Abstract
At birth, the human immune system already contains substantial levels of polymeric IgM, with autoantibodies to neo-epitopes on apoptotic cells (ACs) that are proposed to play homeostatic and anti-inflammatory roles. Yet the biologic origins and developmental regulation of these naturally arising antibodies remain poorly understood. Herein, we report that levels of IgM-antibodies to malondialdehyde (MDA) protein adducts, a common type of in vivo generated oxidative stress-related neoepitope, directly correlate with the relative binding of neonatal-IgM to ACs. While levels of IgM to phosphorylcholine (PC), a natural antibody prevalent in adults, were relatively scant in cord blood, there was significantly greater relative representation of IgM anti-MDA antibodies in newborns compared to adults. To investigate the potential interrelationships between neonatal IgM with pathogenic IgG-autoantibodies, we studied 103 newborns born to autoimmune mothers with IgG anti-Ro (i.e., 70 with neonatal lupus and 33 without neonatal lupus). In these subjects the mean levels of IgM anti-Ro60 were significantly higher than in the newborns from non-autoimmune mothers. In contrast, levels of IgM anti-MDA in IgG anti-Ro exposed neonates were significantly lower than in neonates from non-autoimmune mothers. The presence or absence of neonatal lupus did not influence the total levels of IgM in the anti-Ro exposed newborns. Taken together, our studies provide evidence that the immune development of the natural IgM-repertoire may be affected, and become imprinted by, the transfer of maternal IgG into the fetus.
Keywords: Neonatal immunity, repertoire, neonatal lupus, maternal IgG, malondialdehyde
1. INTRODUCTION
Secreted immunoglobulins play essential roles in host defenses from microbial pathogens, and certain types of spontaneously arising (i.e., natural) antibodies are reported to reinforce fundamental innate pathways for maintaining tissue homeostasis [1–5](reviewed in [6]). Indeed, IgM, the first isotype expressed during immune development (reviewed in [7]), is detectable by the 14th week of human gestation [8, 9]. In both mice and humans autoantibodies are highly represented in the IgM-repertoires [10, 11], and these include natural antibodies that recognize oxidation-associated neo-antigens on apoptotic cells (ACs)[12, 13], such as IgM to phosphorylcholine (PC) in oxidized lipids and malondialdehyde (MDA) protein modifications that have been linked to protective properties [2, 3, 14, 15]. Furthermore, higher levels of IgM to the apoptosis-associated determinant, PC, correlate with protection from atherosclerotic plaque and cardiovascular events [14, 16, 17], as well as with lower overall clinical disease activity in SLE patients in some cohorts [14].
The processes that mold the early development of the IgM-repertoire are poorly understood [18]. During gestation clinical infection can dramatically increase overall IgM levels [19] and induce pathogen-specific antibodies later detectable in umbilical cord blood [20], demonstrating the capacity of the prenatal immune system for antigen-specific responses. Even more relevant, ACs are continuously generated during development, including from the involution of the placenta [21]. Hence the clonal representation within the neonatal IgM-repertoire may in part be selected by oxidative stress-related neo-epitopes on ACs.
In the murine immune system, the earliest arising tier of mature B cells, termed B-1 cells is also the dominant cellular source of natural antibodies. B-1 cells have a specialized repertoire [22] and response profiles believed to result from positive clonal selection of B-1 precursors by some non-protein autoantigens [23, 24]. In humans, an equivalent B-1 cell subset, bearing the CD43+ CD27+ CD70− phenotype, have been detected in the bloodstream of adults and in umbilical cord blood of newborns [25]. Spontaneous production of anti-PC antibodies by these B cells has also been reported [25].
During immune development, despite the indisputable benefits of maternal IgG transfer that augment fetal defenses, there are also uncommon clinical settings in which antibodies from the maternal immune system pose substantial pathogenic threats. Neonatal lupus (NL) represents a well-documented clinical syndrome caused by maternal IgG-autoantibodies reactive with the autoantigens, Ro52 (52 kDa SSA/Ro), Ro60 (60 kDa SSA/Ro), and La (48kDa SSB) that can form immune complexes containing single-stranded (ss)RNA [26]. During gestation, active placental transport mediated by the neonatal Fc receptor (FcRn) introduces maternal IgG that can include IgG anti-Ro antibodies into the fetal circulation. The transplacental transfer of these IgG-autoantibodies can result in cutaneous manifestations, and more seriously in congenital heart block that is associated with high morbidity and 15–30% fetal/neonatal mortality [27, 28]. Cardiac injury in NL has been suggested to be driven by maternal IgG anti-Ro antibodies that bind to RNA-containing complexes exposed on the surface of apoptotic cardiocytes, leading to conduction system defects, and at times global cardiomyopathy. In part, this has been linked to impaired apoptotic cardiocyte clearance, TLR-dependent macrophage activation, and the release of pro-fibrotic active TGF-β [29–34].
Only a small proportion (~2%) of these anti-Ro exposed pregnancies result in heart block in the offspring [26], albeit the recurrence rate in subsequent pregnancies is nearly ten-fold higher [35]. Strikingly, the autoimmune disease status of the mother alone does not predict the development of NL; hence, there are likely other unknown environmental and/or genetic factors that also contribute to pathogenesis [26].
The current study was therefore initiated to characterize the IgM-antibody responses to PC and MDA oxidation-associated neo-determinants and AC binding in newborns. We also sought to determine whether there are differences in the levels of IgM in cord blood samples from healthy neonates of non-autoimmune mothers compared to newborns from mothers with circulating IgG anti-Ro/La antibodies. In addition, we sought to investigate whether NL in the newborn, or the autoimmune process occurring in the mother, can affect the distribution of autoantibodies within the newly emergent neonatal IgM-repertoire.
2. MATERIALS AND METHODS
2.1. Subjects
103 plasma samples were obtained from the umbilical cords of newborns of autoimmune mothers enrolled in the NYU IRB approved Research Registry for Neonatal Lupus (NL), as previously described [27, 36]. Enrollment of a pregnant subject required seropositivity for anti-Ro (Ro52 and/or Ro60) and/or anti-La48 and/or anti-RNP IgG-antibodies by clinical laboratory testing, with later confirmation in our research laboratory, as described [36]. All mothers in the registry provided informed consent. Cord blood samples were from neonates with a diagnosis of documented cardiac NL (n=56), cardiac and cutaneous NL (n=10) or rash alone (n=4), and from unaffected healthy siblings of NL neonates (n=40). In addition, 31 de-identified control cord blood samples from non-autoimmune mothers (with an absence of IgG anti-Ro/La autoantibodies), and 43 healthy unrelated adult donors, were provided through the Blood Bank of the Hospital of the University of Pennsylvania.
