Abstract
Zika virus (ZIKV) is a flavivirus that is responsible for an unprecedented current epidemic in Brazil and the Americas1,2. ZIKV has been causally associated with fetal microcephaly, intrauterine growth restriction, and other birth defects in both humans3–8 and mice9–11. The rapid development of a safe and effective ZIKV vaccine is a global health priority1,2, but very little is currently known about ZIKV immunology and mechanisms of immune protection. Here we show that a single immunization of a plasmid DNA vaccine or a purified inactivated virus vaccine provides complete protection in susceptible mice against challenge with a ZIKV outbreak strain from northeast Brazil. This ZIKV strain has recently been shown to cross the placenta and to induce fetal microcephaly and other congenital malformations in mice11. We produced DNA vaccines expressing full-length ZIKV pre-membrane and envelope (prM-Env) as well as a series of deletion mutants. The full-length prM-Env DNA vaccine, but not the deletion mutants, afforded complete protection against ZIKV as measured by absence of detectable viremia following challenge, and protective efficacy correlated with Env-specific antibody titers. Adoptive transfer of purified IgG from vaccinated mice conferred passive protection, and CD4 and CD8 T lymphocyte depletion in vaccinated mice did not abrogate protective efficacy. These data demonstrate that protection against ZIKV challenge can be achieved by single-shot subunit and inactivated virus vaccines in mice and that Env-specific antibody titers represent key immunologic correlates of protection. Our findings suggest that the development of a ZIKV vaccine for humans will likely be readily achievable.
The World Health Organization declared the clusters of microcephaly and neurological disorders and their association with ZIKV infection to be a global public health emergency on February 1, 2016. ZIKV is believed to cause neuropathology in developing fetuses by crossing the placenta and targeting cortical neural progenitor cells9–14, leading to impaired neurogenesis and resulting in microcephaly and other congenital malformations. ZIKV has also been associated with neurologic conditions in adults such as Guillain-Barre syndrome15.
Vaccines have been developed for other flaviviruses, including yellow fever virus, Japanese encephalitis virus, tick-borne encephalitis virus, and dengue viruses, but no vaccine currently exists for ZIKV. To develop preclinical challenge models for candidate ZIKV vaccines, we obtained low passage ZIKV isolates from northeast Brazil (Brazil/ZKV2015; University of São Paulo)11 and Puerto Rico (PRVABC59; U.S. Centers for Disease Control and Prevention) (Extended Data Fig. 1). We expanded these viruses in Vero cells to generate preclinical challenge stocks, which we termed ZIKV-BR and ZIKV-PR, respectively. These ZIKV strains are part of the Asian ZIKV lineage16 and differ from each other by 5 amino acids in the polyprotein (Extended Data Fig. 2). The Brazil/ZKV2015 strain has also recently been reported to recapitulate key clinical manifestations, including fetal microcephaly and intrauterine growth restriction, in wildtype SJL mice11. Similarly, the related French Polynesian H/PF/2013 strain has been shown to induce placental damage and fetal demise in Ifnar−/− C57BL/6 mice as well as in wildtype C57BL/6 mice following IFN-α receptor blockade10.
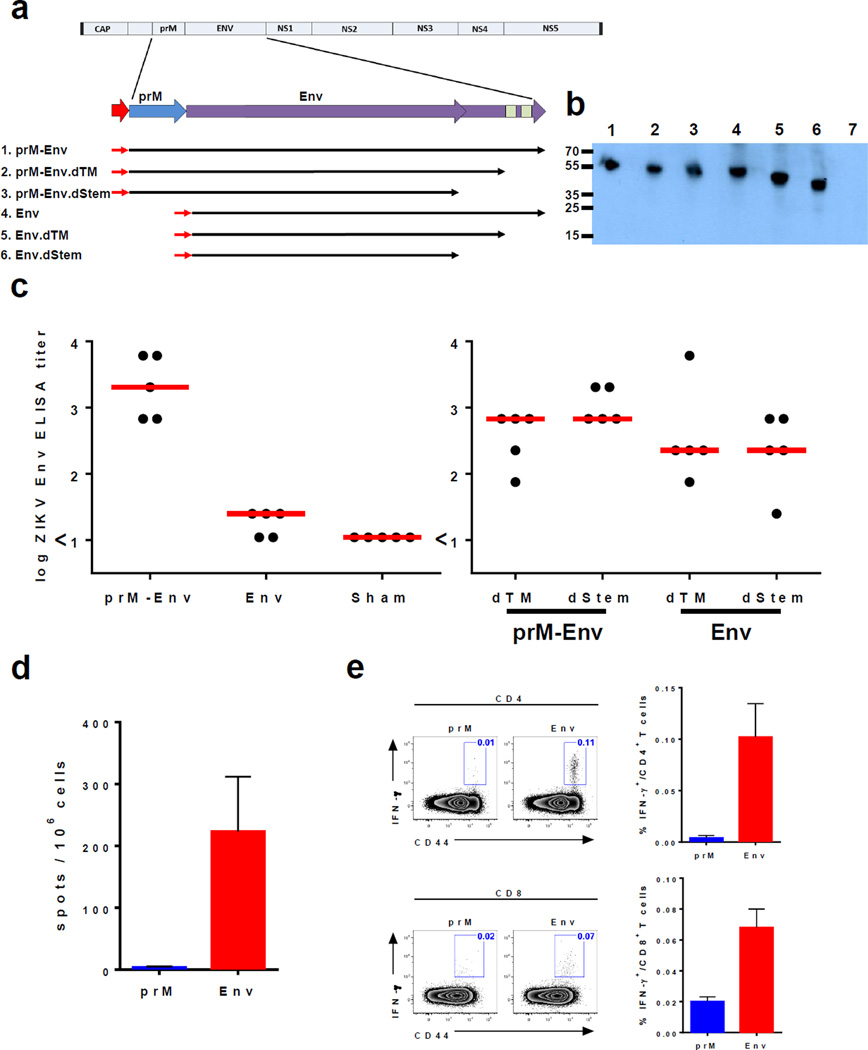
We designed full-length ZIKV pre-membrane and envelope (prM-Env) immunogens from the Brazil BeH815744 strain (Extended Data Fig. 2) and optimized them for increased antigen expression. We also designed deletion mutants lacking prM and/or lacking the transmembrane region (dTM) or the full stem (dStem) of Env (Fig. 1a). Plasmid DNA vaccines encoding these antigens were produced, and transgene expression was verified by Western blot (Fig. 1b). To assess the immunogenicity of these vaccines, groups of Balb/c mice (N=5–10/group) received a single immunization of 50 µg of each DNA vaccine by the i.m. route at week 0. Env-specific antibody responses were evaluated at week 3 by ELISA. The full-length prM-Env DNA vaccine elicited higher Env-specific antibody titers than did the Env DNA vaccine and all the dTM and dStem deletion mutants (Fig. 1c), indicating the importance of including prM as well as the full-length Env sequence. No prM-specific antibody responses were detected (Extended Data Fig. 3). The full-length prM-Env DNA vaccine also induced ZIKV-specific neutralizing antibodies after a single immunization (Table 1), as measured by a virus-specific microneutralization assay17. In addition, the prM-Env DNA vaccine induced Env-specific CD8+ and CD4+ T lymphocyte responses, as assessed by IFN-γ ELISPOT and multiparameter intracellular cytokine staining (ICS) assays (Fig. 1d–e).
Figure 1. Production and immunogenicity of DNA vaccines.
