Abstract
Core executive functions such as working memory, inhibition, and cognitive flexibility are integral to daily life. A growing body of research has suggested that acute stress may impair core executive functions. However, there are a number of inconsistencies in the literature, leading to uncertainty about how or even if acute stress influences core executive functions. We addressed this by conducting a meta-analysis of acute stress effects on working memory, inhibition, and cognitive flexibility. We found that stress impaired working memory and cognitive flexibility, whereas it had nuanced effects on inhibition. Many of these effects were moderated by other variables, such as sex. In addition, we compared effects of acute stress on core executive functions to effects of cortisol administration and found some striking differences. Our findings indicate that stress works through mechanisms aside from or in addition to cortisol to produce a state characterized by more reactive processing of salient stimuli but greater control over actions. We conclude by highlighting some important future directions for stress and executive function research.
Keywords: acute stress, executive function, meta-analysis, working memory, inhibition, cognitive flexibility, cortisol, response inhibition, interference control, set shifting, cognitive control
1. Introduction
Intuitively, most of us believe that stress usually impairs our cognitive abilities. Intuition often fails us, though. As such, developing a scientific understanding of exactly how stress influences cognitive processes is of paramount importance given the ubiquity of stress in most peoples' daily lives (Cohen and Janicki-Deverts, 2012) and the importance of cognition in quality of life (Diamond, 2013). Moreover, what work has been done examining stress effects on cognition has often yielded counterintuitive results. That is, although there are clear cases in which stress disrupts some aspects of cognition there are others in which it clearly benefits cognitive processes. For example, stress generally impairs long term memory retrieval (Gagnon and Wagner, 2016), whereas it can enhance memory encoding (Wiemers et al., 2013), memory retention (Cahill et al., 2003), and decision-making (Shields et al., 2016a). Moreover, although there are many reports of stress impairing executive functions (Alexander et al., 2007; Schoofs et al., 2009), there are other cases in which stress has no effect on executive functions (Quinn and Joormann, 2015), and yet others show that stress can even improve them (Schwabe et al., 2013). Thus, there is a current need for taking a systematic and fine-grained approach to studying stress effects on individual cognitive processes in order to best understand how exactly stress influences cognition.
1.1 Executive Function
One particularly important set of cognitive processes that may be influenced by stress is subsumed under the umbrella term executive function, which refers to the higher cognitive processes that enable planning, forethought, and goal-directed action (Diamond, 2013; Suchy, 2009; Williams et al., 2009). According to an influential theory, performance on complex executive tasks is underpinned by three core executive functions (Diamond, 2013; Miyake et al., 2000). The first of these, working memory, refers to the ability to keep information in mind and update/integrate current contents with new information (e.g., in the verbal n-back task, participants must continually report if the letter/number they are hearing is the same letter/number they heard n letters/numbers ago). The second of these component processes, inhibition, refers to the ability to inhibit thoughts or prepotent responses in order to selectively attend to task-relevant information and engage in goal-directed rather than habitual actions (e.g., in the stop-signal task, participants learn to respond in a particular way to stimuli but on a small proportion of trials they are signaled to withhold that response). The third component process underpinning executive function task performance is cognitive flexibility, which refers to the ability to flexibly shift between cognitive rules or modes of thought (e.g., in the Wisconsin card sorting test, participants categorize cards according to rules that switch throughout the task, requiring participants to switch to a new rule rather than perseverating on an old and incorrect rule).
Although there is some disagreement about the specific tasks that best represent different executive functions, strong evidence that these executive functions are distinct comes from factor analyses (Friedman and Miyake, 2004; Miyake et al., 2001, 2000), brain lesions (Tsuchida and Fellows, 2013), and neuroimaging studies (Smolker et al., 2015). For example, factor analyses indicate that although the latent factors of inhibition, working memory, and cognitive flexibility are related, they are clearly separable as model fit suffers dramatically if one or more of these latent factors are excluded from the model (Miyake et al., 2000). Similarly, although the prefrontal cortex supports each executive function (Yuan and Raz, 2014), performance on executive function tasks can be distinguished at a more fine-grained level of analysis. For example, damage to the left ventrolateral prefrontal cortex is associated with impairments in inhibition and cognitive flexibility, whereas working memory impairments are associated with damage to various areas of the prefrontal cortex but notably not the ventrolateral prefrontal cortex (Tsuchida and Fellows, 2013). Likewise, in healthy young adults, working memory is associated with dorsolateral prefrontal cortex gray matter volume, whereas cognitive flexibility is associated with ventrolateral prefrontal cortex gray matter volume (Smolker et al., 2015). Similarly, functional activation in the left posterior superior parietal cortex and bilateral extrastriate cortex is greater when utilizing cognitive flexibility than when utilizing inhibition, whereas functional activation in the right superior parietal cortex, premotor cortex, and frontopolar cortex is greater when utilizing inhibition than when utilizing cognitive flexibility (Sylvester et al., 2003). Thus, at a neural level, both inhibition and cognitive flexibility appear to rely on the ventrolateral prefrontal cortex but differ in their recruitment of additional regions such as the parietal cortex; by contrast, working memory appears to rely on brain regions other than the ventrolateral prefrontal cortex, such as the dorsolateral prefrontal cortex. In sum, given that each executive function is at least somewhat separable from the other, any attempt to understand how stress might influence executive function should elucidate how stress influences the component cognitive processes underpinning complex executive function task performance.
1.2 Stress
Like executive function, stress can also be divided into various forms with unique effects. One primary distinction is between acute stress, which refers to a recent, transient occurrence of a single stressor, and chronic stress, which refers to an ongoing difficulty facing an individual that may or may not be a constant threat or presence in that individual's life. Because it is extremely difficult, if not unethical, to experimentally manipulate chronic stress in human research participants, prior work has more often examined acute stress effects on core executive functions than chronic stress effects on executive functions. Thus, we will restrict our focus in this review to acute stress effects on core executive functions. For purposes of brevity, we will refer to acute stress as “stress” hereafter unless otherwise specified.
In response to an acute stressor, the body responds in a myriad of ways to successfully handle the current threat. These reactions include activation of the “fight-or-flight” response mediated primarily by the sympathetic-adrenal-medullary (SAM) axis, activation of the hypothalamic-pituitary-adrenal (HPA) axis and subsequent release of adrenal hormones such as cortisol, and upregulation of the immune system and inflammatory activity (Allen et al., 2014). Acute increases in cortisol function to mobilize the body’s energy supply in order to provide readily-available energy for dealing with the current stressor (Munck et al., 1984), whereas acute increases in immune system activity facilitate healing, should injury or infection occur as a result of the current stressor (Dhabhar, 2002). In addition, components of the stress response, such as cortisol, can exert both nongenomic (rapid-acting effects not mediated by alterations in gene expression) and genomic (slow-acting effects mediated by alterations in gene expression) effects (Joëls et al., 2011), which allow time-dependent appropriate adaptations to a stressor. These biological responses are thus adaptive in that they enable an organism to effectively cope with its current unstable circumstances.
1.3 Theories of Stress and Executive Function
Stress is generally thought to impair executive functioning (Arnsten, 2009; Diamond, 2013; Schoofs et al., 2009; Shansky and Lipps, 2013). However, whether stress impairs working memory, inhibition, and cognitive flexibility alike, or whether stress differentially influences these processes is not completely clear.
Currently, the most prevalent theoretical viewpoint of how stress influences executive functions and other cognitive processes argues that stress biases cognition to process information that is most directly related to the current stressor (LeBlanc, 2009; Mather and Sutherland, 2011; Plessow et al., 2011). Stress is thus thought to reallocate finite executive control resources to deal with the stressor at hand; cognitive resources normally devoted to working memory and cognitive flexibility would be funneled to selective attention (i.e., inhibition) in order to enhance the ability to focus on the current stressor. Evidence in support of this theory comes from studies showing that stress impairs working memory (Oei et al., 2006; Schoofs et al., 2009, 2008) and cognitive flexibility (Alexander et al., 2007; Plessow et al., 2011) but enhances inhibition (Schwabe et al., 2013). In addition, a recent meta-analysis of cortisol administration effects found that the rapid-acting effects of cortisol impaired working memory but enhanced inhibition (Shields et al., 2015). Nonetheless, some empirical evidence suggests that individuals are more, rather than less, distracted by interfering information under stress (Sänger et al., 2014) and that stress can enhance working memory under some conditions (Schoofs et al., 2013; Yuen et al., 2009). Thus, a systematic review of the existing evidence is needed to assess the adequacy of this theory.
Recently, another theory of stress and cognitive function has been proposed that argues that stress shifts cognition from top-down control processes to more bottom-up automatic processes (Gagnon and Wagner, 2016; Vogel et al., 2016). Because all core executive functions are thought to be top-down processes, this model would fit with the literature discussed above that has found impairing effects of stress on cognitive inhibition, working memory, and cognitive flexibility. However, it is unclear how this model could accommodate other findings that stress sometimes enhances response inhibition (Schwabe et al., 2013).
At a different level of analysis, another theoretical perspective often adopted in studies of stress and executive functions posits that stress predominately influences executive functions through upregulation of cortisol, since cortisol spikes disrupt typical prefrontal cortical function (Porcelli et al., 2008; Vogel et al., 2016). This theory is appealing because cortisol influences both working memory and inhibition (Henckens et al., 2012, 2011), and because some effects of stress on executive functions (i.e., response inhibition) are abolished by blocking certain receptors for cortisol (Schwabe et al., 2013). However, many effects of stress on executive functions have not been directly compared to effects of cortisol, let alone abolished by blocking the effects of cortisol. In addition, it is possible that cortisol may be necessary, but not sufficient, for stress to exert its effects; perhaps stress-induced cortisol interacts with other factors upregulated by stress—such as noradrenergic or inflammatory activity—to exert effects on executive functions. Thus, it is currently unknown whether stress exerts its effects primarily or entirely through cortisol, or if stress works through other biological mechanisms to influence executive functions.
1.4 Stress and Working Memory
Although it is often thought that stress impairs working memory (e.g., Arnsten, 2009; Schoofs et al., 2009, 2008; Shansky and Lipps, 2013), there are many cases where stress either does not impair or even slightly improves working memory (e.g., Duncko et al., 2009; Giles et al., 2014; Luethi et al., 2008; Schoofs et al., 2013; Yuen et al., 2009). These conflicting results raise the question: under what conditions does stress impair or enhance working memory?
One potentially important moderator of stress effects on working memory is the delay between stress onset and working memory assessment. A recent meta-analysis of cortisol administration on executive functions found that cortisol administration impaired working memory with a short delay between administration and working memory assessment (i.e., less than an hour post-administration). At a longer delay (i.e., slightly over an hour post-administration), however, cortisol administration enhanced working memory (Shields et al., 2015). This time-dependent differential effect can be attributed to the two ways in which cortisol can influence neuronal activity: through nongenomic and genomic mechanisms (Henckens et al., 2011; Joëls et al., 2011). Thus, the delay between stress onset—the start of cortisol upregulation—and working memory assessment may be an important moderator of stress effects on working memory.
Another potentially important moderator of stress effects on working memory is sex. Indeed, two well-powered experiments published in the same paper found that stress impaired working memory in women but tended to enhance it in men (Schoofs et al., 2013). However, these results are in conflict with some prior work, which has found stress-induced working memory impairments in men (Schoofs et al., 2009), even when using the same task as studies which found no impairment (Schoofs et al., 2008). Still, animal work also suggests that stress may impair working memory more in females than males (Shansky et al., 2006). Thus, sex may be an important moderator of stress effects on working memory.
Finally, another potentially important moderator of stress effects on working memory is working memory load. Some evidence suggests that stress effects on working memory may be most apparent when working memory load is high (Oei et al., 2006).
1.5 Stress and Inhibition
Stress effects on inhibition have not been characterized as well as stress effects on working memory. Although some studies have found that stress enhances inhibition (Schwabe et al., 2013), other studies have found that stress impairs inhibition (Sänger et al., 2014). Indeed, there does not seem to be a consensus in the literature about whether stress enhances or impairs inhibitory control (LeBlanc, 2009). Thus, it is unclear whether stress enhances or impairs inhibition, and what conditions might produce these conflicting results.
One important moderator of stress effects on inhibition may be cortisol reactivity to the stressor. As mentioned above, a recent meta-analysis found that cortisol administration enhanced inhibition (Shields et al., 2015). Thus, the extent to which a stressor produces a cortisol increase may moderate stress effects on inhibition.
Another potentially important moderator of stress effects on inhibition is the delay between stress onset and inhibition assessment. A recent meta-analysis of cortisol administration effects on executive functions found that cortisol administration enhanced inhibition with a short delay between cortisol administration and inhibition assessment (e.g., less than an hour), whereas cortisol administration impaired inhibition at a longer delay (e.g., around three hours) between cortisol administration and inhibition assessment (Shields et al., 2015). This time-dependent differential effect can again be attributed to the two ways in which cortisol can influence neuronal activity—through nongenomic and genomic mechanisms.
