Abstract
Over 2 billion people worldwide are infected with helminths (worms). Similarly, infection with Mycobacterium tuberculosis (Mtb) occurs in over a third of the world's population, often with a great degree of geographical overlap with helminth infection. Interestingly, the responses induced by the extracellular helminths and those induced by the intracellular Mtb are often mutually antagonistic and, as a consequence, can result in impaired (or cross-regulated) host responses to either of the infecting pathogens. In this review, we outline the nature of the immune responses induced by infections with helminths and tuberculosis (TB) and then provide data from both experimental models and human studies that illustrate how the immune response engendered by helminth parasites modulates Mtb-specific responses in helminth-TB co-infection.
Overview of Helminth and Mtb Infections
Parasitic helminths are complex eukaryotic organisms, characterized by their ability to maintain long-standing, chronic infections in humans.. Hence, parasitic helminths are a major health care problem worldwide, infecting more than two billion people mostly in resource-limited countries [1]. Common helminth infections, such as gastrointestinal nematodes and filarial infections, are a major medical, social and economic burden to the countries in which these infections are endemic (Table 1). Helminth parasites have characteristically complex life cycles with many developmental stages and often establish chronic infections. Thus, the host is exposed during the course of a single infection to multiple life cycle stages of the parasites, each stage with both a shared and a unique antigenic repertoire. In addition, depending on the anatomical location of the parasite, the responses may be compartmentalized (intestinal mucosa and draining lymph nodes in intestinal nematode infection or skin/subcutaneous tissue and draining lymph nodes in onchocerciasis) or systemic (in lymphatic filariasis) [1]. Moreover, the migration patterns of the parasite might elicit varied cutaneous, pulmonary and intestinal inflammatory pathologies, as seen, for example, in Ascaris or Stronglyloides infections during their migratory phase. Helminth infections can elicit a spectrum of clinical manifestations that reflect the diversity in host immune responses [2]. Another hallmark of all helminth infections is their chronicity, with many helminths surviving in the host for decades. For example, adult filariae may survive in host tissues for up to 20 years, producing eggs and microfilariae throughout most of their lifespan. Similarly, Strongyloides stercoralis due to its ability to auto-infect their host can maintain its life cycle indefinitely [3].
Table 1.
Medically Important Helminth Infections.
| Organism | Numbers infected (in millions)a,b |
|---|---|
| Ascaris lumbricoides | 819 |
| Trichuris trichiura | 465 |
| Hookworm species | 439 |
| Schistosoma mansoni | 252 |
| Wuchereria bancrofti | 120 |
| Strongyloides stercoralis | 30–100 |
| Onchocerca volvulus | 37 |
| Loa loa | 13 |
Hotez PJ, Alvarado M, Basanez MG, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 2014;8:e2865.
Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ and Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest 2008;118:1311-21.
TB is also a major public health care problem and remains a major cause of morbidity and mortality worldwide [4]. In 2014, an estimated 1.5 million people died of tuberculosis, and there were an estimated half a million cases of drug-resistant TB [5]. TB is caused by aerosol infection with the acid-fast bacillus Mtb. It is predominantly a disease of the lungs, with pulmonary TB accounting for 70% of the global burden, although extra-pulmonary TB, most commonly of the lymph nodes, can also occur relatively frequently [6]. Although, ~10 million new active Mtb cases are reported annually, an estimated 2.2 billion people worldwide are infected but remain asymptomatic and are termed latently infected [7]. Latent TB infection (LTBI) is thus defined as infection detectable only through immunological responses without bacterial or radiological confirmation of clinical disease [8]. Finally, control of this major TB epidemic is hampered by the lack of an effective vaccine, compliance with drug treatment given its 6–9 month duration, emergence of drug resistance and the lack of point of care diagnostics [4].
In summary, helminths and TB infections largely overlap at the population (or geographic level) and both elicit distinct and complex immune responses. We will thus focus this review first on the nature of the immune response elicited by each pathogen and then on their potential interaction.
Immune Responses in Helminth Infections
The immunological hallmark of helminth infections is their ability to induce T helper type 2 (Th2)-associated immune responses characterized by the presence of the interleukins IL-4, IL-5, IL-9, IL-10 and IL-13, generalized and localized eosinophilia, goblet and mucosal mast-cell hyperplasia and production of IgE and IgG1 (in mice) and IgE and IgG4 (in humans) [2, 9]. Although initial studies identified CD4+ Th2 cells as an important source of IL-4, IL-5, IL-9, IL-10 and IL-13, it is now abundantly clear that a variety of other cell types including eosinophils, basophils and innate lymphoid cells (ILCs) are capable of producing some of these cytokines in response to helminth infections [9]. Therefore, these responses will be collectively referred to as type 2 immune responses. Similarly, for example in the case of lymphatic filariasis, the immuno-regulatory cytokine IL-10 is now known to be produced by not only Th2 cells but also by Th1 cells and by different subsets of regulatory T cells (Tregs) [10]. While the type 2 immune response induced by helminth parasites is a stereotypical response, the initiation, progression and culmination of this response requires interaction with many different cell types, most notably: (i) epithelial or stromal cells (ii) ILCs (iii) antigen-presenting cells – dendritic cells and macrophages; (iv) T cells; (v) B cells; (vi) eosinophils and (vii) mast cells and basophils [2].
Helminth parasites can stimulate a Type 2 immune response through a variety of mechanisms. Typically, helminths damage tissue during feeding and migration, resulting in the release of danger associated molecular patterns and the alarmin cytokines, thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 [11]. These cytokines in turn can activate ILCs and other cell populations to secrete IL-4, IL-5 and IL-13. This results in activation and differentiation of classical Th2 cells and further amplification of the Type 2 immune response [11]. Helminths can also secrete a variety of excretory/secretory (ES) products that can directly stimulate dendritic cells (DC) and macrophages, which in turn activate naive CD4+ T cells to differentiate into Th2 cells in the presence of IL-4 and/or IL-13 (and in the relative absence of IL-12) [12].
