Abstract
Study Objective
To study whether adolescents with the classical form of polycystic ovary syndrome have alterations in metabolic and vascular structure and function. The effect of metformin was evaluated.
Design
Controlled study
Setting
University outpatient clinic
Participants
Eighteen PCOS non obese adolescents were enrolled. Seventeen healthy age-matched adolescents were recruited as controls.
Interventions
The metabolic profile and the endothelial structure and function were evaluated.
Main Outcome Measure(s)
Hormonal and lipid profile, blood pressure (BP) measurement, fasting glucose and insulin levels, C-reactive protein (CRP), homocysteine, tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1) and plasmin-antiplasmin complexes (PAP) were measured. Flow mediated dilation (FMD), central pulse wave velocity (PWV), radial artery pulse wave (AIx) and common carotid intima-media thickness (IMT) were also assessed. PCOS girls were also studied 6 months after treatment with metformin (850 mg bid).
Results
PCOS adolescents were insulin resistant and/or hyperinsulinemic and they had higher BP values and levels of CRP and PAI-1 than the controls. The levels of t-PA and PAP were similar in both groups. FMD, PWV and IMT were also similar. Metformin significantly (p<0.05) reduced insulin, BP, CRP and PAI-1 levels. The PAP levels significantly (p<0.05) increased. Radial AIx was significantly reduced after metformin. No modifications in FMD, PWV and IMT were observed.
Conclusions
Adolescents with classical PCOS have alterations in some surrogate markers of cardiovascular risk and they are ameliorated by metformin. No deterioration of vascular structure and function has been detected, probably due to the short duration of exposure to the disease.
Keywords: Endothelium, arterial stiffness, fibrinolysis, metformin, PCOS
INTRODUCTION
The Polycystic Ovary Syndrome (PCOS) is a complex endocrine disorder, affecting up to 10% of women of reproductive age, characterized by chronic anovulation, oligomenorrhoea and hyperandrogenism1,2. Polycystic appearance of ovaries may be present. Moreover the existence of a metabolic derangement has been widely documented3-6. Vascular alterations such as endothelial dysfunction, increased arterial stiffness and intima-media thickness, have been shown to be prevalent among women with PCOS7-9. In addition, PCOS women are also characterized by increased plasma levels of PAI-110,11, and therefore by a hypofibrinolytic state. The increased levels of PAI-1 are strongly related to insulin resistance and predictive of the development of diabetes12, and there is substantial experimental and epidemiological evidence that PAI-1 plays a role in the pathogenesis of atherosclerosis and related thrombotic complications13. Most women with PCOS also exhibit some or most components of the metabolic syndrome, including obesity, hypertension, dyslipidemia as well as insulin resistance6. The hyperandrogenic and insulin resistance states with reactive hyperinsulinemia appear to play a critical role6. As a consequence the phenotype with hyperandrogenemia and insulin resistance seems to be at a higher metabolic risk3.
PCOS becomes clinically evident in adolescence and metabolic alterations could be present from the first years after the menarche14. At this age educational programs aimed at promoting diet and physical exercise might play a very important role in the prevention of future cardiovascular events. This approach must be regarded as the first-line therapy but sometimes it is difficult to be followed by adolescents. Despite limited randomized controlled trials of insulin sensitizers in adolescents with PCOS, data from adult studies have led to the use of these medications, specifically metformin, also in young PCOS girls. Thus, in some cases a pharmacological approach with insulin sensitizers may be considered in the presence of a low compliance with or when individualized exercise and eating programs have not been effective.
In the present study, we evaluate if the metabolic and the vascular alterations described in adults are present in adolescents with PCOS from the first years of the fertility age. Moreover in the same study it has been evaluated if a treatment with metformin is able to modify the above parameters.
