Abstract
Healthy neurons have an optimal operating range, coded globally by the frequency of action potentials or locally by calcium. The maintenance of this range is governed by homeostatic plasticity. Here, we discuss how new approaches to treat depression alter synaptic activity. These approaches induce the neuron to recruit homeostatic mechanisms to relieve depression. Homeostasis generally implies that the direction of activity necessary to restore the neuron’s critical operating range is opposite in direction to its current activity pattern. Unconventional antidepressant therapies deep brain stimulation and NMDAR antagonists alter the neuron’s “depressed” state by pushing the neuron’s current activity in the same direction but to the extreme edge. These therapies rally the intrinsic drive of neurons in the opposite direction, thereby allowing the cell to return to baseline activity, form new synapses, and restore proper communication. In this review, we discuss seminal studies on protein synthesis dependent homeostatic plasticity and their contribution to our understanding of molecular mechanisms underlying the effectiveness of NMDAR antagonists as rapid antidepressants. Rapid antidepressant efficacy is likely to require a cascade of mRNA translational regulation. Emerging evidence suggests that changes in synaptic strength or intrinsic excitability converge on the same protein synthesis pathways, relieving depressive symptoms. Thus, we address the question: Are there multiple homeostatic mechanisms that induce the neuron and neuronal circuits to self-correct to regulate mood in vivo? Targeting alternative ways to induce homeostatic protein synthesis may provide, faster, safer, and longer lasting antidepressants.
1. Introduction Engaging homeostatic mechanisms with pharmacological intervention to treat Major Depressive Disorder
The idea of using modern medicine to push “out-of-balance” or diseased neurons to their tipping point seems outrageous. However, basic scientists do so on a regular basis. Experimental paradigms that induce long-lasting changes in synaptic efficacy in one direction are used to study cellular mechanisms of learning and memory (synaptic plasticity). Sustained extremes in neuronal activity, inherent to synaptic plasticity, eventually cause neurons and neuronal circuits to become unstable (Davis and Goodman, 1998; Pozo and Goda, 2010; Turrigiano and Nelson, 2000).To avoid this problem, healthy neurons adjust their synaptic strength to maintain optimal action potential firing rates. This process is known as homeostatic plasticity and requires dynamic molecular changes (Turrigiano, 2008).
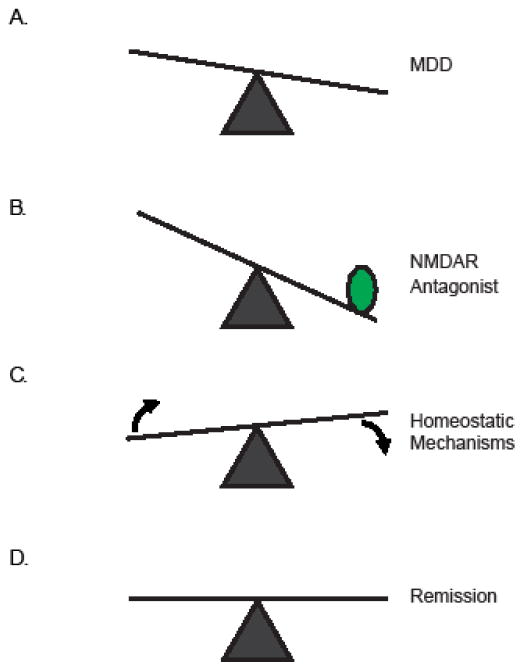
Depression, anxiety disorders, and addiction, result from imbalanced neuronal networks (Figure 1A) (McClung and Nestler, 2008). Such perturbations in neuronal signaling, while severe, may not tip the scale enough for homeostatic mechanisms to kick in. Can modern medicine capitalize on what basic science has taught us about homeostasis? In other words, can pharmacological intervention in vivo push neuronal activity to its limit to induce homeostatic response that will eventually cause neurons and neuronal circuits to self-correct? Studies on rapid antidepressant therapies (i.e. N-methyl-D-aspartate receptor (NMDAR) antagonists argue yes (Figure 1B–C).
Figure 1. Model for rapid antidepressant activation of homeostatic mechanisms.
(A) MDD leads to a network that is outside of the ideal homeostatic range, as indicated by the out of balance scale. (B) NMDAR antagonists (green circle) pushes the network further outside the ideal range activating homeostatic mechanisms (C) that result in relief from MDD and remission (D).
Are new antidepression therapies necessary?
Major Depressive Disorder (MDD) is a severe neuropathophysiology that will affect up to 17% of the population at some point during their lifetimes (Zarate et al., 2010). The most common pharmacological therapies, selective serotonin reuptake inhibitors (SSRIs), target the serotonergic system by blocking serotonin uptake. Unfortunately, only ~37% of individuals afflicted with MDD experience relief from their depressive symptoms with SSRIs (Murrough, 2012). Moreover, with continued use of SSRIs, only ~ 35–50% will go into remission (Murrough and Charney, 2012; Rush et al., 2006; Trivedi et al., 2008; Trivedi et al., 2006). Thus, finding new effective treatments for MDD is imperative. New therapies, such as rapid antidepressants and deep brain stimulation, are hypothesized to work by engaging network and cellular homeostatic mechanisms. Consequently, these therapies that are revolutionizing depression treatment, are predicted to drive the neurons to self-correct.
What are Rapid Antidepressants?
Rapid antidepressants are antagonists of NMDARs (Table 1). NMDAR is a ligand and voltage-gated cation channel that is best characterized for its role in shaping synaptic strength during synaptic plasticity (Blanke and VanDongen, 2009). Behavioral assessment has shown that ketamine, Ro 25-6981and other FDA approved NMDAR antagonists have remarkable efficacy in reversing treatment-resistant depression phenotypes. Additionally, NMDAR antagonists increase synaptogenesis, suggesting that increased neuronal communication arises from NMDAR blockade (Ibrahim et al.; Lepack et al., 2015; Li et al., 2010). While Trullas and Skolnick observed 25 years ago that NMDAR antagonists have antidepressant properties in rodents, recent clinical studies on the efficacy of ketamine and other NMDAR antagonists have renewed the field’s interest in these drugs as potential antidepressants (Skolnick et al., 2009; Zarate et al., 2006). Still, at the molecular level, it is uncertain as to why blocking NMDARs relieves depressive symptoms. This review focuses on the convergence of the clinical application of NMDAR antagonists and the basic science of neuronal homeostasis focusing on protein synthesis-dependent pathways.
Table 1.