Over time, the prototypical host response can lead to the expansion of both thymic and peripheral Tregs and alternatively activated macrophages [13, 14] that in turn modulates immune functions. Moreover, helminth parasites in humans have been shown to: inhibit DC maturation and function, induce alternative activation of macrophages with NO synthase (Nos2) suppression, down regulate the expression of Toll-like receptors on antigen presenting cells (APCs) and T cells, induce apoptosis of DCs, T cells, and natural killer (NK) cells and mediate anergy of cognate T cells [10, 15].
A relatively modulated parasite-specific cellular immune response characterized by decreased T cell proliferation and decreased production of interferon γ (IFN-γ) and IL-2, is common in many parasitic diseases such as lymphatic filariasis [16] and hookworm infections [17]. The reasons for this appear to be multi-factorial and include regulation by IL-10, in utero exposure to parasite antigens, and active suppression by soluble parasite products [18]. Macrophages can also mediate immune-regulatory effects through expression of inhibitory enzymes such as arginase-1, of co-inhibitory molecules such as programmed cell death ligand 1 (PDL1), PDL2, or of other regulators including IL-10 and suppressor of cytokine signaling −1 (SOCS-1) [19]. Macrophages induced in helminth infections are typically of the alternatively activated phenotype and can contribute to tissue remodeling that may be an intricate part of the host response to parasitic infections [20]. Another important regulatory cytokine induced by helminth infections is transforming growth factor β, known to be produced by Treg cells [21]. Although the immune down modulation associated with helminth infections is parasite-antigen specific relatively early in infection, bystander (or spillover) effects on routine vaccinations, allergic processes and autoimmune diseases have been noted [18].
The hygiene hypothesis postulates that the stimulation of the immune system by microbes or microbial products protects from the development of inflammatory and atopic disorders [22, 23]. Human studies have demonstrated that people living in areas endemic for helminth infections have a decreased prevalence of skin tests for allergens and may have less asthma [24]. Experimental animal models also reveal the protective effect of helminth infections against atopy and asthma [25]. Several mechanisms have been proposed for the helminth-induced protection from allergy, chief among them being the induction of regulatory T cell activity and immunosuppressive cytokines, including IL-10 and TGFβ [18]. Similarly, exposure to helminth parasites has been shown to be prevent the onset of Th1 mediated diseases such as multiple sclerosis, diabetes mellitus and inflammatory bowel disease in experimental animal models [26].
The investigation of the relationship between human helminth infections and the immune response to non-helminth antigens is of great public health significance for a number of reasons. If pre-existing infections can influence immune responses against unrelated antigens, the implications for the effectiveness of vaccination programs, especially in resource-limited countries, may be significant. Indeed, a recurring problem of vaccination campaigns in regions of the resource-limited populations has been the poor immunogenicity of vaccines [28, 29] including Mycobacterium bovis BCG (Bacillus Calmette-Guerin) [30], and vaccine failure even where coverage is high for oral polio vaccination [31] and oral cholera vaccine in South Asians of low socioeconomic status [32]. The relevance of this concern has been demonstrated by multiple studies showing poor vaccine responses in regions endemic for onchocerciasis [33, 34], lymphatic filariasis [35], and soil transmitted helminths [36].
In summary, helminth infections lead to modulated immune responses with a strong Th2 bias and a major immunoregulatory component. This regulation can affect not only responses to helminth antigens, but also responses to unrelated antigens.
Immune Responses in Tuberculosis
In the majority of people, infection with Mtb is initially contained by the host immune response, resulting in LTBI [6]. However, individuals with LTBI can progress to active disease at any time, often many years after initial infection. LTBI is defined by a positive tuberculin skin test or a positive IFNγ release assay, which indicates a T cell response to TB antigens [8]. Individuals with LTBI do not have TB bacilli in their sputum and have no clinical or radiological evidence of active disease. Of late, it has been recognized that LTBI is not a single entity, but rather it represents a spectrum of immunological and microbiological changes [37].
Immune responses to Mtb are characterized by the involvement of many different cell types, including T cells, B cells and NK cells [38] with CD4+ T cells being perhaps the major cell type mediating containment of Mtb. These CD4+ Th1 cells secrete IFNγ and tumor necrosis factor α, both of which are crucial for protective immunity against Mtb [6, 38]. The induction of IFNγ is regulated by IL-12, and genetic mutations in the genes coding for IL-12, IL-12R, IFNγR or Stat1 or depletion of CD4+ T cells (by HIV for example), all enhance the susceptibility to disseminated mycobacterial disease [6]. The importance of the Th1 pathway is further emphasized by the increased susceptibility to disease in individuals treated with biologicals targeting TNF [6].
In addition to Th1 cells, it is now increasingly recognized that CD4+ Th17 cells also play an important role in host resistance to TB, at least in terms of the memory immune responses [39]. These Th17 cells secrete IL-17A, IL-17F and IL-22 and are involved in establishing an optimal Th1 response in animal models of TB infection [40]. During activation and differentiation, CD4+ and CD8+ T cells gain the ability to produce more than one cytokine simultaneously [41]. There is some evidence, although not complete, that Mtb antigen-induced multifunctional CD4+ and CD8+ T cells play an important role in host protection against TB [42]. Other cytokine families are also known to influence immunity to TB. Thus, the IL-1 family of cytokines, including IL-1α, IL-1β and IL-18, are considered important for protection against TB disease, while the regulatory cytokines - IL-10 and TGFβ and the Type 1 interferons are thought to promote acquisition of infection and to exacerbate pathology [43].