An additional important moderator of stress effects on inhibition may be the type of inhibition required for performance on a given task. Inhibition is often divided further into response inhibition and cognitive inhibition. Response inhibition refers to the suppression of a prepotent response; cognitive inhibition, which is sometimes called interference control, refers to selectively attending to or ignoring information. Although factor analyses have suggested cognitive and response inhibition are the same process in healthy young adults (Friedman and Miyake, 2004), some evidence suggests that cognitive and response inhibition can be dissociated under certain conditions (Johnstone et al., 2009). Moreover, studies that have found stress-induced enhancements in inhibition have often used tasks requiring response inhibition (Schwabe et al., 2013), whereas studies that have found stress-induced impairments have often used tasks requiring cognitive inhibition (Sänger et al., 2014; Vinski and Watter, 2013).
1.6 Stress and Cognitive Flexibility
Research examining stress effects on cognitive flexibility is relatively new and very limited. Nonetheless, the few studies of stress effects on cognitive flexibility are relatively consistent in showing an impairment in cognitive flexibility following stress (e.g., Alexander et al., 2007; Laredo et al., 2015; Plessow et al., 2011).
Because so few studies have been conducted in this area, it is difficult to determine which factors might moderate stress effects on cognitive flexibility. Even so, one study in humans (Shields et al., 2016b) and another in rodents (Laredo et al., 2015) found that stress-induced impairments of cognitive flexibility are greater for males than females. Although the reason for this sex difference is not completely clear, this sex difference appears to be mediated by sex differences in μ-opioid receptor binding in the orbitofrontal cortex following stress (Laredo et al., 2015). Thus, sex may play an important role in stress effects on cognitive flexibility.
2. Current Research
In this meta-analytic review, we examined the effects of acute stress on each of the three core executive functions (working memory, inhibition, and cognitive flexibility). In addition, we attempted to elucidate potentially important moderators of stress effects on these executive functions using a meta-regression approach. Finally, we contrasted the results of the current meta-analysis with those from a recent meta-analysis of studies that had examined the effects of cortisol administration on executive function (Shields et al., 2015) in order to determine how stress effects on executive functions were related to cortisol effects.
The results of the analysis were expected to be useful in assessing existing theories about whether stress should have detrimental effects on all types of executive functions, or whether some forms, such as inhibition, might actually show stress related enhancements. In addition, we were now in a position to assess claims that the effects of stress were driven primarily by cortisol effects.
In addition to the moderators surveyed in the sections above with theoretical or empirical justification for assessment, in this meta-analysis we also considered a number of moderators that may be important for methodological reasons. These methodological moderators include whether the outcome was an accuracy/error-based outcome or a reaction time outcome, whether the task included an affective/emotional component or not, participant age, the time of day the study began, the severity of the stress manipulation, and the type of stressor used. Similarly, stress effects on all core executive functions are likely to be strongest when confounds modifying stress reactivity or executive function are reduced. For example, acute illnesses, medication use, hormonal contraceptive use, regular cigarette smoking, and high body mass indices all modulate biological responses to stress (Dickerson and Kemeny, 2004; O’Connor et al., 2009), and these variables are often controlled for in studies of stress and cognition by excluding participants with these conditions from the study. Similarly, completing cognitive tasks prior to an executive function task modulates performance on that task (Hagger et al., 2010; Schmeichel, 2007). As such, we examined the association of study precision—the conjunctive reduction of confounds related to stress and executive function assessment—as a potential methodological moderator of stress effects on executive functions.
3. Method
3.1 Study Selection and Inclusion Criteria
3.1.1 Literature review
To obtain studies for use in the meta-analysis, we performed an exhaustive search of the databases PsycINFO, PubMed, and Web of Science for all papers published until March 4, 2016, using the following search string:
(("Trier Social Stress Test" OR "cold-pressor" OR "acute stress" OR "stress was induced" OR "stress induction" OR "stress manipulation") AND ("executive function" OR "executive control" OR "cognitive control" OR "response inhibition" OR "cognitive inhibition" OR "selective attention" OR "executive attention" OR "interference control" OR "emotional interference" OR "sustained attention" OR "working memory" OR "set-shifting" OR "task-switching" OR "cognitive flexibility" OR "n-back" OR "OSPAN" OR "AOSPAN" OR "digit span" OR "Sternberg item recognition" OR "color wheel" OR "change detection task" OR "go/no-go" OR "go no-go" OR "stop signal task" OR "Stroop task" OR "Wisconsin Card Sorting Test" OR "trail making test" OR "letter-number sequencing" OR "d2 test of attention"))
In this search, PubMed returned 503 results, PsycINFO returned 221 results, and Web of Science returned 362 results. References from relevant articles were reviewed, and studies that were potentially relevant were examined from those references. For all articles considered, we followed Dickerson and Kemeny (2004) in reviewing abstracts and examining full texts whenever an article had the potential to include a relevant effect (e.g., if a study incorporated or could have incorporated an acute stressor, the full-text of the article was reviewed).
3.1.2 Inclusion criteria
Our eight inclusion criteria for this study were as follows: Studies had to (1) experimentally manipulate (2) acute stress and assess effects on (3) human participants (4) without a known psychological/psychiatric disorder (5) who then completed a task known or shown to depend upon executive function. (6) To ensure that acute stress was the primary manipulation rather than arousal, the stressor task used had to either be a previously validated stressor, contain components sufficient to elicit a stress response (i.e., a task requiring motivated performance with socio-evaluative threat; Dickerson & Kemeny, 2004), or include a biological measure of stress validation (e.g., cortisol, cytokine reactivity) that is not also sensitive to the effects of acute arousal without stress.1,2 (7) Because stress hormones exert genomic effects on neural processes for hours after cessation of stress, the control condition could not have been subjected to a laboratory stressor on the same day as executive function assessment (e.g., Gärtner et al., 2014). This entails that if a study used a within-subjects, crossover design, the counterbalance of stress and control had to be separated by at least one day. (8) Because dual task performance necessarily involves both working memory and cognitive flexibility, and because stress affects dual task performance (Plessow et al., 2012), we did not include dual-task executive function paradigms (e.g., Scholz et al., 2009) in order to examine stress effects on each core executive function individually. We chose these inclusion criteria to best isolate the effects of acute stress on executive functions.
3.1.3 Selected studies
Our search and study inclusion criteria led to the incorporation of 51 studies, 49 of which were published in 47 peer-reviewed papers. If a study has been presented in both a thesis/dissertation and a published paper, we chose to cite the published paper. Of these 51 studies, 34 assessed effects of stress on working memory, 21 assessed effects of stress on inhibition, and 6 assessed effects of stress on cognitive flexibility.
3.2 Coding of Variables
Tasks that make use of executive function were coded as one of the three core executive functions based upon previous empirical or theoretical literature suggesting that a given task primarily loaded on one of the core executive functions (working memory, inhibition, or cognitive flexibility). See Table 1 for a complete description of task coding.
Table 1.
Coding of Tasks by Core Executive Function
| Working Memory | Inhibition | Cognitive Flexibility | |
|---|---|---|---|
| Tasks coded | Backward span tasks | Response Inhibition: | Wisconsin card sorting test |
| Reading span | Stop-signal task | Anagrams | |
| OSPAN/AOSPAN | Go/no-go | Sequential modulation tasks | |
| n-back | Stroop color reading | Compound remote associates test | |
| Sternberg item recognition | Go/stop task | Task-switching tests | |
| Delayed match-to-sample | Cognitive Inhibition: | Novel cognitive flexibility tasks | |
| Letter-number sequencing | Sustained attention to response task | ||
| WAIS Working Memory Index | Flanker task | ||
| Novel working memory tasks | D2 test of attention | ||
| Stroop word reading | |||
| Emotional Stroop task | |||
| Simple forward span tasks | |||
| Simple reaction time tasks | |||
| Visual attention tasks | |||
| Novel interference control tasks |
The severity of stress manipulations was coded parametrically and based upon prior meta-analyses that determined factors that produced a cortisol response (Dickerson and Kemeny, 2004). We coded a stressor as “low” (0) severity if the stressor did not have any socio-evaluative component and did not apply pain over a moderately-sized area of the body (e.g., watching stressful videos, ice pressed to the forehead). We coded a stressor as “moderate-low” (1) severity if the stressor had one socio-evaluative component or pain was applied to a moderately-sized area, but not both (e.g., the cold pressor task, supposed social evaluation of cognitive tasks through a one-way mirror). We coded a stressor as “moderate” (2) severity if the stressor applied pain to a moderately-sized area and included one component of concurrent social evaluation, or if the stressor had one socio-evaluative component with a monetary incentive for performance (e.g., the socio-evaluative cold pressor task, a video game competition). We coded a stressor as “moderate-high” (3) if the stressor involved two concurrent components of social evaluation (e.g., the Trier Social Stress Test). Finally, we coded a stressor as “high” (4) in severity if the stressor mimicked situations with a high risk of psychological or physical trauma (e.g., a 90min hypothermia induction, a 60min prisoner of war experience).
Study precision was a linear combination of a removal of factors that influence stress responses or cognitive performance such that a higher score indicated better isolation of stress effects on executive functions. Where exclusion of acute illnesses, medication use, hormonal contraceptive use, regular smokers, and hypertension are all dummy-coded as 1 for excluded and 0 for not excluded,3 study acclimation time prior to the stressor is coded as 1 for greater than or equal to 10 minutes and an additional 1 is given when acclimation time is also less than 40 minutes (to avoid boredom or fatigue), the equation is as follows:
Thus, study precision can take on negative values. For example, if a study did not exclude any participants with conditions known to influence stress reactivity, did not include any acclimation time, used a low-severity stressor, and had participants perform 10 cognitive tasks prior to the outcome of interest, study precision would take the value of −10. In contrast, if a study excluded all participants with acute illnesses, on medication, on hormonal contraceptives, who smoked regularly, and had hypertension; provided at least ten minutes for acclimation prior to the stressor but less than 40 minutes; used a high severity stressor; and did not have participants perform any other cognitive tasks prior to the outcome of interest, study precision would take the value of 11.
Stressor type was coded as follows. Stressors were coded as “social” stressors if they included social evaluation but did not include pain (e.g., the Trier Social Stress Test). Stressors were coded as “pain” stressors if they included pain but did not include social evaluation (e.g., the Cold Pressor Task). Stressors were coded as “hybrid” stressors if they included both social evaluation and pain (e.g., the Socially Evaluated Cold Pressor Task). Stressors were coded as “other” if they included none of these characteristics (e.g., skydiving, mock prisoner of war stressor, threat of shock coupled with gruesome pictures).
Working memory load was coded from relatively standard convention. Many tasks (e.g., the n-back, Sternberg item recognition task, etc.) parametrically vary working memory load, but not all do. We coded working memory load as “high” if the working memory task was a task that determined individuals' working memory spans (thereby determining the limit of an individual's working memory), if the “comparison load” in the Sternberg was eight or greater, if the “comparison load” in the delayed match to sample task was sixteen or greater, or if the number back on the n-back was three or greater. We coded all other working memory load as “not high”.
Outcome type (i.e., reaction time or performance based), whether the task contained an affective component, and the type of inhibition task (i.e., response inhibition or cognitive inhibition) were dummy coded. Tasks were considered including an affective component if the task employed affective characteristics, such as using angry faces as stimuli.
The delay between stress onset and assessment of executive functions, percent male participants (i.e., sex), participant age, and time of day the study began were analyzed as continuous variables and centered at their respective lowest obtained values, making the intercept interpretable as the effect size estimate at the lowest obtained value of the moderator. If the average participant age was not given in the article, the median participant age was used if it was reported; if neither of these statistics were listed, the midpoint of the reported participant age range was used.
To assess stress effects on cortisol, we calculated the pretest-posttest-control group effect size (Morris, 2008) and converted from d to g using the correct transformation (Lakens, 2013). We used the baseline samples as the pretest values and the peak reactivity samples (whichever value was the greatest in the stress group and the corresponding sample from the control group at this time) as the posttest values. This effect size provides an unbiased index of the effect of stress on the change in cortisol relative to the change in a control group, thus representing the effect size closest to how cortisol is analyzed in most studies.
The pretest-posttest correlation is required to calculate the variance of the pretest-posttest-control group effect size, and this correlation was unknown to us given that no study reported this. As such, we set the pretest-posttest correlation at .3. Sensitivity analyses from .0 to .8 indicated no differences in stress effects on cortisol with high or low correlations used to derive the variance of the effects.