Homeostatic Response to NMDAR antagonism
| Mechanism | NMDAR antagonist | Brain Region | Effect | References |
|---|---|---|---|---|
| Plasticity | AP5 | HPC | Induces GABABR shift from opening K channels to facilitating L-type calcium channel activity | Workman et al 2013 |
| HPC | Potentiates AMPAR-mediated evoked neurotransmission at rest | Nosyreva et al 2014 | ||
| Ketamine | HPC | Low-dose ketamine promotes low-dose AMPA-induced synaptogenesis (synapsin) | Akinfiresoye and Tizabi, 2013 | |
| PFC | Increases of spine number | Li et al 2010 | ||
| PFC | Increases serotonin-induced EPSC | Li et al 2010 | ||
| MK-801 | PFC | Switches HPC-induced LTD to LTP | Thomases et al 2014 | |
| Ro-25-6981 | HPC | Rescues LTP and gamma oscillation in LTP-deficient Tn65DN mouse | Hanson et al 2013 | |
| HPC | Impairs gamma oscillation in normal mouse | Hanson et al 2013 | ||
| Structural Plasticity | Ketamine | PFC | Promotes spine growth | Li et al 2011 |
| AP5 | HPC cultures | 14-3-3 eta decouples GABABRs from GIRK channels | Workman et al 2015 | |
| Intrinsic excitability | Ketamine | Cortex | Inhibits in Ih current in layer 5 cortical pyramidal cells | Chen et al., 2009 |
| HPC | Knockdown of HCN1 in the dorsal hippocampal produced antidepressant-like behaviors in the FST, similar to ketamine injected rats | Kim et al., 2012 | ||
| AP5 | HPC cultures | Increases expression of HDAC4 mRNA and protein | Chen et al 2014 | |
| Transcription | Ketamine | IC | Increases expression Homer1a mRNA | Bartolomeis et al 2013 |
| AC, SS | Increases expression of Arc mRNA | Bartolomeis et al 2013 | ||
| CP | Decreases expression of PSD95 mRNA | Bartolomeis et al 2013 | ||
| MC | Decreases expression of Homer1b mRNA | Bartolomeis et al 2013 | ||
| MK-801 | CP | Decreases expression of PSD95 mRNA | Bartolomeis et al 2013 | |
| HPC astrocyte cultures | Increases expression of BDNF, GFAP and TrkB mRNAs | Yu et al 2015 | ||
| Translation (eEF2-dependent /Global) | Ketamine | HPC, FC | Increases expression of BDNF | Autry et al 2011 |
| MK-801 | HPC | Increases expression of BDNF | Autry et al 2011 | |
| HPC, FC | Increases expression of Arc | Autry et al 2011 | ||
| Translation (mTOR-dependent) | AP5 | HPC cultures | Baclofen promotes expression of BDNF | Workman et al 2013 |
| Ketamine | PFC | Increases expression of synaptic proteins (GluA1, PSD95, Synapsin 1) | Li et al 2010 | |
| Translation (unknown mechanism) | AP5 | HPC cultures | Increases expression of GABABR2 protein | Workman et al 2015 |
| Ro-25-6981 | HPC | Increases expression of GABABR2 protein | Workman et al 2015 | |
| Apoptosis | AP5 | HPC cultures | Increases expression of pro-apoptotic genes expression | Chen et al 2014 |
| Ro-25-6981 | CTX | Delays the expression of TBI-induced autophagic proteins | Bigford et al 2009 | |
| Autophagy | Neuronal cultures | Inhibits glutamate excitotoxicity-induced cell death | Bigford et al 2009 |
2. NMDAR antagonism leads to homeostatic plasticity
A neuron employs two forms of homeostatic responses global and local synaptic scaling to deal with extreme high or low activity (Turrigiano, 2012). Global synaptic scaling induces a slow, cell-wide compensation in excitatory and inhibitory receptors throughout the soma and dendritic arbor. In contrast, local synaptic scaling occurs within hours as a response to local activity fluctuations, allowing dendrites or groups of synapses to restore operation at an optimal range (Queenan et al., 2012).
NMDAR subunit composition underlies the homeostatic mechanism of Metaplasticity
Metaplasticity is a form of homeostasis describing how cells or circuits reset their baseline activity levels in response to their previous activity (Abraham et al., 2001). Metaplasticity has been studied mostly at the circuit level, albeit it has been reported to occur at a single synapse (Lee et al., 2010a). For example, in seminal studies that deprive rodents of light during a critical period of visual developmental, the absence of visual input alters the threshold for synaptic plasticity in the forms of long-term potentiation (LTP) and long-term depression (LTD) (Kirkwood et al., 1996; Philpot et al., 2003). At the molecular level, light deprivation induces cell-wide forms of synaptic scaling by upregulating excitatory receptors and downregulating inhibitory receptors. Such a shift in the ratio of excitatory and inhibitory inputs will favor excitation and is consistent with a reduced threshold to induce LTP (Turrigiano, 2012). Interestingly, upon a short exposure to light, causing cortical activity to rise, more excitatory synaptic input is required to induce LTP (Philpot et al., 2003). These experiments, as well as many that have come before and after, serve as the basis for metaplasticity (Bear, 1995).
At the molecular level, changes in NMDAR subunit composition that regulate calcium dynamics likewise support metaplasticity (Yashiro and Philpot, 2008). NMDARs are obligate heteromultimers consisting of an NR1 subunit in combination with NR2A-D and NR3A-B (Glasgow et al., 2015). NR2A and B are the most critical subunits in setting the membrane threshold (Yashiro and Philpot, 2008). NR2B-containing NMDARs are slower to deactivate and thus carry more calcium per unit current relative to NR2A-containing receptors (Glasgow et al., 2015; Sobczyk et al., 2005) . A decrease in the NR2A/2B ratio, an adjustment that appears to be seemingly modest in nature, significantly slides the threshold to favor LTP. As expected, NR2B-containing channels are upregulated relative to NR2A in the dark; and upon activation, allow for greater influx of calcium in spines (Yashiro and Philpot, 2008). Thus, one would predict that blocking NR2B activity under conditions of reduced cortical activity would provide the biggest push toward silencing synaptic activity. Furthermore, such a push may be sufficient to engage alternative homeostatic mechanisms to return the neuron back to an optimal operating range.
3. Mammalian Target of Rapamycin (mTOR) serves as a thermostat in NMDAR antagonist-induced homeostatic response
mTOR senses the functional, operating range of a neuron
How does one identify if a neuron is “out-of-range”? Is there a molecular marker whose activity level gauges the general health of a neuron? In MDD, it has been suggested that mTOR activity levels may serve as a molecular thermostat as it can gauge the efficacy of antidepressant drugs (Zarate et al., 2013). mTOR is a serine/threonine kinase that controls protein synthesis, cell growth and metabolism by sensing the levels of nutrients, growth factors, cellular energy, and stress. mTOR is conserved from yeast to human, signifying its critical importance in cellular homeostasis. mTOR consists of two complexes, mTORC1 and C2. mTORC1 regulates protein synthesis through phosphorylation of two proteins that regulate mRNA translation eukaryotic initiation factor 4 binding protein (eIF4BP) and ribosomal S6 kinase (Hay and Sonenberg, 2004). In the brain, mTOR mediates dendritic protein synthesis, critical for synaptic plasticity and memory consolidation (Graber et al., 2013).
In many diseases, mTORC1 is often overactive or underactive, consistent with a neuron operating outside its optimal range. For example, in epilepsy, Alzheimer’s disease, and autism spectrum disorders including Fragile X Syndrome and Tuberous Sclerosis Complex mTORC1 signaling is excessive (Crino, 2015; Gross et al., 2010; Pei and Hugon, 2008; Sahin, 2012; Santini and Klann, 2011; Sharma et al., 2010; Sosanya et al., 2014; Spilman et al., 2010; Zeng et al., 2009; Zeng et al., 2011). Furthermore in MDD and Parkinson’s disease, mTORC1 signaling is reduced (Fragkouli and Doxakis, 2014; Jernigan et al., 2011). It is currently unknown how mTORC1 gets stuck and fails to return to basal levels in these diseases. What can be done to “unstick” mTOR activity, allowing it to return to basal levels? Insights from NMDAR antagonists used for treating MDD may offer an answer. Several labs have shown that mTORC1 activity increases with a single treatment of NMDAR antagonists in vivo. The increase in mTORC1 is required for the antidepressant efficacy of NMDAR antagonists (Li et al., 2010; Workman et al., 2013). In the brain, where the circuitry is intact and the release of neurotransmitters is difficult to control, deciphering how NMDAR blockade increases mTOR activity is challenging. Utilizing a simplified model of cultured hippocampal neurons, where one has tight control over the activation of neurotransmitter receptors, blockade of NMDARs with (2R)-amino-5-phosphonovaleric acid (AP5) reduces basal mTOR activity (Workman et al., 2013). These results indeed suggest that reducing NMDAR signaling pushes mTORC1 activity levels down and would be predicted to induce homeostasis (Figure 1). However as mTORC1 activity remains low, the trigger for the cell to push back and return mTORC1 activity to control levels is absent in these cultures. Collectively, these data provide the first hint that an extrinsic factor, such as a neurotransmitter, is necessary to restore mTORC1 activity to normal basal levels.
