basal sensorimotor or higher cognitive control processes. In a next step, we tested for possible functional connectivity (FC) differences between such regions supporting different aspects of eye movement behavior. In particular, we used meta-analyses to test which regions within the frontal cortex showed consistent involvement during prosaccades across neuroimaging studies and which regions were consistently stronger recruited for antisaccades vs. prosaccades. Then, FC differences between regions selectively involved in prosaccades versus those specifically involved in antisaccades were investigated using task-dependent and task-independent FC. As prosaccades require a direct visuomotor transformation that is strongly reflexive (e.g. Munoz and Everling, 2004) we hypothesized that frontal regions stronger involved in prosaccades would show specific FC with areas that are known to be associated with more basal motor processes. On the other hand, regions specifically involved for antisaccades would be more strongly involved in a network associated with increased demands for cognitive control as antisaccades requires additional control processes to be able to suppress the prepotent motor response and endogenously initiate the incongruent eye movement (McDowell et al., 2008; Munoz and Everling, 2004).
2. Methods
2.1 Meta-analyses
2.1.1 Criteria selection of data used for meta-analysis
The investigations were performed according to the standard procedures for neuroimaging meta-analyses using ALE (cf. e.g. Langner and Eickhoff, 2013; Rottschy et al., 2012). In the present meta-analyses, neuroimaging experiments using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) investigating the neural correlates of the pro- and antisaccade task were included. To be able to investigate consistent differential involvement for different control demands during saccadic eye movements, we included experiments either contrasting prosaccades versus rest or a fixation condition as well as experiments contrasting antisaccades versus prosaccades. Relevant experiments were identified by a PubMed literature search (http://www.pubmed.org), including the ‘related articles’ function of the PubMed database as well as reference tracing of retrieved studies and review articles. Only data from healthy adults were included, while data from children and patients were excluded. Moreover, single-subject reports as well as experiments investigating between-group effects pertaining, for example, to handedness, sex, or genotype were excluded. In the present study only results from whole-brain group analyses reported as coordinates in a standard reference space (Talairach/Tournoux or MNI) were included, while results of region of interest analyses were excluded. However, it has to be noted that in some of the studies included - especially the older ones–the field of view may not always cover the lowest extent of the brain, such as the lowermost part of the cerebellum. Based on these criteria, 12 experiments contrasting prosaccades vs. a baseline condition, which could be either prosaccades vs. rest or prosaccades vs. baseline, were identified eligible for inclusion into the present meta-analysis (see Table 1). Moreover, 12 experiments contrasting antisaccades vs. prosaccades were included to identify those regions that reveal consistent stronger involvement for antisaccades compared to prosaccades and should therefore reflect the additional cognitive processes that are required to correctly perform an antisaccade. We did not include experiments contrasting antisaccades vs. rest or antisaccades vs. baseline as this contrast would also reveal brain regions that are associated with the basal motor aspects of eye movements and we here wanted to focus on those regions that are specifically associated with the cognitive control demands during antisaccades.
Table 1.
Experiments included in the meta-analyses
| Paper | Subjects | Contrast | Design | fMRI/PET | Space |
|---|---|---|---|---|---|
| Aichert et al., 2012 | 54 | Saccades vs Baseline | blocked | fMRI | MNI |
| Aichert et al., 2012 | 54 | Antisaccades vs Saccades | blocked | fMRI | MNI |
| Anderson et al., 1994 | 8 | Saccades vs Baseline | blocked | PET | TAL |
| Brown et al., 2006 | 10 | Saccades vs Baseline | event-related | fMRI | TAL |
| Brown et al., 2006 | 10 | Antisaccades vs Saccades | event-related | fMRI | TAL |
| Brown et al., 2007 | 11 | Antisaccades vs Saccades | event-related | fMRI | TAL |
| Chikazoe et al., 2007 | 24 | Antisaccades vs Saccades | blocked | fMRI | MNI |
| Doricchi et al., 1997 | 10 | Saccades vs Baseline | blocked | PET | TAL |
| Doricchi et al., 1997 | 10 | Antisaccades vs Saccades | blocked | PET | TAL |
| Ettinger et al., 2009 | 24 | Saccades vs Baseline | blocked | fMRI | TAL |
| Ettinger et al., 2009 | 24 | Antisaccades vs Saccades | blocked | fMRI | TAL |
| Ford et al., 2005 | 10 | Antisaccades vs Saccades | blocked | fMRI | TAL |
| Fukumoto-Motoshita et al, 2009 | 18 | Saccades vs Baseline | blocked | fMRI | MNI |
| Law et al., 1997 | 9 | Saccades vs Baseline | blocked | PET | TAL |
| Manoach et al. 2007 | 21 | Antisaccades vs Saccades | event-related | fMRI | TAL |
| Matsuda et al. 2004 | 21 | Saccades vs Baseline | blocked | fMRI | MNI |
| Matsuda et al. 2004 | 21 | Antisaccades vs Saccades | blocked | fMRI | MNI |
| O’Driscoll et al., 1995 | 10 | Antisaccades vs Saccades | blocked | PET | TAL |
| Paus et al., 1993 | 9 | Saccades vs Baseline | blocked | PET | TAL |
| Paus et al., 1993 | 9 | Antisaccades vs Saccades | blocked | PET | TAL |
| Petit et al., 2009 | 27 | Saccades vs Baseline | blocked | fMRI | MNI |
| Schraa-Tam et al., 2009 | 26 | Saccades vs Baseline | blocked | fMRI | MNI |
| Sweeney et al., 1996 | 11 | Saccades vs Baseline | event-related | PET | TAL |
| Sweeney et al., 1996 | 11 | Antisaccades vs Saccades | event-related | PET | TAL |
Coordinates reported in Talairach space were converted into MNI coordinates using a linear transformation (Lancaster et al., 2007) to account for differences in coordinates (MNI vs. Talairach space) between experiments.
2.1.2 Activation likelihood estimation algorithm
The meta-analyses were performed using the revised activation likelihood estimation (ALE) algorithm for coordinate-based meta-analyses of neuroimaging results (Eickhoff et al., 2012; Turkeltaub et al., 2002) implemented as in-house MATLAB tools. This algorithm allows identifying those areas that show a convergence of reported coordinates across experiments, which are higher than expected from a random spatial association. The key idea behind ALE is to treat the reported foci as centers for 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus. Importantly, the width of these uncertainty functions is determined based on empirical data on the between-subject and between-template variance, which represent the main components of this uncertainty. Importantly, the applied algorithm weights the between-subject variance by the number of examined subjects per study, accommodating the notion that larger sample sizes should provide more reliable approximations of the ‘true’ activation effect and should therefore be modeled by ‘smaller’ Gaussian distributions (Eickhoff et al., 2009).
The probabilities of all foci reported in a given experiment were then combined for each voxel, resulting in a modelled activation (MA) map (Turkeltaub et al., 2012). Taking the union across these MA maps yielded voxel-wise ALE scores describing the convergence of results at each particular location of the brain. To distinguish ‘true’ convergence between studies from random convergence (i.e., noise), ALE scores were compared to a null-distribution reflecting a random spatial association between experiments. Hereby, a random-effects inference is invoked, focusing on inference on the above-chance convergence between studies, not clustering of foci within a particular study. The p-value of „true” ALE was then given by the proportion of equal or higher values obtained under the null-distribution. The resulting non-parametric p-values for each meta-analysis were then thresholded at a cluster-level corrected threshold of p<0.05 (cluster-forming threshold at voxel-level p<0.001) and transformed into Z-scores for display.
To test for differences between experimental conditions separate ALE meta-analyses for each condition were performed and subsequently the voxel-wise difference between the ensuing ALE maps was computed (cf. Langner and Eickhoff, 2013; Rottschy et al., 2012). In a next step, all experiments contributing to either analysis were pooled and randomly divided into two groups being of the same size as the two original sets under the null-hypothesis of label-exchangeability. Then, ALE scores for these two randomly assembled groups were substracted from each other voxel-wise. This procedure was repeated 10,000 times yielding an empirical null distribution of ALE-score differences between the two conditions. This empirical null-distribution was used for testing the significance of the observed difference in each voxel’s ALE scores by thresholding at a posterior probability of P > .95 for a true difference between the two samples. Voxels surviving that threshold were inclusively masked by the respective main effect, that is, the significant effect of the ALE analysis for the minuend. Moreover, a cluster-extend threshold of k ≥ 25 voxels was applied (as done in Langner and Eickhoff, 2013).
