Abstract
Esophageal cancer is a deadly disease, ranking sixth among all cancers in mortality. Despite incremental advances in diagnostics and therapeutics, esophageal cancer still carries a poor prognosis, and thus there remains a need to elucidate the molecular mechanisms underlying this disease. There is accumulating evidence that a comprehensive understanding of the molecular composition of esophageal cancer requires attention to not only tumor cells but also the tumor microenvironment, which contains diverse cell populations, signaling factors, and structural molecules that interact with tumor cells and support all stages of tumorigenesis. In esophageal cancer, environmental exposures can trigger chronic inflammation, which leads to constitutive activation of pro-inflammatory signaling pathways that promote survival and proliferation. Anti-tumor immunity is attenuated by cell populations such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), as well as immune checkpoints like programmed death-1 (PD-1). Other immune cells such as tumor-associated macrophages can have other pro-tumorigenic functions, including the induction of angiogenesis and tumor cell invasion. Cancer-associated fibroblasts secrete growth factors and alter the extracellular matrix (ECM) to create a tumor niche and enhance tumor cell migration and metastasis. Further study of how these TME components relate to the different stages of tumor progression in each esophageal cancer subtype will lead to development of novel and specific TME-targeting therapeutic strategies, which offer considerable potential especially in the setting of combination therapy.
Keywords: esophageal cancer, tumor microenvironment, cancer associated fibroblasts, immature myeloid cells
Introduction
Esophageal cancer affects more than 450,000 people worldwide and ranks sixth among all cancers in mortality 1. There are two main subtypes of esophageal cancer—esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)—each with known risk factors and pathological features. ESCC comprises up to 90% of esophageal cancer cases worldwide, but the incidence of EAC is increasing and has surpassed ESCC in several areas of North America and Europe 2. Despite recent advances in diagnostics and therapeutics, the prognosis for esophageal cancer remains poor—the five-year survival rate is approximately 15 to 25 percent—largely due to late diagnosis and propensity for metastasis 1. With standard therapy still limited to surgical or endoscopic resection and chemoradiation, there is a need to understand better the molecular pathogenesis of esophageal cancer for developing novel biomarkers and targeted therapies.
Like other GI tract cancers, initial studies on molecular mechanisms underlying esophageal cancer have focused on tumor cell-intrinsic features, namely the activation of oncogenes (cyclin D1 and EGFR) and inactivation of tumor suppressor genes (TP53, p120catenin, E-cadherin) 3–5. Genomic studies have also been informative in both ESCC and EAC 6,7. However, there is accumulating evidence that tumor cell-extrinsic factors are also integral to esophageal tumorigenesis. These factors, which include immune cells, fibroblasts, endothelial cells, perivascular cells, neurons, adipocytes and extracellular matrix (ECM) components, comprise the tumor microenvironment (TME), which is thought to play a role in inhibiting apoptosis, enabling immune evasion, and promoting proliferation, angiogenesis, invasion and metastasis 8.
A deeper understanding of how tumor cell-TME interactions contribute to esophageal tumorigenesis can direct the development of future therapeutic and diagnostic strategies. In this review we will summarize the current literature on various components of the TME in both ESCC and EAC.
Esophageal tumor initiation is associated with environmental exposures and chronic inflammation
As in several other cancers, esophageal carcinogenesis occurs due to a complex interplay between environmental factors and genetic predisposition. Another common theme in cancer biology is the relationship between inflammation and tumor development 9. In the subtypes of esophageal cancer, both common and unique risk exposures contribute to the generation of inflammation and the transformation of epithelial cells, forming pre-cancerous and eventually cancerous tissue. These specific aspects of tumor initiation in EAC and ESCC are summarized in Table 1 and will be discussed in more detail below.
Table 1. Elements of tumor initiation in EAC and ESCC.
GERD, gastroesophageal reflux disease; HPV, human papilloma virus
| Factor | EAC | ESCC |
|---|---|---|
| Potential cell of origin | Esophageal basal progenitor cells (via transdifferentiation or reprogramming) and/or Gastric cardia progenitor cells (via migration) |
Esophageal basal progenitor cells |
| Precursor lesion | Intestinal metaplasia (Barrett's esophagus) | Squamous dysplasia |
| Location | Distal third of esophagus | Typically proximal two-thirds of esophagus |
| Risk exposures | Age Race (white > black) Gender (males > females) GERD Obesity Cigarette smoking (EAC < ESCC) Diet (high red meat and processed foods; low fruit and vegetables) |
Age Race (black > white) Gender (males > females) Cigarette smoking (ESCC > EAC) Alcohol Nutritional deficiences HPV infection Tylosis palmaris et plantaris (inherited) |
Environmental risk factors and chronic inflammation in EAC
The longstanding model of EAC development involves the exposure of the distal esophageal epithelium to caustic substances, namely refluxed gastric and bile acids (gastroesophageal reflux disease, GERD), which trigger chronic inflammation and the development of intestinal metaplasia (Barrett's esophagus, BE), the precursor lesion to EAC. Other toxic exposures, particularly tobacco, can enhance the degree of tissue damage and inflammation. Interestingly, reflux causes both direct esophageal injury 10,11, as well as the production of reactive oxygen species (ROS) 12,13. Direct injury is thought to lead to intestinal metaplasia by two potential mechanisms. First, aberrant Sonic hedgehog (SHH) signaling between the injured epithelium and adjacent stroma triggers transcription of genes responsible for columnar metaplasia 14. Second, damage to the existing esophageal epithelium can actually make way for migration of epithelial cells from the forestomach, which have a columnar morphology 15. On the other hand, ROS production causes direct DNA damage leading to tumor-initiating mutations 16. Infiltrating inflammatory cells also produce ROS to support the transformation of epithelial cells 17. Furthermore, ROS can activate a number of cancer-associated signaling pathways such as PI3K/Akt, ERK1/2, and NF-κB 13,18. Notably, the presence of both endogenous and exogenous anti-oxidants has been shown to have a protective effect against development of BE and EAC 19–21.
A risk factor unique to EAC is obesity. This association was assumed previously to be related to GERD in the setting of increased intra-abdominal pressure from central adiposity; however, recently, obesity has been linked to increased risk for several other cancers, indicating that it may promote carcinogenesis through mechanisms other than the purely biomechanical consequences of excess body weight 22. One premise is that obesity may constitute a state of chronic inflammation: adipocyte hypertrophy in obesity can cause hypoxia, which leads to infiltration of activated macrophages into adipose tissue and ultimately induces a pro-inflammatory state with the systemic release of cytokines like interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) 23. These cytokines are involved in anti-apoptotic pathways and transcription of oncogenes, respectively, and have been implicated in esophageal cancer 24,25. Furthermore, alteration of adipokine (leptin and adiponectin) signaling has been associated with GI malignancies 26. In BE, increased leptin levels are associated with an elevated risk for EAC, while higher levels of adiponectin were inversely related to EAC. Adipokine receptors such as ObR (leptin receptor) and AdipR2 (adiponectin) are also upregulated in EAC and may correlate with tumor stage and nodal involvement 27,28. Interestingly, while leptin promotes tumor formation by inhibition of apoptosis and stimulation of cell proliferation via Akt, mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription (STAT) pathways 29, adiponectin seems to have anti-inflammatory vasculo-protective effects driven largely by suppression of IL-6 30. Thus, both local (GERD) and systemic (obesity) induction of inflammation can lead to EAC development.
Environmental risk factors and chronic inflammation in ESCC
Although less studied, chronic inflammation is important also for ESCC development, as demonstrated by elevated inflammatory biomarkers, particularly C-reactive protein (CRP), in ESCC 31. Additionally, several well-known risk factors for ESCC, such as smoking and alcohol 2, cause chronic irritation of the esophageal epithelium and subsequent inflammation via direct toxic effect and production of ROS 32,33. Notably alcohol exposure is a risk factor associated with ESCC, but not EAC. Dietary deficiencies (i.e. low fruits and vegetables) and diet itself (as measured by dietary inflammatory index), especially with high red meat and processed food intake, have also been associated with ESCC development via inflammatory mechanisms 34,35. Moreover, certain rare clinical syndromes known to carry increased risk for ESCC, such as tylosis palmaris et plantaris and Plummer-Vinson syndrome, are thought to lead to esophageal dysplasia and later ESCC via chronic inflammation 36. Altogether, this chronic inflammation can trigger the development of esophageal squamous dysplasia and eventually ESCC.
Role of the microbiome in chronic inflammation
The GI tract normally contains commensal bacteria (the microbiome) that live in concert with host cells. Disruption of this relationship, termed dysbiosis, may lead to GI carcinogenesis by disrupting epithelial barriers, triggering inflammation, and inducing subsequent DNA damage or pro-oncogenic signaling 15. The role of microbiota in the esophagus has not been as deeply characterized as that in the distal GI tract; however, some evidence suggests that it may have a role in esophageal carcinogenesis, especially in EAC. First, both esophagitis and BE are characterized by alterations in the esophageal microbiome 37, specifically a significant decrease in Gram(+) bacteria and increase in Gram(−) bacteria 38. Gram(−) production of lipopolysaccharide (LPS) leads to inflammation (via Toll-like receptor 4 and NF-κB activation) and increased reflux (via iNOS-mediated relaxation of the lower esophageal sphincter) 39. Furthermore, analogous to Helicobacter pylori in gastric carcinogenesis, Campylobacter spp. may have a role in causing toxin-mediated inflammation that leads to esophageal cancer 40. Interestingly, H. pylori itself may actually provide a protective effect against EAC 41.
Inflammatory signaling pathways promote cell proliferation and survival
A major mechanism by which inflammation induces esophageal carcinogenesis is by constitutive activation of inflammatory signaling pathways 42. Induction of these pathways leads to downstream activation of gene transcription and enzymatic activity that play a key role in tumor growth and survival. Two of the primary pathways implicated in esophageal carcinoma will be discussed here.
Interleukin-6/STAT3
The IL-6/STAT3 signaling pathway is upregulated in several cancers 43, including esophageal 44. IL-6 is a cytokine that signals via association of its receptor (IL-6Rα) with gp130, which triggers downstream recruitment and activation of several molecules (SHP2, Ras-MAPK, and PI3K) and notably the STAT1 and STAT3 transcription factors 45. In normal physiology, the IL-6/STAT3 pathway allows normal cells to survive in highly toxic inflammatory environments created by the immune system to kill pathogens; however, in carcinogenesis, this pathway is hijacked by neoplastic cells to promote growth, survival, angiogenesis, and metastasis 46. Interestingly, STAT3 signaling is often constitutively activated in cancer, a phenomenon that not only suppresses apoptosis but also inhibits anti-tumor immunity 47.