3.3 Analytic Strategy
The effect size measure of interest was the standardized mean difference between stress and control groups. We used Hedges’ g rather than Cohen’s d as the effect size for analysis, given that the former is a relatively unbiased estimate of the population standardized mean difference effect size while the latter is a biased estimate. Whenever possible, we calculated Hedges’ g from the means, standard deviations, and sample sizes presented in the article. If means and standard deviations were not reported and the design was between-studies, we used t or one-way F statistics—or p values resulting from tests of those two statistics—to calculate the effect size. If none of these statistics were reported, we emailed corresponding authors for these statistics. If we were unable to obtain the necessary statistics for a study from the corresponding author, that study was excluded from analysis. For within-studies designs, we converted effect size estimates and their variances into the between-study effect size metric (Morris and DeShon, 2002).
Given the multifaceted nature of executive function, most studies often report more than one outcome (e.g., effects of stress on positive, negative, or neutral items; effects of stress on recall, cued recall, or recognition; etc.). Multiple outcomes are a problem for conventional meta-analytic methods, as averaging effect sizes within studies without accounting for their correlations can alter or obscure true effect size estimates (Borenstein et al., 2009; Scammacca et al., 2014). Thus, we employed the meta-analytic technique of robust variance estimation, a random-effects meta-regression that can account for dependence between effect size estimates (Hedges et al., 2010; Tanner-Smith and Tipton, 2014). This technique robustly estimates effect size weights and standard errors for the given effects, allowing for multiple outcomes within studies (Hedges et al., 2010). We employed the robu() function of the robumeta package in R, version 3.2.2, to conduct these analyses using the correlated weights given by Hedges et al. (2010), with our primary analyses using the small sample corrections suggested by Tipton (2014). To account for dependency, ρ was set to the recommended .80 (Tanner-Smith and Tipton, 2014). Because we were more interested in understanding factors that influence the effects of stress on executive function than we were interested in understanding factors that contribute to heterogeneity in analyses, we did not separate continuous moderators into within- and between-study continuous moderators.
Degrees of freedom for all primary analyses were estimated using the Satterwaite approximation, where df=2/cv2 and cv represents the coefficient of variation, as simulation studies have indicated that this method of estimating degrees of freedom is most analytically valid with study set sizes under 40 using the RVE meta-analytic technique (Tipton, 2014). Because of how the degrees of freedom are estimated, if the degrees of freedom are less than four, then there is a heightened risk of a Type I error and the analysis results cannot be trusted to represent population values (Tipton, 2014). However, because this estimation of degrees of freedom is extremely sensitive to outliers given a study set size such as in this meta-analysis (since degrees of freedom are divided by the coefficient of variation), one can be relatively confident that when degrees of freedom are greater than four, outlying studies are not driving observed significant effects.
For all of the following analyses, a positive effect size indicates that stress enhanced executive functions relative to a control condition, whereas a negative effect size indicates that stress impaired executive functions relative to a control condition. In addition, because the outcome in these analyses is the standardized mean difference between groups (the effect size), a significant continuous moderator means that the effect size estimate depends upon levels of that continuous variable. In other words, if the coefficient for a continuous moderator is significant, it means that as the continuous variable increases or decreases, the effect of stress on executive functions relative to a control condition increases or decreases.
4. Results
4.1 Preliminary Analyses
4.1.1 Study characteristics
The final sample consisted of 51 studies (i.e., total m=51), assessing stress effects on executive functions in 2,486 participants. There were 223 total effect sizes (i.e., total k=223). The number of effect sizes per study that we obtained is relatively common in social science research (Scammacca et al., 2014) and is similar to the number of effect sizes per study seen in similar meta-analyses (Shields et al., 2015). Stress effects on working memory were examined in 34 studies (k=164) with 1,353 participants. Stress effects on inhibition were examined in 21 studies (k=47) with 1,085 participants. Finally, stress effects on cognitive flexibility were examined in 6 studies (k=11) with 280 participants. Supplementary Table 1 presents each study and its characteristics.
4.1.2 Assessment of publication bias
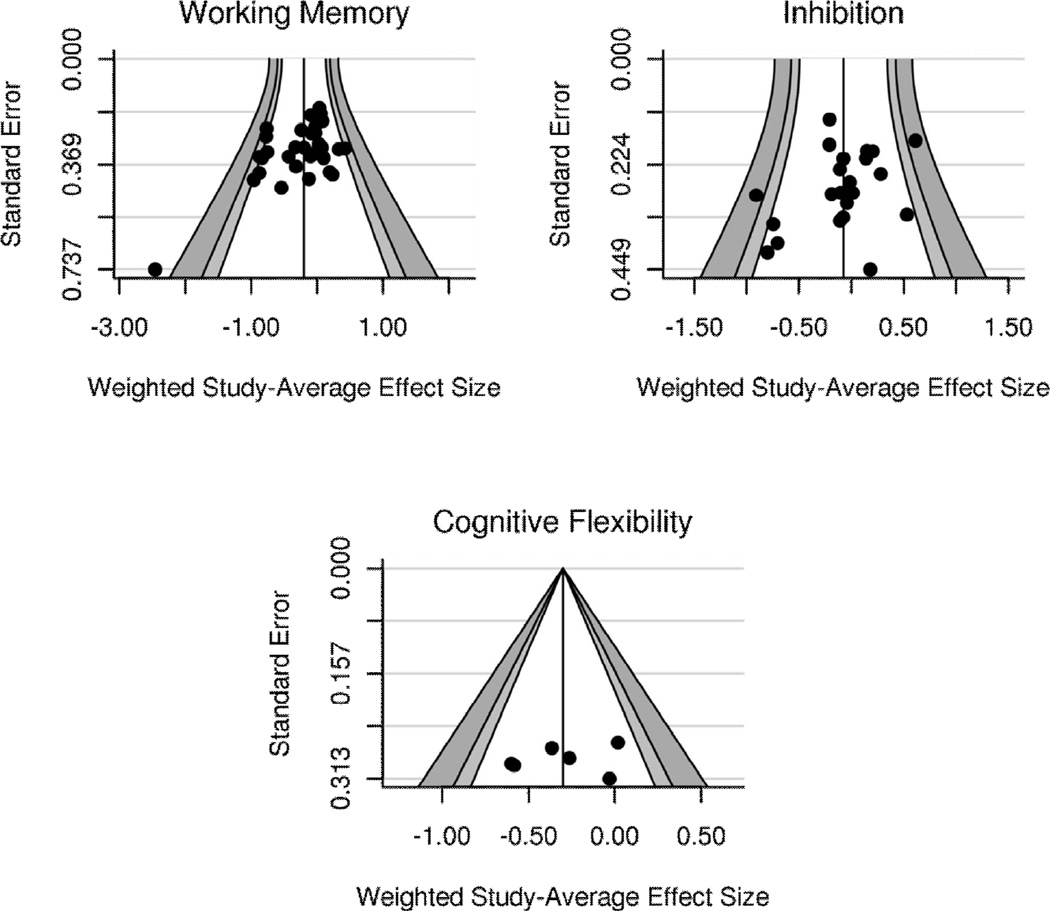
To assess publication bias, we conducted Egger’s test (Egger et al., 1997) for funnel plot asymmetry on each core executive function (Figure 1). Working memory showed marginal evidence for publication bias, t(32)= −1.83, p=.076, but this was driven by one outlying study with a small sample size that produced an extremely large effect size (see Figure 1). With this outlier removed, there was no evidence for publication bias, t(31)= −1.61, p=.118. Removing this outlier did not alter the results of any analyses of working memory. As such, graphs of working memory results are presented without this outlier to enhance clarity. Egger’s test also returned nonsignificant results for inhibition, t(20)= −0.90, p=.377, and cognitive flexibility, t(4)= −0.44, p=.682, although there were not enough studies of cognitive flexibility to make strong claims about publication bias for this core executive function. These results therefore indicate that any effects observed in this meta-analysis are unlikely to be due to publication bias.
Figure 1.
Funnel plots to ascertain evidence for publication bias. Asymmetry of points around the line by the standard error indicates evidence for publication bias. Lightgray bars represent 95% confidence intervals; gray bars represent 99% confidence intervals. Only working memory showed any evidence of publication bias, but this was driven by the outlying study (see graph). Once this outlier was removed, stress effects on working memory showed no evidence of publication bias.
4.2 Primary Analyses
4.2.1 Working memory
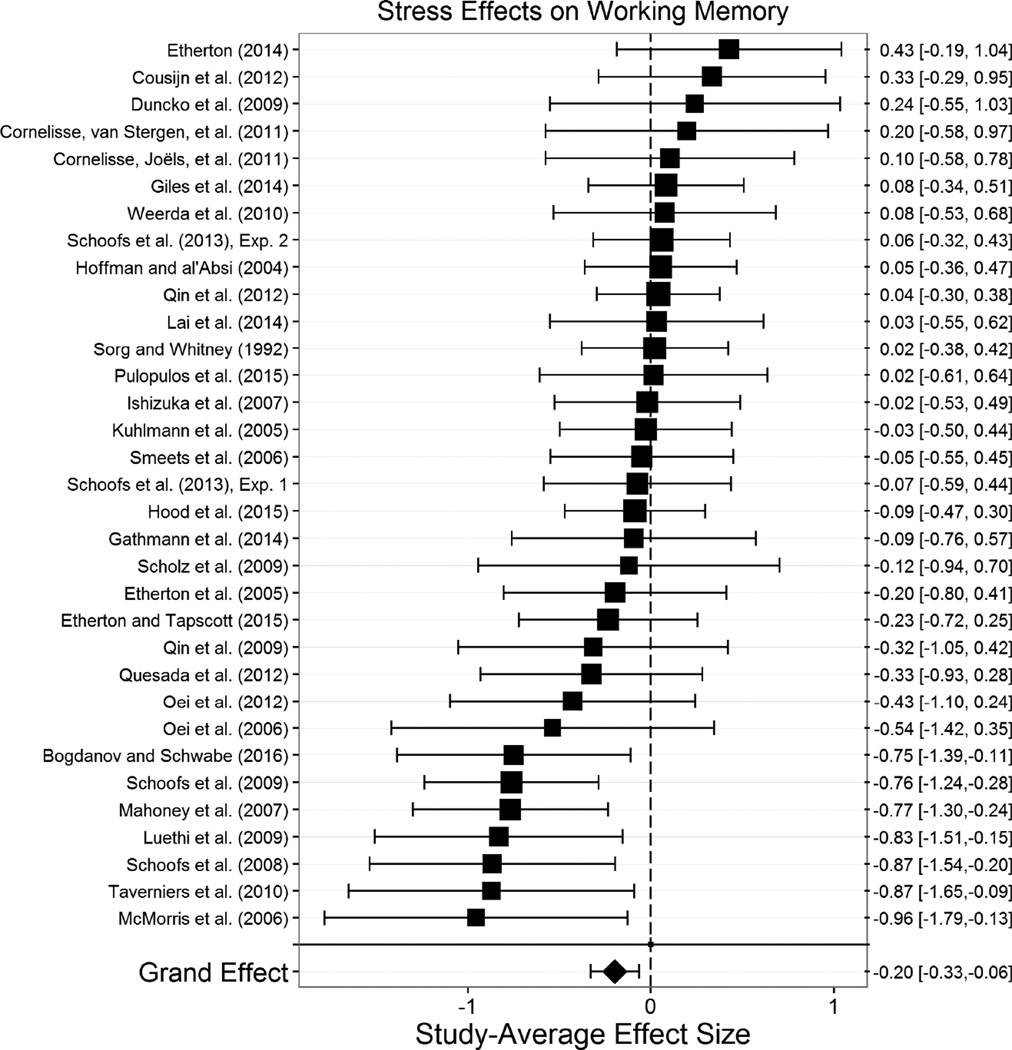
The overall effect of acute stress on working memory (m=34, k=164, N=1,353) was significant, g+= −.197, t(31.1)= −3.05, p=.005, 95% CI [−.330, −.064] (Figure 2), such that acute stress impaired working memory. There was low heterogeneity across these studies’ effects, τ2=0.11, indicating that the impairing effect of stress on working memory is relatively consistent across various conditions. Nonetheless, we explored the effects of moderators expected a priori to play an important role in the effects of stress on working memory. The effects of all potential moderators we considered of stress effects on working memory are displayed in Table 2.
Figure 2.
Effect of stress effect on working memory. Acute stress significantly impaired working memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent 95% confidence intervals of the effect size.
Table 2.
