Abstract
Background
Phthalate exposure is widespread. Prior research suggests that prenatal phthalate exposure may influence birth size and gestational duration, but published results have been inconsistent.
Objective
We quantified the relationship between maternal urinary phthalate concentrations and infant birth weight z-scores, length, head circumference, and gestational duration.
Methods
In a cohort of 368 women from the HOME Study, based in Cincinnati, OH, we measured nine phthalate metabolites representing exposure to six parent phthalate diesters in urine collected at approximately 16 and 26 weeks gestation. Infant birth size and gestational duration were abstracted from medical records. We used multivariable linear regression to estimate covariate adjusted associations between urinary phthalate metabolite concentrations and infant outcomes.
Results
In unadjusted models, we observed a negative association between monoethyl phthalate (MEP) and birth weight z-scores, while mono-3-carboxypropyl phthalate (MCPP) was positively associated with gestational duration. After covariate adjustment, phthalate metabolite concentrations were no longer associated with birth size or gestational duration.
Conclusions
In this cohort, urinary phthalate metabolites concentrations during pregnancy were not associated with infant birth size or gestational duration. Additional research is needed to determine if exposures during earlier periods of fetal development are associated with infant health.
Introduction
Phthalic acid diesters, or phthalates, are found in a wide variety of consumer products. Phthalates like diethyl phthalate (DEP), di-n-butyl phthalate and di-iso-butyl phthalate are used to retain scents in personal care products, such as lotions and perfumes (Braun et al. 2013; Koo and Lee 2004). Other phthalates, namely di(2-ethylhexyl) phthalate (DEHP) and benzylbutyl phthalate, are used as plasticizers in polyvinyl chloride plastics, food processing equipment, adhesives, and rainwear (Calafat et al. 2006; Hauser and Calafat 2005). Phthalate exposure is widespread, including among pregnant women (Braun et al. 2012; Philippat et al. 2012). Phthalates diesters are metabolized quickly into hydrolytic and/or oxidative monoester metabolites, conjugated to glucuronide or sulfate, and excreted in urine. Urinary concentrations of phthalate monoester metabolite can be used to assess exposure to phthalates. However, exposures are variable over time, possibly leading to exposure misclassification (Braun et al. 2012).
Phthalate monoester metabolites have been detected in amniotic fluid, umbilical cord blood, and meconium (Latini et al. 2003; Silva et al. 2004; Zhang et al. 2003). Some phthalates also have well documented anti-androgenic activity in rats and the potential to affect other hormonal pathways like the hypothalamic-pituitary-adrenal axis and thyroid axis, which are important for growth and development (Boas et al. 2012; Howdeshell et al. 2008; Ma et al. 2011).
Prenatal phthalate exposure has been reported to have an effect on birth size in some animal studies (Sharpe 2005; Tanaka 2002, 2003, 2005; Tyl et al. 2004) but not in others (Arcadi et al. 1998; Hoshino et al. 2005). Similarly, associations between prenatal phthalate exposure and infant size at birth or gestational duration in humans have been inconsistent. Some studies reported that increased prenatal phthalate exposure was associated with smaller size at birth and shorter gestation (Ferguson et al. 2014b; Meeker et al. 2009; Whyatt et al. 2009), while others reported that exposure was associated with longer gestation (Adibi et al. 2009; Wolff et al. 2008).
The purpose of this study was to examine the relation between prenatal phthalate exposure and infant size at birth (birth weight z-score, length, and head circumference) and gestational duration using a population-based prospective cohort of pregnant women from Cincinnati, OH, who provided up to two urine samples during the 2nd and 3rd trimesters of pregnancy.
Methods
Study Participants
We analyzed data collected from an ongoing prospective pregnancy and birth cohort, the Health Outcomes and Measures of the Environment (HOME) Study. Eligibility requirements included that women were ≥ 18 years of age, less than 19 weeks of gestation, living in the Cincinnati, OH area, and living in a home built before 1978 (Braun et al. 2010; Braun et al. 2016). Women in this study were recruited from seven prenatal care clinics affiliated with three Cincinnati, OH hospitals between 2003–2006. Our analysis included women who gave birth to a live singleton infant. Our final sample size was 368 mother-infant pairs after excluding two infants with genetic or chromosomal abnormalities and women missing covariate information (n=18).
Prenatal Phthalate Exposure Assessment
Participants provided two spot urine samples at approximately 16 (range: 10.4–22.6) and 26 (range: 19.1–34.6) weeks gestation. Urine was collected into polypropylene specimen cups, refrigerated until processing, and stored at or below −20°C until chemical analysis. Nine urinary phthalate metabolites were measured using previously described analytic methods; the limits of detection ranged from 0.1–1.0 ng/ml (Silva et al. 2004). To account for urine dilution, phthalate metabolite concentrations were creatinine-standardized by dividing metabolite concentrations (ng/mL) by creatinine concentrations (mg/dL) and multiplying by 100. These creatinine-standardized values were then log10-transformed. If more than one urine sample was provided, as was the case for 353 women (96%), the log10-transformed creatinine-standardized values from the 16- and 26-week samples were averaged. We created a summary measure for the four monoester metabolites of DEHP by dividing each metabolite by its molar mass and summing the metabolite concentrations, such that (ΣDEHP) was the molar sum of mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP).