MATERIAL AND METHODS
Study population
In this study, 18 adolescents (age 18±1 years) with PCOS were consecutively recruited from the newly diagnosed outpatient cases in the Reproductive Endocrinology Clinic at the Department of Obstetrics and Gynaecology, seventeen healthy subjects without PCOS being recruited from students as controls (age 20±1 years). Because of the young age and the enrolled population of PCOS being adolescents, diagnosis of PCOS was made according to Rotterdam criteria taking into account the suggestions of Carmina et al15. Accordingly only girls with a contemporary presence of clinical and/or biochemical evidence of hyperandrogenism (defined as total testosterone (tT) more than 0.8 ng/ml, and/or androstenedione (A) more than 3.1 ng/ml, after exclusion of other pathologies), oligomenorrhea (intermenstrual interval of 36 days or longer) and ovarian volume >10 cc were enrolled. All girls with elevated T levels had the free androgen index (FAI) over normal values. As a consequence all PCOS girls had the classic and more severe form of PCOS16. As for pelvic ultrasound, when the transvaginal route was not feasible, the examination was performed abdominally. Only subjects with insulin resistance or hyperinsulinemia evidenced with an oral glucose tolerance test (OGTT) were selected for the study. Hyperinsulinemia was defined as a value of the area under the curve (AUC) for insulin after a 75 g of glucose load over the cut-off of our lab obtained in normal women. Insulin-resistance was defined as an homeostasis model assessment of insulin resistance index (HOMA-IR) >than 2.5. No subjects had an abnormal glucose response to the OGTT.
Subjects with congenital adrenal hyperplasia, hyperprolactinaemia, thyroid disease or Cushing syndrome were excluded.
To avoid the possibility that other conditions might affect the results, exclusion criteria included history of cardiovascular disease, diabetes mellitus and use of any pharmacological treatment in the previous 6 months. Information on current smoking status (smoker/non-smoker) was also collected. Girls smoking more than 5 cigarettes a day were excluded from the study.
Study design
All the PCOS adolescents and healthy subjects were studied during the follicular phase (3-7 days after the onset of a spontaneous or progestin-induced menstrual cycle). After baseline evaluations (see below), patients with PCOS were treated with metformin 850 mg (Glucophage, Merck, Italy), twice daily, for 6 months. Patients were instructed to take tablets with their meals. Throughout the study no changes in lifestyle were implemented, and patients were instructed to follow their habitual diet and physical activity and to use a barrier contraceptive.
Experimental procedures
All the experimental procedures here described were performed on each patient with PCOS before (baseline evaluation) and during the 6th month of metformin treatment.
At entry, height and weight were measured and the BMI was calculated. Blood samples were obtained between 08.00 and 08.30 a.m. after an overnight fast for the determination of LH, FSH, estradiol (E2), sex hormone-binding globulin (SHBG), 17-hydroxyprogesterone (17OHP), tT, DHEA-S, and A. Moreover, blood samples for the determination of the levels of glucose, insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides, homocysteine, protein C reactive (CRP) were collected. The tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1) and plasmin-antiplasmin complexes (PAP) were also measured. The endothelial function and vascular structure parameters were also evaluated.
The endothelium-dependent response was assessed as dilation of the brachial artery to increased flow (flow mediated dilation, FMD) as previously described17. Briefly, a B-mode scan of the right brachial artery was obtained in longitudinal section between 5 cm and 10 cm above the elbow using a 7.5 MHz linear array transducer, held by a stereotactic clamp to ensure a constant image. B-mode images were triggered to the ECG signal to obtain only end-diastolic frames. The arterial flow velocity was obtained by a pulsed Doppler signal at 70° to the vessel with the range gate (1.5 mm) in the centre of the artery. A cuff was placed around the forearm just below the elbow and was inflated for five minutes at 250 mmHg and then deflated to induce reactive hyperaemia.
Endothelium-independent dilation was obtained by the administration of a low dose (25 μg) of sublingual glyceril trinitrate (GTN). Brachial artery diameter (BAD) measurements were performed after studying the acquired frames by the computerized edge detection system18. The baseline vessel size was considered as the mean of measurements obtained during the first minute. The FMD and response to GTN were calculated as the maximal percent increase in diameter above the baseline. The coefficient of variation for the FMD in repeated studies was 15%18. The doppler flow velocity was measured at the baseline and within 15 seconds after cuff release. The volume blood flow was calculated by multiplying the Doppler flow velocity (corrected for the angle) by heart rate and vessel cross-sectional area (πr2). Reactive hyperaemia (RH) was calculated as the maximum percent increase in flow after cuff release as compared to the baseline flow.