(a) Schema of ZIKV prM-Env immunogens and deletion mutants. (b) Western blot of transgene expression from (1) prM-Env, (2) prM-Env.dTM, (3) prM-Env.dStem, (4) Env, (5) Env.dTM, (6) Env.dStem, and (7) sham DNA vaccines transfected in 293T cells. Balb/c mice (N=5/group) received a single immunization with 50 µg of these DNA vaccines by the i.m. route. (c) Humoral immune responses were assessed at week 3 following vaccination by Env-specific ELISA. Red bars reflect medians. Cellular immune responses were assessed by (d) IFN-γ ELISPOT assays and (e) multiparameter intracellular cytokine staining assays. Error bars reflect s.e.m.
Table 1. ZIKV-specific neutralizing antibody titers.
Balb/c mice received a single immunization with 50 µg of various DNA vaccines (Fig. 1–2) or 1 µg purified inactivated virus (PIV) vaccines with alum (Fig. 4), and pooled serum was assessed for ZIKV-specific neutralizing antibodies at week 4. 50% microneutralization (MN50) titers are shown. Also shown are MN50 titers in serum from mice following two immunizations with DNA-prM-Env (boost) and an anti-flavivirus human polyclonal antibody.
| Vaccine | ZIKV MN50 Titer |
|---|---|
| DNA-prM-Env | 22 |
| DNA-prM-Env.dTM | <10 |
| DNA-prM-Env.dStem | <10 |
| DNA-Env | <10 |
| DNA-Env.dTM | <10 |
| DNA-Env.dStem | <10 |
| DNA-prM-Env (boost) | 1,022 |
| PIV/alum i.m. | 15 |
| PIV/alum s.q. | 15 |
| Sham/alum i.m. | <10 |
| Sham/alum s.q. | <10 |
| Anti-flavivirus Ab | 232 |
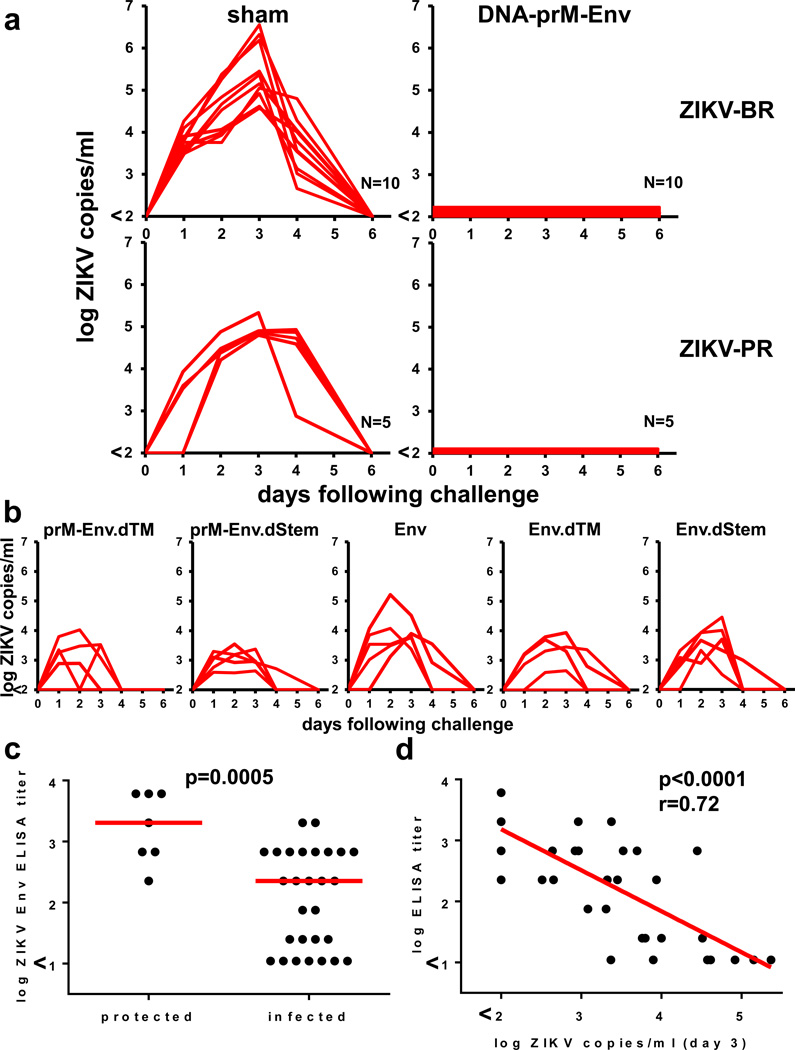
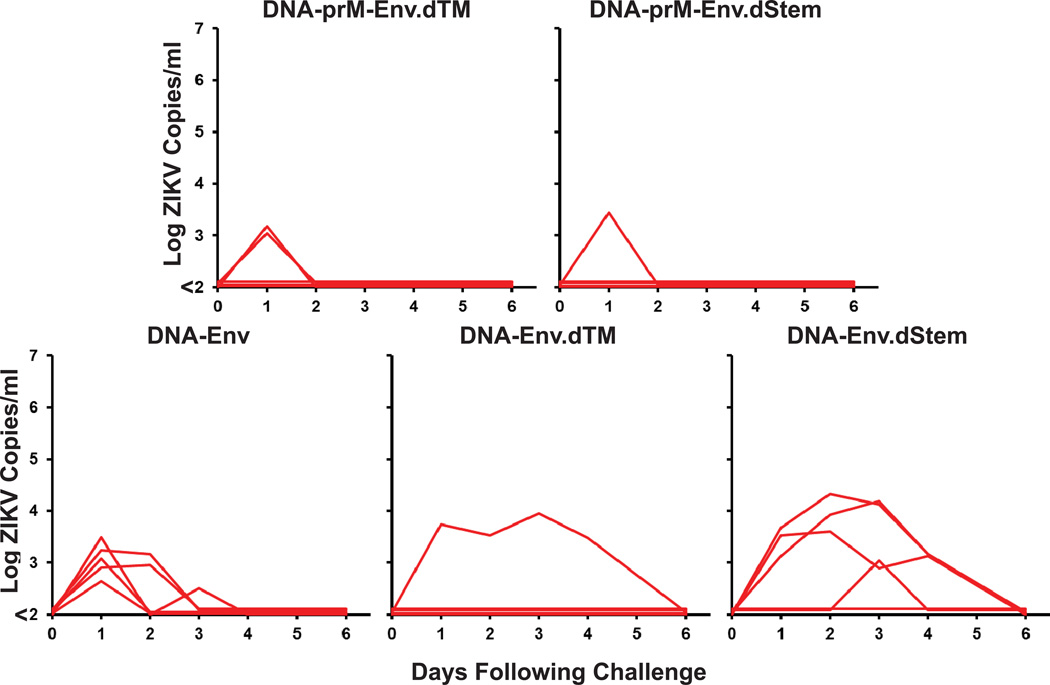
To assess the protective efficacy of these DNA vaccines against ZIKV challenge, we infected vaccinated or sham control Balb/c mice at week 4 by the i.v. route with 105 viral particles (VP) [102 plaque-forming units (PFU)] of ZIKV-BR or ZIKV-PR. Viral loads following ZIKV challenge were quantitated by RT-PCR18. Sham vaccinated mice inoculated with ZIKV-BR developed approximately 6 days of detectable viremia with a mean peak viral load of 5.42 log copies/ml (range 4.55–6.57 log copies/ml; N=10) on day 3 following challenge (Fig. 2a). In contrast, a single immunization with the prM-Env DNA vaccine provided complete protection against ZIKV-BR challenge with no detectable viremia (<100 copies/ml) at any timepoint (N=10). Complete protection was also observed when vaccinated mice were challenged at week 8 (data not shown). The prM-Env DNA vaccine also afforded complete protection against ZIKV-PR challenge (N=5). ZIKV-PR replicated to slightly lower levels (mean peak viral load 4.96 log copies/ml; range 4.80–5.33 log copies/ml; N=5) than did ZIKV-BR in sham controls. In contrast with the full-length prM-Env DNA vaccine, the DNA vaccines lacking prM as well as the dTM and dStem deletion mutants did not provide complete protection against ZIKV-BR challenge, although viral loads were still reduced in these animals as compared with sham controls (Fig. 2b).