Infant Anthropometrics and Gestational duration
We abstracted infant birth anthropometry, including birth weight (g), length (cm), and head circumference (cm), from medical records. Gestational duration (weeks) was calculated using mothers’ self-report of last menstrual period (n=360), ultrasound (n=6), or Ballard scores (n=2). We calculated birth weight z-scores, a standardized measure of birth weight for gestational age, from United States reference data (Oken et al. 2003).
Covariates
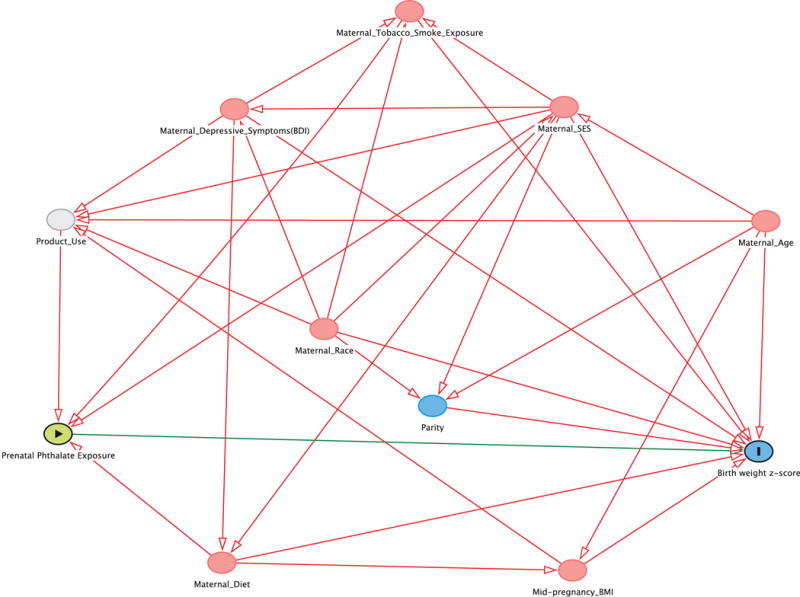
A directed acyclic graph (DAG) was drawn a priori to assess potential confounders associated with both phthalate exposure and birth outcomes (Supplemental Figure 1) (Greenland et al. 1999; Textor et al. 2011). We considered socio-demographic, nutritional, environmental, and perinatal factors. Socio-demographic factors including maternal age, race, income, marital status, and insurance status were assessed using standardized interviews administered by trained research assistants. Nutritional factors were assessed during the 2nd or 3rd trimester of pregnancy using standardized interviews and included maternal food security, prenatal vitamin use, and frequency of fruit, vegetable, and fish consumption. Serum cotinine, a sensitive and specific marker of active and secondhand tobacco smoke exposure, was measured using previously described methods (Bernert et al. 1997; Braun et al. 2010). Perinatal factors including parity and maternal mid pregnancy body mass index (BMI) at ~16 weeks gestation were abstracted from medical records. Depressive symptoms at 20 weeks gestation were assessed using the Beck Depression Inventory-II (Beck 1996). Our final adjusted models included maternal race, age, income, education, marital status, insurance status, parity, cotinine, food security, BMI, prenatal vitamin use, fish consumption, fruit/vegetable consumption, and depressive symptoms. When assessing head circumference, mode of delivery was added to the model.
Statistical Analysis
We described mean birth weight z-scores according to covariates. We then examined univariate characteristics of urinary phthalate metabolite concentrations, calculated Spearman correlations between repeated measures at 16 and 26 weeks gestation for all phthalate metabolites, and examined correlations between different phthalate metabolites. We used linear regression to estimate the unadjusted and adjusted difference in size at birth or gestational duration for each ten-fold increase in urinary phthalate metabolite concentration. We also considered preterm birth (gestational duration <37 weeks) as a dichotomous outcome. We evaluated the presence of non-linear relationships of phthalate metabolites with birth weight z-score and gestational duration using restricted cubic splines (Desquilbet and Mariotti 2010).
We performed additional analyses removing nutritional exposures and only including socio-demographic covariates in our multivariable models. We also examined whether maternal smoking (serum cotinine >3 ng/mL vs. < 3 ng/mL) (Benowitz et al. 2009), infant sex, or race (Black vs. White) modified the association between urinary phthalate metabolite concentrations and birth outcomes by including product interaction terms between urinary phthalate metabolite concentrations and these potential modifiers. We examined the magnitude and precision of associations within strata, as well as the product interaction term p-value.