Tonometry was performed by a trained operator (C.G.) according to international recommendations19. After an overnight fast, measurements were performed with the subjects in the supine position in a quiet, air-conditioned room (22-24°C). A hand-held probe was placed on the artery and 10-15 subsequent images were recorded. The pulse wave analysis (PWA) (SphygmoCor, AtCor Medical, Sydney, Australia) was used to transform the radial pressure waveform into an aortic pressure one by using a validated transfer function. Three successive measurements were recorded. Augmented pressure was calculated as the difference between the second and the first systolic peak and the augmentation index was calculated as the ratio between the augmented pressure and pulse pressure (PP). Time to reflection (Tr) and central blood pressure were also obtained from the PWA. Since the augmentation index correlated with the heart rate, values were normalized at a heart rate of 75 beats/min. Peripheral and central pulse wave velocities (PWV) were assessed with the same device, recording waveforms at the radial or femoral and carotid site sequentially (Radial Aix). The surface distance between the two recording sites was measured (SphygmoCor®, AtCor Medical, Sydney, Australia). A simultaneously recorded ECG was used as a reference frame to calculate wave transit time.
In our laboratory the coefficient of variation in repeated studies was 14% and 13% for the AIx and PWV, respectively20.
Finally, the IMT was assessed at the baseline by high resolution B-mode ultrasound with a 7.5-10 MHz linear array transducer (MyLab25; ESAOTE). Longitudinal scans of the left and right common carotid arteries were examined by a certified operator (CG). The IMT was measured in the posterior (far) wall of the common carotid artery, 1 cm proximal to the carotid bulb by utilizing an automatic edge detection system 18.
Biochemical Assays
Blood samples were collected in adequate tubes and immediately placed on ice. Plasma was immediately centrifuged at −4°C at 3000 rpm for 15 minutes and stored at −70 °C until assayed. The samples were assayed in duplicate all together in order to avoid inter-assay errors.
Total serum cholesterol, triglycerides, HDL-cholesterol and glucose were assessed by enzymatic methods (Roche, Diagnostic). LDL-cholesterol was calculated by Friedewalds’ equation. Insulin was determined by an immunoradiometric assay (DiaSorin S.pA., Vercelli, Italy). The intra-assay and inter-assay CV for the insulin assay were 2.1% to 2.6% and 2.9% to 4.7%, respectively. Plasma homocysteine levels were measured by HPLC (Chromosystem Instruments & Chemicals. GmbH, Germany). High sensitive CRP plasma levels were measured by enzyme-linked immunosorbent assay (DRG Diagnostics. GmbH, Germany). The concentrations of t-PA, PAI-1 and PAP were measured by ELISA (Technoclone GmbG, Wien, Austria). All of the samples were assayed in duplicate on the same test plate. Intra-assay and inter-assay variation coefficients were <10%.
Plasma LH, FSH and E2 concentrations were determined by immunometric assays (Johnson&Johnson S.p.A-Ortho Clinical Inc., Rochester, NY). Plasma levels of A were determined by using a radioimmunoassay (Biosource Europe S.A, Nivelles, Belgium). The intra-assay and inter-assay CV for the A assay were 3.2% to 4.5% and 5.9% to 9.0%, respectively. Total T concentrations were determined by using a competitive immunoassay (Johnson & Johnson S.p.A-Ortho Clinical Inc.). The intra-assay and inter-assay CV of tT were 2.3% to 3.1% and 4.9% to 7.0%, respectively. The concentrations of DHEA-S were determined by using a radioimmunoassay (Orion Diagnostica, Espoo, Finland). The intra-assay and inter-assay CV for the DHEAS assay were 3.5% to 6.5% and 4.0% to 8.1%, respectively. 17OHP concentrations were determined by using a radioimmunoassay (MP Biomedicals, Orangeburg, NY). The intra-assay and inter-assay CV of the 17OHP assay were 3.5% to 6.5% and 4.0% to 8.1%, respectively.
