Abstract
This study tested the hypothesis that optimized microneedle patch formulations can stabilize trivalent subunit influenza vaccine during long-term storage outside the cold chain and when exposed to potential stresses found during manufacturing and storage. Formulations containing combinations of trehalose/sucrose, sucrose/arginine, and arginine/heptagluconate were successful at retaining most or all vaccine activity during storage at 25°C for up to 24 months as determined by ELISA assay. The best formulation of microneedle patches contained arginine/heptagluconate, which showed no significant loss of vaccine activity during the study. To validate these in vitro findings, mice were immunized using trivalent influenza vaccine stored in microneedle patches for more than one year at 25°C, which elicited antibody titers greater than or equal to fresh liquid vaccine delivered by intradermal injection, indicating the retention of immunogenicity during storage. Finally, influenza vaccine in microneedle patches lost no significant activity during exposure to 60°C for four months, multiple freeze-thaw cycles, and electron beam irradiation. We conclude that optimally formulated microneedle patches can retain influenza vaccine activity during extended storage outside the cold chain and during other environmental stresses, which suggests the possibility of microneedle patch storage on pharmacy shelves without refrigeration.
Background
Influenza is estimated to cause up to five million illnesses and five hundred thousand deaths each year.(1) Annual seasonal influenza vaccination is recommended by the Centers for Disease Control and Prevention(2), and more than 148 million people received the vaccine in the United States during the 2014-15 influenza season.(3) As a result, there is significant cost, complex logistics and vaccine spoilage due to the need to distribute and store the vaccine in the cold-chain .(4) Elevated temperatures as well as exposure to freeze-thaw cycles have been shown to adversely affect the stability of seasonal influenza vaccine.(5)
One method to maintain the activity of vaccines, or proteins in general, is lyophilization. For many proteins, preservation in a dry state enables storage for longer time or without refrigeration.(6) In a dry state, protein mobility is greatly reduced,(7) and there is less water available for many decomposition reactions.(8) Lyophilized biopharmaceuticals must still be reconstituted with an appropriate diluent and administered via a hypodermic needle and syringe. All current influenza vaccines are stored and delivered in the liquid state and also require the use of the cold-chain. The cold-chain can cost hundreds of million dollars per vaccine program(4), thus an influenza vaccine stable at room temperature could be of significant financial benefit.
We and others are developing microneedle patches for vaccination against influenza and other diseases.(9-14) Microneedle patches contain an array of sub-millimeter projections composed primarily of water-soluble sugars and polymers that encapsulate a payload of drug or vaccine.(15) Since these patches hold the vaccine in a dry state, once produced, microneedle patches can maintain stability similar to lyophilized vaccine. To achieve this stable state, formulation excipients must be optimized to maintain vaccine activity during manufacturing and subsequent storage. During manufacturing, a vaccine and excipient mixture is cast and dried into molds and allowed to dry, which can damage the vaccine. There has been work to develop formulation strategies to stabilize influenza vaccine during microneedle fabrication. For example, silk(16) and trehalose(17) have been incorporated into microneedle formulations to stabilize influenza vaccine during drying. Aside from stability enhancement, microneedles could increase the acceptance of vaccine administration(18) and improve the immune response in recipients(19, 20).
Formulation excipients that function as lyoprotectants of proteins are often small sugars.(21-24) There are thought to be two different, yet possibly simultaneous, mechanisms by which sugars and polyols maintain protein stability in a dried state.(22, 23) One is the formation of an amorphous sugar glass phase with such a high viscosity that molecular mobility is severely restricted.(25) This restricted mobility decreases kinetics of possible degradation pathways, both chemical and physical.(7) The other mechanism is the replacement of removed water molecules’ hydrostatic interactions by hydroxyl groups from the sugars,(26) which is needed because water molecules form a stabilizing hydration shell around proteins in solution.(27) Polysaccharides, such as inulin(28), and amino acids, such as arginine(29), have also been used to stabilize proteins, due also to advantageous excipient/protein interactions and the creation of a favorable dry matrix.
In a previous study, we screened 61 different formulation excipients and some of their combinations to find three pairwise excipient combinations that were especially promising at stabilizing a trivalent subunit influenza vaccine: trehalose/sucrose, sucrose/arginine, and arginine/heptagluconate.(30) In this study, we further examined these formulations for stability at 25°C and 40°C over periods up to 24 months and during environmental stresses such as exposure to 60°C for four months, multiple freeze-thaw cycles, and electron beam irradiation.
Materials and Methods
Vaccine and reagents
Monovalent vaccine stocks were generously provided by Novartis Vaccines and Diagnostics (Cambridge, MA). Studies with monovalent vaccine dried on chips and in microneedle patches stored for eighteen months utilized B/Brisbane/60/2008 influenza antigen, while studies testing stability at 60°C, freeze conditions, and irradiation utilized A/Brisbane/10/2010 (H1N1) influenza antigen. Studies with trivalent vaccine utilized B/Brisbane/60/2008, A/Brisbane/59/2007 (H1N1) and A/Victoria/210/2009 (H3N2) influenza antigens. All excipients were obtained from Sigma-Aldrich (St. Louis, MO) and used as received.
Formulations
Vaccine stock solutions were received in phosphate buffered saline (PBS). To buffer exchange into ammonium acetate buffer, monovalent stock solutions of the desired strain were concentrated at least 10- fold using Vivaspin centrifuge filters (Sartorius AG, Göttingen, Germany) and then diluted with 150 mM ammonium acetate buffer. This process was repeated 2-3 times. Finally, additional ammonium acetate buffer was used to adjust the vaccine solution to the appropriate concentration for experiments.
To formulate vaccine for casting onto molds, dry excipients were weighed and placed into Type 1 glass vials (Wheaton, Millville, NJ), to which buffer-exchanged vaccine stock solution was added. All formulations contained 1% w/v 250 kDa sodium carboxymethyl cellulose (NaCMC). All formulations contained a total stabilizing excipient concentration of 10% w/v, where the concentration was split amongst up to four compounds.