Although the role played by CD8+ T cells and NK cells is less well established than CD4+ cells in Mtb infection, these cell populations are generally considered important for the optimal host immune response to TB [44]. Thus, CD8+ T cells can eliminate Mtb-infected target cells, and cytolytic molecules (i.e., perforin, granzyme B and granulysin) are considered important in host resistance [6, 42]. NK cells, an important source of IL-22, can activate phagocyte defense mechanisms and directly kill Mtb-infected target cells [6, 42]. Moreover, an important group of non-classical T cells including Human Leukocyte Antigen - E restricted CD8+ T cells; lung associated mucosal invariant - T cells (MAIT) and CD1-restricted T cells, can alos contribute to host defense against Mtb [6, 42].
In summary, immunity to Mtb infection requires a predominant Th1 response with minor contributions from Th17 cells and other accessory cells. In addition, TB is typically characterized by a strong pro-inflammatory milieu.
Helminth - Tuberculosis Co-Infection
Epidemiological Outcomes of Co-Infection
Human epidemiological studies in helminth-TB co-infection have focused mainly on three aspects of the immune response to TB, those being: (1) the effect on diagnostic tests of LTBI; (2) the effect on the acquisition of active disease from LTBI; and (3) the effect on the efficacy of BCG vaccination.
Helminth infections appear to play an important role in modulating the immune response to Mtb purified protein derivative (PPD) and other Mtb antigens and, therefore, indirectly affect the diagnostic tests used for diagnosing LTBI [45]. Onchocerciasis has also been shown to modulate delayed type hypersensitivity to tuberculin skin testing in adults in Mali [46] and in Chad [47] and to Mtb antigens (in vitro) in children [48]. Helminth infections were also associated with inconclusive results in the IFNγ release assays (IGRAs) in pregnant mothers in Ethiopia [49] and in children in Bangladesh [50]. Similarly, in South Africa, children with IgE antibodies to Ascaris appeared to be less likely to mount a positive response to the tuberculin skin test [51]. In contrast, coincident lymphatic filarial infection had no significant influence on tuberculin skin test positivity in a population endemic for Wuchereria bancrofti and hookworm infections and tuberculosis in South India [52]. Similar findings were seen in a study of patients in Brazil [53] and Peru [54], in which there was no difference in skin test reactivity between those with intestinal parasites and those without. A more recent study in South Africa similarly revealed no significant effect of antihelmintic treatment on tuberculin skin test or IGRAs in children [55]. Thus, helminth infections appear to influence the reactivity to TB antigens (albeit not in all studies) that form the basis of diagnostic tests to detect LTBI. Moreover, the discrepancy in the results in terms of skin test reactivity or IGRA's appears to be due to either the type of helminth parasites studied (tissue invasive versus intestinal), the method used for diagnosing the helminth infection (stool microscopy versus serology), the cutoff used for skin test reactivity (12 mm versus 10 mm) and the differences in geographic regions.
The influence of helminth infection on the development of active tuberculosis or of outcome following anti-tuberculous treatment is not completely clear. It has been shown that patients with active TB were more likely to have intestinal helminths than were controls in a hospitalized population in Brazil [56]. Similarly, in Ethiopia, a significant association was observed between the incidence of TB disease and the presence of intestinal helminth infection [57]. However, in HIV positive populations in both Uganda and Brazil, neither gastrointestinal parasitic infections nor Mansonella perstans (a filarial nematode) was associated with progression to active TB, though in Uganda there was a relationship between having schistosomiasis and progression from LTBI to active TB [53, 58]. Interestingly, eosinophilia, that typically accompanies helminth infections, was found to be strongly associated with the risk of active TB in Africa [59]. These data imply that the correlation between helminth infections and active TB breaks down in the presence of HIV, which is not surprising since the influence of HIV on the immune responses to Mtb is conceivably many fold higher than the effects of helminths on TB.
Individuals with helminth - TB co-infection also present with more advanced TB disease compared to helminth-uninfected TB patients [60]. A more recent study in Ethiopia, however, indicated that, in patients with asymptomatic helminth infection, active TB is associated with lower rates of sputum smear positivity, leading to the conclusion that there was a beneficial effect of helminth infection on bacterial burdens [61]. Moreover, a randomized double blind clinical trial examining the effect of anti-helmintic treatment on clinical improvement of TB after 2 months showed no significant effect of anti-helmintic (albendazole) treatment over placebo [62]. Finally, a large study in South India with >5000 individuals followed over a period of 10 years showed no significant effect of the presence of helminth co-infection on the rate of progression from latent to active TB nor on the severity of TB disease in those individuals developing TB during the course of study [63]. This study does suffer from the limitation that, although adequately powered to detect effects on progression and pathology, the helminth status was only determined at baseline and not during the subsequent longitudinal follow ups. Thus, it is quite possible that the rate of helminth acquisition during the follow up period could have influenced the outcome of the study. Overall, most clinical studies appear to indicate that helminth infections had little to no effect on pathogenesis or pathology of active TB. However, it is important to note that with the exception of the study mentioned above [63], no longitudinal study with adequate power has been performed to directly address the influence of helminths on progression from LTBI to active TB and that very few clinical studies have been carried out in active TB - helminth co-infection to tackle the issue of disease severity.
The process by which helminth infections are likely to exert the most dominant effect on TB is by interfering with vaccine-induced responses to TB. The protective efficacy of BCG against pulmonary TB varies greatly in different regions of the world. Thus, while its efficacy in the United Kingdom (and other parts of northern Europe) is in the range of 80%, it varies between 0–50% in low- to middle-income countries where helminths are often co-endemic [64]. It has been reported previously that cellular immune responses to Mtb antigens are decreased in individuals with concurrent helminth infections, explaining at least in part, the reduced efficiency of BCG in helminth endemic regions of the world [65]. Later, it was demonstrated that helminth-induced immune sensitization during gestation persists into childhood and skews subsequent childhood immune responses away from protective Th1 immune responses [66]. Finally, a more recent study has clearly demonstrated that concomitant helminth infections significantly impair the immunogenicity of BCG vaccines, an impairment associated with increased TGFβ production [67].
