Abstract
Objective
Posttraumatic stress disorder (PTSD) has been linked to elevated heart rate (HR) and reduced heart-rate variability (HRV) in cross-sectional research. Using ecological momentary assessment (EMA) and minute-to-minute HRV/HR monitoring, we examined whether cross-sectional associations between PTSD symptom severity and HRV/HR were due to overall elevations in distress levels or to attenuated autonomic regulation during episodes of acute distress.
Methods
Two hundred nineteen young adults (18–39 years old), 99 with PTSD, underwent one day of Holter monitoring and concurrently reported distress levels via EMA. Using multilevel modeling, we examined the associations between momentary distress and the 5-minute means for low-frequency (LF) and high-frequency (HF) HRV and HR immediately following distress ratings, and whether PTSD symptom severity moderated these associations.
Results
Compared to controls, participants with PTSD recorded higher ambulatory distress (M=1.7±SD=0.5 vs. 1.2±0.3, p < .001) and HR (87.2±11.8 vs. 82.9±12.6 bpm, p = .011), and lower ambulatory LF HRV (36.9±14.7 vs. 43.7±16.9 ms, p = .002) and HF HRV (22.6±12.3 vs. 26.4±14.6 ms, p = .043). Overall distress level was not predictive of HR or HRV (ps > .27). However, baseline PTSD symptom severity was associated with elevated HR, t(1257) = 2.76, p = .006, and attenuated LF, t(1257) = −3.86, p < .001, and HF, t(1257) = −2.62, p = .009, in response to acute momentary distress.
Conclusions
Results suggest that PTSD is associated with heightened arousal following situational distress and could explain prior findings associating PTSD with HR/HRV. Implications for treatment and cardiovascular risk are discussed.
Keywords: posttraumatic stress disorder, heart rate variability, autonomic functioning, distress
Introduction
A hallmark of posttraumatic stress disorder (PTSD) is autonomic nervous system imbalance, characterized by a hypoactive parasympathetic nervous system (PNS) and hyperactive sympathetic nervous system (SNS) (1). Indeed, PTSD diagnosis and symptom severity have been associated with higher baseline heart rate (HR) (2) and reduced heart-rate variability (HRV) (3), a non-invasive index of autonomic control of cardiac rhythm (4). This is concerning because elevated HR and attenuated HRV are not only indicators of poor cardiovascular health, they are also precursors, conveying heightened risk for arrhythmia (5) and artherosclerosis (6). However, almost all previous research linking PTSD with HR and HRV has relied on cross-sectional analyses. Thus, it is unclear whether HR and HRV are chronically or situationally atypical in individuals with PTSD.
Emerging evidence from ambulatory recordings of HRV suggests that there are particular contexts in which autonomic recovery from excitation is attenuated in PTSD. In one study, we found that PTSD-related contrasts in minute-to-minute high-frequency (HF) HRV were greatest during periods of sleep, indicating that sleep disturbance, possibly from nightmares, was a major contributing factor to the association between PTSD and reduced HRV (7). In another analysis from the same study, we found that momentary PTSD symptom severity was negatively associated with minute-to-minute low-frequency (LF) HRV, suggesting that periods of acute symptomatology are characterized by delayed or attenuated autonomic recovery (8). One possible interpretation of these findings is that increased emotional distress, whether during sleep or waking hours, may in part account for previously observed cross-sectional associations between PTSD and HRV.
Drawing from these findings, we hypothesized that individuals with severe PTSD symptoms experience a higher baseline of emotional distress than individuals with mild or no PTSD symptoms and thus may experience overall heightened physiological arousal, characterized by high HR and low HRV. As an alternative to this hypothesis, we also examined a second contextual model of HR and HRV. Specifically, we hypothesized that individuals with severe PTSD symptoms would experience heightened physiological arousal following emotional distress in comparison to individuals with no to mild symptoms. Importantly, we wished to compare support for both hypotheses to determine whether individual differences in distress level drive the association between PTSD symptom severity and HRV/HR, or whether high-distress situations are primarily responsible for that link. To test these hypotheses, we used the same dataset from which the prior two minute-to-minute HRV findings were generated. In addition to baseline measurements of PTSD symptom severity, these data included ecological momentary assessments (EMA) of self-reported distress and one day of Holter monitoring of HR and HRV.