Vaccine stability testing on chips
As previously described(30), throughput of formulation screening can by increased by drying droplets of vaccine solution on surfaces representative of molds used to make microneedles. For this purpose, a thin film of polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning, Midland, MI) was cured, from which 6 mm-diameter circular chips were removed with a hammer punch. Droplets of approximately 2 µL of formulated vaccine solution were placed onto each chip. Droplet weights were measured (Sartorius SE2 Ultra Micro Balance, Sartorius AG, Göttingen, Germany) to more precisely determine the amount of solution applied. Chips were then packaged in aluminum pouches (Oliver-Tolas Healthcare, Grand Rapids, MI) and sealed with an impulse heat-sealer (AIE-300, American International Electric, Industry, CA). All samples were stored with desiccant (Hammond Drierite, Xenia, OH). Chips were then stored in environmental test chambers maintained at 40°C (Model 6020, Caron, Marietta, OH).
Vaccine stability testing in microneedle patches
Microneedle patches were prepared using two different casting solutions. First, a PDMS mold in the shape of the microneedle patch was prepared, using methods described previously.(31) The vaccine casting solution consisted of influenza vaccine, 1% w/v NaCMC, and 10% w/v of stabilizer in 150 mM ammonium acetate buffer. The matrix casting solution consisted of polyvinyl alcohol (PVA), sucrose, and water in a mass ratio of 8:6:15. To make microneedle patches, vaccine casting solution was placed on a mold to fill the microneedle cavities with the aid of vacuum. Excess vaccine solution was removed from the mold surface with a flat blade. The vaccine casting solution was then allowed to dry into the tips of the microneedle mold cavities under vacuum. Next, matrix casting solution was placed on the mold and allowed to dry under vacuum, thereby forming a complete microneedle patch. Each patch was then stored in a desiccator at room temperature (~21 - 25°C) for two days before demolding with Scotch tape (3M, St. Paul, MN).
Demolded patches were packaged with desiccant in aluminum pouches and stored at a given temperature (i.e., 4°C, 25°C or 40°C) in stability chambers. For patches subjected to freeze-thaw cycles, each cycle consisted of two hours at −20°C followed by two hours at 4°C. Patches subjected to electron-beam sterilization were irradiated using a 10 MeV (20 kW) system at room temperature for a total exposure of 10 or 20 kGy. The non-irradiated controls were subjected to the same travel and storage conditions as the test samples.
ELISA assay
Reconstituted vaccine samples were assayed for hemagglutinin activity using a sandwich enzyme-linked immunosorbent assay (ELISA). Polyclonal, strain-specific antibodies were received from the Center for Biologics Evaluation and Research of the Food and Drug Administration (Silver Spring, MD). Antibodies were conjugated to horseradish peroxidase with a Lightning Link conjugation kit (Innova Biosciences, Cambridge, UK). Unformulated vaccine stock was serially diluted in PBS with 0.5% Tween-20 (PBST) and used to generate a reference standard curve. After the prescribed storage time, chips and microneedle patches were placed into PBST so that the dried vaccine and excipients were redissolved. This solution was run parallel to the reference standard curve on an Immulon 2HB 96-well microplate (Thermo Scientific, Waltham, MA). The microplate was washed three times between each step with PBST containing 3% w/v bovine serum albumin. The horseradish peroxidase (HRP) substrate reaction involved SureBlue Reserve TMB (3,3’,5,5’-tetramethylbenzidine) solution (KPL, Gaithersburg, MD), which was stopped with TMB Stop Solution (KPL). The microplate absorbance at 620 nm was read using an iMark plate reader (BioRad, Hercules, CA). The standard curve was fit to a four-parameter function using the Microplate Manager 6 software (BioRad).
Immunization of mice
Groups of female BALB/c mice (n=8 per group), all 6-8 weeks of age, were immunized via either an intradermal (ID) injection at the base of the tail or by application of a microneedle patch to the back. In the first study, animals were vaccinated with 3.8 µg, 2.8 µg, and 3.8 µg of H1N1, H3N2, and B strains, respectively, administered either by ID injection of fresh stock vaccine or using microneedle patches stored at 25 °C for 13 months. In the second study, animals were vaccinated with 1 µg, 0.6 µg, and 0.6 µg of H1N1, H3N2, and B strains, respectively, by ID injection of fresh stock vaccine, by ID injection of vaccine reconstituted from microneedle patches stored at 25 °C for 13 months or using freshly made microneedle patches. All patches were made using arginine and heptagluconate at a mass ratio of 50:50 as the stabilizers in the vaccine casting solution.
Hair was removed one day prior to immunization using electric clippers and a depilatory cream (Nair; Ewing, NJ). Patches were applied by pressing the patch against a pinch of hair-free skin using the thumb and forefinger for 1 min. Adhesive on the patch backing held the patch in place for a further 20 min to ensure microneedle dissolution in the skin. Blood was drawn via the jugular vein at days 0, 14, and 28. Whole blood was then spun down in serum separation tubes (Becton Dickinson, Franklin Lakes, NJ) to collect the serum. All studies involving animals were approved by the Institutional Animal Care and Use Committees (IACUC) of Georgia Tech.
Hemagglutination inhibition titers
Production of influenza-specific antibodies was determined by hemagglutination inhibition (HAI) assay. Serum was treated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C. Samples were then incubated at 56°C for 30 min before being incubated overnight with packed red blood cells at 4°C. After centrifugation, supernatant was collected, serially diluted in PBS, and mixed with separate strain-specific influenza viruses for 30 min. Finally, 0.5% chicken red blood cells was added. The reciprocal of highest dilution titer that prevented hemagglutination was read as the HAI titer.
Electron beam irradiation
Electron beam irradiation was conducted by Synergy Health (Lima, OH) using a 10 MeV (20 kW) system that exposed microneedle patches to 10 or 20 kGy irradiation. Irradiation was conducted at ambient temperature. The non-irradiated controls were subjected to the same travel and storage conditions as the test samples.