Sensitivity Analyses
We performed several sensitivity analyses to examine the robustness of results to various assumptions and adjustments. First, we excluded 49 women with one or more of the following medical conditions that could possibly affect phthalate metabolite excretion and fetal growth: gestational diabetes (n=10), pregnancy induced hypertension (n=18), preeclampsia (n=21), chorioamnionitis (n=5), placenta previa (n=2), or placental abruption (n=9). Then we performed analyses using birth weight, instead of birth weight z-scores, both with and without adjusting for gestational duration in the model. Next, we conducted analyses without creatinine-standardizing phthalate metabolite concentrations, instead adding creatinine to the model as a covariate. We also re-conducted our analyses restricting to women with urinary creatinine levels between 30–300 mg/dl. We ran analyses adjusting for maternal weekly weight gain during pregnancy. We also examined urinary phthalate concentrations separately at 16 and 26 weeks gestation by estimating the adjusted association between birth weight z-scores or gestational age and each phthalate metabolite, at each time point with separate regression models. Finally, we assessed whether adjusting for other environmental exposures affected the magnitude or precision of our associations. These other environmental factors included bisphenol A (BPA), organophosphate (OP) pesticides, and self-reported alcohol consumption. Urinary BPA and six dialkyl phosphate (DAP) metabolites (biomarker of OP pesticide exposures) were measured at 16 and 26 weeks gestation using previously described analytic chemistry methods (Braun et al. 2009; Bravo et al. 2004; Ye et al. 2005). Prenatal alcohol consumption was assessed at 20 weeks gestation and 4 weeks postpartum using a questionnaire.
Results
Women in this cohort were predominantly white (63%), married (66%), and had household income >$40,000 per year (62%) (Table 1). Characteristics of women included in the analyses did not significantly differ from characteristics of the full sample of women in the study (results not shown).
Table 1.
Birth weight z-score by covariates among HOME Study women and their infants (2003–2006)
| n (%) | Mean Birth Weight Z-score (SD) | |
|---|---|---|
| Overall | 368 | 0.06 (1) |
| Maternal age (years) | ||
| <25 | 85 (23) | −0.45 (0.85) |
| 25–35 | 222 (60) | 0.23 (0.98) |
| >35 | 61 (17) | 0.17 (1.22) |
| Household Income ($/year) | ||
| <20,000 | 78 (21) | −0.47 (0.80) |
| 20–<40,000 | 63 (17) | −0.07 (1.00) |
| 40–<80,000 | 124 (34) | 0.39 (1.10) |
| ≥80,000 | 103 (28) | 0.16 (0.97) |
| Race | ||
| Non-Hispanic White | 231 (63) | 0.28 (1.06) |
| Black | 111 (30) | −0.33 (0.89) |
| Other | 26 (7) | −0.20 (0.87) |
| Marital Status | ||
| Married | 241 (65) | 0.25 (1.05) |
| Unmarried, cohabiting | 51 (14) | −0.17 (0.84) |
| Unmarried, living alone | 76 (21) | −0.37 (0.94) |
| Education | ||
| <High School | 38 (10) | −0.45 (0.81) |
| High School or some college | 140 (38) | −0.09 (0.99) |
| Bachelors or more | 190 (52) | 0.27 (1.06) |
| Serum Cotinine Concentrations | ||
| <0.015 ng/mL (Unexposed) | 112 (30) | 0.18 (1.11) |
| 0.015–3 ng/mL (Second hand) | 217 (59) | 0.05 (0.98) |
| >3 ng/mL (Active smoker) | 39 (11) | −0.25 (1.01) |
| Depressive Symptoms | ||
| Minimal | 288 (78) | 0.09 (1.03) |
| Mild | 51 (14) | −0.06 (1.09) |
| Moderate/severe | 29 (8) | −0.04 (0.93) |
| Parity at Enrollment | ||
| Nulliparous | 166 (45) | −0.11 (1.04) |
| 1–2 | 176 (48) | 0.21 (1.04) |
| 3+ | 26 (7) | 0.08 (0.80) |
| Insurance | ||
| Private | 265 (72) | 0.21 (1.05) |
| Public/uninsured | 103 (28) | −0.32 (0.88) |
| Prenatal Vitamin use | ||
| None | 52 (14) | −0.20 (0.93) |
| Any | 316 (86) | 0.11 (1.05) |
| Food Security | ||
| Enough | 283 (77) | 0.12 (1.06) |
| Enough, but not kinds of food wanted | 68 (18) | −0.04 (0.96) |
| Not enough | 17 (5) | −0.61 (0.43) |
| Fish Consumption | ||
| Weekly | 78 (21) | −0.01 (0.97) |
| Monthly | 124 (34) | 0.22 (1.10) |
| Infrequent | 166 (45) | 0.02 (1.00) |
| Fruit/Vegetable Consumption | ||
| Daily | 145 (39) | −0.02 (0.98) |
| Weekly | 181 (49) | 0.13 (1.09) |
| Monthly | 42 (11) | 0.00 (0.96) |
| Alcohol consumption during pregnancy | ||
| Never | 206 (56) | 0.00 (1.06) |
| <1/month | 111 (30) | 0.16 (0.97) |
| >1/month | 21 (6) | 0.01 (0.92) |
| Binge | 30 (8) | 0.13 (1.15) |
| Infant Sex | ||
| Female | 199 (54) | −0.02 (0.97) |
| Male | 169 (46) | 0.16 (1.10) |
| Maternal Body Mass Index (Kg/m2) at 16 weeks gestation | ||
| Underweight-normal (≤24.99) | 155 (42) | −0.12 (0.99) |
| Overweight (25–29.9) | 123 (33) | 0.15 (1.02) |
| Obese(≥30) | 90 (24) | 0.25 (1.09) |
| Delivery Method | ||
| Vaginal | 229 (62) | −0.03 (0.99) |
| Vaginal (forceps, vacuum) | 31 (8) | 0.26 (1.06) |
| Cesarean | 108 (29) | 0.19 (1.11) |
Maternal urine samples were collected at an average (± standard deviation) of 16 ± 1.9 and 26.5 ± 2.1 weeks gestation. Metabolite concentrations are described in Table 2. Log10-transformed monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP) and mono-iso-butyl phthalate (MiBP), and monobenzyl phthalate (MBzP) concentrations were moderately correlated (r=0.41, 0.44, 0.48, 0.52, respectively) across the 16- and 26-week visits, while log 10-transformed ΣDEHP and mono-3-carboxypropyl (MCPP) concentrations were weakly correlated (r=0.24 and 0.38, respectively) (Supplemental Table 1). Correlations between different mean urinary metabolite concentrations ranged between 0.02 (MEP and ΣDEHP) and 0.44 (MBzP and MBP) (Supplemental Table 2).