Data analysis
Descriptive data are expressed as means ± SD. Characteristics of the PCOS girls and healthy controls were compared using the Student’s t-test or the Wilcoxon signed-rank test for non-parametric values, as appropriate. General linear model analysis of variance was used for repeated measurement comparisons. All statistical procedures were performed using the NCSS statistical package, (NCSS, Kaysville, Utah, USA). Differences were considered statistically significant when p< 0.05. The sample size of the present study was calculated to reach a power ≥ 0.8 to detect a significant change in all parameters.
Ethical approval
The local Institutional Review Board approved the protocol and all study participants or their parents (if the age was less than 18 yrs) gave written informed consent for the study.
RESULTS
The clinical characteristics of the study population at the baseline are shown in Tables 1 and 2. Age and smoking status were not significantly different between the two groups. As expected the PCOS girls showed higher plasma levels of A, tT and DHEAS and a higher FAI. Moreover, lower values of SHBG were found.
Table 1.
Clinical Characteristics and Hormonal Parameters of the Study Population
| Healthy subjects (n=17) |
PCOS (n=18) |
PCOS during metformin (n=18) |
|
|---|---|---|---|
| Age, years | 20±1 | 18±1 | 18±1 |
| SBPa, mmHg | 112.7±6.7 | 123.1±10.0* | 116.1±9.1† |
| DBPb, mmHg | 67.0±4.9 | 75.6±8.8* | 70.9±8.7† |
| BMIc, Kg/m2 | 22.5±1.5 | 26.8±6.3* | 25.8±5.6 |
| Androstenedione, ng/dL | 2.0±0.7 | 3.33±0.7** | 2.7±0.3† |
| TotalTestosterone, ng/dL | 60.1±11 | 123.1±12 ** | 84.7±19† |
| SHBGd, nmol/L | 26.4±4.4 | 20.3±8.3** | 24.7±9.8† |
| DHEASe, μg/dL | 142±34 | 321±90** | 267±58† |
| FAIf, % | 2.4±0.9 | 9.3±3.2** | 4.1±1.8† |
Data are presented as the mean ± SD or number of subjects.
systolic blood pressure;
diastolic blood pressure;
body mass index;
sex hormone-binding globulin;
dehydroepiandrosterone sulfate;
free androgen index;
p<0.01 vs. healthy subjects;
p<0.001 vs. healthy subjects,
p<0.05 vs PCOS patients at baseline.
Table 2.
Metabolic, Inflammatory and Fibrinolytic Parameters of Healthy Subjects and of PCOS Adolescents before and after treatment with metformin
| Healthy subjects (n=17) |
PCOS (n=18) |
PCOS during metformin (n=18) |
|
|---|---|---|---|
| Total cholesterol, mg/dL | 157.3±10.1 | 170.1±22.3* | 168.5±33.1 |
| HDLa cholesterol, mg/dL | 58.2±10.9 | 53.6±9.1 | 53.7±14.5 |
| LDLb cholesterol, mg/dL | 86.2±9.6 | 106.7±7.1* | 95.8±14.2† |
| Triglycerides, mg/dL | 63.8±8.2 | 68.9±11.2 | 67.6±11.1 |
| Fasting glucose, mg/dL | 76.5±9.6 | 78.1±7.5 | 77.6±8.3 |
| Fasting insulin, U/mL | 11.7±8.7 | 19.4±8.8* | 13.4±6.8† |
| HOMA IRc | 2.2±1.1 | 3.9±1.8* | 2.6±1.5† |
| Homocysteine, μmol/L | 8.5±1.7 | 8.7±1.3 | 8.9±2.1 |
| CRPd, mg/L | 1.2±0.5 | 5.6±1.5** | 3.9±1.3† |
| t-PAe, ng/mL | 1.8±0.3 | 1.9±0.7 | 2.8±1.2 |
| PAI-1f, ng/mL | 15.9±4.7 | 38.2±7.9** | 25.2±9.2† |
| PAPg, ng/mL | 12.4±1.6 | 9.3±1.4** | 14.3±10.1† |
Data are presented as the mean value ± SD.
high density lipoprotein;
low density lipoprotein;
homeostasis model assessment for insulin resistance.
C-Reactive Protein;
tissue-type plasminogen activator;
Plasminogen activator inhibitor-1;
plasmin-antiplasmin complexes.
p<0.01 vs. healthy subjects;
p<0.001 vs. healthy subjects;
p<0.05 vs PCOS at baseline.