Statistics
All statistics were calculated using Excel 2013 software (Microsoft, Redmond, WA). Reported averages represent the arithmetic mean of the tested samples, except for HAI titers, which are presented as the geometric mean titer for each group. Seropositivity was considered to be an HAI titer greater than or equal to 40. Comparisons between individual samples were performed using an unpaired t-test with a significance cutoff of p<0.05.
Results
Formulation screening to stabilize monovalent influenza vaccine
Our previous study indicated that vaccine casting solution formulations containing two-way combinations of trehalose, sucrose, arginine and heptagluconate stabilized influenza vaccine during storage at elevated temperature.(30) We therefore studied the ability of these excipients to stabilize influenza vaccine in greater detail by screening for stability using droplets of formulated monovalent vaccine dried onto PDMS chips to represent drying on a microneedle patch mold and then stored at 40°C.
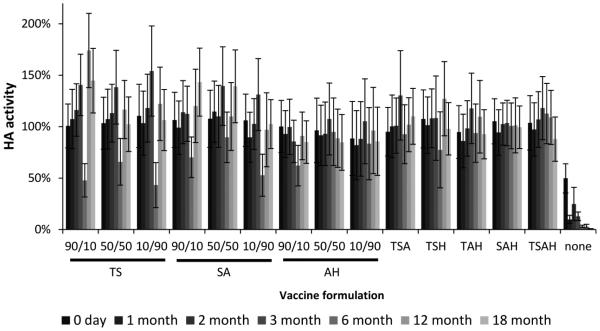
As shown in Figure 1, vaccine dried with no formulation stabilizers lost at least half of its activity during drying (day 0) and then lost almost all activity within one month of storage. The three two-way combinations of stabilizers found previously to be optimal at a mass ratio of 50:50,(30) were studied additionally at mass ratios of 90:10 and 10:90. Comparisons between 18 month activity and initial activity showed no change in vaccine stability for all of these formulations, independent of mass ratio (Fig. 1, Student’s t-test, p > 0.05). We also examined the four possible three-way combinations of these excipients as well as their four-way combination, and again found not significant change in vaccine activity after 18 months (Fig. 1, Student’s t-test, p > 0.05).
Fig. 1.
Effect of stabilizing excipient formulation on hemagglutinin (HA) activity after drying influenza vaccine on PDMS chips and storing with desiccant for up to twelve months at 40°C. Vaccine (B/Brisbane/60/2008) was formulated with combinations of two, three, or four stabilizing excipients at a total stabilizer concentration of 10% w/v in the vaccine casting solution. For the two-stabilizer combinations, the concentration ratio is shown, where the first number corresponds to the relative content of the first excipient listed below it on the x axis. The three-way and four-way formulation had equal amounts of each excipient. T = trehalose, S = sucrose, A = arginine, H = sodium heptagluconate. HA activity is expressed as a percentage of HA activity in the liquid casting solution. Data represent averages of n = 12 replicates, with standard deviation bars shown.
Stability of influenza vaccine patches over time at room temperature
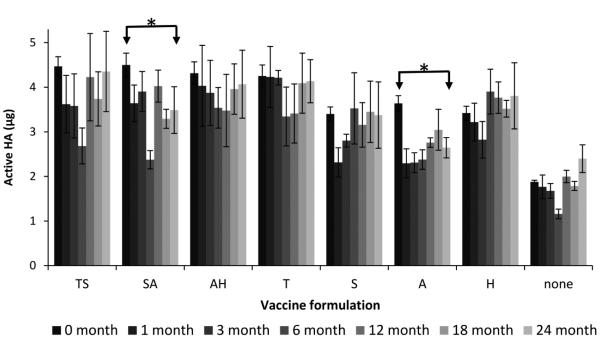
Guided by the screening results generated on chips showing that all of the two-, three- and four-way combinations of stabilizers effectively stabilized the vaccine, we made microneedle patches containing monovalent vaccine using the three optimal two-way combinations and tested their stability during storage at 25°C for 24 months. These patches were made by first casting a vaccine solution containing the antigen and stabilizing excipients and, after drying, casting a matrix solution containing sucrose and PVA to fill any remaining space not occupied by the dried vaccine solution and to form the patch backing. Among the microneedle patches with two-way combinations of stabilizing excipients, both the trehalose/sucrose and arginine/heptagluconate formulations exhibited no significant loss of vaccine activity after 24 months (Fig. 2, Student’s t-test, p > 0.05).
Fig. 2.
Effect of formulation on hemagglutinin (HA) activity after fabricating microneedle patches and storing with desiccant for up to 24 months at 25°C. Monovalent microneedle patches were fabricated with B/Brisbane/60/2008 influenza vaccine formulated with stabilizing excipients at a total concentration of 10% w/v in the vaccine casting solution. The two-stabilizer combinations contained equal amounts of each excipient. T = trehalose, S = sucrose, A = arginine, H = sodium heptagluconate. HA activity is shown as the mass of active HA in each patch. Asterisk (*) indicates a significant difference in vaccine activity between the initial time point and the furthest time point (Student’s t-test, p < 0.05). Data represent averages of n = 6 replicates, with standard deviation bars shown.
Using just the individual stabilizers showed that formulation with any of the saccharides resulted in no significant loss of vaccine activity after 24 months (Fig. 2, Student’s t-test, p > 0.05), but there was a significant decrease in vaccine activity at 24 months when arginine was used as the only stabilizer (Fig. 2, Student’s t-test, p = 0.03). The unformulated vaccine also had no loss of activity after 24 months (Fig. 2, Student’s t-test, p > 0.05). However, the patches using unformulated vaccine and vaccine formulated with the single stabilizers sucrose, arginine or heptagluconate had significant losses in activity during fabrication on Day 0 by comparison with vaccine activity in patches formulated with arginine-heptagluconate as a positive control (Fig. 2, Student’s t-test, p < 0.05). This is probably due to differences in vaccine stabilization associated with vaccine drying during patch fabrication. Comparison of these findings in microneedle patches to the previous findings on chips indicates that the excipients in the matrix casting solution used to make the microneedle patch backing provided additional stabilization.