For anatomic labelling of resulting effects in reference to probabilistic cytoarchitectonic maps the SPM Anatomy Toolbox (Eickhoff et al., 2007; Eickhoff et al., 2005), version 2.0, was used.
2.2 Functional connectivity analyses
2.2.1 Task-dependent functional connectivity: Meta-analytic connectivity modeling
To identify the co-activation profile of the foci obtained from the meta-analysis that showed differential stronger convergence for either the pro- or the antisaccade task, meta-analytic connectivity modeling (MACM) based on the BrainMap database (www.brainmap.org) was used. Details on the basic concept and methodology can be found in Eickhofff et al., (2010) as well as Laird et al., (2009) and Robinson et al., (2010).
In a first step, all experiments that showed at least one focus of activation in the seed region were identified. We restricted the search to fMRI and PET experiments reporting results from healthy adults only and did not consider any experiments involving pharmacological interventions or reported group comparisons (e.g. male vs. female; left-handed vs. right-handed participants). Then, a coordinate-based meta-analysis was performed in order to identify consistent co-activations across experiments by using the revised Activation Likelihood Estimation (ALE) algorithm (Eickhoff et al., 2012).
In a next step, for each voxel, the probabilities of all foci of a given experiment were aggregated, yielding a modeled activation (MA) map (Turkeltaub et al., 2012). The union of all MA maps then resulted in voxel-wise ALE scores, which reflect the convergence of results at each particular location of the brain. The ensuing ALE maps were then tested for statistical significance as described above for the meta-analyses. To investigate differences in task-based FC between functionally divergent seeds a similar approach as for the meta-analyses was performed. That is, difference maps calculating the voxel-wise difference of the Z-scores obtained from the seed specific MACM maps were calculated. Experiments contributing to either analysis were then pooled and randomly divided into two groups of the same size as the sets of contrasted experiments (Eickhoff et al. 2011). Voxelwise ALE scores for these two randomly assembled groups were subtracted from each other and recorded. Repeating this process 10,000 times yielded an empirical null distribution of ALE-score differences between the two conditions. Based on this permutation procedure, the map of true differences was then thresholded at a posterior probability of p>0.95 for a true difference between the two samples. The resulting maps were then masked with the respective main effect of the minuend connectivity map to avoid obtaining significant connectivity in voxels of the difference map that do not show significant co-activation on the underlying connectivity map. Furthermore, only regions with at least 25 cohesive voxels were considered in the resulting difference maps.
2.2.2 Task-independent functional connectivity: Resting-State
For the task-independent FC analyses resting state data from the Nathan Kline Institute ‘Rockland’ sample (online available as part of the International Neuroimaging Datasharing Initiative (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html)) were examined. In the present study only healthy subjects between 18 and 85 years were included resulting in a sample of 132 subjects (mean age: 42.3 ± 18.08 years; 78 male, 54 female). Images were acquired on a Siemens TrioTim 3T scanner using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, repetition time (TR)=2.5 s, echo time (TE)=30 ms, flip angle=80°, in-plane resolution=3.0 × 3.0 mm, 38 axial slices (3.0 mm thickness), covering the entire brain]. Data was processed using SPM8 (Welcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). From the 260 echo-planar imaging (EPI) images per subject the first four scans were discarded prior to further analyses, allowing for magnetic field saturation, followed by motion correction using a two-pass affine registration. Then, the mean EPI image of each subject was spatially normalized to the MNI152 space using the “unified segmentation” approach (Ashburner and Friston, 2005) and the ensuing deformations applied to the individual EPI volumes. Images were smoothed by a 5-mm full width at half maximum Gaussian kernel. The effect of the following nuisance variables were then removed from the BOLD signal time series of each voxel: i) the six motion parameters derived from the image realignment; ii) the first derivatives of these motion parameters; ii) mean grey matter, white matter and cerebrospinal fluid signal intensity per time point as obtained by separately averaging across voxels attributed to the respective tissue class in the SPM8 segmentation. All nuisance variables entered the model as first and also as second order terms (cf. Satterthwaite et al., 2013). Finally, data was band-pass filtered with the cut-off frequencies of 0.01 and 0.08 Hz as meaningful resting-state correlations will be predominantly found in these frequencies since the BOLD response acts as a low-pass filter (Biswal et al., 1995; Fox and Raichle, 2007). Then, linear (Pearson) correlation coefficients were computed between the ensuing times series of the respective seed regions and those of all other gray matter voxels of the brain. These voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores and fed into a second-level analysis of variance (ANOVA) including an appropriate non-sphericity correction as implemented in SPM8. To investigate differences in task-independent FC between functionally divergent seeds, we contrasted FC between seeds by computing linear contrasts within SPM (e.g. FC of seed A > FC of seed B). In order to only identify regions showing positive FC with the seed these contrasts were always restricted to regions found in the positive main effect of the respective seed by applying minimum conjunction analysis (i.e. (FC of region A > FC of region B) ∩ positive FC of region A).
Results were thresholded at a cluster-level FWE corrected threshold of p<0.05 (cluster-forming threshold at voxel-level p<0.001). Only regions with at least 25 cohesive voxels were considered in the resulting difference maps. Hence, the same threshold was used for MACM and resting state analyses.
3. Results
3.1 Meta-analytic results
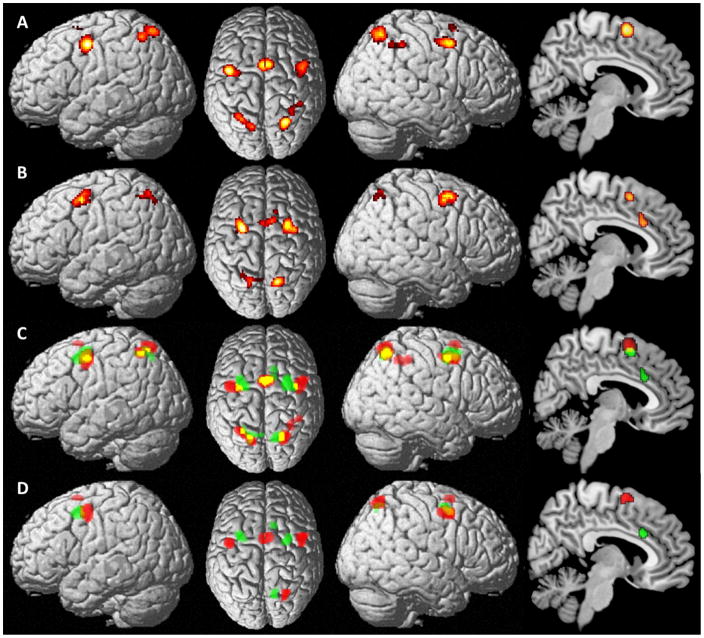
Performing a meta-analysis across the prosaccade task (i.e. experiments contrasting “prosaccades vs. rest” and “prosaccades vs. fixation”) revealed consistent involvement of the SEF, the left and right lateral FEF (covering mainly the precentral gyrus), the bilateral superior parietal lobule (SPL, area 7A) (Scheperjans et al., 2008a; Scheperjans et al., 2008b) and the right intraparietal sulcus (IPS, hlP3 and hIP2) (Choi et al., 2006; Scheperjans et al., 2008b). Moreover, subcortically, consistent involvement of the left thalamus was shown for the prosaccade task (Fig. 1A, Table S1). To identify those regions consistently stronger activated for antisaccades, a meta-analysis across experiment contrasting antisaccades vs. prosaccades was performed. This approach revealed consistent involvement of the bilateral medial FEF (covering mainly superior and middle frontal gyrus), the SEF, bilateral SPL (area 7A, 7P) (Scheperjans et al., 2008a; Scheperjans et al., 2008b), as well as the anterior midcingulate cortex (aMCC) (Fig. 1B, Table S2). Overlaying the results of the two meta-analyses as depicted in Fig. 1C revealed spatial overlap within bilateral SPL, FEF and the SEF (Fig. 1C).