Figure 2. Protective efficacy of DNA vaccines.
(a) Balb/c mice (N=5 or 10/group) received a single immunization by the i.m. route with 50 µg full-length prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 VP (102 PFU) ZIKV-BR or ZIKV-PR. Serum viral loads are shown. (b) Mice (N=5/group) received a single immunization with 50 µg of various DNA vaccines and were challenged with ZIKV-BR. Correlates of (c) protective efficacy and (d) day 3 viral loads are shown. Red bars reflect medians. P-values reflect t-tests and Spearman rank-correlation tests.
The varying degrees of protection obtained with this set of DNA vaccines allowed for an analysis of immune correlates of protection. Protective efficacy correlated with Env-specific binding antibody titers (P=0.0005 comparing protected vs infected animals; Fig. 2c) as well as ZIKV-specific neutralizing antibody titers >10 (Table 1). In addition, peak viral loads on day 3 were inversely correlated with antibody titers (P<0.0001, R=0.72; Fig 2d). These data suggest that Env-specific antibodies were critical for the protective efficacy of DNA vaccines against ZIKV-BR challenge. Mice that received two immunizations with the prM-Env DNA vaccine at week 0 and week 4 developed high neutralizing antibody titers of 1,022 at week 8 (Table 1) and were also protected against ZIKV-BR challenge (data not shown).
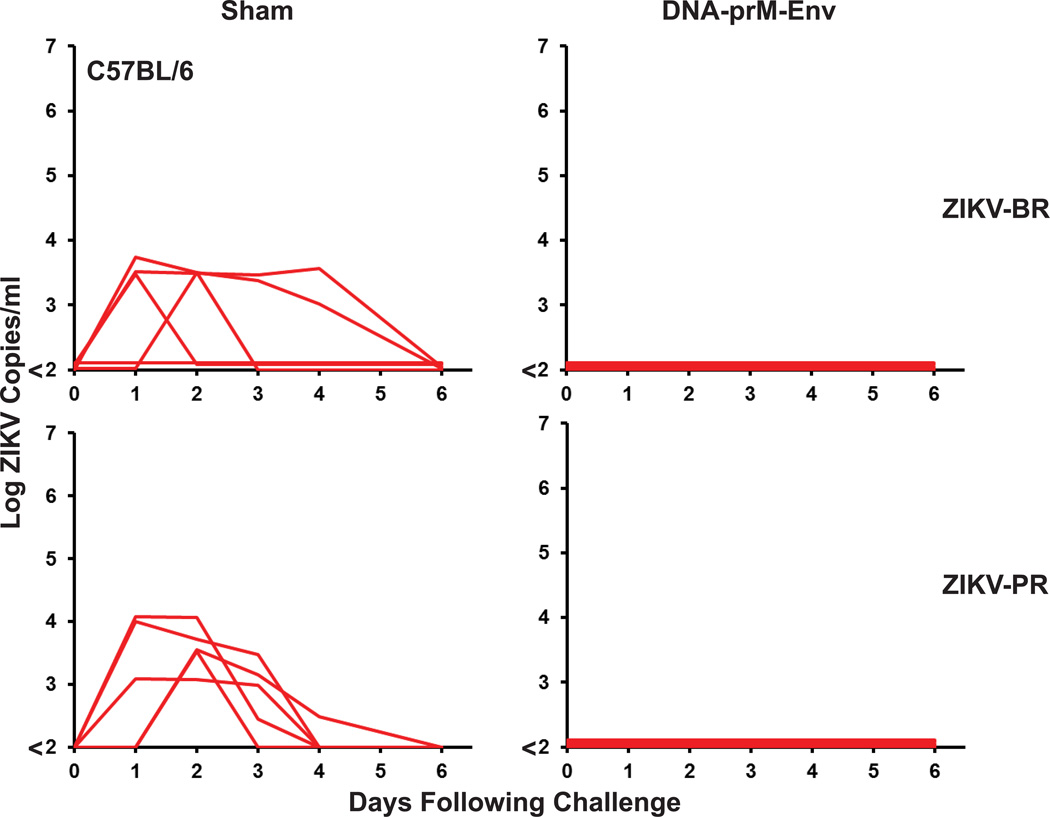
The prM-Env DNA vaccine also provided complete protection against ZIKV-BR challenge in SJL mice (Extended Data Fig. 4) and against both ZIKV-BR and ZIKV-PR challenge in C57BL/6 mice (Extended Data Figs. 5–6). ZIKV-BR replicated efficiently in SJL mice, consistent with a prior study11, although at slightly lower levels (mean peak viral load 4.70 log copies/ml; range 3.50–5.92 log copies/ml; N=5) than in Balb/c mice (Fig. 2a). In contrast, both ZIKV-BR and ZIKV-PR replicated poorly in C57BL/6 mice (Extended Data Fig. 5), also consistent with prior reports, potentially as a result of robust IFN-α mediated innate immune restriction in this strain of mice10,11,19,20.