Functional Outcomes of Co-Infections
The major immunological effects of helminth co-infection on immune responses to TB antigens in humans has been primarily demonstrated in vitro. Thus, a variety of studies from different endemic areas of the world and using different co-infecting helminth species have repeatedly shown a significant effect of the presence of helminth infection on the quality of immune response engendered by the mycobacteria. In vitro studies have shown that pre-exposure of APCs to filarial parasites limits the maturation and pro-inflammatory responses of APCs (DC and macrophages) in response to subsequent TB infection [68]. In LTBI, co-incident filarial infections have been shown to have a major immunological impact on the Mtb-antigen specific immune responses; thus, Th1 and Th17 responses to PPD and Mtb secreted proteins were significantly lower in LTBI individuals with concomitant filarial infections compared to those without filarial infections in an ex vivo study [69]. This downmodulation is at least partially mediated through the increased expression of the negative costimulatory molecules - cytotoxic T lymphocyte associated antigen - 4 (CTLA-4) and programmed cell death protein −1 (PD-1) [69]. Similarly, several studies have documented a poor in vitro response to PPD in onchocerciasis [70, 71]. Filarial infections also have the additional effect of downmodulating the expression and function of Toll-like receptors (TLR), specifically TLR2 and TLR9 [72]. Filarial infections are also known to modulate the immune response to Mtb antigens through an expansion of CD4+IL-4+ memory T cells, which presumably down regulates the expansion of Th1 cells, via a Th1-Th2 crosstalk [73].
Hookworm infections have also been shown to exert a profound inhibitory effect on protective Th1 and Th17 responses, thus potentially modulating the cytokine environment in which TB is controlled in LTBI [74]. Similarly, Strongyloides and Mtb co-infection has also been shown to be associated with significantly lower systemic levels of type 1 (IFNγ, TNFα and IL-2) and Th17 (IL-17A and IL-17F) cytokines and significantly elevated systemic levels of regulatory (IL-10 and TGFβ) and type 2 (IL-4, IL-5 and IL-13) cytokines ex vivo [75]. In addition, a study of immigrants to the United Kingdom demonstrated that helminth infections (mainly Schistosoma mansoni and S. stercoralis) were associated with a decreased frequency of CD4+ IFNγ secreting T cells and an increased frequency of CD4+ Tregs in those with LTBI in comparison to helminth-uninfected controls [76]. Interestingly, following anthelmintic treatment, the frequency of Th1 cells increased and that of Tregs decreased, indicating that the modulation of T cell responses was helminth-mediated. These studies, in aggregate, suggest that helminth infections can modulate a variety of Mtb-specific responses. Whether this modulation leads to the development of active tuberculosis awaits clarification.
Very few studies have been performed examining the immunological effects of helminth co-infections on active pulmonary TB, although these few studies indicate that helminths also modulate the host immune response in active disease. For example, it has been shown that intestinal helminth co-infection is accompanied by lowered in vitro production of IFNγ and elevated production of IL-10 in individuals with active pulmonary tuberculosis [60]. Similarly, it was also recently shown that helminth infections with coincident active TB have marked decreases in dual-functional Th1 and Th17 cell frequencies [77] which, in one study at least, may be associated with increased Treg and Th2 responses [61].
Thus, human in vitro studies clearly demonstrate a major influence of helminth infections on host responses to TB. In large part, cytokine regulation appears to be the main focus of the studies conducted so far on this topic (schematized in Figure 1). There could be multiple reasons as to why the major clinical and epidemiological studies have been unable to demonstrate reflect an influence of helminth infection on latent or active TB that include: (1) most clinical or epidemiological studies have been underpowered to detect clinical and epidemiological endpoints of significance; (2) cross-sectional studies, while useful to highlight the immunological effects are not useful for studies clinical outcomes and few longitudinal studies have been performed; (3) the type (lung migrating versus non-lung migrating) or intensity (the higher the intensity of infection the more the potential impact on outcomes) of helminth infection; and (4) the differences in methodologies used for diagnosis of a given helminth infection That being said, more detailed studies on the effect of helminths on innate cells, including neutrophils and ILCs, activation and function of DCs, monocytes/macrophages and B cells need to be conducted.
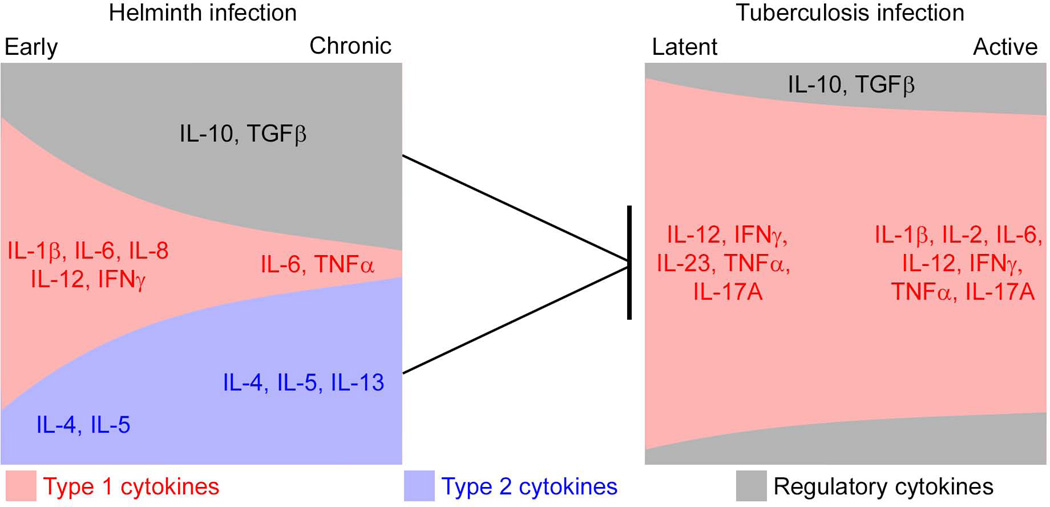
Figure 1. Cytokine Regulation of Tuberculosis Infection by Helminths.