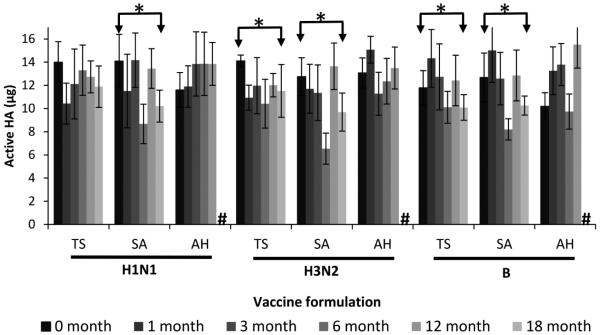
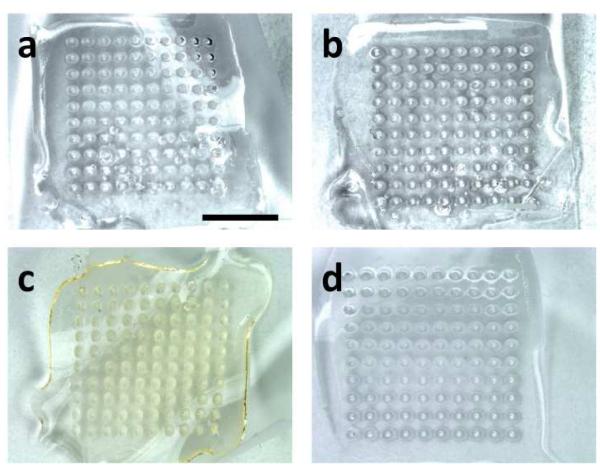
Based on these findings with monovalent patches, we assessed the stability of trivalent patches using the three two-way combinations of stabilizers. There was no significant change in vaccine activity for any of the three vaccine strains using the arginine/heptagluconate formulation after storage for 12 months at 25°C, as well as the H1N1 strain when formulated with trehalose/sucrose and stored for 18 months (Fig. 3, Student’s t-test, p > 0.05). There was also no noticeable change in the appearance of the patches after storage compared to freshly prepared patches (Fig. 4a-b). These findings further indicate the superiority of the arginine/heptagluconate formulation as a stabilizer.
Fig. 3.
Effect of formulation on hemagglutinin (HA) activity after fabricating microneedle patches and storing with desiccant for up to 18 months at 25°C. Trivalent microneedle patches were fabricated with A/Brisbane/59/2007 (H1N1), A/Victoria/210/2009 (H3N2) and B/Brisbane/60/2008 (B) influenza vaccine formulated with two-way combinations of stabilizing excipients at a ratio of 50:50 with a total stabilizer concentration of 10% in the vaccine casting solution. T = trehalose, S = sucrose, A = arginine, H = sodium heptagluconate, # = data not available. Asterisk (*) indicates a significant difference in vaccine activity between the initial time point and the furthest time point (Student’s t-test, p < 0.05). Data represent averages of n = 6 replicates, with standard deviation bars shown.
Fig. 4.
Images of representative microneedle patches (a) immediately after production, (b) after storage at 25°C for 18 months, (c) after storage at 60°C for two months, and (d) after five freeze-thaw cycles. Scale bar represents 5 mm.
Immunogenicity of stored influenza vaccine patches
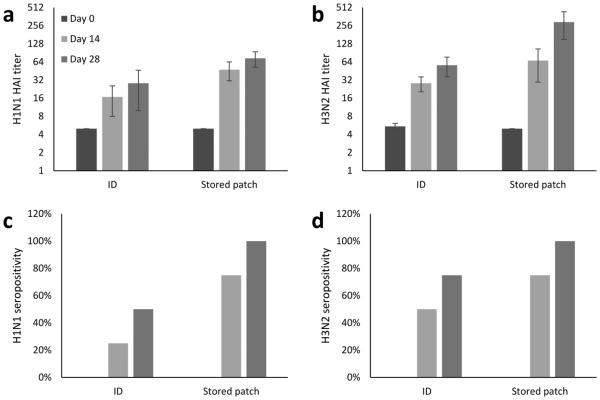
To determine if the in vitro vaccine stability of microneedle patches correlated with in vivo immunogenicity, mice were immunized using microneedle patches containing trivalent influenza vaccine that had been stored for 13 months at 25°C and compared to ID injection of fresh vaccine. There was no significant difference in immune response to the H1N1 influenza strain between the mice immunized with a stored microneedle patch and by an ID injection (Fig 5a). Immune response to the H3N2 strain was higher for stored microneedle patches than ID injection (Fig. 5b). For both strains, all mice were seropositive after vaccination with stored microneedle patches, but only 50% – 70% were seropositive after ID vaccination (Fig 5c – 5d). However, immune responses to the B strain of influenza could not be measured due to lack of access to the virus needed for the assay.
Fig. 5.
Immune responses of BALB/c mice immunized with trivalent influenza vaccine. Strain-specific hemagglutination inhibition (HAI) titers are represented as geometric means of n = 8 mice, with ± standard error of mean bars shown for (a) H1N1 and (b) H3N2 influenza vaccination. Asterisk (*) indicates a significant difference in antibody titers (Student’s t-test, p < 0.05). Seropositivity data for (c) H1N1 and (d) H3N2 influenza vaccination are represented as the percentage of each experimental group that achieved an antibody titer ≥ 40. Trivalent microneedle patches were fabricated with A/Brisbane/59/2007 (H1N1), A/Victoria/210/2009 (H3N2) and B/Brisbane/60/2008 (B) influenza vaccine formulated with a two-way combination of arginine and heptagluconate each at a concentration of 5% w/v in the vaccine casting solution. ID = intradermal vaccination, Stored patch = vaccination with a microneedle patch after storage at 40°C with desiccant for 13 months. Data from vaccination with B/Brisbane/60/2008 are not available due to technical limitations.