Methods
Participants and Procedure
Participants were 230 young adults (18–39 years old), 105 of whom met DSM-IV criteria for PTSD as assessed by the Clinician Administered PTSD Scale (CAPS) (9). Participants were recruited from hospital clinics and waiting rooms as well as via online ads. The study was approved by both the Durham Veterans Affairs and Duke University Medical Center Institutional Review Boards. All patients gave written informed consent prior to participation. Data was collected between August 2009 and September 2013. Further information on participant recruitment, exclusion criteria, and study procedures are described elsewhere (10).
Baseline Davidson Trauma Scale (DTS) (11) scores were used to quantify PTSD symptom severity. Smoking status and demographic information, including age, gender, racial minority status, pack-year history, and the use of prescription medications were collected during the baseline session. Seven participants (n = 3 with PTSD) using beta or calcium-channel blockers were dropped from the analyses on account of potential effects on HRV/HR. Similarly, four participants (n = 3 with PTSD) using tricyclic antidepressants or norepinephrine reuptake inhibitors were also dropped.
One week after baseline, participants were fitted with a digital Holter monitor (Lifecard CF, Del Mar Reynolds, Irvine, CA) and given a PalmPilot to complete EMA ratings of mood in response to random alarms. Monitoring sessions began at approximately 2:00 PM and lasted 24 hours. One diary item captured participants’ current level of distress (“Indicate to what extent you have felt this way [distressed] over the last five (5) minutes before the alarm”) using a scale ranging from 0 (“very slightly/not at all”) to 4 (“extremely”). Participants also recorded their current activity level: lying down, sitting, standing up and engaging in light activity (e.g., walking slowly), or standing up and engaging in heavy activity (e.g., running).
Minute-by-minute changes in the amplitude of the LF and HF components of HRV were assessed by complex demodulation, a nonlinear time-domain method for assessing time-dependent changes in nonstationary oscillatory components within a predefined frequency band. The detail of this method has been reported previously (12). Briefly, time-dependent changes in LF and HF amplitudes were extracted continuously by demodulating the frequency bands of 0.04–0.15 Hz and 0.15–0.45 Hz, respectively, and the amplitude time series of LF and HF components were averaged over every 1-minute segment.
Given our interest in analyzing interindividual differences in intraindividual processes, we used multilevel modeling (MLM) to test the study hypotheses. MLM is appropriate for analyzing unbalanced repeated-measures data and can accommodate person-level and reading-level predictors (13). Three sets of models were specified, two each corresponding to LF HRV, HF HRV, and HR. In each model, momentary distress was used to predict mean HRV/HR levels recorded during the 5-minute span immediately following the corresponding EMA entry.
To disentangle between-person associations between distress level and HRV/HR from within-person associations, grand-mean standardized (GMS) distress scores were generated by calculating each individual’s mean distress level across the observation period and z-scoring these in relation to those of the other participants in the sample. Resulting GMS scores captured interindividual differences in distress level. Individual-mean standardized (IMS) scores were then calculated by using each individual’s mean distress level and corresponding standard deviation to z-score the distress levels recorded at each reading. These IMS scores captured intraindividual variability in distress levels independent of between-person differences. Namely, each participant had a mean IMS distress score of 0 and a standard deviation of 1.
In a first step, both GMS and IMS scores were entered as predictors of HRV/HR as were baseline total DTS scores. These main-effects models, in accordance with MacKinnon’s approach for testing mediation (14), would determine whether there was a significant unique effect of interindividual differences in distress scores on HRV/HR and thus the possibility that higher overall distress levels mediates the association between PTSD symptom severity and elevated physiological arousal. In a second step, the first-order interactions of PTSD symptom severity with the GMS and IMS distress levels were added to determine whether the association between PTSD symptom severity and HRV/HR is contextual, such that higher PTSD symptom severity is associated with reduced autonomic recovery following moments of acute distress. Age, smoking status, and momentary activity level were entered as covariates given their known association with HRV/HR.