Several studies have correlated increased epithelial IL-6/STAT3 activity with cell proliferation and apoptotic resistance in BE and EAC 48–50. Furthermore, evidence from mouse models and human tissues suggests that exposure to bile acid and low pH induces this pathway in the esophagus 15,51. In fact, in vitro exposure of Seg-1 cells (EAC cell line) to a bile acid cocktail and pH of 4 increased IL-6 secretion and activated STAT3 51. Also, in the L2-IL-1β mouse model of BE/EAC, exposure to bile acids accelerated development of BE and EAC by an IL-6 dependent mechanism, with failure of carcinogenesis in the setting of IL-6 deficiency 15. In addition, patients with EAC had higher serum levels of IL-6 than normal controls 52, and increased serum IL-6 was associated with progression from BE to EAC 53. IL-6 is also one of the primary inflammatory mediators produced by adipose tissue and thus may be important in obesity-related inflammation 54.
In ESCC, several studies have reported increased expression of IL-6, IL-6Rα, and STAT3 in vitro and in ESCC patients 25,55,56. Moreover, high serum levels and tumor expression of IL-6 correlate with a poor prognosis in ESCC patients receiving neoadjuvant chemoradiotherapy 57–60, while overexpression of STAT3 similarly indicated a poor prognosis in those who had undergone surgical resection 61. Mechanistically, IL-6 has been shown to drive expansion of pro-tumorigenic myeloid-derived suppressor cells (MDSCs) 60,62, while STAT3 activation leads to production of anti-apoptotic molecules like myeloid cell differentiation protein-1 (Mcl-1) 55.
Recent evidence indicates that the IL-6/STAT3 pathway is an actionable target. First, siRNA-mediated IL-6 inhibition in ESCC cell lines resulted in enhanced chemosensitivity and increased cell death, decreased angiogenesis and less epithelial-to-mesenchymal transition (EMT) 59,63. Furthermore, inhibition of STAT3 signaling by small molecules like stattic radio-sensitized ESCC cells in vivo 64. Stattic also induced apoptosis in BE and EAC cells and restored chemo- and radio-sensitivity 65,66.
Nuclear factor-kappaB
Nuclear factor-kappaB (NF-κB) is a family of structurally related transcription factors that regulate important cell functions like survival, proliferation, and cytokine production. Under normal conditions, NF-κB is maintained in an inactive state by the binding of inhibitory IκB protein 67. Following stimulation by environmental insults such as oxidative or inflammatory stimuli, chemotherapy or radiation, proteasomal degradation of IκB leads to release of NF-κB dimers and translocation to the nucleus, where they activate transcription of critical genes involved in tumorigenesis, immune evasion and treatment resistance 67,68. NF-κB is overexpressed in many liquid and solid tumors, including both EAC and ESCC 69. Activation of this mechanism is thought to be a key link between an inflammatory microenvironment and cancer development 68.
In BE and EAC, bile and gastric acid induce NF-κB expression in esophageal epithelial cells and may enhance cell survival 70,71. NF-κB is also postulated to have a role in cell cycle regulation, as it was found to spatially co-localize and be dually upregulated with cyclin D1 in EAC 72. Interestingly, NF-κB overexpression was specific to BE and EAC, but not reflux esophagitis 73, which suggests that it may be a marker of metaplasia-dysplasia-adenocarcinoma progression rather than simple inflammation. NF-κB was also associated with shortened disease-free and overall survival in patients with EAC 74.
Overexpression of NF-κB has also been noted in ESCC. Previously our laboratory showed increased NF-κB expression in the p120-catenin conditional knockout mouse model of ESCC 75. Other in vitro studies on ESCC have shown activation of NF-κB signaling by modulation of upstream mediators, such as upregulation of the transcription factor Id-1 (inhibitor of differentiation/DNA binding) and downregulation of the tumor suppressor Nkx2-8 76,77. NF-κB activation in these settings led to resistance to TNF-α induced apoptosis and angiogenesis 76,77. As in EAC, NF-κB overexpression was also associated with a poor prognosis in ESCC 78.
Two major downstream effectors of NF-κB involved in esophageal carcinoma are IL-8 and IL-1β. IL-8, also known as CXCL-8, is a chemokine best known for its neutrophil chemotactic properties. However, it has recently been implicated in breast, lung, prostate, and pancreatic cancers, where it had effects on angiogenesis, survival, tumor cell stemness, migration, metastasis, and immune cell infiltration 79,80. IL-1β is a pro-inflammatory cytokine (known to also be a potent inducer of NF-κB) that is abundantly secreted at tumor sites, where it promotes invasiveness, tumor-mediated immune suppression, cancer stem cell self-renewal 81,82. Expression of both of these inflammatory mediators has been demonstrated in esophageal carcinogenesis. For instance, the bile acid deoxycholic acid (DCA) was shown to induce IL-8 expression via NF-κB activation in esophageal cells in vitro 71, and this expression was directly correlated with progression to BE and EAC. Meanwhile, elimination of reflux via Nissen fundoplication led to a decrease in IL-8 expression 83. Furthermore, both IL-8 and IL-1β were elevated in BE and markedly elevated in EAC, and overexpression was localized to the site of tumorigenesis 73,84. In ESCC, IL-8 was associated with tumor progression, metastasis, inflammation, and poor prognosis in ESCC patients 85.
As with STAT3, blocking NF-κB activity enhances sensitivity of esophageal cancer cells to paclitaxel 86 and 5-fluorouracil 87. Curcumin, a plant-derived anti-NF-κB compound (interestingly, the STAT3 inhibitor stattic was derived from curcumin), suppresses the esophageal inflammatory response to bile and acid in BE and EAC 88. In addition, inhibition of IL-8 and IL-1β reduces tumor invasiveness as well as tumor-induced immunosuppression 81.
Cyclooxygenase-2
Although STAT3 and NF-κB converge on several common targets, cyclooxygenase-2 (COX-2) is particularly prominent in esophageal carcinogenesis. COX-2 is an inflammatory enzyme that is responsible for the production of prostaglandin E(2), which has been implicated in GI cancer-related inflammation 89. COX-2 expression is induced by exposure to bile acids 13,90, and its levels are elevated in BE and EAC 91. Studies in ESCC tissues have revealed a positive correlation between COX-2 expression and the degree of dysplasia 92. High COX-2 expression in ESCC was also associated with poor prognosis and chemotherapy resistance 93.
COX-2 inhibitors have shown potential in esophageal cancer. Several studies have demonstrated that both selective and nonselective COX-2 inhibitors suppress inflammation and cell growth while inducing apoptosis in BE and EAC 94–96. Furthermore, chronic intake of NSAIDs is associated with a decreased incidence of EAC, suggesting a role in prevention as well 96. In ESCC, COX-2 inhibition leads to decreased cell proliferation, PGE2 production and overall tumor progression in vitro and in vivo 92. It should be noted that STAT3 and NF-κB are not simply parallel pathways but actually have a complex, interdependent relationship. In fact, NF-κB/IL-6/STAT3 are thought to form a self-sustaining positive feedback loop for signal amplification 97. Additionally, STAT3 and NF-κB share several downstream effectors, exemplifying redundancy that enhances the resilience of cancer cells even if one of these pathways is inhibited 98.
Immune modulation promotes tumor evasion and survival
Tumor escape from anti-tumor immunity is critical for tumor survival and progression. Tumor cells can suppress the anti-tumor immune response via recruitment of various immune cell populations or expression of inhibitory molecular factors (Figure 1). The specific cell types and factors implicated in esophageal cancer will be discussed below.
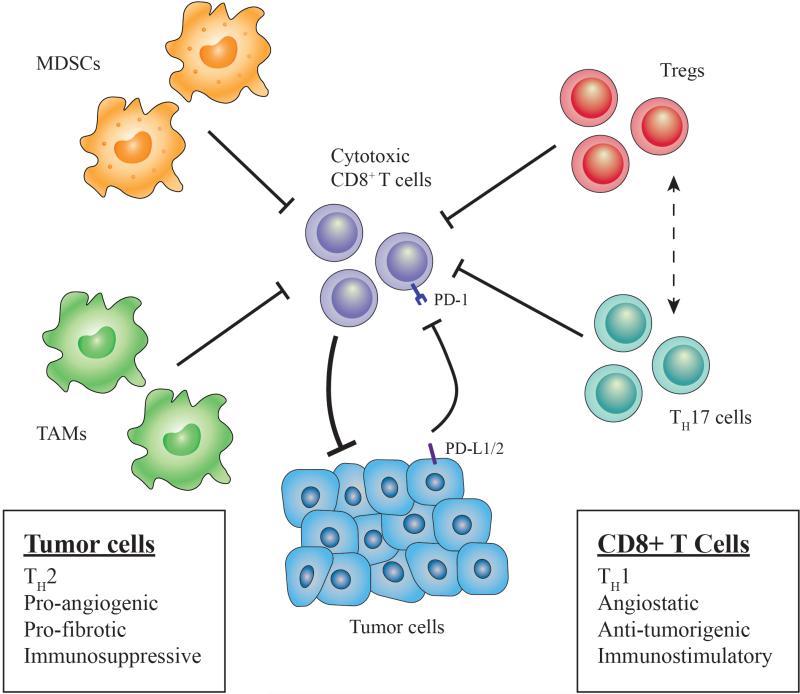
Figure 1. Immune landscape in esophageal cancer.
Several immune cell types disrupt anti-tumor immunity (cytotoxic CD8+ T cells) in the tumor microenvironment (TME). Tregs expressing CCR4 are recruited by chemokines CCL17 and CCL22 that are secreted by tumor cells (and TAMs). Tregs exert immunosuppressive function via direct contact with effector T cells or by molecules such as adenosine or immunosuppressive cytokines (IL-10, IL-35). Th17 cells are stimulated by TGF-β and IL-6 and have the ability to convert into Tregs (dashed line) and release adenosine by ectoenzymatic (CD39, CD73) function. Expansion of myeloid derived suppressor cells (MDSC), or immature myeloid cells, is stimulated by inflammation and tumor-derived factors (i.e. VEGF), and these cells directly inhibit T cell activation and NK cell cytotoxicity, while also inducing Tregs. TAM expansion (M2 polarization) occurs in presence of Th2 cytokines (i.e. IL-4, IL-13), and these cells are recruited via chemokines such as MCP-1. Furthermore, TAMs and tumor cells both express PD-L1/2 to inhibit T cell activation via the PD-1 receptor. Altogether, these cells suppress anti-tumor immunity while also promoting tumor growth and progression by various mechanisms.