Adolescents with PCOS showed higher systolic and diastolic blood pressure, BMI, levels of fasting insulin, CRP and LDL-cholesterol compared to the controls (Table 2). Higher values of HOMA-IR were found in the PCOS adolescents compared to the controls. In contrast, levels of fasting glucose, total cholesterol, HDL-cholesterol and triglycerides were similar in both the PCOS patients and healthy women (Table 2). Plasma levels of PAI-1 were significantly higher in the PCOS adolescents compared to healthy women, while levels of t-PA were similar in both groups. PAP levels were significantly reduced in the PCOS adolescents compared to the controls (Table 2). In the PCOS adolescents, PAI-1 levels were significantly related to CRP (r=0.58; p<0.01) and to HOMA-IR (r=0.59; p<0.001).
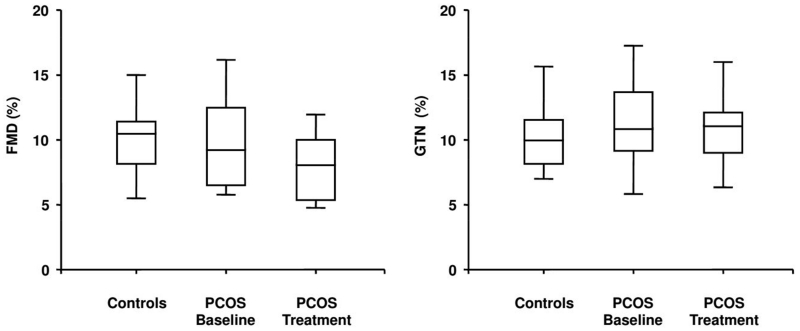
The FMD was similar in the PCOS adolescents compared to the healthy controls, as well as the response to GTN (Figure 1). No correlation was found between IR and FMD. No significant difference was found in RH (497±189 vs 503±211 %), and basal BAD in both the PCOS adolescents and controls (3.1±0.2 vs 2.9±0.2 mm, respectively).
Figure 1.
Box plots show endothelium-dependent (Flow mediated dilation: FMD) and independent (GTN) vasodilation in the controls and in the PCOS adolescents at the baseline and after 6 months of treatment with metformin.
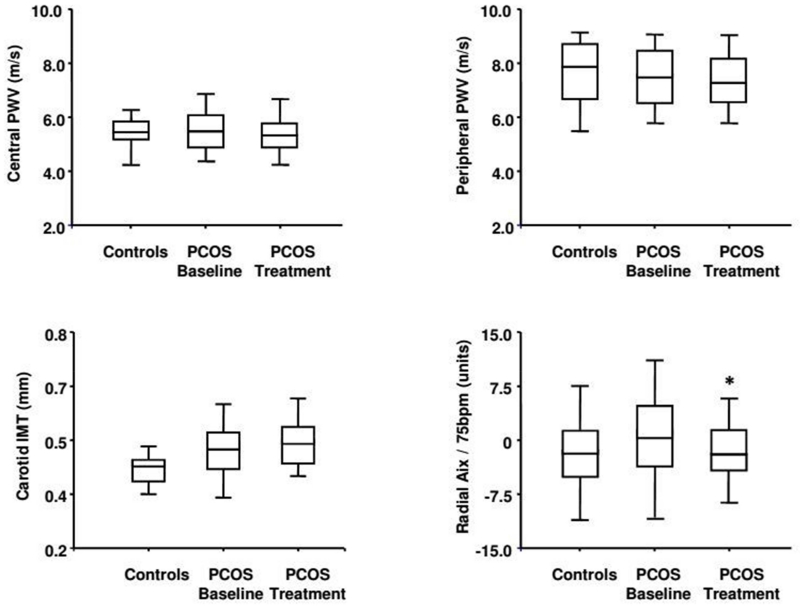
Peripheral and central PWV were not different between the PCOS patients and controls, in the same way with carotid IMT (Figure 2). Radial AIx was higher, although not significantly, in the PCOS adolescents compared to the controls.
Figure 2.