The cytokine responses engendered by relatively acute (early) and chronic helminth infections are depicted on the left, while the cytokine responses induced in latent or active tuberculosis is shown on the right. Type 1 cytokines are represented in red, type 2 cytokines in blue and regulatory cytokines in black. The size of the box represents the magnitude of the response. Thus, helminth infections predominantly induce type 2 and regulatory responses, while tuberculosis infection and disease is predominantly type 1, or pro-inflammatory, with different degrees of magnitude. IFN: Interferon, TGF, Transforming growth factor; TNF, Tumor necrosis factor
Experimental Models
Experimental animal models probably provide the best opportunity to study mechanisms underlying the interaction between helminths and TB. A number of studies have shown that mice with an persistent type 2 cytokine response induced by filarial parasites [78] or S. mansoni [79] exhibit a type 2-domininated response to Mtb antigens following Mtb infection. Mice with Mtb [80] or M. bovis BCG [81] and concomitant S. mansoni infections exhibited higher mycobacterial burdens in vivo compared to S. mansoni -uninfected mice, suggesting that helminth infections directly influence bacterial growth in these experimental settings. Similarly, mice infected with either Nippostrogylus brasiliensis [82] or Strongyloides venezuelensis [83] also demonstrated impaired bacterial control and higher bacterial burdens in the lungs. And a recent study has also demonstrated that mice co-infected with Heligmosomoides polygyrus bakeri and M. bovis BCG resulted in higher mycobacterial loads [84]. Despite these findings, there have been other studies in which there was little to no effect of pre-existing helminth infection on TB. In one mouse study, infection with Toxocara canis was not associated with increased susceptibility to pulmonary tuberculosis [85]. Similarly, mice co-infected with N. braziliensis and M. bovis BCG were able to clear the BCG infection at the same rate as the helminth-uninfected controls [86]. Finally, a cotton rat model of Mtb-helminth co-infection also failed to detect an influence of the helminth on TB disease or bacterial burdens [87]. One possible explanation for the differences in the findings in animal models is the use of different helminths that in turn may exert different effects on mycobacterial co-infection. Since filariae, strongylids and schistosomes all reside in different tissue spaces, have different life cycles and release different excretory-secretory products, their influence on coexisting Mtb infection could potentially be very different. Another possible explanation is the difference in the species of mycobacteria used (M. bovis BCG versus Mycobacterium avium versus Mtb), the strain of Mtb used (Erdman versus H37Rv) and the route of infection (some studies use the iv route and others the aerosol route). Similarly, lung migrating helminths have the propensity to induce changes in the local inflammatory milieu of Mtb infection (for example, inducing alternative activation of macrophages in the lung) and hence have the potential to exert more direct effects on co-infection. Finally, the setting in which co-infections were studied (acute helminth infection versus chronic helminth infection) could also play a role in the different in vivo results.
In terms of mechanisms of modulation, there have been four recent studies that have uncovered differing mechanisms underlying the helminth-induced influence on host immunity to TB. One mechanism by which helminths modulate immunity to TB is by the establishment of a type 2 cytokine milieu in the lung that facilitates the development of alternatively activated macrophages [82]. These macrophages then mediate through an IL-4R dependent process impairment of immune responses and bacterial control. Another mechanism by which helminthes modulate the immune response to TB is by the production of TGFβ, that can mediate the suppression of Th1 responses in mice resulting in higher bacterial burdens and decreased delayed type hypersensitivity responses [84]. The third mechanism at play is a direct effect on T cells with decreased Th17 responses and increased expression of CTLA-4 [83]. Finally, the expression of arginase-1 during co-infections has been shown to mediate impaired Th1 responses to Mtb antigens and exacerbated pulmonary pathology [88]. Thus, animal models exploring the mechanisms underlying immune interactions in co-infection clearly reveal the complexity of this process. The various pathways through which helminths can modulate immune responses have been summarized in Figure 2.