Figure 1.
Figure 1A: Foci of brain activity with significant convergence across all prosaccade experiments included in the meta-analysis (cluster-level p < .05, family-wise error-corrected for multiple comparisons; cluster-forming threshold p < .001 at voxel level).
Figure 1B: Foci of brain activity showing consistent stronger involvement for antisaccades versus prosaccades (cluster-level p < .05, family-wise error-corrected for multiple comparisons; cluster-forming threshold p < .001 at voxel level).
Figure 1C: Overlay (in yellow) of results for the meta-analyses across prosaccades (in red) and across antisaccades versus prosaccades (in green).
Figure 1D: Brain regions showing significantly stronger across-experiment convergence of prosaccade-related activity compared to antisaccade-specific convergence are shown in red. Brain regions showing significantly stronger across-experiment convergence of antisaccade-specific activity compared to prosaccade-specific convergence are shown in green.
As the number of included experiments per meta-analysis (n=12) was rather small in the supplementary results we provide a list of the experiments that contributed to the specific regional effects, showing that the results were very stable and not driven by only a small portion of experiments. In particular, for the meta-analysis across prosaccades at least seven experiments (i.e. more than half of all included experiments) contributed to the effect in each cortical region. Less convergence was found for the thalamus, where only four experiments were contributing to the effect. For antisaccades vs. prosaccades there were at least six experiments contributing for the effect in each region, but for most regions it was even more (eight to ten experiments).
Furthermore, we directly contrasted the above results, to test for differential convergence in pro- and antisaccades, respectively. This analysis revealed stronger convergence of activity for prosaccades in the SEF, the more lateral parts of the FEF as well as the right SPL (area 7A) and the left thalamus (Fig. 1D in red, Table S3). In contrast, antisaccade-specific consistency of activity was found in more medial parts of the FEF, the right aMCC and the right precuneus extending into SPL (Fig. 1D in green, Table S4). That is, within the frontal lobes we found a functional differentiation within the FEF and the posterior medial frontal cortex for pro- and antisaccades.
3.2. Task-dependent and task-independent functional connectivity analyses
Meta-analytic results revealed a spatial dissociation within the frontal cortex. In particular, within the posterior medial frontal cortex the SEF was more consistently found for prosaccades while the aMCC revealed consistent stronger involvement for antisaccades vs. prosaccades. An analog dissociation was found within the lateral frontal cortex, particularly within the FEF, with lateral FEF more often involved in prosaccades and medial FEF more often associated with antisaccades vs. prosaccades. To further examine if the different spatial locations of clusters within the FEF and posterior medial frontal cortex reflect distinct functional regions that can also be distinguished in respect to their functional connectivity profile with other regions, the frontal oculomotor regions resulting from the latter meta-analytic contrast were used as seed regions for MACM and RS connectivity analysis. The general approach was identical for MACM and RS analyses. To test for regions showing increased FC with prosaccade-specific frontal regions, we first performed a contrast between lateral vs. medial FEF’s FC as well as a contrast between SEF’s vs. aMCC’s FC. Then, a conjunction analysis across the specific FC profile of the lateral FEF and the SEF was performed to reveal those regions commonly integrated with prosaccade-specific frontal regions. An analog approach was performed to test for regions functionally coupled with antisaccade-specific regions.
3.2.1 Task-dependent functional connectivity
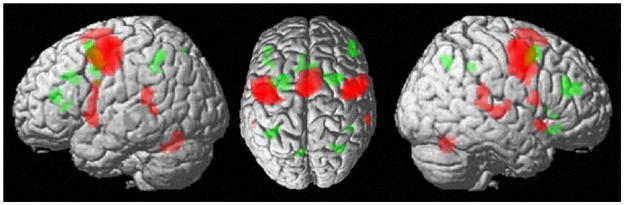
We first performed a contrast between lateral versus medial FEF FC-profile as well as between SEF versus aMCC, to identify regions that showed stronger MACM-FC with prosaccade-related regions. In a next step, a minimum conjunction analysis across these two contrasts was conducted to reveal those regions that show conjoint stronger task-dependent FC with lateral FEF and SEF. This approach revealed a network consisting of bilateral precentral gyrus, superior temporal gyrus (STG), Area 44, the SEF extending into the supplementary motor area (SMA), right temporal pole as well as bilateral putamen, left thalamus and the cerebellum (Fig. 2, in red, Table S5).
Figure 2.
Specific conjoint MACM functional connectivity of the lateral FEF and SEF (in red) and medial FEF and aMCC (in green). That is, regions depicted in red revealed increased FC with the lateral vs. medial FEF as well as increased FC with SEF vs. aMCC. Regions shown in green revealed increased FC with medial vs. lateral FEF as well as with aMCC vs. SEF.
The same rational was done for antisaccade-specific regions. First, a contrast between medial versus lateral FEF and aMCC versus SEF MACM-FC was performed, with subsequent conjunction analysis across specific lateral FEF and aMCC MACM-FC. This analysis revealed a network consisting of bilateral medial frontal gyrus, IPS, dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), the aMCC, left SPL and right anterior insula (Fig. 2, in green, Table S6).
3.2.2 Task-independent functional connectivity
Again, we first performed a contrast between lateral versus medial FEF and SEF versus aMCC RS-FC maps respectively and then performed a conjunction analysis across these contrasts to identify regions that showed specific RS-FC with prosaccade-related regions. This approach revealed a network consisting of bilateral precentral gyrus, middle (MTG) extending into superior temporal gyrus (STG), SEF extending into SMA, as well as left IFG (in particular area 44) (Figure 3, in red, Table S7). In contrast, medial FEF and aMCC showed conjoint specific RS-FC with bilateral DLPFC, IPL, posterior cingulate cortex, the aMCC and right superior frontal gyrus (Figure 3, in green, Table S8).
Figure 3.
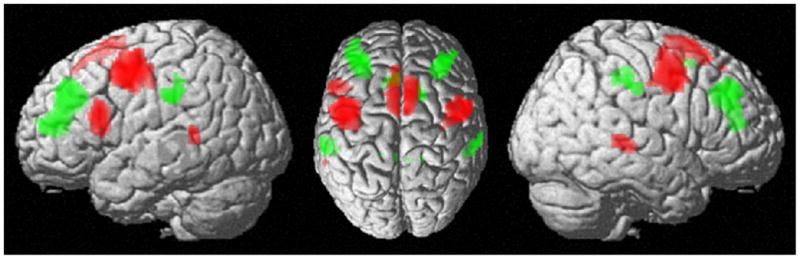
Specific conjoint RS functional connectivity of lateral FEF and SEF (in red) and medial FEF and aMCC (in green). That is, regions depicted in red revealed increased FC with the lateral vs. medial FEF as well as increased FC with SEF vs. aMCC. Likewise, regions shown in green revealed increased FC with medial vs. lateral FEF as well as with aMCC vs. SEF.
4. Discussion
The aim of the present study was to examine frontal involvement for different demands of eye movement control during visually guided prosaccades compared to antisaccades. This was done by integrating results from previous neuroimaging studies using coordinate-based meta-analyses. Within the frontal cortex specific consistent involvement of lateral FEF and posterior SEF was found for prosaccades, while antisaccade performance revealed consistent stronger recruitment of the medial FEF and aMCC. Follow-up task-dependent and task-independent FC analyses revealed differential involvement of saccade-specific frontal regions with motor-related areas as well as subcortical regions. In contrast, antisaccade-specific regions were functionally embedded in a dorsal fronto-parietal network.
The results hence show that differential association of frontal regions with either more basal aspects of eye movement control during the performance of prosaccades or higher cognitive demands during antisaccade performance are corroborated by specific involvement in differential neural networks.
4.1. Meta-analysis
In a first step we performed a meta-analysis across experiments contrasting prosaccades vs. baseline or fixation revealing consistent involvement of bilateral SPL, lateral FEF, SEF, the right IPS and left thalamus (see Fig. 1A). A second meta-analysis was performed across experiments contrasting antisaccades vs. prosaccades and showed consistent stronger involvement of bilateral SPL, medial FEF, SEF and aMCC for antisaccades (Fig. 1B). Finally, commonalities as well as differences between these two experiment classes were delineated by conjunction and contrast analysis. In the following we will discuss each of the regions contributing to saccadic eye movements as well as potential spatial differentiation particularly within the frontal cortex.