Potential Moderators of Stress Effects on Working Memory
| Moderator | ||||
|---|---|---|---|---|
| Continuous Variables | B | β | df | p |
| Time Study Began (min) | <−.001 | −.043 | 8.2 | .520 |
| Percentage Male Participants | −.004 | −.158 | 19.2 | .022 |
| Participant Age (years) | .005 | .027 | 1.4 | .325 |
| Stress Severity | −.137 | −.125 | 14.9 | .014 |
| Study Precision | −.040 | −.112 | 6.9 | .005 |
| Stress Effects on Cortisol | −.062 | −.091 | 4.3 | .151 |
| Stress to Working Memory Delay (min) | −.006 | −.102 | 6.0 | .044 |
| Categorical Variables | F | g | df | p |
| Stress Type | 3.11 | 1, 4.4 | .140 | |
| Social | −.165 | 17.2 | .037 | |
| Pain | −.284 | 7.3 | .122 | |
| Hybrid (i.e., social/pain) | .047 | 1.0 | .252 | |
| Other | −.263 | 3.7 | .336 | |
| Outcome Type | 1.73 | 1, 15.5 | .208 | |
| Reaction Time Based | −.081 | 10.2 | .422 | |
| Accuracy Based | −.234 | 26.0 | .005 | |
| Working Memory Load | 5.87 | 1, 24.9 | .023 | |
| High Load | −.303 | 19.7 | .005 | |
| Not High Load | −.049 | 13.0 | .404 | |
Note: Significant or marginal (p<.10) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator; negative effects mean that stress impaired working memory, whereas positive means that stress enhanced it. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator or effect size in question.
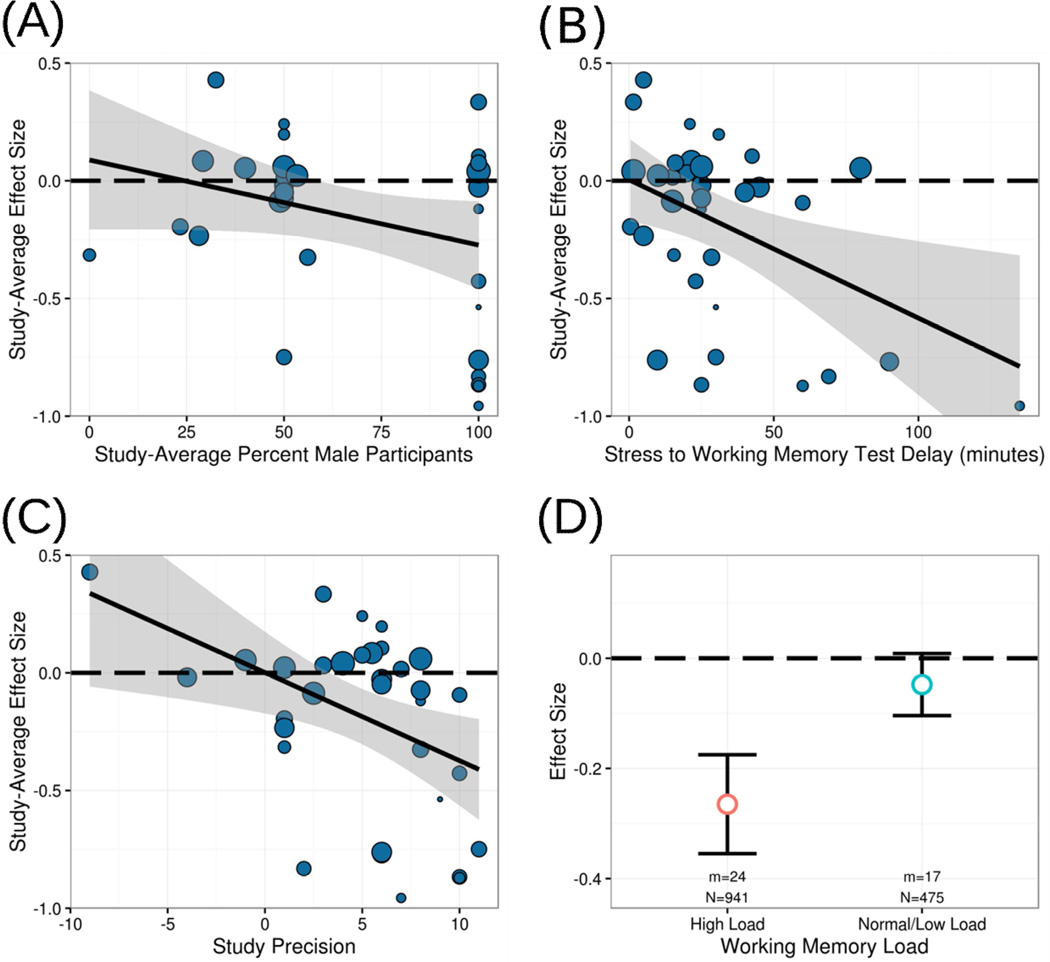
We expected sex to moderate effects of stress on working memory, as previous studies have suggested that stress may impair working memory to a lesser degree in men than women (Schoofs et al., 2013). Surprisingly, however, we found that stress-induced impairments were greater as the percent of males increased in analyses, B= −.0044, t(19.2)= −2.50, p=.022 (Figure 3). That is, stress impaired working memory more in men than women. Given that a two-study empirical paper (Schoofs et al., 2013) found the opposite of this result, we restricted analyses to the task used in that paper—the n-back—and the outcome measure (reaction time). Although the percent of male participants was no longer a significant moderator in this restricted analysis, B= −.0029, p=.321, the direction of the effect remained such that stress appeared to impair working memory more in men than women.
Figure 3.
Significant moderators of stress effects on working memory. As (A) the percent of male participants, (B) the delay between stress onset and working memory testing, (C) study precision, and (D) working memory load increased, stress effects on working memory became more impairing. However, only (C) study precision—the reduction of confounds related to assessment of acute stress and/or working memory—and (D) working memory load remained significant when accounting for covariance of other moderators.
We also expected the delay between stress onset and working memory assessment to moderate stress effects on working memory, given that a meta-analysis of cortisol administration effects found that the impairing effects of cortisol on working memory reversed over time to become an enhancing effect (Shields et al., 2015). Surprisingly, however, we found that effects of stress on working memory actually became more impairing as the delay between stress onset and working memory assessment increased, B= −.006, t(6.0)= −2.55, p=.044 (Figure 3).
Further, we expected study precision to moderate stress effects on working memory, as we expected that studies which removed more confounds related to both stress effects and working memory testing would show a relatively greater effect of stress on working memory. As expected, study precision significantly moderated stress effects on working memory, B= −.039, t(7.1)= −3.99, p=.005 (Figure 3), such that as study precision increased, the impairing effect of stress on working memory increased in magnitude. Although there was one notable outlier with a large negative value in study precision (see Figure 3), removing this outlier did not affect the results; study precision remained a significant predictor of stress effects on working memory without this outlier included in the analysis, B= −.370, t(9.9)= −2.83, p=.018.
Additionally, we expected working memory load to moderate stress effects on working memory, given empirical literature suggesting stress effects on working memory might be strongest at high loads (Oei et al., 2006). As expected, stress effects on working memory when working memory load was high (g+= −.303, p=.005) were significantly greater than stress effects when working memory load was not high (g+= −.049, p=.404), t(25.3)=2.24, p=.023.
Similarly, we expected that stress effects on cortisol would moderate stress effects on working memory given that a prior meta-analysis found that cortisol administration influenced working memory (Shields et al., 2015). Surprisingly, however, stress effects on cortisol did not moderate stress effects on working memory, B= −.062, t(4.3)= −1.75, p=.151. Moreover, stress effects on cortisol did not interact with any of the above factors to moderate stress effects on working memory, ps>.217.
Finally, we examined a number of potential methodological moderators of stress effects on working memory. Of these moderators, stress severity predicted a greater stress-induced impairment of working memory as it increased, B= −.129, t(15.6)= −2.71, p=.016.
To better assist future research with study design, we attempted to elucidate the simultaneously significant and controllable moderators of stress effects on working memory. Using a forward stepwise regression, we entered the strongest moderator of stress effects on working memory at each step and proceeded until there were no more moderators with p<.10 and df≥4. In this model, study precision emerged as a significant moderator, B= −039, t(4.8)= −3.40, p=.021, and working memory load emerged as a marginally significant moderator, B= −.205, t(23.8)= −2.03, p=.054. When centering these moderators at their highest reliably obtained values (i.e., study precision of 8—see section 3.2 for coding of study precision—and high working memory load), the effect of stress on working memory was moderate and significant, g+= −519, t(16.3)= −4.40, p<.001, 95% CIg [−769, −.269]. To achieve 80% power to detect this effect, a sample size of 114 (57 stress, 57 control) is needed for a two-tailed test, whereas a sample size of 60 (30 stress, 30 control) is needed for a one-tailed test.
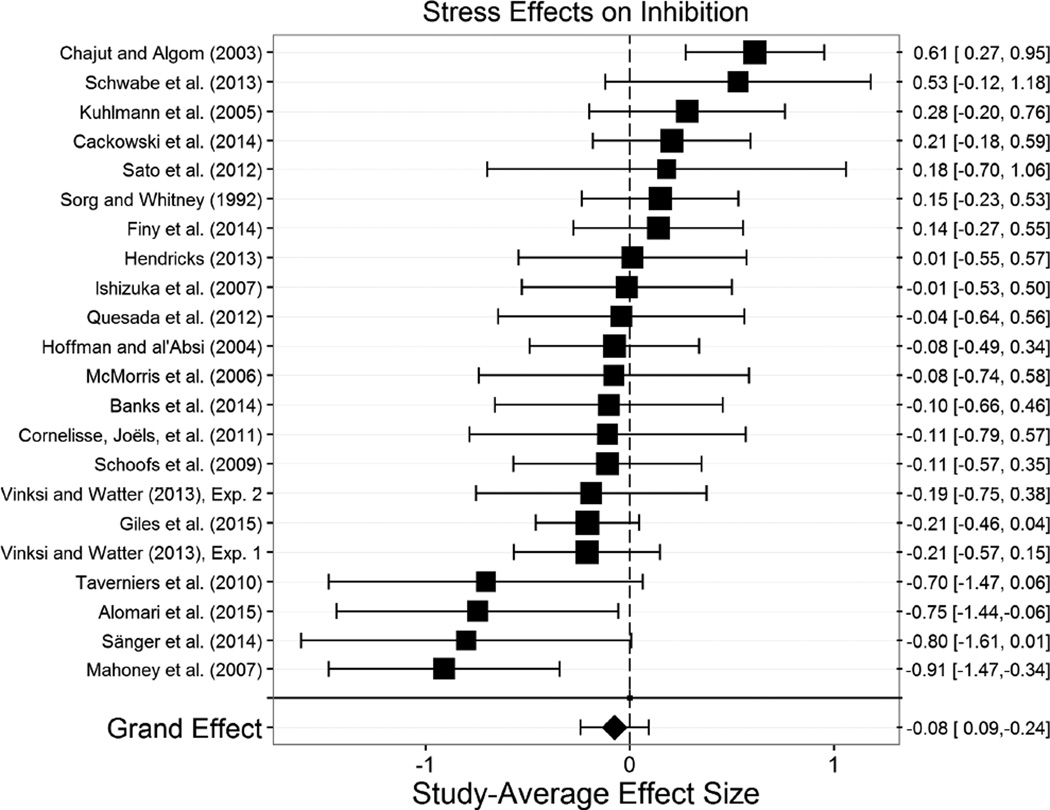
4.2.2 Inhibition
The overall effect of acute stress on inhibition (m=22, k=48, N=1,156) was not significant, g+= −.076, t(20.5)= −0.94, p=.358, 95% CI [−.243, .092] (Figure 4). There was, however, moderate heterogeneity across these studies’ effects, τ2=0.19, indicating that the effect of stress on inhibition was likely moderated by one or more variables. As such, we explored the effects of moderators expected a priori to play an important role in the effects of stress on inhibition. The effects of all potential moderators we considered of stress effects on inhibition are displayed in Table 3.
Figure 4.
Effect of stress effect on inhibition. Acute stress did not influence inhibition overall. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent 95% confidence intervals of the effect size.
Table 3.
Potential Moderators of Stress Effects on Inhibition
| Moderator | ||||
|---|---|---|---|---|
| Continuous Variables | B | β | df | p |
| Time Study Began (min) | <−.001 | −.055 | 4.3 | .533 |
| Percentage Male Participants | −.001 | −.032 | 9.2 | .682 |
| Participant Age (years) | .004 | .012 | 2.9 | .843 |
| Stress Severity | −.126 | −.104 | 8.5 | .189 |
| Study Precision | −.030 | −.086 | 5.7 | .276 |
| Stress Effects on Cortisol | −.040 | −.122 | 3.0 | .353 |
| Stress to Inhibition Test Delay (min) | −.005 | −.140 | 4.0 | .252 |
| Categorical Variables | F | g | df | p |
| Stress Type | 0.66 | 1, 4.9 | .614 | |
| Social | .047 | 10.8 | .557 | |
| Pain | −.331 | 2.0 | .350 | |
| Hybrid (i.e., social/pain) | −.250 | 3.0 | .476 | |
| Other | −.138 | 2.0 | .652 | |
| Outcome Type | 0.20 | 1, 9.3 | .667 | |
| Reaction Time Based | −.017 | 6.3 | .925 | |
| Accuracy Based | −.098 | 15.8 | .255 | |
| Inhibition Type | 14.4 | 1, 7.6 | .006 | |
| Response Inhibition | .296 | 4.7 | .041 | |
| Cognitive Inhibition | −.208 | 15.1 | .021 | |
Note Significant or marginal (p<.10) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator; negative effects mean that stress impaired inhibition, whereas positive means that stress enhanced it. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator or effect size in question.