Table 2.
Percentiles of maternal phthalate and creatinine concentrations of individual sample measurements taken at 16 and 26 weeks of gestation among HOME Study women (n=368a, 2003–2006)
| 5th percentile | 25th percentile | 50th percentile | 75th percentile | 95th percentile | |
|---|---|---|---|---|---|
| 16 Week | |||||
| ΣDEHP (nmol/mL) | 0.04 | 0.14 | 0.31 | 0.72 | 4.38 |
| MBP (ng/mL) | 2.90 | 11.70 | 26.90 | 53.90 | 142.00 |
| MiBP (ng/mL) | 0.40 | 2.20 | 5.70 | 11.10 | 25.20 |
| MBzP (ng/mL) | 0.86 | 4.18 | 10.01 | 23.98 | 71.35 |
| MCPP (ng/mL) | 0.40 | 1.40 | 2.80 | 5.10 | 9.80 |
| MEHP (ng/mL) | 0.85 | 1.30 | 4.50 | 13.00 | 81.00 |
| MECPP (ng/mL) | 4.90 | 15.80 | 37.10 | 89.30 | 398.00 |
| MEHHP (ng/mL) | 2.40 | 11.60 | 26.90 | 61.50 | 445.00 |
| MEOHP (ng/mL) | 1.80 | 9.10 | 19.80 | 45.80 | 293.00 |
| MEP (ng/mL) | 13.53 | 53.72 | 131.34 | 357.06 | 1089.00 |
| Creatinine (mg/dL) | 23.10 | 61.30 | 111.70 | 171.40 | 284.10 |
| 26 Week | |||||
| ΣDEHP (nmol/mL) | 0.03 | 0.10 | 0.24 | 0.52 | 2.78 |
| MBP (ng/mL) | 2.90 | 9.10 | 23.10 | 46.90 | 124.00 |
| MiBP (ng/mL) | 0.21 | 1.60 | 4.50 | 10.40 | 22.10 |
| MBzP (ng/mL) | 0.79 | 3.31 | 7.70 | 19.51 | 84.24 |
| MCPP (ng/mL) | 0.20 | 0.80 | 1.70 | 3.30 | 8.40 |
| MEHP (ng/mL) | 0.85 | 1.60 | 3.60 | 9.80 | 46.50 |
| MECPP (ng/mL) | 3.40 | 12.70 | 28.60 | 63.50 | 279.00 |
| MEHHP (ng/mL) | 2.20 | 8.30 | 20.30 | 46.60 | 258.00 |
| MEOHP (ng/mL) | 1.90 | 7.00 | 16.50 | 37.50 | 203.00 |
| MEP (ng/mL) | 9.04 | 37.62 | 112.86 | 295.68 | 1953.60 |
| Creatinine (mg/dL) | 14.70 | 47.30 | 91.40 | 148.50 | 255.70 |
For 16 week samples N=367 for phthalate metabolites and N=365 for creatinine. For 26 week samples N=355 for phthalate metabolites and 354 for creatinine.
Monoethyl phthalate (MEP), Mono-n-butyl phthalate (MBP) and Mono-iso-butyl phthalate (MiBP), Mono-3-carboxypropyl phthalate (MCPP), Monobenzyl phthalate (MBzP). Di(2-ethylhexyl) phthalate (ΣDEHP) metabolites is the sum of Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), Mono(2-ethylhexyl) phthalate (MEHP), Mono(2-ethyl-5-oxohexyl) phthalate (MEHOP) and Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP).