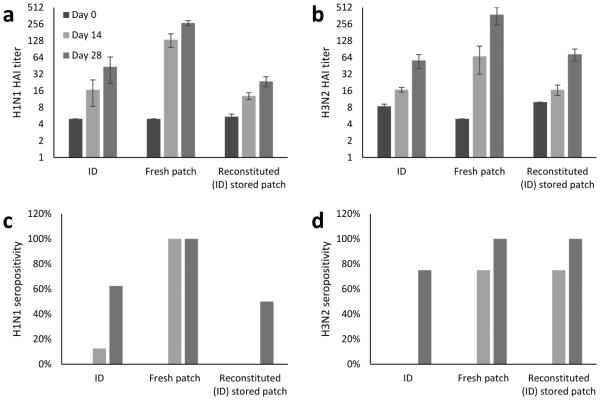
The superior immune response of stored microneedle patch vaccination compared to ID vaccination may at first be surprising. However, comparison of fresh microneedle patch vaccination to ID vaccination similarly showed improved immune response after microneedle patch vaccination (Fig. 6), indicating that the superior response was not associated with storage, but was instead associated with the microneedle patch method of vaccination, which is consistent with prior literature(20, 32). Moreover, ID injection of vaccine reconstituted from stored microneedle patches generated immune responses not significantly different from ID injection of fresh vaccine (Fig. 6), further suggesting that stored vaccine was as immunogenic as fresh vaccine and that improved immunogenicity was associated with microneedle patch administration.
Fig. 6.
Immune responses of BALB/c mice immunized with trivalent influenza vaccine. Strain-specific hemagglutination inhibition (HAI) titers are represented as geometric means of n = 8 mice, with ± standard error of mean bars shown for (a) H1N1 and (b) H3N2 influenza vaccination. Asterisk (*) indicates a significant difference in antibody titers (Student’s t-test, p < 0.05). Seropositivity data for (c) H1N1 and (d) H3N2 influenza vaccination are represented as the percentage of each experimental group that achieved an antibody titer ≥ 40. Trivalent microneedle patches were fabricated with A/Brisbane/59/2007 (H1N1), A/Victoria/210/2009 (H3N2) and B/Brisbane/60/2008 (B) influenza vaccine formulated with a two-way combination of arginine and heptagluconate each at a concentration of 5% w/v in the vaccine casting solution ID = intradermal vaccination, Fresh patch = vaccination with a microneedle patch, Reconstituted (ID) stored patch = intradermal vaccination using a reconstituted microneedle patch after storage at 40°C with desiccant for 13 months. Data from vaccination with B/Brisbane/60/2008 are not available due to technical limitations.
Stability of influenza vaccine patches at elevated temperature
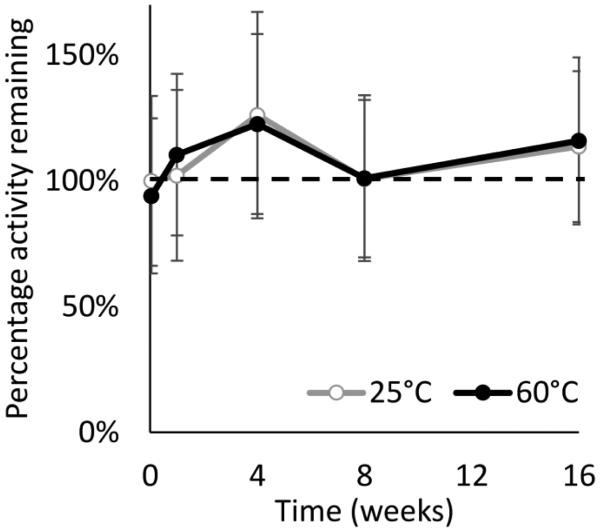
While microneedle patches stored at 25°C simulates vaccine storage in a climate-controlled pharmacy or warehouse, we wanted to determine if microneedle patches remained stable at higher temperatures. Microneedle patches containing monovalent influenza vaccine were therefore stored at 60°C for four months. There was no significant loss of vaccine activity for these patches as a function of time (Fig. 7, ANOVA, p > 0.05) or in comparison to patches stored in parallel at 25°C (Fig. 7, ANOVA, p > 0.05). Visual inspection of the patches indicated minor discoloration of the patch after storage at 60°C, suggesting that patch excipients may have been affected (Fig. 4c).
Fig. 7.
Stability of influenza vaccine in microneedle patches stored at 60°C with desiccant for up to four months compared to patches stored at 25°C under the same conditions. Monovalent microneedle patches were fabricated with A/Brisbane/10/2010 influenza vaccine formulated with a two-way combination of arginine and heptagluconate, each at a concentration of 5% w/v in the vaccine casting solution. Data from patches stored at 60°C are shown, as well as control patches which were stored at 25°C and tested simultaneously. HA activity is shown as the percentage of active HA remaining in each patch. Data represent averages of n = 6 replicates, with standard deviation bars shown.