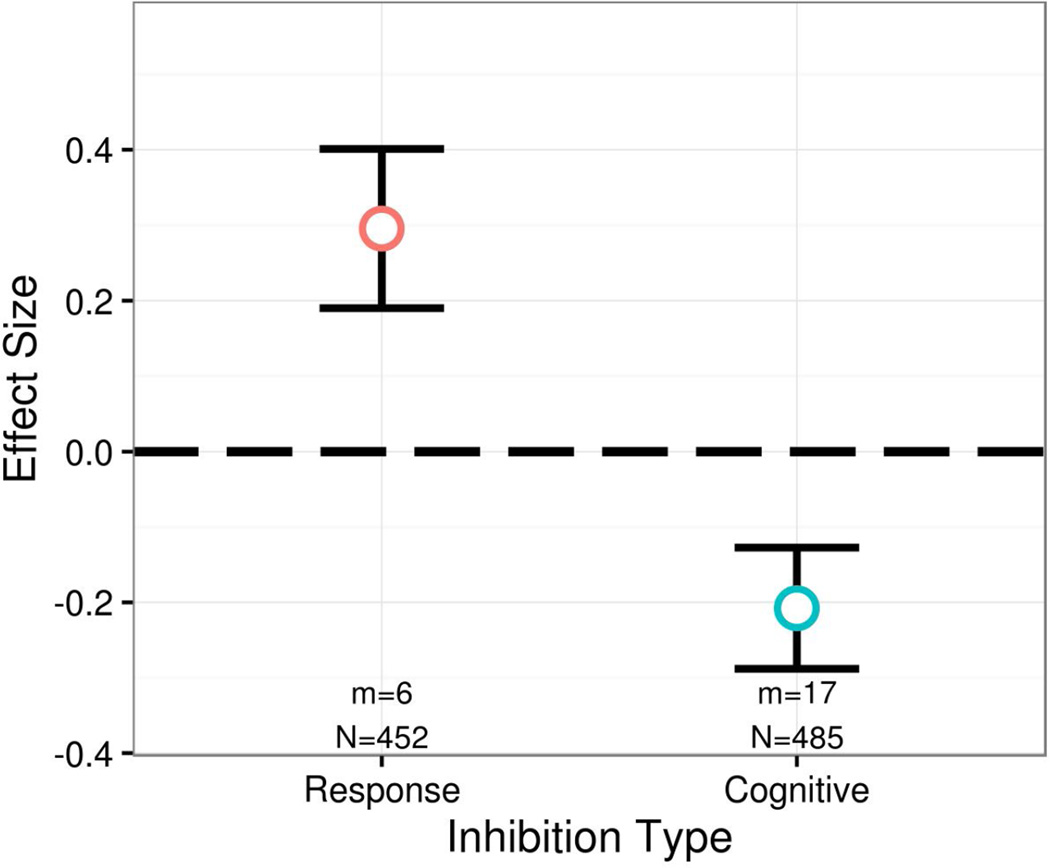
We examined whether the type of inhibition (i.e., cognitive or response inhibition) required by the task moderated stress effects on inhibition, given evidence that these forms of inhibition can be dissociated under some conditions (Johnstone et al., 2009; Sänger et al., 2014; Schwabe et al., 2013). As expected, we found that the type of inhibition moderated stress effects on inhibition, t(8.3)= −3.80, p=.005 (Figure 5). Specifically, stress significantly enhanced response inhibition, g+= .296, t(4.7)=2.80, p=.041, whereas stress significantly impaired cognitive inhibition, g+= −.208, t(14.2)= −2.58, p=.021.
Figure 5.
Inhibition type significant moderates stress effects on inhibition. Stress significantly impaired cognitive inhibition (i.e., interference control) but enhanced response inhibition.
We also expected effects of stress on cortisol to moderate stress effects on inhibition because a prior meta-analysis found cortisol administration influenced inhibition (Shields et al., 2015). Surprisingly, however, stress effects on cortisol did not moderate stress effects on inhibition, B= −.041, t(3.0)= −1.10, p=.353. Effects of stress on cortisol also did not interact with type of inhibition, p=.420, or with the delay between stress onset and inhibition testing (see the paragraph below), p=.115, to moderate stress effects on inhibition.
In addition, we expected that the delay between stress onset and inhibition assessment would moderate stress effects on inhibition, given that a meta-analysis of cortisol administration effects found that the initial enhancing effects of cortisol on inhibition reversed over time to become an impairing effect (Shields et al., 2015). Although the effect was in the expected direction, the delay between stress onset and inhibition assessment did not moderate stress effects on inhibition, B= −.005, t(4.0)= −1.34, p=.252. In addition, the delay between stress onset and inhibition did not interact with type of inhibition to moderate stress effects on inhibition, p=.780.
We also expected that study precision would moderate stress effects on inhibition, as we expected that studies which removed more confounds related to both stress effects and inhibition testing would show a relatively greater effect of stress on inhibition. Surprisingly, however, study precision did not moderate stress effects on inhibition, B= −.030, t(5.7)= −1.20, p=.276, nor did study precision interact with type of inhibition to moderate stress effects on inhibition, p=.545.
We examined a number of potential methodological moderators of stress effects on inhibition (see Table 3). None of these additional potential moderating effects were significant.
To better assist future research with study design, we attempted to elucidate the simultaneously significant and controllable moderators of stress effects on inhibition. Using a forward stepwise regression, we entered the strongest moderator of stress effects on inhibition at each step and proceeded until there were no more moderators with p<.10 and df≥4. In this model, only type of inhibition emerged as a significant moderator, t(8.6)=3.75, p=.005. For cognitive inhibition, stress produced a significant impairment, g+= −.208, t(15.1)= −2.58, p=.021, 95% CIg [−.379, −.035]. To achieve 80% power to detect this effect, a sample size of 688 (344 stress, 344 control) is needed for a two-tailed test, whereas a sample size of 348 (174 stress, 174 control) is needed for a one-tailed test. For response inhibition, stress produced a significant enhancement, g+=.296, t(4.7)=2.80, p=.041, 95% CIg [.018, .573]. To achieve 80% power to detect this effect, a sample size of 350 (175 stress, 175 control) is needed for a two-tailed test, whereas a sample size of 178 (89 stress, 89 control) is needed for a one-tailed test.
4.2.3 Cognitive Flexibility
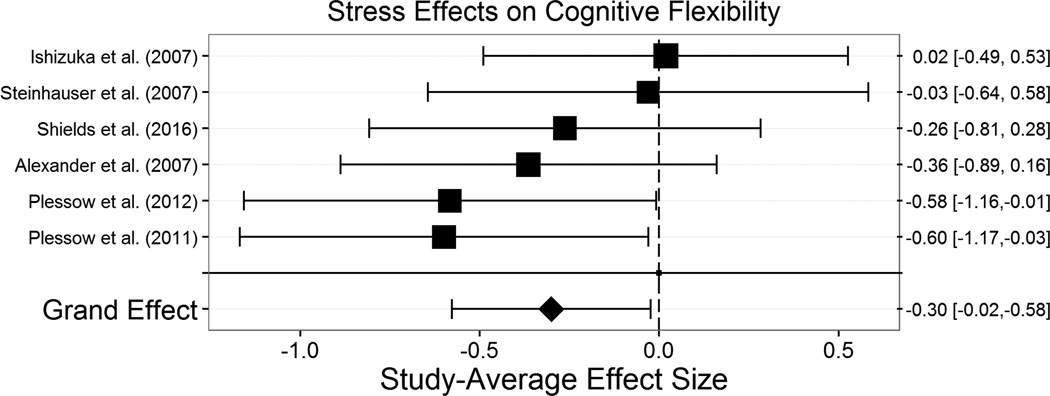
The overall effect of acute stress on cognitive flexibility (m=6, k=11, N=280) was significant, g+= −.300, t(5.0)= −2.79, p=.039, 95% CI [−.577, −.023] (Figure 6), such that acute stress impaired cognitive flexibility. There was low heterogeneity across these studies’ effects, τ2=0.05, indicating that the impairing effect of stress on cognitive flexibility is relatively consistent across various conditions. Nonetheless, we explored the effects of moderators expected a priori to play an important role in the effects of stress on cognitive flexibility. The effects of all potential moderators we considered of stress effects on cognitive flexibility are displayed in Table 4.
Figure 6.
Effect of stress effect on cognitive flexibility. Acute stress significantly impaired cognitive flexibility. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent 95% confidence intervals of the effect size.
Table 4.
Potential Moderators of Stress Effects on Cognitive Flexibility
| Moderator | ||||
|---|---|---|---|---|
| Continuous Variables | B | β | df | p |
| Time Study Began (min) | NA | |||
| Percentage Male Participants | −.009 | −.195 | 1.0 | .299 |
| Participant Age (years) | .049 | .068 | 1.8 | .682 |
| Stress Severity | −.222 | −.232 | 2.1 | .032 |
| Study Precision | −.040 | −.217 | 2.5 | .026 |
| Stress Effects on Cortisol | NA | |||
| Stress to Cognitive Flexibility Delay (min) | −.007 | −.082 | 3.0 | .427 |
| Categorical Variables | F | g | df | p |
| Stress Type | NA | |||
| Social | ||||
| Pain | ||||
| Hybrid (i.e., social/pain) | ||||
| Other | ||||
| Outcome Type | 4.04 | 1, 2.5 | .155 | |
| Reaction Time Based | −.110 | 1.8 | .403 | |
| Accuracy Based | −.380 | 3.8 | .032 | |
Note: Significant or marginal (p<.10) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator; negative effects mean that stress impaired cognitive flexibility, whereas positive means that stress enhanced it. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator or effect size in question. When there were not enough studies to estimate a moderating effect, NA is listed in the column for B or F.
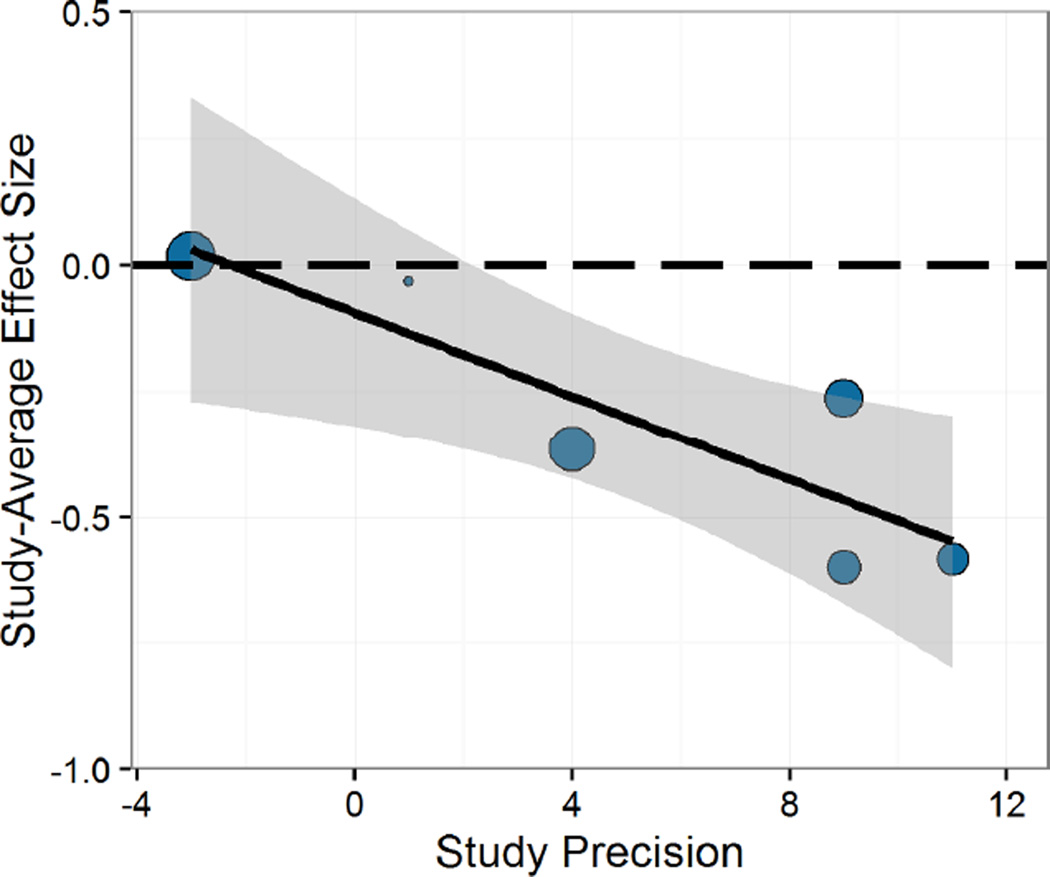
We expected study precision to moderate stress effects on cognitive flexibility, as we expected that studies which removed more confounds related to both stress effects and cognitive flexibility testing would show a relatively greater effect of stress on cognitive flexibility. As expected, study precision significantly moderated stress effects on cognitive flexibility, B= −.040, t(2.5)= −4.85, p=.026 (Figure 7), such that as study precision increased, the impairing effect of stress on cognitive flexibility increased in magnitude.4
Figure 7.