NMDAR activity at rest shapes the ability of metabotropic GABABR signaling to turn mTORC1 activity up
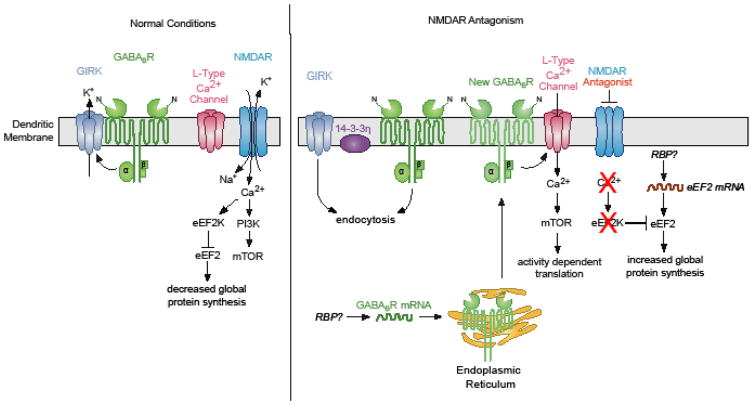
What turns mTOR activity on with NMDAR blockade? One clue came from studies showing that NMDARs and γ-aminobutyric acid B receptors (GABABR), that are metabotropic G-protein-coupled receptor, extensively interact in a feedback loop (Guetg et al., 2010; Terunuma et al., 2010; Workman et al., 2013). GABA is considered as the major inhibitory neurotransmitter in the brain (Jembrek and Vlainic, 2015). Under normal physiological conditions, GABABRs inhibit neuronal activity through pre- and postsynaptic mechanisms. Postsynaptically, GABABRs mediate the slow inhibitory postsynaptic potentials via the activation of postsynaptic, G-protein-regulated, inwardly rectifying potassium (GIRK) channels (Padgett and Slesinger). Stimulating NMDARs in cultured hippocampal neurons leads to endocytosis of GABABRs (Guetg et al., 2010; Terunuma et al., 2010). Likewise, blocking NMDAR signaling in vitro increases the dendritic surface expression of GABABRs (Workman et al., 2013). This reciprocal relationship between synaptic activity and GABABR surface expression, at first glance, would appear to provide positive feedback less inhibition with synaptic stimulation and more inhibition with reduced synaptic activity. Surprisingly, NMDAR blockade causes GABABR function to shift from signaling that opens GIRK channels to signaling that increases dendritic Ca2+ requiring L-type Ca2+ channel activity (Workman et al., 2013). This increase in Ca2+, in turn, activates mTORC1 in primary apical dendrites that are enriched in GABAergic synapses (Megias et al., 2001; Workman et al., 2013). Moreover, GABABR signaling through mTORC1 promotes the synthesis of the brain derived neurotropic factor (BDNF), which is required for new synapse formation with rapid antidepressants (Figure 2) (Li et al., 2010; Workman et al., 2013). These results emphasize that restoring neuronal communication leads to the sustained antidepressive efficacy of NMDAR antagonists (Li et al., 2010; Li et al., 2011; Liu et al., 2012).
Figure 2. Cartoon describing molecular pathways triggered by NMDAR antagonists.
(left panel) Activation of GABABRs signal to open GIRK channels reducing dendritic calcium levels. Calcium entry through NMDARs activates the translation elongation factor kinase eEF2K that in turn phosphorylates eEF2, inhibiting global translation. (right panel) In the presence of NMDAR antagonists GABABR signaling shift to facilitating L-type calcium channel activity and activates mTOR-dependent protein synthesis. This requires the protein synthesis and insertion of new GABABRs into the dendritic membrane. What RNA binding protein (RBP) mediates GABABR mRNA repression (left) and or translation (right) is an open question. In addition, Reduced NMDAR signaling to eEF2K increases the activity of eEF2 allowing for global translation. eEF2 protein expression increases when mTOR activity is reduced. If new eEF2 mRNA translation and the RBPs that regulate its expression is unknown.
4. Rapid antidepressant efficacy requires distinct stages of neuronal protein synthesis
Evoked versus miniature NMDAR currents
One perplexing concept regarding the effectiveness of NMDAR antagonists as rapid antidepressant is the requirement that the membrane be depolarized for NMDARs to open. NMDARs require the binding of two neurotransmitters, glutamate and glycine. Even in the presence of these molecules the channel remains closed due to Mg2+ blocking the ion-conducting pathway. Depolarization of the membrane dislodges Mg2+, and facilitates the movement of cations, particularly Ca2+, in and out of the cell (Burnashev et al., 1992). Considering the strict biophysical properties of the channel, one would predict that NMDAR antagonists quiet active neurons and neuronal circuits. However, some studies suggest that the Mg2+ block of NMDARs is partial at the resting membrane potential (Reese and Kavalali, 2015). Fortunately, since NMDAR antagonists come in many flavors, pharmacological approaches can dissect NMDAR resting current blockade from evoked and total NMDAR blockade. For example, MK-801, which selectively blocks open NMDARs, and memantine, only blocks NMDARs when Mg2+ is dispelled from the pore conducting pathway, are widely used. It is generally considered that MK-801 blocks both evoked and resting NMDAR currents, whereas memantine only blocks NMDARs engaged in firing Remarkably, rodents treated with MK-801 display improvement on behavioral despairs, while with memantine they do not (Autry et al., 2011; Gideons et al., 2014). Thus, NMDAR antagonists that are used as rapid antidepressants (e.g. MK-801) are effective at reducing the activity of neurons engaged in firing, as well as a population of NMDARs that are open at rest via spontaneous activity.
NMDAR blockade-induced translation initiates two phases of antidepressant efficacy
Studies suggest that antidepressant efficacy requires two phases induction and sustained. While it is clear that mTORC1-dependent protein synthesis is required for the long-lasting effects of rapid antidepressants, the molecular changes that initiate the process remain unclear. Does the initiation phase require protein synthesis? Work on local homeostatic synaptic scaling demonstrates that acute blockade of spontaneous NMDAR activity increases the miniature excitatory synaptic current (mEPSC) mediated by the glutamate receptor that contains α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) 1 subtype (GluA1). Such AMPAR-mediated synaptic scaling requires de novo dendritic protein synthesis of GluA1 (Sutton et al., 2006).