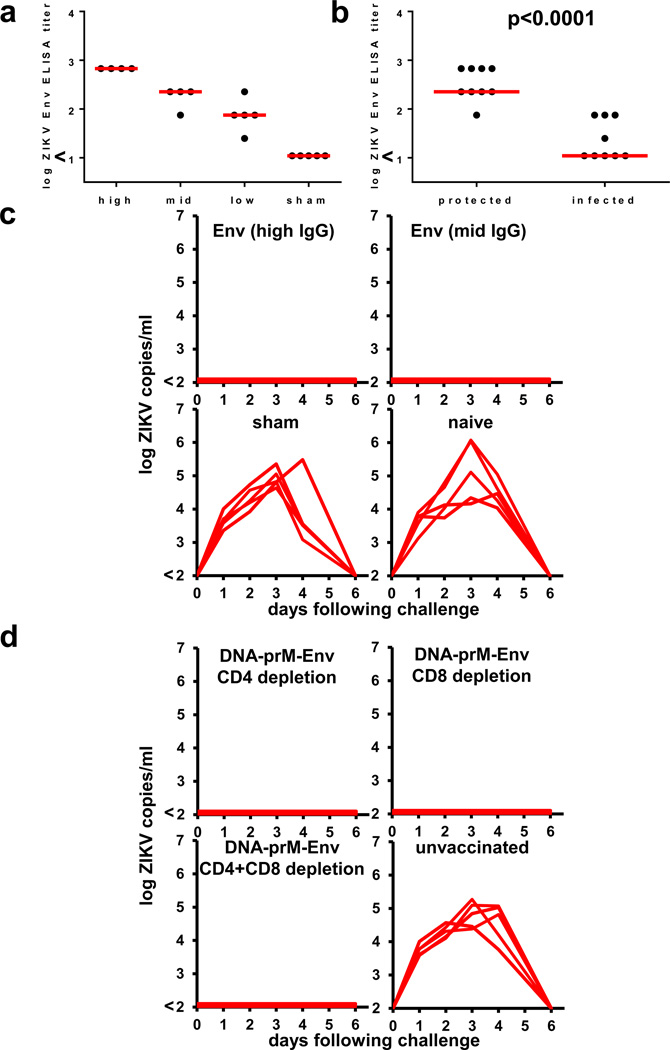
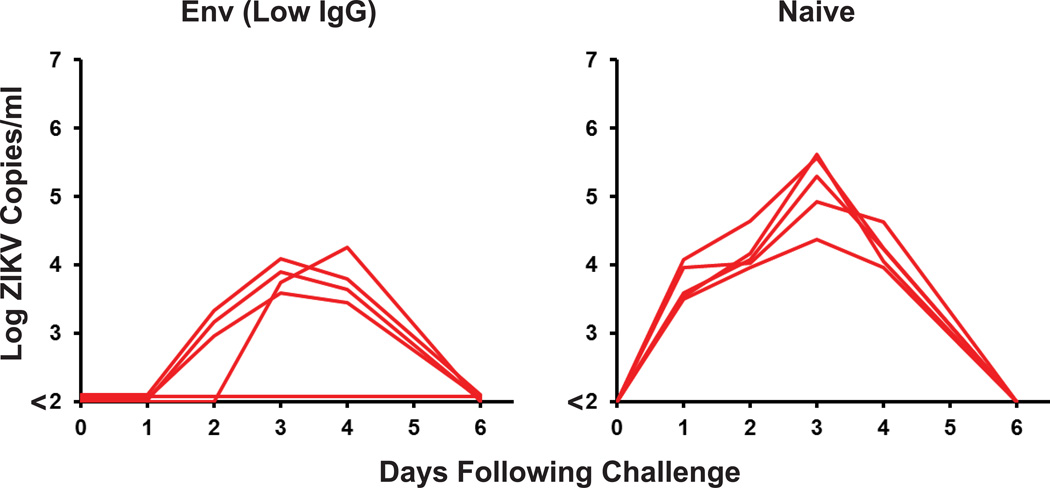
To investigate the immunologic mechanism of protection against ZIKV-BR challenge, we purified IgG from serum from prM-Env DNA vaccinated Balb/c mice. Passive infusion of varying quantities of purified IgG by the i.v. route resulted in median Env-specific log serum antibody titers of 2.82 (high), 2.35 (mid), and 1.87 (low) in recipient mice following adoptive transfer (Fig. 3a). All recipient mice with log serum antibody titers of 2.35 or higher were protected against ZIKV-BR challenge (Fig. 3b–c), demonstrating that protection can be mediated by vaccine-elicited IgG alone and confirming that the magnitude of Env-specific antibody titers correlates with protective efficacy (P<0.0001, Fig. 3b). In contrast, only 1 of 5 recipient mice that received low levels of Env-specific IgG were protected, although they still exhibited reduced viral loads compared with sham controls (Extended Data Fig. 7). These data define the minimum threshold of Env-specific antibody titers required for protection in this model.
Figure 3. Mechanistic studies.
(a) Env-specific serum antibody titers in recipient Balb/c mice (N=5/group) following adoptive transfer of varying amounts (high, mid, low) of IgG purified from serum from prM-Env DNA vaccinated mice or naïve mice (sham). (b) Correlates of protective efficacy. (c) Serum viral loads in mice that received adoptive transfer of purified IgG from vaccinated mice and were challenged with ZIKV-BR. (d) Serum viral loads in prM-Env DNA vaccinated mice that were depleted of CD4+ and/or CD8+ T lymphocytes prior to challenge with ZIKV-BR. Red bars reflect medians. P-values reflect t-tests.
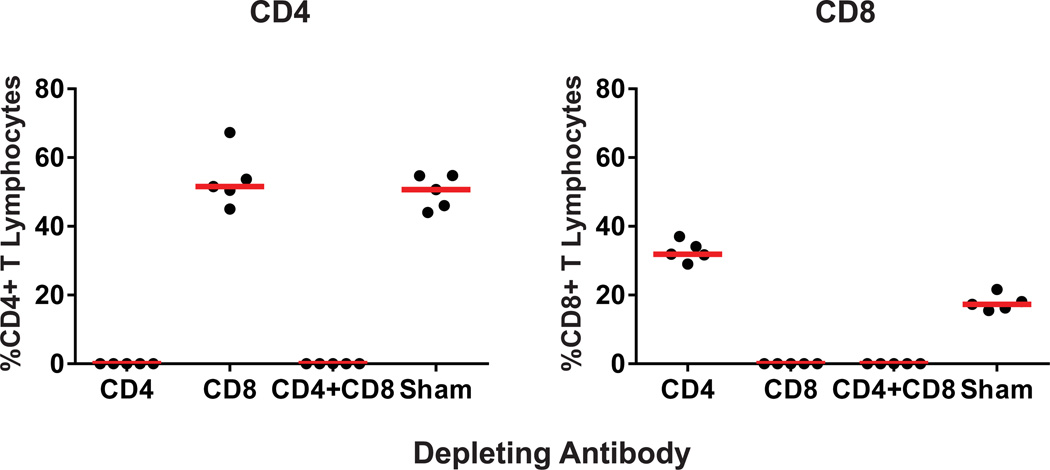
We next depleted CD4+ and/or CD8+ T lymphocytes in prM-Env vaccinated mice on day −2 and day −1 prior to challenge (>99.9% efficiency; Extended Data Fig. 8). Depletion of these T lymphocyte subsets did not detectably abrogate the protective efficacy of the prM-Env DNA vaccine against ZIKV-BR challenge (Fig. 3d). These data indicate that Env-specific T lymphocyte responses were not required for protection in this model, although these findings do not exclude the possibility that ZIKV-specific cellular immune responses may be beneficial in other settings.