Stability of influenza vaccine patches after multiple freeze-thaw cycles
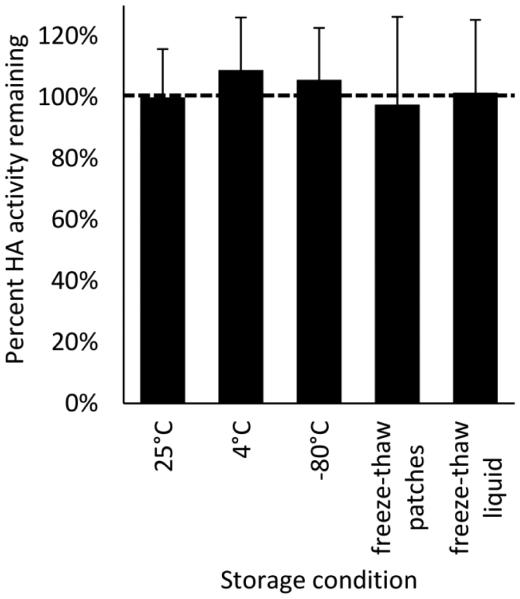
Vaccines can be exposed to freeze-thaw cycles if stored without environmental controls or during poorly-controlled refrigeration. To address such conditions, microneedle patches containing monovalent influenza vaccine were subjected to five freeze-thaw cycles, alternating between −20°C and 4°C, over the course of 24 h, after which there was no significant change in vaccine activity compared to patches maintained at 25°C (Fig. 8, Student’s t-test, p = 0.95). Patches maintained at 4°C or at −80°C also had no significantly loss of activity compared to patches stored at 25°C (Fig. 8, Student’s t-test, p > 0.05). Interestingly, unformulated liquid vaccine subjected to identical freeze-thaw cycles also showed no loss in vaccine activity (Fig. 8, Student’s t-test, p = 0.88). The appearance of patches after the freeze-thaw cycles showed no apparent differences from fresh patches (Fig. 4d).
Fig. 8.
Stability of influenza vaccine in microneedle patches subjected to five freeze-thaw cycles. Monovalent microneedle patches were fabricated with A/Brisbane/10/2010 influenza vaccine formulated with a two-way combination of arginine and heptagluconate each at a concentration of 5% w/v in the vaccine casting solution. All samples were stored for a total of 24 h. Freeze-thawed samples were subjected to five freeze-thaw cycles each of 2 h at 4°C followed by 2 h at −20°C. Patches stored at 25°C served as the positive control to which all other HA levels were normalized. Patches were also stored at 4°C and −80°C for comparison. Finally, liquid vaccine was subjected to the same freeze-thaw cycles. Data represent averages of n = 6 replicates, with standard deviation bars shown.
Stability of influenza vaccine patches after exposure to electron-beam sterilization cycle
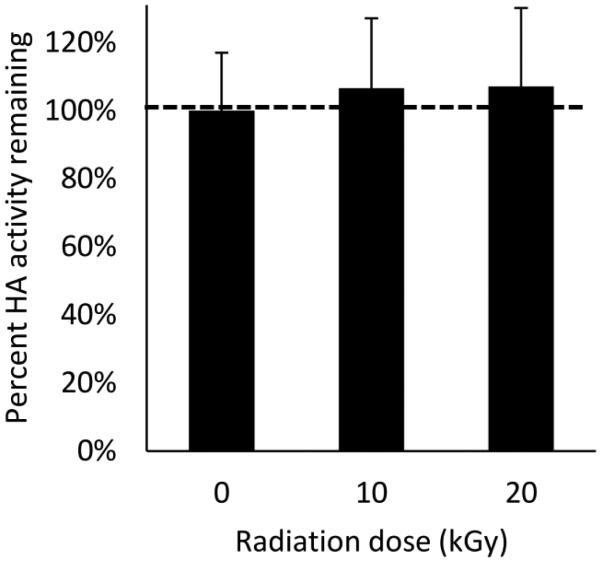
Terminal sterilization of microneedle patches could provide cost savings relative to aseptic manufacturing. Microneedle patches containing monovalent influenza vaccine were therefore subjected to electron beam sterilization at two different radiation doses. Compared to untreated patches, there was no significant loss of vaccine activity at either radiation dose (Fig. 9, Student’s t-test, p > 0.05). Patch appearance after irradiation showed no apparent changes from fresh patches (image not available).
Fig. 9.
Stability of influenza vaccine in microneedle patches exposed to electron beam irradiation. Monovalent microneedle patches were fabricated with A/Brisbane/10/2010 influenza vaccine formulated with a two-way combination of arginine and heptagluconate each at a concentration of 5% w/v in the vaccine casting solution. HA activity is shown as the mass of active HA in each patch. Data represent averages of n = 6 replicates, with standard deviation bars shown.
Discussion
This work builds upon previous work that included a screen of microneedle formulation excipients to stabilize influenza vaccine.(30) The stability of dried vaccine on chips as well as in patches stored for up to six months at 25°C was reported in that work. The work reported here shows further evidence of stability when influenza vaccine was incorporated into dissolving microneedle patches and maintained activity after storage at 25°C for at least 24 months, 60°C for at least four months, five freeze-thaw cycles and electron beam irradiation. The ability to withstand this wide array of stressful conditions suggests that a properly formulated microneedle patch could enable influenza vaccine to be removed from the cold chain. Resistance to electron beam irradiation suggests that terminal sterilization may be possible, as opposed to the more costly scenario of aseptic manufacturing, which could enable significant cost savings.
Influenza vaccine stability, however, requires proper formulation. Influenza vaccine dried on a PDMS surface without a stabilizer lost essentially all of its activity after any storage period studied at 40°C. The optimized formulations studied here all provided significant stabilization of the vaccine, and the arginine/ heptagluconate formulation maintained full vaccine activity at all of the conditions studied. It was interesting to note that the ratio of excipient concentration of the two-fold combinations of stabilizers had no significant effect on stabilization, which provides additional formulation flexibility.