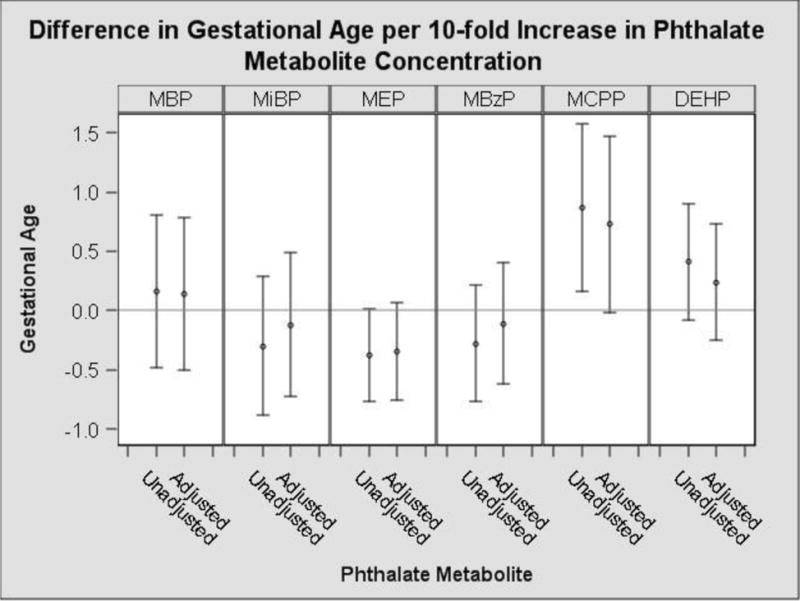
In our unadjusted model, a ten-fold increase in urinary MEP concentrations was associated with a 0.23 standard deviation reduction in birth weight z-score (95% CI: −0.46, −0.01) (Figure 1, Supplemental Table 3). Non-significant negative associations of MEP were also observed with gestational duration, birth length and head circumference (Supplemental Table 3, Figure 2). In the unadjusted model, a ten-fold increase in urinary MCPP concentrations was associated with a 0.91 week increase in gestational duration (95% CI: 0.19, 1.62) (Figure 2). We observed non-significant positive associations of urinary MCPP concentrations with birth weight z-score, birth length and head circumference (Supplemental Table 3).
Figure 1.
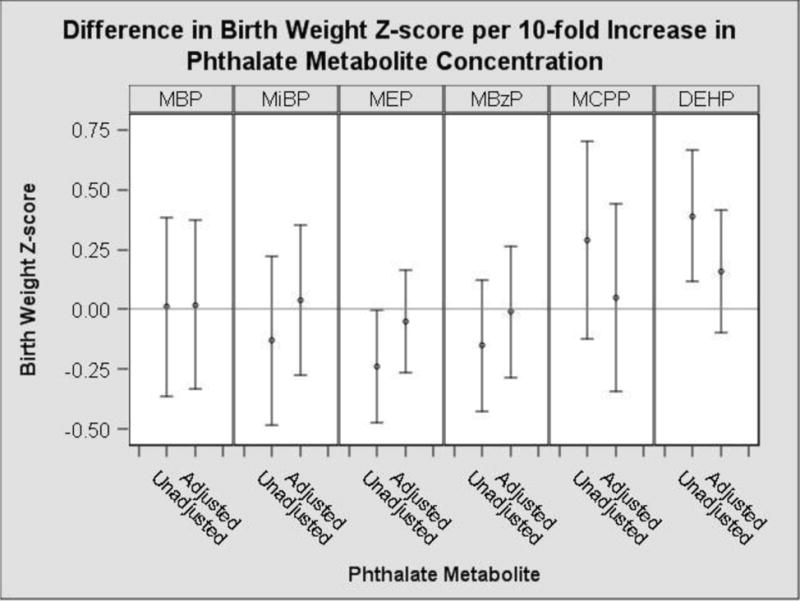
Unadjusted and adjusted change in infant birth weight z-score with a 10-fold increase in average prenatal urinary phthalate metabolite concentrationsa
Monoethyl phthalate (MEP), Mono-n-butyl phthalate (MBP) and Mono-iso-butyl phthalate (MiBP), Mono-3-carboxypropyl phthalate (MCPP), Monobenzyl phthalate (MBzP). Di(2-ethylhexyl) phthalate (ΣDEHP) metabolites is the sum of Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), Mono(2-ethylhexyl) phthalate (MEHP), Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP).
aAdjusted for: maternal race, age, income, education, marital status, insurance, parity, food security, prenatal vitamin use, fish consumption, fruit/vegetable consumption, BMI, BDI score and serum cotinine level using multivariable linear regression.
Figure 2.
Unadjusted and adjusted change in gestational age with a 10-fold increase in average prenatal urinary phthalate metabolite concentrationsa
Monoethyl phthalate (MEP), Mono-n-butyl phthalate (MBP) and Mono-iso-butyl phthalate (MiBP), Mono-3-carboxypropyl phthalate (MCPP), Monobenzyl phthalate (MBzP). Di(2-ethylhexyl) phthalate (ΣDEHP) metabolites is the sum of Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), Mono(2-ethylhexyl) phthalate (MEHP), Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP).
aAdjusted for: maternal race, age, income, education, marital status, insurance, parity, food security, prenatal vitamin use, fish consumption, fruit/vegetable consumption, BMI, BDI score and serum cotinine level using multivariable linear regression.
