Abstract
In the past decade, significant advances have been made in the design and optimization of novel biomaterials and microfabrication techniques to generate vascularized tissues. Novel microfluidic systems have facilitated the development and optimization of in vitro models for exploring the complex pathophysiological phenomena that occur inside a microvascular environment. To date, most of these models have focused on engineering of increasingly complex systems, rather than analyzing the molecular and cellular mechanisms that drive microvascular network morphogenesis and remodeling. In fact, mutual interactions among endothelial cells (ECs), supporting mural cells and organ-specific cells, as well as between ECs and the extracellular matrix, are key driving forces for vascularization. This review focuses on the integration of materials science, microengineering and vascular biology for the development of in vitro microvascular systems. Various approaches currently being applied to study cell-cell/cell-matrix interactions, as well as biochemical/biophysical cues promoting vascularization and their impact on microvascular network formation, will be identified and discussed. Finally, this review will explore in vitro applications of microvascular systems, in vivo integration of transplanted vascularized tissues, and the important challenges for vascularization and controlling the microcirculatory system within the engineered tissues, especially for microfabrication approaches. It is likely that existing models and more complex models will further our understanding of the key elements of vascular network growth, stabilization and remodeling to translate basic research principles into functional, vascularized tissue constructs for regenerative medicine applications, drug screening and disease models.
1. Introduction
The generation of vascularized tissue constructs to mimic the structure and function of native tissues is still in the early stages of development. Inadequate mass transport has often impaired the development of densely populated and metabolically functional tissues by leading to the formation of necrotic cores (Radisic et al., 2004). Simple microvascular systems have failed to deliver sufficient oxygen and nutrients to complex tissues (Asakawa et al., 2010, Jain et al., 2005, Melero-Martin et al., 2008). Consequently, the slow growth of the host vessels often failed to maintain the viability of the implanted tissue constructs and failed to facilitate integration of the implants into the host tissues. Ausprunk et al. reported the average speed of sprouting invasion was in the range of 0.25 to 0.5 mm/day (Ausprunk and Folkman, 1977), which implied considerable time is required for vessel ingrowth, colonization of the implanted tissue construct and anastomosis with the host vasculature. Therefore, the development of functional and perfusable microvascular networks is crucial for generating thick tissue constructs with physiologically relevant cell densities and improving the survival rate and function of implanted, tissue-engineered constructs (Miller et al., 2012). Furthermore, the presence of a vascular compartment plays a key role in the design, usability and optimization of advanced in vitro models that mimic complex biological phenomena involving functional and perfusable microvascular networks (Bersini and Moretti, 2015).
Over the last decade, significant advancements were made both in the field of microfabrication and materials science, which led to the design of vascularized tissues with properties that could be adjusted according to their specific application (Bersini et al., 2015). Vasculogenesis and angiogenesis-based techniques replicated physiological processes occurring during typical vascular growth, development and remodeling through better replication of 3D microvascular structures, and these techniques have led to the generation of highly branched and interconnected microvascular networks (Lim et al., 2013). In contrast, bioartificial networks can be developed by assembling simple building blocks (e.g., monolayers of ECs) (Zhang et al., 2013) or directly bioprinting cell-laden structures with precise shapes and dimensions (Huang et al., 2011, Khademhosseini and Langer, 2007, Malda et al., 2013). Current strategies employed for the generation of vascularized tissue constructs rely on multi-material combinations with tunable physicochemical properties (Nichol et al., 2010). Naturally derived biomaterials, such as collagen and its derivative gelatin, as well as fibrin, elastin, chitosan, alginate, and hyaluronic acid, are appealing for biological applications due to their high biocompatibility as well as their cell signaling and cell-interactive properties, whereas synthetic biomaterials, such as poly(ethylene glycol) (PEG), poly(glycerol sebacate) (PGS), poly(vinyl alcohol) (PVA), have been extensively studied as promising alternatives due to their generally superior mechanical properties and higher control of biodegradation (Annabi et al., 2014, Gaharwar et al., 2014, Thiele et al., 2014). In addition, new classes of hybrid hydrogels based on the optimal properties of both synthetic and natural biomaterials have been developed, such as collagen-PEG composites or elastin-based hydrogels with embedded PEG sequences (Jia and Kiick, 2009, Kopecek and Yang, 2012).
The combination of novel hydrogels, fabrication techniques and microfluidic technologies has the potential to overcome some of the technical limitations of traditional in vitro models, such as the Boyden chamber or the scratch-wound assay (Simpson et al., 2008). Microfluidic systems are characterized by their ability to biochemically and biophysically control the local microenvironment to investigate the complicated interactions between diverse types of cells and signal molecules within their neighboring microenvironment while significantly reducing the costs of reagents and cells (Selimovic et al., 2013, Shin et al., 2012). Microvascular systems were employed to develop organ-on-a-chip platforms (Huh et al., 2010, Jusoh et al., 2015, Lee et al., 2007, Shin et al., 2014, Ye et al., 2013), study cancer cell dissemination through intra- and extravasation (Bersini et al., 2014a, Bersini et al., 2014b, Jeon et al., 2015, Song et al., 2009, Zervantonakis et al., 2012), assess toxicity and screen for efficacy during drug development (Kim et al., 2014, Lee et al., 2013, Zhang, Peticone, 2013), develop vascular disease models (Tsai et al., 2012) and analyze mass transfer and diffusion phenomena within tissue constructs (Baker et al., 2013, Mu et al., 2013).
Despite major advances for various models (Cuchiara et al., 2012, Wang et al., 2014, Yeon et al., 2012), there are still challenges in optimizing materials and facilitating accurate microfabrication techniques for creating complex systems. These models often suffer from the lack of a detailed analysis of the biomolecular kinetics and cellular dynamics underlying vascularization, such as the interaction between endothelial cells and 3D matrices, or the biochemical/biophysical stimuli promoting vascular network remodeling. In particular, while EC contact and paracrine secretion influence the phenotype of interacting stromal cells, e.g., mesenchymal stem cells (MSCs) promoting differentiation into mural-like cells (Goerke et al., 2012), different stromal cell types, such as pericyte subpopulations, contribute to the stabilization of the microvascular network (Armulik et al., 2005, Armulik et al., 2011, Au et al., 2008). In fact, both biochemical and biophysical cues affect microvascular network development, such as EC migration and capillary sprouting (Carmeliet and Jain, 2011, Chien et al., 1998, Risau, 1997). Understanding the possibilities offered by current designs and microfabrication strategies would allow us to exploit the advantages offered by these models to effectively analyze cell-cell and cell-matrix interactions.
1.1. Scope and originality of the review
The generation of vascularized tissues requires an interdisciplinary approach combining materials science, biology and microfabrication. Several literature reviews have largely focused on identifying critical aspects for successful development of vascularized models. Many of them were found to be mainly focused on the fundamental principles of engineering, microfabrication techniques (Auger et al., 2013) and microfluidic technologies (Inamdar and Borenstein, 2011, Wong et al., 2012), while other reviews highlighted the pivotal role of cell sources (Baldwin et al., 2014), scaffolds and pro-angiogenic factors (Bae et al., 2012, Kaully et al., 2009, Palumbo et al., 2014, Park and Gerecht, 2014) with low emphasis on microfabrication strategies. This review attempts to critically summarize and connect engineering principles and biological considerations as well as highlight how design of the system architecture and selection of the most suitable microfabrication technique are the first steps for analyzing biological phenomena occurring in a specific environment. Additionally, the endothelium is a dynamic tissue with properties modulated by a complex network of biological stimuli coming from the surrounding organ-specific microenvironment that affect both the physical structure of the endothelium and endothelial gene expression. Understanding how biochemical, biophysical and biomechanical cues contribute to the development and stabilization of vascular networks would help researchers to identify the most suitable system architecture and foster the development of new approaches, as well as future breakthroughs in the field.
2. Structural, Mechanical and Microfabrication Considerations for Generating Microvascular Systems
Unique combinations of the structural and mechanical properties of materials, microfabrication technique, cell type, growth factors and nutrients can promote the generation of microvascular tissue constructs. The focus of this section is discussing the materials and microfabrication approaches to generate microvascular systems. Other review papers are recommended for detailed analyses of cell sources and biochemical modification of scaffolds (Bae, Puranik, 2012, Baldwin, Antille, 2014).
2.1.Microfabrication
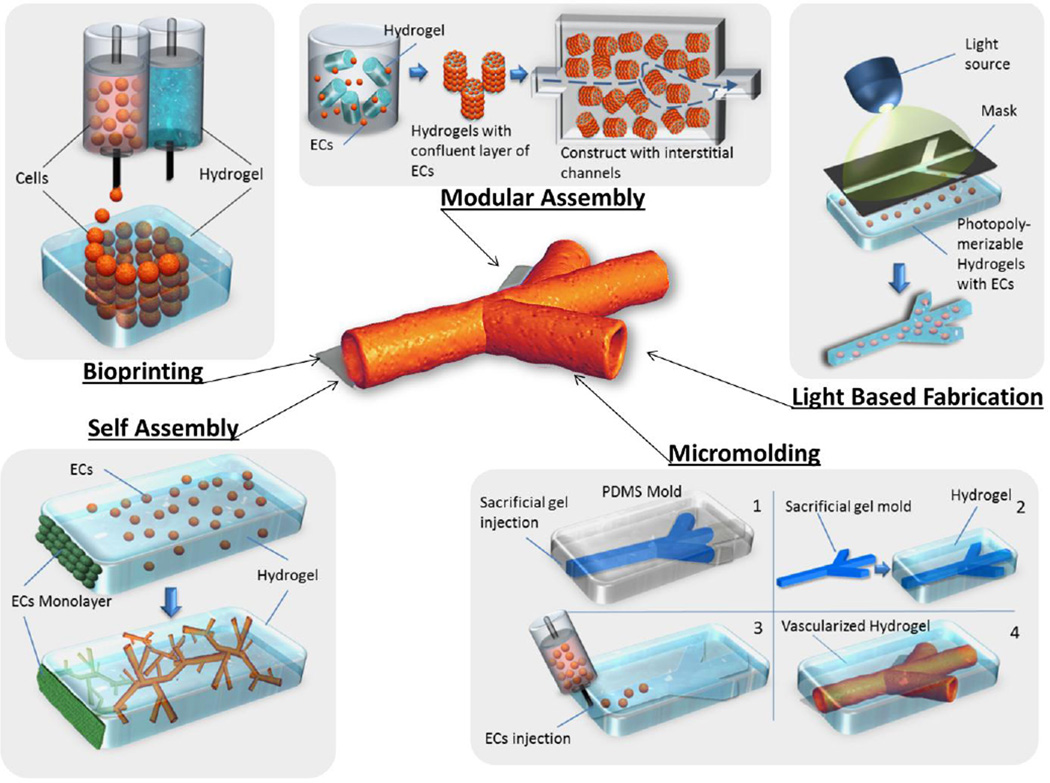
Microfabrication techniques can be classified into different categories. The most common classification of microfabrication methods differentiates between vasculogenesis/angiogenesis-based techniques and the design of bioartificial microvascular networks through bioprinting, light-based microfabrication, micromolding and modular assembly (Fig. 1). Microvascular networks can be generated within 3D macroscale hydrogels or embedded within microfluidic devices, which are generally made of poly(dimethyl-siloxane) (PDMS).
Fig. 1.
Schematic representation of current microfabrication approaches for the generation of microvascular networks.
2.1.1. Self-assembly through vasculogenesis/angiogenesis-based techniques
Vasculogenesis/angiogenesis-based approaches rely on naturally occurring processes within the body. Vasculogenesis refers to the initial formation of a vascular plexus during embryo development in which endothelial precursor cells organize themselves in vascular islands that gradually fuse together and differentiate into mature ECs. Next, ECs migrate and begin developing into endothelial tubes, which constitute the basic units for generating a complete vascular system. Finally, pericytes, smooth muscle cells and other mural-like cells are recruited by nascent vessels to promote stabilization and maturation into larger arteries and veins (Jain, 2003). Remarkably, vasculogenesis can also occur within adult tissues as a result of severe damage or tumor development. Whereas vasculogenesis is defined as de novo morphogenesis of a vascular plexus, angiogenesis comprises two different mechanisms: endothelial sprouting and intussusceptive microvascular growth (which is remodeling of existing vascular networks by forming a new vessel from an existing one). This process can be driven by growth factors, e.g., vascular endothelial growth factor (VEGF) gradients, hypoxic conditions, the presence of tumors or transendothelial flow (Carmeliet and Jain, 2011). Vasculogenesis/angiogenesis-based approaches were successfully employed within macroscale hydrogels and microfluidic devices (Chen, Lin, 2012, Jeon, Bersini, 2014, Kim et al., 2013, Song et al., 2012). For example, microfluidic models exploited paracrine and juxtacrine signaling between ECs and MSCs (Jeon, Bersini, 2014) or ECs and fibroblasts to generate vascular networks (Fig. 2A and 2C) (Kim, Lee, 2013, Moya et al., 2013, Whisler et al., 2013, Yeon, Ryu, 2012). EC+MSC co-cultures were employed to vascularize cell spheroids for therapeutic neovascularization (Fig. 2B) (Chen et al., 2013, Lee et al., 2011). Other studies embedded EC coated microcarrier beads within 3D fibrin gels (Fig. 2D) (Carrion et al., 2013, Ghajar et al., 2008) to demonstrate that cellular bodies with homogeneously dispersed cells or tissue constructs characterized by a core of MSCs and an external shell of ECs could be generated. Finally, "cell sheet tissue engineering" represents a peculiar type of self-assembly technique based on layer-by-layer stacking of intact cell monolayers with embedded ECs, which then organize into microvascular networks (Sekine et al., 2013, Shimizu et al., 2003) (Tanaka et al., 2014).