It is well known that trehalose and sucrose are good lyoprotectants(25, 28, 33-35), and arginine, a basic amino acid, has been shown to both inhibit aggregation(29) and dry into an amorphous state(36). Calcium heptagluconate has received limited attention as a stabilizer, but has been shown to stabilize hemoglobin in the liquid state,(37) which was the reason we included this excipient in our initial stabilizer screen. A possible reason for the success of multiple-stabilizer formulations relative to formulations using single stabilizers (i.e., in chips, without the presence of additional formulation excipients used in the complete microneedle patch production) is the inhibition of crystallization of one excipient by the other excipient(s).(38)
Some microneedle patches were made without a stabilizer in the first mold fill. These patches showed significantly less active vaccine after fabrication, but did not have additional losses once dried and placed in storage. This indicates that the stabilizers included in the vaccine casting solution were needed predominately for stability during the fabrication process. Once the patches were completed, dried, and stored, the sucrose and PVA that compromise the bulk of the microneedles and backing provides sufficient stabilization to maintain vaccine activity during long-term storage. These findings of monovalent vaccine stability also held true for microneedle patches containing trivalent influenza vaccine. A dose comparable to the full human dose in clinical use was incorporated into these patches, which suggests that these findings can be readily translated into microneedle patches used to administer seasonal influenza vaccine to people.
As further tests of influenza vaccine stability in microneedle patches, patches utilizing the arginine/heptagluconate formulation were subjected to very high temperature, freeze-thaw cycling, and electron beam irradiation. Over a period of four months, vaccine in microneedle patches lost no significant activity when stored at 60°C. While we do not envision patches needing to withstand such a high temperatures for so long, it suggests that the microneedle patch could survive most any expected elevated thermal exposure that a seasonal influenza vaccine might experience during its one-year shelf life. The excellent stability during freeze-thaw cycling suggests the ability of influenza vaccine in microneedle patches to survive freezing that could occur due to handling errors or faulty cold-chain equipment. The ability of microneedle patches to preserve vaccine activity at both −80°C and after five consecutive freeze-thaw cycles could be associated with the relatively low moisture content of a few percent (data not shown). This small amount of water may be bound water that does not form ice crystals(39) or it may be phase-separated or otherwise in poor contact with the vaccine antigen, so that they do not interact.
Of relevance to the anticipated future use of microneedle patches for clinical influenza vaccination, maintenance of vaccine activity after exposure to sterilizing irradiation by electron beam suggests that terminal sterilization of microneedle patches could be use. This would avoid the high cost of aseptic manufacturing and therefore represent a significant cost savings. These findings, however, should be tempered by the fact that there is evidence that damaging effects of electron beam irradiation may not be apparent until after storage(40) (which we did not study) and that the sterilization cycle used in this study is expected to be suitable for microneedle patch sterilization, but sterility of these patches was not verified.
The primary test of vaccine activity in this work was performed by ELISA, which is a reasonable measure of vaccine stability, but is not the gold standard method, which is single radial immunodiffusion (SRID).(41) We did not use SRID in this study because it is a complex assay that is difficult to operate reproducibly and is relatively low throughput. Instead, we verified our in vitro findings with in vivo studies of immunogenicity in mice. When compared to ID injection of a comparable vaccine dose, stored microneedle patches stored for more than one year at 25°C elicited an equal or stronger immune response after vaccination. This increase in antibody response when compared to ID delivery was seen in this study when using freshly made microneedles patch and has been noted before in the literature.(20, 32) To remove the effect of delivery route, stored microneedles were dissolved in water and injected ID. Mice receiving either fresh vaccine or stored vaccine delivered ID generated indistinguishable antibody responses, showing that the vaccine in the stored patches had retained its immunological activity.
When considered in the context of the broader microneedle patch literature, we believe this study further suggests that influenza vaccination using microneedle patches could be a valuable improvement over current vaccination practice. Previous studies indicate that vaccination using microneedle patches provides improved immune responses, probably due to targeting of the vaccine to the skin.(42) In addition, microneedle patches are small, which facilitates storage, distribution and storage, and because they dissolve in the skin, microneedles generate no biohazardous sharps waste.(9-14) In part because they are painless and can be self-administered, human subjects have indicated a strong preference for microneedle patches over hypodermic needle injections.(18) This study adds one more reason why influenza vaccination could benefit from microneedle patch administration; the remarkable thermostability demonstrated here suggests that influenza vaccine could be taken out of the cold chain to decrease costs, decrease vaccine spoilage and increase convenience of vaccination.
Conclusion
This study shows that influenza vaccine formulated into microneedle patches remained stable after storage for 24 months at 25°C, storage for four months at 60°C, exposure to five freeze-thaw cycles and irradiation during electron-beam sterilization. Most stability measurements were made by assessing activity of monovalent vaccine by ELISA; additional studies using trivalent influenza vaccine assessed by ELISA and measuring antibody responses in mice gave similar results. A microneedle patch for influenza vaccination with this high level of stability could enable vaccine storage and distribution outside the cold chain to simplify and reduce costs of annual influenza vaccination campaigns and vaccine stockpiling for emergencies.
Acknowledgements
We thank Novartis for generously providing monovalent influenza vaccine stock. This work was supported in part by the National Institutes of Health. We thank Polo Gaputan and Miraj Desai for their work on this project. The work was carried out in the Center for Drug Design, Development and Delivery, and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology. Matthew Mistilis, Andreas Bommarius and Mark Prausnitz are inventors of patent(s) that have been or may be licensed to companies developing microneedle-based products, and Mark Prausnitz is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products. This potential conflict of interest has been disclosed and is overseen by Georgia Tech and Emory University.
References
- 1.World Health Organization Influenza (Seasonal) Fact Sheet 2014 [10/24/15] Available from: http://www.who.int/mediacentre/factsheets/fs211/en/index.html.
- 2.Grohskopf L, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2014– 15 Influenza Season. Morbidity and Mortality Weekly Report. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 3.CDC Flu Vaccination Coverage, United States, 2014-15 Influenza Season: United States Centers for Disease Control and Prevention. 2015 [cited 2015 December]. Available from: http://www.cdc.gov/flu/fluvaxview/coverage-1415estimates.htm.