After adjustment for potential confounders, the associations between all the phthalate metabolites and birth weight z-scores were attenuated towards the null (Figure 1). The associations with MEP and birth weight z-score, gestational duration, birth length and head circumference were attenuated but still negative in direction (Figure 1 & 2, Supplemental Table 3). Associations between MCPP and gestational duration were attenuated after adjustment as well although they still demonstrated a positive trend (0.77 week, 95%CI: −0.03, 1.52) (Figure 2, Supplemental Table 3).
We also considered preterm birth (born before 37 weeks) as a dichotomous outcome and consistent with the increase in gestational duration observed with increasing maternal urinary MCPP, we observed a decrease in the odds of preterm birth associated with a 10-fold increase in urinary MCPP concentrations (OR: 0.08, 95%CI: 0.01, 0.52) (Table 3).
Table 3.
Adjusted odds ratio of preterm birth with 10-fold Increase in average prenatal urinary phthalate metabolite concentration (2003–2006) a,b
| Phthalate Metabolite | Odds Ratio (95% CI) |
|---|---|
| MBP | 0.20 (0.04, 0.96) |
| MiBP | 1.41 (0.36, 5.58) |
| MEP | 1.35 (0.56, 3.27) |
| MBzP | 0.57 (0.19, 1.74) |
| MCPP | 0.08 (0.01, 0.52) |
| ΣDEHP | 0.62 (0.20, 1.90) |
Adjusted for: maternal race, age, income, education, marital status, insurance, parity, food security, prenatal vitamin use, fish consumption, fruit/vegetable consumption, BMI, BDI score and serum cotinine level using multivariable linear regression.
32 cases and 357 non-cases.
Monoethyl phthalate (MEP), Mono-n-butyl phthalate (MBP) and Mono-iso-butyl phthalate (MiBP), Mono-3-carboxypropyl phthalate (MCPP), Monobenzyl phthalate (MBzP). Di(2-ethylhexyl) phthalate (ΣDEHP) metabolites is the sum of Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), Mono(2-ethylhexyl) phthalate (MEHP), Mono(2-ethyl-5-oxohexyl) phthalate (MEHOP) and Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP).
All p-values for non-linearity were >0.05 except for the p-value for the association between MEP and birth weight z-score (p<0.02). However, the confidence intervals were very imprecise at very low and high MEP concentrations, and the significant non-linearity p-value may have been driven by influential points at the extremes of the exposure/outcome distribution. We found no evidence to suggest that the associations between urinary phthalate metabolite concentrations and birth weight z-score were modified by infant sex (all product interaction p-values > 0.13), maternal race (all product interaction p-values > 0.07) or smoking (all product interaction p-values > 0.18). Further, there was no evidence to suggest that the associations between urinary phthalate metabolite concentrations and gestational duration were modified by maternal race (all product interaction p-values >0.14) or smoking (all product interaction p-values>0.42). However, we did find that the association between maternal MBzP urinary concentrations and gestational duration was modified by infant sex (P=0.05). A ten-fold increase in urinary MBzP concentrations was associated with a 0.20 week increase in gestational duration in boys (95% CI: −0.54, 0.94) and a 0.82 week decrease in gestational duration in girls (95% CI: −1.56, −0.08).
Sensitivity Analyses
When birth weight was considered as the outcome adjusted for other covariates but without adjustment for gestational duration, we observed that each ten-fold increase in urinary MEP phthalate metabolite concentration was associated with a 95 gram reduction in birth weight (95% CI: −226, −36). However, this association was attenuated when we adjusted for gestational duration (−21g, 95%CI: −122, 80) (Supplemental Table 3). Our results were not substantively changed when we added creatinine to the model as opposed to standardizing by creatinine or restricted our analyses to women with creatinine levels between 30–300 mg/dl (Supplemental Table 4). Further, the results were not substantially changed after excluding women with medical conditions, adjusting for maternal urinary BPA or DAPs concentrations, adjusting for self-reported prenatal alcohol use, or adjusting for maternal weekly weigh gain during pregnancy (Supplemental Table 4). We found no evidence that the birth weight z-score associations differed with respect to the timing of exposure (16 vs. 26 weeks gestation) (Supplemental Table 5). Similarly, there was little evidence that associations between gestational age and phthalate metabolites differed based on timing of exposure, although associations with MEP appeared to be slightly stronger for exposure at 16 weeks and associations with MCPP appeared to be slightly stronger for exposure at 26 weeks (Supplemental Table 6). We also found similar results when restricting to a smaller set of covariates that included only demographic and socioeconomic variables or a larger set including all the covariates in Table 1 (results not shown).