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that play a key role in immune suppression and other tumor-promoting processes, such as fibroblast activation and angiogenesis 99. Activation and expansion of MDSCs are triggered by inflammation, namely pro-inflammatory molecules like IL-1β, IL-6, and PGE2 100,101, as well as other tumor-secreted factors like VEGF 102. MDSCs suppress anti-tumor immunity by several mechanisms—direct inhibition of T cell activation 103, inhibition of NK cell cytotoxicity 104, depletion of the amino acids arginine and cysteine 105, and the induction of regulatory T cells 106. The notion that MDSCs have a role in tumor progression is supported by several animal models 107. In fact, our laboratory showed that MDSCs were greatly expanded in the p120-catenin deficient mouse model of ESCC and could activate fibroblasts to induce desmoplasia 75. Furthermore, elevated levels of MDSCs were observed in esophageal cancer patients and were associated with advanced disease, a poor prognosis and therapeutic resistance 60,108.
MDSCs present a therapeutic challenge because of their heterogeneous nature. In an effort to better define the factors that mark and drive MDSC-mediated immunosuppression in esophageal cancer, we recently demonstrated that MDSCs with greater immunosuppressive and pro-tumorigenic capacity express high levels of CD38 62. CD38 expression was driven by factors such as IL-6, IFN-γ, TNF-α, IGFBP-3 and CXCL16, and crosslinking of CD38 with a monoclonal antibody daratumumab (now FDA-approved for treatment of multiple myeloma 109) decreased esophageal tumor growth in vivo 62. Importantly, the expansion of CD38-positive MDSCs was also found in the peripheral blood of advanced stage cancer patients 62. Certainly, further investigation into MDSC biology, particularly the functions of various subsets of this population, will provide direction for therapeutic development.
Regulatory T cells
Regulatory T cells (Tregs) also possess immunosuppressive capacity in cancer. In normal physiology, Tregs regulate the expansion and activation of B and T cells, as well as NK cell cytotoxicity; however, in cancer they suppress anti-tumor immune responses 110. Interestingly, Tregs may have a dual role in tumorigenesis, initially suppressing inflammation that leads to carcinogenesis, but later attenuating anti-tumor immunity via mechanisms such as the secretion of immunosuppressive cytokines, interference with tumor-associated antigen presentation and inhibition of cytotoxic cell function and granule release 111.
Expansion of Tregs has been noted both in the peripheral blood and esophageal mucosa of esophageal cancer patients (relative to healthy donors) 112,113. Increased recruitment of Tregs in esophageal cancer, particularly ESCC, is at least partially mediated by the chemokines CCL17 and CCL22, which are secreted by tumor cells and macrophages to recruit Tregs via the CCR4 receptor 114. Furthermore, Treg infiltration was found to have prognostic significance, with higher amounts of Tregs associated with deeper tumor invasion 115, metastasis 116, overall disease severity 117, and decreased survival post-chemotherapy 118. In addition, a recent report on esophageal cancer patients receiving neoadjuvant chemoradiation showed that the density of Tregs in the residual tumor (post-treatment) was correlated not only with pathological response but also with cancer-specific survival 119. A number of strategies for interfering with Treg differentiation, recruitment, and function have been outlined previously 111. Like MDSCs, however, Tregs are heterogeneous and possess several context-dependent functions that are not well-characterized, presenting a challenge for the field.
Th-17 cells
Th17 cells are a subset of T-helper cells more recently implicated in tumor immunomodulation. As such, their precise role in regulating tumor immunity is still being debated 120. In some cases, Th17 cells seem to have anti-tumor properties, while in others they appear to promote tumor growth through suppression of anti-tumor immunity 121. For instance, when cultured with the cytokines TGF-β and IL-6, Th17 cells express the ectonucleotidases CD39 and CD73, which release adenosine, leading to CD8+ T cell suppression 122. Th17 cells also possess the capacity to convert into Tregs 123. Furthermore, through secreted cytokines such as IL-17 and IL-22, Th17 cells can also induce angiogenesis and promote tumor growth via STAT3 activation 121.
There is some evidence supporting Th17 cell involvement in esophageal cancer. Increased proportions of Th17 cells have been observed in the peripheral blood and tumor tissues of EAC and ESCC patients, and the degree of Th17 infiltration was correlated with disease stage 124,125. However, the significance of Th17 cells in cancer remains controversial, and the factors that influence Th17 behavior are not currently well defined 120,121. Thus, there is a clear need for a deeper understanding of the role of Th17 cells in esophageal cancer to determine its potential as a therapeutic target.
Programmed death-1
There has recently been increased interest in other immunosuppressive mechanisms, particularly immune checkpoints like the programmed cell death protein 1 (PD-1) pathway. PD-1 is a negative co-stimulatory receptor that is part of the CD28 family, expressed primarily on activated T cells 126. Upon engagement with its ligands, programmed cell death ligand 1 or 2 (PD-L1 or PD-L2), PD-1 inhibits T cell activation 127. These ligands can be expressed by tumor cells, immune cells (i.e. macrophages), and endothelial cells to suppress T-cell mediated tumor immunity 128.
In esophageal cancer, multiple studies have reported elevated expression of both PD-L1 and PDL2 129,130. In fact, increased expression of either PD-L1 or PD-L2 in cancer cells was associated with decreased survival in ESCC patients 129, and increased PD-L1 expression was correlated with greater depth of tumor invasion and worse survival in ESCC 130. Interestingly, only PD-L2 expression was correlated with decreased CD8+ T cell infiltration 129. Additionally, a recent study demonstrated increased expression of PD-L2 in BE and EAC, with a weaker relationship in PD-L1 126. The increase in PD-L2 expression was induced by pro-tumorigenic Th2 cytokines such as IL-4/IL-13 126. This evidence suggests that PD-1 blocking agents, which have shown promise in melanoma, renal and lung cancer 131, may have utility in esophageal cancer.
Tumor-associated macrophages
Tumor-associated macrophages (TAMs) facilitate a variety of pro-tumorigenic mechanisms. Macrophages exist on a phenotypic spectrum, ranging from an M1 to M2 state: whereas M1 represents “classically” activated macrophages that produce type I pro-inflammatory cytokines, present antigens, fight infections and have anti-tumor qualities, M2 macrophages produce type II cytokines and have many pro-tumorigenic attributes 132. M2 polarization can be induced by hypoxia as well as via activation of the COX-2/PGE2 pathway 89,107,133. Once M2-polarized, TAMs produce growth factors and proteases that enhance tumor initiation, invasion, angiogenesis, metastasis, and immunosuppression 107,134. Interactions between macrophages and epithelial cells play a critical role in esophageal carcinogenesis. In a rat model of reflux esophagitis, M1 macrophages recruited to the inflammation site activated the STAT3 pathway in epithelial and stromal cells, promoting subsequent M2 macrophage polarization and progression to both ESCC and EAC 135. Furthermore, in tissues obtained from EAC patients, tumor cell upregulation of Th2 cytokines like IL-4 and IL-13 promoted M2 macrophage infiltration, which was associated with MDSC-mediated immunosuppression 136. In ESCC patients, increased secretion of tumor-derived macrophage chemoattractant protein-1 (MCP-1) resulted in TAM infiltration and production of angiogenic enzymes like thymidine phosphorylase 137. TAM infiltration was also associated with poor responses to chemotherapy and overall poor prognosis in ESCC 138.
Stromal components and signaling facilitate tumor progression
Cancer-associated fibroblasts
Many cancers, including esophageal cancer, are derived from chronic injury and inflammation—wounds that do not properly heal. Unsurprisingly, cells that normally respond to injury, such as fibroblasts, have a prominent role in the initiation, progression, and eventual spread of tumors. Indeed, there is substantial evidence that a specific subset of fibroblasts, called cancer-associated fibroblasts (CAFs), are integral to all stages of cancer 139. CAFs have an activated phenotype—marked by expression of proteins like fibroblast activation protein-α (FAP) and α-smooth muscle actin (α-SMA)—that is thought to be induced by factors like transforming growth factor-β (TGF-β), secreted by cancer cells 139. Recent studies have also implicated miRNAs in the conversion of fibroblasts to CAFs 140, a finding that has been corroborated in ESCC 141. Once in this state, CAFs can modulate the TME by communicating with tumor and other stromal cells via secreted factors, activating pro-inflammatory pathways, disrupting immune surveillance and altering the extracellular matrix (ECM) (Figure 2) 107,139.
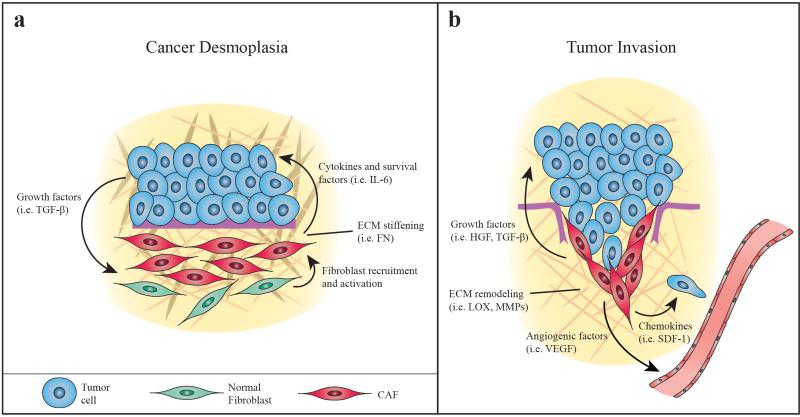
Figure 2. Stromal compartment of the esophageal TME.
A. Neoplastic cells secrete growth factors to activate quiescient fibroblasts designated as cancer associated fibroblasts (CAFs). CAFs can proliferate to contribute to desmoplasia, secreting extracellular matrix (ECM) components such as fibronectin (FN) to enhance the development of the primary tumor niche. CAFs also secrete cytokines that promote tumor cell survival (anti-apoptosis). B. Later in tumorigenesis, CAFs remodel the ECM with enzymes like lysyl oxidase (LOX) and matrix metalloproteinases (MMPs) as well as ECM components like dermatan sulfate (DS) and hyaluronan (HA) to promote invasion. CAFs also secrete growth factors that trigger tumor cells to undergo epithelial-mesenchymal transition (EMT) and chemokines that induce tumor cell migration. CAFs can also promote angiogenesis via VEGF secretion.