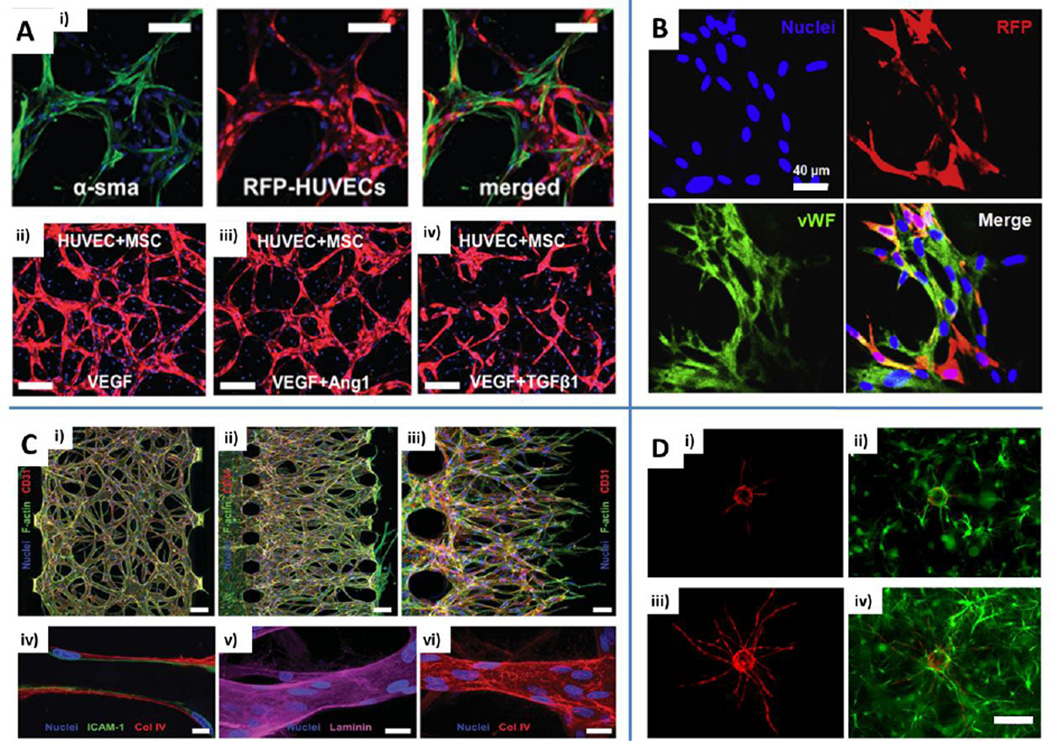
Fig. 2.
Engineering microvascular networks through vasculogenesis and angiogenesis-based techniques. A) Human umbilical vein endothelial cell (HUVEC)-human bone marrow-derived mesenchymal stem cell (MSC) co-culture demonstrating direct contact is necessary to promote MSC phenotypic transition toward mural-like cells expressing alpha smooth muscle actin (α-SMA). RFP: red fluorescent protein. Scale bars: 100 µm (i). Different microvascular network architectures obtained with the addition of vascular endothelial growth factor (VEGF), angiopoietin (Ang)-1 or transforming growth factor (TGF)-β1. Scale bars: 200 µm (ii, iii, iv). Blue: nuclei (DAPI). Adapted from Jeon et al. (2014) by permission of the Royal Society of Chemistry. B) Immunofluorescent staining of microvascular networks formed by 3D aggregates of HUVECs and RFP cord blood derived MSCs. HUVECs stained with von Willebrand factor (vWF, green). Scale bar: 40 µm. Reproduced from Chen et al. (2013) by permission of Elsevier. C) Engineered microvascular networks by means of vasculogenesis (i) or angiogenesis-based (ii) approach. Red: CD31. Green: F-actin. Tumor necrosis factor (TNF)-α stimulated vessels stained for intercellular cell adhesion molecule (ICAM)-1 (iv, green, scale bar: 10 µm). Endothelial cells secrete basic components of the basement membrane such as laminin (v, purple, scale bar: 20 µm) and collagen type IV (vi, red, scale bar: 20 µm). Blue: nuclei (Hoechst 33342). Reproduced from Kim et al. (2013) by permission of the Royal Society of Chemistry. D) HUVECs coated on microcarrier beads (i and iii, red) were cultured in fibrin gels containing bone marrow MSCs (ii and iv, green) and the sprouting process was imaged at day 3 (i and ii) and day 7 (iii and iv). Reproduced from Carrion et al. (2013) by permission of Elsevier.
2.1.1.1. Advantages and drawbacks
Although the generation of self-organized and stable capillaries is generally a slow process depending on multiple biological mechanisms, vasculogenesis/angiogenesis-based systems have shown to be the best models for mimicking the angiogenic steps that occur under physiological and/or pathological conditions as well as the morphogenesis of in vivo microvasculature. These steps include intracellular vacuole generation and fusion, development of complete lumens, branching and pruning, and recruitment of supportive mural cells. For this reason, other techniques (including bioprinting and micromolding) are often employed to quickly develop perfusable microvascular networks, which have simplified structures compared to vasculogenesis/angiogenesis approaches. Overall, vasculogenesis/angiogenesis-based methods appear to be the optimal strategy for analyzing vascular cell-matrix interactions, both during network development and homeostasis.
2.1.2. Bioprinting
Bioprinting has been employed by several research groups to develop vascularized tissue constructs or build whole organ-like structures. It is defined as the process of patterning cell suspensions embedded within a biocompatible material, such as hydrogels, i.e., bio-ink, with a hierarchically defined 3D spatial organization (Auger, Gibot, 2013). Two different bioprinting methods can be applied: direct contact and contactless bioprinting. The first approach implies a direct contact between the substrate and the bio-ink dispenser (Jakab et al., 2004, Norotte et al., 2009, Smith et al., 2004). Wu et al. developed 3D vascular networks through omnidirectional printing (Fig. 3A). First, these researchers printed fugitive ink filaments within a photocrosslinkable gel and filled the voids left by the nozzle translation with photocrosslinked gel and fluid filler. Finally, they liquefied and removed the fugitive ink by applying a low vacuum (Wu et al., 2011). Contactless bioprinting is based on the transfer of bio-inks in the form of bio-droplets onto a substrate without any contact with the dispenser (Phillippi et al., 2008, Xu et al., 2005). Cui et al. used fibrin and ECs to print 3D scaffolds through the use of modified inkjet printers and demonstrated the ability to generate aligned tubular structures (Cui and Boland, 2009). Finally, a unique type of bioprinting can be achieved with the digital mask or maskless projection printing, which employs digital micro-mirror devices to photopolymerize a pre-hydrogel solution (Fozdar et al., 2011).
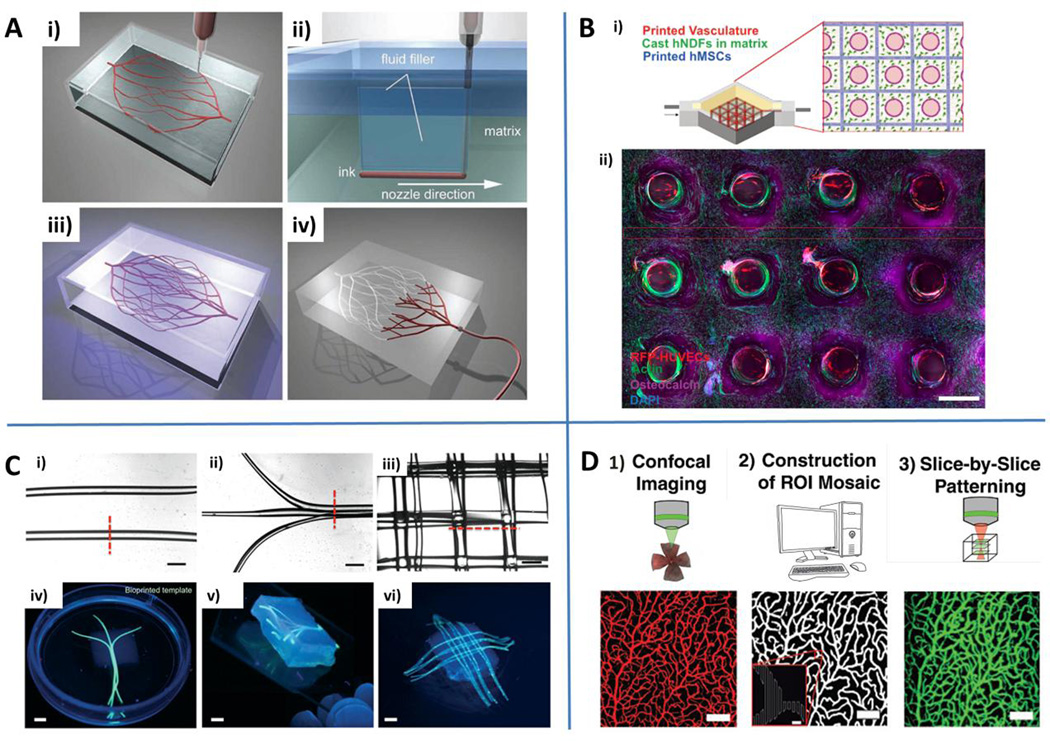
Fig. 3.
Engineering microvascular networks through bioprinting and light-based approaches. A) Omnidirectional bioprinting of 3D vascular networks. Deposition of a fugitive ink (i), filling of gaps generated by the nozzle translation during bioprinting (ii), photopolymerization (iii) and liquefaction/removal of the template to generate microvascular channels (iv). Reproduced from Wu et al. (2011) by permission of John Wiley and Sons. B) Schematic of a bioprinted multicellular tissue (i) and confocal image through a cross-section of 1 cm thick vascularized tissue cultured under perfusion for 30 days (ii). Red: HUVECs. Green: F-actin. Purple: osteocalcin. Blue: nuclei (DAPI). Reproduced by permission from Kolesky DB, Homan KA, Skylar-Scott MA and Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016, epub ahead of print. C) Gelatin methacryloyl (GelMA) hydrogels embedding bioprinted vascular networks. Linear parallel (i), planar bifurcating (ii) or 3D lattice architecture (iii) of agarose template fibers. Scale bars: 1 mm. Photographs of bioprinted templates: parallel bifurcating (iv), 3D branching template (v) and 3D lattice architecture (vi). Scale bars: 3 mm. Reproduced from Bertassoni et al. (2014) by permission of the Royal Society of Chemistry. D) Light-based vascular network patterning. Synthetic description of the main steps of the process: confocal imaging, reconstruction of cross-sectional structures, and slice-by-slice light patterning of vascular structures. Scale bar: 100 µm (5 µm for inset). Reproduced from Culver et al. (2012) by permission of John Wiley and Sons.
Miller et al. developed optically transparent and mechanically stable sucrose-glucose-dextran carbohydrate glasses as sacrificial templates to generate 3D complex microchannel networks within matrices made of PEG, agarose, fibrin or other materials. Glass fibers reinforced by incorporating dextrans were drawn with a 3D printer to create glass lattices with complex, multiscale architectures (Miller, Stevens, 2012). Microchannels within bulk hydrogels were also developed through bioprinted agarose fiber templates embedded within photocrosslinkable systems (Bertassoni, Cecconi, 2014). Agarose fibers could be easily removed by applying low vacuum because dissolution of their network template could generate cytotoxic by-products, which can alter the matrix composition or cause osmolarity damage in the cells. This approach was made possible through low adhesion between the template and surrounding gel (gelatin methacryloyl (GelMA), PEG diacrylate, PEG dimethacrylate, star poly(ethylene glycol-co-lactide) acrylate) (Fig. 3C). Recently, 1-cm-thick vascularized bone tissue constructs containing perfusable endothelialized channels were successfully bioprinted and maintained in culture for up to 6 weeks, which raised the barin the field of vascular bioprinting (Kolesky et al., 2016).
2.1.2.1. Advantages and drawbacks
Although the term “bioprinting” is defined as depositing cells, ECM and other biologically relevant materials in defined patterns to build tissue constructs de novo, the broadest definition of bioprinting includes studies that employed techniques to print acellular sacrificial templates made of fugitive inks or microfibers (Bertassoni, Cecconi, 2014, Wu, DeConinck, 2011). The use of microfibers represents a reliable alternative compared to dissolving sacrificial templates to easily generate hollow microvascular channel networks within a bulk matrix. However, for complex and highly branched structures, several limitations remain unless a modular approach can be employed to combine simple building blocks. Currently, bioprinting has a lower resolution compared to microfluidic techniques such as micromolding or photolithography. Nonetheless, bioprinting requires high initial costs to purchase necessary equipment and train operators to properly set and optimize the technical parameters. Additionally, the high speed of cell deposition during ejection and/or impact of fluid drops with the substrate can lead to cell damage and low cell survival.
2.1.3. Light-based microfabrication
Light-based techniques include the well-known approaches photolithography (Nichol, Koshy, 2010) and two-photon laser lithography (Culver et al., 2012). Photolithography is based on pre-polymer solutions, which are exposed to UV light through custom-built photomasks. Upon exposure to UV light, these prepolymers undergo crosslinking while the unexposed area can be easily washed away. This technique is based on the reaction between a photoinitiator and a photosensitive pre-polymer solution, which generally contains acrylate or methacrylate groups (Nikkhah et al., 2012). Two-photon laser lithography was successfully employed by Culver and co-authors (Culver, Hoffmann, 2012) using a confocal microscopy to capture 3D images of the vasculature of native tissues such as the retina, cerebral cortex and heart. Based on a collection of cross-sections of the vasculature segments, they micropatterned a photocrosslinkable PEG hydrogel to form blood vessel-like structures for organized vasculatures using physical cues to guide and align embedded ECs into in vivo-like structures (Fig. 3D) (Culver, Hoffmann, 2012).