2.2. ELISA assays
Adapting previously described methods [14], high-binding ELISA plates were coated with PC16-bovine serum albumin (BSA) (Biosearch Technologies), MDA-modified BSA (MDA-BSA, Academy Biomedical), or Ro60 from calf thymus (Fitzgerald Industries) at 3 μg/ml in PBS. Samples were analyzed for IgM anti-PC and anti-Ro60 at 1:200 and 1:1,000 dilutions, and IgM anti-MDA at 1:500 and 1:2,500 dilutions, detected with biotinylated goat anti-human IgM (Jackson Immunoresearch), followed by streptavidin-polyHRP80 (Fitzgerald). In adults, IgM reactivities were assessed at 1:6,000 and 1:30,000 for MDA, or 1:10,000 and 1:50,000 for PC. IgG anti-Ro60 reactivity was assessed at dilutions of 1:3,000 and 1:30,000, and IgG anti-MDA at 1:200 and 1:1,000, and detected with goat anti-human IgG-HRP (Jackson Immunoresearch), using modified from [14]. Assays for IgM-rheumatoid factor (RF) used Quanta Lite® kits (INOVA Diagnostics), and sensitivity of detection was enhanced with biotinylated goat Fab′2 anti human μ-specific antibody (Jackson Immunoresearch), using streptavidin poly HRP80 (Fitzgerald Industries). All assays included standard curves of control samples, for quantitation of reactivities in relative activity units (RU)/ml. For example, when measuring IgM anti-MDA in cord blood plasma at 1:500 dilution, a reference SLE pool at 1:5,000 was set as 100 RU/ml, and a seven point standard curve was prepared by subsequent 2-fold dilution of the reference sample for the interpolation of the values for the neonatal serum samples. Similarly, a reference pool from patients with Sjögren’s syndrome was used for quantification of anti-Ro60 antibody reactivity. Levels of total IgM were also measured, which used a human monoclonal IgM (Jackson Immunoresearch) for the ELISA standard. In this highly quantitative immunoassay, 100 RU/ml corresponded to IgM at 38 mg/dl as determined by nephelometry (NYU Clinical Laboratory).
2.3. Binding to apoptotic cells
Using a standard assay [5,6], apoptotic death of murine thymocytes was induced by incubation with 1μM dexamethasone (Sigma Aldrich) at 37°C for 4 hrs in RPMI media supplemented with 10% FBS, penicillin, streptomycin, and L-glutamine, at 2×106 cells/ml. Apoptosis in the human T-cell line, Jurkat, was induced by incubation with anti-CD95/Fas (EOS9.1, Biolegend) at 100 ng/ml for 2 hrs. Plasma samples were each separately incubated with ACs in 3% BSA in PBS for 45 min on ice, at indicated dilutions. Binding was detected with biotinylated goat anti-human IgM μ-chain specific antibodies (Jackson Immunoresearch), followed by streptavidin conjugated to phycoerythrin (PE) (BD Biosciences). Human myeloma IgM (Jackson Immunoresearch) at 1 or 10 ug/ml was used as isotype control. Before the flow cytometry studies, cells were co-stained with Annexin V (BD Biosciences) and 7AAD (BD Biosciences), according to the manufacturer’s instructions. For antigen inhibition studies, diluted cord blood samples were pre-incubated with 100 ug/ml in-house prepared MDA-modified BSA or control mock-treated BSA 1 hr at 4C, before flow cytometry binding studies. MDA modification was performed according to established methods. Briefly, MDA was generated by acid hydrolysis of tetramethoxypropane (Sigma Aldrich) and molecular grade BSA (NEB) was modified by 50 mM MDA in PBS for 24 hrs at 37C, followed by extensive dialysis. Data were acquired using a FACSCalibur instrument or BD Accuri (BD Biosciences) and analyzed with FlowJo software (Tristar).
2.4. Statistical analysis
For statistical analysis, Prism (Graphpad) was used to assess for differences between groups and for correlations between measurements. For groups with unequal variances, we applied two-tailed Student’s t-tests with Welch correction. Pearson or Spearman correlations were used to for evaluation of correlation between measurements as indicated. P-values <0.05 were considered statistically significant.
3. RESULTS
3.1. IgM levels in neonates compared to healthy adults
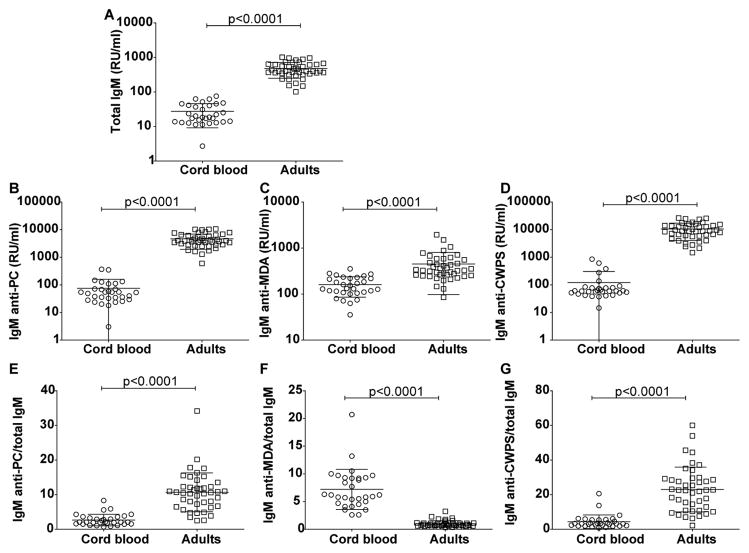
In our studies of circulating natural IgM-antibodies, we first analyzed cord blood samples from unaffected neonates of non-autoimmune mothers. As expected, all neonates had detectable levels of total IgM, but these levels were a mean 17-fold lower than found in the plasma of adults (mean ± SD; 27.6 ±18.4 vs. 479 ± 230 RU/ml, p<0.0001) (Figure 1A) corresponding to 180 mg/dl in adults and 11 mg/dl in neonates. We next measured levels of IgM antibodies that bound the oxidative-stress related PC- and MDA- antigens, and found that these levels in neonates were significantly lower than in healthy adults (for anti-PC 75.9± 15 vs. 4642 ±390 RU/ml, p<0.0001; for anti-MDA 161±14 vs. 450±54 RU/ml, p<0.0001)(Figure 1B and C). Strikingly, there was a much greater difference between neonatal and adult levels for IgM anti-PC (~60-fold) than for IgM anti-MDA (~2.8 fold) (Figure 1B and C). For comparison, we also investigated IgM reactivity with another PC-containing reagent, pneumococcal cell wall polysaccharide (CWPS), in which the PC epitopes are also immunodominant on these teichoic acid polysaccharide molecules. Here, the binding patterns were similar to anti-PC conjugate, as IgM-antibodies to CWPS were also at a relative (~85-fold) lower level in cord blood than in adult sera (122±181 vs 10,567±6526 RU/ml, p<0.0001)(Figure 1D). These findings support the notion that IgM reactivity with apoptosis-associated antigens is common at birth and persists following immune maturation into adulthood although the representation of fine specificities shifts.
Figure 1. Levels of IgM in newborn and adult blood samples.
Analysis of IgM levels in 31 cord blood samples compared to 43 healthy adults. Panels depict results from ELISA immunoassays for; Total IgM (A) or IgM that recognize: phosphorylcholine (PC) (B); malondialdehyde (MDA) protein adducts (C); pneumococcal cell wall polysaccharide, CWPS (D). Panels (E)–(G) illustrate the ratios of IgM anti-PC/total IgM, IgM anti-MDA/total IgM and IgM anti-CWPS/total IgM. Binding assays were performed by ELISA. P-values were derived from two-sided un-paired t test with Welch’s correction.