Notably, several groups have observed increased expression of GluA1 with administration of NMDAR antagonists in vivo (Autry et al., 2011; Li et al., 2010; Nosyreva et al., 2013; Workman et al., 2013). A separate set of studies have also demonstrated that blocking spontaneous NMDAR activity increases evoked AMPAR-mediated synaptic potentiation in the CA1 region of hippocampal slices. This form of potentiation was sustained and further amplified an hour after the antagonist had been washed away (Nosyreva et al., 2013). Interestingly in a BDNF knockout mouse, the initial synaptic potentiation due to NMDAR antagonists was present and similar to wildtype slices; however, the sustained potentiation was absent. In contrast, the inclusion of a protein synthesis inhibitor blocked the early and sustained potentiation induced by NMDAR blockade, indicating a requirement for protein synthesis in both plasticities (Nosyreva et al., 2013). These results confirm the necessity of BDNF for the long-lasting effects on synaptic potentiation due to NMDAR blockade and perhaps hint at a separate requirement for protein synthesis for the initial potentiation phase.
If mTORC1 activity mediates translation of mRNAs that are required for the sustained antidepressant effects of NMDAR antagonists, what mediates protein synthesis in the initiation phase? Schuman and colleagues proposed that Ca2+ entry through open NMDARs promotes the kinase activity of the eukaryotic elongation factor 2 kinase (eEF2K), which inhibits eEF2 an elongation factor that promotes global mRNA translation. As they had predicted, NMDAR blockade with AP5 in the presence of tetrodotoxin (TTX), which inhibits action potential, reduced Ca2+ entry, inactivated eEF2K, and increased local protein synthesis. These results led the authors to conclude that NMDAR-mediated mEPSCs inhibit local protein synthesis (Sutton et al., 2006; Sutton and Schuman, 2006; Sutton et al., 2004). These studies, for the first time, assigned a function to spontaneous release and mEPSCs, as a critical regulator of homeostatic protein synthesis (Sutton et al., 2004). In light of MDD, do NMDAR-mediated mEPSCs occur more often or have a greater impact in “depressed” neurons or neuronal circuits? There is no direct experimental evidence that addresses this question. However, work on rapid antidepressants demonstrates that eEF2-dependent translation is upregulated with NMDAR antagonists in vivo. Furthermore, NMDAR-antagonist-induced antidepressant response can be bypassed completely by treating rodents with drugs that directly activate eEF2 (Autry et al., 2011).
5. NMDAR antagonists induce structural homeostasis that correlates with antidepressant efficacy
One of the most striking effects of rapid antidepressants is increased spine density (Ohgi et al., 2015). Dendritic spines are the primary location of excitatory synapses in the adult brain. Spine shape, size, and number vary over the normal lifetime of an individual, and many neuropathologies display atypical spine distribution, shape, and number (Bourne and Harris, 2008). Structural homeostasis is defined by neuronal architecture changes in response to long-term fluctuations in activity (Yin and Yuan, 2014). Over the years, sensory paradigms that prompt homeostatic plasticity also induce morphological changes in spines (Butz et al., 2009; Keck et al., 2011). For example, sensory deprivation results in enlarged spine heads and increased postsynaptic AMPAR expression (Keck et al., 2013; Wallace and Bear, 2004). Moreover, whisker trimming paradigms that reduce sensory inputs to the cortex decreased spine elimination (Zuo et al., 2005).
In 1999, Kirov and Harris found that blocking NMDARs in an acute hippocampal slice preparation significantly increased the spine density in dendrites (Kirov and Harris, 1999). Duman and colleagues, a decade later, also reported that NMDAR antagonism increased dendritic spines in vivo by using mice that sparsely expressed yellow fluorescent protein in the prefrontal cortex (Li et al., 2010; Li et al., 2011). With new synapse formation one would expect increased pre- to postsynaptic signaling. As predicted, mEPSC frequency in layer II/III cortical neurons increased 24 hours post-rapid antidepressant injection (ketamine or Ro 25-6981) (Miller et al., 2014).
mTOR mediates NMDAR antagonist-induced spine formation
mTOR activity increases spine density. In neurons with hyperactive mTOR signaling, elevated spine number can be reduced with the mTORC1 inhibitor rapamycin (Tavazoie et al., 2005). It has been suggested that mTORC1-dependent protein synthesis is required for new spine formation and may be the key link for remission from depression (Li et al., 2010; Li et al., 2011). In support of this view, direct rapamycin infusion into the prefrontal cortex prevents the ketamine-induced increase in spine number (Li et al., 2010; Li et al., 2011). Of note, BDNF, which is translated via eEF2 and mTORC1, is necessary for the increased expression, maturation and stability of spines (Bennett and Lagopoulos, 2014). In light of how BDNF is translated, speculations abound that BDNF synthesis is required for the expression of the early and late phases of NMDAR antagonist-mediated antidepressant response, whereby the “early” synthesized BDNF is secreted to act upon postsynaptic TrkB receptors that bind BDNF with high affinity (Autry et al., 2011). Upon BDNF binding, TrkB signals cascade to turn mTOR on (Kavalali and Monteggia, 2012, 2015; Takei et al., 2004). BDNF secretion from the postsynaptic neuron is likely to require L-type calcium activity, perhaps through the activation of GABABRs (Lepack et al., 2015; Workman et al., 2013). Further work is needed to test this hypothesis and to identify the intermediate events that lead to new synapse formation. In spite of incomplete mechanistic details, these data suggest that altering neuronal activity with natural stimuli such as light, experimentally with whisker trimming, or pharmacologically with NMDAR antagonists promotes changes in spine shape, size and density to maintain homeostasis.
6. Evidence for alternative ways of inducing homeostatic plasticity to remedy depression
Intrinsic Excitability
If NMDAR antagonists exert their action by lowering synaptic activity to engage homeostatic mechanisms, then directly altering ion channel function or density (intrinsic excitability) of the dendritic membrane may serve the same function as NMDAR antagonism. For example, knockdown of the HCN1 channel produces an antidepressant behavioral phenotype and increases mTOR and BDNF, two molecular mediators of rapid antidepressant efficacy (Kim et al., 2012). While ketamine is best known for its ability to block NMDARs, it has been shown to also block HCN channels (Chen et al., 2009). Moreover, as mentioned above, switches in receptor-channel signaling are involved in formation and reversal of depressive symptoms. Specifically upon NMDAR antagonism, GABABR signaling to GIRK channels decreases while L-type channel activity increases. This switch in turn enhances mTOR activity and BDNF expression (Workman et al., 2013). Blocking the decoupling of GABABR from GIRK in the presence of NMDAR antagonists restores GABABR-mediated hyperpolarization and prevents rapid antidepressant efficacy in vivo (Workman et al., 2015). Interestingly, sleep deprivation also mediates a rapid antidepressant effect (McClung, 2007). In fruit flies, a screen designed to find mutants with a short sleeping phenotype identified the shaker locus (Kv1) (Cirelli et al., 2005), a channel whose expression is repressed by mTORC1 activity (Niere et al., 2016; Raab-Graham et al., 2006; Sosanya et al., 2015a; Sosanya et al., 2015b; Sosanya et al., 2013). It will be interesting if like HCN, reduced levels of Kv1.1 feedback to increase mTORC1/BDNF signaling resulting in antidepressant behaviors. All in all, these findings suggest that changes in intrinsic excitability can regulate the mTOR/BDNF antidepressant pathway.