2.1.3.1. Advantages and drawbacks
Light-based microfabrication has great promise due to the ability to reproduce complex microvasculature with high fidelity. The main limitation is that it requires expensive two photon laser microscopy or photolithography facilities, which are not common in biological laboratories. Another drawback is that the learning curve to design custom-built photomasks and use these technologies could be steep for a beginning user.
2.1.4. Micromolding
Micromolding can be considered as a basic method to develop micropatterned hydrogel slabs for layer-by-layer assembly or to prepare PDMS features within microfluidic devices. It relies on a master mold, which is often created on a silicon wafer or other mounting supports, that is used to prepare a transfer mold by casting and curing a polymer solution. Finally, the transfer mold can be bonded to a flat substrate or used to print a micropattern onto a hydrogel (Price et al., 2008, Shin, Han, 2012) with a resolution limit based on nanometer-scale electron beam-induced deposition (Rogers and Nuzzo, 2005).
Golden and Tien encapsulated micromolded gelatin within a second hydrogel, such as collagen type I or fibrin gel (Golden and Tien, 2007). The gelatin was used as a template channel, and it was then removed by heating and flushing it away to generate a microchannel network within a bulk matrix. Interestingly, these researchers demonstrated the possibility to create multiplanar networks by stacking several hydrogel layers and embedded fibroblasts within abulk gel or microvascular ECs on the inner surface of the microchannel network (Golden and Tien, 2007). More recently, another study had similar results that were obtained using sodium alginate to generate mono or multilayered structures of endothelialized microchannels. This method was employed to develop networks of different sizes and shapes, and it demonstrated the adaptive responses of seeded ECs to different levels of shear stress (Wang, Jin, 2014).
In a different study, instead of generating sacrificial hydrogel or glass templates, an interesting micromolding approach was proposed combining self-assembled monolayer (SAM)-based EC deposition and photocrosslinking to rapidly engineer micrometric tubular structures within a matrix (Inaba et al., 2009). The researchers chemically adsorbed a thiol-based oligopeptide sequence on gold rods and seeded ECs on the rod surface. The endothelialized gold rod was then embedded in a photocrosslinked GelMA hydrogel, and the endothelial monolayer was transferred from the rod onto the gel by electrochemical cleavage of the oligopeptide. Subsequently, the rod was removed and the macroscale hydrogel was connected to a syringe pump to facilitate a steady flow (Sadr et al., 2011). An interesting application of this technique involves transferring double layers of cells, such as an inner layer of ECs and an outer layer of perivascular cells, thus creating a relevant model for the study of EC-mural cell interactions. An alternative strategy to using sacrificial templates or disposable polymeric fibers is using syringe needles. The cell-laden hydrogel is polymerized around the needle, which is subsequently removed to leave perfusable microchannels that can be endothelialized to generate vascularized tissue constructs (Nichol, Koshy, 2010). These systems were successfully employed to analyze leukocyte adhesion (Chrobak et al., 2006) and tumor cell-EC interactions under flow conditions (Buchanan et al., 2014).
Baranski et al. developed a microtissue molding technique based on the generation of aligned structures with embedded ECs and 10T1/2 cells within collagen cord templates (Fig. 4A). Cells self-assembled in a few hours and contracted the encasing collagen gel significantly. The cords were subsequently removed from the PDMS mold, embedded within a fibrin gel and implanted within the peritoneal cavity of nude mice (Baranski et al., 2013). Similar to this approach, Leong et al. developed the interfacial polyelectrolyte complexation (IPC) fiber assembly technique to create aligned vessels within multicellular tissue constructs (Fig. 4B). Polyionic droplets containing cells were placed on a substrate and IPC fibers were drawn in parallel and fused by rotating the template around a single point. The resulting secondary structures were based on a central fiber that was surrounded by external fibers with different embedded cell types (Leong et al., 2013, Wan et al., 2004). This system has promising applications for the study of vascular cell interactions and potentially can be used to analyze the effect of different matrices in microvessel generation. Finally, there is a micromolding technique that is based on the stacking and bonding of two adjacent layers. A network of channels was obtained by joining the two layers by applying pressure within a cage or by chemical sealing. Micropatterned calcium alginate hydrogels containing living cells were separately micromolded and then bonded together to create a microfluidic scaffold for controlling temporospatial distribution of biomolecules (Choi et al., 2007). Mu et al. reported an additional example of layer-by-layer microfabrication in which they combined fibrillogenesis and liquid molding to generate 3D microvascular networks within a collagen/alginate gel (Fig. 4C). Compared to conventional techniques employed to bond hydrogel layers, such as heating, ions and chaotropes (Cabodi et al., 2005, Ling et al., 2007, Price, Chu, 2008), they harnessed the assembly of collagen molecules into insoluble fibers (i.e., fibrillogenesis), at the interface between a gelled and a liquid hydrogel to tightly bond the two hydrogel layers. In addition, water was employed as a liquid mold to maintain the geometry of the gelled hydrogel layer until the hydrogel solution was fully polymerized (Mu, Zheng, 2013).
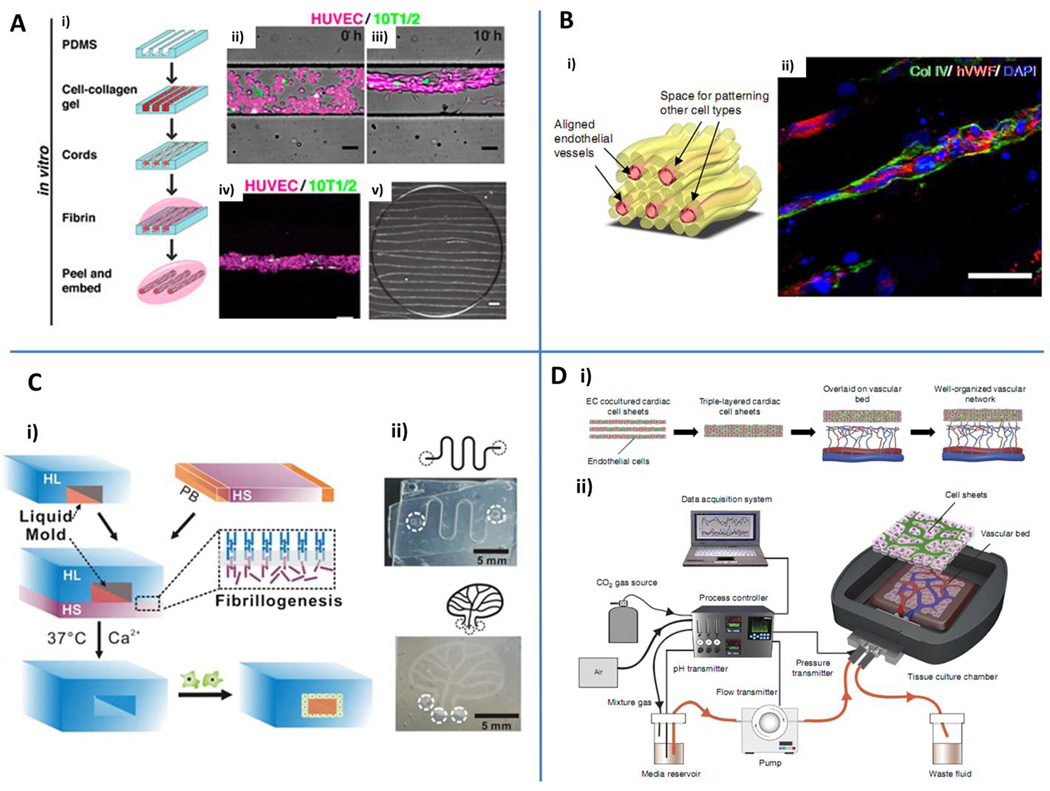
Fig. 4.
Engineering microvascular networks through micromolding and layer-by-layer assembly. A) Schematic representation of the cord generation process (i). Cord formation at 0 h (ii) and 10 h (iii) within poly-dimethyl-siloxane (PDMS) microchannels (HUVECs red, 10T1/2 green). Maximum intensity z-projection of a generated cord (iv). Cord constructs after removal from the PDMS substrate and embedded in fibrin gel (v). Scale bars: 50 µm (ii, iii, iv); 500 µm (v). Reproduced by permission from Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A. 2013;110:7586-91 (Baranski et al. (2013)). B) Pre-vascularized hydrogels obtained through assembly of adjacent fibers. Schematic of a typical tissue construct with endothelial cell embedding fibers surrounded by additional supporting, organ-specific cell types (i). Confocal microscopy of human vWB+ human microvascular endothelial cell (hMVEC, red) aligned vessels characterized by collagen type IV containing basement membrane (green). Scale bar: 50 µm (ii). Blue: nuclei (DAPI). Reproduced from Leong et al. (2013) by permission of the Nature Publishing Group. C) Fibrillogenesis and liquid molding are employed to generate hydrogel embedded microchannels (i). Different circuit shapes can be designed, generating serpentine or tree-like vascular networks (ii). HL: hydrogel layer. HS: hydrogel solution. PB: PDMS barrier. Reproduced from Mu et al. (2013) by permission of the Royal Society of Chemistry. D) Stacked vascularized cardiac cell sheets overlaid on a vascular bed (i) and perfused by means of a custom made bioreactor (ii). Reproduced from Sekine et al. (2013) by permission of the Nature Publishing Group.
2.1.4.1. Advantages and drawbacks
Micromolding is the simplest and most widely used strategy to create vessels with a predefined size and shape due to the lower costs compared to light-based or bioprinting techniques. Nevertheless, it does not result in the generation of physiological-like and hierarchycal microvascular networks unless an hybrid approach combining micromolding and vasculogenesis/angiogenesis can be used, as recently demonstrated by Arrigoni et al. (Arrigoni et al., 2016).
2.1.5. Modular assembly
An interesting approach to develop vascularized tissue constructs is the modular assembly of small basic units to create complex systems. For example, sub-millimeter-sized collagen cylindrical building blocks were covered with a monolayer of ECs, assembled within larger tubes and perfused with media or blood to form interconnected channels in the interstitial spaces between the building blocks (McGuigan and Sefton, 2006). Alternatively, cells can be embedded within shape-controlled microgels for developing multicomponent constructs (Du et al., 2008).
2.1.5.1. Advantages and drawbacks
Modular assembly is a simple and effective approach to creating complex networks of endothelialized channels within the interstitial space of self-assembled building blocks. This technique could be significantly improved if the building blocks were filled with EC. In fact, the combination of vasculogenesis/angiogenesis and modular assembly would allow researchers to generate main channels in the interstitial space among building blocks, and these channels have been shown to rapidly anastomose with self-assembled microvascular networks within the hydrogel. The main disadvantage of this approach is that ECs generate an endothelium that does not completely cover the interstitial space and forms a leaky vascular network.
2.2. Biomaterials
Collagen or Matrigel® hydrogels, due to their unique biocompatibility and range of constituents, provide suitable microenvironments for tissue growth and are widely used in the field (Goerke, Plaha, 2012, Lee et al., 2009b). However, these gels are characterized by limited mechanical stability and suboptimal durability, which generally compromise in vivo implantation (Annabi et al., 2013). Antoine et al. provided a detailed discussion on fabrication and characterization of collagen type I hydrogels (Antoine et al., 2014). Collagen gels alone or in combination with other materials, such as fibrin gels, were successfully employed within microfluidic devices to generate microvascularized in vitro models (Jeon, Bersini, 2014, Kim, Lee, 2013). In addition, collagen and fibrin gels were used to load cells within porous poly(D,L-lactic-co-glyclic acid) (PLGA) scaffolds and obtain vascularized 3D constructs with superior mechanical properties compared to conventional hydrogels but the quality of the microvascular network was lower (Zhao et al., 2014). Compared to natural polymers, synthetic hydrogels, such as PEG, have superior mechanical properties that are counterbalanced by limited cellular adhesion and low degradation rate, which impair cell organization and movement within the gel (Zhu, 2010). Interestingly, arginine-glycine-aspartic acid (RGD) sequences and matrix metalloprotease (MMP)-sensitive degradation motifs can significantly improve cell adhesion and spreading as well as remodeling of the synthetic hydrogel matrix (Benton et al., 2009, Cuchiara, Gould, 2012). A promising alternative is gelatin, which is obtained from thermal denaturation of collagen. Additionally, the poor mechanical properties of its original form can be easily adjusted by adding methacryloyl groups to the reactive side groups of the polymer and making it suitable for photopolymerization under exposure to UV light (Nichol, Koshy, 2010). Nichol et al. seeded ECs on substrates with adjustable mechanical properties that were created with various gelatin concentrations and methacryloyl modification. These substrates generated compressive modulus values up to 30 kPa in the presence of 15% w/v GelMA and an 80% methacryloyl modification with significantly lower cell viability within the 3D constructs compared to lower GelMA concentrations (Nichol, Koshy, 2010). The same material composition was then employed by Chen et al. to develop functional microvascular networks stabilized by supporting perivascular cells (Chen, Lin, 2012). Overall, GelMA hydrogels showed significantly higher mechanical properties compared to pure collagen (2 mg/ml, 0.1 kPa) or fibroin microfiber hydrogels (0.2 kPa) (de Moraes et al., 2012). Another promising photocrosslinkable material, methacryloyl-modified tropoelastin (MeTro), was recently developed by Annabi et al. for micropatterning. They tested different concentrations of the polymer from 5% to 15% w/v and demonstrated superior cell adhesion compared to GelMA and a considerably increase in mechanical properties, such as extensibility up to 400% and an elastic modulus in the range of 2.8 to 14.8 kPa (Annabi, Tsang, 2013). Similarly, other groups developed synthetic elastin by mixing recombinant tropoelastin and bis(sulfosuccinimidyl) suberate to obtain a Young's modulus value of up to 280 kPa and extensibility up to 370% (Mithieux et al., 2004). In addition, Hanjaya-Putra et al. have modified 5% w/v hyaluronic acid, which is known to promote angiogenesis (Hanjaya-Putra et al., 2011), by adding methacrylate groups and used it to encapsulate cells within hydrogel and create cell docking templates for cell microarrays (Khademhosseini et al., 2006). Chitosan/β-glycerophosphate hydrogels were employed to deliver MSCs within decellularized arterial scaffolds (Sheridan et al., 2014), whereas gellan gum-hyaluronic acid spongy-like hydrogels with embedded human adipose stem cells and microvascular ECs were developed to promote neovascularization and wound closure (Cerqueira et al., 2014). Using a combination of two natural hydrogels, fibrinogen+thrombin and gelatin+transglutaminase, the quattroGel was developed by Aberle et al., and its structural and mechanical properties were optimized to promote cell adhesion even though the proliferation rates of several cell types, including ECs, were generally unaffected. Both neurons and ECs failed to extend neurites or create vascular tubes, respectively (Aberle et al., 2014). That study provided a clear example of the possibilities offered by combining different materials to achieve properties that can be adjusted for specific applications. In particular, this approach could launch new avenues for optimizing new classes of materials suitable for the generation of 3D functional vascularized tissue constructs.