Parietal lobe
The dorsal parietal lobe along the IPS, extending dorsomedially into the SPL, as well as the dorsal frontal cortex along the FEF, has commonly been found to be activated in tasks involving covert attentional shifts to peripheral targets as well as when attention and saccades are shifted into the same direction (Dyckman et al., 2007; McDowell et al., 2005). This finding is well in line with the hypothesis that the neural correlates of oculomotor and attentional processes overlap and both processes are highly interrelated (Corbetta, 1998; Corbetta et al., 1998). Spatial and object location information travels from the visual cortex to the posterior parietal cortex (PPC) (Baizer et al., 1991) where visual space information is retinotopically mapped preferentially in the contralateral hemisphere (Sereno et al., 2001). Non-human primate studies have shown that stimulus location is coded within a specific region of the PPC, the parietal eye field (PEF), in terms of distance and direction from the current center of gaze (Colby and Duhamel, 1996) and that this region is connected to the FEF (Ferraina et al., 2002; Schall, 1997). The human homologue of the parietal eye field has been proposed to lie within the deep region of the IPS (Muri et al., 1996). Interestingly, in the present meta-analyses, consistent involvement of the IPS (particularly area hIP2, hIP3) was only found within the right hemisphere for prosaccades. In contrast, a more posterior region of the PPC - area 7A within the SPL - was consistently found across prosaccades, but also for experiments contrasting antisaccades vs. prosaccades (see Figure 1A–C). That is, while area 7A seems to be involved whenever participants have to perform an eye movement towards a visually coded spatial location, it furthermore increases its activity during the performance of the cognitively more demanding antisaccade. Furthermore, a spatial dissociation was observed within right SPL, as more lateral parts were more often involved for prosaccades while medial parts were more consistently involved for antisaccades vs. prosaccades (Fig. 1D).
One possible reason for strong involvement of the SPL for pro- as well as antisaccades in the present study, while the IPS was only found for prosaccades in the right hemisphere, might be that the IPS is known to respond preferentially to visual targets in the contralateral field (Capotosto et al., 2013; Cieslik et al., 2010). As in fMRI paradigms investigating the antisaccade task, trials are rarely analyzed separately for left and right-sided eye movements, less consistent involvement of the IPS might be found. The SPL, in contrast, has been found to play a crucial role in shifting the locus of spatial attention, such as when a spatial displacement occurs in the location of a target or the focus of attention independent of its direction (Capotosto et al., 2013; Molenberghs et al., 2007). This region could therefore be found more consistently irrespective of analyzing eye movements to opposite directions separately or together. The present study hence provides evidence for a strong involvement of area 7A in saccadic eye movements which is most possibly associated with the shifting of attentional resources towards a particular location that takes place when performing a prosaccade but even more so when participants have to endogenously reorient their attention away from the target to the mirror-symmetrical position.
Frontal eye fields
In humans the FEF are located in the precentral gyrus around the intersection with the caudalmost part of the superior frontal gyrus (cf. Paus, 1996) with exact location varying between studies and sometimes also covering the middle frontal gyrus (cf. Amiez and Petrides, 2009; Blanke et al., 2000). Discrepancies between imaging and the few electrical stimulation studies that are available in humans further complicate reliable anatomical delineations of the FEF in humans (cf. Amiez and Petrides, 2009). Lesion and transcranial stimulation studies revealed a key role for the FEF in the initiation of internally-triggered intentional or exploratory saccades (Heide and Kompf, 1998; Wipfli et al., 2001). Anatomical and functional connectivity between vestibular regions and the FEF indicate that these two systems interact during the generation of smooth pursuit eye movements (Fukushima et al., 2006; zu Eulenburg et al., 2012). Furthermore, the FEF have not only been related to the generation but also the preparation of eye movements. For example, Connolly and colleagues (Connolly et al., 2002) used an antisaccade task with a variable gap period before appearance of the target stimulus and participants were instructed before the gap period to make either a pro- or antisaccade. Using this setup the authors found that activation within the FEF began to increase during the gap period and moreover revealed higher activity for anti-than prosaccadic trials. Thereby it seems that the FEF are already involved at an early stage when the intention to perform an eye movement occurs and not only during the generation process itself.
The present meta-analyses revealed consistent involvement of the frontal eye fields for prosaccades as well as consistent higher activity for antisaccades compared to prosaccades. Interestingly, even though there was some overlap between the FEF clusters revealed from the two meta-analyses, the exact location of the FEF clusters differed between both meta-analyses. While the FEF foci consistently found for prosaccades were located on the precentral sulcus, the antisaccade-specific foci lay more medial covering mainly the superior and middle frontal gyrus (Fig. 1). Different functional properties of medial and lateral eye fields have previously been proposed based on imaging studies revealing specific involvement of medial vs. lateral FEF during newly learned saccade sequences compared to familiar ones (Grosbras et al., 2001), or saccades to unpredictable targets that require increased spatial attention demands (Simo et al., 2005). Similarly, a combined MEG-/EEG-study showed increased medial FEF activity for antisaccades compared to prosaccades prior to saccade generation, while the lateral FEF showed a divergent pattern (McDowell et al., 2005). Here, activity for antisaccades showed an initial rise but decelerated close to the antisaccade response while for prosaccades a continuous activity increase up until response generation was observed. Hence, it may be argued that the lateral part of the FEF is involved in the actual motor output, while the medial part is more strongly associated with higher cognitive processes that come into play e.g. when eye-movements have to be initiated endogenously (Jamadar et al., 2013; McDowell et al., 2008). Our results are well in line with this hypothesis. Importantly, the contrast analysis provided statistical evidence for a spatial dissociation within the FEF, with the lateral parts being consistently involved in prosaccades while the medial FEF were consistently stronger associated with antisaccade performance (Fig. 1D).
Summarizing, the present meta-analytic approach reveals meta-analytic evidence for a spatial dissociation within the frontal eye fields. While the lateral aspects are involved in more basal processes of eye movement preparation the more medial parts are specifically recruited for higher control of movement preparation that is necessary when selection of motor behavior has to be chosen based on specific task instructions. This spatial dissociation may then also explain some of the variance found for anatomical location of the FEF in different studies, which may in some cases be explained by different functional demands and hence differential involvement of subregions of the FEF.
Posterior medial frontal cortex
Within the posterior medial frontal cortex the SEF as well as the aMCC were identified being associated with different aspects of eye movement control. The SEF is located at the paracentral sulcus, rostral to the supplementary motor area (SMA) (Grosbras et al., 1999). Neural stimulation studies in primates as well as lesion and functional imaging studies in humans indicate a crucial role for the SEF in various saccadic tasks such as e.g. visually guided saccades (Luna et al., 1998), smooth pursuit (Missal and Heinen, 2001), monitoring of errors and reward or when there is conflict between several ongoing, competing saccadic responses (Parton et al., 2007). The present finding of consistent activity within the SEF for experiments contrasting prosaccades versus fixation or baseline provides further evidence that the SEF is involved in visually guided saccades. Moreover, consistent with the view that the SEF is strongly recruited when conflict between competing oculomotor response plans arises (cf. Husain et al., 2003; Parton et al., 2007) the same region was also consistently found for increased control demands during antisaccade performance as revealed by overlapping the two results (see Fig. 1C). In line with that Miller and colleagues (Miller et al., 2005) found two regions lying very close to our right medial FEF and SEF (which they labeled preSMA) to show greater coherence of the respective fMRI time series during antisaccades vs. prosaccades providing further evidence that these two regions strongly interact when increased control processes become necessary for accurate eye movement behavior. Interestingly however, the present contrast analysis revealed the SEF to be more consistently involved in prosaccadic eye movements. It has to be noted that stronger consistency for the SEF for prosaccades is not contradicting the finding that activity within the SEF increases when the control demands for eye movements increase. With meta-analytic contrasts we merely test which brain regions are more consistently involved in a specific process. The present results let us infer that the SEF is already strongly involved in the more reflexive processes of eye movements during prosaccades and would thereby reflect a ubiquitous component in the saccadic motor circuit that is involved whenever an eye movement has to be made. Additional increases of activity during more complex eye movements, such as antisaccades, may then be observed depending on the level of difficulty increases.