Regulation of Autophagy
Aberrant autophagy has been suggested to underlie depression (Abelaira et al., 2014). Autophagy is triggered when the cell is deprived of nutrients or placed in stressed conditions and serves to recycle organelles, clear protein aggregates, and eliminate non-essential macromolecules in order to aid in cell survival. In certain cases, cell signaling events that induce autophagy eventually lead to apoptosis (Marino et al., 2014). Tissue studies in human and animal models have shown that depression is characterized by the loss of neural and glial cells due to chronic stress (Banasr and Duman, 2008; Krishnan and Nestler, 2008; Pittenger and Duman, 2008). Various stress paradigms in rats have been shown to upregulate levels of pro-apoptotic proteins that are released through mitochondrial outer membrane permeablization as well as the release of caspase 3 (Bachis et al., 2008; Kosten et al., 2008). Furthermore, a transcriptome-wide profile of post-mortem human prefrontal cortex brain tissue from 14 pairs of subjects analyzed by microarray revealed the over-expression of apoptosis factors and inflammatory cytokines (Shelton et al., 2011). Notably, antidepressants have been shown to upregulate levels of anti-apoptotic proteins, particularly members of the Bcl-2 family involved in the suppression of mitochondrial outer membrane permeabilization, a key step in the intrinsic apoptosis pathway (McKernan et al., 2009).
mTOR is known to control the homeostasis between cell growth (synthesis of new molecules) and autophagy (breakdown of current cell structures and macromolecules). mTOR is a negative regulator of autophagy but its mechanism of control is poorly understood. mTOR phosphorylation of ULK1 prevents activation of the ULK1 complex, a regulator of autophagy and inducer of apoptosis (Ganley et al., 2009; Jung et al., 2009; Lee and Tournier, 2011; Mukhopadhyay et al., 2015). Importantly, a reduction in both phosphorylation and expression of components of the mTOR signaling pathway has been observed in a postmortem examination of prefrontal cortex tissue obtained from depressed subjects in a series of studies. These proteins include NR2A, NR2B, mGluR5, PSD-95 as well as mTOR and its downstream targets involved in the control of translation p70SK6, eIF4E, p-eIF4E, eIF4B, and p-eIF4B (Deschwanden et al., 2011; Feyissa et al., 2009; Jernigan et al., 2011). All of this suggests an important role for mTOR in the homeostatic balance between autophagy and protein synthesis that may be relevant in the context of depression.
Finally, the NMDAR antagonists, 3-methyladenine (3-MA) and Ro-25-6981, have been shown to suppress the induction of autophagy and neuronal cell death (Bigford et al., 2009; Sadasivan et al., 2010; Xin et al., 2011). Bigford and colleagues demonstrated that Ro-25-6981 blocked the induction of autophagy and lowered the levels of autophagic proteins both in vitro and in vivo. This and previously discussed work suggest the possibility that the Ro-25-6981-mediated repression of NR2B signaling may by neuroprotective through the mTOR pathway. Thus, mTOR may be uniquely positioned (1) to promote the growth of new spines, (2) to regenerate dendritic architecture through protein synthesis, and (3) to suppress autophagy-mediated apoptosis in neurons injured by chronic stress (Jia and Le, 2015). Future studies will be needed to determine the manner in which NR2B-specific antagonists are able to trigger these effects.
7. Perspectives and Open Questions
Insight into depression and antidepressant therapies through the identification of proteins synthesized when mTORC1 is inhibited
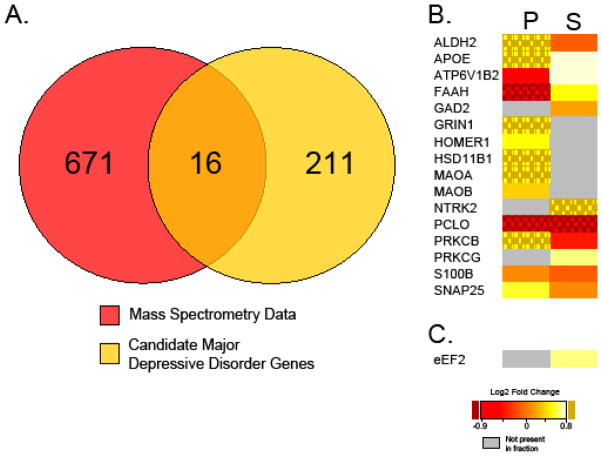
Are there other ways to coax the compromised or diseased neuron to self-correct? Clues may come from recent work that examines dendritic protein synthesis in the presence of the mTORC1 inhibitor rapamycin in vivo (Niere et al., 2015). While the role of mTORC1 activity to promote translation of mRNAs is grounded in its established function of phosphorylating eIF4BP and ribosomal S6 kinase (Hay and Sonenberg, 2004), a few studies suggest a separate and likely just as important role for mTORC1 activity in repressing mRNA translation of specific transcripts (Niere et al., 2015; Raab-Graham et al., 2006; Sosanya et al., 2013). Interestingly, Niere et al., using the unbiased approach of mass spectrometry, demonstrates that the number of proteins that increase at the synapse with mTORC1 inhibition is approximately equal in number to those whose expression is reduced (Niere et al., 2015). Is it possible that some of these mRNAs are homeostatic in nature, and that the proteins they encode may predispose the neuron to turn mTORC1 back on? Notably, some of the proteins that are synthesized with brief mTORC1 inhibition overlap with proteins previously associated with depression and antidepressant properties (Figure 3A-C (Aragam et al., 2011; De Vry et al., 2016; Dekker et al., 2012; Dlugos et al., 2009; Fatemi et al., 2001; Galeotti and Ghelardini, 2011; Gatt et al., 2015; Gray et al., 2015; Lee et al., 2010b; Liu et al., 2015; Luo et al., 2010; Ogawa and Kunugi, 2015; Ray et al., 2014; Rietschel et al., 2010; Sakaida et al., 2013; Shyn et al., 2011; Skoog et al., 2015; Stachowicz et al., 2015; Tadic et al., 2007; Unschuld et al., 2009; Wagner et al., 2015; Yoshimasu et al., 2015)). For example, Homer 1a was found to be upregulated in several paradigms of antidepression therapy including sleep deprivation, electroconvulsive shock, ketamine, imipramine, and fluoxetine (Serchov et al., 2015). Moreover, the Homer1 knockout mouse displays phenotypes consistent with anxiety and depression (Szumlinski et al., 2005). To date, it is unclear as to what protein synthesis pathways and translation factors are utilized to translate these homeostatic mRNAs. One would predict that eEF2 is involved but may not be the only factor. Notably, eEF2 is a protein whose expression is differentially regulated by mTORC1 activity, in a subcellular specific manner (Figure 3c). Is synthesis of eEF2 protein required to turn mTORC1 activity on with NMDAR blockade (Figure 2)? Such a result would suggest that additional translational mechanisms upstream of eEF2 are required for antidepressant efficacy. Nevertheless, coordinated stages of protein synthesis utilizing different translational mechanisms such as eEF2 and mTORC1 are required for rapid antidepressant efficacy, and mTORC1-dependent translation of BDNF is critical for the sustained effects of NMDAR antagonists (Lepack et al., 2015).
Figure 3. Protein expression regulated by mTORC1 activity overlap with depression.