Different biomaterials could heavily impact the morphogenesis of capillary networks. The detailed analysis of the relationship between structural/mechanical properties of the matrix and cellular components would greatly advance our knowledge of vascular biology and lead to novel pro-angiogenic materials. The high-throughput nature of some current systems could play a critical role in this process.
2.3. Overall considerations
This section has provided a concise and simple review of the technical instruments engineers, biomaterial scientists and chemists have been continuously designing and optimizing to better mimic the structure and function of living tissues for both in vitro modeling and regenerative medicine applications. We would like to highlight that for most of the models discussed above, a specific combination of biomaterial and microfabrication techniques were employed. Only a few investigators characterized the biomaterial mechanical properties (Bertassoni, Cecconi, 2014, Nichol, Koshy, 2010) or tested different matrix compositions (Miller, Stevens, 2012) to demonstrate the versatility of their systems. Carrion et al. analyzed a signaling mechanism between ECs and stromal cells during vascular network development (Carrion, Kong, 2013), whereas Chen et al. showed matrix deposition within spherical cellular aggregates (Chen, Wei, 2013). Nonetheless, only a limited number of these systems were actually employed to investigate vascular cell-matrix interactions as well as network morphogenesis and remodeling.
3. Biological Considerations for Generating Microvascular Systems
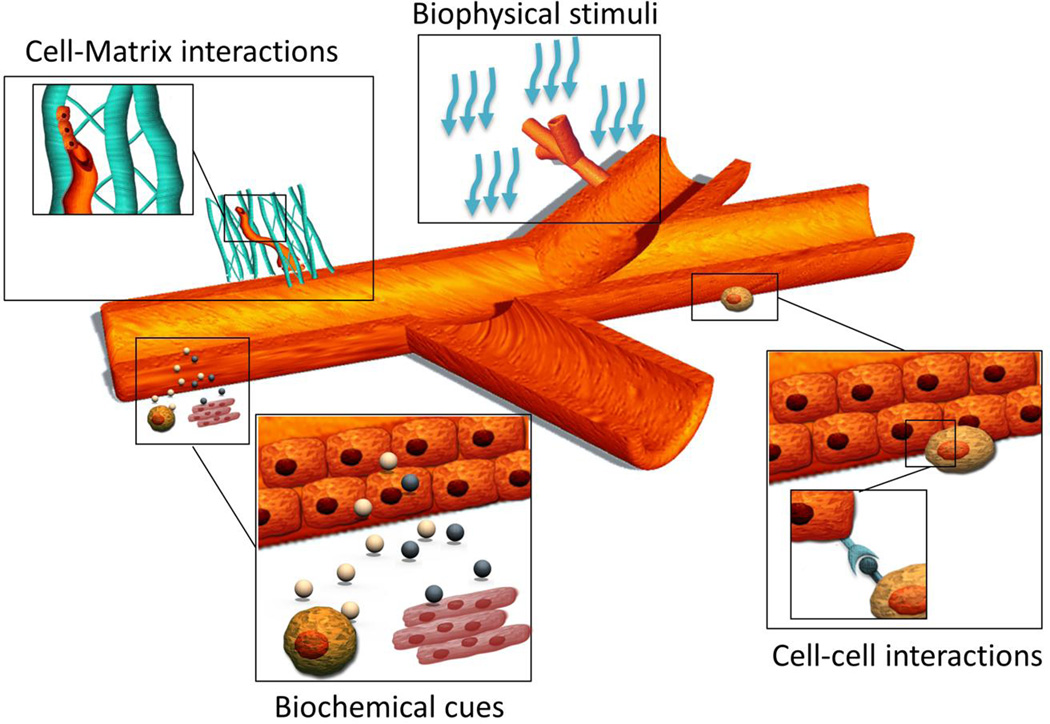
A broad range of biological stimuli affect the development of microvascular networks, including cell-cell interactions with supporting mural cells, cell-matrix interactions, biochemical cues from organ-specific microenvironments and mural cells, and biophysical stimuli (Fig. 5).
Fig. 5.
Biological cues driving microvascular network morphogenesis, stabilization and remodeling. These ones include biochemical stimuli from organ-specific microenvironments, biophysical stimuli, cell-matrix interactions and cell-cell interactions with supporting mural cells.
3.1. EC-mural cell interactions
The maturation and stabilization of nascent blood vessels occurs due to the mutual interaction between ECs and supporting mural cells, such as smooth muscle cells, pericytes and fibroblasts (Jain, 2003). Several molecular pathways are involved in the recruitment and differentiation of mural cells, including platelet derived growth factor (PDGF)-B, sphingosine 1-phosphate (S1P), angiopoietin-1(Ang-1), and transforming growth factor (TGF)-β. PDGF-B is secreted by ECs in response to VEGF and promotes mural cell proliferation and migration during microvasculature maturation (Hellstrom et al., 2001). Signaling molecules involved with S1P secreted by ECs and mural cells play a critical role in both mural cell recruitment (Armulik, Abramsson, 2005) and EC interaction with surrounding mural cells through the expression of N-cadherins (Paik et al., 2004). Mural cells are the main source of Ang-1, which reduces vessel permeability and promotes vessel stabilization through interaction with the EC Tie-2 receptor (Thurston et al., 1999, Yancopoulos et al., 2000). The role of angiopoietin-2 (Ang-2) remains unclear because it acts as a promoter of vascular sprouting in the presence of VEGF, yet it functions as an antagonist of Ang-1 and induces vessel regression in the absence of VEGF (Jain, 2003). Jeon et al. used a 3D microfluidic model that demonstrated direct contact between ECs and bone marrow derived MSCs was critical for promoting MSC differentiation into a mural-like phenotype. The addition of Ang-1 significantly increased the expression of the mural marker α-SMA and induced partial pericyte differentiation (Jeon, Bersini, 2014). In another study, Holnthoner et al. demonstrated that adipose derived stem cells (ASCs) can develop pericyte characteristics when the cells are cultured with ECs, and ASCs directly contribute to microvascular network formation and stabilization (Holnthoner et al., 2015, Rohringer et al., 2014). The role of TGF-β seems less clear because it had shown pro- or anti-angiogenic properties depending on the specific microenvironment and concentration. In addition, TGF-β is involved in several vascular processes, including mesenchymal cell differentiation towards a mural cell phenotype (Gohongi et al., 1999, Pepper, 1997). It was also shown that heparin-binding epidermal growth factor (HB-EGF) produced by EC was able to recruit smooth muscle cells via ErbB1 and ErbB2 receptors (Iivanainen et al., 2003). Additionally, Ang-1 was reported to induce HB-EGF secretion by ECs and create a positive loop to promote pericyte migration (Stratman et al., 2010). Other molecular pathways connected to pericyte recruitment include stromal-derived factor 1-a (SDF-1α)/C-X-C chemokine receptor type 4 (CXCR4) and sonic hedgehog (Shh)/Patched (Ptc) (Armulik, Genove, 2011), whereas Notch 3 was associated with the regulation of mural cell recruitment through a mechanism involving Ang-2 (Jin et al., 2008, Liu et al., 2010). It is interesting to note that knocking down ephrin-B2 in mural cells resulted in poor association between ECs and smooth muscle cells/pericytes(Foo et al., 2006). Different groups designed in vitro models to investigate mesenchymal cell differentiation into mural cells when co-cultured with ECs (Goerke, Plaha, 2012) or pericyte recruitment by means of EC secreted factors (Stratman et al., 2009). These models were used to develop a better understanding of how pericytes in the vessel basement membrane facilitate and integrate cell-cell communications through adherens, tight and gap junctions (Armulik, Abramsson, 2005), and how they contribute to vessel stabilization and endothelial basement membrane deposition (Stratman, Malotte, 2009). Pericytes covering capillaries, pre-capillary arterioles and post-capillary venules are characterized by peculiar morphologies and different ratios of ECs according to the specific tissues where they reside (Shepro and Morel, 1993, Sims, 1986). Furthermore, pericytes were shown to differentiate into smooth muscle cells during vessel growth and remodeling (Nehls and Drenckhahn, 1993). Impairment of either ECs or pericytes led to damage of other cell types (Armulik, Abramsson, 2005), which demonstrated the critical interaction between these two cell populations for vascular homeostasis (Armulik, Abramsson, 2005, Jain, 2003).
3.1.1. Remarks
Different mural cell types can contribute to specific steps in vessel wall formation and remodeling, e.g., stabilization, branching and pruning through the secretion of cell-specific factors. Most importantly, the type of mural cell and endothelial-to-mural ratio modulate microvascular network features. As a result, the mural cell component should be carefully defined during the design of the system both in terms of biomaterial choice and microfabrication technique.
3.2. Cell-matrix interactions influencing microvascular network generation
3.2.1. Structural and mechanical properties of the matrix influence microvascular network development
Several studies demonstrated that ECs remodel the local microenvironment by generating bundles of ECM (2D assay) (Vernon, Lara, 1995) or producing sprouts between neighboring 3D spheroids (Korff and Augustin, 1999). Researchers have also suggested that traction forces generated by ECs lead to local matrix remodeling, which assists with cell migration and microvascular network generation (Kniazeva, Weidling, 2012, Korff and Augustin, 1999, Lee, Yeh, 2009a). McLeod et al. used suspended ECs within collagen gels to show a significant retraction over time coupled with alignment/gathering of collagen fibers between adjacent ECs (Fig. 6A) (McLeod et al., 2013).These fibers allowed the transmission of mechanical forces among neighboring cells by providing contact guidance for vascular network development. These experimental observations correlated with computational models that suggest extensive EC migration and network formation occur after matrix remodeling (Ma et al., 2013, Reinhardt et al., 2013, Utzinger et al., 2015). Finite element models were used to simulate the contraction of 3D gels with specific boundary conditions and demonstrated that cell alignment was a consequence of the anisotropic deformation of the matrix (Underwood et al., 2014).Overall, the experiments showed that microvascular network formation is related to microscopic and macroscopic changes within the collagen architecture. Force propagation, gathering of protein fibers and remodeling of the matrix are critical elements for microvascular network development, which explains why highly stiff materials or specific matrix microstructures do not allow interconnected microvasculatures to form. In general, increasing cell density promotes force generation and reduces cell-cell distances, which consequently contributes to improving the quality of the microvascular network (McLeod, Higgins, 2013). Sieminski et al. compared the behavior of different EC types, such as human umbilical vein ECs, human microvascular ECs and human outgrowth ECs, in the formation of microvascular networks within collagen gels. They found superior network length and increased gel contraction in presence of outgrowth ECs. The superior performance of outgrowth ECs was attributed to their ability to produce higher forces that affect the formation of tubular structures (Sieminski et al., 2005).
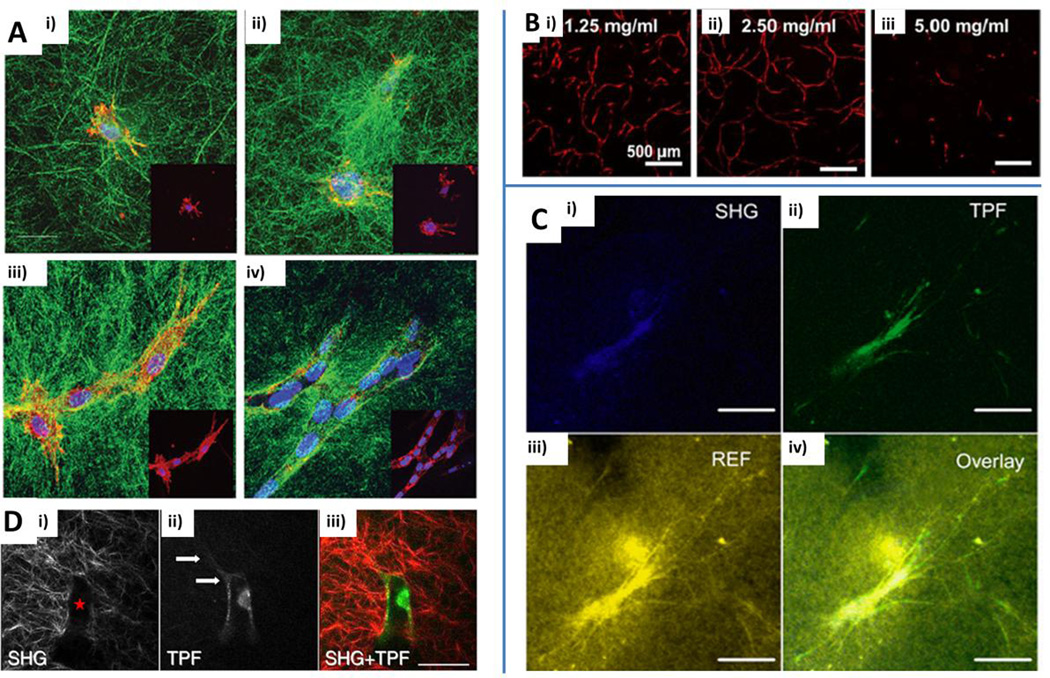
Fig. 6.