Within the posterior medial frontal cortex another region showed differential involvement for antisaccades compared to prosaccades. Consistent increased activity for antisaccades was observed in the aMCC, which has previously often been labeled dorsal anterior cingulate cortex based on Brodmann’s coarser labelling despite evidence of a structurally and functionally distinct middle cingulate cortex (cf. Palomero-Gallagher et al., 2009; Vogt, 2004, 2005). Interestingly, this region did not overlap with the cingulate eye field that has been proposed to be located more posteriorly (Amiez and Petrides, 2009). Previous lesion and imaging studies revealed evidence for a crucial role of the aMCC specifically in higher complex saccades, such as memory guided saccades or antisaccades (Brown et al., 2006; Gaymard et al., 1998; Matsuda et al., 2004). Moreover, the aMCC has been found to increase its activity in error antisaccade trials compared to correct antisaccade trials (Ford et al., 2005), specifically later in the trial (Polli et al., 2005). These results are well in line with the hypothesis of a specific role of the aMCC for error detection and conflict monitoring (for a review see Botvinick et al., 2004). More generally, the anterior portion of the midcingulate cortex has been consistently found in various tasks that require suppression of a routine action in favour of another task-dependent one (Cieslik et al., 2015). The role of the aMCC might then correspond to what Stuss and collegues (Stuss et al., 1995) conceptualized as “energization of the appropriate task schemata” and what would come into play whenever a non-dominant task schemata would need to be “energized” or activated (cf. Cieslik et al., 2015). This process would also come into play when generating the non-dominant antisaccade in the contralateral direction of the stimulus. Following this interpretation, the region within the aMCC found to be consistently stronger activated during the performance of antisaccades compared to prosaccades in the present study may not be selectively involved in eye movement control, but more generally in the activation of the appropriate task schema necessary to perform the required action.
Subcortical areas
The only subcortical region that was found to be consistently involved in the generation of eye movements in the present meta-analyses was the thalamus, showing consistent involvement for prosaccade generation (Fig. 1). Cells within the thalamus form a link between subcortical areas and the cortex. Within the cortico-basal ganglia oculomotor network it is generally believed that the signals that reach the basal ganglia from the cortical eye fields are sent back to the cortex via the oculomotor thalamus (for a review see Isoda and Hikosaka, 2011 as well as Tanaka and Kunimatsu, 2011). While the thalamus has been found during the performance of visually guided saccades in human and non-human primates (e.g. Aichert et al., 2012; Baker et al., 2006), some other authors have related the thalamus mainly to self-initiated and voluntarily triggered saccades (Grosbras et al., 2005; Tanaka, 2007). We did not find evidence for consistent stronger thalamic involvement for antisaccades versus prosaccades in the present meta-analysis, which is in line with a previous meta-analysis from Jamadar and colleagues (2013). We therefore would suggest, that the thalamic involvement during eye movement performance is mainly related to the motor processes that are triggered whenever an eye movement is initiated and that would come into play to some extent whenever an oculomotor movement is initiated, no matter if it is visually driven or internally initiated.
No other subcortical region of the cortico-basal ganglia network was found in the present meta-analysis even though relatively precise models of basal ganglia involvement in saccadic eye movements have been established. Within the basal ganglia circuit the caudate nucleus receives input from the frontal cortex and projects either directly on the substantia nigra (SN) or through an indirect pathway - via the globus pallidus and subthalamic nucleus - on the SN. From the SN information is transmitted back to the cortex via the thalamus or forwarded to brain stem structures such as the superior colliculus (Hikosaka et al., 2000; Smith et al., 1998). The lack of basal ganglia involvement in the present study might most possibly be due to methodological reasons of imaging techniques. In particular, deep brain areas are difficult to investigate with standard fMRI protocols due to their small size and close proximity to vascular structures which may be causing physiological noise in the brain stem or midbrain (Guimaraes et al., 1998) and hence making it difficult to reliably measure hemodynamic responses in these areas.
Summarizing, the present meta-analytic results revealed consistent involvement of a frontoparietal network known to be involved in oculomotor control (e.g. Jamadar et al., 2013; McDowell et al., 2008; Munoz and Everling, 2004). Consistent increased activity during antisaccade performance was mainly identified in those cortical regions that were also recruited during prosaccades as revealed by overlaying the results of both meta-analyses. Furthermore, consistent with our hypothesis, a spatial dissociation was found particularly within the premotor and the posterior medial frontal cortex for different aspects of saccadic eye movement control (Fig. 1D). While the medial FEF and aMCC showed antisaccade-specific involvement, lateral FEF and SEF were stronger related to visually guided prosaccades. We would hypothesize that if these results reflect differential involvement in the control of eye movements these regions should also be integrated in distinct neural networks. To investigate potential involvement in differential neural networks FC analyses were performed using task-dependent and task-independent FC which will be discussed in the following paragraphs.
4.2 Functional connectivity analyses
As outlined in the introduction, task-dependent FC enables us to derive hypotheses about regions that form functional networks based on their common co-activation profile across different tasks (cf. Cieslik et al., 2013). Task-independent FC in contrast allows inferences about co-activation of spontaneous BOLD signal time series in individuals, in the absence of an externally structured task (for a review see van den Heuvel and Hulshoff Pol, 2010). In the present study we used task-dependent FC based on MACM to delineate differences in the functional connectivity pattern between the lateral and medial FEF as well as between the SEF and aMCC. Task-independent FC was then used as a second approach to confirm the differences found in task-based connectivity.
4.2.1 Task-dependent and task-independent FC
Performing a conjunction analysis across the FC maps for the prosaccade-related seeds revealed specific task-dependent FC of lateral FEF and SEF with the precentral gyrus and SEF/SMA surrounding the original seed regions (Fig. 2). Beside areas within and closely surrounding the original seed regions, specific task-dependent FC was found with bilateral area 44 and STG as well as bilateral putamen, left thalamus and the cerebellum. A very similar cortical pattern was found using RS analyses, which revealed specific task-independent FC with bilateral precentral gyrus and SEF/SMA as well as the middle extending into superior temporal gyrus and left area 44. However, no specific RS-FC with subcortical areas was found.
Importantly, we only report specific FC; that is, FC with regions that were stronger for lateral FEF vs. medial FEF and also for SEF vs. aMCC. It can hence be concluded that lateral FEF and SEF show stronger FC with each other compared to the medial FEF and aMCC. Interestingly, while the SEF is anatomically connected not only to the lateral but also the medial FEF (Schall et al., 1993) it seems that functionally the SEF is stronger linked to the lateral FEF.
Further frontal cortex involvement was found in the IFG, particularly area 44. While this region has not commonly been associated with eye movements in the past, a recent parcellation study of Clos and colleagues (2013) functionally characterized subregions within left area 44 based on BrainMap meta-data and showed that the posterior portion of area 44 is more strongly related to action processes, such as action execution as well as action imagination. Clos and colleagues furthermore showed that all identified subregions within area 44 are functionally connected to the IFG and adjacent precentral gyrus, the SMA as well as the thalamus and putamen subcortically, providing further evidence that this region is functionally embedded in a motor network.
Besides the frontal cortex, specific FC with other cortical regions was only found with the bilateral posterior STG extending into the MTG. This region is known to respond to biologically valid information, specifically to eye movements of another person (Pelphrey et al., 2005; Puce et al., 1998). In line with that, the STG and adjacent STS have been found to increase their activity during joint attention, in particular when a person is following someone else’s gaze, i.e. is responding to joint attention (Redcay et al., 2012). The STG may therefore play a role in the integration of biologically valid direction information into eye movement motor plans.