Discussion
In this prospective cohort, maternal urinary phthalate metabolite concentrations were not associated with infant birth weight z-score, birth length, head circumference, or gestational duration after adjustment for potential confounders. There was some evidence that MCPP, a nonspecific metabolite of several high molecular weight phthalates, was associated with an increase in gestational duration. Further, there was some evidence that MEP was associated with reductions in birth weight, but these associations were attenuated after adjusting for gestational duration. Given the observed association between some prenatal urinary phthalate concentrations and gestational age in these data, gestational age may be an intermediate between prenatal phthalate exposure and birthweight. Thus, adjusting for gestational age may be inappropriate and the more appropriate association may be from the results that are not adjusted for gestational age (Supplemental Table 3). Phthalate concentrations among women in our cohort were similar in magnitude to concentrations reported in a sample of pregnant women from the general US population in 2003–2004 (Woodruff et al. 2011) and, with few exceptions, to concentrations in other studies that have evaluated the association between prenatal phthalate exposure and infant birth size or gestational duration (Adibi et al. 2003; Ferguson et al. 2014b; Philippat et al. 2012; Whyatt et al. 2009; Wolff et al. 2008). Differences in urinary phthalate metabolite concentrations may reflect differences across studies in demographic composition, lifestyle, years of sample collection, diet, and personal care product use.
Results are discrepant among the four studies reporting results for MEP. Philippat et al. (2012) found no association between maternal urinary MEP metabolite concentrations and size at birth. Wolff et al. (2008) found positive associations between maternal urinary MEP concentrations and both head circumference and gestational duration. Both Meeker et al. (2009) and Ferguson et al. (2014) reported slightly elevated, although not statistically significant, odds of preterm birth with increasing maternal urinary MEP concentrations.
Among studies examining DEHP phthalate metabolites and birth outcomes, results have been inconsistent, reporting inverse (Ferguson et al. 2014b; Meeker et al. 2009; Whyatt et al. 2009), null (Philippat et al. 2012; Wolff et al. 2008), and positive (Adibi et al. 2003) associations between urinary prenatal DEHP phthalate metabolite concentrations and infant birth outcomes. Among studies examining MCPP, no significant associations were found with gestational duration (Ferguson et al. 2014b; Wolff et al. 2008) or birth size (Philippat et al. 2012; Wolff et al. 2008).
A possible explanation for discrepant results could be differences in the timing or number of urine samples used to quantify phthalate exposure in each study. Ferguson et al. (2014) collected 3 to 4 urine samples per woman, while the other studies used a single maternal urine sample to assess prenatal phthalate exposure (Meeker et al. 2009; Philippat et al. 2012; Wolff et al. 2008). Due to the relatively short half-life of phthalate metabolites and episodic nature of exposure, there is considerable within-person variability of some urinary phthalate metabolites, especially ΣDEHP and MCPP, making accurate exposure assessment difficult and potentially leading to exposure misclassification. Assuming the misclassification is non-differential, our study may be more likely to find associations than prior studies that had just one urine sample per woman.
The exact timing of urine collection varied between studies, with collection predominantly occurring in the third trimester. While results in our study did not substantially vary based on trimester of maternal urine collection, analyses by Ferguson and colleagues suggest that the risk of preterm birth associated with prenatal exposure to some phthalates may be dependent on timing (Ferguson et al. 2014a). If there are time-specific associations between prenatal phthalate exposure and birth outcomes, the discrepant results across studies may be due to differences in the timing of phthalate measurements and future studies will need to consider collecting serial measures during pregnancy.
Our study has several strengths including a prospective study design with rich covariate information including information on co-exposures of interest. Another strength of our study is that we collected two urine samples from almost all women (96%), enabling us to better characterize exposure and reduce exposure misclassification. However, the degree of exposure misclassification likely varies according to the within-person variability of phthalate exposure sources. Serial concentrations of MBP, MiBP, MBzP, and MEP in 16- and 26-week urine samples were more strongly correlated than repeated concentrations of ΣDEHP and MCPP. This is likely due to the parent diesters of MBP, MiBP, and MEP coming predominately from personal care product use and exposure to DEHP and the precursors of MCPP coming predominately from the diet (Hauser and Calafat 2005).
We adjusted for a variety of potential confounders, including socio-demographic, nutritional, environmental, and perinatal factors. After covariate adjustment results were attenuated towards the null, indicating that there was confounding by these factors. However, our measures of diet were crude, and it is possible that there is residual confounding from diet, as maternal diet may be associated with exposure to phthalates like DEHP and diet quality may be associated with fetal growth. There is also the potential for residual confounding from other unmeasured factors associated with both phthalate exposure and fetal growth or gestational duration.
We found evidence that child sex modified the association between urinary MBzP concentrations and gestational duration, with higher maternal urinary MBzP concentrations associated with shorter gestational duration in female infants but not male infants. In this cohort, we previously observed an association between MBzP concentrations and increased diastolic blood pressure in pregnant women (Werner et al. 2015), a risk factor associated with preterm birth (Bramham et al. 2014). However, it is unclear why we would only see decreased gestational duration in female infants and it is possible that this association is spurious. Our sample size may not have been large enough to test for effect modification by maternal smoking, maternal race, or infant sex. However, it is important to consider these modifiers because the effects of prenatal phthalate exposure could differ within each of those categories. For example, prior studies have shown sex-specific associations of phthalates on infant and child outcomes, including anogenital distance and behavior (Engel et al. 2010; Swan 2006).
While our study population was not demographically representative of women in the United States, concentrations of phthalate metabolites in our sample were similar to other US studies of pregnant women within the same timeframe (Whyatt et al. 2009; Wolff et al. 2008; Woodruff et al. 2011). In addition, sociodemographic factors in this cohort were associated with birth weight in the directions found in other studies, suggesting that these findings may be generalizable to other populations of pregnant women.