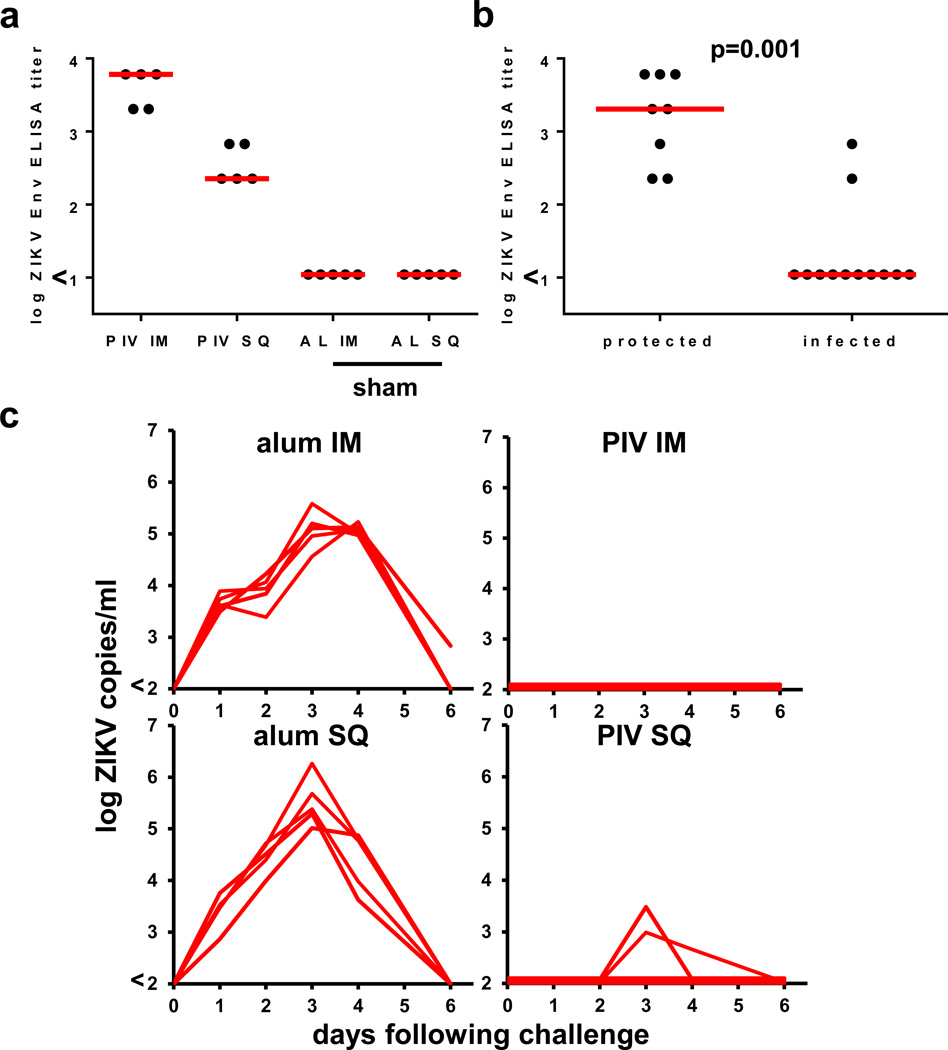
To extend these observations to a vaccine platform that has historically provided clinical efficacy against other flaviviruses, we explored the immunogenicity and protective efficacy of a ZIKV purified inactivated virus (PIV) vaccine derived from the Puerto Rico PRVABC59 strain. Groups of Balb/c mice (N=5/group) received a single immunization of 1 µg of the PIV vaccine with alum or alum alone by the i.m. or s.q. routes. Antibody titers were higher in the group that received the PIV vaccine by the i.m. route as compared with the s.q. route by ELISA (Fig. 4a). The PIV vaccine by both routes also induced ZIKV-specific neutralizing antibodies after a single immunization (Table 1). At week 4, all mice were challenged with ZIKV-BR by the i.v. route as described above. Complete protection was observed in the group that received the PIV vaccine by the i.m. route (Fig. 4b–c). Two mice that received the PIV vaccine by the s.q. route showed brief low levels of viremia (Fig. 4c), consistent with the lower Env-specific binding antibody titers in this group (Fig. 4b).
Figure 4. Immunogenicity and protective efficacy of PIV vaccine.
Balb/c mice (N=5/group) received a single immunization by the i.m. or s.q. route with 1 µg PIV vaccine with alum or alum alone and were challenged at week 4 by the i.v. route with 105 VP (102 PFU) ZIKV-BR. (a) Humoral immune responses were assessed at week 3 following vaccination by Env-specific ELISA. (b) Correlates of protective efficacy. (c) Serum viral loads are shown following ZIKV-BR challenge. Red bars reflect medians. P-values reflect t-tests.
Our data demonstrate that a single immunization with a DNA vaccine or a PIV vaccine provided complete protection against parenteral ZIKV challenges in mice. The prM-Env DNA vaccine afforded protection in three strains of mice and against both ZIKV-BR and ZIKV-PR challenges, suggesting the generalizability of these observations. Protective efficacy was mediated by vaccine-elicited Env-specific antibodies, as evidenced by (i) statistical analyses of immune correlates of protection (Figs. 2c–d, 4b), (ii) adoptive transfer studies with purified IgG from vaccinated mice (Fig. 3a–c), and (iii) T lymphocyte depletion studies in vaccinated mice (Fig. 3d). The adoptive transfer studies also defined the threshold of Env-specific antibody titers required for protection in this model.
It is difficult to extrapolate directly the results from these vaccine studies in mice to potential clinical efficacy in humans. Nevertheless, the robust protection observed in the present studies and the clear immune correlate of protection suggest a path forward for ZIKV vaccine development in humans. Of note, similar antibody-based correlates of protection, including neutralizing antibody titers >10, have been reported for other flavivirus vaccines, including yellow fever virus, tick-borne encephalitis virus, and Japanese encephalitis virus21–23. Moreover, the ZIKV-BR challenge isolate used in the present study has been shown in wildtype SJL mice to recapitulate certain key clinical findings of ZIKV infection in humans, including fetal microcephaly and intrauterine growth retardation11. ZIKV-BR did not lead to a fatal outcome in wildtype Balb/c and SJL mice, as has been observed in Ifnar−/− C57BL/6 mice10,19,20, but the magnitude and duration of viremia in Balb/c and SJL mice appear comparable with that in humans2, suggesting the potential relevance of this model. It is notable that ZIKV-BR replicated efficiently in wildtype Balb/c and SJL mice (Fig. 2a, Extended Data Fig. 4), but replicated poorly in wildtype C57BL/6 mice (Extended Data Fig. 5), which is consistent with prior observations10,11 and indicates important strain-specific differences for ZIKV infectivity. Further investigation into the immunologic mechanisms underlying these differences may lead to insights into innate immune control of ZIKV. Moreover, further characterization of the susceptible Balb/c and SJL murine models may facilitate future studies of ZIKV pathogenesis and the development of antiviral interventions.
The explosive epidemiology of the current ZIKV outbreak1,2 and the devastating clinical consequences for fetuses in pregnant women who become infected3–8 demand the urgent development of a ZIKV vaccine. Our data demonstrate that complete protection against ZIKV challenge was reliably and robustly achieved with both DNA vaccines and purified inactivated virus vaccines in susceptible mice. These vaccine platforms have previously been utilized at comparable doses to develop vaccines for other flaviviruses, including West Nile virus24,25, dengue viruses26,27, tick-borne encephalitis virus28,29, and Japanese encephalitis virus30, and may offer safety advantages over live attenuated and replicating flavivirus vaccines, particularly for pregnant women. Moreover, the magnitude of Env-specific antibody titers that provide complete protection against ZIKV challenge in mice should be readily achievable by DNA vaccines and purified inactivated virus vaccines in humans. Taken together, our findings provide substantial optimism that the development of a safe and effective ZIKV vaccine for humans will likely be feasible.
Methods
Animals
Balb/c, SJL, and C57BL/6 female mice at 6–8 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were vaccinated with 50 µg DNA vaccine in saline without adjuvant by the i.m. route or with 1 µg PIV vaccines with 100 µg alum (Alhydrogel; Brenntag Biosector, Denmark) adjuvant by the i.m. or s.q. routes in a 100 µl volume and were then challenged at week 4 by the i.v. route with 105 viral particles (VP) [102 plaque-forming units (PFU)] ZIKV-BR or ZIKV-PR. Animals were randomly allocated to groups. Immunologic and virologic assays were performed blinded. All animal studies were approved by the BIDMC Institutional Animal Care and Use Committee (IACUC).
DNA vaccines
ZIKV strain BeH815744 (accession number KU365780) was used to design transgenes, which were produced synthetically. Sequences were optimized for enhanced transgene expression. Full-length pre-membrane and envelope (prM-Env; defined as amino acids 216–794 of the polyprotein) or Env alone were cloned into the mammalian expression plasmid pcDNA3.1+ (Invitrogen, CA, USA). Deletion mutants lacked the transmembrane (dTM) or stem (dStem) regions of Env. A Kozak sequence and the Japanese encephalitis virus leader sequence were included24. Plasmids were produced with Machery-Nagel endotoxin-free gigaprep kits. Sequences were confirmed by double stranded sequencing.