Biological considerations for the generation of microvascular networks: cell-matrix interactions. A) Time-course of collagen gel microscopic remodeling at 0 h (i), 4 h (ii), 24 h (iii), 48 h (iv) of endothelial cell culture. Collagen fibers: green. Red: endothelial cells. Blue: nuclei (DAPI). Reproduced from McLeod et al. (2013) by permission of the American Society of Mechanical Engineers (ASME). B) Effect of protein concentration (1.25 mg/ml (i), 2.5 mg/ml (ii) and 5 mg/ml (iii)) on vascular network development within collagen/fibrin hydrogels after 7 days of culture. Reproduced from Rao et al. (2012) by permission of Springer. C) Confocal image of a fibroblast within a fibrin gel matrix. Second harmonic generation (SHG) revealing intracellular collagen (i), two-photon fluorescence (TPF) (ii), reflectance (REF) image showing cell-matrix interaction (iii) and overlay of SGH, TPF and REF acquisitions (iv). Scale bars: 50 µm. Reproduced from Ghajar et al. (2008) by permission of Elsevier. D) Interaction between invading endothelial cells and collagen matrix: SHG showing a collagen-free space (i, red star), TPF highlighting an invading endothelial cell with extending processes (ii, white arrows) and combination of the two images (iii). Scale bars: 50 µm. Reproduced from Lee et al. (2009a) by permission of Elsevier.
Rao et al. also investigated the correlation between mechanical properties of the matrix and microvascular network generation by testing multiple EC/MSC ratios and matrix compositions. The authors analyzed the correlation between microvascular network features and structural/mechanical properties of the matrix in depth and demonstrated an inverse correlation between gel stiffness and network formation. Moreover, the total network length was decreased by increasing the total protein concentration (Fig. 6B) or changing the degree of crosslinking of the matrix. Overall, despite gel composition modifications, both total protein concentration and the degree of crosslinking altered the generation of microvascular networks due to changes in matrix stiffness (Rao et al., 2012). Remarkably, another study showed that the reduction in the formation of microvascular networks in presence of high matrix density was not due to increased stiffness of the matrix but due to reduced mass transport within the construct. EC coated microspheres were embedded within a fibrin gel and fibroblasts were seeded on top of the gel or within the matrix (Fig. 6C). The matrix density impaired the diffusion of pro-angiogenic factors secreted by seeded fibroblasts on top of the gel, whereas co-cultured fibroblasts with ECs inhibited the effect of the increased matrix density (Ghajar, Chen, 2008). These observations expand and complement the Rao et al. findings on the different key factors that affect the development of microvascular networks.
3.2.2. Molecular and cellular mechanisms of microvascular network development in a 3D matrix
Investigation of the molecular and cellular mechanisms involved in the generation of microvascular networks was performed by Hanjaya-Putra et al. Because ECs gradually break down ECM proteins using matrix metalloprotease (MMPs) (Kniazeva, Weidling, 2012, Sacharidou et al., 2010), the investigators created acrylated hyaluronic acid hydrogels with RGD sequences and peptide crosslinkers that can be enzymatically degraded by MMP-1 and MMP-2 to analyze vascular morphogenesis (Hanjaya-Putra, Bose, 2011). Another study showed vacuole and lumen formation were RGD-dose dependent (Montano et al., 2010). The results demonstrate the unique role of critical integrins, such as αvβ3 and α5β1, in lumen formation, while exhibiting no effects from integrin α2β1. This outcome is consistent with several other studies that demonstrated the importance of the former two integrins in lumen formation and matrix degradation/invasion while showing that α2β1 can act both as an activator of vascular processes as well as a stabilizer (Davis and Senger, 2005). Sieminski et al. reported that only blocking α2β1 and not αvβ3 impaired network formation in both outgrowth ECs and umbilical vein ECs (Sieminski, Hebbel, 2005). For the expression of proteases, MMP-1 and MMP-2 were both discovered in close proximity of the cell membrane during sprouting, whereas the formation of vascular guidance tunnels was driven by the action of membrane-type (MT)-MMPs, such as MT1-MMP. Cells expressed hyaluronidase (Hyal)-2 and Hyal-3 coupled with MT1-MMP, MMP-1 and MMP-2 in the first three days of culture. Following the stabilization of vascular networks, ECs switched their secretion to tissue inhibitors of metalloproteinases (TIMPs), such as TIMP-1, TIMP-2 and TIMP-3 (Hanjaya-Putra, Bose, 2011). Using second harmonic generation and two-photon excited fluorescence, endothelial sprouting morphogenesis was analyzed in 3D collagen matrices (Lee, Yeh, 2009a) (Fig. 6D). Increased collagen density was found among bifurcations points and around the perimeter of luminal structures, but not close to the sprouting tips. Blocking Rho-associated kinases, which are proteins related to alteration of cell morphology, cell migration and focal adhesion formation, proved that the generation of cell tensions increased collagen matrix alterations. Additionally, the investigators found that peripheral sprouts extended along fibrous collagen structures, which led them to speculate that angiogenic sprouts could exert tension and reorganize the matrix.
3.2.2.1. Remarks
The mechanical properties of the local microenvironment are known to influence cell function and differentiation (Engler, Sen, 2006). The dynamic interaction between the ECs and the ECM is often underestimated and can be critical during microvascular development because EC migration, elongation and assembly into tubular structures directly affect the matrix composition and mechanical properties (Sieminski, Hebbel, 2004). Overall, a delicate balance between RGD sequence density, integrin activity, protease expression/secretion and kinase activity is important to regulate microvascular morphogenesis (Zaman et al., 2006).
3.3. Effect of biochemical cues on the microvascular system
Several microfluidic platforms with embedded microvascular networks were employed to investigate biochemical factors affecting EC migration/activation and capillary sprouting. Researchers using microfluidic platforms generally rely on multiple gel microchambers to study cell-cell/cell-matrix interactions as well as cell migration under controlled biochemical conditions (Shin, Han, 2012). Chung et al. investigated basic capillary growth from a confluent monolayer lining a gel microchamber under multiple conditions. They demonstrated that different cancer cell types could either attract or have minimal influence on ECs. Additionally, they noticed that ECs tend to sprout towards a VEGF gradient. This 3D microfluidic model represented one of the first clear examples of miniaturized systems paving the way for a more systematic approach to study complex heterotypic interactions involving ECs (Chung et al., 2009). In fact, subsequent studies analyzed the process of angiogenesis from a pre-existing EC monolayer under combined gradients of chemoattractant (VEGF) and stabilizing angiopoietin-1 (Ang-1) and revealed 3D cooperative migration among tip and stalk cells (Shin et al., 2011). Collagen-patterned microfluidic vascular networks were developed to recapitulate vascular phenomena, including angiogenesis and thrombosis. The interaction between perivascular cells seeded within the collagen bulk and ECs was investigated and showed that pericytes can promote endothelial sprouting due to fibroblast growth factor (FGF) and VEGF secretion or induce retraction of the endothelium from the walls of the microchannels (Morgan et al., 2013, Zheng et al., 2012). Other studies addressed the role of specific molecules on the structural properties of the endothelium and quantified the effect of histamine, tumor necrosis factor-alpha (TNF-α) or anti-VEGF treatment on vascular permeability. Both TNF-α and histamine treatment had a significant increase in vessel permeability (Lee, Kim, 2013). A novel angiogenesis model was designed by Nguyen et al. to study capillary sprouting by connecting a main endothelialized microchannel and a lateral microchannel as a source of growth factors. Several factors promoting angiogenesis, including VEGF, sphingosine-1-phosphate (S1P) and phorbol 12-myristate 13-acetate (PMA), were identified. The system was tested with anti-angiogenic drugs, including VEGFR2 and S1PR inhibitors, which reduced or even abrogated the total sprouting length by arresting cellular protrusion length and number. This result demonstrates that biochemical cues alone are capable of promoting sprouting angiogenesis but raises new questions about the role of fluid shear stress in the angiogenesis process (Nguyen et al., 2013). This specific study also represents a clear example of how the design of system architecture is critical to analyze the role of complex 3D biochemical gradients in an environment similar to physiological conditions. Other studies have focused on the role of paracrine and juxtacrine signaling between ECs and supporting mural cells. Jeon et al. demonstrated that ECs co-cultured with human bone marrow-derived MSCs supplemented with VEGF and Ang-1 not only promoted an increase in total network length and branching, and reduced microvessel diameter compared to the EC monoculture but also strongly induced MSC differentiation toward a supporting mural-like phenotype (Jeon, Bersini, 2014) (Fig. 1A). Whisler et al. showed that S1P in combination with VEGF played a critical role in microvascular network development when ECs were cultured with human lung fibroblasts (no contact) (Whisler, Chen, 2013).
3.3.1. Remarks
A wide range of growth factors have been identified to modulate microvascular network properties, and several studies showed that different concentrations and combinations of two or more of these factors can have unique effects on the microvascular network. System architecture and most suitable biomaterials should be carefully designed to analyze the role of complex 3D biochemical gradients in a physiological-like environment.
3.4. Effect of biophysical stimuli on the microvascular system
The influence of flow on sprouting angiogenesis has been a heavily researched topic in the field of vascular research. Basal to apical transendothelial flow but not the opposite flow was shown to induce vascular endothelial (VE)-cadherin delocalization and EC activation through focal adhesion kinase signaling, which led to angiogenic sprouting (Vickerman and Kamm, 2012). Microfluidic models were developed to better investigate the combined role of shear stress, interstitial flow and biochemical gradients on sprouting angiogenesis (Song and Munn, 2011). In addition to showing that VEGF induced sprouting, the results also demonstrated that shear stress blocked angiogenesis and flow-induced sprouting inhibition was mediated by nitric oxide (NO) synthesis. Additionally, ECs migration either moved against the interstitial flow direction (in the presence of positive VEGF gradients) or along the flow direction (in the presence of negative VEGF gradients). These results indicated that more filopodia were present when ECs were sprouting against the interstitial flow direction or along VEGF gradients. Conversely, sprouting along the flow direction or towards negative VEGF gradients resembled vessel dilation rather than angiogenic sprouting (Song and Mun, 2011). In a similar study, Song et al. showed that ECs migrated and sprouted along the interstitial flow direction to generate anastomoses between sprouting vessels (Song, Bazou, 2012). Galie et al. also showed through an experimental/computational approach that the sprouting angiogenesis process was directed by mechanical stimuli, such as shear stress, rather than chemical gradients or oxygen tensions (Galie et al., 2014). Both intraluminal shear stress over the endothelium and transmural flow through the endothelium above a shear stress threshold triggered capillary sprouting and invasion of the surrounding ECM. Additionally, a wide panel of MMPs were analyzed, and various flow conditions induced MMP-1 expression. However, a continuous flow appeared to be essential to promote sustained MMP-1 expression and capillary sprouting. Remarkably, an apical to basal transmural flow induced sprouting angiogenesis. This observation confirms previous findings (Song, Bazou, 2012) even though the role of transmural flow is still controversial because other studies have demonstrated that basal to apical flow was required to promote sprouting angiogenesis (Song and Munn, 2011, Vickerman and Kamm, 2012). Other studies also highlighted the importance of a shear stress threshold in promoting angiogenesis for both transmural and intraluminal flow (Galie, Nguyen, 2014), which contrasts previous studies demonstrating that luminal flow limits capillary sprouting (Chouinard-Pelletier, Jahnsen, 2013). Rather than focusing on angiogenic sprouting, several studies designed microfluidic platforms to investigate the effects of interstitial flow on the de novo generation of microvascular networks. Co-cultures of endothelial progenitor cells and fibroblasts were maintained for up to 40 days. Because paracrine factors secreted by co-cultured fibroblasts promoted the generation of microvascular networks when coupled with interstitial flow stimulation, no growth factors (VEGF or bFGF) were externally supplemented during the culture. In this approach, only supra-physiological interstitial flow conditions determined anastomosis between microvascular networks embedded within fibrin gel-filled microchambers and lateral microfluidic channels (Hsu et al., 2013a, Hsu et al., 2013b, Moya, Hsu, 2013). Several justifications were provided to describe this particular behavior, including superior mass transport of nutrients towards cells. In addition, interstitial flow was demonstrated to induce matrix anisotropy while matrix alignment was previously shown to guide capillary sprouting (Morin and Tranquillo, 2011, Ng et al., 2004). Using this system architecture could allow the researchers to monitor the dynamic remodeling of the matrix due to interstitial flow. The same researchergroup recently reported a novel microfluidic model that mimicked the transport phenomena from artery to vascularized tissue and then to vein through the anastomosis between microvessels and endothelialized microchannels (Wang et al., 2015). This system can potentially provide useful insights on the biophysical cues driving capillary sprouting and anastomosis within formed microvascular networks.