Fibroblasts have been shown to play a critical role in esophageal cancer, especially ESCC. In fact, we previously showed that activated fibroblasts promote ESCC cell invasion in a 3-D organotypic cell culture (OTC) model system 142, via the secretion of hepatocyte growth factor (HGF) 143. Furthermore, our lab demonstrated that in the p120-catenin knockout mouse model of ESCC, invasion by tumor cells was accompanied by a marked desmoplastic reaction due to activation of fibroblasts in the tumor stroma by infiltrating MDSCs 75. Our findings have been supported by a number of other studies. For example, several in vitro studies in ESCC cell lines have reported that cancer cell proliferation, angiogenesis, and mobility are largely dependent on the presence of activated fibroblasts 144,145. Moreover, the presence of CAFs in ESCC patients was associated with increased microvessel density, increased TAMs, and EMT, which is vital to cancer progression and metastasis 146. CAFs were also associated with poor 3-year survival and disease recurrence after chemoradiation 147. Further investigation showed that irradiation led to increased expression of HGF and β-catenin by fibroblasts, with concomitant downregulation of E-cadherin in co-cultured ESCC cells, indicating a more invasive phenotype 148.
CAFs are involved in EAC as well. Hayden et al. observed that CAF conditioned media supported EAC cell growth despite the presence of cisplatin and 5-FU and led to a twofold increase in EAC cell invasion in OTC compared to normal fibroblast conditioned media 149. Meanwhile, Underwood et al. found that the vast majority of EAC cases (93%) contained activated CAFs in resected esophageal tissue, with absence of CAFs associated with improved survival 150.
Transforming growth factor-β
Transforming growth factor-β (TGF-β) signaling regulates tumor initiation, progression and metastasis 151. Classically, TGF-β family ligands bind the extracellular domain of the TGF-β receptor, which triggers downstream activation of canonical Smad protein signaling, leading to transcription of genes important for tissue homeostasis, neoplastic growth and progression 151. Interestingly, TGF-β signaling appears to have a dual role in regulating tumorigenesis: in early stages it is a growth suppressor, but later it promotes EMT and metastasis 151,152.
This dual role has been described in both EAC and ESCC. Early in esophageal carcinogenesis, TGF-β signaling appears to have an inhibitory effect on tumor growth, with both EAC and ESCC cell lines showing decreased TGF-β responsiveness via downregulation of Smad4 or TGF-β-resistant c-Myc expression 153. Consistent with this, Smad4 expression was progressively decreased in the metaplasia-dysplasia-adenocarcinoma sequence of EAC with recovery of the antiproliferative response upon Smad4 restoration 154. Interestingly, ESCC-specific studies have had mixed findings. Whereas TGF-β downregulation by DACH1 methylation or decreased Smad4 expression were associated with increased depth of invasion, later tumor stage and poor differentiation 155,156, TGF-β downregulation by proteasomal degradation actually suppressed growth and invasion in vivo 157. Still, ESCC patient studies have supported a tumor-suppressive role for TGF-β, with decreased signaling correlated with more aggressive tumor characteristics and a worse prognosis 158.
Later in tumorigenesis, TGF-β seems to have a pro-tumorigenic effect. This “switch” is thought to be mediated largely by the loss of adaptor proteins, which are required for proper control of TGF-β tumor suppressor function. For example, β2-spectrin (β2-SP) is an adaptor protein that plays an essential role in cell-cell interactions and maintenance of epithelial cell polarity. In EAC, loss of β2-SP in tumor cells led to increased expression of SOX9 and c-Myc but reduced expression of other TGF-β targets like E-cadherin and the cell-cycle regulators p21 and p27 159. TGF-β signaling also triggers fibroblast activation, which also contributes to tumor invasion, angiogenesis, and EMT 144. Together, these changes allow TGF-β to promote tumor progression and eventual metastasis via EMT. In fact, in both EAC and ESCC, increased TGF-β signaling is associated with advanced tumor stage, metastasis, and treatment resistance 160,161.
Hepatocyte growth factor
Hepatocyte growth factor (HGF), also known as scatter factor, is a growth factor involved in embryogenesis and organ regeneration and wound healing in adults. By binding its tyrosine kinase receptor c-Met, HGF induces the activation of oncogenic signaling pathways and facilitates invasion, angiogenesis and scattering of cells leading to metastasis 162. Importantly, overexpression of HGF has been noted in both ESCC and EAC as compared to non-dysplastic tissues 163–165. HGF overexpression has also been correlated with decreased survival, poor differentiation, depth of tumor invasion, pathologic stage, metastasis and recurrence 165,166.
Several studies have highlighted the mechanisms by which HGF promotes tumor progression. To start, we previously showed that fibroblast-derived HGF was essential for tumor cell invasion of the ECM in an organotypic culture model of ESCC 143. Both fetal esophageal fibroblasts (FEFs) and ESCC-derived CAFs secreting HGF were able to promote invasion of EPC-hTERT-EGFR-p53R175H (genetically transformed primary esophageal cells) cells, TE12 and TE7 cells, whereas HGF-deficient fibroblasts did not promote invasion 143. Moreover, siRNA and pharmacological inhibition of HGF/c-Met signaling each prevented invasion 143. Interestingly, radiation exposure, a known risk factor for ESCC, induced fibroblasts to increase secretion of HGF, leading to enhanced growth, invasion, EMT and metastasis in vitro 167. Additionally, HGF was shown to upregulate VEGF expression and promote angiogenesis in ESCC 164,168. In EAC, upregulation of HGF/c-Met signaling led to increased PI3K/Akt pathway activation, decreased E-cadherin, and increased β-catenin signaling 169.
Vascular endothelial growth factor
Angiogenesis is generally accepted as a key mechanism of continued survival and progression in solid tumors, and has been shown to play a role in both ESCC and EAC 170,171. A key mediator of angiogenesis is vascular endothelial growth factor (VEGF), which comprises a family of structurally similar proteins that primarily trigger endothelial cells to proliferate, migrate, and break down the extracellular matrix to establish new vessels 172. Both tumor and stromal cells, notably fibroblasts, secrete active VEGF under the influence of environmental conditions such as hypoxia 173. Accordingly, a significant subset (30-60%) of esophageal carcinomas have increased VEGF-A expression 174–176, though several studies have suggested that VEGF-A upregulation has prognostic significance in only ESCC 175,176. Several studies have also confirmed that VEGF-C, a lymphangiogenic factor, is associated with survival, tumor depth, stage, and lymph node metastasis in ESCC 177,178. In EAC, there is evidence of progressively increasing VEGF-A expression in the metaplasia-dysplasia-adenocarcinoma sequence, with more advanced cancers showing still higher levels 179,180.
Stromal cell-derived factor-1
In addition to growth factors, fibroblasts also secrete chemokines, particularly stromal cell-derived factor-1 (SDF-1), also known as CXCL12 139. Binding of SDF-1 to its receptors CXCR4 and CXCR7 on tumor cells has been shown to induce tumor cell growth, promote angiogenesis, stimulate motility and invasiveness, and recruit tumor cells to metastatic sites 181.
SDF-1/CXCR4/CXCR7 expression has been noted in both ESCC and EAC 182,183, and activity of this axis is associated with survival as well as tumor invasion and metastasis 182, though independent analyses of each of these components as prognostic indicators have yielded inconsistent results 183–185. Nonetheless, in EAC, SDF-1 was shown to mediate the migration of CXCR4-positive tumor cells in vitro and in vivo, where daily stimulation by SDF-1 led to the dose-dependent development of liver, lung, peritoneal and retroperitoneal metastases in NMRI/nu mice 186. In ESCC, siRNA knockdown of CXCR4 suppressed proliferation, invasion, and metastasis of KYSE-150 and TE-13 cell lines in vitro and in vivo 187.
Extracellular matrix remodeling
Extracellular matrix (ECM) remodeling is thought to play a key role in tumor formation and progression, particularly invasion 188. Fibroblasts and other stromal cells secrete ECM remodeling enzymes, such as lysyl oxidase (LOX) and matrix metalloproteinases (MMPs), which contribute to formation of a primary tumor or metastatic niche and downregulation of cellular adhesion to enable invasion, migration, and intravasation 189,190. ECM remodeling has been implicated in esophageal cancer, especially ESCC. For example, LOX-L2 was overexpressed in over 90% of ESCCs 191. In addition, several matrix metalloproteinases (MMPs), including MMP-2, MMP-7, and MMP-9, were upregulated in ESCC and associated with tumor stage 192,193. MMPs like MMP-1 and MMP-7 may also have a role in progression of BE to EAC 194,195. Interestingly, several MMPs are known to be downstream of STAT3 and NF-κB signaling 192,196.
Several molecular components of the ECM have also been reported to be important in supporting tumor progression. For example, our laboratory previously showed that periostin (POSTN), a matricellular protein secreted by fibroblasts in response to TGF-β 188, cooperates with mutant p53 to induce STAT1 activation and facilitate ESCC cell invasion 197. Intriguingly, both shRNA-mediated knockdown of POSTN and restoration of wildtype p53 decreased STAT1 activation and tumor invasion in vivo 197. OTC-based investigation of POSTN in EAC yielded similar results, with downregulation of POSTN leading to total loss of EAC cell invasion 149. Other ECM components have also been implicated, particularly in ESCC. Fibronectin (FN), for example, is an ECM glycoprotein that is upregulated in ESCC and associated with depth of tumor invasion 198. In addition, the proteoglycan dermatan sulfate (DS) was increased five-fold in human biopsies of ESCC 199. Subsequent study of DS in vitro showed that knockdown of iduronic acid, a component of DS, led to decreased migration and invasion of ESCC cells and was correlated with decreased HGF binding and pERK1/2 activity 199. Lastly, hyaluronan (HA), a glycosaminoglycan in the ECM with pro-tumorigenic properties, has been shown to be upregulated in the stroma surrounding ESCC tissues, especially in tumors with significant desmoplasia 200. Importantly, inhibition of HA synthesis by either 4-methylumbelliferone or lentiviral knockdown of HA-synthase suppressed tumor progression and promoted a more differentiated phenotype in ESCC xenografts 201.