PIV vaccine
The ZIKV purified inactivated virus (PIV) vaccine was produced at the Pilot Bioproduction Facility, Walter Reed Army Institute of Research, Silver Spring, MD, USA. The PIV vaccine was based on the Puerto Rican PRVABC59 isolate, which was obtained from the U.S. Centers for Disease Control and Prevention, Fort Collins, CO, USA. The Vero cells used for passage and vaccine production were a derivative of a certified cell line manufactured at The Salk Institute, Swiftwater, PA. After inoculation, virus was harvested on days 5 and 7, clarified by centrifugation and depth filter (0.45–0.2 µm), and treated with benzonase. The viral harvest was concentrated with an ultrafilter followed by purification using Captocore chromatography resin. The purified ZIKV was then inactivated with formalin (0.05%) at 22°C for 7 days. Following inactivation, formalin was removed by dialysis, and the antigen concentration was adjusted. The final PIV vaccine was assessed for infectivity by passage in Vero cells followed by plaque assays to demonstrate inactivation.
ZIKV challenge stocks
ZIKV stocks were provided by University of São Paulo, Brazil (Brazil ZKV2015; ZIKV-BR11) and the U.S. Centers for Disease Control and Prevention, USA (Puerto Rico PRVABC59; ZIKV-PR). Both strains were passage number 3. Low passage number Vero cells were then infected at an MOI of 0.01 PFU/cell. Supernatant was screened daily for viral titers and harvested at peak growth. Culture supernatants were clarified by centrifugation, and fetal bovine serum was added to 20% final concentration (v/v) and stored at −80°C. The concentration and infectivity of the stocks were determined by RT-PCR and PFU assays. The viral particle (VP) to plaque-forming unit (PFU) ratio of both stocks was approximately 1,000.
RT-PCR
Cap genes of available ZIKV genomes were aligned using Megalign (DNAstar, WI, USA), and primers and probes to a highly conserved region were designed using primer express v3.0 (Applied Biosystems, CA, USA). Primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA) and probes by Biosearch Technologies (Petaluma, CA, USA). To assess viral loads, RNA was extracted from serum with a QIAcube HT (Qiagen, Germany). Reverse transcription and RT-PCR were performed as previously described18. The wildtype ZIKV BeH815744 Cap gene was utilized as a standard and was cloned into pcDNA3.1+, and the AmpliCap-Max T7 High Yield Message Maker Kit was used to transcribe RNA (Cellscript, WI, USA). RNA was purified using the RNA clean and concentrator kit (Zymo Research, CA, USA), and RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per ml. Assay sensitivity was 100 copies/ml. The infectivity of virus in peripheral blood from ZIKV challenged mice was confirmed by PFU assays.
PFU assay
Vero WHO cells were seeded in a MW6 plate to reach confluency at day 3. Cells were infected with log dilutions of ZIKV for 1 h and overlayed with agar. Cells were stained after 6 days of infection by neutral red staining. Plaques were counted, and titers were calculated by multiplying the number of plaques by the dilution and divided by the infection volume.
Western blot
To assess transgene expression from DNA vaccines, cell lysates obtained 48 h following lipofectamine 2000 (Invitrogen, CA, USA) transient transfection of 293T cells were mixed with reducing sample buffer, heated for 5 min at 100°C, cooled on ice, and run on a precast 4–15% SDS-PAGE gel (Biorad, CA, USA). Protein was transferred to PVDF membranes using the iBlot dry blotting system (Invitrogen, CA, USA), and the membranes were blocked overnight at 4°C in PBS-T (Dulbeco’s Phosphate Buffered Saline + 0.2% V/V Tween 20 + 5% W/V non-fat milk powder). Following overnight blocking, the membranes were incubated for 1 h with PBS-T containing a 1:5000 dilution of mouse anti-ZIKV Env mAb (BioFront Technologies, FL, USA). Membranes were then washed 3 times with PBS-T and incubated for 1 h with PBS-T containing a 1:1000 dilution of rabbit anti-mouse HRP (Jackson ImmunoResearch, PA, USA). Membranes were then washed 3 times with PBS-T and developed using the Amersham ECL plus Western blotting detection system (GE Healthcare, Chicago, USA).
ELISA
Mouse ZIKV Env ELISA kits (Alpha Diagnostic International, TX, USA) were used to determine endpoint antibody titers using a modified protocol. 96-well plates coated with ZIKV Env protein were first equilibrated at room temperature with 300 µl of kit working wash buffer for 5 min. 6 µl of mouse serum was added to the top row, and 3-fold serial dilutions were tested in the remaining rows. Samples were incubated at room temperature for 1 h, and plates washed 4 times. 100 µl of anti-mouse IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 µl of 3,3’,5,5’–tetramehylbenzidine (TMB) substrate, and stopped by the addition of 100 µl of stop solution. Plates were analyzed at 450nm/550nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices, CA, USA). ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance >2-fold over background values.
Neutralization assay
A high-throughput ZIKV microneutralization (MN) assay was developed for measuring ZIKV-specific neutralizing antibodies as a modified version of a qualified dengue virus microneutralization assay used in clinical dengue vaccine trials17. Briefly, serum samples were serially diluted three-fold in 96-well micro-plates, and 100 µl of ZIKV-PR containing 100 PFU were added to 100 µl of each serum dilution and incubated at 35°C for 2 h. Supernatants were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 d, cells were fixed with absolute ethanol: methanol for 1 h at −20°C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from JT Roehrig, CDC) was then added to each well, incubated at 35°C for 2 h, and washed with PBS. Plates were washed, developed with 3,3’,5,5’–tetramehylbenzidine (TMB) substrate for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be ≤ 0.5, and virus-only control wells had to be ≥ 0.9. Normalized absorbance values were calculated, and the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized ≥ 50% of ZIKV. Seropositivity was defined as a titer ≥ 1:10.
ELISPOT
ZIKV-specific cellular immune responses were assessed by interferon-γ (IFN-γ) ELISPOT assays using pool of overlapping 15-amino-acid peptides covering the prM or Env proteins (JPT, Berlin, Germany). 96-well multiscreen plates (Millipore, MA, USA) were coated overnight with 100 µl/well of 10 µg/ml anti-mouse IFN-γ (BD Biosciences, CA, USA) in endotoxin-free Dulbecco's PBS (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 2 h with D-PBS containing 5% FBS at 37°C, washed three times with D-PBS-Tween, rinsed with RPMI 1640 containing 10% FBS to remove the Tween 20, and incubated with 2 µg/ml of each peptide and 5 × 105 murine splenocytes in triplicate in 100 µl reaction mixture volumes. Following an 18 h incubation at 37°C, the plates were washed nine times with PBS-Tween and once with distilled water. The plates were then incubated with 2 µg/ml biotinylated anti-mouse IFN-γ (BD Biosciences, CA, USA) for 2 h at room temperature, washed six times with PBS-Tween, and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, AL, USA). Following five washes with PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, IL, USA), stopped by washing with tap water, air dried, and read using an ELISPOT reader (Cellular Technology Ltd., OH, USA). The numbers of spot-forming cells (SFC) per 106 cells were calculated. The medium background levels were typically <15 SFC per 106 cells.