Box plots show peripheral and central pulse wave velocity (PWV), radial augmentation index at 75 beat per minutes (Aix/75bpm) and carotid intima-media thickness (IMT) in the controls and in the PCOS adolescents at the baseline and after 6 months of treatment with metformin. * p<0.05 versus baseline
A significant reduction of tT, A, DHEAS and FAI was observed in the PCOS girls during treatment with metformin (Table 1). The plasma levels of SHBG significantly increased.
As for the metabolic parameters, after 6 months of treatment with metformin BMI, total cholesterol, triglycerides and fasting glucose were unchanged in the PCOS adolescents (Tables 1, 2). In contrast, systolic and diastolic blood pressure, LDL cholesterol, insulin levels and HOMA-IR significantly decreased (Tables 1, 2). Among the fibrinolytic parameters, the PAI-1 levels significantly reduced following 6 months of treatment, while the t-PA levels were unchanged (Table 2). In contrast, the PAP levels were significantly higher following treatment with metformin. Finally, the CRP levels, but not homocysteine, significantly decreased after treatment (Table 2). No significant modifications in FMD, GTN (Figure 1) and RH (497±189 vs 501±190 %) were observed after 6 months of treatment compared to the baseline.
No significant modification was observed in central and peripheral PWV, while radial AIx was significantly reduced after metformin (Figure 2) reaching the basal values of the control group.
DISCUSSION
During adolescence the possibility of making the diagnosis of PCOS is controversial. In fact, the same criteria (anovulation, hyperandrogenism and polycystic ovaries) that in adults are used for diagnosis, in adolescents may be transitory or in evolution21-23. According to previous suggestions15, to overcome errors in diagnosis only subjects after at least 2 years from the menarche were considered. Moreover only subjects with oligoamenorrhea, hyperandrogenism and ovarian volume >10 cc were enrolled. Then all hyperandrogenic adolescents had features similar to the classical phenotype of PCOS.
Controversial data are present in the literature regarding the presence of an elevation in cardiovascular risk factors in adolescents with PCOS. The heterogeneity of the syndrome may explain these contrasting findings11,14,24-27. The present study shows that adolescents with classical PCOS and insulin resistance and/or hyperinsulinemia are characterized by an inflammatory state as suggested by the presence of higher values of CRP, compared to the controls. No change in CRP was observed by Ganie et al25 in a larger sample of PCOS adolescents. Because of the correlation observed between CRP and BMI, the higher BMI of our adolescents may explain the different results confirming that the heterogeneity of this endocrine disorder may lead to different findings. The insulin resistance and elevation of CRP found in our group of adolescents may account for a hypofibrinolytic state, characterized by higher levels of PAI-1 and reduced PAP levels. Experimental data showed that CRP, other than insulin resistance, may promote PAI-1 expression in endothelial cells28 inducing high levels of PAI-1. In agreement with the above a positive correlation was observed between PAI-1 and CRP and PAI-1 and insulin resistance.
These results are in line with previous findings showing increased levels of PAI-110,11 and a reduced global fibrinolytic capacity29 in young women with PCOS.
In contrast to data of other authors7-9,30, we did not find changes in the vascular function, the IMT and peripheral and central arterial stiffness being similar to the controls. Moreover, an abnormal endothelial function was not detectable in these patients. Previous studies of Carmassi et al31 and Kuboki et al32 showed an impairment of insulin-mediated vasodilation in insulin-resistant states suggesting that insulin-resistance may affect insulin-induced nitric oxide release by endothelial cells. Moreover, Baron33 also demonstrated that endothelial-dependent vasodilation, but not endothelial-independent vasodilation, is impaired in insulin resistant states. In our study, in spite of the hyperinsulinemia and inflammatory state, our population of PCOS adolescents has the endothelium-dependent vasodilation at the level of peripheral conduit artery similar to that observed in the healthy controls. Endothelium-independent vasodilation is also similar. Differences in the methodology used may only partially account for the different results. However, the relatively young age of these PCOS patients may explain these findings. In fact Orio et al7 and Guleira et al30 reported an increased carotid IMT in young women with PCOS but the mean age of the subjects in these studies was higher than in our group. Moreover Kelly et al8 evidenced an increased arterial PWV in PCOS patients at the level of the brachial artery. However, also in this study the patients included were older and had a higher BMI, two conditions that might explain the different results.