Perspectives
Targeting the TME in esophageal cancer
A number of observations have highlighted the TME as a potential therapeutic target. First, cells within the TME are much more genetically stable than cancer cells, with less selection pressure, fewer mutations, and a lower chance of developing resistance. Furthermore, because they cannot rely on genetic mutations to drive behavior, cells in the TME are highly dependent on factors in their environment for their pro-tumorigenic features. Consequently, they can be manipulated by disrupting environmental factors and other interactions that drive functional changes observed in tumorigenesis.
The TME frequently provides a tumor-protective niche that contributes to treatment resistance. For example, the ability to blunt responses to conventional chemoradiation was a common function among several of the TME components in esophageal cancer. On the whole, disruption of these components in preclinical studies restored sensitivity to chemoradiation, suggesting considerable promise for TME-directed therapeutics in combination with tumor cell-directed agents. This combinatorial approach is now being explored in several clinical trials (Table 2). To date, the most popular treatment approaches in esophageal cancer targeting the TME generally include inhibition of angiogenesis (anti-VEGF) or immune checkpoint blockade (PD-1, CTLA-4)—both potential options for treatment of esophageal cancer due to their availability and early promise in other solid malignancies. TME-targeting treatment approaches specific to individual subtypes of esophageal cancer—analogous to targeting human epidermal growth factor receptor-2 (HER2) in EAC over ESCC, since HER2 gene amplification is far more common in EAC 202—have not yet been pursued, but further identification of differential factors uniquely important to each subtype may reveal more specific strategies. One possibility would be to target the stromal compartment of the TME (i.e. CAFs and ECM components) specifically in ESCC, which has a stronger association with desmoplasia and more evidence showing the upregulation of these factors in tumor progression.
Table 2. Clinical trials with agents targeting the esophageal TME.
In some cases agents may also target tumor cells directly. Under “molecular target,” all agents are inhibitors except for those in parentheses, which are molecular mimics of endogenous agonists. For combinatorial approaches, combined agent is standard chemotherapy unless another agent is specified. CCR4, C-C chemokine receptor 4; CD137, cluster of differentiation antigen 137; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; IL-6, interleukin-6; IL-12, interleukin-12; IL-15, interleukin-15; LAG3, lymphocyte-activation gene 3; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PDGFR, platelet-derived growth factor receptor; SMO, smoothened; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
| TME component | Molecule | Sponsor | Molecular Target or (mimic) | Treatment modality | Phase |
|---|---|---|---|---|---|
| Angiogenesis | Apatinib | Hangzhou Cancer Hospital | VEGFR-2 | Single Agent | II (NCT02544737) |
| Bevacizumab | Dana-Farber Cancer Institute | VEGF-A | Combination | II (NCT01191697) | |
| Fox Chase Cancer Center | Combination | II (NCT01212822) | |||
| Memorial Sloan Kettering Cancer Center | Combination | II (NCT00354679) | |||
| Vanderbilt University Medical Center | Single Agent (after chemoradiation) | 0 (NCT02072720) | |||
| Genentech | Combination | I (NCT01633970) | |||
| Endostar | Jiangsu Simcere Pharmaceutical Co. | VEGFR-2 | Combination (radiotherapy) | II (NCT01368419) | |
| Sunitinib | Roswell Park Cancer Institute | PDGFRs and VEGFRs | Combination | I (NCT00524186) | |
| Ziv-aflibercept | Dana-Farber Cancer Institute | VEGF | Combination | II (NCT01747551) | |
| Immune checkpoint | Avelumab | EMD Serono | PD-L1 | Single Agent | I (NCT01772004) |
| BMS-986016 | ONO/Bristol-Meyers Squibb | LAG3 | Combination (nivolumab) | I (NCT01968109) | |
| Iplimumab | MD Anderson Cancer Center | CTLA-4 | Combination (imatinib) | I (NCT01738139) | |
| MEDI4736 | MedImmune/AstraZeneca | PD-1 | Single Agent | I/II (NCT01693562) | |
| MPDL3280A | Roche/Genentech | PD-1 | Single Agent | I (NCT01375842) | |
| Nivolumab | ONO/Bristol-Meyers Squibb | PD-1 | Single Agent | II (JapicCTI-142422) | |
| Bristol-Meyers Squibb | Combination (lirilumab) | I (NCT01714739) | |||
| Pembrolizumab | Merck | PD-1 | Single Agent | I (NCT02054806) | |
| PF-05082566 | Pfizer | CD137 | Single Agent | I (NCT01307267) | |
| Urelumab | Bristol-Meyers Squibb | CD137 | Single Agent | I (NCT01471210) | |
| Immune (other) | Mogamulizumab | Aichi Medical University | CCR4 | Single Agent | I (NCT01929486) |
| Siltuximab | Janssen Biotech | IL-6 | Single Agent | I/II (NCT00841191) | |
| NHSIL-12 | NCI | (recombinant IL-12) | Single Agent | I (NCT01417546) | |
| rhIL-15 | NCI | (recombinant IL-15) | Single Agent | I (NCT01572493) | |
| Thymalfasin | Hangzhou Cancer Hospital | (synthetic thymosin alpha-1) | Combination (radiotherapy) | II (NCT02545751) | |
| Stroma/ECM | LDE225 | MD Anderson Cancer Center/Novartis | SMO | Combination (everolimus) | I (NCT02138929) |
| Other | Thalidomide | Changzhou No. 2 People's Hospital | broadly targets vasculature and immune components | Combination | II (NCT01551641) |
Still, certain characteristics of the TME can make effective intervention quite challenging. For instance, the TME has the paradoxical capacity to both promote and impede tumor growth and progression. Additionally, despite relative genetic stability, the TME contains cell populations that are quite plastic and heterogeneous in nature. What is more, there are likely important differences between the microenvironments of different cancers 142,203, and even a single cancer's microenvironment likely changes in response to the tumor's mutational landscape or simply during different stages of disease.
These characteristics can make targeting the TME quite challenging. There are strategies to potentially overcome this heterogeneity, however. For example, because the TME possesses both pro-tumor and anti-tumor capabilities, identifying the specific drivers of each of these behaviors could allow for reprogramming of the cells in the TME to actively impair tumor progression 107. This strategy has been explored in cell populations like TAMs, where various agents from receptor inhibitors to miRNAs have been used to re-polarize TAMs into M1-like macrophages 204,205. Another method for combating heterogeneity involves identifying subsets of cell populations that are predominant drivers of protumorigenic behavior, as we demonstrated with CD38high MDSCs. A third approach for overcoming this complexity is to identify and target factors involved in the crosstalk between cells—that is, targeting cell- cell interactions rather than cells directly. Several of the factors reviewed here, such as IL-6 and TGF-β, fall into this category. Certainly, continued efforts identifying novel TME interactions and characterizing context-dependent variations in the TME will lead to refinement of these treatment approaches.
Future horizons
Recent efforts to characterize the TME in esophageal cancer have provided a glimpse into the vast landscape of cell types and factors that contribute to esophageal carcinogenesis. However, considering recent findings in other cancers, there are areas of TME research in esophageal cancer that will likely evolve in the coming years. First, we anticipate that the mechanism by which risk factors (i.e. obesity) predispose to esophageal cancer will be better characterized. In fact, recent studies in other cancers have implicated fibroblast activation, increased ECM stiffness, and altered gut microbiota as ways by which obesity can lead to malignancy 206,207. Furthermore, there are relatively nascent areas of TME research yet to be explored in esophageal cancer. For example, a recent study in gastric cancer showed that tumor growth could be inhibited by blocking cholinergic signaling, demonstrating the presence of a “neural niche” for gastric tumorigenesis 208. Lastly, there remains a need for further elucidation of factors in the TME that could potentially drive the divergence of EAC and ESCC. For example, it is possible that risk factors unique to EAC (i.e. GERD and obesity) create a microenvironment that specifically contributes to the development of EAC (and not ESCC), and we imagine that continued study will reveal mediators—such as cytokines and chemokines—that may be differentially important for each subtype of esophageal cancer. In the end, it is likely that a complex, dynamic interaction between cell of origin and microenvironment leads to the divergence of these subtypes, despite arising in the same organ. Importantly, a better understanding of the idiosyncrasies of the TME in EAC versus ESCC could have broad implications for the treatment and prevention of each of these cancers.
Conclusions
In this review, we have discussed several of the major cell populations, molecular factors, and signaling pathways of the TME that have been studied specifically in ESCC and EAC (Fig. 1, 2). The TME is intricately involved in all stages of tumorigenesis, from creating a niche for initial development to modulating immune function, promoting angiogenesis, and inducing metastasis. Going forward, it will be critical to gain further insights into what defines and drives the heterogeneity of the TME. Understanding how the TME promotes each subtype of esophageal cancer, how specific TME components alter response to therapy, and how the TME adapts to different tumor oncogenic profiles will also be key. Despite tremendous variability and certain differences, however, there is still a great deal of similarity between the different TMEs. As we have shown here, many risk factors, pathways, signaling factors, and cell types are conserved among EAC and ESCC. Ultimately, insights into both similarities and differences of TMEs from various cancer types will be crucial for the future development of TME-targeted therapies, as well as determining who should receive them. In the case of esophageal cancer, progress in these areas will hopefully lead to improved treatment options and better outcomes for this deadly disease.