3.4.1. Remarks
The role of biophysical stimuli is less studied compared to other biological factors affecting microvascular network formation. Recently designed microfluidic platforms allow for fine control of the flow even though there is still not a widely accepted theory of flow effects. Once flow parameters affecting microvascular network development are identified, other key factors, such as mural cells, should be introduced to study the combinatorial effects of multiple biological factors.
3.5. Overall considerations
In the past decade, considerable research has been focused on biochemical cues, such as growth factors and cytokines, as well as biophysical stimuli, such as fluid shear stress and interstitial flow, as driving forces for angiogenesis and vasculogenesis processes. The effects of biochemical cues go beyond the effects of VEGF or other molecules secreted by supporting mural cells because recent advances have clearly demonstrated that organ-specific microenvironmental cues influence not only the physical parameters of a vascular network, e.g., branching and length (Bongio et al., 2016) but also endothelium gene expression (Nolan et al., 2013). In addition, it is evident that structural and mechanical properties of the ECM and dynamic remodeling of the ECM by perivascular cells, such as pericytes, contribute to vascular morphogenesis, maturation and stabilization. Microfluidic models could provide valuable insights in the analysis of bi-directional dynamic interactions between ECs and the surrounding 3D extracellular matrix, and these models would enhance our understanding of the role of supporting mural cells. These models could be developed by combining highly controlled biophysical and biochemical cues, adjustable matrix composition properties and real-time high resolution microscopy techniques. However, macroscale systems are not affected by size limitations and allow researchers to reproduce biological events occurring in thicker tissues, such as oxygen diffusion. This approach can potentially complement microfluidic data through the quantification of protein and gene expression, which is generally challenging within microsystems. In summary, biochemical/biophysical cues, cell-cell and cell-matrix interactions, and microenvironment cues synergistically modulate the properties of engineered microvascular networks. The system architecture should be carefully defined according to the specific interaction that needs to be investigated. While an endothelialized tubular structure could be sufficient to study the influence of mural cells on vessel contraction/dilation, a bioprinting approach would be essential to precisely locate different cell types within a 3D matrix. Finally, optimal biomaterial and the most suitable system in terms of imaging and long-term stability should be selected to study matrix remodeling and protein deposition by mural cells.
4. Biological Applications
This final section will provide examples of biological applications to microvascular systems, both in vitro and in vivo, to demonstrate how the presence of a functional endothelium can promote the development of in vitro models to mimic physiological conditions and improve the outcome of implanted tissue constructs (Fig. 7).
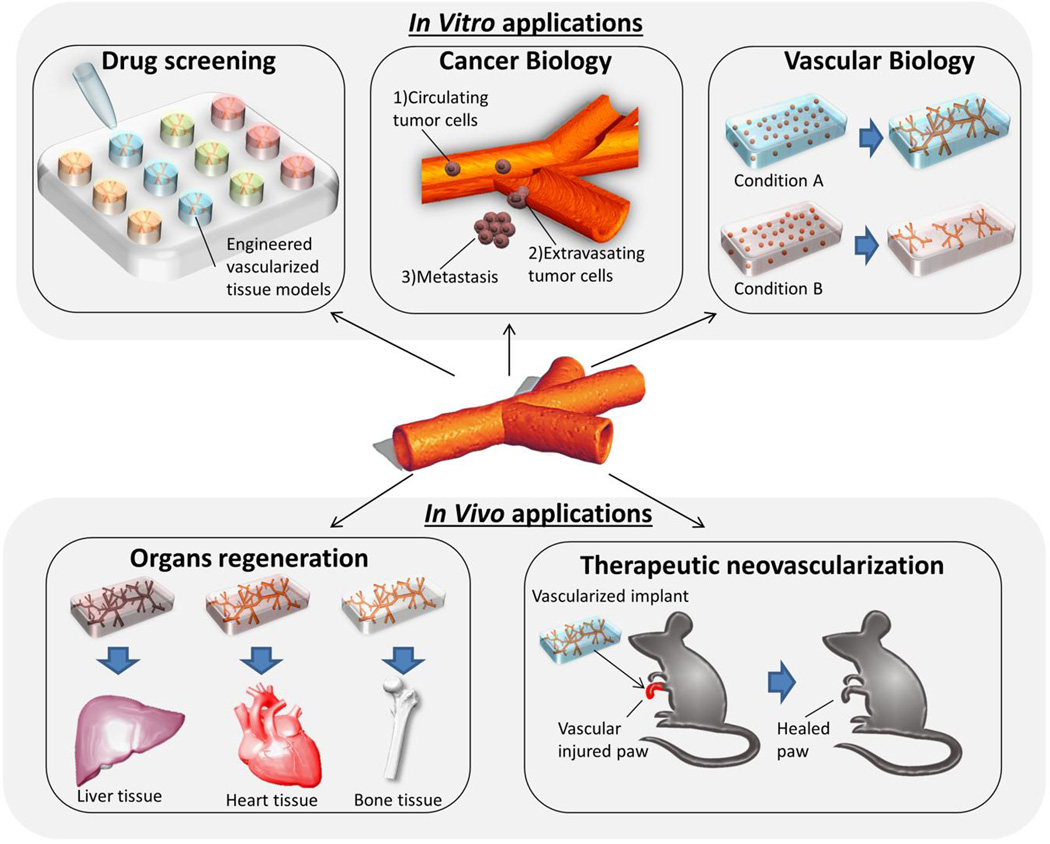
Fig. 7.
Biological applications. Microvascular networks as building blocks for the development of vascularized tissues/organs including heart, liver and bone.
4.1. In vitro models
Sucrose-glucose-dextran carbohydrate sacrificial templates designed by Miller et al. were employed to develop endothelialized microchannels embedded within a hepatocyte-laden 3D matrix. Albumin secretion and urea synthesis appeared to be significantly higher compared to the slab gels without perfused microchannels, which reflected the key role of microvasculature in delivering oxygen and nutrients to the thick tissue construct. The architecture of this system was based on a complex sacrificial template, which led to the generation of an immediately perfusable network of endothelialized microchannels that nourished a high number of embedded hepatocytes. Taking different approaches would have caused specific problems. For example, a vasculogenesis-based approach would not have allowed researchers to quickly generate perfusable microvessels with factors related to the survival of embedded cells. The use of sacrificial fibers instead of carbohydrates would not allow investigators to create complex and interconnected microchannels at the millimeter-scale. A further extension of this study could include a characterization of potential differences in EC adhesion/proliferation as well as an analysis of matrix degradation and EC protease secretion in these 3D matrices using various compositions and stiffness properties. Other studies developed endothelialized microchannels through 3D printing of agarose fiber templates. Mouse pre-osteoblast cell lines embedded within GelMA matrices were grown on sacrificial fibers that were removed to create hollow channels (Fig. 3C). An alkaline phosphatase assay was used to demonstrate superior viability and osteogenic differentiation of these cells compared to control hydrogels without microchannels (Bertassoni, Cecconi, 2014). The investigators also considered different photopolymerizable materials with acrylate or methacrylate groups that had a wide range of hydrogel concentrations. This approach represents a valuable alternative to the use of sacrificial templates because it prevents potential osmotic damage to the cells from template dissolution. However, the extraction of the fibers could damage the surrounding gel and would require materials with the proper mechanical properties. Putnam et al. employed EC-coated microspheres to investigate the role of MSCs expressing α6β1 integrin in EC angiogenic sprouting within 3D fibrin gels containing embedded MSCs. They found that blocking α6β1 integrin not only inhibited EC sprouting but also limited MSC proliferation, laminin deposition and alpha SMA expression. Surprisingly, no effects were detected when α6β1 knockout MSCs were cultured as a monolayer on top of a 3D fibrin gel loaded with EC-coated microbeads. These results imply that α6β1 integrin plays a key role in promoting EC sprouting when ECs reside in close proximity with MSCs without significantly affecting their paracrine secretion of pro-angiogenic molecules (Carrion, Kong, 2013). Based on these promising findings, it would be interesting to highlight potential changes in EC/MSC behavior by using different matrix concentrations or compositions. In fact, different ECM proteins could activate alternative integrin pathways without inhibiting EC sprouting. This approach was employed by Putnam et al. to investigate the role of integrins during angiogenesis through a simple but effective strategy that did not require special equipment, e.g., a bioprinter, microfluidic technologies or other complex experimental procedures. One of the advantages of microfluidic models is the ability to precisely control different stimuli, including biophysical cues. With this control, several microfluidic models were developed to investigate biological phenomena occurring at the microscale in the presence of a functional endothelium, which cannot be effectively quantified within traditional microfabricated systems (Bersini and Moretti, 2015). Microvascular networks were generated within microfluidic systems through vasculogenesis/angiogenesis-based techniques that showed cytoskeleton rearrangements with actin re-distribution and NO synthesis under flow conditions. Moreover, intercellular cell adhesion molecules (ICAMs)-1 were detected when microvascular networks were treated with inflammatory cytokines (Kim, Lee, 2013). In another study, a microfluidic model was developed to analyze the adhesion of cancer cells on a monolayer of microvascular ECs under physiological flow conditions (Song, Cavnar, 2009). In addition to the pivotal role of CXCL12 in cancer cell migration, the researchers found this chemokine could also promote breast cancer cell adhesion in the endothelium (Song, Cavnar, 2009).
Microfluidic models allow for fine tuning of biochemical cues within microenvironment through the development of complex gradients or controlled paracrine secretions from compartmentalized cell populations. In a recent study, interconnected hollow microchannels were created through the so-called viscous finger patterning technique to study the single and combined effects of growth factors and paracrine secretion in smooth muscle cells. First, a pre-polymerized hydrogel solution was pipetted into each microchannel. Next, culture media was added to the hydrogel solution through surface tension-based passive pumping. Following polymerization, a patterned lumen was developed in the hydrogel (Bischel et al., 2013). One of the advantages of this technique was that ECs lining the channel wall were completely surrounded by the ECM and were not exposed to the presence of other materials, such as PDMS. Additionally, most microfluidic devices are based on the endothelialization of glass or PDMS surfaces, which do not mimic the physiological ECM. Microfluidic vascular models can also be used to study cancer biology. For example, Bersini et al. developed organ-specific endothelialized microchannels that mimic a bone tissue microenvironment. They demonstrated that endothelial permeability can be modified due to the presence of cancer cells (Bersini, Jeon, 2014a). A similar platform was employed to quantify the effects of specific drugson morphological changes of EC, cell-cell junctions and EC angiogenic properties (Kim, Kasuya, 2014).
Although current microvascular systems have shown beneficial properties, the next generation of microvascular systems within organ-specific tissues will provide significant advantages for analyzing the structural and functional properties of organ-specific endothelia. These systems will be embedded within more complex microenvironments for the study of vascular-related phenomena, such as cancer metastases.
4.2. In vivo implantation
Cell spheroids are commonly used as building blocks in modular assembly strategies. In a recent study, spherical cell aggregates containing ECs and MSCs were seeded on Matrigel surfaces and their behavior was compared to dissociated ECs/MSCs. Cell spheroids showed an increased and sustained ability to generate tubular structures stabilized by smooth muscle cell-like differentiated MSCs. The study showed the deposition of multiple ECM proteins, including collagen I-III, laminin and fibronectin, and demonstrated that vascular cells gradually create and tune the surrounding microenvironment. Interestingly, transplantation in a mouse model of hindlimb ischemia demonstrated superior reperfusion of the injured site compared to control animals treated with dissociated ECs/MSCs or saline. They also found perfusable von Willebrand factor positive capillaries and smooth muscle actin (SMA) positive arterioles within the injured limb, which provided strong evidence of a functional connection between host cells and in vitro generated cell aggregates. Nevertheless, the study failed to clarify if the SMA signal was due to host or transplanted cells, and there was no indication of the dynamic remodeling of the matrix structure during network development. Overall, this study clearly demonstrated the possibility of rescuing damaged tissues from degeneration following ischemia (Chen, Wei, 2013, Lee, Tsai, 2011). In a different study conducted by Chen et al., the researchers showed the effect of multiple degrees of methacryloyl modification in 5% GelMA hydrogels encapsulated with ECs and MSCs on the generation of perfusable microvasculatures. They analyzed several structural parameters of the network, such as total length, average capillary length, number of branches and number of branch points. Additionally, they implanted acellular hydrogels into nude mice and analyzed their behavior post-implantation (Fig. 8A). Tissue constructs did not generate a significant inflammatory response compared to control collagen group and were still present post-implantation. Murine erythrocytes were detected within human CD31+ vessels, which demonstrated functional anastomoses between host circulation and implanted constructs. Overall, the degree of methacryloyl modification influenced vessel number, distribution and size. Hydrogels with a lower crosslinking degree appeared to show improved performance over highly crosslinked constructs (Chen, Lin, 2012). Both cell spheroids and cell-laden GelMA hydrogels demonstrated successful anastomosis with the host vasculature and perfusable vessel development. However, other microfabrication approaches could be necessary to create highly oriented and perfusable capillaries. Microtissue molding allowed researchers to develop aligned cords with embedded ECs and study in vivo remodeling. Stable capillaries were found at early time points while mature vessels surrounded by alpha SMA+ cells were detected a few weeks post-implantation. Chimeric structures containing both human and murine cells were clearly observed, which demonstrated integration with the host vasculature (Fig. 8B). Moreover, human hepatocyte aggregates were implanted within tissue constructs containing aligned cords and compared with tissues characterized by randomly organized ECs. Increased metabolic activity of implanted hepatocytes was detected by measuring albumin secretion with geometrically aligned endothelial cords. These findings proved the efficacy of a specific pre-vascularization technique for the generation of engineered hepatic tissues. The generation of cell spheroids or cell-laden hydrogels would not have provided a sufficient level of vessel orientation and hepatic tissue maturation. As discussed by Baranski et al., modulating vessel distribution and tissue perfusion is critical for improving tissue integration (Baranski, Chaturvedi, 2013). Highly aligned vascular structures were also developed through the polyelectrolyte complexation technique and employed to design pre-vascularized adipose and hepatic tissues. Following co-culture of hepatocyte precursor cells derived from human embryonic stem cells and ECs, tissue constructs were implanted into the livers of severe combined immunodeficiency (SCID) mice (Fig. 8C). Immunofluorescence images showed the presence of albumin-positive human hepatocytes surrounded by human CD31+ ECs, which was a clear indication of tissue construct survival and integration within the host (Leong, Toh, 2013, Wan, Yim, 2004).