Subcortical involvement was revealed by the task-based FC analysis that showed specific MACM-FC of lateral FEF and SEF with bilateral putamen and left thalamus. It has long been proposed that within the basal ganglia-thalamocortical circuit the putamen receives input from cortical areas such as motor and somatosensory cortices and itself projects on the pallidum. This area, in turn, innervates the thalamus, which projects back to a specific cortical area (cf. Alexander et al., 1986; DeLong and Wichmann, 2007). Data from non-human primates have shown that the thalamus is reciprocally connected with the FEF and SEF (for a review see Tanaka and Kunimatsu, 2011). In the present study we could identify the mediodorsal thalamic part which showed specific MACM-FC with the SEF and lateral FEF as thal-prefrontal, i.e. a region that is anatomically connected with the prefrontal cortex as revealed by diffusion data in humans (Behrens et al., 2003). The same region in the left hemisphere was found to be consistently activated for prosaccades in the present meta-analysis, a region that we would suggest to be involved in the basal aspects of eye movement initiation, no matter if it is a visually guided or endogenous initiated saccade.
Even though lesion and ataxia studies indicate a crucial role of the cerebellum for both accurate and adaptive smooth pursuit eye movements and horizontal as well as vertical saccades (Moschner et al., 1999; for a review see Robinson and Fuchs, 2001 and Voogd et al., 2012) neuroimaging studies have found less consistent involvement of the cerebellum in pro- and antisaccade behavior, which was also reflected in our meta-analyses. Potential reasons for this might be the lack of complete cerebellar coverage particularly in older imaging studies. However, in the task-based FC analyses specific MACM-FC was observed particularly with lobule VI of the cerebellum. A tracer study in non-human primates showed that this part of the cerebellum is reciprocally connected with M1 (Kelly and Strick, 2003). Furthermore, a recent RS-FC analysis in humans that aimed to investigate cerebellar contributions to several previously identified networks found this region to participate in the sensorimotor network including amongst others the sensorimotor cortex, premotor cortex, SMA, aMCC but also subcortical regions such as the thalamus and putamen (Habas et al., 2009). We would hence suggest that lobule VI of the cerebellum that showed specific MACM-FC with the lateral FEF and SEF in the present study reveals an adaptive control function to afford saccade mechanisms with adaptability through interaction with motor related cortical regions as well as subcortically the thalamus and putamen.
Summarizing, investigation of lateral FEF’ and SEF’s functional connectivity profile revealed that these regions are commonly integrated in a network related to motor output control including the basal ganglia-thalamocortical motor circuit as well as the more motor related part of area 44, the STG and cerebellum.
In contrast, the medial FEF and aMCC were integrated in a (dorsal) fronto-parietal network. In particular, they showed conjoint MACM-FC with a bilateral network consisting of the middle frontal gyrus and posterior medial frontal cortex surrounding the original seed region, the DLPFC, IFG, IPS, the left SPL, right angular gyrus and right anterior insula (Fig. 2). The RS analyses revealed a slightly different connectivity profile, particularly with bilateral DLPFC, IPL, PCC, the aMCC and right superior frontal gyrus (Fig. 3). A very similar set of fronto-parietal regions, including premotor regions of the precentral gyrus, posterior parts of the IFG and adjacent insula, the middle frontal gyrus, the posterior medial frontal cortex as well as parietal regions including the IPS and adjacent IPC, has been proposed to respond in a domain- and process-general fashion to a wide variety of cognitive demanding tasks and has hence been called the multiple demand network (for a review see Duncan, 2010; Federenko et al., 2013). In line with that these regions have been implicated in a variety of tasks not only involving cognitive action control (Cieslik et al., 2015), but also working memory (Rottschy et al., 2012) or vigilant attention (Langner and Eickhoff, 2013). Moreover, signal fluctuations in these regions strongly correlate with each other during specific task demands but also during rest conditions when participants are lying in the scanner without a specific task to perform (Blank et al., 2014). Most of these regions, specifically in the PFC, have also been found to be important for the increased cognitive control demands during antisaccade performance. Even though we could not find consistent stronger activation for antisaccades versus prosaccades within the PFC in our meta-analysis, a crucial role of the lateral PFC in antisaccade performance has been revealed by lesion (Pierrot-Deseilligny et al., 2003) as well as neuroimaging studies (Chikazoe et al., 2007; Ettinger et al., 2008; Ford et al., 2005) particularly in the late preparation period. While the involvement of the lateral PFC in tasks such as the antisaccade as well as the go/no-go or stop signal task has usually been associated with specific inhibitory processes, recent evidence suggests that this region might be more strongly related to the maintenance and implementation of the adequate task set including the activation of the appropriate behavioral alternative (Everling and Johnston, 2013; Munakata et al., 2011).
While both, task-dependent and task-independent, FC analyses revealed specific involvement with the lateral PFC, FC connectivity with the parietal cortex differed between both methods. In particular, while MACM-FC revealed FC with IPS and SPL, that is regions that were also found in the meta-analytic approach and play a crucial role in attentional orienting, RS-FC was found with the IPC. Moreover, specific FC with the PCC was found in the RS analysis that was not present in the MACM analysis. These differences may most possibly be related to methodological reasons. As the multiple demand network has been initially characterized based on task-fMRI data (Duncan, 2010; Duncan and Owen, 2000) that all used a structured task, differences to RS-FC are actually not surprising but may be explained by the lack of specific task instructions that most commonly require participants to process a visual or another sensory input and process it in a task-specific manner. In contrast, in RS sessions participants are laying in the scanner without performing a structured task, hence attentional resources will be directed more internally and thoughts being more self-referential concerning e.g. autobiographic contents (Mazoyer et al., 2001). In line with that, besides its crucial role in attentional capturing and reorienting to salient and behaviorally relevant stimuli the IPC–and particularly the supramarginal gyrus - has been associated with episodic memory retrieval (for a review see Hutchinson et al., 2009; Skinner and Fernandes, 2007). Furthermore, the dorsal aspect of the PCC that was also found in the RS analysis has been shown to feature FC with regions strongly related to cognitive control such as the dorsal attention network, the fronto-parietal control network, the salience network but also the default mode network (Leech et al., 2011; for a review see Leech and Sharp, 2014). It has hence been proposed to modulate the interaction between cognitive control and default mode networks, thereby playing a key role in mediating the attentional focus between internal representations and external changes in the environment, which might play a constant role when participants are lying in the scanner without a specific task to perform (cf. Leech et al., 2011).
Summarizing, the medial FEF and aMCC that were found to be more consistently associated with increased demands for antisaccades versus prosaccades seem to be integrated in a network that is associated with a diversity of cognitive control processes. Higher cognitive control specifically necessary when a participant has to respond to a visual stimulus in an incongruent manner could then be facilitated through interactions of medial FEF and aMCC with other top-down regions, specifically those regions that play a dominant role in the multiple demand network.
4.3 Integrating meta-analytic and FC results
Bringing together the connectivity results with those from the meta-analyses, the hypothesis of a functional dissociation within the FEF and posterior medial frontal cortex as suggested by the meta-analytic findings is corroborated by the FC analyses that showed divergent functional profiles of the respective subregions. That is, different spatial locations within the posterior medial frontal cortex and FEF cannot be explained simply by spatial uncertainty, but rather reflect different functional modules that are integrated in distinct neural networks. In particular, involvement of lateral FEF and SEF for the more basal aspects of eye movements thereby goes along with FC with a motor output network, while involvement of medial FEF and aMCC for increased control demands during antisaccade performance is associated with FC with the multiple demand network. Interestingly, FC was not only observed with those regions found in the respective meta-analysis but revealed a much broader network of cortical and subcortical regions. Most of these regions are part of the saccade network as supported by non-human primate and human studies (for a review see Munoz and Everling, 2004). Different factors might explain why not all of these regions were found in the meta-analytic results. First, as already noted some regions such as subcortical regions or the cerebellum may be difficult to investigate reliably with fMRI due to technical reasons (Guimaraes et al., 1998; McDowell et al., 2008) and may only be found when authors specifically focus on that area and adapt the imaging protocol accordingly. Moreover, specific regional involvement may depend on what kind of information has to be integrated to drive an eye movement towards the respective direction; e.g. the STG might be selectively involved when biologically valid stimuli such as eye movements are presented but not commonly in every antisaccade task. Moreover, it has to be kept in mind that the original set of experiments included in each meta-analysis was rather small (n=12) compared to other large-scale meta-analyses that investigate widely-used fMRI paradigms, such as working memory experiments. Therefore, regions that show higher spatial variability across studies such as the DLPFC may be found less consistently using meta-analyses across a relatively small number of studies.