References
- 1.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 3.Sunpaweravong P, Sunpaweravong S, Puttawibul P, Mitarnun W, Zeng C, Barón AE, et al. Epidermal growth factor receptor and cyclin D1 are independently amplified and overexpressed in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:111–9. doi: 10.1007/s00432-004-0610-7. [DOI] [PubMed] [Google Scholar]
- 4.Hollstein MC, Metcalf RA, Welsh JA, Montesano R, Harris CC. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci U S A. 1990;87:9958–61. doi: 10.1073/pnas.87.24.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung Y, Lam AKY, Luk JM, Law S, Chan K-W, Lee P-Y, et al. Altered E-Cadherin Expression and p120 Catenin Localization in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2007;14:3260–3267. doi: 10.1245/s10434-007-9511-8. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Li L, Ou Y, Gao Z, Li E, Li X, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–5. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 7.Stachler MD, Taylor-Weiner A, Peng S, McKenna A, Agoston AT, Odze RD, et al. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nat Genet. 2015;47:1047–55. doi: 10.1038/ng.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison EW, De Castro VA, Nyhus LM, Kusakari K, Bombeck CT. The significance of bile in reflux esophagitis. Surg Gynecol Obstet. 1972;134:419–24. [PubMed] [Google Scholar]
- 11.Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525–31. doi: 10.1097/00000658-199522240-00010. discussion 531–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007;56:763–71. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S, Guha S, Liu K, Buttar NS, Bresalier RS. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2007;56:1512–21. doi: 10.1136/gut.2007.121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, et al. Aberrant epithelialmesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology. 2010;138:1810–22. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quante M, Bhagat G, Abrams J a., Marache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poulsen HE, Prieme H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev. 1998;7:9–16. [PubMed] [Google Scholar]
- 17.Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett's esophagus, and the latter's progression toward esophageal cancer? Am J Gastroenterol. 2002;97:22–6. doi: 10.1111/j.1572-0241.2002.05444.x. [DOI] [PubMed] [Google Scholar]
- 18.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 19.Sihvo EIT, Salminen JT, Rantanen TK, Rämö OJ, Ahotupa M, Färkkilä M, et al. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551–5. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, et al. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res Mol Mech Mutagen. 2001;480-481:189–200. doi: 10.1016/s0027-5107(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 21.Martin RCG, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007;13:5176–82. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 22.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 23.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, McConkey C, et al. Tumour necrosis factor-alpha in Barrett's oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–81. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- 25.Oka M, Iizuka N, Yamamoto K, Gondo T, Abe T, Hazama S, et al. The influence of interleukin-6 on the growth of human esophageal cancer cell lines. J Interferon Cytokine Res. 1996;16:1001–6. doi: 10.1089/jir.1996.16.1001. [DOI] [PubMed] [Google Scholar]
- 26.Kant P, Hull MA. Excess body weight and obesity--the link with gastrointestinal and hepatobiliary cancer. Nat Rev Gastroenterol Hepatol. 2011;8:224–38. doi: 10.1038/nrgastro.2011.23. [DOI] [PubMed] [Google Scholar]
- 27.Duggan C, Onstad L, Hardikar S, Blount PL, Reid BJ, Vaughan TL. Association between markers of obesity and progression from Barrett's esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:934–43. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, Reynolds J V. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–7. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 29.Ogunwobi O, Mutungi G, Beales ILP. Leptin stimulates proliferation and inhibits apoptosis in Barrett's esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology. 2006;147:4505–4516. doi: 10.1210/en.2006-0224. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein JH, Kao JY, Madanick RD, Zhang M, Wang M, Spacek MB, et al. Association of adiponectin multimers with Barrett's oesophagus. Gut. 2009;58:1583–9. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei X, Wang F, Zhang D, Qiu M, Ren C, Jin Y, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radojicic J, Zaravinos A, Spandidos DA. HPV, KRAS mutations, alcohol consumption and tobacco smoking effects on esophageal squamous-cell carcinoma carcinogenesis. Int J Biol Markers. 2012;27:1–12. doi: 10.5301/JBM.2011.8737. [DOI] [PubMed] [Google Scholar]
- 33.Kubo N, Morita M, Nakashima Y, Kitao H, Egashira A, Saeki H, et al. Oxidative DNA damage in human esophageal cancer: clinicopathological analysis of 8-hydroxydeoxyguanosine and its repair enzyme. Dis Esophagus. 2014;27:285–93. doi: 10.1111/dote.12107. [DOI] [PubMed] [Google Scholar]
- 34.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hébert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer Causes Control. 2015 doi: 10.1007/s10552-015-0636-y. doi:10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- 35.Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, et al. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2012;31:4550–8. doi: 10.1038/onc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman L, Schafer AI. Goldman-Cecil Medicine: Expert Consult - Online. [4 Aug 2015];Elsevier Health Sciences. 2015 https://books.google.com/books?id=40Z9CAAAQBAJ&pgis=1.
- 37.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–97. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker MM, Talley NJ. Review article: bacteria and pathogenesis of disease in the upper gastrointestinal tract--beyond the era of Helicobacter pylori. Aliment Pharmacol Ther. 2014;39:767–79. doi: 10.1111/apt.12666. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138–44. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macfarlane S, Furrie E, Macfarlane GT, Dillon JF. Microbial colonization of the upper gastrointestinal tract in patients with Barrett's esophagus. Clin Infect Dis. 2007;45:29–38. doi: 10.1086/518578. [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein JH, Inadomi JM, Scheiman J, Schoenfeld P, Appelman H, Zhang M, et al. Association between Helicobacter pylori and Barrett's esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2014;12:239–45. doi: 10.1016/j.cgh.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Latif MMM, Duggan S, Reynolds J V, Kelleher D. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol. 2009;9:396–404. doi: 10.1016/j.coph.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 44.Groblewska M, Mroczko B, Sosnowska D, Szmitkowski M. Interleukin 6 and C-reactive protein in esophageal cancer. Clin Chim Acta. 2012;413:1583–90. doi: 10.1016/j.cca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 48.Dvorakova K. Increased Expression and Secretion of Interleukin-6 in Patients with Barrett's Esophagus. Clin Cancer Res. 2004;10:2020–2028. doi: 10.1158/1078-0432.ccr-0437-03. [DOI] [PubMed] [Google Scholar]
- 49.Dvorak K, Dvorak B. Role of interleukin-6 in Barrett's esophagus pathogenesis. World J Gastroenterol. 2013;19:2307–2312. doi: 10.3748/wjg.v19.i15.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E, et al. Cancer-related inflammation and Barrett's carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G454–60. doi: 10.1152/ajpgi.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, et al. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: Relevance to Barrett's esophagus. Clin Cancer Res. 2007;13:5305–5313. doi: 10.1158/1078-0432.CCR-07-0483. [DOI] [PubMed] [Google Scholar]
- 52.Łukaszewicz-Zając M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Szmitkowski M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis Esophagus. 2012;25:242–9. doi: 10.1111/j.1442-2050.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 53.Hardikar S, Onstad L, Song X, Wilson AM, Montine TJ, Kratz M, et al. Inflammation and oxidative stress markers and esophageal adenocarcinoma incidence in a Barrett's esophagus cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:2393–403. doi: 10.1158/1055-9965.EPI-14-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–36. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- 55.Leu C-M, Wong F-H, Chang C, Huang S-F, Hu C. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22:7809–18. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 56.Yan S, Zhou C, Zhang W, Zhang G, Zhao X, Yang S, et al. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett. 2008;271:85–97. doi: 10.1016/j.canlet.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 57.Makuuchi Y, Honda K, Osaka Y, Kato K, Kojima T, Daiko H, et al. Soluble interleukin-6 receptor is a serum biomarker for the response of esophageal carcinoma to neoadjuvant chemoradiotherapy. Cancer Sci. 2013;104:1045–51. doi: 10.1111/cas.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoneda M, Fujiwara H, Furutani A, Ikai A, Tada H, Shiozaki A, et al. Prognostic impact of tumor IL-6 expression after preoperative chemoradiotherapy in patients with advanced esophageal squamous cell carcinoma. Anticancer Res. 2013;33:2699–705. [PubMed] [Google Scholar]
- 59.Chen M-F, Chen P-T, Lu MS, Lin PY, Chen W-C, Lee K-D. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. 2013;12:26. doi: 10.1186/1476-4598-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen M-F, Kuan F-C, Yen T-C, Lu M-S, Lin P-Y, Chung Y-H, et al. IL-6-stimulated CD11b + CD14 + HLA-DR − myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716–8728. doi: 10.18632/oncotarget.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Xiao W, Ma J, Zhang Y, Li R, Ye J, et al. Dual high expression of STAT3 and cyclinD1 is associated with poor prognosis after curative resection of esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:7989–98. [PMC free article] [PubMed] [Google Scholar]
- 62.Karakasheva TA, Waldron TJ, Eruslanov E, Lee J-S, O'Brien S, Hicks PD, et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-3639. doi:10.1158/0008-5472.CAN-14-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suchi K, Fujiwara H, Okamura S, Okamura H, Umehara S, Todo M, et al. Overexpression of Interleukin-6 suppresses cisplatin-induced cytotoxicity in esophageal squamous cell carcinoma cells. Anticancer Res. 2011;31:67–75. [PubMed] [Google Scholar]
- 64.Zhang Q, Zhang C, He J, Guo Q, Hu D, Yang X, et al. STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumour Biol. 2015;36:2135–42. doi: 10.1007/s13277-014-2823-y. [DOI] [PubMed] [Google Scholar]
- 65.Yu C, Zhang Q, Zhang HY, Zhang X, Huo X, Cheng E, et al. Targeting the intrinsic inflammatory pathway: honokiol exerts proapoptotic effects through STAT3 inhibition in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol. 2012;303:G561–9. doi: 10.1152/ajpgi.00033.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q, Zhang C, He J, Guo Q, Hu D, Yang X, et al. STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumour Biol. 2014 doi: 10.1007/s13277-014-2823-y. doi:10.1007/s13277-014-2823-y. [DOI] [PubMed] [Google Scholar]