To further investigate the patterns of expression of these natural antibody specificities, we assessed the representation of each of the antigen-specific IgM within the total IgM pool. These ratios showed that IgM anti-PC were relatively underrepresented in neonates than in adults (2.7±1.7 vs 10.7±5.6, p<0.0001)(Figure 1E). Similarly, the ratio of IgM anti-CWPS/total IgM was also significantly lower in the newborns than adults (4.3±3.9 vs. 23±12)(Figure 1G). In contrast, the ratio of IgM anti-MDA/total IgM was significantly higher in neonates compared to adults (7.2±3.6 vs 1.0±0.6, p<0.0001) (Figure 1F). However, we unexpectedly demonstrated that the relative reactivity of anti-MDA IgM-antibodies is disproportionately greater at birth than later in life (i.e., in adult plasma) (Figure 1). We speculate that this may be a surrogate for preferential display of a subset of oxidative-stress related antigens, arising in vivo in the hypoxic uterine environment. Furthermore, the representation of IgM-antibodies to the two distinct oxidation-associated neo-antigens under examination appeared to become differentially shifted with the immune maturation of the subject, with much greater representation of IgM anti-MDA at birth (Figure 1).
3.2. Apoptotic cell binding of neonatal IgM
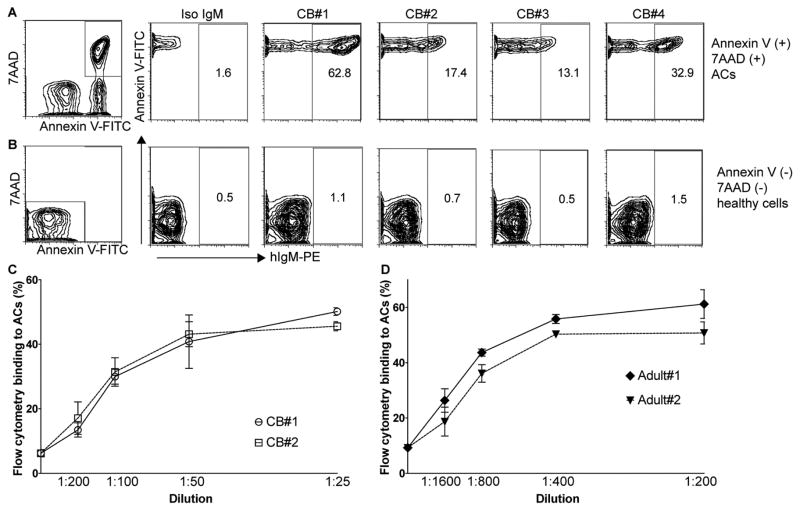
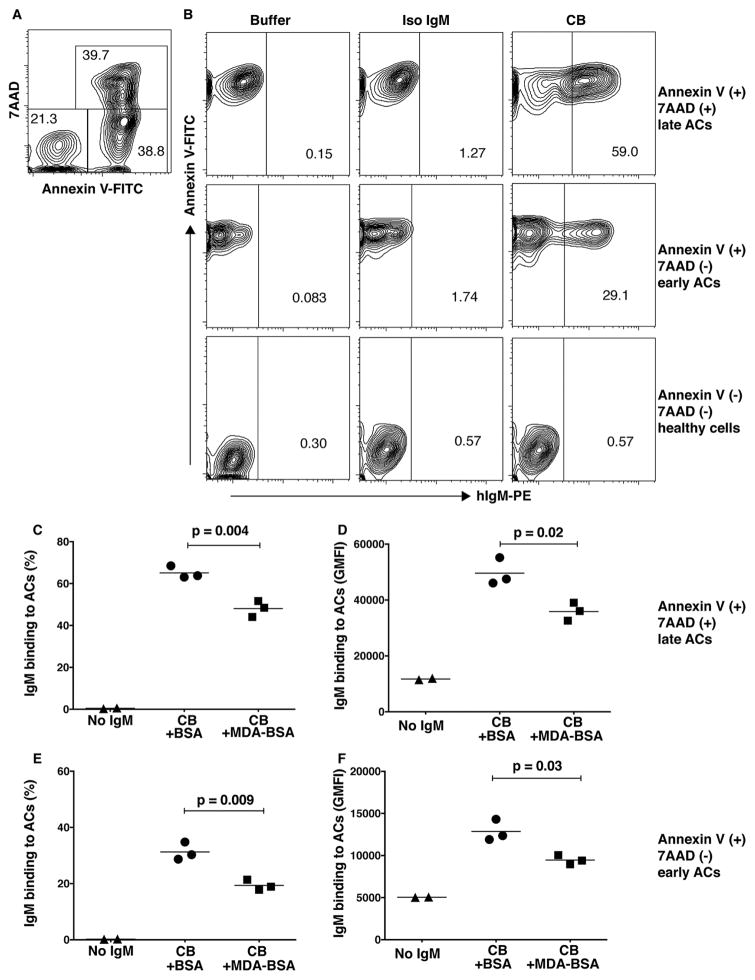
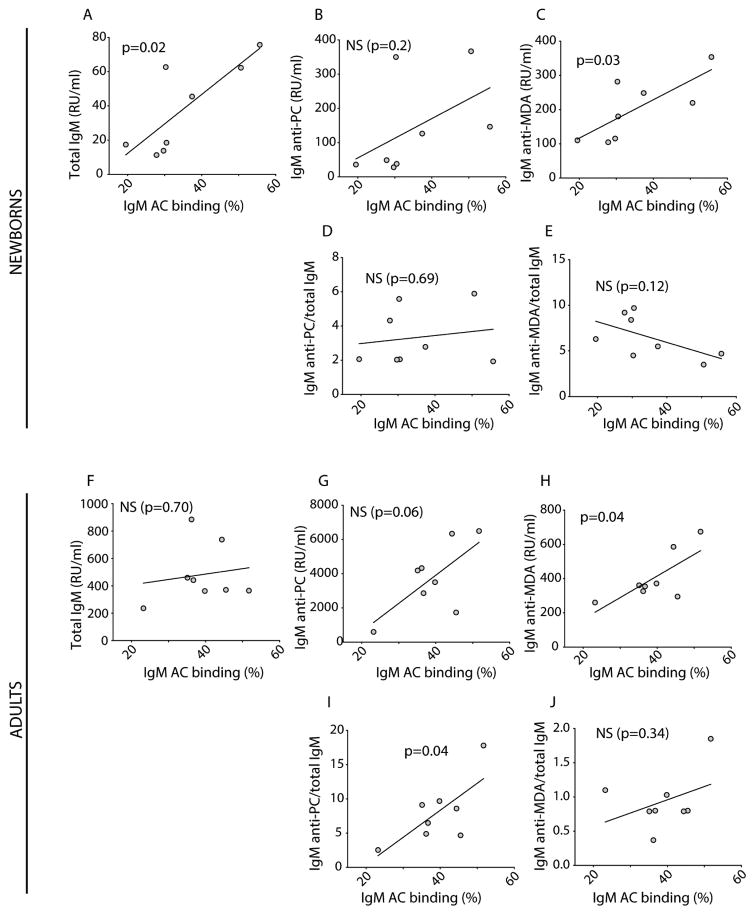
Antibody recognition of the PC- and MDA- neo-determinants that arise on the membranes of mammalian cells undergoing apoptotic death has been shown to enable the immune discrimination of apoptotic from healthy cells and to enhance host homeostatic functions [1–3, 37]. We therefore investigated the binding of IgM-antibodies in cord blood with ACs by a standard flow cytometric assay. All tested cord blood samples showed IgM binding to 7AAD(+) Annexin V(+) ACs and low or undetectable binding to freshly isolated 7AAD(−) Annexin V(−) “healthy” thymocytes in which apoptosis-associated neo-epitopes are not exposed (Figure 2). The cord blood IgM apoptotic thymocyte binding pattern was confirmed by studies of anti-Fas induced apoptotic human Jurkat cells, where binding to both early and late apoptotic cells was detected (Figure 3). We detect higher levels of IgM binding to late apoptotic cells 7AAD(+) Annexin V(+) compared to early stage apoptotic cells 7AAD(−) Annexin V(+), presumably due to an accumulation of apoptosis markers on these cells during the apoptosis process. Notably, in the eight cord blood samples from neonates of non-autoimmune mothers, the level of IgM-binding to ACs directly correlated with the level of total IgM (p=0.02, Pearson R=0.80) and IgM anti-MDA reactivity (p=0.03, Pearson R=0.75), as measured by ELISA (Figure 4A and C). When normalizing the IgM anti-MDA signal for total IgM (IgM anti-MDA/total IgM) we found no significant correlation with AC-binding (Figure 4D and E). We postulate that this significant correlation between total IgM and IgM anti-MDA provides evidence that a major proportion of the total IgM in the neonate has binding specificity for this oxidation-associated epitope. Indeed, in the antigen-inhibition studies, following pre-incubation with MDA-BSA we detected a significant reduction in the binding of neonatal IgM to both early and late apoptotic cells, compared to control-treated BSA (Figure 3).