Study precision significantly moderated stress effects on cognitive flexibility. As study precision improved, stress effects on cognitive flexibility became more impairing.
We also expected that sex would moderate stress effects on cognitive flexibility, given some evidence suggesting stress impairs cognitive flexibility more in males than females (Laredo et al., 2015). Although the effect was in the expected direction, with a greater proportion of males descriptively associated with a greater impairment, percentage male participants did not emerge as a significant moderator of stress effects on cognitive flexibility, B= −.009, t(1.0)= −1.96, p=.299. Nonetheless, because of the small study set size, we are not able to make strong claims about the lack of association here.
We attempted to determine whether stress effects on cortisol moderated stress effects on cognitive flexibility. However, only three studies of stress and cognitive flexibility included cortisol data; as such, we were unable to analyze this variable as a potential moderator.
We also examined a number of potential methodological moderators of stress effects on cognitive flexibility. Of these moderators, only stress severity moderated stress effects on cognitive flexibility, B= −.222, t(2.1)= −5.23, p=.032, predicting a greater impairment in cognitive flexibility as stress severity increased. Because df are less than four in that moderator analysis, though, there is a twofold greater risk of making a Type I error if inferring this to be a true effect.
To better assist future research with study design, we attempted to elucidate the simultaneously significant and controllable moderators of stress effects on cognitive flexibility using a forward stepwise regression model as we did for working memory and inhibition. In this model, study precision emerged as the only significant moderator, B= −.040, t(2.5)= −4.85, p=.026. Because df are less than four in the prior analysis, we present power analyses with and without study precision as a moderator. When centering study precision at its highest reliably obtained value (i.e., study precision of 8—see section 3.2 for coding of study precision), the effect of stress on cognitive flexibility was moderate and significant, g+= −.541, t(2.6)= −5.73, p=.016, 95% CIg [−.870, −.211]. To achieve 80% power to detect this effect, a sample size of 106 (53 stress, 53 control) is needed for a two-tailed test, whereas a sample size of 56 (28 stress, 28 control) is needed for a one-tailed test. When study precision is not included as a moderator (g+= −.300, t(5.0)= −2.79, p=.039), to achieve 80% power to detect the effect of stress on cognitive flexibility, a sample size of 330 (165 stress, 165 control) is needed for a two-tailed test, whereas a sample size of 168 (84 stress, 84 control) is needed for a one-tailed test.
4.3 Comparison of Stress with Cortisol Administration Effects
By conducting a secondary analysis of a recent meta-analysis of cortisol administration effects on executive functions (Shields et al., 2015), we are able to determine whether stress effects on executive functions significantly differ from cortisol administration effects. This will elucidate whether stress exerts effects on executive functions primarily through cortisol or whether stress exerts additional influences on executive functions.
At a global level (i.e., across all executive function tasks), stress effects on executive functions significantly differed from cortisol administration effects, t(58.4)=2.69, p=.009. Stress impaired executive functions overall, g+= −.151, t(47.0)= −2.92, p=.005, 95% CIg [−.256, −.047], whereas cortisol administration did not, g+=.030, t(26.4)=0.69, p=.495, 95% CIg [−.058, .117]. However, cortisol administration differentially influenced working memory and inhibition, and these effects also depend upon the time since cortisol administration (Shields et al., 2015); as such, a more fine-grained analysis is appropriate.
4.3.1 Working memory
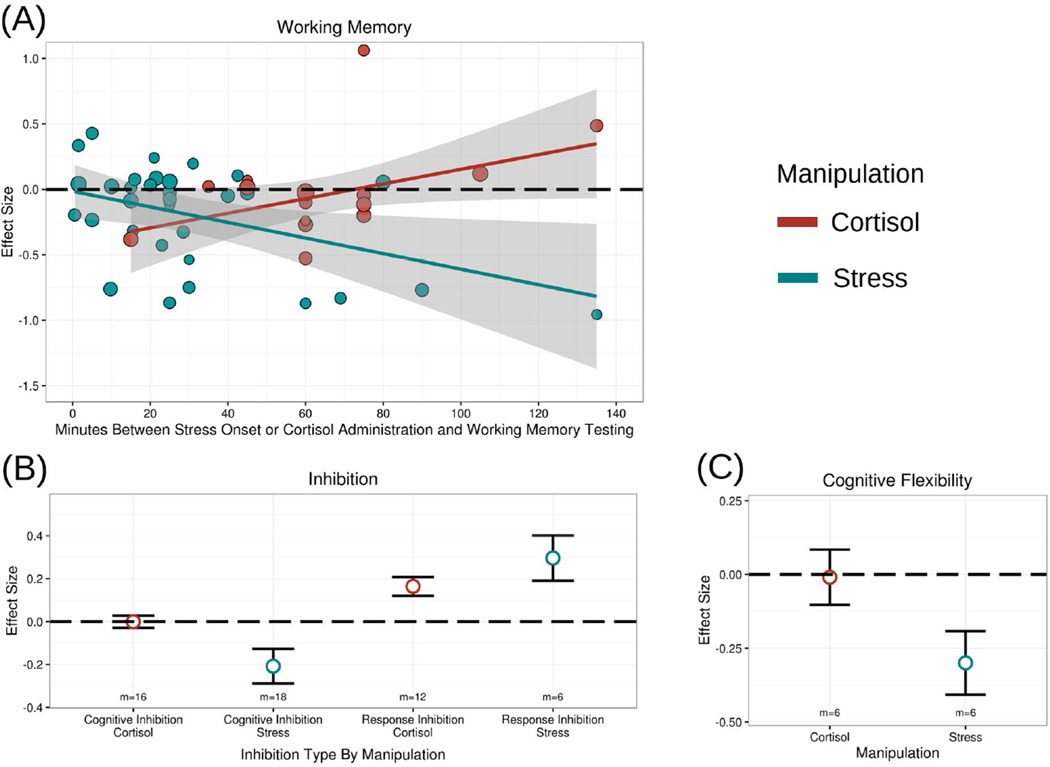
Because cortisol administration either enhanced or impaired working memory depending upon the delay between administration and testing, we examined whether the effect of delay (following either cortisol administration or stress onset) differed between cortisol and stress. Indeed, as Figure 8a shows, the moderating effect of delay significantly differed between cortisol administration and stress, B=.011, t(5.5)=4.51, p=.005. For studies of stress (as discussed further in section 4.2.1), the delay between stress onset and working memory testing contributed to a greater impairing effect of stress on working memory, B= −.006, p=.044, whereas for cortisol administration, the delay between cortisol administration and testing reversed the impairing effect and contributed to an enhancement of working memory, B=.005, p=.032.
Figure 8.
Comparison of effects of acute stress and cortisol administration on core executive functions. (A) The time-dependent effects of acute stress on working memory significantly differed from the time-dependent effects of cortisol on working memory. (B) The effects of acute stress on cognitive inhibition (i.e., interference control) significantly differed from the effects of cortisol administration on cognitive inhibition; no significant difference was observed in response inhibition, although stress effects tended to be larger. (C) The effects of acute stress on cognitive flexibility were marginally more impairing than the effects of cortisol administration on cognitive flexibility.
4.3.2 Inhibition
Because cortisol administration either enhanced or impaired inhibition depending upon the delay between administration and testing, we examined whether the effect of delay (following either cortisol administration or stress onset) differed between cortisol and stress. The moderating effect of delay on inhibition did not differ between cortisol administration and stress, B=.004, t(4.7)=1.14, p=.310. However, with or without controlling for the delay between administration and testing, Figure 8b illustrates that cortisol administration significantly differed from stress effects in how it influenced cognitive inhibition (i.e., interference control), t(25.1)=2.37, p=.026. Specifically, cortisol administration did not impair cognitive inhibition (g+=.047, p=.290), whereas stress did (g+= −.208, p=.021).
4.3.3 Cognitive flexibility
Despite the small study set size for cognitive flexibility, cortisol administration effects still emerged marginally different from stress effects on cognitive flexibility, t(9.2)=2.03, p=.072 (see Figure 8c). While stress impaired cognitive flexibility (g+= −.300, p=.039), cortisol administration had no effect on cognitive flexibility (g+= −.010, p=.923).
In sum, stress effects each core executive function differed from effects of cortisol administration alone. Thus, stress appears to exert effects on executive functions through more pathways than cortisol alone, if stress exerts actions on executive functions through cortisol at all.
5. Discussion
5.1 Discussion of Results
Stress is a ubiquitous force in our daily lives. Despite the frequency of its occurrence and its impact on health, academic achievement, and career success, little is known about how acute stress (i.e., stress) systematically influences higher cognitive processes. In this meta-analysis we attempted to address that gap in the literature by systematically examining stress effects on the three core executive functions: working memory, inhibition, and cognitive flexibility. We found that, overall, stress impaired working memory and cognitive flexibility but did not exert a main effect on inhibition. However, within inhibition we discovered that stress impaired cognitive inhibition but enhanced response inhibition. In addition, by contrasting cortisol administration effects on executive functions with stress effects, we determined that stress influenced executive functions through additional pathways rather than through cortisol alone.
These main effects were qualified by some important moderators. Study precision—the extent to which a study removed confounds related to acute stress or executive function assessment—moderated stress effects on both working memory and cognitive flexibility, such that as study precision increased, the magnitude of the impairing effect of stress on working memory and cognitive flexibility increased. Similarly, stress effects on working memory were greater in magnitude as the percentage of male participants, working memory load, and delay between stress onset and working memory assessment increased—though we found that only working memory load remained significant when simultaneously considering study precision, indicating some overlap between study precision and percentage male participants, and the delay between stress onset and working memory assessment.
Our results thus highlight the importance of methodology in assessing stress effects on cognition. Studies that best controlled for variables influencing either stress reactivity or executive functions showed the strongest effects of stress on working memory and cognitive flexibility. Thus, future research aimed at examining the effects of stress on executive functions should attempt to carefully control for factors influencing stress and executive functions.
Although most effects were in the expected directions, three moderators—sex, the stress to working memory delay, and cortisol reactivity—exhibited notable differences from what was expected based upon prior literature. In particular, as the percent of male participants or the delay between stress and working memory testing increased, stress effects on working memory became more impairing, which was opposite of what was expected (more on this in subsequent paragraphs). In addition, despite expectations, stress effects on cortisol did not moderate stress effects on working memory or inhibition (and could not be examined in cognitive flexibility).
Two studies from the same lab have suggested that stress may impair working memory performance more in women than in men (Schoofs et al., 2013), and this finding also coincides with some animal work (Shansky et al., 2006).5 Why then, in contrast to these aforementioned experiments, did we find that stress actually impaired working memory more as the percentage of males increased? We believe the answer to this question lies in study precision, as we will explain. Because stress research has consistently found that sex hormones can alter stress responses, many studies most concerned about isolating stress effects on working memory were conducted with only male participants. Thus, the studies with the best ability to isolate stress effects on working memory tended to have the greatest percentage of male participants. Indeed, the moderating effect of sex was no longer significant once study precision was considered. Thus, perhaps effects of stress on working memory in women are greater than men in studies that achieve equivalent levels of study precision, but more research is necessary to address this issue.
Although a prior meta-analysis of cortisol administration effects found that as the delay between administration and working memory assessment increased, the impairing effect of cortisol administration decreased and even became an enhancement over time (Shields et al., 2015). However, we found that the delay between stress onset (i.e., the initial endogenous cortisol increase) and working memory actually contributed to a greater impairing effect of stress on working memory as the delay increased. The reason for this discrepancy, though, is likely that stress is exerting effects on working memory through mechanisms beyond cortisol alone, as our analysis in section 4.3.1 shows. For example, stress increases circulating proinflammatory cytokines (Steptoe et al., 2007), which are known to impair working memory (Marsland et al., 2006; Sparkman et al., 2006). Moreover, stress-induced cytokine increases follow a different timecourse than cortisol, peaking and returning to baseline after cortisol peaks and returns to baseline. Thus, because cytokines impair working memory but are delayed in doing so, a stress-induced impairment may increase as the delay between stress onset and working memory assessment increases.