Taking together the above results, the findings of the present study show that during the first years of reproduction, the classical PCOS phenotype is not associated with vascular alterations. In fact, despite the alteration of the metabolic and the androgen profile, adolescents with classical PCOS show no significant alteration of vascular structure and function. This is relevant also considering that all PCOS adolescents enrolled with the classical form had elevated androgen levels and the phenotype with increased androgens and insulin were considered as a higher metabolic risk16. As previously discussed, the possible explanation could be an effect of young age and then the duration of the disease. If this hypothesis is true, the duration of the disease and therefore the time of exposure to hormonal, metabolic and fibrinolytic alterations as well as to inflammation might play a crucial role in determining vascular alterations in the future. The fact that all our teenagers were not obese could also play a role.
A premature intervention focused at improving diet and promoting physical activity is obviously of great importance to modify the possible metabolic future of these young women and should be regarded as the first-line therapy mainly for overweight/obese patients34. However, in subjects with normal weight or with a low compliance to lifestyle interventions, insulin-sensitizers are proposed to ameliorate the metabolic profile. Despite limited randomized controlled trials with insulin sensitizers in adolescents with PCOS, data from adult studies have led to the use of these medications, specifically metformin, also in adolescents. In this study metformin was used. All girls with PCOS were insulin resistant and/or hyperinsulinemic. Concerning the effect of treatment with the insulin-sensitizer metformin, a significant reduction in fasting insulin levels and HOMA-IR was observed, in agreement with a previous result35. No significant modification of BMI was evidenced, suggesting a specific effect on the metabolic profile not dependent on BMI variation. In addition, metformin significantly reduced CRP levels as well as PAI-1 levels and significantly increased PAP levels. Overall, these findings demonstrate that in adolescents with PCOS metformin effectively produces a reduction of insulin resistance and of the inflammatory state as well as the drug having benefits on fibrinolysis.
Moreover, metformin treatment induced a significant reduction of systolic and diastolic blood pressure. When considering the well-known link between insulin resistance and blood pressure36, the reduction of blood pressure values in treated PCOS patients is not surprising. A reduction in peripheral wave reflection was also observed after metformin treatment, which could contribute to blood pressure reduction. These results may also suggest a major role of treatment at the microcirculatory site19,37 and a selective effect of treatment on peripheral wave reflection. One interesting finding is that the vascular and endothelial function continued to be normal and similar to the controls also after 6 months from the first determination. Because of the limited time of observation a possible role of metformin in avoiding a progression of the disease cannot be hypothesized.
The present study demonstrates that as previously described a derangement of metabolic, inflammatory, fibrinolityc parameters as well as blood pressure and peripheral wave reflections can be evidenced also in teenagers with PCOS. Conversely, in spite of the presence of hyperandrogenemia and insulin resistance no deterioration of vascular structure and function has been detected at this young age. This could be related to the duration of the disease and then to exposure to the disease. Treatment with metformin ameliorates hormonal, metabolic, inflammatory, fibrinolytic parameters as well as blood pressure. Future larger longitudinal studies performed by the use of standardized techniques, will help to clarify long term vascular consequences and whether the improvement of insulin resistance and therefore of metabolic, inflammatory and fibrinolytic parameters might protect from vascular alterations in women with PCOS, possibly modifying the progression of the disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin action associated with an increased risk of non-insulin-dependent diabetes mellitus. Am J Med. 1995;98:33S–39S. doi: 10.1016/s0002-9343(99)80057-6. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Carmina E. Cardiovascular risk and events in polycystic ovary syndrome. Climateric. 2009;12(Suppl 1):22–25. doi: 10.1080/13697130903003842. [DOI] [PubMed] [Google Scholar]