Results
Participants underwent combined EMA and HRV monitoring for a mean of 18.31 hours (SD = 6.14) and recorded a mean of 7.40 diary entries (SD = 2.94) during that time. Participants with PTSD reported higher distress during these readings than participants without PTSD and demonstrated lower mean LF and HF HRV and higher mean HR during the 5-minute intervals following the EMA readings (see Table 1). In bivariate analyses, the prediction that PTSD severity would be positively associated with mean distress levels, r(217) = .61, p < .001, and heart rate, r(214) = .26, p < .001, and negatively associated with mean LF, r(214) = −.30, p < .001, and HF HRV, r(214) = −.22, p < .001, was borne out.
Table 1.
Participant Characteristics by PTSD Status
| Variable | No PTSD (n = 120) | PTSD (n = 99) | Difference Test |
|---|---|---|---|
| Age (years) | 27.80 (5.47) | 30.32 (5.42) | t(217) = 3.41, p < .001 |
| Females | 64 (53%) | 49 (49%) | X2(1) = 0.32, p = .57 |
| Minority Status | 57 (49%) | 60 (61%) | X2(1) = 3.36, p = .067 |
| DTS Total | 17.06 (23.96) | 70.32 (32.14) | t(217) = 14.03, p < .001 |
| Smoking Status | X2(3) = 16.08, p = .001 | ||
| 0 - Non-smoker | 75 (63%) | 37 (37%) | |
| 1 - Former smoker | 17 (14%) | 19 (19%) | |
| 2 - Smokes ≤ 10 cigs/day | 11 (9%) | 24 (24%) | |
| 3 - Smokes >10 cigs/day | 17 (14%) | 19 (19%) | |
| Mean EMA Distress | 1.20 (0.33) | 1.65 (0.54) | t(217) = 7.41, p < .001 |
| Mean EMA LF HRV (ms) | 43.70 (16.86) | 36.92 (14.69) | t(214) = 3.12, p = .002 |
| Mean EMA HF HRV (ms) | 26.36 (14.58) | 22.58 (12.33) | t(214) = 2.03, p = .043 |
| Mean EMA Heart Rate (bpm) | 82.93 (12.56) | 87.24 (11.78) | t(214) = 2.58, p = .011 |
Note. DTS Total = Davidson Trauma Scale total score; EMA = ecological momentary assessment; LF HRV = low-frequency heart-rate variability; HF HRV = high-frequency heart-rate variability.
Turning to MLM, analysis of null models (i.e., models without predictors) indicated that 60% of the variance in LF HRV, 60% of the variance in HF HRV, and 51% of the variance in HR were attributable to interindividual differences as opposed to intraindividual variability. Results from full models with predictors are depicted in Table 2. According to the first-step main-effects models, interindividual differences in distress (GMS) were not significantly predictive of HRV/HR after controlling for intraindividual variability in distress (IMS) and baseline PTSD symptom severity. This suggests that greater overall psychological distress does not account for previously observed associations between PTSD symptom severity and HRV/HR. In the second-step interaction models, each of the interactions between baseline PTSD symptom severity and momentary distress were significant, whereas none of the interactions between baseline PTSD symptoms severity and interindividual differences in distress were significant.
Table 2.