Figure 2. Binding of cord blood IgM to apoptotic cells.
IgM binding to 7AAD(+) Annexin V(+) apoptotic murine thymocytes (A), or freshly isolated 7AAD(−) Annexin V(−) healthy thymocytes (B), in four representative cord blood samples at 1:100 dilution compared to human monoclonal IgM isotype control (Iso IgM). Dose-dependent binding of cord blood IgM (C) or adult serum IgM (D) to 7AAD (+) Annexin V (+) apoptotic thymocytes. Studies were performed by multiparameter flow cytometry with values for geometric mean fluorescence intensity (MFI) depicted. Results are representative of surveys of 8 cord blood and 8 unrelated healthy adult samples, performed in duplicate.
Figure 3. Antigen-inhibition of cord blood IgM binding to apoptotic cells.
A. Distribution in percentage of Annexin V and 7AAD positive apoptotic Jurkat cells after induction of apoptosis with anti-Fas (100 ng/ml 2 hrs 370C).
B. Binding of cord blood (CB) plasma IgM to late apoptotic cells 7AAD (+) Annexin V (+) (top panel) early apoptotic cells 7AAD (−) Annexin V (+) (middle panel) or healthy cells (bottom panel). Pre-incubation with MDA-BSA at 100 ug/ml gave blocking of neonatal IgM binding to late 7AAD (+) Annexin V (+) (C, D) and early apoptotic cells 7AAD (−) Annexin V (+) (E, F) and is shown by % binding (C, E) or geometric mean fluorescence intensity (GMFI; D, F). MDA-BSA was in-house produced and the control BSA was mock-treated using the same method but without addition of MDA. Cord blood plasma was diluted 1:100 and all samples were analyzed in triplicates. P-values were derived by two-sided unpaired t-test with Welch’s correction.
Figure 4. Correlation between serum IgM and IgM apoptotic cell binding in cord blood and healthy adults.
Correlation between serum IgM levels and IgM binding to 7AAD(+) Annexin V(+) apoptotic thymocytes, using eight cord blood samples (% binding at 1:100 dilution) in top panel, and eight adult samples (% binding at 1:800 dilution). Correlation with total IgM levels (A, F), IgM anti-PC (B, G), IgM anti-MDA (C, H), the ratio of IgM anti-PC/total IgM (D, I), and the ratio of IgM anti-MDA/total IgM (E, J) are shown. Serum IgM levels were determined by ELISA, and IgM binding to apoptotic cells by flow cytometry. R and p-values were derived from Pearson correlation analysis.
There was no correlation between the level of AC binding with the level of IgM anti-PC, which were relatively low compared to anti-MDA in these neonates (Figure 4B). In studies of samples from healthy adults, the levels of IgM AC-binding significantly correlated with the levels of IgM anti-MDA antibodies (p=0.04, Pearson R=0.74) but not with total IgM levels (Figure 4F–J). There was also a trend towards a direct correlation between AC-binding and serum IgM anti-PC levels (p=0.06, Pearson R=0.68) (Figure 4G). However, the correlation with binding to ACs was statistically significance when the levels of the IgM anti-PC were normalized for total IgM levels (p=0.04, Pearson R=0.72)(Figure 4I). These findings therefore confirm earlier evidence that in adults the IgM anti-PC antibody is a primary mediator for binding of AC membranes [38]. In addition, these studies provide further support for the notion that during the maturation of the human immune system there is a shift in IgM-fine binding specificities responsible for AC recognition.
3.3. IgM in neonates from autoimmune mothers
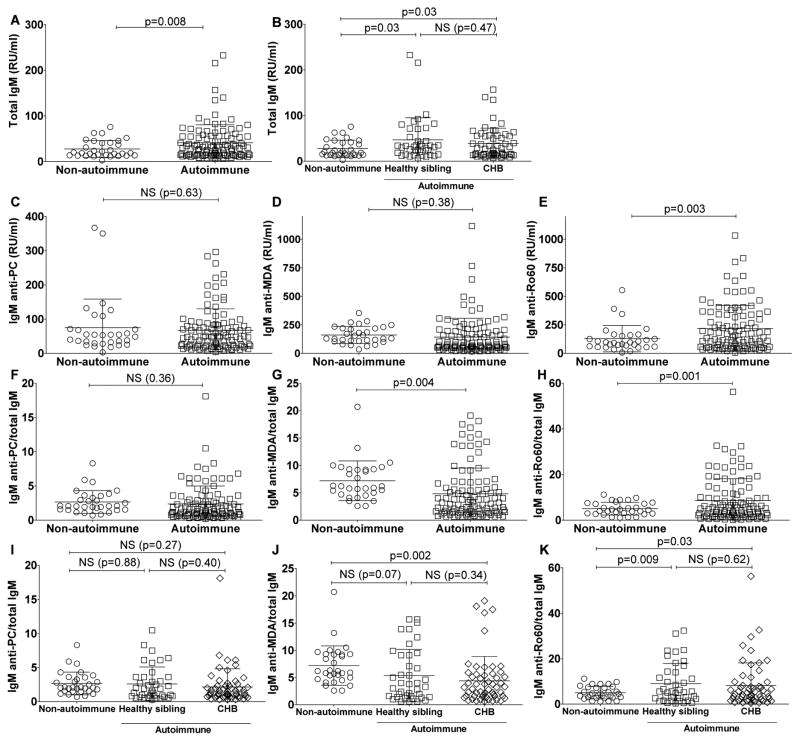
We next investigated whether the neonatal IgM-repertoire is affected by gestation in an environment containing maternal IgG-autoantibodies that can complex with autoantigens and ssRNA molecules (Figure 5). Herein, we evaluated IgM levels in 103 cord blood samples from the neonates exposed to maternal IgG anti-Ro/La autoantibodies (i.e., from autoimmune mothers) and 31 unrelated neonates from non-autoimmune mothers. The total IgM in the autoimmune-exposed cord bloods were significantly higher than in the cord blood samples from non-autoimmune mothers (41±39 RU/ml vs. 27± 18 RU/ml; p=0.008) (Figure 5A & B). Yet, these IgM elevations in the offspring of autoimmune mothers were much below the levels that are considered diagnostic for congenital infection [19]. IgM anti-PC and IgM anti-MDA levels did not significantly account for the overall increase in total IgM in autoimmune neonates as the mean levels of these IgM specificities were not significantly different between the cord blood samples from those exposed or not exposed to maternal IgG anti-Ro antibodies (Figure 5C & D).