- 67.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 68.Karin M, Cao Y, Greten FR, Li Z-W. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 69.Izzo JG, Correa AM, Wu T-T, Malhotra U, Chao CKS, Luthra R. Pretherapy nuclear factor-kappaB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. 29 Mol Cancer Ther. 2006;5:2844–50. doi: 10.1158/1535-7163.MCT-06-0351. [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Latif MMM, O'Riordan J, Windle HJ, Carton E, Ravi N, Kelleher D, et al. NF-kappaB activation in esophageal adenocarcinoma: relationship to Barrett's metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenkins GJS, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, et al. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–23. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 72.Malhotra U, Hittelman WN, Wu TT, Luthra R, Swisher S, Luthra M, et al. Association of activated NF-kB, altered cyclin D1 and poor outcome in esophageal adenocarcinoma. Cancer Res. 2005;65:548–a. [Google Scholar]
- 73.O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GSA, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–64. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 74.Izzo JG, Malhotra U, Wu T-T, Luthra R, Correa AM, Swisher SG, et al. Clinical biology of esophageal adenocarcinoma after surgery is influenced by nuclear factor-kappaB expression. Cancer Epidemiol Biomarkers Prev. 2007;16:1200–5. doi: 10.1158/1055-9965.EPI-06-1083. [DOI] [PubMed] [Google Scholar]
- 75.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of p120-Catenin Results in a Tumor Microenvironment with Inflammation and Cancer that Establishes It as a Tumor Suppressor Gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Cheung PY, Wang X, Tsao SW, Ling MT, Wong YC, et al. Id-1 activation of PI3K/Akt/NF??B signaling pathway and its significance in promoting survival of esophageal cancer cells. Carcinogenesis. 2007;28:2313–2320. doi: 10.1093/carcin/bgm152. [DOI] [PubMed] [Google Scholar]
- 77.Lin C, Song L, Gong H, Liu A, Lin X, Wu J, et al. Nkx2-8 downregulation promotes angiogenesis and activates NF-κB in esophageal cancer. Cancer Res. 2013;73:3638–3648. doi: 10.1158/0008-5472.CAN-12-4028. [DOI] [PubMed] [Google Scholar]
- 78.Hatata T, Higaki K, Tatebe S, Shomori K, Ikeguchi M. Immunohistochemical study of nuclear factor-κB expression in esophageal squamous cell carcinoma: Prognostic significance and sensitivity to treatment with 5-FU. Dis Esophagus. 2012;25:716–722. doi: 10.1111/j.1442-2050.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 79.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 80.Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P, et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. 2014;4:5911. doi: 10.1038/srep05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh DS, DeMeester SR, Vallbohmer D, Mori R, Kuramochi H, Hagen JA, et al. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett's esophagus with antireflux surgery. Arch Surg. 2007;142:554–9. doi: 10.1001/archsurg.142.6.554. discussion 559–60. [DOI] [PubMed] [Google Scholar]
- 84.Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002;51:316–22. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogura M, Takeuchi H, Kawakubo H, Nishi T, Fukuda K, Nakamura R, et al. Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery. 2013;154:512–20. doi: 10.1016/j.surg.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen DM, Chen GA, Reddy R, Tsai W, Schrump WD, Cole G, et al. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg. 2004;127:365–75. doi: 10.1016/j.jtcvs.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 87.Tian F, Zang W-D, Hou W-H, Liu H-T, Xue L-X. Nuclear factor-kB signaling pathway constitutively activated in esophageal squamous cell carcinoma cell lines and inhibition of growth of cells by small interfering RNA. Acta Biochim Biophys Sin (Shanghai) 2006;38:318–26. doi: 10.1111/j.1745-7270.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 88.Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG, et al. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2009;296:G388–98. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- 89.Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97–106. doi: 10.1007/s00535-011-0523-6. [DOI] [PubMed] [Google Scholar]
- 90.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–96. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 91.Morris CD, Armstrong GR, Bigley G, Green H, Attwood SE. Cyclooxygenase-2 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990–6. doi: 10.1111/j.1572-0241.2001.03599.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhi H, Wang L, Zhang J, Zhou C, Ding F, Luo A, et al. Significance of COX-2 expression in human esophageal squamous cell carcinoma. Carcinogenesis. 2006;27:1214–21. doi: 10.1093/carcin/bgi304. [DOI] [PubMed] [Google Scholar]
- 93.Akutsu Y, Hanari N, Yusup G, Komatsu-Akimoto A, Ikeda N, Mori M, et al. COX2 expression predicts resistance to chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18:2946–51. doi: 10.1245/s10434-011-1645-z. [DOI] [PubMed] [Google Scholar]
- 94.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective Inhibition of Cyclooxygenase- 2 Suppresses Growth and Induces Apoptosis in Human Esophageal Adenocarcinoma Cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 95.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101–12. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 96.Bardou M, Barkun A, Ghosn J, Hudson M, Rahme E. Effect of chronic intake of NSAIDs and cyclooxygenase 2—selective inhibitors on esophageal cancer incidence. Clin Gastroenterol Hepatol. 2004;2:880–887. doi: 10.1016/s1542-3565(04)00389-1. [DOI] [PubMed] [Google Scholar]
- 97.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong J, Xie J, Bedolla R, Rivas P, Chakravarthy D, Freeman JW, et al. Combined targeting of STAT3/NF-κB/COX-2/EP4 for effective management of pancreatic cancer. Clin Cancer Res. 2014;20:1259–73. doi: 10.1158/1078-0432.CCR-13-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: Targets for therapy. Oncoimmunology. 2013;2:e24117. doi: 10.4161/onci.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, et al. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol. 2012;188:5365–76. doi: 10.4049/jimmunol.1103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 104.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quail DF, Joyce J a. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373:1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 110.Ha T-Y. The role of regulatory T cells in cancer. Immune Netw. 2009;9:209–35. doi: 10.4110/in.2009.9.6.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 112.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 113.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–71. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 115.Nabeki B, Ishigami S, Uchikado Y, Sasaki K, Kita Y, Okumura H, et al. Interleukin-32 expression and Treg infiltration in esophageal squamous cell carcinoma. Anticancer Res. 2015;35:2941–7. [PubMed] [Google Scholar]
- 116.Xia M, Zhao MQ, Wu K, Lin XY, Liu Y, Qin YJ. Investigations on the clinical significance of FOXP3 protein expression in cervical oesophageal cancer and the number of FOXP3+ tumourinfiltrating lymphocytes. J Int Med Res. 2013;41:1002–1008. doi: 10.1177/0300060513488504. [DOI] [PubMed] [Google Scholar]
- 117.Osaki T, Saito H, Fukumoto Y, Yamada Y, Fukuda K, Tatebe S, et al. Inverse correlation between NKG2D expression on CD8+ T cells and the frequency of CD4+CD25+ regulatory T cells in patients with esophageal cancer. Dis Esophagus. 2009;22:49–54. doi: 10.1111/j.1442-2050.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 118.Xu T, Duan Q, Wang G, Hu B. CD4 + CD25high regulatory T cell numbers and FOXP3 mRNA expression in patients with advanced esophageal cancer before and after chemotherapy. Cell Biochem Biophys. 2011;61:389–92. doi: 10.1007/s12013-011-9197-1. [DOI] [PubMed] [Google Scholar]
- 119.Vacchelli E, Semeraro M, Enot DP, Chaba K, Poirier Colame V, Dartigues P, et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget. 2015;6:20840–50. doi: 10.18632/oncotarget.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Végran F, Hichami A, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–73. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 123.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211:529–43. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L, et al. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cell Immunol. 2012;272:166–74. doi: 10.1016/j.cellimm.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 125.Jiao ZJ, Gao JJ, Hua SH, Chen DY, Wang WH, Wang H, et al. Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer. World J Gastroenterol. 2012;18:5454–5461. doi: 10.3748/wjg.v18.i38.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial PD-L2 expression marks Barrett's Esophagus and Esophageal Adenocarcinoma. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0046. doi:10.1158/2326-6066.CIR-15-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193:3835–41. doi: 10.4049/jimmunol.1401572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 130.Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, et al. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:6015–6023. [PMC free article] [PubMed] [Google Scholar]
- 131.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 133.Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 135.Miyashita T, Tajima H, Shah FA, Oshima M, Makino I, Nakagawara H, et al. Impact of inflammation-metaplasia-adenocarcinoma sequence and inflammatory microenvironment in esophageal carcinogenesis using surgical rat models. Ann Surg Oncol. 2014;21:2012–9. doi: 10.1245/s10434-014-3537-5. [DOI] [PubMed] [Google Scholar]
- 136.Gao J, Wu Y, Su Z, Amoah Barnie P, Jiao Z, Bie Q, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9:e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 138.Shigeoka M, Urakawa N, Nakamura T, Nishio M, Watajima T, Kuroda D, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1112–1119. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 140.Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–8. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanaka K, Miyata H, Sugimura K, Fukuda S, Kanemura T, Yamashita K, et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis. 2015;36:894–903. doi: 10.1093/carcin/bgv067. [DOI] [PubMed] [Google Scholar]
- 142.Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grugan KD, Miller CG, Yao Y, Michaylira CZ, Ohashi S, Klein-Szanto AJ, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107:11026–11031. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noma K, Smalley KSM, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–93. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, et al. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–4027. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 146.Ha SY, Yeo S-Y, Xuan Y, Kim S-H. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma. PLoS One. 2014;9:e99955. doi: 10.1371/journal.pone.0099955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y, Li X, Yang H, Xia Y, Guo L, Wu X, et al. Expression of basic fibroblast growth factor, CD31, and α-smooth muscle actin and esophageal cancer recurrence after definitive chemoradiation. Tumour Biol. 2014;35:7275–82. doi: 10.1007/s13277-014-1987-9. [DOI] [PubMed] [Google Scholar]