Multilevel models of low- and high-frequency HRV, heart rate
| LF Amplitude (ms) | HF Amplitude (ms) | Heart Rate (BPM) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Parameter | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 |
| Within-Person | ||||||
| Intercept | 67.40** (5.49) | 67.74** (5.61) | 56.35** (4.49) | 57.52** (4.58) | 71.84** (4.46) | 71.97** (4.53) |
| Sittinga | −1.51 (0.95) | −1.73† (0.96) | −4.20** (0.78) | −4.39** (0.79) | 5.17** (0.78) | 5.23** (0.77) |
| Light activity (standing up)a | −2.56* (1.05) | −2.83** (1.06) | −8.65** (0.86) | −9.01** (0.87) | 11.91** (0.86) | 11.99** (0.86) |
| Heavy activity (standing up)a | −11.01** (2.06) | −11.50** (2.08) | −13.65** (1.69) | −13.94** (1.71) | 18.09** (1.68) | 18.18** (1.68) |
| Distress (IMS) | −1.14** (0.37) | −0.97* (0.38) | −0.57† (0.31) | −0.44 (0.31) | 0.49 (0.30) | 0.39 (0.31) |
| Between-Person | ||||||
| Age | −0.80** (0.18) | −0.82** (0.19) | −0.85** (0.15) | −0.89** (0.15) | 0.14 (0.15) | 0.14 (0.15) |
| Smoking status | −2.41* (0.96) | −2.49* (0.97) | −2.28** (0.78) | −2.38** (0.79) | 2.40** (0.78) | 2.39** (0.78) |
| PTSD Sx | −2.50† (1.33) | −2.24 (1.36) | −1.34 (1.09) | −1.25 (1.11) | 2.67* (1.09) | 2.57* (1.10) |
| Distress (GMS) | 0.44 (1.24) | −0.37 (1.56) | 1.06 (1.02) | 1.00 (1.27) | −0.94 (1.01) | −0.60 (1.25) |
| PTSD Sx X Distress (GMS) | - | 0.98 (1.07) | - | 0.13 (0.87) | - | −0.39 (0.86) |
| Cross-Level Interaction | ||||||
| PTSD Sx X Distress (IMS) | - | −0.97* (0.39) | - | −0.64* (0.32) | - | 0.73* (0.32) |
Note. Model coefficients and standard errors (in parentheses). LF = low-frequency; HF = high-frequency; Distress (IMS) = individual-mean standardized distress score; PTSD Sx = PTSD symptom severity; Distress (GMS) = grand-mean standardized distress score.
Prostrate position used as reference value.
p < .10,
p < .05,
p < .01
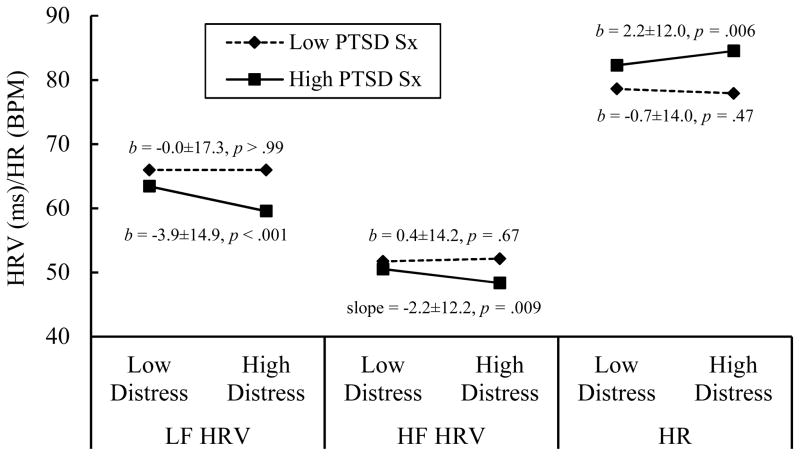
Follow-up analyses revealed that individuals with higher PTSD symptom severity demonstrated reduced HRV and elevated HR following high levels of momentary distress (see Figure 1). No differences in HRV and HR were observed across distress levels for people with low PTSD symptom severity, reflecting minimal physiological arousal following acute distress. Moreover, PTSD symptom severity was not associated with HRV/HR in moments of low distress, ps > .10. Thus, these findings support our hypothesis that interindividual differences in PTSD symptom severity are associated with distinct autonomic responses to momentary distress rather than generalized patterns of autonomic activation.
Figure 1.
Modeled levels of HRV and HR as a function of continuous baseline PTSD symptom severity and continuous momentary distress level. Low and high PTSD symptom severity were calculated as 1-standard deviation offsets from the sample mean. Low and high distress were calculated as 1-standard deviation offsets from individuals’ personal means. Simple slopes (b) plus/minus their standard deviations and p-values are presented. PTSD Sx = PTSD symptom severity; LF HRV = low-frequency heart-rate variability; HF HRV = high-frequency heart-rate variability; HR = heart rate.
Discussion
In this study, we employed EMA measurement of subjective momentary emotional distress and ambulatory monitoring of minute-to-minute HR and HRV to explore the relationship of PTSD symptom severity with physiological arousal and autonomic recovery. We examined two hypotheses concerning these linkages. The first hypothesis was that baseline PTSD symptom severity would be associated with higher mean distress levels, which would in turn be associated with higher mean physiological arousal (i.e., higher HR and lower LF and HF HRV). The second alternative hypothesis was that interindividual differences in PTSD symptom severity would be evident primarily during moments of acute distress. Specifically, we predicted that individuals with high, as opposed to low, PTSD symptom severity would demonstrate heightened physiological arousal and thus minimal autonomic recovery following moments of high self-reported distress.