- 4.Conway GS, Agrawal R, Betteridge DJ, et al. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol Oxf. 1992;37:119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 5.Talbott E, Clerici A, Berga SL, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998;51:415–422. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orio F, Jr, Palomba S, Cascella T, et al. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:4588–4593. doi: 10.1210/jc.2003-031867. [DOI] [PubMed] [Google Scholar]
- 8.Kelly CJ, Lyall H, Petrie JR, et al. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–746. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 9.Kravariti M, Naka KK, Kalantaridou SN, et al. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 10.Tarkun I, Cantürk Z, Arslan BC, et al. The plasminogen activator system in young and lean women with polycystic ovary syndrome. Endocr J. 2004;51:467–472. doi: 10.1507/endocrj.51.467. [DOI] [PubMed] [Google Scholar]
- 11.Carmassi F, De Negri F, Fioriti R, et al. Insulin resistance causes impaired vasodilation and hypofibrinolysis in young women with polycystic ovary syndrome. Thromb Res. 2005;116:207–214. doi: 10.1016/j.thromres.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 14.Fruzzetti F, Perini D, Lazzarini V, et al. Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril. 2009;92:626–634. doi: 10.1016/j.fertnstert.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201–205. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Carmina E, Chu MC, Longo RA, et al. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–2549. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 17.Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–1286. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 18.Gemignani V, Faita F, Ghiadoni L, et al. A system for real-time measurement of the brachial artery diameter in B-mode ultrasound images. IEEE Trans Med Imaging. 2007;26:393–404. doi: 10.1109/TMI.2006.891477. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 21.Hickey M, Balen A. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update. 2003;9:493–495. doi: 10.1093/humupd/dmg038. [DOI] [PubMed] [Google Scholar]
- 22.Van Hoof MH, Voorhorst FJ, Kaptein MB, et al. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries for oligomenorrhea at age 18 years. Hum Reprod. 2004;19:383–392. doi: 10.1093/humrep/deh079. [DOI] [PubMed] [Google Scholar]
- 23.Viksten-Almstromer M, Hirschberg AL, Hagenfeltk K. Prospective follow-up of menstrual disorders in adolescence and prognostic features. Acta Obstet Gynecol Scand. 2007;87:1162–1168. doi: 10.1080/00016340802478166. [DOI] [PubMed] [Google Scholar]
- 24.Fruzzetti F, Perini D, Lazzarini V, et al. Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:335–343. doi: 10.1080/09513590802630146. [DOI] [PubMed] [Google Scholar]
- 25.Ganie MA, Hassan S, Nisar S, et al. High-sensitivity C-reactive protein (hs-CRP) levels and its relationship with components of polycystic ovary syndrome in Indian adolescent women with polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2014;30:781–784. doi: 10.3109/09513590.2014.924099. [DOI] [PubMed] [Google Scholar]
- 26.Baer TE, Milliren CE, Walls C, Di Vasta AD. Clinical Variability in Cardiovascular Disease Risk Factor Screening and Management in Adolescent and Young Adult Women with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol. 2015;28:317–323. doi: 10.1016/j.jpag.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 29.Yildiz BO, Haznedaroğlu IC, Kirazli S, et al. Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J Clin Endocrinol Metab. 2002;87:3871–3875. doi: 10.1210/jcem.87.8.8716. [DOI] [PubMed] [Google Scholar]
- 30.Guleria AK, Syal SK, Kapoor A, et al. Cardiovascular disease risk in young Indian women with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30:26–29. doi: 10.3109/09513590.2013.831835. [DOI] [PubMed] [Google Scholar]
- 31.Carmassi F, Morale M, Ferrini L, et al. Local insulin infusion stimulates expression of plasminogen activator inhibitor-1 and tissue-type plasminogen activator in normal subjects. Am J Med. 1999;107:344–350. doi: 10.1016/s0002-9343(99)00240-5. [DOI] [PubMed] [Google Scholar]
- 32.Kuboki K, Jiang ZY, Takahara N, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 33.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 34.Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4655–4663. doi: 10.1210/jc.2013-2385. [DOI] [PubMed] [Google Scholar]
- 35.Orio F, Jr, Palomba S, Cascella T, et al. Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: results of a 6-month study. J Clin Endocrinol Metab. 2005;90:6072–6076. doi: 10.1210/jc.2005-0965. [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 37.Izzo JL., Jr Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19:341–345. doi: 10.1097/01.hco.0000126581.89648.10. [DOI] [PubMed] [Google Scholar]