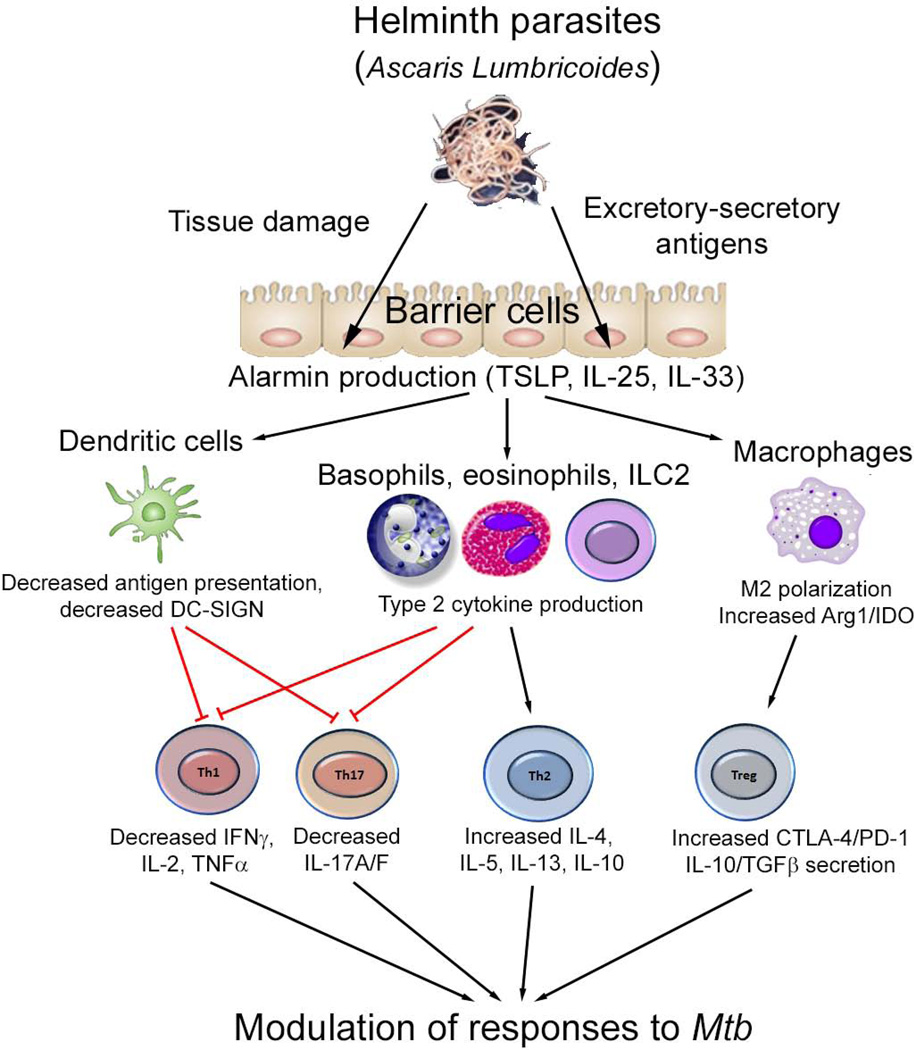
Figure 2. Modulation of Immune Response to Tuberculosis Infection by Helminths Based on Murine Models.
Helminth parasites (for example Ascaris Lumbricoides) induce the release of alarmin cytokines, including thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 from barrier cells including epithelial cells. These cytokines then activate a variety of secondary cell types. In addition, helminth parasites or their excretory-secretory products can directly interact with dendritic cells, macrophages, basophils and eosinophils to induce activation and initiation of the type 2 immune response. This subsequently modulates the T cell response to effect influence on Mycobacterium tuberculosis (Mtb) infection and disease through a variety of mechanisms described. TSLP, thymic stromal lymphopoietin; Arg-1, arginase-1; IDO, indoleamine 2,3 dioxygenase; DC-SIGN, dendritic cell specific intracellular adhesion molecule-3 grabbing non-integrin; PD-1, programmed cell death protein -1; CTLA-4, cytotoxic T lymphocyte associated protein-4, TNF: tumor necrosis factor, TGF: transforming growth factor
Concluding Remarks
It is clear that there is great degree of interaction in the initiation and maintenance of host immune responses to concomitant or co-existing helminth infections and TB. Whether this antigen-specific crosstalk actually alters clinical outcomes remains an important question. Longitudinal studies involving large populations are required to definitively understand the interface between helminth infections and TB (be it latent or active) infections (see Outstanding Questions box). The area of greatest impact of the modulation of the immune response by helminth infections likely involves attenuation of vaccine-induced immune responses. Given that vaccines to prevent either TB infection or TB disease is a major need, it is therefore imperative that the contribution of coincident helminth infection be considered during the conduct of vaccine studies in helminth-endemic areas of the world. Finally, studies (be they systems biology focused or experimental models) are required to gain insight into the fundamental nature of the immune responses engendered by co-infecting multicellular pathogens.
OUTSTANDING QUESTIONS BOX.
-
In the context of helminth/TB co-infection
What is/are the mechanism(s) by which helminths mediate the modulation of effector responses to TB antigens?
What effects do helminth infections have on the clinical course of TB disease?
What effects do helminth infections have on bacterial burdens and/or severity of TB disease?
Do helminths increase the risk of reactivation of latent TB or the risk of acquiring new infections?
Can anti-helmintic treatment modulate the effects of helminths on TB antigen responses?
Would anti-helmintic treatment increase TB vaccine efficacy in helminth endemic areas?
TRENDS BOX.
Helminths appear to influence the immune response to tuberculosis (TB) by modulating both innate and adaptive responses to the TB pathogen.
T cells are the predominant cell type studied in the context of helminth-TB co-infections and appear to exhibit both dampened effector responses and expanded regulatory responses.
Amongst the various regulatory mechanisms examined, IL-10 appears to play the most profound role in influencing the T cell responses to mycobacterial antigens in the context of helminth-TB co-infection.