- 148.Bao C-H, Wang X-T, Ma W, Wang N-N, Un Nesa E, Wang J-B, et al. Irradiated fibroblasts promote epithelial-mesenchymal transition and HDGF expression of esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2015;458:441–7. doi: 10.1016/j.bbrc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Hayden AL, Derouet MF, Noble F, Primrose JN, Blaydes JP, Thomas G, et al. OC-121 Fibroblast activation in the tumour microenvironment promotes tumour cell invasion and resistance to chemotherapy in oesophageal adenocarcinoma. Gut. 2012;61:A52–A53. [Google Scholar]
- 150.Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 2015;235:466–77. doi: 10.1002/path.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 152.Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–99. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lebman DA, Edmiston JS, Chung TD, Snyder SR. Heterogeneity in the transforming growth factor beta response of esophageal cancer cells. Int J Oncol. 2002;20:1241–6. [PubMed] [Google Scholar]
- 154.Onwuegbusi BA, Aitchison A, Chin S-F, Kranjac T, Mills I, Huang Y, et al. Impaired transforming growth factor beta signalling in Barrett's carcinogenesis due to frequent SMAD4 inactivation. Gut. 2006;55:764–74. doi: 10.1136/gut.2005.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fukuchi M, Masuda N, Miyazaki T, Nakajima M, Osawa H, Kato H, et al. Decreased Smad4 expression in the transforming growth factor-beta signaling pathway during progression of esophageal squamous cell carcinoma. Cancer. 2002;95:737–43. doi: 10.1002/cncr.10727. [DOI] [PubMed] [Google Scholar]
- 156.Wu L, Herman JG, Brock M V, Wu K, Mao G, Yan W, et al. Silencing DACH1 promotes esophageal cancer growth by inhibiting TGF-β signaling. PLoS One. 2014;9:e95509. doi: 10.1371/journal.pone.0095509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hou J, Liao L-D, Xie Y-M, Zeng F-M, Ji X, Chen B, et al. DACT2 is a candidate tumor suppressor and prognostic marker in esophageal squamous cell carcinoma. Cancer Prev Res (Phila) 2013;6:791–800. doi: 10.1158/1940-6207.CAPR-12-0352. [DOI] [PubMed] [Google Scholar]
- 158.Fukai Y, Fukuchi M, Masuda N, Osawa H, Kato H, Nakajima T, et al. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer. 2003;104:161–6. doi: 10.1002/ijc.10929. [DOI] [PubMed] [Google Scholar]
- 159.Song S, Maru DM, Ajani JA, Chan C-H, Honjo S, Lin H-K, et al. Loss of TGF-β adaptor β2SP activates notch signaling and SOX9 expression in esophageal adenocarcinoma. Cancer Res. 2013;73:2159–69. doi: 10.1158/0008-5472.CAN-12-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.von Rahden BHA, Stein HJ, Feith M, Pühringer F, Theisen J, Siewert JR, et al. Overexpression of TGF-beta1 in esophageal (Barrett's) adenocarcinoma is associated with advanced stage of disease and poor prognosis. Mol Carcinog. 2006;45:786–94. doi: 10.1002/mc.20259. [DOI] [PubMed] [Google Scholar]
- 161.Kim AH, Lebman DA, Dietz CM, Snyder SR, Eley KW, Chung TD. Transforming growth factor- β is an endogenous radioresistance factor in the esophageal adenocarcinoma cell line OE-33. Int J Oncol. 2003;23:1593–1599. [PubMed] [Google Scholar]
- 162.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 163.Dreikhausen L, Blank S, Sisic L, Heger U, Weichert W, Jäger D, et al. Association of angiogenic factors with prognosis in esophageal cancer. BMC Cancer. 2015;15:121. doi: 10.1186/s12885-015-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11:6190–7. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 165.Takada N, Yano Y, Matsuda T, Otani S, Osugi H, Higashino M, et al. Expression of immunoreactive human hepatocyte growth factor in human esophageal squamous cell carcinomas. Cancer Lett. 1995;97:145–148. doi: 10.1016/0304-3835(95)03967-2. [DOI] [PubMed] [Google Scholar]
- 166.Tuynman JB, Lagarde SM, Ten Kate FJW, Richel DJ, van Lanschot JJB. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer. 2008;98:1102–8. doi: 10.1038/sj.bjc.6604251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Patel ZS, Grugan KD, Rustgi AK, Cucinotta FA, Huff JL. Ionizing radiation enhances esophageal epithelial cell migration and invasion through a paracrine mechanism involving stromal-derived hepatocyte growth factor. Radiat Res. 2012;177:200–8. doi: 10.1667/rr2790.1. [DOI] [PubMed] [Google Scholar]
- 168.Xu Z, Wang S, Wu M, Zeng W, Wang X, Dong Z. TGFβ1 and HGF protein secretion by esophageal squamous epithelial cells and stromal fibroblasts in oesophageal carcinogenesis. Oncol Lett. 2013;6:401–406. doi: 10.3892/ol.2013.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Anderson MR, Harrison R, Atherfold PA, Campbell MJ, Darnton SJ, Obszynska J, et al. Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res. 2006;12:5936–43. doi: 10.1158/1078-0432.CCR-06-1208. [DOI] [PubMed] [Google Scholar]
- 170.Kitadai Y, Onogawa S, Kuwai T, Matsumura S, Hamada H, Ito M, et al. Angiogenic switch occurs during the precancerous stage of human esophageal squamous cell carcinoma. Oncol Rep. 2004;11:315–319. [PubMed] [Google Scholar]
- 171.Couvelard A, Paraf F, Gratio V, Scoazec JY, Hénin D, Degott C, et al. Angiogenesis in the neoplastic sequence of Barrett's oesophagus. Correlation with VEGF expression. J Pathol. 2000;192:14–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH709>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 172.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ito T-K, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 174.Ekman S, Bergqvist M, Heldin C-H, Lennartsson J. Activation of growth factor receptors in esophageal cancer--implications for therapy. Oncologist. 2007;12:1165–77. doi: 10.1634/theoncologist.12-10-1165. [DOI] [PubMed] [Google Scholar]
- 175.Möbius C, Freire J, Becker I, Feith M, Brücher BLDM, Hennig M, et al. VEGF-C expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J Surg. 2007;31:1768–72. doi: 10.1007/s00268-006-0373-1. discussion 1773–4. [DOI] [PubMed] [Google Scholar]
- 176.Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1126–34. doi: 10.1158/1055-9965.EPI-12-0020. [DOI] [PubMed] [Google Scholar]
- 177.Xu W, Yang G, Zhou L, Xie Y, Zhang L. Prognostic value of VEGF expression in primary esophageal squamous cell carcinoma. Chinese J Cancer Res. 2004;16:85–89. [Google Scholar]
- 178.ZHANG H, ZHANG L, CHEN K, GAO D, HE F, ZHANG Y. Expression of Vascular Endothelial Growth Factor C and Its Clinical Significance in Human Esophageal Squamous Cell Carcinoma. Cancer Biol. Med. 2007;4:83–88. [Google Scholar]
- 179.Lord RVN, Park JM, Wickramasinghe K, DeMeester SR, Oberg S, Salonga D, et al. Vascular endothelial growth factor and basic fibroblast growth factor expression in esophageal adenocarcinoma and Barrett esophagus. J Thorac Cardiovasc Surg. 2003;125:246–53. doi: 10.1067/mtc.2003.203. [DOI] [PubMed] [Google Scholar]
- 180.Prins MJD, Verhage RJJ, ten Kate FJW, van Hillegersberg R. Cyclooxygenase isoenzyme-2 and vascular endothelial growth factor are associated with poor prognosis in esophageal adenocarcinoma. J Gastrointest Surg. 2012;16:956–66. doi: 10.1007/s11605-011-1814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–22. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. 2005;97:1840–7. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 183.Tachezy M, Zander H, Gebauer F, von Loga K, Pantel K, Izbicki JR, et al. CXCR7 expression in esophageal cancer. J Transl Med. 2013;11:238. doi: 10.1186/1479-5876-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sasaki K, Natsugoe S, Ishigami S, Matsumoto M, Okumura H, Setoyama T, et al. Expression of CXCL12 and its receptor CXCR4 in esophageal squamous cell carcinoma. Oncol Rep. 2009;21:65–71. [PubMed] [Google Scholar]
- 185.Wang D-F, Lou N, Zeng C-G, Zhang X, Chen F-J. Expression of CXCL12/CXCR4 and its correlation to prognosis in esophageal squamous cell carcinoma. Ai Zheng. 2009;28:154–8. [PubMed] [Google Scholar]
- 186.Gros SJ, Graeff H, Drenckhan A, Kurschat N, Blessmann M, Rawnaq T, et al. CXCR4/SDF-1α-mediated chemotaxis in an in vivo model of metastatic esophageal carcinoma. In Vivo. 2012;26:711–8. [PubMed] [Google Scholar]
- 187.Wang T, Mi Y, Pian L, Gao P, Xu H, Zheng Y, et al. RNAi targeting CXCR4 inhibits proliferation and invasion of esophageal carcinoma cells. Diagn Pathol. 2013;8:104. doi: 10.1186/1746-1596-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 188.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fong SFT, Dietzsch E, Fong KSK, Hollosi P, Asuncion L, He Q, et al. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46:644–55. doi: 10.1002/gcc.20444. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Y, Wang Q, Ma A, Li Y, Li R, Wang Y. Functional expression of TLR9 in esophageal cancer. Oncol Rep. 2014;31:2298–2304. doi: 10.3892/or.2014.3095. [DOI] [PubMed] [Google Scholar]
- 193.Gu Z-D, Li J-Y, Li M, Gu J, Shi X-T, Ke Y, et al. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol. 2005;100:1835–43. doi: 10.1111/j.1572-0241.2005.50018.x. [DOI] [PubMed] [Google Scholar]
- 194.Salmela MT, Karjalainen-Lindsberg ML, Puolakkainen P, Saarialho-Kere U. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett's oesophageal adenocarcinoma. Br J Cancer. 2001;85:383–392. doi: 10.1054/bjoc.2001.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grimm M, Lazariotou M, Kircher S, Stuermer L, Reiber C, Höfelmayr A, et al. MMP-1 is a (pre- )invasive factor in Barrett-associated esophageal adenocarcinomas and is associated with positive lymph node status. J Transl Med. 2010;8:99. doi: 10.1186/1479-5876-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carpenter RL, Lo H-W. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel) 2014;6:897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wong GS, Lee J-S, Park Y-Y, Klein-Szanto a J, Waldron TJ, Cukierman E, et al. Periostin cooperates with mutant p53 to mediate invasion through the induction of STAT1 signaling in the esophageal tumor microenvironment. Oncogenesis. 2013;2:e59. doi: 10.1038/oncsis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang J, Zhi H, Zhou C, Ding F, Luo A, Zhang X, et al. Up-regulation of fibronectin in oesophageal squamous cell carcinoma is associated with activation of the Erk pathway. J Pathol. 2005;207:402–9. doi: 10.1002/path.1846. [DOI] [PubMed] [Google Scholar]
- 199.Thelin M a., Svensson KJ, Shi X, Bagher M, Axelsson J, Isinger-Ekstrand A, et al. Dermatan sulfate is involved in the tumorigenic properties of esophagus squamous cell carcinoma. Cancer Res. 2012;72:1943–1952. doi: 10.1158/0008-5472.CAN-11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang C, Tammi M, Guo H, Tammi R. Hyaluronan distribution in the normal epithelium of esophagus, stomach, and colon and their cancers. Am J Pathol. 1996;148:1861–9. [PMC free article] [PubMed] [Google Scholar]
- 201.Twarock S, Freudenberger T, Poscher E, Dai G, Jannasch K, Dullin C, et al. Inhibition of oesophageal squamous cell carcinoma progression by in vivo targeting of hyaluronan synthesis. Mol Cancer. 2011;10:30. doi: 10.1186/1476-4598-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang J-X, Zhao K, Lin M, Wang Q, Xiao W, Lin M-S, et al. HER2 gene amplification in esophageal squamous cell carcinoma is less than in gastroesophageal junction and gastric adenocarcinoma. Oncol Lett. 2013;6:13–18. doi: 10.3892/ol.2013.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bertos NR, Park M. Breast cancer - one term, many entities? J Clin Invest. 2011;121:3789–96. doi: 10.1172/JCI57100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang C-Y, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4:341–3. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 206.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130–301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 208.Zhao C-M, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]