Figure 5. IgM levels in cord blood from control newborns compared to newborns with autoimmune mothers.
IgM levels were measured in 31 control cord bloods (from non-autoimmune mothers) and 103 cord blood samples from the NL registry from newborns with autoimmune anti-Ro60/52/La positive mothers (autoimmune). Within the autoimmune cohort 56 samples came from neonates with congenital heart block (CHB) and 40 from clinically healthy siblings. A. Total IgM levels. B. Total IgM levels for autoimmune neonates with or without heart block. C. IgM anti-PC levels. D. IgM anti-MDA levels. E. IgM anti-Ro60 levels. F. The ratio of IgM anti-PC/total IgM. G. The ratio of IgM anti-MDA/total IgM. H. The ratio of IgM anti-Ro60/total IgM. I. The ratio of IgM anti-PC/total IgM for neonates, from autoimmune mothers, with or without heart block. J. The ratio of IgM anti-MDA/total IgM for neonates, from autoimmune mothers, with or without heart block. K. The ratio of IgM anti-Ro60/total IgM for neonates from autoimmune mothers, with or without heart block. Binding assays were performed by ELISA. P-values were derived from 2-sided unpaired t-test with Welch’s correction.
We found that in non-autoimmune newborns IgM anti-MDA was relatively over-represented in the total pool of IgM, as the mean values for IgM anti-MDA/total IgM were significantly higher in the non-autoimmune neonates compared to the autoimmune cohort (7.2±3.6 vs. 4.8±4.7, p=0.004) (Figure 5G). Yet there were no significant differences between the groups for anti-PC binding reactivity that was present in neonates at low or undetectable levels (Figure 5F).
As Ro60 has been implicated in NL pathogenesis, we next investigated expression in the neonate of antibody reactivity to Ro60, one of the autoantigens targeted by IgG-autoantibodies in autoimmune mothers. Most neonates had substantial levels of IgM reactive with Ro60, however the mean levels of IgM anti-Ro60 were significantly higher in neonates of autoimmune mothers compared to those from non-autoimmune mothers (218±203 RU/ml vs. 129±115 RU/ml, p=0.003) (Figure 5E). In contrast to our findings for IgM anti-MDA, we found that IgM anti-Ro60 was over-represented in the total IgM pool from neonates exposed to maternal IgG anti-Ro/La autoantibodies, compared to non-autoimmune newborns (8.6± 9.6 vs. 5.0±2.8, p=0.001) (Figure 5H). In addition, IgM anti-PC and IgM anti-MDA each significantly correlated with total IgM levels in both cord blood cohorts, while IgM anti-Ro60 only correlated with total IgM in the cord blood samples from the control neonates with non-autoimmune mothers (Supplemental Table 1).
Based on the postulation that maternal IgM are not actively transported into the fetus and do not contribute to the immunoglobulin in the fetal circulation, these findings suggest that exposure to maternal IgG anti-Ro autoantibodies can alter the specificity of circulating neonatally expressed autoreactive IgM antibodies.
Due to the concern that these reactivity patterns could reflect the in vivo formation of immune complexes of neonatal IgM-autoantibodies with maternal IgG-autoantibodies, we also assayed for rheumatoid factor (RF) activity (i.e., IgM anti-IgG Fc). RF were readily detected, although cord blood samples from both non-autoimmune and autoimmune mothers generally contained only low levels of RF, and in only 1/23 of the selected cord blood samples the RF level was greatly elevated (Supplemental Figure 1). Notably, we found no associations between the level of IgM-RF and other types of IgM-antibodies, including the IgM anti-Ro60 (Supplemental Figure 1). Hence the finding in our assays of elevated IgM anti-Ro60 in neonates from autoimmune mothers was unlikely due to the influence of RF-autoantibodies.
3.4 Autoantibody levels are not affected by overt clinical NL
To determine whether the development of cardiac neonatal lupus in the fetus affects IgM levels and specificity we next analyzed the data according to fetal diagnosis. We found no significant differences for IgM antibodies between anti-Ro/La-exposed fetuses that developed congenital heart block and their unaffected siblings (Figure 5B, I, J and K). Interestingly, there appeared to be trends for concordance for IgM autoantibody reactivity levels between related siblings from individual autoimmune mothers (Supplemental Figure 2), which may suggest these levels are influenced by shared familial genetic and/or environmental factors.
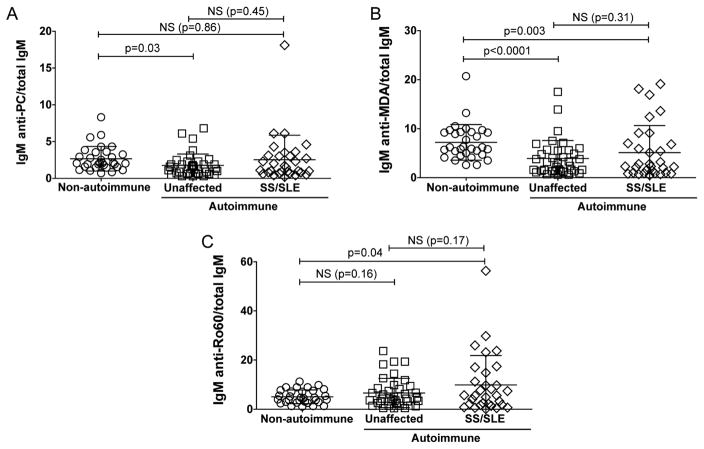
Having determined that neonatal lupus is not associated with IgM levels or binding specificity, we next studied whether maternal diagnosis has an impact on newborn IgM. In the research registry for NL cohort, 30 of the mothers had previously been diagnosed with SLE and/or Sjögren’s syndrome (SS) based on clinical criteria, while a separate set of 31 of the mothers in this cohort did not have a clinical history of autoimmune disease or symptoms when their child(ren) were born with heart block. While the IgM levels in the neonates from autoimmune mothers were generally increased compared to those from non-autoimmune mothers, there were no differences between the offspring from clinically autoimmune yet unaffected mothers (i.e., without signs or symptoms of disease) with those from the autoimmune mothers with overt SS/SLE disease (Figure 6).
Figure 6. IgM levels in cord blood correlate with maternal autoimmunity but not a diagnosis of SLE or Sjögrens syndrome.
IgM levels were compared between 31 control cord blood (non-autoimmune) and 67 cord blood samples from the NL registry from newborns with IgG anti-Ro60/52 positive (autoimmune) mothers. Within the autoimmune cohort 37 of the neonates with mothers that did not have signs or symptoms of autoimmune disease at the time of sample collection (unaffected) and 30 had mothers with a diagnosis of SLE or SS or SLE with secondary SS (SS/SLE). A. The ratio of IgM anti-PC/total IgM. B. The ratio of IgM anti-MDA/total IgM. C. The ratio of IgM anti-Ro60/total IgM. Binding assays were performed by ELISA. P-values were derived from two-sided unpaired t test with Welch’s correction.