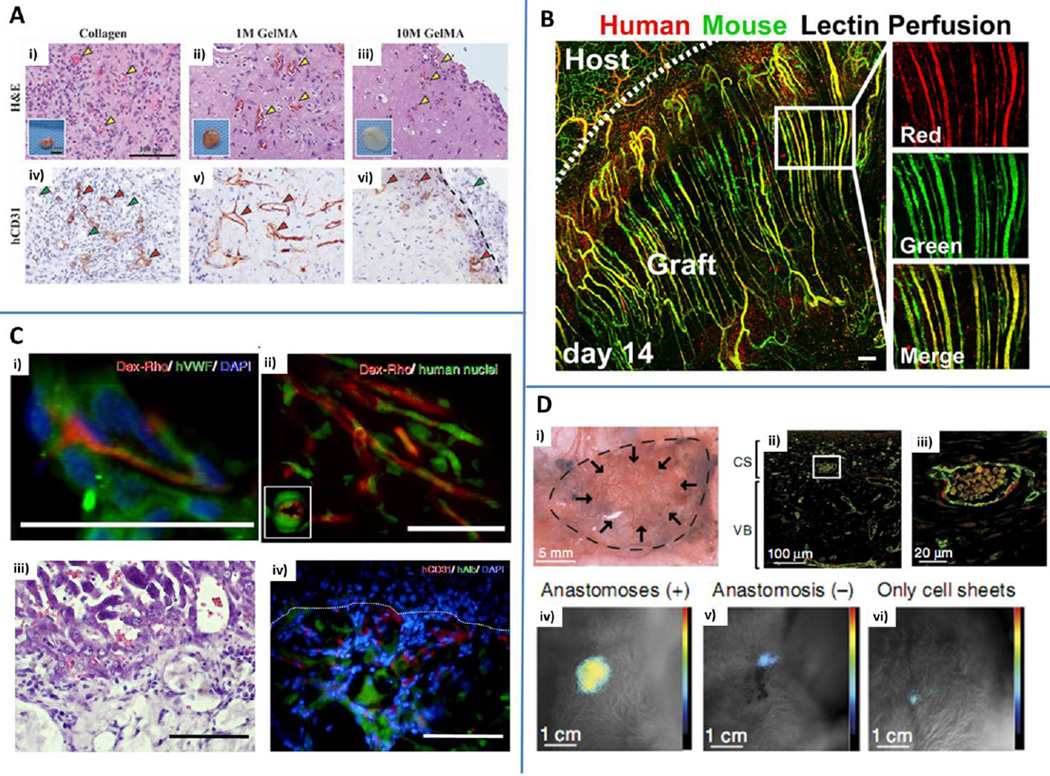
Fig. 8.
In vivo applications. A) 1M and 10 M GelMA hydrogels with embedded human endothelial colony forming cells and mesenchymal stem cells were in vitro pre-cultured for 24 h and implanted in mice. Collagen gels were used as controls. Hematoxylin&eosin (H&E) staining revealed the presence of blood vessels containing mouse erythrocytes (yellow arrowheads) within collagen (i), 1M GelMA (ii) and 10 M GelMA (iii). Explants are shown in the insets. Human CD31 staining (iv, v, vi) highlights the presence of human capillaries (red arrowheads) while murine vessels are not stained (green arrowheads). Reproduced from Chen et al. (2012) by permission of John Wiley and Sons. B) Parallel arrays of EC cords anastomosed with the mouse vasculature. Human-specific lectin (red) and mouse-specific lectin (green) were infused via tail vein injection demonstrating the presence of patent vessels chimeric in composition. Scale bar: 150 µm. Reproduced by permission from Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A. 2013;110:7586-91 (Baranski et al. (2013)). C) Hydrogels obtained with interfacial polyelectrolyte complexation anastomosed with the host vasculature after 14 days, as demonstrated by dextran rhodamine (Dex-Rho) injection (i and ii, red). Green: human vWF (i) and human nuclei (ii). H&E (iii) and immunofluorescent staining (iv) of hepatocyte constructs. Human serum albumin (hAlb, green) and human CD31 (red) were identified within tissue constructs. Scale bar: 50 µm (i and ii) and 100 µm (iii and iv). Blue: nuclei (DAPI). Adapted from Figure 2 and Figure 4 (Leong et al. (2013)) by permission of the Nature Publishing Group. D) Vascularized grafts obtained by cell sheet engineering anastomosed with the host circulatory system and maintained their functionality for at least 14 days following implantation (i). Immunofluorescence staining of CD31 (green) and calponin (red) showing uniform distribution of vessels inside the tissue construct. CS: cell sheets. VB: vascular bed (ii and iii (enlargement)). Bioluminescent image representative of the integration of the tissue construct with the host. Higher signals were detected for anastomosed tissues (iv) compared to non-anastomosed (v) or constructs embedding cell sheets without a connecting vascular bed (vi). Reproduced from Sekine et al. (2013) by permission of the Nature Publishing Group.
Finally, layer-by-layer deposition techniques led to the development of a multi-layer vascular graft containing cardiac cells and ECs, which was successfully implanted in mice through anastomoses within the carotid artery and the jugular vein (Fig. 8D). Vascularized grafts with anastomoses maintained vascular structures and heartbeat activity for two weeks after the procedure compared to tissue constructs that were not anastomosed within the host vasculature or simple cell sheets without any connection to a vascular bed (Sekine, Shimizu, 2013). Overall, these results demonstrated that a critical level of pre-vascularization coupled with surgical anastomosis within the host circulatory system could significantly improve viability, functionality and integration of the implant. The main limitation was that the approach requires the presence of a vascular bed obtained from femoral tissue resection and prolonged perfusion in a bioreactor to promote maturation of the tissue.
5. Conclusions and Future Perspectives
This review highlights the integration of materials science, microengineering and biology for the design, development and analysis of microvascular systems. In recent years, significant advances have been made through the optimization of original microfabrication techniques and the development of new synthetic and hybrid hydrogels. Microfluidic technologies have progressed and facilitate analysis of multiple biological processes occurring at the microscale under biochemically and biophysically controlled microenvironments. Most of these phenomena involve the presence of a functional endothelium. A full understanding of the cellular and molecular mechanisms underlying microvasculature morphogenesis and homeostasis is necessary to develop vascularized tissue constructs, design vascular disease models or organ-on-a-chip platforms. However, despite our vast knowledge of the biochemical cues driving vascular morphogenesis, study of the interactions between vascular cells and surrounding 3D matrix and perivascular cells is a field that has plenty of room for additional research and development. Consequently, researchers envision that the wide set of current microvascular system architectures will be used to better clarify the influence of physical factors on microvessel growth and remodeling and to potentially analyze their specific roles with respect to biochemical cues. Overall, biological interactions, such as biophysical stimuli and organ-specific microenvironments, as well as biomaterial selection and microfabrication technique are equally critical to adjusting microvascular network features.
Functional and perfusable microvascular networks can be applied to the field of cancer biology because most of the events occurring in the metastatic cascade involve the presence of a microvascular system. Cancer cells must cross the endothelial barrier to spread from a primary tumor, survive in the circulation and finally extravasate to colonize a distant organ. At the same time, the study of the immune response would greatly benefit from the use of physiological microvascular networks, such as analysis of leukocyte extravasation or macrophage interaction within the endothelium. In addition, functional endothelia will be critical to model mass transport and pharmacokinetics phenomena, whereas the integration of microvascular networks into organ-on-a-chip platforms would contribute to the development of more realistic models in the field of developmental biology. This development would enable further investigation of the heterotypic interaction between ECs and organ-specific cell types, which are critical for promoting tissue growth and homeostasis. Using induced pluripotent stem cells (iPSCs) would also be fundamental for developing patient-specific models for both diagnosis and prognosis (Kusuma et al., 2013, Samuel et al., 2013). These models could be utilized to identify disease-specific biomarkers, such as molecular signatures or cellular behaviors that lead to cancer metastases in specific organs, or to design and optimize highly tailored personalized therapies, such as testing various combinations of drugs at particular ratios and concentrations within a microenvironment recreated using patient specific cells.
The endothelium does not represent a mere wall separating blood and tissues, but it is critical for mediating a wide range of pathophysiological processes within the body. The goal of the next generation of models is to recreate this intrinsic dynamic nature.
Highlights.
Generating functional microvascular networks is critical for both tissue engineering and in vitro modeling
Microfabrication techniques and novel Biomaterials have been developed to generate physiological microvascular networks
Complex interactions exist between endothelial cells, supporting mural cells and extracellular matrix
Biochemical and biophysical cues drive microvascular network formation
Structural/mechanical properties of the ECM and its remodeling by endothelial cells contribute to vascular morphogenesis and vessel stabilization
Acknowledgments
Funding from the Italian Ministry of Health is greatly acknowledged (MM). This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program under Award No. W81XWH-15-1-0092. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense (MM). This work was supported by the Institute for Soldier Nanotechnology, National Institutes of Health (HL092836, EB02597, AR057837, HL099073), the National Science Foundation (DMR0847287), the Office of Naval Research Young Investigator award, ONR PECASE (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Simone Bersini, Cell and Tissue Engineering Lab, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy
Dr. Iman K. Yazdi, Biomaterials Innovation Research Center, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA, USA Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Cambridge, MA 02139, USA.
Ing. Giuseppe Talò, Cell and Tissue Engineering Lab, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy
Dr. Su Ryon Shin, Biomaterials Innovation Research Center, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA, USA Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Cambridge, MA 02139, USA.
Dr. Matteo Moretti, Cell and Tissue Engineering Lab, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy; Regenerative Medicine Technologies Lab, Ente Ospedaliero Cantonale, Lugano, Switzerland; Swiss Institute for Regenerative Medicine, Lugano, Switzerland; Cardiocentro Ticino, Lugano, Switzerland.
Prof. Ali Khademhosseini, Biomaterials Innovation Research Center, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Cambridge, MA 02139, USA; Department of Physics, King Abdulaziz University, Jeddah 21569, Saudi Arabia; College of Animal Bioscience and Technology, Department of Bioindustrial Technologies, Konkuk University, Hwayang-dong, Kwangjin-gu, Seoul 143-701, Republic of Korea.