Mechanistic studies examining the mechanism by which helminths influence pathogenesis or pathology in active TB are currently ongoing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Nutman TB. Lymphatic filariasis: new insights and prospects for control. Curr Opin Infect Dis. 2001;14:539–546. doi: 10.1097/00001432-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Barry CE, 3rd, Maartens G. Tuberculosis. Lancet. 2016;387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global Tuberculosis Report. 2013
- 6.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 7.Getahun H, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis Infection. N Engl J Med. 2015;373:1179–1180. doi: 10.1056/NEJMc1508223. [DOI] [PubMed] [Google Scholar]
- 8.Salgame P, Geadas C, Collins L, Jones-Lopez E, Ellner JJ. Latent tuberculosis infection--Revisiting and revising concepts. Tuberculosis (Edinb) 2015;95:373–384. doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Grencis RK. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu Rev Immunol. 2015;33:201–225. doi: 10.1146/annurev-immunol-032713-120218. [DOI] [PubMed] [Google Scholar]
- 10.Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol. 2013 doi: 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Metenou S, Nutman TB. Regulatory T cell subsets in filarial infection and their function. Front Immunol. 2013;4:305. doi: 10.3389/fimmu.2013.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semnani RT, Law M, Kubofcik J, Nutman TB. Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J Immunol. 2004;172:6229–6238. doi: 10.4049/jimmunol.172.10.6229. [DOI] [PubMed] [Google Scholar]
- 15.Semnani RT, Nutman TB. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol Rev. 2004;201:127–138. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 16.King CL, Mahanty S, Kumaraswami V, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaze S, Bethony JM, Periago MV. Immunology of experimental and natural human hookworm infection. Parasite Immunol. 2014;36:358–366. doi: 10.1111/pim.12088. [DOI] [PubMed] [Google Scholar]
- 18.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 19.Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014;7:753–762. doi: 10.1038/mi.2014.23. [DOI] [PubMed] [Google Scholar]
- 20.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol. 2013;34:847–861. doi: 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strachan DP. Lifestyle and atopy. Lancet. 1999;353:1457–1458. doi: 10.1016/S0140-6736(99)90038-7. [DOI] [PubMed] [Google Scholar]
- 23.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 24.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- 26.Weinstock JV, Elliott DE. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol. 2014;193:3239–3247. doi: 10.4049/jimmunol.1400927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmby H. Human helminth therapy to treat inflammatory disorders - where do we stand? BMC Immunol. 2015;16:12. doi: 10.1186/s12865-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelman R. Perspective on the development and deployment of rotavirus vaccines. Pediatr Infect Dis J. 1987;6:704–710. doi: 10.1097/00006454-198708000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Levine MM, Kaper JB. Live oral vaccines against cholera: an update. Vaccine. 1993;11:207–212. doi: 10.1016/0264-410x(93)90019-t. [DOI] [PubMed] [Google Scholar]
- 30.Baily GV. Tuberculosis prevention Trial, Madras. Indian J Med Res. 1980;(72 Suppl):1–74. [PubMed] [Google Scholar]
- 31.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 32.Suharyono, Simanjuntak C, Witham N, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5-9-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 33.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 34.Prost A, Schlumberger M, Fayet MT. Response to tetanus immunization in onchocerciasis patients. Ann Trop Med Parasitol. 1983;77:83–85. doi: 10.1080/00034983.1983.11811675. [DOI] [PubMed] [Google Scholar]
- 35.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott AM, Mawa PA, Webb EL, et al. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 40.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 42.Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- 43.Mayer-Barber KD, Sher A. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev. 2015;264:264–275. doi: 10.1111/imr.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottenhoff TH. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 2012;20:419–428. doi: 10.1016/j.tim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum . Curr Opin HIV AIDS. 2012;7:231–238. doi: 10.1097/COH.0b013e3283522c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rougemont A, Boisson-Pontal ME, Pontal PG, Gridel F, Sangare S. Tuberculin skin tests, B.C.G vaccination in hyperendemic area of onchocerciasis. Lancet. 1977;1:309. doi: 10.1016/s0140-6736(77)91857-8. [DOI] [PubMed] [Google Scholar]
- 47.Buck AA, Anderson RI, Kawata K, Hitchcock JC., Jr Onchocerciasis: some new epidemiologic clinical findings Results of an epidemiologic study in the Republic of Chad. Am J Trop Med Hyg. 1969;18:217–230. doi: 10.4269/ajtmh.1969.18.217. [DOI] [PubMed] [Google Scholar]
- 48.Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–523. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebreegziabiher D, Desta K, Howe R, Abebe M. Helminth infection increases the probability of indeterminate QuantiFERON gold in tube results in pregnant women. Biomed Res Int. 2014;2014:364137. doi: 10.1155/2014/364137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas TA, Mondal D, Noor Z, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. 2010;126:e1522–e1529. doi: 10.1542/peds.2010-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Soelen N, Mandalakas AM, Kirchner HL, et al. Effect of Ascaris Lumbricoides specific IgE on tuberculin skin test responses in children in a high-burden setting: a cross-sectional community-based study. BMC Infect Dis. 2012;12:211. doi: 10.1186/1471-2334-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipner EM, Gopi PG, Subramani R, et al. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am J Trop Med Hyg. 2006;74:841–847. [PubMed] [Google Scholar]
- 53.Neto LM, Oliveira Rde V, Totino PR, et al. Enteroparasitosis prevalence and parasitism influence in clinical outcomes of tuberculosis patients with or without HIV co-infection in a reference hospital in Rio de Janeiro (2000–2006) Braz J Infect Dis. 2009;13:427–432. doi: 10.1590/s1413-86702009000600008. [DOI] [PubMed] [Google Scholar]