Summarizing, the present study revealed a spatial dissociation within the frontal oculomotor regions. While volitional saccade paradigms have provided evidence for two different foci of activity within the FEF (Dyckman et al., 2007; Jamadar et al., 2013; McDowell et al., 2008) a spatial dissociation has rarely been tested directly. Furthermore, to our knowledge this is the first study that could show using multimodal FC analyses that this spatial dissociation is driven by different neural network involvement. An analog spatial dissociation was present within the anterior-posterior axis of the posterior medial frontal cortex.
Relating our results to the well characterized saccade network let us assume that input of frontal oculomotor areas to the subcortical motor system and cerebellum might be mainly implemented through the lateral FEF and SEF. In contrast, the medial FEF and aMCC specifically communicate with regions of the multiple demand network and together might implement higher cognitive control processes necessary in the antisaccade task. It has to be kept in mind that the present meta-analytic and FC approaches do not allow to make any inference about the directionality between the respective regions, which has however rarely been investigated in the literature. One previous study has investigated effective connectivity within the saccade network and found top-down connections from cortical regions, such as the IPS, FEF, SEF and anterior insula to the thalamus and putamen during pro- and antisaccades (Hwang et al., 2010). Moreover, during antisaccades the medial frontal gyrus and IFG were additionally integrated in the network and revealed top-down influences on the cortical eye fields as well as subcortical areas. This is in line with the hypothesis that additional PFC areas come into play when complexity of saccadic movements increase (Everling and Fischer, 1998; Munoz and Everling, 2004; Sweeney et al., 2007). To better understand the complexity of interregional communication during saccadic eye movements, future studies should try to further disentangle the flow of information between regions using e.g. effective connectivity.
In conclusion, it seems that spatially neighboring regions within the frontal lobe may represent transition zones where different functional roles are driven by distinct involvement in neural networks. Within the saccade network the FEF and the posterior medial frontal cortex may hence represent an interface between motor output and cognitive control and mediate between the different control demands necessary for accurate eye movements.
4.4 Limitations and future directions
As already mentioned earlier it has to be noted that in comparison to other large-scale meta-analyses that focus on more widely used neuroimaging paradigms such as working memory or face processing, in the present study only a limited number of experiments (n=12) met the inclusion criteria for each meta-analysis. This drawback might influence how representative the resulting seed regions are which were then used for further connectivity analyses. Moreover, this point may particularly have an impact on regions that are known to show higher spatial variability across studies such as the DLPFC or IFC. These regions may therefore be found less consistently using meta-analyses across a relatively small number of studies. At the same time, the contributions reported in the supplementary results revealed that the regional effects that could be identified in the present meta-analyses were very stable. In particular, for each meta-analysis at least half of the experiments (but mostly even eight to ten experiments) contributed to the effect in each cortical region. Less convergence was found only for the thalamus. Thus, we could show that even though the total number of included experiments was rather small, results were not driven by only a small portion of the studies included.
Another limitation is that we here only included prosaccades and antisaccades but no other eye movements such as e.g. smooth pursuit or memory-guided saccades. Therefore, our results of a functional dissociation within the FEF and the posterior medial frontal cortex for either more basal motor processes versus higher cognitive control processes are dependent on the type of eye movement included. As studies in macaque monkeys have revealed evidence for a specific involvement of the caudal parts of the FEF for smooth pursuit eye movements (Fukushima et al., 2011; Fukushima et al., 2013), the question arises if the FEF may be subdivided into even more finely graduated submodules than previously thought and if there might also be a rostro-caudal functional gradient within the FEF. Future imaging studies could hence try to disentangle further potential subdivisions within the FEF by comparing the neural correlates of saccades, antisaccades, memory-guided saccades as well as smooth pursuit eye movements within the same participants.
Conclusion
In the present study functional differentiations within the frontal cortex for eye movement control were investigated by integrating results from neuroimaging studies analyzing pro- and antisaccade performance in healthy participants. We found that the lateral FEF and the SEF were more often involved in prosaccade performance whereas the medial FEF and the aMCC showed consistent increased involvement for antisaccades vs. prosaccades. This functional dissociation was furthermore mirrored by a differential involvement in neural networks as revealed by subsequent MACM- and RS-FC analyses. While the frontal regions stronger recruited for prosaccades were embedded in a motor output network, the frontal regions associated with higher control mechanisms for eye movements showed stronger FC with a multiple demand network that comes into play whenever task difficulty increases.
The present results reveal quantitative functional as well as multimodal network evidence that specific regions within the frontal cortex are differentially embedded in the saccade network. In particular, we would propose a functional gradient within the FEF and posterior medial frontal cortex with medial parts of the FEF and anterior parts of the posterior medial frontal cortex being strongly involved in higher cognitive control mechanisms necessary in the antisaccade task while lateral parts of the FEF and the posterior medial frontal cortex are associated with the motor output.
Supplementary Material
Highlights.
Meta-analyses show differential involvement of frontal cortex for eye movements
Lateral FEF and posterior SEF reveal stronger convergence for prosaccades
Medial FEF and aMCC reveal consistent stronger involvement for antisaccades
Lateral FEF and posterior SEF show specific FC with a motor network
Medial FEF and aMCC show specific FC with a cognitive control network
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1, LA 3071/3, EI816/6-1), the National Institute of Mental Health (R01-MH074457) and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (Human Brain Project).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. Journal of neurophysiology. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Aichert DS, Williams SC, Moller HJ, Kumari V, Ettinger U. Functional neural correlates of psychometric schizotypy: an fMRI study of antisaccades. Psychophysiology. 2012;49:345–356. doi: 10.1111/j.1469-8986.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amiez C, Petrides M. Anatomical organization of the eye fields in the human and non-human primate frontal cortex. Progress in neurobiology. 2009;89:220–230. doi: 10.1016/j.pneurobio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RS, Kennard C. Cortical control of saccades and fixation in man. A PET study. Brain : a journal of neurology. 1994;117(Pt 5):1073–1084. doi: 10.1093/brain/117.5.1073. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Babapoor-Farrokhran S, Hutchison RM, Gati JS, Menon RS, Everling S. Functional connectivity patterns of medial and lateral macaque frontal eye fields reveal distinct visuomotor networks. Journal of neurophysiology. 2013;109:2560–2570. doi: 10.1152/jn.01000.2012. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Patel GH, Corbetta M, Snyder LH. Distribution of activity across the monkey cerebral cortical surface, thalamus and midbrain during rapid, visually guided saccades. Cereb Cortex. 2006;16:447–459. doi: 10.1093/cercor/bhi124. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. The Journal of comparative neurology. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blank I, Kanwisher N, Fedorenko E. A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. Journal of neurophysiology. 2014;112:1105–1118. doi: 10.1152/jn.00884.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Spinelli L, Thut G, Michel CM, Perrig S, Landis T, Seeck M. Location of the human frontal eye field as defined by electrical cortical stimulation: anatomical, functional and electrophysiological characteristics. Neuroreport. 2000;11:1907–1913. doi: 10.1097/00001756-200006260-00021. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Tosoni A, Spadone S, Sestieri C, Perrucci MG, Romani GL, Della Penna S, Corbetta M. Anatomical segregation of visual selection mechanisms in human parietal cortex. J Neurosci. 2013;33:6225–6229. doi: 10.1523/JNEUROSCI.4983-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of cognitive neuroscience. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. The Journal of comparative neurology. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23:2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB. Dissociating bottom-up and top-down processes in a manual stimulus-response compatibility task. Journal of neurophysiology. 2010;104:1472–1483. doi: 10.1152/jn.00261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB. Tackling the multifunctional nature of Broca’s region meta-analytically: Co-activation-based parcellation of area 44. Neuroimage. 2013;83C:174–188. doi: 10.1016/j.neuroimage.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Spatial representations for action in parietal cortex. Brain research Cognitive brain research. 1996;5:105–115. doi: 10.1016/s0926-6410(96)00046-8. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nature neuroscience. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of neurology. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Incoccia C, Grassi F, Cappa SF, Bettinardi V, Galati G, Pizzamiglio L, Fazio F. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Experimental brain research. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in cognitive sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clinical EEG and neuroscience. 2011;42:107–121. doi: 10.1177/155005941104200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Williams SC, Patel D, Michel TM, Nwaigwe A, Caceres A, Mehta MA, Anilkumar AP, Kumari V. Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–561. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Johnston K. Control of the superior colliculus by the lateral prefrontal cortex. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2013;368:20130068. doi: 10.1098/rstb.2013.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraina S, Pare M, Wurtz RH. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. Journal of neurophysiology. 2002;87:845–858. doi: 10.1152/jn.00317.2001. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. Journal of neurophysiology. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fukumoto-Motoshita M, Matsuura M, Ohkubo T, Ohkubo H, Kanaka N, Matsushima E, Taira M, Kojima T, Matsuda T. Hyperfrontality in patients with schizophrenia during saccade and antisaccade tasks: a study with fMRI. Psychiatry and clinical neurosciences. 2009;63:209–217. doi: 10.1111/j.1440-1819.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Kurkin S, Kaneko CR, Fukushima K. The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. Journal of vestibular research : equilibrium & orientation. 2006;16:1–22. [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Shichinohe N, Kurkin S, Kaneko CR, Fukushima K. Neuronal activity in the caudal frontal eye fields of monkeys during memory-based smooth pursuit eye movements: comparison with the supplementary eye fields. Cereb Cortex. 2011;21:1910–1924. doi: 10.1093/cercor/bhq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Fukushima J, Warabi T, Barnes GR. Cognitive processes involved in smooth pursuit eye movements: behavioral evidence, neural substrate and clinical correlation. Frontiers in systems neuroscience. 2013;7:4. doi: 10.3389/fnsys.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Cassarini JF, Dubard T, Rancurel G, Agid Y, Pierrot-Deseilligny C. Effects of anterior cingulate cortex lesions on ocular saccades in humans. Experimental brain research. 1998;120:173–183. doi: 10.1007/s002210050391. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Leonards U, Lobel E, Poline JB, LeBihan D, Berthoz A. Human cortical networks for new and familiar sequences of saccades. Cereb Cortex. 2001;11:936–945. doi: 10.1093/cercor/11.10.936. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Lobel E, Van de Moortele PF, LeBihan D, Berthoz A. An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex. 1999;9:705–711. doi: 10.1093/cercor/9.7.705. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Melcher JR, Talavage TM, Baker JR, Ledden P, Rosen BR, Kiang NY, Fullerton BC, Weisskoff RM. Imaging subcortical auditory activity in humans. Hum Brain Mapp. 1998;6:33–41. doi: 10.1002/(SICI)1097-0193(1998)6:1<33::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Heide W, Kompf D. Combined deficits of saccades and visuo-spatial orientation after cortical lesions. Experimental brain research. 1998;123:164–171. doi: 10.1007/s002210050558. [DOI] [PubMed] [Google Scholar]
- Herweg NA, Weber B, Kasparbauer A, Meyhofer I, Steffens M, Smyrnis N, Ettinger U. Functional magnetic resonance imaging of sensorimotor transformations in saccades and antisaccades. Neuroimage. 2014;102(Pt 2):848–860. doi: 10.1016/j.neuroimage.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological reviews. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nature neuroscience. 2003;6:117–118. doi: 10.1038/nn1005. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learning & memory. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Cortico-basal ganglia mechanisms for overcoming innate, habitual and motivational behaviors. The European journal of neuroscience. 2011;33:2058–2069. doi: 10.1111/j.1460-9568.2011.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar SD, Fielding J, Egan GF. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Frontiers in psychology. 2013;4:749. doi: 10.3389/fpsyg.2013.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychological bulletin. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law I, Svarer C, Holm S, Paulson OB. The activation pattern in normal humans during suppression, imagination and performance of saccadic eye movements. Acta physiologica Scandinavica. 1997;161:419–434. doi: 10.1046/j.1365-201X.1997.00207.x. [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR American journal of neuroradiology. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. The Anatomical record. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 3rd. Oxford University Press; New York: 1999. [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Tian JR. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Progress in brain research. 2006;151:461–501. doi: 10.1016/S0079-6123(05)51015-X. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, Barton JJ. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci. 2007;27:1791–1798. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry research. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain research bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- Miller LM, Sun FT, Curtis CE, D’Esposito M. Functional interactions between oculomotor regions during prosaccades and antisaccades. Hum Brain Mapp. 2005;26:119–127. doi: 10.1002/hbm.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. Journal of neurophysiology. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17:2703–2712. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- Moschner C, Crawford TJ, Heide W, Trillenberg P, Kompf D, Kennard C. Deficits of smooth pursuit initiation in patients with degenerative cerebellar lesions. Brain : a journal of neurology. 1999;122( Pt 11):2147–2158. doi: 10.1093/brain/122.11.2147. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in cognitive sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Muri RM, Iba-Zizen MT, Derosier C, Cabanis EA, Pierrot-Deseilligny C. Location of the human posterior eye field with functional magnetic resonance imaging. Journal of neurology, neurosurgery, and psychiatry. 1996;60:445–448. doi: 10.1136/jnnp.60.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci U S A. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, Morgan PS, Jackson S, Rees G, Husain M. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia. 2007;45:997–1008. doi: 10.1016/j.neuropsychologia.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. Journal of neurophysiology. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cereb Cortex. 2005;15:1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Petit L, Zago L, Vigneau M, Andersson F, Crivello F, Mazoyer B, Mellet E, Tzourio-Mazoyer N. Functional asymmetries revealed in visually guided saccades: an FMRI study. Journal of neurophysiology. 2009;102:2994–3003. doi: 10.1152/jn.00280.2009. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain : a journal of neurology. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Kleiner M, Saxe R. Look at this: the neural correlates of initiating and responding to bids for joint attention. Frontiers in human neuroscience. 2012;6:169. doi: 10.3389/fnhum.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annual review of neuroscience. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall J. Visuomotor Areas of the Frontal Lobe. In: Rockland K, Kaas J, Peters A, editors. Extrastriate Cortex in Primates. Springer; US: 1997. pp. 527–638. [Google Scholar]
- Schall JD, Morel A, Kaas JH. Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Visual neuroscience. 1993;10:385–393. doi: 10.1017/s0952523800003771. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008a;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008b;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schraa-Tam CK, van Broekhoven P, van der Geest JN, Frens MA, Smits M, van der Lugt A. Cortical and cerebellar activation induced by reflexive and voluntary saccades. Experimental brain research. 2009;192:175–187. doi: 10.1007/s00221-008-1569-4. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: a review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. The Journal of comparative neurology. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. Neuroimage. 2007;36(Suppl 2):T54–60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Cognitive signals in the primate motor thalamus predict saccade timing. J Neurosci. 2007;27:12109–12118. doi: 10.1523/JNEUROSCI.1873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kunimatsu J. Contribution of the central thalamus to the generation of volitional saccades. The European journal of neuroscience. 2011;33:2046–2057. doi: 10.1111/j.1460-9568.2011.07699.x. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. Cingulate Gyrus. In: Paxinos G, Mai JK, editors. The Human Nervous System. Elsevier; Amsterdam: 2004. pp. 915–949. [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410. doi: 10.1007/s12311-010-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener SP, Johnston K, Everling S. Microstimulation of monkey dorsolateral prefrontal cortex impairs antisaccade performance. Experimental brain research. 2008;190:463–473. doi: 10.1007/s00221-008-1488-4. [DOI] [PubMed] [Google Scholar]
- Wipfli M, Felblinger J, Mosimann UP, Hess CW, Schlaepfer TE, Muri RM. Double-pulse transcranial magnetic stimulation over the frontal eye field facilitates triggering of memory-guided saccades. The European journal of neuroscience. 2001;14:571–575. doi: 10.1046/j.0953-816x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, Luo YL, Dilks DD, Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J Neurosci. 2011;31:10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60:162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.