Several babies in the NL cohort were born prematurely (i.e., at less than 36 weeks). We therefore investigated whether Ig/antibody levels in the neonate correlated with the gestational age at delivery (Supplemental Figure 3). Yet we found neither an association between gestational age at birth with total IgM levels (Supplemental Figure 3A), nor with IgG anti-Ro or IgG anti-MDA (data not shown). However, there was a significant direct correlation between gestational age and levels of IgM anti-PC (p=0.02, Spearman R=0.26), IgM anti-MDA (p=0.005, Spearman R=0.32) and IgM anti-Ro60 (p=0.01, Spearman R=0.28)( Supplemental Figure 3B, C & D). When IgM anti-MDA levels were normalized to total IgM levels we found significant direct correlations with the gestational age at birth (p=0.02, Spearman R=0.26), suggesting that the proportion of the representation of IgM may be increasing during in utero development. These findings may therefore suggest that these antibodies may first be produced during different points during in utero development and/or there is accumulation over time as a function of the maturation of the fetus/neonate.
3.5. IgG-autoantibodies of maternal origin
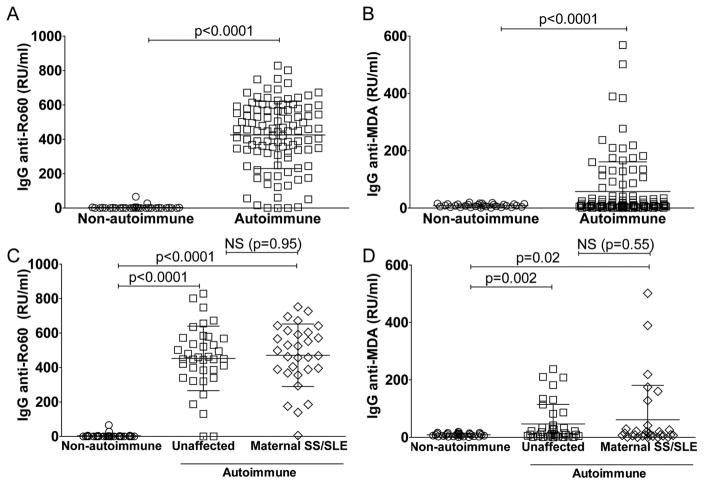
We previously reported that levels of IgG anti-MDA were strongly associated with lupus clinical disease activity, as determined by SLEDAI, while in healthy adults the levels of IgG anti-MDA antibodies were either much lower or undetectable [14]. Therefore, we evaluated maternal IgG anti-MDA in the NL cohort, with IgG anti-Ro60 as a comparison. Levels of IgG anti-MDA were significantly higher in autoimmune than in non-autoimmune mothers (9.1±5.3 RU/ml, p<0.0001)(Figure 7B). However, we were surprised to find that levels of IgG anti-MDA in the neonates from autoimmune but clinically unaffected mothers (47±68 RU/ml) were comparable with those in the SLE/SS affected mothers (61±120)(Figure 7D). While there was no correlation between levels of the maternally transferred IgG anti-Ro60 and neonatal IgM anti-Ro60, we did detect a significant correlation between maternally transferred IgG anti-MDA and neonatal IgM anti-MDA (p<0.0001, Spearman R=0.67; Supplemental Figure 4).
Figure 7. Detection of maternal IgG autoantibodies in cord blood.
A–B. Levels of maternally transferred IgG-autoantibodies to Ro60 or the oxidation-associated malondialdehyde (MDA) in 31 control cord bloods (non-autoimmune) or 103 cord blood samples from the neonatal registry cohort of mothers with IgG anti-Ro/La autoantibodies (autoimmune). C–D. Levels of IgG-autoantibodies to Ro60 and MDA in 31 control cord blood samples (non-autoimmune), compared to 33 cord blood samples of newborns with mothers enrolled in the NL cohort but without documented signs and/or symptoms or prior diagnosis of autoimmune disease at the time of birth (unaffected), or 30 newborns from mothers with a preceding history of systemic lupus erythematosus (SLE), primary Sjögren’s syndrome (SS), or both (SS/SLE). P-values were derived two-sided unpaired t-test with Welch’s correction.
Importantly, there were no differences in the levels of IgG anti-MDA or IgG anti-Ro60 in neonates with congenital heart block from autoimmune mothers compared to their clinically healthy siblings (not shown). Furthermore, there were no significant differences in cord blood levels of IgM-antibodies or IgG-autoantibodies from the neonates of mothers who had received in utero dexamethasone treatment for the fetal development of congenital heart block compared to autoimmune mothers whom had not received dexamethasone (data not shown).
4. DISCUSSION
In our studies of neonatal humoral immunity we investigated the expression of natural IgM reactive with PC- or MDA neo-determinants that recognize cells undergoing apoptotic death [2, 37]. IgM-autoantibodies to both PC and to MDA were detected in cord blood/neonatal samples, albeit with a higher representation of IgM anti-MDA, and lower representation of IgM anti-PC, within neonatal compared to adult responses. We also analyzed samples from subjects with NL to investigate for effects of maternal autoimmunity at the fetal immune interface. While maternal IgG-antibodies that cross the placenta can initiate fetal cardiac and cutaneous injury, there is no known active transport mechanism in the placenta for maternal IgM. Therefore, IgM detected in cord blood, including anti-Ro60 IgM antibodies should originate from the fetal immune system. Indeed, the representation of IgM anti-Ro60 antibodies was found to be increased in the offspring from anti-Ro60 positive mothers, although these antibodies did not discriminate between neonates with NL vs. healthy siblings, or were related to maternal clinical status. We also considered the potential contribution of IgM-RF autoantibodies to evidence of elevated IgM anti-Ro60 reactivity in the offspring of autoimmune mothers, and we performed specific assays that removed this concern. Taken together, we interpret our findings as evidence that maternal transfer of IgG anti-Ro/La autoantibodies is likely to have secondary effects on the neonatal IgM-repertoire regardless of whether there is clinically overt NL and/or clinically apparent autoimmune disease in the mother.
The tissue injury associated with cardiac NL is known to first develop at 18–24 weeks of gestation [28], a time when circulating IgM antibodies are certainly present [8, 9]. The process that results in congenital heart block may also be associated with an increase in local cell death. We hypothesized that this could modulate circulating levels of neonatal IgM to AC-associated determinants, akin to changes in IgM anti-PC in adults reported following TNFα infusions [38]. Indeed we found evidence of decreased representation of IgM anti-MDA within the overall IgM pool in the cord blood samples of neonates from autoimmune mothers, compared to cord blood from non-autoimmune mothers (p=0.004)(Figure 5G). Hence our studies may provide evidence of selective consumption of the potentially protective IgM anti-MDA natural antibodies. In contrast, we have previously shown that adult SLE patients have significantly elevated levels of IgM anti-MDA compared to controls [14], this may be due to persistent stimulation of anti-MDA B cells by factors released due to chronic inflammation, apoptotic death, and/or oxidative stress.
The healthy adult relatives of autoimmune patients have been reported to often have detectable anti-Ro60 IgM-antibodies [39], and it was hypothesized that this could predispose these individuals to later IgG class-switched anti-Ro60 responses and the development of overt clinical disease [39]. In an earlier report, 4–5 week old neonates from autoimmune mothers were found to have only low or non-detectable levels of IgM anti-Ro60 [40]. Yet it is uncertain how to reconcile differences between these observations, and it could be that we detected IgM anti-Ro60 in a greater number of subjects simply due to increased sensitivity of our methods. There could also be subsequent decreases in levels of IgM anti-Ro60 that occur weeks to months after birth [41, 42].