- 54.Zevallos K, Vergara KC, Vergara A, Vidal C, Garcia HH, Evans CA. Tuberculin skin-test reactions are unaffected by the severity of hyperendemic intestinal helminth infections and co-infections. Am J Trop Med Hyg. 2010;83:319–325. doi: 10.4269/ajtmh.2010.10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Zalm MM, van Soelen N, Mandalakas AM, et al. The Effect of Deworming on Tests of Tuberculosis Infection in Children with Recent Tuberculosis Exposure - A Randomized Controlled Trial. Pediatr Infect Dis J. 2016;35:706–708. doi: 10.1097/INF.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tristao-Sa R, Ribeiro-Rodrigues R, Johnson LT, Pereira FE, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev Soc Bras Med Trop. 2002;35:533–535. doi: 10.1590/s0037-86822002000500020. [DOI] [PubMed] [Google Scholar]
- 57.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11:551–558. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 58.Brown M, Miiro G, Nkurunziza P, et al. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. Am J Trop Med Hyg. 2006;74:819–825. [PubMed] [Google Scholar]
- 59.Elliott AM, Kyosiimire J, Quigley MA, et al. Eosinophilia and progression to active tuberculosis in HIV-1-infected Ugandans. Trans R Soc Trop Med Hyg. 2003;97:477–480. doi: 10.1016/s0035-9203(03)90096-4. [DOI] [PubMed] [Google Scholar]
- 60.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abate E, Belayneh M, Idh J, et al. Asymptomatic Helminth Infection in Active Tuberculosis Is Associated with Increased Regulatory and Th-2 Responses and a Lower Sputum Smear Positivity. PLoS Negl Trop Dis. 2015;9:e0003994. doi: 10.1371/journal.pntd.0003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abate E, Elias D, Getachew A, et al. Effects of albendazole on the clinical outcome and immunological responses in helminth co-infected tuberculosis patients: a double blind randomised clinical trial. Int J Parasitol. 2015;45:133–140. doi: 10.1016/j.ijpara.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee S, Kolappan C, Subramani R, et al. Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One. 2014;9:e94603. doi: 10.1371/journal.pone.0094603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 67.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 68.Talaat KR, Bonawitz RE, Domenech P, Nutman TB. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis . J Infect Dis. 2006;193:196–204. doi: 10.1086/498912. [DOI] [PubMed] [Google Scholar]
- 69.Babu S, Bhat SQ, Kumar NP, et al. Human Type 1 and 17 Responses in Latent Tuberculosis Are Modulated by Coincident Filarial Infection through Cytotoxic T Lymphocyte Antigen-4 and Programmed Death-1. J Infect Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soboslay PT, Dreweck CM, Hoffmann WH, et al. Ivermectin-facilitated immunity in onchocerciasis. Reversal of lymphocytopenia, cellular anergy and deficient cytokine production after single treatment. Clin Exp Immunol. 1992;89:407–413. doi: 10.1111/j.1365-2249.1992.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steel C, Lujan-Trangay A, Gonzalez-Peralta C, Zea-Flores G, Nutman TB. Immunologic responses to repeated ivermectin treatment in patients with onchocerciasis. J Infect Dis. 1991;164:581–587. doi: 10.1093/infdis/164.3.581. [DOI] [PubMed] [Google Scholar]
- 72.Babu S, Bhat SQ, Kumar NP, et al. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl Trop Dis. 2009;3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. Filarial infection modulates the immune response to Mycobacterium tuberculosis through expansion of CD4+ IL-4 memory T cells. J Immunol. 2015;194:2706–2714. doi: 10.4049/jimmunol.1402718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190:5161–5168. doi: 10.4049/jimmunol.1203311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George PJ, Pavan Kumar N, Jaganathan J, et al. Modulation of pro- and anti-inflammatory cytokines in active and latent tuberculosis by coexistent Strongyloides stercoralis infection. Tuberculosis (Edinb) 2015;95:822–828. doi: 10.1016/j.tube.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toulza F, Tsang L, Ottenhoff TH, Brown M, Dockrell HM. Mycobacterium tuberculosis-specific CD4(+) T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur J Immunol. 2016;46:752–761. doi: 10.1002/eji.201545843. [DOI] [PubMed] [Google Scholar]
- 77.George PJ, Anuradha R, Kumar NP, et al. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog. 2014;10:e1004375. doi: 10.1371/journal.ppat.1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearlman E, Kazura JW, Hazlett FE, Jr, Boom WH. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 79.Sacco R, Hagen M, Sandor M, Weinstock JV, Lynch RG. Established T(H1) granulomatous responses induced by active Mycobacterium avium infection switch to T(H2) following challenge with Schistosoma mansoni. Clin Immunol. 2002;104:274–281. doi: 10.1006/clim.2002.5263. [DOI] [PubMed] [Google Scholar]
- 80.Frantz FG, Rosada RS, Peres-Buzalaf C, et al. Helminth coinfection does not affect therapeutic effect of a DNA vaccine in mice harboring tuberculosis. PLoS Negl Trop Dis. 2010;4:e700. doi: 10.1371/journal.pntd.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elias D, Akuffo H, Thors C, Pawlowski A, Britton S. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin Exp Immunol. 2005;139:398–404. doi: 10.1111/j.1365-2249.2004.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dias AT, de Castro SB, Alves CC, et al. Lower production of IL-17A and increased susceptibility to Mycobacterium bovis in mice coinfected with Strongyloides venezuelensis . Mem Inst Oswaldo Cruz. 2011;106:617–619. doi: 10.1590/s0074-02762011000500015. [DOI] [PubMed] [Google Scholar]
- 84.Obieglo K, Feng X, Bollampalli VP, et al. Chronic Gastrointestinal Nematode Infection Mutes Immune Responses to Mycobacterial Infection Distal to the Gut. J Immunol. 2016;196:2262–2271. doi: 10.4049/jimmunol.1500970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frantz FG, Rosada RS, Turato WM, et al. The immune response to toxocariasis does not modify susceptibility to Mycobacterium tuberculosis infection in BALB/c mice. Am J Trop Med Hyg. 2007;77:691–698. [PubMed] [Google Scholar]
- 86.Rafi W, Bhatt K, Gause WC, Salgame P. Neither primary nor memory immunity to Mycobacterium tuberculosis infection is compromised in mice with chronic enteric helminth infection. Infect Immun. 2015;83:1217–1223. doi: 10.1128/IAI.03004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hubner MP, Killoran KE, Rajnik M, et al. Chronic helminth infection does not exacerbate Mycobacterium tuberculosis infection. PLoS Negl Trop Dis. 2012;6:e1970. doi: 10.1371/journal.pntd.0001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monin L, Griffiths KL, Lam WY, et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest. 2015;125:4699–4713. doi: 10.1172/JCI77378. [DOI] [PMC free article] [PubMed] [Google Scholar]