5.2 Comparison of Effects of Stress and Cortisol on Executive Functions
As the previous paragraph suggests and our analyses in section 4.3 make clear, stress effects on executive functions differed markedly from cortisol effects on executive functions. Indeed, stress effects on working memory, cognitive inhibition, and cognitive flexibility all significantly or marginally differed from cortisol effects. Similarly, although effects of stress did not significantly differ from effects of cortisol on response inhibition, this lack of difference may have simply been due to insufficient power, as effects of stress on response inhibition were nearly twice as large as effects of cortisol. Moreover, these differences were paralleled by a lack of association of cortisol reactivity with any stress effects on executive functions.
Although it might be tempting to think that stress is simply a more forceful manipulation of cortisol levels than exogenous administration of cortisol, two lines of evidence argue against this. First, cortisol administration usually increases cortisol levels much more than stress (for example, compare Henckens et al., 2011; Weerda et al., 2010). For example, a 10mg dose of cortisol is relatively small in the cortisol administration literature (Shields et al., 2015), yet it still produces cortisol increases on the order of four to five times greater than a stress-induced increase (e.g., Terfehr et al., 2011). Higher doses of cortisol used in cortisol administration studies, such as 100mg, can produce cortisol increases of approximately 500 nmol/L, which is about 50 times greater than a normal stress-induced increase in cortisol (e.g., Hsu et al., 2003). As such, it is difficult—if not impossible—to argue that stress simply results in greater circulating cortisol than cortisol administration. Second, the dramatic dissociation in the temporal effects of stress and cortisol administration on working memory argue that the effects of stress and cortisol on working memory are distinct. Stress effects do not look like cortisol effects on working memory over time; indeed, they look qualitatively different. In addition, whereas stress impaired cognitive flexibility and cognitive inhibition, cortisol administration does not appear to impact cognitive flexibility or cognitive inhibition. Therefore, it appears that stress is acting through more biological mechanisms than cortisol alone to accomplish its effects.
Presumably, the disagreement between effects of stress and effects of cortisol administration on executive functions arises because stress exerts effects on multiple biological processes aside from cortisol. For example, stress upregulates sex hormones (Lennartsson et al., 2012a), alters immune system activity (Segerstrom and Miller, 2004; Steptoe et al., 2007), and upregulates other adrenal hormones such as DHEA or noradrenaline (Allen et al., 2014; Lennartsson et al., 2012b; Shields et al., 2016a; Thoma et al., 2012), and many of these hormones and immune system factors exert effects on cognition (Allen et al., 2014; Mehta and Josephs, 2010; Shields et al., 2016a; Sparkman et al., 2006). Similarly, stress alters catecholaminergic activity and corticotropin-releasing hormone (CRH), both of which have known and important effects on executive function (Arnsten, 2009; Shansky and Lipps, 2013; Uribe-Mariño et al., 2016). As such, effects of stress on any one of these biological processes may be responsible in part for stress effects on executive functions, and a failure to consider them may result in an incomplete picture of how stress influences executive functions. Indeed, our results make clear that cortisol, at least by itself, does not appear to be responsible for producing stress effects on executive functions. These results therefore suggest that a more systematic approach—simultaneously examining multiple hormones or immune system processes—is necessary in order to understand the biological mechanisms behind the effects of stress on executive functions.
On a different level of explanation, various psychological and social factors may directly contribute to effects of stress on executive function, irrespective of the HPA axis, SAM axis, or immune system responses to stress. For example, common stressors include negative social evaluation, and it is possible that following this social evaluation participants would begin to ruminate on their presumed poor performance (De Lissnyder et al., 2012). Rumination in turn diminishes executive control (Philippot and Brutoux, 2008), and so stress-induced rumination may lead to worse performance on subsequent executive function tasks by diminishing executive control. Alternatively, laboratory stress manipulations may simply reduce motivation to be a good study participant due to stress-induced anger directed at the study or experimenter, and worse performance on executive function tasks may be a function of this reduced motivation—though it would be difficult to imagine why response inhibition would improve if this were the case. Although these are not an exhaustive list of potential psychological mechanisms, this list serves to illustrate that the effects of stress on executive function are complex due to conjunctive effects of biological processes resulting from HPA axis, SAM axis, and immune system activation as well as possible psychological factors not necessarily dependent upon the activation of these biological systems. Nonetheless, we are careful to note that most of the effects of stress on executive functions that we observed appear to have strong biological components, as it is difficult to imagine why, for example, sex or the delay between stress and executive function testing would moderate effects of stress without reference to a biological process.
5.3 Theoretical Implications
Our results have important implications for theoretical perspectives on stress and executive functions. First, our results provide only partial support for the perspective that stress reallocates executive control resources from working memory and cognitive flexibility to selective attention in order to focus processing on current stress-relevant information (e.g., LeBlanc, 2009; Mather and Sutherland, 2011). Although cortisol administration appears to bias cognitive processing in this way, we found that stress impaired cognitive inhibition. Second, our results provide only partial support for the perspective that stress impairs executive control and shifts cognition to a state of reactive or habitual action to facilitate adaptation to current circumstances (Gagnon and Wagner, 2016; Vogel et al., 2016). Although stress impaired almost all executive functions, stress enhanced response inhibition, which is inconsistent with the idea that stress impairs all executive control.
Based upon our results and building off of prior theoretical perspectives, we suggest that stress shifts higher cognitive processing in a way that facilitates both engagement with and/or avoidance of the current stressor (i.e., fight or flight). By impairing executive control of cognition (i.e., working memory, cognitive inhibition, and cognitive flexibility), stress contributes to a reactive cognitive state that is fine-tuned to rapidly consider highly salient (i.e., stressor-related) information (Gagnon and Wagner, 2016; Vogel et al., 2016). Our perspective is thus in agreement with models that suggests stress impairs executive control in order to force attention toward highly salient information (Vogel et al., 2016), but our perspective differs from these models by arguing that it is not all top-down control that is impaired by stress—only executive control of cognition is impaired, leaving executive control of motor actions intact. We suggest that by enhancing executive motor control (i.e., response inhibition), stress produces a state of enhanced control over actions—ideal for either fighting with or fleeing from a current stressor. Our perspective is thus in agreement with models that suggest stress reallocates limited executive resources in adaptive ways (LeBlanc, 2009), although our perspective differs from these models regarding what executive function receives these reallocated resources and why. Thus, although approach or avoidance responses to a stressor can vary in their effects (Moons and Shields, 2015; Moons et al., 2010; Shields and Moons, 2016), we propose that stress produces a cognitive phenotype conducive to both approach and avoidance by impairing executive control over thoughts but improving executive control over motor actions.
5.4 Limitations and Future Directions
Despite its strengths, this meta-analysis has limitations. First, the small number of studies examining stress effects on cognitive flexibility limited our ability to make inferences, especially with regard to moderators. As such, when more studies examine stress effects on cognitive flexibility, an additional meta-analysis of stress effects on cognitive flexibility will be warranted. Second, there may be moderators of stress effects of executive functions that are unaccounted for in our analyses. For example, because of the variety of stressors coupled with relatively small study set sizes, we were unable to examine whether certain effects of stress on executive functions are either restricted to or do not occur within particular stressor paradigms, such as the Trier Social Stress Test (TSST). As such, we do not claim to present a complete picture of moderators of stress effects on executive functions. Indeed, future research should attempt to determine whether additional factors moderator effects of stress on executive functions. Third, some paradigms may have used a previously validated stressor paradigm incorrectly without reporting it and so failed to induce stress. Because we included all studies using a previously validated stressor paradigm, it is possible that including these studies in analyses may have underestimated actual effects of stress. Although analyses suggest against this possibility (see footnote #2), we note here that our results should be considered to be a conservative estimate of stress effects on executive functions. Indeed, the strength of stress effects on executive functions may be greater in magnitude than what we found here because we may have included studies that did not actually induce stress, given our inclusion criteria that tried to find a balance between verifying that stress was induced and study inclusivity. Fourth, some paradigms may have elicited a stress response without the study using a previously validated stressor paradigm, including a biological validation of stress, or containing the components of socio-evaluative threat and motivated performance—and meeting one of these three conditions was one of our study inclusion criteria. As such, it is also possible that we have underestimated stress effects on executive functions by failing to include these studies in analyses, as we may have missed out on the additional power including these studies would have provided—due to our inclusion criteria that tried to find a balance between verifying that stress was induced and study inclusivity. Finally, we were not able to examine the potential role of emotional stimuli in moderating effects of stress on executive functions. Although we initially coded this variable in our dataset, only four studies used an executive function task that included emotional content—across all types of executive function tasks. As such, we were unable to analyze the contribution of this variable. Thus, future research could examine whether stress effects on “hot” executive function tasks incorporating emotional stimuli differ from the effects of stress on traditional “cool” executive function tasks.
Some (e.g., McEwen and Sapolsky, 1995) have argued that aging exacerbates effects of stress on cognition due in part to the exaggerated biological effects stress hormones have on neurons in older animals. We did not observe any moderating effect of participant age on stress effects on executive functions, but this may have been due to our study set. Although not all studies of stress effects on executive function were on college students, only three studies had average participant ages not between 18 and 29, with two studies examining children and one study examining older adults. Thus, our ability or lack thereof to detect age effects is properly limited to young adults. Indeed, most of the studies included in our analyses primarily examined effects of stress on executive function in Western, educated, industrialized, rich, and democratic (so-called “WEIRD”) samples, which limits our generalizability to this population (Henrich et al., 2010). Future research should attempt to extend effects of stress on executive functions to older and/or non-WEIRD samples.
The number of significant moderators of stress effects on working memory substantially exceeded the number of significant moderators of stress effects on inhibition or cognitive flexibility. This begs the question: is there something different about how stress affects working memory, or did the relatively greater study set size for working memory simply provide more power for us to elucidate moderators? We believe the answer is that both of the above are true. Working memory can be distinguished from other executive functions on a neurobiological level, and because it does not exert a uniform effect on every brain circuit, there is reason to suspect that stress might influence working memory differently than other executive functions. However, the relatively greater statistical power certainly allowed us to elucidate more moderators of stress effects on working memory than moderators of stress effects on cognitive inhibition, response inhibition, and cognitive flexibility. Thus, as more studies that examine stress effects on cognitive inhibition, response inhibition, and cognitive flexibility are conducted, another meta-analysis aimed at deriving additional moderators of these effects will be warranted.
Although our analyses of stress effects on inhibition were nuanced, we want to make explicitly clear that we do not believe effects of stress on working memory or cognitive flexibility are completely straightforward. It is certainly possible that stress might influence component working memory processes, such as maintenance or updating, in different ways; similarly, stress may influence cognitive flexibility restricted to more local changes in a different way than cognitive flexibility of more global changes. However, not enough published literature separated components or types of working memory or cognitive flexibility to permit these analyses, so we were unable to examine stress effects on the particularities of these constructs further. As such, the effects of stress on particular processes within working memory or cognitive flexibility remains an interesting avenue for future research.
Understanding the biological mechanisms behind the cognitive effects of stress is extremely important. In this meta-analysis we were able to demonstrate that effects of stress differed from effects of cortisol on core executive functions, with the possible exception of response inhibition. However, because hormones and immune system processes are intimately related to each other and often regulate one another, studying effects of isolated biological processes may not provide a complete picture of how stress influences executive functions. As such, we suggest that future research aimed at understanding the biological mechanisms behind stress effects on executive functions use factorial manipulations of individual biological mechanisms (Schwabe et al., 2012). This approach will allow an understanding of both the main and interactive effects of stress-related biological changes on executive functions, and should thus provide further insight into the mechanisms behind the effects of stress on cognition.
In addition, although the behavioral results of and relevance of some hormones to stress effects on executive functions are becoming clear, the neurobiological mechanisms behind stress effects on executive functions remain largely unknown. For example, some studies have found that stress leads to a deactivation in the dorsolateral prefrontal cortex (DLPFC) during a working memory task (Qin et al., 2009), whereas others have found that stress leads to increased activation in the DLPFC during a working memory task (Porcelli et al., 2008; Weerda et al., 2010); however, this discrepancy may be explained by genetic differences between participants in these studies (Qin et al., 2012). Still, stress may influence activity throughout the brain during working memory tasks, but currently there are not enough studies to conduct a meta-analysis in order to determine what the true effects are of stress on neural activity during a working memory task. Additionally, to our knowledge, no studies have examined stress effects on neural activity related to inhibition or cognitive flexibility. Nonetheless, understanding how stress influences neural activity during executive function tasks could have important implications for both understanding the basis of executive functions and preventing stress from influencing executive functions. Thus, how stress influences neural systems supporting executive functions is a fruitful avenue for future research.