References
- Aberle T, Franke K, Rist E, Benz K, Schlosshauer B. Cell-type specific four-component hydrogel. PLoS One. 2014;9:e86740. doi: 10.1371/journal.pone.0086740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85–123. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, et al. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater. 2013:23. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine EE, Vlachos PP, Rylander MN. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng Part B Rev. 2014;20:683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Arrigoni C, Bongio M, Talo G, Bersini S, Enomoto J, Fukuda J, et al. Rational Design of Prevascularized Large 3D Tissue Constructs using Computational Simulations and Biofabrication of Geometrically Controlled Microvessels. Advanced Healthcare Materials. 2016 doi: 10.1002/adhm.201500958. in press. [DOI] [PubMed] [Google Scholar]
- Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31:3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger FA, Gibot L, Lacroix D. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng. 2013;15:177–200. doi: 10.1146/annurev-bioeng-071812-152428. [DOI] [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, et al. Building vascular networks. Sci Transl Med. 2012;4:160ps23. doi: 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Trappmann B, Stapleton SC, Toro E, Chen CS. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13:3246–3252. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J, Antille M, Bonda U, De-Juan-Pardo EM, Khosrotehrani K, Ivanovski S, et al. In vitro pre-vascularisation of tissue-engineered constructs A co-culture perspective. Vasc Cell. 2014;6:13. doi: 10.1186/2045-824X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A. 2013;110:7586–7591. doi: 10.1073/pnas.1217796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30:6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Gilardi M, Arrigoni C, Talo G, Zamai M, Zagra L, et al. Human in vitro 3D co-culture model to engineer vascularized bone-mimicking tissues combining computational tools and statistical experimental approach. Biomaterials. 2015;76:157–172. doi: 10.1016/j.biomaterials.2015.10.057. [DOI] [PubMed] [Google Scholar]
- Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014a;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Jeon JS, Moretti M, Kamm RD. In vitro models of the metastatic cascade: from local invasion to extravasation. Drug Discov Today. 2014b;19:735–742. doi: 10.1016/j.drudis.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Moretti M. 3D functional and perfusable microvascular networks for organotypic microfluidic models. J Mater Sci Mater Med. 2015;26:5520. doi: 10.1007/s10856-015-5520-5. [DOI] [PubMed] [Google Scholar]
- Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongio M, Lopa S, Gilardi M, Bersini S, Moretti M. A 3D vascularized bone remodeling model combining osteoblasts and osteoclasts in a CaP nanoparticles-enriched matrix. Nanomedicine: nanotechnology, biology, and medicine. 2016 doi: 10.2217/nnm-2015-0021. in press. [DOI] [PubMed] [Google Scholar]
- Buchanan CF, Voigt EE, Szot CS, Freeman JW, Vlachos PP, Rylander MN. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng Part C Methods. 2014;20:12. doi: 10.1089/ten.tec.2012.0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion B, Kong YP, Kaigler D, Putnam AJ. Bone marrow-derived mesenchymal stem cells enhance angiogenesis via their alpha6beta1 integrin receptor. Exp Cell Res. 2013;319:2964–2976. doi: 10.1016/j.yexcr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira MT, da Silva LP, Santos TC, Pirraco RP, Correlo VM, Reis RL, et al. Gellan Gum-Hyaluronic Acid Spongy-like Hydrogels and Cells from Adipose Tissue Synergize Promoting Neoskin Vascularization. ACS Appl Mater Interfaces. 2014 doi: 10.1021/am504520j. [DOI] [PubMed] [Google Scholar]
- Chen DY, Wei HJ, Lin KJ, Huang CC, Wang CC, Wu CT, et al. Three-dimensional cell aggregates composed of HUVECs and cbMSCs for therapeutic neovascularization in a mouse model of hindlimb ischemia. Biomaterials. 2013;34:1995–2004. doi: 10.1016/j.biomaterials.2012.11.045. [DOI] [PubMed] [Google Scholar]
- Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM, et al. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv Funct Mater. 2012;22:2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. doi: 10.1007/s10456-012-9300-2. [DOI] [PubMed] [Google Scholar]
- Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- Cuchiara MP, Gould DJ, McHale MK, Dickinson ME, West JL. Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels. Adv Funct Mater. 2012;22:4511–4518. doi: 10.1002/adfm.201200976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Culver JC, Hoffmann JC, Poche RA, Slater JH, West JL, Dickinson ME. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv Mater. 2012;24:2344–2348. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- de Moraes MA, Paternotte E, Mantovani D, Beppu MM. Mechanical and biological performances of new scaffolds made of collagen hydrogels and fibroin microfibers for vascular tissue engineering. Macromol Biosci. 2012;12:1253–1264. doi: 10.1002/mabi.201200060. [DOI] [PubMed] [Google Scholar]
- Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Fozdar DY, Soman P, Lee JW, Han LH, Chen S. Three-Dimensional Polymer Constructs Exhibiting a Tunable Negative Poisson's Ratio. Adv Funct Mater. 2011;21:2712–2720. doi: 10.1002/adfm.201002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111:441–453. doi: 10.1002/bit.25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94:1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke SM, Plaha J, Hager S, Strassburg S, Torio-Padron N, Stark GB, et al. Human endothelial progenitor cells induce extracellular signal-regulated kinase-dependent differentiation of mesenchymal stem cells into smooth muscle cells upon cocultivation. Tissue Eng Part A. 2012;18:2395–2405. doi: 10.1089/ten.TEA.2012.0147. [DOI] [PubMed] [Google Scholar]
- Gohongi T, Fukumura D, Boucher Y, Yun CO, Soff GA, Compton C, et al. Tumor-host interactions in the gallbladder suppress distal angiogenesis and tumor growth: involvement of transforming growth factor beta1. Nat Med. 1999;5:1203–1208. doi: 10.1038/13524. [DOI] [PubMed] [Google Scholar]
- Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- Hanjaya-Putra D, Bose V, Shen YI, Yee J, Khetan S, Fox-Talbot K, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holnthoner W, Hohenegger K, Husa AM, Muehleder S, Meinl A, Peterbauer-Scherb A, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9:127–136. doi: 10.1002/term.1620. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Moya ML, Abiri P, Hughes CC, George SC, Lee AP. Full range physiological mass transport control in 3D tissue cultures. Lab Chip. 2013a;13:81–89. doi: 10.1039/c2lc40787f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, Moya ML, Hughes CC, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip. 2013b;13:2990–2998. doi: 10.1039/c3lc50424g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Zhou LH, Zhang QC, Chen YM, Sun W, Xu F, et al. Microfluidic hydrogels for tissue engineering. Biofabrication. 2011;3:012001. doi: 10.1088/1758-5082/3/1/012001. [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- Inaba R, Khademhosseini A, Suzuki H, Fukuda J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials. 2009;30:3573–3579. doi: 10.1016/j.biomaterials.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Inamdar NK, Borenstein JT. Microfluidic cell culture models for tissue engineering. Curr Opin Biotechnol. 2011;22:681–689. doi: 10.1016/j.copbio.2011.05.512. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb) 2014;6:555–563. doi: 10.1039/c3ib40267c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol Biosci. 2009;9:140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- Jusoh N, Oh S, Kim S, Kim J, Jeon NL. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab Chip. 2015;15:3984–3988. doi: 10.1039/c5lc00698h. [DOI] [PubMed] [Google Scholar]
- Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization--the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, 3rd, Langer R, et al. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79:522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kim C, Kasuya J, Jeon J, Chung S, Kamm RD. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip. 2014 doi: 10.1039/c4lc00866a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- Kniazeva E, Weidling JW, Singh R, Botvinick EL, Digman MA, Gratton E, et al. Quantification of local matrix deformations and mechanical properties during capillary morphogenesis in 3D. Integr Biol (Camb) 2012;4:431–439. doi: 10.1039/c2ib00120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecek J, Yang J. Smart self-assembled hybrid hydrogel Biomaterials. Angew Chem Int Ed Engl. 2012;51:7396–7417. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:12601–12606. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim S, Chung M, Kim JH, Jeon NL. A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res. 2013 doi: 10.1016/j.mvr.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Lee PF, Yeh AT, Bayless KJ. Nonlinear optical microscopy reveals invading endothelial cells anisotropically alter three-dimensional collagen matrices. Exp Cell Res. 2009a;315:396–410. doi: 10.1016/j.yexcr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng. 2007;97:1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009b;30:1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Lee WY, Tsai HW, Chiang JH, Hwang SM, Chen DY, Hsu LW, et al. Core-shell cell bodies composed of human cbMSCs and HUVECs for functional vasculogenesis. Biomaterials. 2011;32:8446–8455. doi: 10.1016/j.biomaterials.2011.07.061. [DOI] [PubMed] [Google Scholar]
- Leong MF, Toh JK, Du C, Narayanan K, Lu HF, Lim TC, et al. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat Commun. 2013;4:2353. doi: 10.1038/ncomms3353. [DOI] [PubMed] [Google Scholar]
- Lim SH, Kim C, Aref AR, Kamm RD, Raghunath M. Complementary effects of ciclopirox olamine, a prolyl hydroxylase inhibitor and sphingosine 1-phosphate on fibroblasts and endothelial cells in driving capillary sprouting. Integr Biol (Camb) 2013;5:1474–1484. doi: 10.1039/c3ib40082d. [DOI] [PubMed] [Google Scholar]
- Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010;107:860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Schickel ME, Stevenson MD, Sarang-Sieminski AL, Gooch KJ, Ghadiali SN, et al. Fibers in the extracellular matrix enable long-range stress transmission between cells. Biophys J. 2013;104:1410–1418. doi: 10.1016/j.bpj.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malda J, Visser J, Melchels FP, Jungst T, Hennink WE, Dhert WJ, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod C, Higgins J, Miroshnikova Y, Liu R, Garrett A, Sarang-Sieminski AL. Microscopic matrix remodeling precedes endothelial morphological changes during capillary morphogenesis. J Biomech Eng. 2013;135:71002. doi: 10.1115/1.4023984. [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithieux SM, Rasko JE, Weiss AS. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25:4921–4927. doi: 10.1016/j.biomaterials.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Montano I, Schiestl C, Schneider J, Pontiggia L, Luginbuhl J, Biedermann T, et al. Formation of human capillaries in vitro: the engineering of prevascularized matrices. Tissue Eng Part A. 2010;16:269–282. doi: 10.1089/ten.TEA.2008.0550. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, et al. Formation of microvascular networks in vitro. Nat Protoc. 2013;8:1820–1836. doi: 10.1038/nprot.2013.110. [DOI] [PubMed] [Google Scholar]
- Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials. 2011;32:6111–6118. doi: 10.1016/j.biomaterials.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Moya ML, Hsu YH, Lee AP, Hughes CC, George SC. In Vitro Perfused Human Capillary Networks. Tissue Eng Part C Methods. 2013 doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Zheng W, Xiao L, Zhang W, Jiang X. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13:1612–1618. doi: 10.1039/c3lc41342j. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110:6712–6717. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012;33:9009–9018. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo VD, Bruno A, Tomasello G, Damiano G, Lo Monte AI. Bioengineered vascular scaffolds: the state of the art. International Journal of Artificial Organs. 2014;37:503–512. doi: 10.5301/ijao.5000343. [DOI] [PubMed] [Google Scholar]
- Park KM, Gerecht S. Harnessing developmental processes for vascular engineering and regeneration. Development. 2014;141:2760–2769. doi: 10.1242/dev.102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells. 2008;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- Price GM, Chu KK, Truslow JG, Tang-Schomer MD, Golden AP, Mertz J, et al. Bonding of macromolecular hydrogels using perturbants. J Am Chem Soc. 2008;130:6664–6665. doi: 10.1021/ja711340d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt JW, Krakauer DA, Gooch KJ. Complex matrix remodeling and durotaxis can emerge from simple rules for cell-matrix interaction in agent-based models. J Biomech Eng. 2013;135:71003. doi: 10.1115/1.4024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Nuzzo RG. Recent progress in soft lithography. Materials Today. 2005;8:7. [Google Scholar]
- Rohringer S, Hofbauer P, Schneider KH, Husa AM, Feichtinger G, Peterbauer-Scherb A, et al. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014;17:921–933. doi: 10.1007/s10456-014-9439-0. [DOI] [PubMed] [Google Scholar]
- Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010;115:5259–5269. doi: 10.1182/blood-2009-11-252692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadr N, Zhu M, Osaki T, Kakegawa T, Yang Y, Moretti M, et al. SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures. Biomaterials. 2011;32:7479–7490. doi: 10.1016/j.biomaterials.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110:12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimovic S, Dokmeci MR, Khademhosseini A. Organs-on-a-chip for drug discovery. Curr Opin Pharmacol. 2013;13:829–833. doi: 10.1016/j.coph.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Sheridan WS, Grant OB, Duffy GP, Murphy BP. The application of a thermoresponsive chitosan/beta-GP gel to enhance cell repopulation of decellularized vascular scaffolds. J Biomed Mater Res B Appl Biomater. 2014;102:1700–1710. doi: 10.1002/jbm.b.33138. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, et al. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip. 2011;11:2175–2181. doi: 10.1039/c1lc20039a. [DOI] [PubMed] [Google Scholar]
- Shin Y, Yang K, Han S, Park HJ, Seok Heo Y, Cho SW, et al. Reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix. Adv Healthc Mater. 2014;3:1457–1464. doi: 10.1002/adhm.201300569. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Hebbel RP, Gooch KJ. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11:1332–1345. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- Sims DE. The pericyte--a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10:1566–1576. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- Song JW, Bazou D, Munn LL. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr Biol (Camb) 2012;4:857–862. doi: 10.1039/c2ib20061a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Cavnar SP, Walker AC, Luker KE, Gupta M, Tung YC, et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Ota H, Fukumori K, Miyake J, Yamato M, Okano T. Micro-patterned cell-sheets fabricated with stamping-force-controlled micro-contact printing. Biomaterials. 2014;35:9802–9810. doi: 10.1016/j.biomaterials.2014.08.043. [DOI] [PubMed] [Google Scholar]
- Thiele J, Ma Y, Bruekers SM, Ma S, Huck WT. 25th anniversary article: Designer hydrogels for cell cultures: a materials selection guide. Adv Mater. 2014;26:125–147. doi: 10.1002/adma.201302958. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CJ, Edgar LT, Hoying JB, Weiss JA. Cell-generated traction forces and the resulting matrix deformation modulate microvascular alignment and growth during angiogenesis. Am J Physiol Heart Circ Physiol. 2014;307:H152–H164. doi: 10.1152/ajpheart.00995.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger U, Baggett B, Weiss JA, Hoying JB, Edgar LT. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis. 2015;18:219–232. doi: 10.1007/s10456-015-9461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RB, Lara SL, Drake CJ, Iruela-Arispe ML, Angello JC, Little CD, et al. Organized type I collagen influences endothelial patterns during "spontaneous angiogenesis in vitro": planar cultures as models of vascular development. In Vitro Cell Dev Biol Anim. 1995;31:120–131. doi: 10.1007/BF02633972. [DOI] [PubMed] [Google Scholar]
- Vickerman V, Kamm RD. Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol (Camb) 2012;4:863–874. doi: 10.1039/c2ib00184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan AC, Yim EK, Liao IC, Le Visage C, Leong KW. Encapsulation of biologics in self-assembled fibers as biostructural units for tissue engineering. J Biomed Mater Res A. 2004;71:586–595. doi: 10.1002/jbm.a.30158. [DOI] [PubMed] [Google Scholar]
- Wang X, Phan DT, Sobrino A, George SC, Hughes CC, Lee AP. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip. 2015 doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Jin ZH, Gan BW, Lv SW, Xie M, Huang WH. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip. 2014;14:2709–2716. doi: 10.1039/c4lc00069b. [DOI] [PubMed] [Google Scholar]
- Whisler JA, Chen M, Kamm R. Control of Perfusable Microvascular Network Morphology Using a Multi-Culture Microfluidic System. Tissue Eng Part C Methods. 2013 doi: 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater. 2011;23:H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Ye X, Lu L, Kolewe ME, Park H, Larson BL, Kim ES, et al. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials. 2013;34:10007–10015. doi: 10.1016/j.biomaterials.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip. 2012;12:2815–2822. doi: 10.1039/c2lc40131b. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Peticone C, Murthy SK, Radisic M. A standalone perfusion platform for drug testing and target validation in micro-vessels networks. Biomicrofluidics. 2013;7:13. doi: 10.1063/1.4818837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu L, Wang J, Xu Y, Zhang W, Khang G, et al. In vitro vascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1863. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]