(A) Venn diagram illustrating several proteins identified by mass spectrometry of synapses with brief in vivo mTORC1 inhibition overlap with proteins associated with depression. (B) Heat map of 16 overlapping proteins in the postsynaptic density fraction (P) and soluble fraction (S) of synaptoneurosomes isolated from cortices of control and rapamycin injected rats. Note, many of those proteins increase with mTORC1 inhibition (yellow). (C) Heat map of EEF2, demonstrating increased expression in soluble fraction of synaptoneurosomes with brief mTORC1 inhibition. Data obtained from Niere et al., 2015 and (Aragam et al., 2011; De Vry et al., 2016; Dekker et al., 2012; Dlugos et al., 2009; Fatemi et al., 2001; Galeotti and Ghelardini, 2011; Gatt et al., 2015; Gray et al., 2015; Lee et al., 2010b; Liu et al., 2015; Luo et al., 2010; Ogawa and Kunugi, 2015; Ray et al., 2014; Rietschel et al., 2010; Sakaida et al., 2013; Shyn et al., 2011; Skoog et al., 2015; Stachowicz et al., 2015; Tadic et al., 2007; Unschuld et al., 2009; Wagner et al., 2015; Yoshimasu et al., 2015) for bioinformatics analysis.
The unexplored role of RNA-binding proteins in rapid antidepression therapy
Targeting new protein synthesis pathways as a means to treat depression is a relatively new idea. As noted above, protein synthesis of specific proteins is orchestrated to mediate short- and long-term effects. Spatial accumulation of mRNAs in dendrites is widely believed to be a mechanism that regulates temporal gene expression. Cis-acting elements such as noncoding RNAs and RNA binding proteins (RBP) reversibly bind to the mRNA and ensure that the message is made into protein only when needed (Fernandez-Moya et al., 2014).To date, little work has been done to determine the role of RNA binding factors in antidepression efficacy. Considering the importance of new synaptic connections, RBPs that regulate synapse structure and function are likely to be important for long-lasting antidepressant effects with NMDAR antagonism. Here we will highlight some of the RBPs that are critical for new synapse formation and synaptic scaling.
NMDAR-mediated RNA granule formation
mRNA-silencing foci are RNA granules that contain silenced mRNAs and RBPs that repress translation. Distinct mRNA-silencing foci are formed and dispersed with different forms of synaptic plasticity (Thomas et al., 2014). One particular foci that forms with NMDAR stimulation is the synaptic XRN1 bodies (SX-bodies). While XRN1 is an RBP that is best known for its role in mRNA decay, SX-bodies lack decapping activity and therefore serve to sequester mRNAs (Luchelli et al., 2015). Since these bodies increase upon NMDAR stimulation, it is interesting to ask, what happens to SX-bodies with NMDAR antagonism? Moreover, are the sequestered mRNAs needed for the induction of rapid antidepressant efficacy?
mRNA trafficking
A network of RBPs have been implicated in dendritic and synapse morphology. Remarkably, there is little functional redundancy among them. A single knockdown or a mutation in any one RBP disrupts translational regulation of its target mRNAs resulting in dendritic/spine defects. Some of the most compelling data for the importance of RBPs in synapse formation is the knockdown of the RBPs that affect mRNA trafficking (Doyle and Kiebler, 2011). The Staufen proteins are best known for their role in mRNA trafficking into the dendrites and are required for synaptic plasticity (Tang et al., 2001).
Knockdown of Stau2 results in 40% fewer mRNAs in dendrites that are retained in the soma. Moreover, Stau2-knockdown neurons show a significant reduction in synapse number (Goetze et al., 2006). Many depression studies have examined total mRNA expression; however synaptic changes in mRNA levels may be obscured. To the best of our knowledge, none have examined synaptic mRNA levels. Future studies regarding RBP-mediated mRNA trafficking deficits with MDD and with rapid antidepressant treatment are needed.
Dendrite and Spine Morphology
Neurodegenerative diseases with synaptic deficiencies may serve as a clue as to which RBPs may be required for new synapse formation with rapid antidepressants. For example, mutations in two nuclear proteins TAR DNA-binding protein 43 (TDP-43) and fused in sarcoma (FUS) are associated with frontotemporal dementia and amyotrophic lateral sclerosis (ALS). Newly discovered roles for TDP-43 and FUS in dendritic branching and synapse formation, respectively, are emerging (Lu et al., 2009; Sephton et al., 2014). For example, FUS accumulates in dendritic spines to regulate morphology upon mGluR5 stimulation (Fujii and Takumi, 2005). Mutations in FUS result in reduced dendritic branching and spine maturation (Sephton et al., 2014). One might predict that even modest changes in the expression or subcellular localization of TDP-43 and FUS may contribute to the observed synaptic dysfunction, synapse loss, and neuronal atrophy in MDD.
Synaptic Scaling
Two RBPs have been implicated in homeostatic synaptic scaling, the retinoic acid receptor (RAR) α and fragile X mental retardation protein (FMRP). Both RBPs repress translation of their target mRNAs. During synaptic scaling the increase in GluA1 synthesis arises from the upstream intracellular synthesis of retinoic acid (RA), which in turn, causes dissociation of RARα from GluA1 mRNA, allowing it to be translated (Aoto et al., 2008). Interestingly, Soden and Chen demonstrated that AMPAR-mediated synaptic scaling is absent in CA1 hippocampal neurons of Fmr1 knockout mouse. Moreover, RA-induced mRNA translation of GluA1, GluA2, and eEF2 observed in hippocampal neurons isolated from wildtype mice does not occur in Fmr1 knockout neurons (Soden and Chen, 2010). How FMRP regulates RA-induced synaptic scaling is still an open question. Furthermore, it is unknown whether FMRP- and RA-mediated mRNA translation is required for the rapid antidepressant efficacy induced by NMDAR antagonists.
The RBPs discussed here is a representative sample of RBPs that may be essential to restore proper communication between neurons that will relieve depressive symptoms. Many more are likely to be required. Future work examining the roles of RBPs with NMDAR antagonists is therefore crucial. Moreover, targeting specific RBPs may be critical in revealing more specific therapies for MDD.
8. Conclusion
Rapid antidepressants activate local homeostatic processes. The efficacy of these drugs relies on their ability to engage homeostatic mechanisms that trigger protein synthesis pathways. Interestingly the antidepressant efficacy remains long after the drug has left the patient’s system (Abdallah et al., 2015). The acute dose of NMDAR antagonists engages numerous synaptic mechanisms that produce a sustained and lasting increase in synaptic connections that require protein synthesis. Perhaps these new synapses can reestablish an activity state in the brain that remediates a depressed system. Utilizing native mechanisms of homeostasis, rapid antidepressants offer a novel treatment that may have many applications outside of MDD.
Highlights.
Herein, we review seminal studies on protein synthesis dependent homeostatic plasticity underlying the effectiveness of NMDAR antagonists as rapid antidepressants.
Rapid antidepressant efficacy is likely to require a cascade of mRNA translational regulation.
Future studies regarding the role of RNA binding proteins are discussed.
Acknowledgments
This study was supported by the National Science Foundation IOS 1026527 and IOS 1355158 (KRG), Postdoctoral Research Fellowship in Biology DBI-1306528 (FN); a NIH-NIAAA pilot grant provided by the Integrated Neuroscience Initiative for Alcoholism (KRG); Department of Defense, United States Army Medical Research and Materiel Command USAMRMC Award W81XWH-14-1-0061 (KRG).
Footnotes
Authors Contributions: KRG, ERW, SN and FN wrote and edited the manuscript. ERW prepared Figure 1 and SN prepared Figure 2 and 3. SN and FN prepared Table 1.