Intracellular cytokine staining
ZIKV-specific CD4+ and CD8+ T lymphocyte responses were assessed using splenocytes and analyzed by flow cytometry. Cells were stimulated for 1 h at 37°C with 2 µg/ml of overlapping 15-amino-acid peptides covering the prM or Env proteins (JPT, Berlin, Germany). Following incubation, brefeldin-A and monensin (BioLegend, CA, USA) were added, and samples were incubated for 6 h at 37°C. Cells were then washed, stained, permeabilized with Cytofix/Cytoperm (BD Biosciences, CA, USA). Data was acquired using an LSR II flow cytometer (BD Biosciences, CA, USA) and analyzed using FlowJo v.9.8.3 (Treestar, OR, USA). Monoclonal antibodies included: CD4 (RM4-5), CD8α (53-6.7), CD44 (IM7), and IFN-γ (XMG1.2). Antibodies were purchased from BD Biosciences, eBioscience, or BioLegend, CA, USA. Vital dye exclusion (LIVE/DEAD) was purchased from Life Technologies, CA, USA.
IgG purification and adoptive transfer
Serum was collected from prM-Env DNA vaccinated mice or naïve mice, and polyclonal IgG was purified using protein G purification kits (Thermo Fisher Scientific, MA, USA). Varying amounts of purified IgG was infused by the i.v. route into naïve recipient mice prior to ZIKV challenge.
CD4+ and CD8+ T lymphocyte depletion
Anti-CD4 (GK1.5) and/or anti-CD8 (2.43) (Bio X Cell, NH, USA) mAbs were administered at doses of 500 µg/mouse to prM-Env DNA vaccinated mice by the i.p. route on day -2 and day -1 prior to ZIKV challenge. Antibody depletions were >99.9% efficient as determined by flow cytometry.
Statistical analyses
Analysis of virologic and immunologic data was performed using GraphPad Prism v6.03 (GraphPad Software, CA, USA). Comparisons of groups was performed using t-tests and Wilcoxon rank-sum tests. Correlations were assessed by Spearman rank-correlation tests.
Extended Data
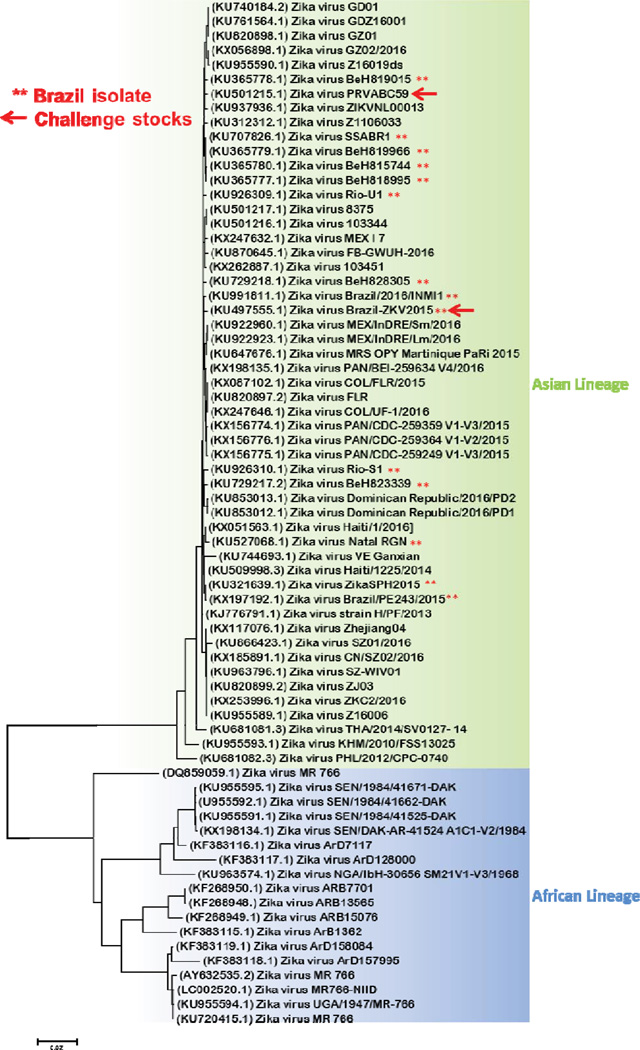
Extended Data Figure 1. ZIKV maximum likelihood phylogenetic tree.
The ZIKV-BR and ZIKV-PR challenge isolates are depicted with red arrows.
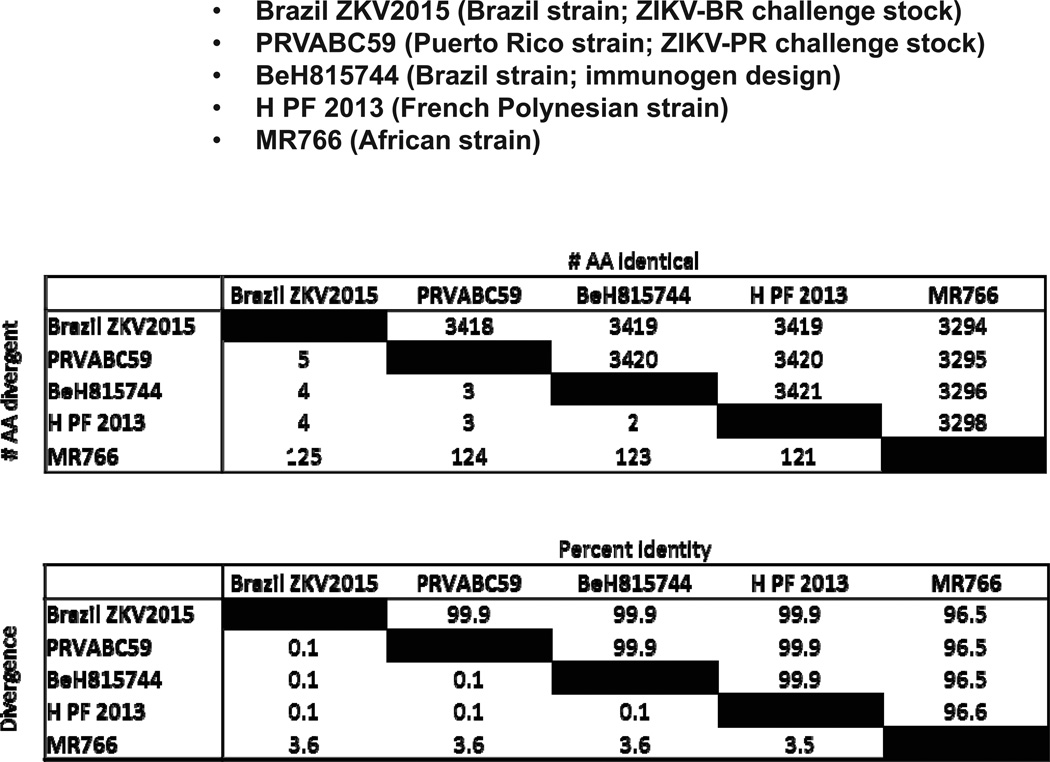
Extended Data Figure 2. ZIKV amino acid sequence comparisons.
Number of and percentage amino acid differences in the polyprotein are shown for the following ZIKV isolates: Brazil/ZKV2015 (Brazil strain; ZIKV-BR challenge stock), PRVABC59 (Puerto Rico strain; ZIKV-PR challenge stock), BeH815744 (Brazil strain; immunogen design), H/PF/2013 (French Polynesian strain), and MR766 (African strain).
Extended Data Figure 3. prM-specific antibody responses in DNA vaccinated mice.
In the experiment described in Figure 2, humoral immune responses were assessed at week 3 following vaccination by prM-specific ELISA. Red bars reflect medians.