The design of our current studies was influenced by our prior observation that experimental infusions of large numbers of apoptotic cells into adult mice can nearly double overall IgM levels [2], with preferential induction of IgM-antibodies reactive with PC- or MDA-containing antigens [2]. We postulate that when these antibodies arise in the fetus there is the potential for protective effects.
In the murine repertoire, anti-PC natural antibodies are also infrequent at birth while there is a subsequent post-natal expansion [43]. We have also previously documented a similar age-dependent switch from an early dominance of anti-MDA compared to a later emergence of anti-PC antibodies that are responsible for natural antibody mediated anti-inflammatory effects in weanlings compared adult mice, respectively [3]. Hence, we have now found evidence to support the classic immunologic theory that mice and humans share a conserved programmed development of antibodies [41, 42], which our findings suggest are directed against different neo-determinants on the cell membranes of ACs. We wonder whether the early repertoire is in part a reflection of oxidative stress and the hypoxic environment in utero [44], and/or is also influenced by variations of (auto)antigenic exposures during in utero development. After birth, the representation of these natural antibody-secreting B cells may also be affected by postnatal microbiome colonization of the gut [45] or under the influence of dietary antigens [46]. Intriguingly, neonatal IgM is believed to be primarily from B-1 cells. Based our current findings, we hypothesize that human IgM anti-MDA antibodies are commonly produced by B-1 cells that arise during the prenatal period, while most IgM anti-PC may instead derive from B-cells that arise later, akin to what has been described in mice [43].
Whereas IgG-antibodies to MDA are not commonly present in healthy adults, levels of these antibodies are often increased in individuals following the development of autoimmunity. In fact, in cross-sectional studies elevated levels of IgG anti-MDA levels directly correlated with higher lupus disease activity [14, 47]. It was therefore predictable that maternal IgG anti-MDA would be detected in a major proportion of the cord blood samples from autoimmune mothers but not in neonatal samples from non-autoimmune mothers. However, the immunobiologic implications remain unclear. An increase in expression of IgG anti-MDA may arise in a setting of an altered redox state, in which increased oxidative stress and lipid peroxidation may lead to the release of free, chemically-reactive MDA that leads to adduct protein modifications, and induces an anti-MDA IgG response [48, 49]. While this could represent a bystander effect of inflammation, murine experimental models have suggested that serum levels of free MDA can reflect the overall level of oxidative injury, and induced IgG anti-MDA antibodies may further contribute to autoimmune pathogenesis [50–52]. We therefore wonder whether the increased levels of IgG anti-MDA antibodies found in a subset of asymptomatic autoimmune mothers reflects a subclinical systemic pathologic process. Furthermore, the IgG anti-MDA arising in SLE patients are overwhelmingly of the IgG1 and IgG3 subclasses [14], which are more efficient at triggering the complement cascade and engaging activating FcγR [53]. We therefore speculate that IgG anti-MDA levels in these autoimmune mothers could provide a predictive biomarker for later development of clinically active disease. Longitudinal follow-up studies of these at-risk individuals are therefore warranted.
We have recently shown that anti-PC antibodies are present in adult bone marrow, the primary antibody production site of plasma cells derived from follicular B cells [54]. An independent report with BCR cloning from single human B cells suggested that adult human anti-PC responses may not be restricted to the B-1 cell compartment [55]. Yet there are also data supporting the notion that in diverse adult populations there are dominant and recurrent anti-PC clonotypic sets that are generated by convergent somatic mechanisms to display part of the B-cell common public repertoire [46, 55].
In conclusion, natural IgM-antibodies to AC determinants are present in health at birth and the relative representation of specific antibody reactivities appears to be differentially expressed during development. While natural IgM have housekeeping functions to increase apoptotic cell clearance in adults, our studies cannot address whether natural IgM-antibodies are also commonly involved in AC phagocytosis during fetal development, a period associated with high levels of apoptosis that are integral to organogenesis. Furthermore, it is currently unknown whether natural IgM antibodies play homeostatic roles in the newborn and if a distortion in the neonatal IgM repertoire may influence the health of the child or affect subsequent immune development. We speculate that the increased overall IgM levels in newborns from autoimmune mothers may reflect a response to exaggerated inflammation-associated antigenic stimulation [2]. This could also be a part of a feedback system involved in the resolution of the inflammatory process linked to the maternal transfer of the IgG anti-Ro/La antibodies that appears to occur whether or not overt NL is clinically detected. In mice, maternal antigenic exposure can change the immune response of the newborn [56]. Our studies appear to characterize a different type of prenatal immune challenge. Indeed, we found possible evidence of the selective IgM anti-MDA depletion in the offspring of autoimmune mothers, which may reflect a locally consumptive process. Our data do strongly support the notion that in utero exposure to maternally transferred IgG-autoantibodies (along with associated immune complexes and possibly other inflammation-associated factors), can influence the immune development of the human fetus (i.e., immunologic imprinting) even in the absence of pathologic neonatal cardiac or cutaneous manifestations [15, 57].
Supplementary Material
HIGHLIGHTS.
Natural IgM binding of apoptosis-associated epitopes and Ro60 is common in neonates
Binding of cord IgM to apoptotic cells correlates with reactivity with malondiadehyde
Newborns from IgG anti-Ro/La(+) mothers have higher IgM anti-Ro60 than other neonates
Newborns from IgG anti-Ro/La(+) mothers have lower IgM anti-MDA than other neonates
Maternal autoimmunity may influence the congenital IgM-repertoire of the newborn
Acknowledgments
This work was supported by National Institutes of Health Grants; R01AI090118, R01AI068063, R01-AR42455, N01-AR-4-2271, an American Recovery and Reinvestment Act supplement; the American College of Rheumatology Research Education Foundation Within Our Reach campaign; the Alliance for Lupus Research; the Lupus Research Institute; the Arthritis Foundation; and The P. Robert Majumder Charitable Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AC
Apoptotic Cell
- CWPS
Cell Wall Polysaccharide
- MDA
Malodialdehyde
- NL
Neonatal lupus
- PC
Phosphorylcholine
- RF
Rheumatoid Factor
- SS
Sjögrens syndrome
- SLE
Systemic Lupus Erythematosus
- SLE
Disease Actvity Index (SLEDAI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–59. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–43. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grönwall C, Chen Y, Vas J, Khanna S, Thiel S, Corr M, et al. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A. 2012;109:19745–50. doi: 10.1073/pnas.1211868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman GJ, Vas J, Grönwall C. Protective autoantibodies in the rheumatic diseases: lessons for therapy. Nat Rev Rheumatol. 2013;9:291–300. doi: 10.1038/nrrheum.2013.30. [DOI] [PubMed] [Google Scholar]
- 5.Vas J, Grönwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64:3388–98. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grönwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–86. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 8.Berg T, Nilsson BA. The foetal development of serum levels of IgG and IgM. Acta Paediatr Scand. 1969;58:577–83. doi: 10.1111/j.1651-2227.1969.tb04765.x. [DOI] [PubMed] [Google Scholar]