Conflict of Interest
Authors declare that the research was conducted in the absence of any commercial or financial interests that could be considered a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelaira HM, Reus GZ, Neotti MV, Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014;101:10–14. doi: 10.1016/j.lfs.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Abraham MT, Kuriakose MA, Sacks PG, Yee H, Chiriboga L, Bearer EL, Delacure MD. Motility-related proteins as markers for head and neck squamous cell cancer. Laryngoscope. 2001;111:1285–1289. doi: 10.1097/00005537-200107000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragam N, Wang KS, Pan Y. Genome-wide association analysis of gender differences in major depressive disorder in the Netherlands NESDA and NTR population-based samples. J Affect Disord. 2011;133:516–521. doi: 10.1016/j.jad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Nosheny RL, Mocchetti I. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008;442:104–108. doi: 10.1016/j.neulet.2008.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. doi: 10.1016/0896-6273(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Progress in neurobiology. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Bigford GE, Alonso OF, Dietrich D, Keane RW. A novel protein complex in membrane rafts linking the NR2B glutamate receptor and autophagy is disrupted following traumatic brain injury. J Neurotrauma. 2009;26:703–720. doi: 10.1089/neu.2008.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke ML, VanDongen AMJ. Activation Mechanisms of the NMDA Receptor. In: Van Dongen AM, editor. Biology of the NMDA Receptor. Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual review of neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Gunther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Butz M, Worgotter F, van Ooyen A. Activity-dependent structural plasticity. Brain research reviews. 2009;60:287–305. doi: 10.1016/j.brainresrev.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Crino PB. mTOR Signaling in Epilepsy: Insights from Malformations of Cortical Development. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- De Vry J, Vanmierlo T, Martinez-Martinez P, Losen M, Temel Y, Boere J, Kenis G, Steckler T, Steinbusch HW, Baets MD, et al. TrkB in the hippocampus and nucleus accumbens differentially modulates depression-like behavior in mice. Behav Brain Res. 2016;296:15–25. doi: 10.1016/j.bbr.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Tiemeier H, Luijendijk HJ, Kuningas M, Hofman A, de Jong FH, Stewart PM, Koper JW, Lamberts SW. The effect of common genetic variation in 11beta-hydroxysteroid dehydrogenase type 1 on hypothalamic-pituitary-adrenal axis activity and incident depression. J Clin Endocrinol Metab. 2012;97:E233–237. doi: 10.1210/jc.2011-0601. [DOI] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos AM, Palmer AA, de Wit H. Negative emotionality: monoamine oxidase B gene variants modulate personality traits in healthy humans. J Neural Transm (Vienna) 2009;116:1323–1334. doi: 10.1007/s00702-009-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Kiebler MA. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J. 2011;30:3540–3552. doi: 10.1038/emboj.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12:3257–3262. doi: 10.1097/00001756-200110290-00023. [DOI] [PubMed] [Google Scholar]
- Fernandez-Moya SM, Bauer KE, Kiebler MA. Meet the players: local translation at the synapse. Front Mol Neurosci. 2014;7:84. doi: 10.3389/fnmol.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, Doxakis E. miR-7 and miR-153 protect neurons against MPP(+)-induced cell death via upregulation of mTOR pathway. Front Cell Neurosci. 2014;8:182. doi: 10.3389/fncel.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R, Takumi T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci. 2005;118:5755–5765. doi: 10.1242/jcs.02692. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C. Antidepressant phenotype by inhibiting the phospholipase Cbeta(1)--protein kinase Cgamma pathway in the forced swim test. Neuropharmacology. 2011;60:937–943. doi: 10.1016/j.neuropharm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8649–8654. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow NG, Siegler Retchless B, Johnson JW. Molecular bases of NMDA receptor subtype-dependent properties. The Journal of physiology. 2015;593:83–95. doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze B, Tuebing F, Xie Y, Dorostkar MM, Thomas S, Pehl U, Boehm S, Macchi P, Kiebler MA. The brain-specific double-stranded RNA-binding protein Staufen2 is required for dendritic spine morphogenesis. J Cell Biol. 2006;172:221–231. doi: 10.1083/jcb.200509035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Mem. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetg N, Abdel Aziz S, Holbro N, Turecek R, Rose T, Seddik R, Gassmann M, Moes S, Jenoe P, Oertner TG, et al. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13924–13929. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA., Jr Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jembrek MJ, Vlainic J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr Pharm Des. 2015;21:4943–4959. doi: 10.2174/1381612821666150914121624. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Le W. Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci Bull. 2015;31:427–434. doi: 10.1007/s12264-015-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hubener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–334. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, Bonhoeffer T, Hubener M. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron. 2011;71:869–882. doi: 10.1016/j.neuron.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Kim CS, Chang PY, Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron. 2012;75:503–516. doi: 10.1016/j.neuron.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nature neuroscience. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Galloway MP, Duman RS, Russell DS, D'Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology. 2008;33:1545–1558. doi: 10.1038/sj.npp.1301527. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Tournier C. The requirement of uncoordinated 51-like kinase 1 (ULK1) and ULK2 in the regulation of autophagy. Autophagy. 2011;7:689–695. doi: 10.4161/auto.7.7.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010a;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Hahn CY, Lee JF, Huang SY, Chen SL, Kuo PH, Lee IH, Yeh TL, Yang YK, Chen SH, et al. MAOA interacts with the ALDH2 gene in anxiety-depression alcohol dependence. Alcohol Clin Exp Res. 2010b;34:1212–1218. doi: 10.1111/j.1530-0277.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Huang L, Luo XJ, Wu L, Li M. MAOA Variants and Genetic Susceptibility to Major Psychiatric Disorders. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9374-0. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ferris J, Gao FB. Frontotemporal dementia and amyotrophic lateral sclerosis-associated disease protein TDP-43 promotes dendritic branching. Mol Brain. 2009;2:30. doi: 10.1186/1756-6606-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchelli L, Thomas MG, Boccaccio GL. Synaptic control of mRNA translation by reversible assembly of XRN1 bodies. J Cell Sci. 2015;128:1542–1554. doi: 10.1242/jcs.163295. [DOI] [PubMed] [Google Scholar]
- Luo KR, Hong CJ, Liou YJ, Hou SJ, Huang YH, Tsai SJ. Differential regulation of neurotrophin S100B and BDNF in two rat models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1433–1439. doi: 10.1016/j.pnpbp.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacology & therapeutics. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Dinan TG, Cryan JF. “Killing the Blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–263. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Das DN, Panda PK, Sinha N, Naik PP, Bissoyi A, Pramanik K, Bhutia SK. Autophagy protein Ulk1 promotes mitochondrial apoptosis through reactive oxygen species. Free Radic Biol Med. 2015;89:311–321. doi: 10.1016/j.freeradbiomed.2015.07.159. [DOI] [PubMed] [Google Scholar]
- Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Charney DS. Is there anything really novel on the antidepressant horizon? Curr Psychiatry Rep. 2012;14:643–649. doi: 10.1007/s11920-012-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Namjoshi S, Song E, Dilly GA, Schoenhard G, Zemelman BV, Mechref Y, Raab-Graham KF. Analysis of proteins that rapidly change upon mTORC1 repression identifies PARK7 as a novel protein aberrantly expressed in Tuberous Sclerosis Complex. Mol Cell Proteomics. 2015 doi: 10.1074/mcp.M115.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Namjoshi S, Song E, Dilly GA, Schoenhard G, Zemelman BV, Mechref Y, Raab-Graham KF. Analysis of Proteins That Rapidly Change Upon Mechanistic/Mammalian Target of Rapamycin Complex 1 (mTORC1) Repression Identifies Parkinson Protein 7 (PARK7) as a Novel Protein Aberrantly Expressed in Tuberous Sclerosis Complex (TSC) Mol Cell Proteomics. 2016;15:426–444. doi: 10.1074/mcp.M115.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Kunugi H. Inhibitors of Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase: New Targets for Future Antidepressants. Curr Neuropharmacol. 2015;13:760–775. doi: 10.2174/1570159X13666150612225212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K. Glutamate Signaling in Synaptogenesis and NMDA Receptors as Potential Therapeutic Targets for Psychiatric Disorders. Current molecular medicine. 2015;15:206–221. doi: 10.2174/1566524015666150330143008. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Hugon J. mTOR-dependent signalling in Alzheimer's disease. Journal of cellular and molecular medicine. 2008;12:2525–2532. doi: 10.1111/j.1582-4934.2008.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan BN, Lee KJ, Pak DT. Wherefore art thou, homeo(stasis)? Functional diversity in homeostatic synaptic plasticity. Neural plasticity. 2012;2012:718203. doi: 10.1155/2012/718203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry. 2014;4:e389. doi: 10.1038/tp.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese AL, Kavalali ET. Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife. 2015;4 doi: 10.7554/eLife.09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, Steffens M, Mier D, Esslinger C, Walter H, et al. Genome-wide association, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol Psychiatry. 2010;68:578–585. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sadasivan S, Zhang Z, Larner SF, Liu MC, Zheng W, Kobeissy FH, Hayes RL, Wang KK. Acute NMDA toxicity in cultured rat cerebellar granule neurons is accompanied by autophagy induction and late onset autophagic cell death phenotype. BMC Neurosci. 2010;11:21. doi: 10.1186/1471-2202-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Current opinion in neurobiology. 2012;22:895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaida M, Sukeno M, Imoto Y, Tsuchiya S, Sugimoto Y, Okuno Y, Segi-Nishida E. Electroconvulsive seizure-induced changes in gene expression in the mouse hypothalamic paraventricular nucleus. J Psychopharmacol. 2013;27:1058–1069. doi: 10.1177/0269881113497612. [DOI] [PubMed] [Google Scholar]
- Santini E, Klann E. Dysregulated mTORC1-Dependent Translational Control: From Brain Disorders to Psychoactive Drugs. Frontiers in behavioral neuroscience. 2011;5:76. doi: 10.3389/fnbeh.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Tang AA, Kulkarni A, West J, Brooks M, Stubblefield JJ, Liu Y, Zhang MQ, Green CB, Huber KM, et al. Activity-dependent FUS dysregulation disrupts synaptic homeostasis. Proc Natl Acad Sci U S A. 2014;111:E4769–4778. doi: 10.1073/pnas.1406162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serchov T, Heumann R, Calker DV, Biber K. Signaling pathways regulating Homer1a expression: implications for antidepressant therapy. Biol Chem. 2015 doi: 10.1515/hsz-2015-0267. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, Garriock HA, Yokoyama JS, McGrath PJ, Peters EJ, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends in pharmacological sciences. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Skoog I, Waern M, Duberstein P, Blennow K, Zetterberg H, Borjesson-Hanson A, Ostling S, Guo X, Kern J, Gustafson D, et al. A 9-Year Prospective Population-Based Study on the Association Between the APOE*E4 Allele and Late-Life Depression in Sweden. Biol Psychiatry. 2015;78:730–736. doi: 10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosanya NM, Brager DH, Wolfe S, Niere F, Raab-Graham KF. Rapamycin reveals an mTOR-independent Repression of Kv1.1 Expression during Epileptogenesis. Neurobiology of disease. 2014 doi: 10.1016/j.nbd.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Sosanya NM, Brager DH, Wolfe S, Niere F, Raab-Graham KF. Rapamycin reveals an mTOR-independent repression of Kv1.1 expression during epileptogenesis. Neurobiol Dis. 2015a;73:96–105. doi: 10.1016/j.nbd.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Sosanya NM, Cacheaux LP, Workman ER, Niere F, Perrone-Bizzozero NI, Raab-Graham KF. Mammalian Target of Rapamycin (mTOR) Tagging Promotes Dendritic Branch Variability through the Capture of Ca2+/Calmodulin-dependent Protein Kinase II alpha (CaMKIIalpha) mRNAs by the RNA-binding Protein HuD. J Biol Chem. 2015b;290:16357–16371. doi: 10.1074/jbc.M114.599399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone-Bizzozero NI, Raab-Graham KF. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. J Cell Biol. 2013;202:53–69. doi: 10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PloS one. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowicz A, Glombik K, Olszanecki R, Basta-Kaim A, Suski M, Lason W, Korbut R. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda-1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes, brain, and behavior. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Tadic A, Rujescu D, Muller MJ, Kohnen R, Stassen HH, Dahmen N, Szegedi A. A monoamine oxidase B gene variant and short-term antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1370–1377. doi: 10.1016/j.pnpbp.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Meulemans D, Vazquez L, Colaco N, Schuman E. A role for a rat homolog of staufen in the transport of RNA to neuronal dendrites. Neuron. 2001;32:463–475. doi: 10.1016/s0896-6273(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nature neuroscience. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MG, Pascual ML, Maschi D, Luchelli L, Boccaccio GL. Synaptic control of local translation: the plot thickens with new characters. Cell Mol Life Sci. 2014;71:2219–2239. doi: 10.1007/s00018-013-1506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Hollander E, Nutt D, Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. The Journal of clinical psychiatry. 2008;69:246–258. doi: 10.4088/jcp.v69n0211. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor perspectives in biology. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Specht M, Erhardt A, Ripke S, Heck A, Kloiber S, Straub V, Brueckl T, Muller-Myhsok B, et al. Polymorphisms in the GAD2 gene-region are associated with susceptibility for unipolar depression and with a risk factor for anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1100–1109. doi: 10.1002/ajmg.b.30938. [DOI] [PubMed] [Google Scholar]
- Wagner KV, Hartmann J, Labermaier C, Hausl AS, Zhao G, Harbich D, Schmid B, Wang XD, Santarelli S, Kohl C, et al. Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology. 2015;40:1222–1233. doi: 10.1038/npp.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman ER, Haddick PC, Bush K, Dilly GA, Niere F, Zemelman BV, Raab-Graham KF. Rapid antidepressants stimulate the decoupling of GABA receptors from GIRK/Kir3 channels through increased protein stability of 14-3-eta. Molecular psychiatry. 2015 doi: 10.1038/mp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman ER, Niere F, Raab-Graham KF. mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology. 2013;73:192–203. doi: 10.1016/j.neuropharm.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, Chen SD. 2-methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci. 2011;38:631–638. doi: 10.1017/s031716710001218x. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Yuan Q. Structural homeostasis in the nervous system: a balancing act for wiring plasticity and stability. Frontiers in cellular neuroscience. 2014;8:439. doi: 10.3389/fncel.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Mure K, Hashimoto M, Takemura S, Tsuno K, Hayashida M, Kinoshita K, Takeshita T, Miyashita K. Genetic alcohol sensitivity regulated by ALDH2 and ADH1B polymorphisms is strongly associated with depression and anxiety in Japanese employees. Drug Alcohol Depend. 2015;147:130–136. doi: 10.1016/j.drugalcdep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H. New paradigms for treatment-resistant depression. Ann N Y Acad Sci. 2013;1292:21–31. doi: 10.1111/nyas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Human molecular genetics. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]