- 4.Das P. Revolutionary vaccine technology breaks the cold chain. The Lancet Infectious Diseases. 2004;4(12):719. doi: 10.1016/s1473-3099(04)01222-8. [DOI] [PubMed] [Google Scholar]
- 5.Patois E, Capelle MAH, Gurny R, Arvinte T. Stability of seasonal influenza vaccines investigated by spectroscopy and microscopy methods. Vaccine. 2011;29(43):7404–13. doi: 10.1016/j.vaccine.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Cicerone MT, Soles CL. Fast dynamics and stabilization of proteins: binary glasses of trehalose and glycerol. Biophys J. 2004;86(6):3836–45. doi: 10.1529/biophysj.103.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L, Pikal MJ. Mechanisms of protein stabilization in the solid state. Journal of Pharmaceutical Sciences. 2009;98(9):2886–908. doi: 10.1002/jps.21825. [DOI] [PubMed] [Google Scholar]
- 8.Lee VHL. Changing needs in drug delivery in the era of peptide and protein drugs. In: Lee VHL, editor. Peptide and Protein Drug Delivery. Marcel Dekker, Inc.; New York: 1991. pp. 1–56. [Google Scholar]
- 9.Chen X, Fernando GJP, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. Journal of Controlled Release. 2011;152(3):349–55. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Pearton M, Kang SM, Song JM, Kim YC, Quan FS, Ivory M, et al. Immune stimulation following microneedle delivery of influenza virus-like particle (VLP) vaccines to human skin. Drug Discovery Today. 2010;15(23-24):A87. [Google Scholar]
- 11.Kommareddy S, Baudner BC, Bonificio A, Gallorini S, Palladino G, Determan AS, et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31(34):3435–41. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Delivery. 2010;17(4):187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath MG, Vrdoljak A, O'Mahony C, Oliveira JC, Moore AC, Crean AM. Determination of parameters for successful spray coating of silicon microneedle arrays. International Journal of Pharmaceutics. 2011;415(1-2):140–9. doi: 10.1016/j.ijpharm.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 14.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. Journal of Controlled Release. 2012;161(2):645–55. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, et al. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res. 2011;28(1):135–44. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. Journal of Infectious Diseases. 2010;201(2):190–8. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug delivery and translational research. 2015;5(4):360–71. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 22.Wang W. Lyophilization and development of solid protein pharmaceuticals. International Journal of Pharmaceutics. 2000;203(1-2):1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 23.Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal stability of vaccines. Journal of Pharmaceutical Sciences. 2003;92(2):218–31. doi: 10.1002/jps.10296. [DOI] [PubMed] [Google Scholar]
- 24.Cicerone MT, Pikal MJ, Qian KK. Stabilization of proteins in solid form. Advanced Drug Delivery Reviews. 2015;93:14–24. doi: 10.1016/j.addr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71(4):2087–93. doi: 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasmeijer N, Stankovic M, de Waard H, Frijlink HW, Hinrichs WLJ. Unraveling protein stabilization mechanisms: Vitrification and water replacement in a glass transition temperature controlled system. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2013;1834(4):763–9. doi: 10.1016/j.bbapap.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Monkos K. Studies of protein solution conformations using viscometric measurements. In: Uversky VN, Permyakov EA, editors. Conformational Stability, Size, Shape and Surface of Protein Molecules. Nova Science Publishers; 2007. p. 363. [Google Scholar]
- 28.Geeraedts F, Saluja V, ter Veer W, Amorij J-P, Frijlink H, Wilschut J, et al. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. The AAPS Journal. 2010;12(2):215–22. doi: 10.1208/s12248-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtake S, Kita Y, Arakawa T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev. 2011;63(13):1053–73. doi: 10.1016/j.addr.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. Journal of Pharmaceutical Sciences. 2015;104(2):740–9. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. Journal of Pharmaceutical Sciences. 2010;99(10):4228–38. doi: 10.1002/jps.22140. [DOI] [PubMed] [Google Scholar]
- 32.Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific Reports. 2012:2. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowe JH, Spargo BJ, Crowe LM. Preservation of dry liposomes does not require retention of residual water. Proc Natl Acad Sci U S A. 1987;84(6):1537–40. doi: 10.1073/pnas.84.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantino HR, Carrasquillo KG, Cordero RA, Mumenthaler M, Hsu CC, Griebenow K. Effect of excipients on the stability and structure of lyophilized recombinant human growth hormone. Journal of Pharmaceutical Sciences. 1998;87(11):1412–20. doi: 10.1021/js980069t. [DOI] [PubMed] [Google Scholar]
- 35.Choi HJ, Bondy BJ, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. Journal of Controlled Release. 2013;166(2):159–71. doi: 10.1016/j.jconrel.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattern M, Winter G, Kohnert U, Lee G. Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems. Pharmaceutical Development And Technology. 1999;4(2):199–208. doi: 10.1081/pdt-100101354. [DOI] [PubMed] [Google Scholar]
- 37.Hai TT, Nelson DJ. Stabilization of therapeutic hemoglobin compositions Patent WO1997039027 A1. 1997 [Google Scholar]
- 38.Roe KD, Labuza TP. Glass transition and crystallization of amorphous trehalose-sucrose mixtures. International Journal of Food Properties. 2005;8(3):559–74. [Google Scholar]
- 39.Wolfe J, Bryant G, Koster KL. What is 'unfreezable water', how unfreezable is it, and how much is there? Cryo letters. 2002;23(3):157–66. [PubMed] [Google Scholar]
- 40.Ameri M, Wang XM, Maa YF. Effect of irradiation on parathyroid hormone PTH(1-34) coated on a novel transdermal microprojection delivery system to produce a sterile product-adhesive compatibility. Journal of Pharmaceutical Sciences. 2010;99(4):2123–34. doi: 10.1002/jps.21985. [DOI] [PubMed] [Google Scholar]
- 41.Williams MS. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Veterinary Microbiology. 1993;37(3-4):253–62. doi: 10.1016/0378-1135(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 42.Skountzou I, Compans RW. Skin immunization with influenza vaccines. Curr Top Microbiol Immunol. 2015;386:343–69. doi: 10.1007/82_2014_407. [DOI] [PMC free article] [PubMed] [Google Scholar]