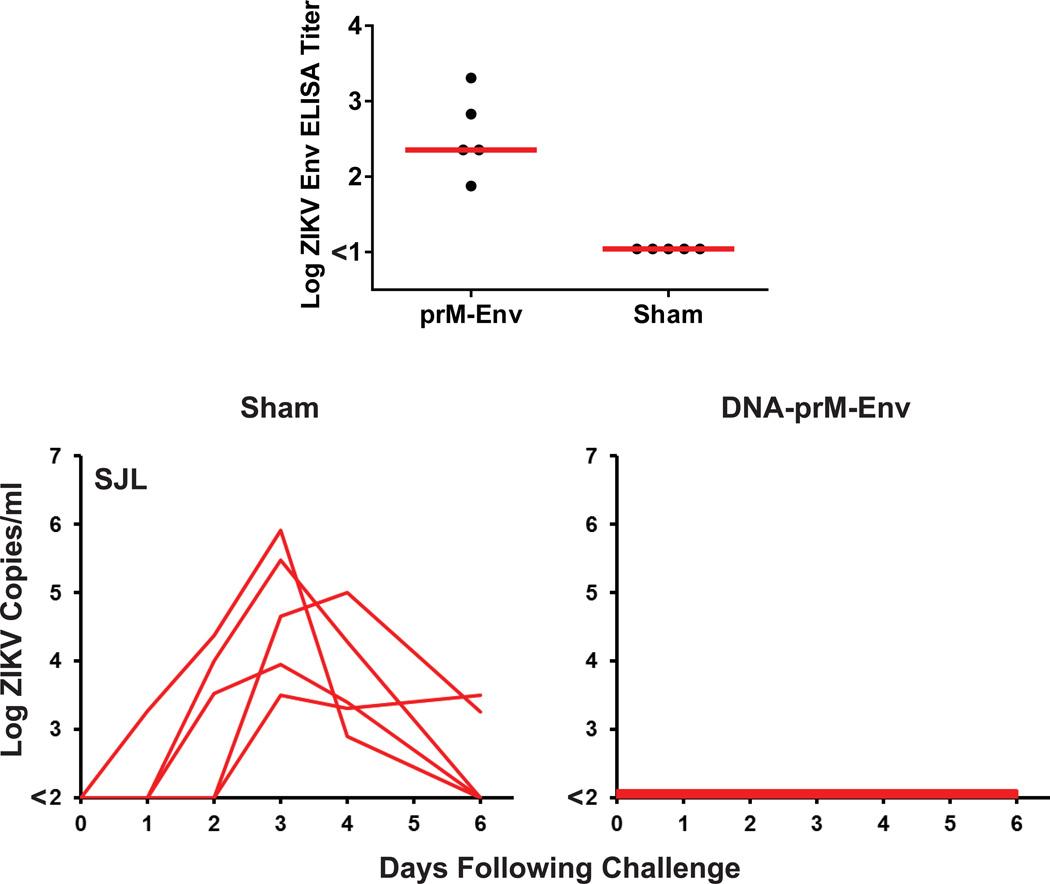
Extended Data Figure 4. Immunogenicity and protective efficacy of prM-Env DNA vaccine in SJL mice.
SJL mice (N=5/group) received a single immunization by the i.m. route with 50 µg full-length prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 VP (102 PFU) ZIKV-BR. Humoral immune responses were assessed at week 3 following vaccination by Env-specific ELISA (top). Red bars reflect medians. Serum viral loads are shown following ZIKV-BR challenge (bottom).
Extended Data Figure 5. Protective efficacy of prM-Env DNA vaccine in C57BL/6 mice.
C57BL/6 mice (N=5/group) received a single immunization by the i.m. route with 50 µg full-length prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 VP (102 PFU) ZIKV-BR or ZIKV-PR. Serum viral loads are shown following challenge.
Extended Data Figure 6. Protective efficacy of various DNA vaccines in C57BL/6 mice.
C57BL/6 mice (N=5/group) received a single immunization by the i.m. route with 50 µg various DNA vaccines and were challenged at week 4 by the i.v. route with 105 VP (102 PFU) ZIKV-BR. Serum viral loads are shown following challenge.
Extended Data Figure 7. Adoptive transfer of low titers of Env-specific IgG.
Serum viral loads in mice that received adoptive transfer of low titers of Env-specific IgG (as defined in Figure 3a) and were then challenged with ZIKV-BR.
Extended Data Figure 8. CD4+ and CD8+ T lymphocyte depletion.
CD4+ and/or CD8+ T lymphocyte depletion following mAb treatment of prM-Env DNA vaccinated Balb/c mice.
Acknowledgments
We thank K. Modjarrad, R. Olson, K. Kabra, C. Kannadka, C. Springer, G. Ballarini, G. Alter, K. Stephenson, F. Stephens, B. Lee, J. Jimenez, A. Chandrashekar, P. Penaloza, and C. Cabral for generous advice, assistance, and reagents. We acknowledge support from the Ragon Institute of MGH, MIT, and Harvard, the National Institutes of Health (AI095985, AI096040, AI100663, AI124377), and the São Paulo Research Foundation (FAPESP 2011/18703-2, 2014/17766-9). The views expressed in this manuscript are those of the authors and do not represent the official views of the Department of the Army or the Department of Defense.
Footnotes
Author Contributions
R.A.L., P.A., and D.H.B. designed the studies. J.P.S.P. and P.M.A.Z. developed the challenge virus. P.A., M.B., D.N., M.K., R.N., N.B.M., and Z.L. produced the DNA vaccines and conducted the virologic assays. R.A.B., R.G.J., K.H.E., N.L.M., and S.J.T. produced the PIV vaccines. R.A.L., M.J.I., and A.B.Z. conducted the mouse studies. R.A.L., C.A.B., E.T.M., E.N.B., P.B.G., D.J., G.N., J.N.P., L.F.M., R.A.B., and R.G.J. conducted the immunologic assays. D.H.B. wrote the paper with all co-authors.
The authors declare no competing financial interests.
References
- 1.Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 3.Mlakar J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 4.Calvet G, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 5.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driggers RW, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016 doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 8.Johansson MA, Mier YTRL, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Miner JJ, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 13.Qian X, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang H, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brasil P, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 16.Faria NR, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas SJ, et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. Am J Trop Med Hyg. 2013;88:73–88. doi: 10.4269/ajtmh.2012.12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tartaglia LJ, et al. Production of Mucosally Transmissible SHIV Challenge Stocks from HIV-1 Circulating Recombinant Form 01_AE env Sequences. PLoS Pathog. 2016;12:e1005431. doi: 10.1371/journal.ppat.1005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazear HM, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi SL, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016 doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997;110:358–361. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Applied microbiology. 1973;25:539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JE, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledgerwood JE, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez LJ, et al. Safety and Immunogenicity of a Dengue Virus Serotype-1 Purified-Inactivated Vaccine: Results of a Phase 1 Clinical Trial. Am J Trop Med Hyg. 2015;93:454–460. doi: 10.4269/ajtmh.14-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez S, et al. An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques. Am J Trop Med Hyg. 2015;92:698–708. doi: 10.4269/ajtmh.14-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demicheli V, Debalini MG, Rivetti A. Vaccines for preventing tick-borne encephalitis. The Cochrane database of systematic reviews. 2009 doi: 10.1002/14651858.CD000977.pub2. CD000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demicheli V, Graves P, Pratt M, Jefferson T. Vaccines for preventing tick-borne encephalitis. The Cochrane database of systematic reviews. 2000 doi: 10.1002/14651858.CD000977. CD000977. [DOI] [PubMed] [Google Scholar]
- 30.Erra EO, Kantele A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev Vaccines. 2015;14:1167–1179. doi: 10.1586/14760584.2015.1061939. [DOI] [PubMed] [Google Scholar]