6. Conclusion
Despite the ubiquity of stress and the importance of executive functions for daily life, the exact influence of stress on executive functions has been unclear. We addressed this ambiguity by conducting a meta-analysis of acute stress effects on executive functions. We found that stress impaired working memory, cognitive flexibility, and cognitive inhibition, whereas stress enhanced response inhibition. These findings suggest that stress contributes to a cognitive state of reactive and automatic processing while also enhancing executive motor control, which should facilitate engagement with or escape from the current stressor. Notably, this cognitive phenotype differed markedly from effects of cortisol administration. Thus, future research aimed at uncovering the biological mechanisms behind stress effects on executive functions should utilize factorial manipulations of hormones and immune system processes, which will allow us to better understand main and interactive effects of stress-induced biological mechanisms on cognition. In addition, the neural mechanisms behind stress effects on executive functions remain largely unclear and present a promising avenue for future research.
Supplementary Material
Highlights.
Acute stress impaired working memory, cognitive flexibility, and interference control.
Acute stress enhanced response inhibition.
Acute stress effects on executive functions were moderated by a number of variables.
Acute stress effects differed markedly from cortisol effects on executive functions.
Acknowledgments
This research was supported by NIH MH059352 and NEI EY025999 to Andrew Yonelinas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Only two studies (Chajut and Algom, 2003; Steinhauser et al., 2007) included in analyses used the same stressor that had not been previously validated with a biological index of stress and did not report biological validation of stress. Both of these studies, however, included a difficult task requiring motivated performance that also included socio-evaluative threat, which are condition sufficient to elicit a stress response (Dickerson and Kemeny, 2004).
Because we wanted to ensure we had included all studies that examined stress effects on executive functions, we adopted a criterion that would include a study that included a previously-validated stressor paradigm even if that study did not report biological validation of their stressor paradigm within their specific study, which could be the case due to funding reasons. However, we acknowledge that it would be good to determine whether studies that validated their current stress induction differed in effects from studies that did not for whatever reason. To examine this, we dummy coded all studies that validated their current stress induction as 1 and all studies that did not validate their current stress induction as 0 and tested for differences. We found that there were no differences between studies that validated their current stress induction protocol and those that did not across all studies, t(30.7)= −1.07, p=.295, across studies examining working memory, t(15.6)= −0.35, p=.734, and across studies examining inhibition, t(14.8)= −0.61, p=.549. Not enough studies of cognitive flexibility reported cortisol for us to examine this contrast in only studies of cognitive flexibility. Thus, it appears stress effects on executive functions were equivalent in magnitude between studies that validated their current stress inductions and those that did not.
Note that studies only examining men necessarily excluded women taking hormonal contraceptives as well as women on their menstrual period and were thus coded as “excluded” (i.e., 1) for each of these variables.
It should be noted, however, that because df are less than four in the prior analysis, the risk of making a Type I error increases approximately twofold (Tipton, 2014). In this case, however, df are less than four in the prior analysis not primarily due to outlying observations or variability within the moderator, but instead because with only six studies, given any variability in effects whatsoever df will necessarily be less than four. Thus, skepticism of results with df<4 may not be as warranted here as in other analyses with larger study set sizes.
Some (Shansky and Lipps, 2013) have suggested that stress may exert its sex-specific effects on working memory through actions of sex hormones. As such, we conducted a secondary analysis (data not shown) to examine whether excluding women taking hormonal contraceptives or women currently on their menstrual period moderated stress effects on working memory. These emerged as marginal and significant moderators, respectively, although the effects disappeared when moderators such as sex or study precision were included in the model. Thus, although we found some evidence for effects of sex hormones in stress effects on working memory, it is unclear whether these effects are true effects or if they emerged as significant due to commonality with other participant selection variables.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J. Cogn. Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn. Mem. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. J. Pers. Soc. Psychol. 2003;85:231–248. doi: 10.1037/0022-3514.85.2.231. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J. Appl. Soc. Psychol. 2012;42:1320–1334. [Google Scholar]
- De Lissnyder E, Koster EHW, Goubert L, Onraedt T, Vanderhasselt M-A, De Raedt R. Cognitive control moderates the association between stress and rumination. J. Behav. Ther. Exp. Psychiatry. 2012;43:519–525. doi: 10.1016/j.jbtep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integr. Comp. Biol. 2002;42:556–564. doi: 10.1093/icb/42.3.556. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Duncko R, Johnson L, Merikangas K, Grillon C. Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiol. Learn. Mem. 2009;91:377–381. doi: 10.1016/j.nlm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent variable analysis. J. Exp. Psychol. Gen. 2004;133 doi: 10.1037/0096-3445.133.1.101. SESE. [DOI] [PubMed] [Google Scholar]
- Gagnon SA, Wagner AD. Acute stress and episodic memory retrieval: Neurobiological mechanisms and behavioral consequences. Ann. N. Y. Acad. Sci. 2016 doi: 10.1111/nyas.12996. [DOI] [PubMed] [Google Scholar]
- Gärtner M, Rohde-Liebenau L, Grimm S, Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology. 2014;43:105–113. doi: 10.1016/j.psyneuen.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Giles GE, Mahoney CR, Brunyé TT, Taylor HA, Kanarek RB. Stress effects on mood, HPA axis, and autonomic response: comparison of three psychosocial stress paradigms. PLoS One. 2014;9:e113618. doi: 10.1371/journal.pone.0113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychol. Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- Henckens MJaG, van Wingen Ga, Joëls M, Fernández G. Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Front. Integr. Neurosci. 2012;6:66. doi: 10.3389/fnint.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernández G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5801–5806. doi: 10.1073/pnas.1019128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. Most people are not WEIRD. Nature. 2010;466:29. doi: 10.1038/466029a. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Garside MJ, Massey aE, McAllister-Williams RH. Effects of a single dose of cortisol on the neural correlates of episodic memory and error processing in healthy volunteers. Psychopharmacology (Berl) 2003;167:431–442. doi: 10.1007/s00213-003-1413-2. [DOI] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: A matter of timing. Trends Cogn. Sci. 2011 doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: a visual ERP investigation. Int. J. Psychophysiol. 2009;72:145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur. J. Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc VR. The effects of acute stress on performance: Implications for health professions education. Acad. Med. 2009;84:S25–S33. doi: 10.1097/ACM.0b013e3181b37b8f. [DOI] [PubMed] [Google Scholar]
- Lennartsson A-K, Kushnir MM, Bergquist J, Billig H, Jonsdottir IH. Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. Int. J. Psychophysiol. 2012a;84:246–253. doi: 10.1016/j.ijpsycho.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lennartsson A-K, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012b;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front. Behav. Neurosci. 2008;2:5. doi: 10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom. Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect. Psychol. Sci. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm. Behav. 2010;58:898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. J. Exp. Psychol. Gen. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain. Behav. Immun. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Moons WG, Shields GS. Anxiety, not anger, induces inflammatory activity: An avoidance/approach model of immune system activation. Emotion. 2015;15:463–476. doi: 10.1037/emo0000055. [DOI] [PubMed] [Google Scholar]
- Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods. 2008;11:364–386. [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain. Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Philippot P, Brutoux F. Induced rumination dampens executive processes in dysphoric young adults. J. Behav. Ther. Exp. Psychiatry. 2008;39:219–227. doi: 10.1016/j.jbtep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: Acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J. Cogn. Neurosci. 2011;23:3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- Plessow F, Schade S, Kirschbaum C, Fischer R. Better not to deal with two tasks at the same time when stressed? Acute psychosocial stress reduces task shielding in dual-task performance. Cogn. Affect. Behav. Neurosci. 2012;12:557–570. doi: 10.3758/s13415-012-0098-6. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, Rypma B. The effects of acute stress on human prefrontal working memory systems. Physiol. Behav. 2008;95:282–289. doi: 10.1016/j.physbeh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Qin S, Cousijn H, Rijpkema M, Luo J, Franke B, Hermans EJ, Fernández G. The effect of moderate acute psychological stress on working memory-related neural activity is modulated by a genetic variation in catecholaminergic function in humans. Front. Integr. Neurosci. 2012;6:16. doi: 10.3389/fnint.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Quinn ME, Joormann J. Control when it counts: Change in executive control under stress predicts depression symptoms. Emotion. 2015;15:522–530. doi: 10.1037/emo0000089. [DOI] [PubMed] [Google Scholar]
- Sänger J, Bechtold L, Schoofs D, Blaszkewicz M, Wascher E. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. Neurosci. 2014;8:353. doi: 10.3389/fnbeh.2014.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammacca N, Roberts G, Stuebing KK. Meta-analysis with complex research designs: Dealing with dependence from multiple neasures and multiple group comparisons. Rev. Educ. Res. 2014;84:328–364. doi: 10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J. Exp. Psychol. Gen. 2007;136:241–255. doi: 10.1037/0096-3445.136.2.241. [DOI] [PubMed] [Google Scholar]
- Scholz U, La Marca R, Nater UM, Aberle I, Ehlert U, Hornung R, Martin M, Kliegel M. Go no-go performance under psychosocial stress: Beneficial effects of implementation intentions. Neurobiol. Learn. Mem. 2009;91:89–92. doi: 10.1016/j.nlm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behav. Brain Res. 2013;241:144–153. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav. Neurosci. 2009;123:1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Höffken O, Tegenthoff M, Wolf OT. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology. 2013;38:2319–2326. doi: 10.1016/j.psyneuen.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, Wolf OT. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J. Neurosci. 2012;32:10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Front. Hum. Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AFT. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav. Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Bonner JC, Moons WG. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology. 2015;58:91–103. doi: 10.1016/j.psyneuen.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Shields GS, Lam JCW, Trainor BC, Yonelinas AP. Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrinology. 2016a;67:51–60. doi: 10.1016/j.psyneuen.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Moons WG. Avoidance-related EEG asymmetry predicts circulating interleukin-6. Emotion. 2016;16:150–154. doi: 10.1037/emo0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Trainor BC, Lam JCW, Yonelinas AP. Acute stress impairs cognitive flexibility in men, not women. Stress Advance on. 2016b doi: 10.1080/10253890.2016.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker HR, Depue BE, Reineberg AE, Orr JM, Banich MT. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct. Funct. 2015;220:1291–1306. doi: 10.1007/s00429-014-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser M, Maier M, Hübner R. Cognitive control under stress: How stress affects strategies of task-set reconfiguration. Psychol. Sci. 2007;18:540–545. doi: 10.1111/j.1467-9280.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain. Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Suchy Y. Executive functioning: overview, assessment, and research issues for non-neuropsychologists. Ann. Behav. Med. 2009;37:106–116. doi: 10.1007/s12160-009-9097-4. [DOI] [PubMed] [Google Scholar]
- Sylvester CYC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tanner-Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: Practical considerations including a software tutorial in Stata and SPSS. Res. Synth. Methods. 2014;5:13–30. doi: 10.1002/jrsm.1091. [DOI] [PubMed] [Google Scholar]
- Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, Beblo T, Driessen M, Spitzer C, Löwe B, Wingenfeld K. Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology (Berl) 2011;215:71–79. doi: 10.1007/s00213-010-2117-z. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol. Psychol. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol. Methods. 2014;20:375–393. doi: 10.1037/met0000011. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are core component processes of executive function dissociable within the frontal lobes? Evidence from humans with focal prefrontal damage. Cortex. 2013;49:1790–1800. doi: 10.1016/j.cortex.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, Dournes C, Fries GR, Masana M, Labermeier C, Wang X-D, Hafner K, Schmid B, Rein T, Chen A, Deussing JM, Schmidt MV. Prefrontal cortex corticotropin-releasing hormone receptor 1 conveys acute stress-induced executive dysfunction. Biol. Psychiatry Advance on. 2016 doi: 10.1016/j.biopsych.2016.03.2106. [DOI] [PubMed] [Google Scholar]
- Vinski MT, Watter S. Being a grump only makes things worse: A transactional account of acute stress on mind wandering. Front. Psychol. 2013;4:730. doi: 10.3389/fpsyg.2013.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends Cogn. Sci. 2016;20:192–203. doi: 10.1016/j.tics.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Hum. Brain Mapp. 2010;31:1418–1429. doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT. What we remember from a stressful episode. Psychoneuroendocrinology. 2013;38:2268–2277. doi: 10.1016/j.psyneuen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: Implications for stress regulation. Ann. Behav. Med. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014;42:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.