- 9.Vick DJ, Hogge WA, Normansell DE, Burkett BJ, Harbert GM., Jr Determination of normal human fetal immunoglobulin M levels. Clin Diagn Lab Immunol. 1995;2:115–7. doi: 10.1128/cdli.2.1.115-117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dighiero G, Lymberi P, Holmberg D, Lundquist I, Coutinho A, Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985;134:765–71. [PubMed] [Google Scholar]
- 11.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–8. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Turunen SP, Kummu O, Veneskoski M, Lehtimaki J, Nissinen AE, et al. Natural antibodies of newborns recognize oxidative stress-related malondialdehyde acetaldehyde adducts on apoptotic cells and atherosclerotic plaques. Int Immunol. 2013;25:575–87. doi: 10.1093/intimm/dxt022. [DOI] [PubMed] [Google Scholar]
- 14.Grönwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–8. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman GJ, Srikrishnan R, Germar K, Goodyear CS, Andrews KA, Ginzler EM, et al. Genetic imprinting of autoantibody repertoires in systemic lupus erythematosus patients. Clinical and experimental immunology. 2008;153:102–16. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–50. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 17.Grönwall C, Reynolds H, Kim JK, Buyon J, Goldberg JD, Clancy RM, et al. Relation of carotid plaque with natural IgM antibodies in patients with systemic lupus erythematosus. Clin Immunol. 2014;153:1–7. doi: 10.1016/j.clim.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meffre E, Salmon JE. Autoantibody selection and production in early human life. J Clin Invest. 2007;117:598–601. doi: 10.1172/JCI31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daffos F, Forestier F, Grangeot-Keros L, Capella Pavlovsky M, Lebon P, Chartier M, et al. Prenatal diagnosis of congenital rubella. Lancet. 1984;2:1–3. doi: 10.1016/s0140-6736(84)91993-7. [DOI] [PubMed] [Google Scholar]
- 20.Dent PB, Finkel A. Intrauterine infection and cord immunoglobulin M. 3. Serological analysis of infants with elevated cord serum immunoglobulin M. Can Med Assoc J. 1974;110:1354–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–69. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feeney AJ. Predominance of VH-D-JH junctions occurring at sites of short sequence homology results in limited junctional diversity in neonatal antibodies. J Immunol. 1992;149:222–9. [PubMed] [Google Scholar]
- 23.Baumgarth N. Innate-like B cells and their rules of engagement. Adv Exp Med Biol. 2013;785:57–66. doi: 10.1007/978-1-4614-6217-0_7. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–6. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 25.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buyon JP, Clancy RM. Neonatal lupus: basic research and clinical perspectives. Rheum Dis Clin North Am. 2005;31:299–313. vii. doi: 10.1016/j.rdc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 28.Friedman D, Duncanson L, Glickstein J, Buyon J. A review of congenital heart block. Images Paediatr Cardiol. 2003;5:36–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Briassouli P, Rifkin D, Clancy RM, Buyon JP. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-beta and potentiates fibrosis. J Immunol. 2011;187:5392–401. doi: 10.4049/jimmunol.1101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clancy RM, Alvarez D, Komissarova E, Barrat FJ, Swartz J, Buyon JP. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol. 2010;184:2148–55. doi: 10.4049/jimmunol.0902248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 32.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda-Carus ME, Boutjdir M, Tseng CE, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161:5886–92. [PubMed] [Google Scholar]
- 34.Reed JH, Sim S, Wolin SL, Clancy RM, Buyon JP. Ro60 requires Y3 RNA for cell surface exposure and inflammation associated with cardiac manifestations of neonatal lupus. J Immunol. 2013;191:110–6. doi: 10.4049/jimmunol.1202849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed JH, Clancy RM, Lee KH, Saxena A, Izmirly PM, Buyon JP. Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken) 2012;64:1373–81. doi: 10.1002/acr.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padilla ND, Ciurana C, van Oers J, Ogilvie AC, Hack CE. Levels of natural IgM antibodies against phosphorylcholine in healthy individuals and in patients undergoing isolated limb perfusion. J Immunol Methods. 2004;293:1–11. doi: 10.1016/j.jim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira R, Barreto M, Santos E, Pereira C, Martins B, Andreia R, et al. Heritable factors shape natural human IgM reactivity to Ro60/SS-A and may predispose for SLE-associated IgG anti-Ro and anti-La autoantibody production. J Autoimmun. 2005;25:155–63. doi: 10.1016/j.jaut.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Klauninger R, Skog A, Horvath L, Winqvist O, Edner A, Bremme K, et al. Serologic follow-up of children born to mothers with Ro/SSA autoantibodies. Lupus. 2009;18:792–8. doi: 10.1177/0961203309103188. [DOI] [PubMed] [Google Scholar]
- 41.Perlmutter RM. Programmed development of the antibody repertoire. Curr Top Microbiol Immunol. 1987;135:95–109. doi: 10.1007/978-3-642-71851-9_7. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–3. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 43.Sigal NH, Pickard AR, Metcalf ES, Gearhart PJ, Klinman NR. Expression of phosphorylcholine-specific B cells during murine development. J Exp Med. 1977;146:933–48. doi: 10.1084/jem.146.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clancy RM, Zheng P, O’Mahony M, Izmirly P, Zavadil J, Gardner L, et al. Role of hypoxia and cAMP in the transdifferentiation of human fetal cardiac fibroblasts: implications for progression to scarring in autoimmune-associated congenital heart block. Arthritis Rheum. 2007;56:4120–31. doi: 10.1002/art.23061. [DOI] [PubMed] [Google Scholar]
- 45.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501:112–5. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman GJ. Protective natural autoantibodies to apoptotic cells: evidence of convergent selection of recurrent innate-like clones. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12788.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62:2064–72. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan SZ, Gheita TA, Kenawy SA, Fahim AT, El-Sorougy IM, Abdou MS. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: relationship to disease manifestations and activity. Int J Rheum Dis. 2011;14:325–31. doi: 10.1111/j.1756-185X.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 49.Shah D, Sah S, Wanchu A, Wu MX, Bhatnagar A. Altered redox state and apoptosis in the pathogenesis of systemic lupus erythematosus. Immunobiology. 2013;218:620–7. doi: 10.1016/j.imbio.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Li H, Firoze Khan M. Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic Res. 2012;46:1472–81. doi: 10.3109/10715762.2012.727209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Wang J, Fan X, Ansari GA, Khan MF. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology. 2012;292:113–22. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yahya MD, Pinnas JL, Meinke GC, Lung CC. Antibodies against malondialdehyde (MDA) in MRL/lpr/lpr mice: evidence for an autoimmune mechanism involving lipid peroxidation. J Autoimmun. 1996;9:3–9. doi: 10.1006/jaut.1996.0002. [DOI] [PubMed] [Google Scholar]
- 53.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–75. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 54.Grönwall C, Charles ED, Dustin LB, Rader C, Silverman GJ. Selection of apoptotic cell specific human antibodies from adult bone marrow. PLoS One. 2014;9:e95999. doi: 10.1371/journal.pone.0095999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiskesund R, Steen J, Amara K, Murray F, Szwajda A, Liu A, et al. Naturally Occurring Human Phosphorylcholine Antibodies Are Predominantly Products of Affinity-Matured B Cells in the Adult. J Immunol. 2014;192:4551–9. doi: 10.4049/jimmunol.1303035. [DOI] [PubMed] [Google Scholar]
- 56.Fusaro AE, Maciel M, Victor JR, Oliveira CR, Duarte AJ, Sato MN. Influence of maternal murine immunization with Dermatophagoides pteronyssinus extract on the type I hypersensitivity response in offspring. Int Arch Allergy Immunol. 2002;127:208–16. doi: 10.1159/000053865. [DOI] [PubMed] [Google Scholar]
- 57.Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la Konrad Lorenz? Scand J Immunol. 1999;50:348–54. doi: 10.1046/j.1365-3083.1999.00620.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.