The results of this study suggest that maternal urinary phthalate metabolite concentrations are not associated with birth size and gestational duration in our cohort of women in Cincinnati, Ohio after covariate adjustment. While there is potential for phthalate exposure misclassification, 96% of women in our cohort provided two urine samples, enabling us to better characterize exposure than most prior studies. Additional research is needed in larger studies assessing phthalate exposure earlier in pregnancy to confirm these findings and determine if exposures during earlier periods of fetal development are associated with infant health.
Supplementary Material
Highlights.
After adjustment, phthalate metabolite concentrations were not associated with gestational age
After adjustment, phthalate metabolite concentrations were not associated with birth size
Acknowledgments
This work was supported by NIEHS grants R00 ES020346, R01 ES 024381, P01 ES11261, R01 ES014575, and R01 ES020349. We acknowledge the technical assistance of M. Silva, E. Samandar, J. Preau, and J. Tao (Centers for Disease Control and Prevention, Atlanta, GA) in measuring the urinary concentrations of phthalate metabolites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
The authors have no competing financial interests.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure
Dr. Lanphear has served as an expert witness and a consultant to the California Attorney General’s Office for the plaintiffs in a public nuisance case related to childhood lead poisoning, but he has not personally received any compensation for these services. Dr. Lanphear has also served as a paid consultant on a US Environmental Protection Agency research study related to childhood lead poisoning. Dr. Braun was financially compensated for conducting a re-analysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. None of these activities are directly related to the present study.
References
- Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, et al. Prenatal exposures to phthalates among women in new york city and krakow, poland. Environmental health perspectives. 2003;111:1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a us multicenter pregnancy cohort study. Am J Epidemiol. 2009;169:1015–1024. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, et al. Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the long-evans rat. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1998;36:963–970. doi: 10.1016/s0278-6915(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for the beck depression inventory-ii. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the united states between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clinical chemistry. 1997;43:2281–2291. [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol a exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: The correlation between serum and meconium and their association with infant birth weight. Environ Health. 2010;9:53. doi: 10.1186/1476-069X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. Journal of exposure science & environmental epidemiology. 2013 doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. Cohort profile: The health outcomes and measures of the environment (home) study. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, et al. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. Journal of exposure analysis and environmental epidemiology. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Brock JW, Silva MJ, Gray LE, Jr, Reidy JA, Barr DB, et al. Urinary and amniotic fluid levels of phthalate monoesters in rats after the oral administration of di(2-ethylhexyl) phthalate and din-butyl phthalate. Toxicology. 2006;217:22–30. doi: 10.1016/j.tox.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014a;70C:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014b;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino N, Iwai M, Okazaki Y. A two-generation reproductive toxicity study of dicyclohexyl phthalate in rats. The Journal of toxicological sciences. 2005;30:79–96. doi: 10.2131/jts.30.s79. Spec No. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environmental research. 2008;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A. 2004;67:1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. Exposure to di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biology of the neonate. 2003;83:22–24. doi: 10.1159/000067012. [DOI] [PubMed] [Google Scholar]
- Ma X, Lian QQ, Dong Q, Ge RS. Environmental inhibitors of 11beta-hydroxysteroid dehydrogenase type 2. Toxicology. 2011;285:83–89. doi: 10.1016/j.tox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. Urinary phthalate metabolites in relation to preterm birth in mexico city. Environ Health Perspect. 2009;117:1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a united states national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environmental health perspectives. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. Phthalate exposure during pregnancy and lower anogenital index in boys: Wider implications for the general population? Environmental health perspectives. 2005;113:A504–505. doi: 10.1289/ehp.113-a504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the u.S. Population from the national health and nutrition examination survey (nhanes) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Prenatal phthalate exposure and anogenital distance in male infants. Environ Health Perspect. 2006;114:A88–89. doi: 10.1289/ehp.114-a88b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (dehp) administered to mice in the diet. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2002;40:1499–1506. doi: 10.1016/s0278-6915(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Effects of bis(2-ethylhexyl) phthalate (dehp) on secondary sex ratio of mice in a cross-mating study. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2003;41:1429–1432. doi: 10.1016/s0278-6915(03)00162-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural effects of bis(2-ethylhexyl) phthalate (dehp) in a cross-mating toxicity study of mice. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43:581–589. doi: 10.1016/j.fct.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Textor J, Hardt J, Knuppel S. Dagitty: A graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Fail PA, Seely JC, Brine DR, et al. Reproductive toxicity evaluation of dietary butyl benzyl phthalate (bbp) in rats. Reprod Toxicol. 2004;18:241–264. doi: 10.1016/j.reprotox.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The home study. Environmental health: a global access science source. 2015;14:75. doi: 10.1186/s12940-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, et al. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124:e1213–1220. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the united states: Nhanes 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol a, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Chen BH, Zheng LX, Wu XY. study on the level of phthalates in human biological samples. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2003;37:429–434. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


