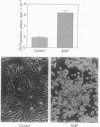
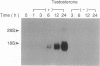
Abstract
An androgen-dependent mouse mammary carcinoma cell line (SC-3) requires androgen for growth stimulation. We have shown previously that androgen acts on SC-3 cells to induce secretion of a fibroblast growth factor (FGF)-like growth factor, which in turn stimulates growth of the cells in an autocrine manner. In this study, the androgen-induced growth factor (AIGF) was purified from a conditioned medium of SC-3 cells stimulated with testosterone. cDNA cloning of AIGF by use of its partial amino acid sequence data revealed that AIGF is a distinctive FGF-like growth factor. An AIGF cDNA (pSC17) encodes a 215-amino acid protein with a putative signal peptide, which shares 30-40% homology with known members of the FGF family. The AIGF mRNA was markedly induced by 10 nM testosterone in Northern blot analysis. Expression of AIGF cDNA in mammalian cells clearly showed remarkable stimulatory effects of AIGF on growth of SC-3 cells in the absence of androgen. Thus, it is clear that the androgen-induced growth of SC-3 cells is mediated in an autocrine manner by AIGF, which is secreted by the tumor cells themselves in response to hormonal stimuli.
Full text
PDF

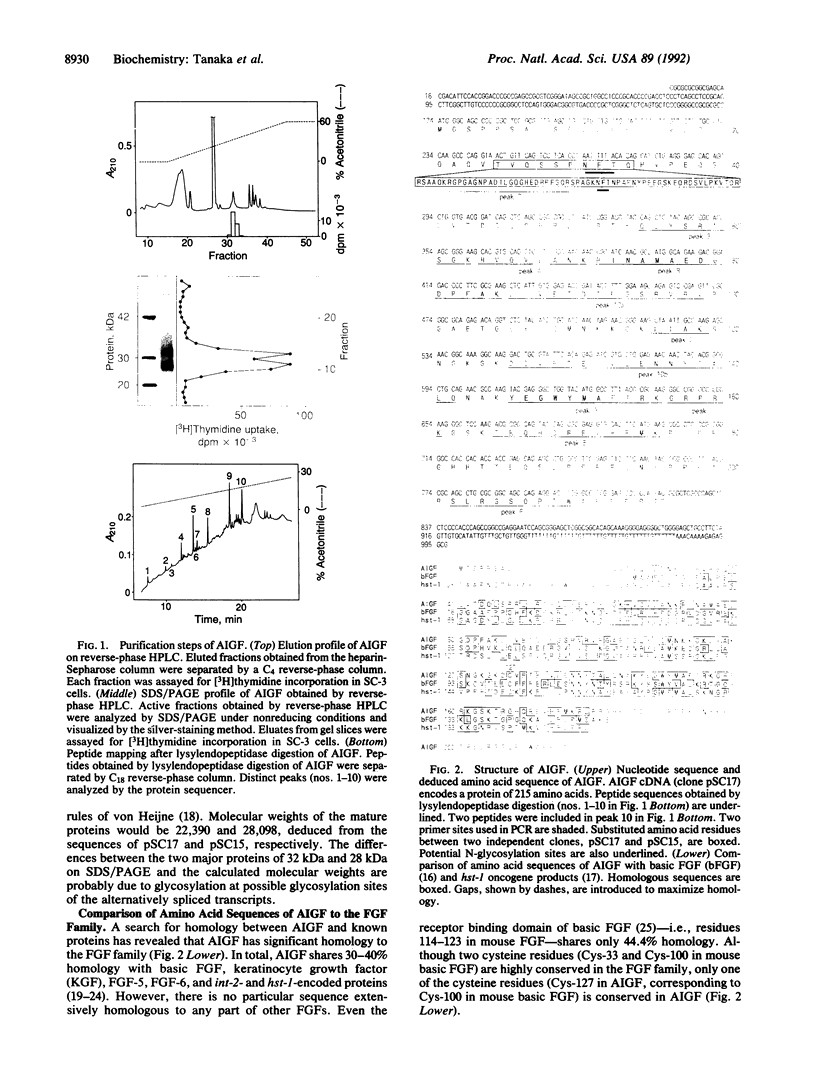
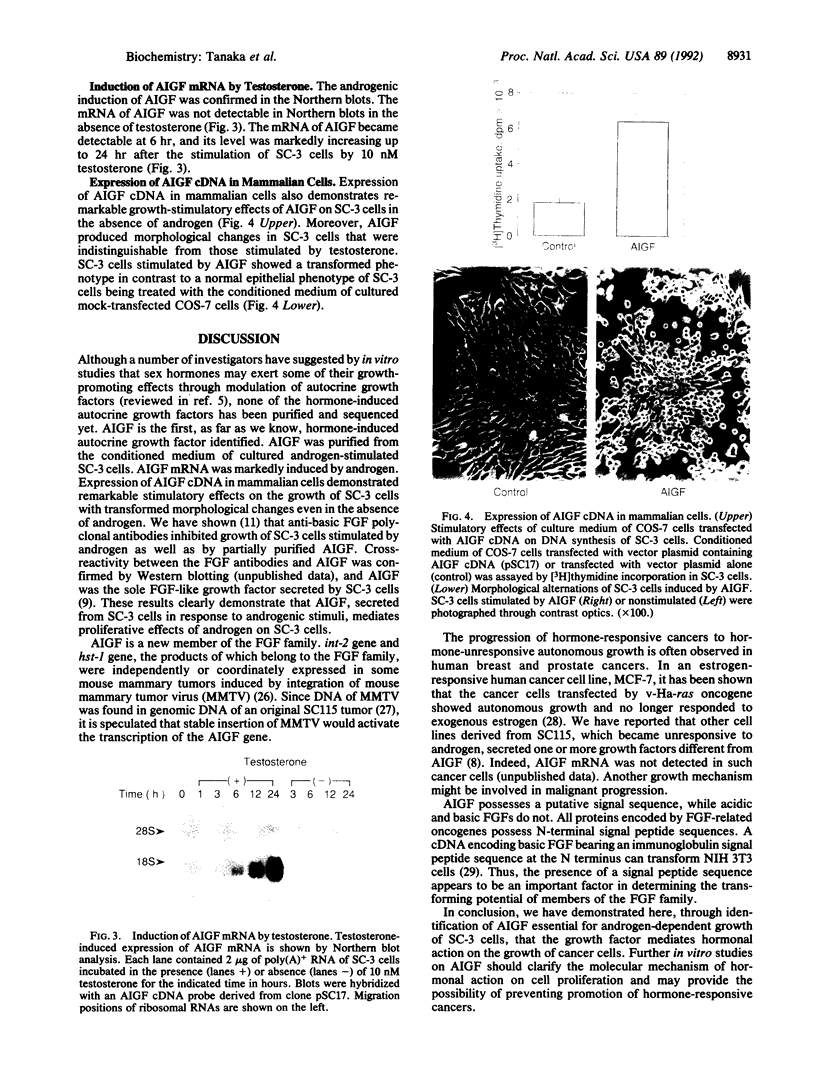

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes S., Smith R., Thurlow J., Dickson C., Peters G. The mouse homologue of hst/k-FGF: sequence, genome organization and location relative to int-2. Nucleic Acids Res. 1989 Jun 12;17(11):4037–4045. doi: 10.1093/nar/17.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darbre P., Dickson C., Peters G., Page M., Curtis S., King R. J. Androgen regulation of cell proliferation and expression of viral sequences in mouse mammary tumour cells. Nature. 1983 Jun 2;303(5916):431–433. doi: 10.1038/303431a0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Huff K. K., Spencer E. M., Lippman M. E. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986 Jan;118(1):138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Thompson E. W., Lippman M. E. Regulation of proliferation, invasion and growth factor synthesis in breast cancer by steroids. J Steroid Biochem Mol Biol. 1990 Nov 20;37(3):305–316. doi: 10.1016/0960-0760(90)90479-5. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Cousens L. S., Weaver L. H., Matthews B. W. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C. Toward the primary prevention of cancer. Science. 1991 Nov 22;254(5035):1131–1138. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- Huff K. K., Knabbe C., Lindsey R., Kaufman D., Bronzert D., Lippman M. E., Dickson R. B. Multihormonal regulation of insulin-like growth factor-I-related protein in MCF-7 human breast cancer cells. Mol Endocrinol. 1988 Mar;2(3):200–208. doi: 10.1210/mend-2-3-200. [DOI] [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Kasid A., Lippman M. E., Papageorge A. G., Lowy D. R., Gelmann E. P. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science. 1985 May 10;228(4700):725–728. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- Lu J., Nishizawa Y., Tanaka A., Nonomura N., Yamanishi H., Uchida N., Sato B., Matsumoto K. Inhibitory effect of antibody against basic fibroblast growth factor on androgen- or glucocorticoid-induced growth of Shionogi carcinoma 115 cells in serum-free culture. Cancer Res. 1989 Sep 15;49(18):4963–4967. [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Nakamura N., Nishizawa Y., Noguchi S., Uchida N., Sato B., Matsumoto K. Action mechanisms of physiological doses of androgen or pharmacological doses of estrogen in growth stimulation of Shionogi carcinoma 115 in mice. J Steroid Biochem. 1987;27(1-3):459–464. doi: 10.1016/0022-4731(87)90340-2. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Nakamura N., Uchida N., Noguchi S., Sato B., Sonoda T., Matsumoto K. Growth-stimulatory effect of androgen-induced autocrine growth factor(s) secreted from Shionogi carcinoma 115 cells on androgen-unresponsive cancer cells in a paracrine mechanism. Cancer Res. 1988 Sep 1;48(17):4904–4908. [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Placzek M., Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5678–5682. doi: 10.1073/pnas.86.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yamanishi H., Nonomura N., Tanaka A., Nishizawa Y., Terada N., Matsumoto K., Sato B. Proliferation of Shionogi carcinoma 115 cells by glucocorticoid-induced autocrine heparin-binding growth factor(s) in serum-free medium. Cancer Res. 1991 Jun 1;51(11):3006–3010. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M. Autocrine transformation by chimeric signal peptide-basic fibroblast growth factor: reversal by suramin. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5346–5350. doi: 10.1073/pnas.87.14.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]