Although we found evidence in bivariate analysis of aggregate data that baseline PTSD symptom severity was associated with overall higher distress levels observed during EMA monitoring, MLM analyses revealed that overall distress levels did not drive the association of PTSD symptom severity and HRV/HR. Specifically, individual differences in distress were not significantly associated with LF HRV, HF HRV, or HR, whether by main effect or via interaction with baseline PTSD symptom severity. However, momentary distress levels were associated with subsequent HRV/HR via an interaction with baseline PTSD symptom severity such that, as PTSD symptom severity increased, so too did physiological arousal following periods of acute distress. Thus, whereas people with low PTSD symptom severity did not demonstrate increased HR and decreased HRV subsequent to reporting acute distress, people with high PTSD symptom severity did. This is consistent with the notion that the PNS acts as a “brake” on fight-or-flight responses, but that traumatic experiences can disable this brake (15).
These findings have a number of potentially important implications. If the crux of autonomic dysfunction in PTSD resides in the intervals following acute distress, developing interventions that help patients gain greater control over their physiological arousal during these moments should theoretically reap greater benefits than those focusing on generalized distress levels. There are already several interventions that aim to do just this, including biofeedback (16) and mindfulness-based interventions (17). However, applying these principles in situ can prove challenging (16). A real potential lies in tapping mobile-health technology to aid in the detection of adverse states of arousal and to prompt patients in those moments to apply skills learned in therapy sessions (18). Beyond enhancing patients’ capacity to manage acute psychological distress, such interventions also have the potential to modulate deleterious patterns of autonomic dysfunction. Increased HR and attenuated HRV are known to convey cardiovascular risk (19, 20). If specific episodes of acute distress are the primary contributors to increased HF and attenuated HRV, as suggested by our findings, targeting these episodes could also return substantial health benefits.
Of course, interpretation of these findings must take into account certain limitations. Subjective distress was measured in response to random prompts during the monitoring period; as such, the antecedents of acute distress were unknown, as were the onset and trajectory of distress levels. By using HR and HRV measurements taken directly after each diary entry, we made an assumption that any variance in the trajectory of distress levels across the readings was, apart from individual differences in distress management, randomly distributed across readings and participants. Given that we were expressly modeling individual differences in autonomic functioning following distress, we stand by our interpretation of the present findings. Also, whereas HF HRV is strongly associated with PNS activity, the mechanisms underlying LF HRV are less clear (21). Thus, interpretation of that finding is limited. Ultimately, we believe that this study provides unique information regarding the complex relationship between PTSD, distress, and autonomic recovery from physiological arousal, with potentially important implications for treatment.
Acknowledgments
Source of Funding: Preparation of this work was supported by the National Institute of Mental Health (2R01MH062482), the Durham, NC Veterans Affairs Medical Center; and the Department of Veterans Affairs office of Research and Development Clinical Science. This project was also supported by Award Number 1IK2CX000718 to Dr. Dedert from the CSR&D Service of the VA Office of Research and Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Abbreviations
- PTSD
posttraumatic stress disorder
- PNS
parasympathetic nervous system
- SNS
sympathetic nervous system
- HR
heart rate
- HRV
heart-rate variability
- HF
high-frequency
- LF
low-frequency
- EMA
ecological momentary assessment
- CAPS
Clinician-Administered PTSD Scale
- DTS
Davidson Trauma Scale
- MLM
multilevel modeling
- GMS
grand-mean standardized
- IMS
individual-mean standardized
Footnotes
Conflicts of Interest: The authors have no financial disclosures to make or conflicts of interest to report.
References
- 1.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–43. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 2.Orr SP, Meyerhoff JL, Edwards JV, Pitman RK. Heart rate and blood pressure resting levels and responses to generic stressors in Vietnam veterans with posttraumatic stress disorder. J Trauma Stress. 1998;11:155–64. doi: 10.1023/A:1024421502881. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers JA, Quintana DS, Maree J, Abbott A, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry. 2014;5:1–11. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force of the European Society of Cardiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–81. [PubMed] [Google Scholar]
- 5.Bikkina M, Alpert MA, Mukerji R, Mulekar M, Cheng B-Y, Mukerji V. Diminished short-term heart rate variability predicts inducible ventricular tachycardia. Chest Journal. 1998;113:312–6. doi: 10.1378/chest.113.2.312. [DOI] [PubMed] [Google Scholar]
- 6.Huikuri HV, Jokinen V, Syvänne M, Nieminen MS, Airaksinen KJ, Ikäheimo MJ, Koistinen JM, Kauma H, Kesäniemi AY, Majahalme S. Heart rate variability and progression of coronary atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1979–85. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 7.Rissling MB, Dennis PA, Watkins LL, Calhoun PS, Dennis MF, Beckham JC, Ulmer C. Circadian contrasts in heart rate variability associated with PTSD symptomatology in a young adult cohort. Journal of Traumatic Stress. doi: 10.1002/jts.22125. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green KT, Dennis PA, Neal LD, Hobkirk AL, Hicks TA, Watkins LL, Hayano J, Sherwood A, Calhoun PS, Beckham JC. Exporing the relationship between posttraumatic stress disorder symptoms and momentary heart rate variability. J Psychosom Res. doi: 10.1016/j.jpsychores.2016.01.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake DD, Weathers FS, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 10.Dennis PA, Watkins L, Calhoun PS, Oddone A, Sherwood A, Dennis MF, Rissling MB, Beckham JC. Posttraumatic stress disorder, heart-rate variability, and the mediating role of behavioral health risks. Psychosom Med. 2014;76:629–37. doi: 10.1097/PSY.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg MA, Mellman T, Beckham JC, Smith RD, Davidson RM, Katz R, Feldman ME. Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychol Med. 1997;27:153–60. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- 12.Hayano J, Taylor JA, Yamada A, Mukai S, Hori R, Asakawa T, Yokoyama K, Watanabe Y, Takata K, Fujinami T. Continuous assessment of hemodynamic control by complex demodulation of cardiovascular variability. American Journal of Physiology-Heart and Circulatory Physiology. 1993;264:H1229–H38. doi: 10.1152/ajpheart.1993.264.4.H1229. [DOI] [PubMed] [Google Scholar]
- 13.Searle SR, Casella G, McCulloch CE. Variance components. New York: Wiley; 1992. [Google Scholar]
- 14.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale LP, Carroll LE, Galen G, Hayes JA, Webb KW, Porges SW. Abuse history is related to autonomic regulation to mild exercise and psychological wellbeing. Applied Psychophysiology and Biofeedback. 2009;34:299–308. doi: 10.1007/s10484-009-9111-4. [DOI] [PubMed] [Google Scholar]
- 16.Peira N, Fredrikson M, Pourtois G. Controlling the emotional heart: Heart rate biofeedback improves cardiac control during emotional reactions. Int J Psychophysiol. 2014;91:225–31. doi: 10.1016/j.ijpsycho.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Satyapriya M, Nagendra HR, Nagarathna R, Padmalatha V. Effect of integrated yoga on stress and heart rate variability in pregnant women. International Journal of Gynecology and Obstetrics. 2009;104:218–22. doi: 10.1016/j.ijgo.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Gaggioli A, Cipresso P, Serino S, Campanaro DM, Pallavicini F, Wiederhold BK, Riva G. Positive technology: A free mobile platform for the self-management of psychological stress. Annual Review of Cybertherapy and Telemedicine. 2014;199:25–9. [PubMed] [Google Scholar]
- 19.Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: A cardiovascular risk factor? European Heart Journal. 2006;27:2387–93. doi: 10.1093/eurheartj/ehl259. [DOI] [PubMed] [Google Scholar]
- 20.Dekker JM, Crow RS, Folsom A, Hanna PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC study. Circulation. 2000:102. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 21.Heathers JA. Everything Hertz: Methodological issues in short-term frequency-domain HRV. Frontiers in Physiology. 2014;5:1–15. doi: 10.3389/fphys.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]