Abstract
Our recent studies have displayed the protective functions of aspirin against heat stress (HS) in chicken myocardial cells, and it may be associated with heat shock proteins (HSPs). In this study, we further investigated the potential role of HSPs in the aspirin-induced heat stress resistance. Four of the most important HSPs including HspB1 (Hsp27), Hsp60, Hsp70, and Hsp90 were induced by aspirin pretreatment and were suppressed by BAPTA-AM. When HSPs were induced by aspirin, much slighter HS injury was detected. But more serious damages were observed when HSPs were suppressed by BAPTA-AM than those cells exposed to HS without BAPTA-AM, even the myocardial cells have been treated with aspirin in prior. Comparing to other HSPs, HspB1 presented the largest increase after aspirin treatments, 86-fold higher than the baseline (the level before HS). These findings suggested that multiple HSPs participated in aspirin’s anti-heat stress function but HspB1 may contribute the most. Interestingly, during the experiments, we also found that apoptosis rate as well as the oxidative stress indicators (T-SOD and MDA) was not consistently responding to heat stress injury as expected. By selecting from a series of candidates, myocardial cell damage-related enzymes (CK-MB and LDH), cytopathological tests, and necrosis rate (measured by flow cytometry assays) are believed to be reliable indicators to evaluate heat stress injury in chicken’s myocardial cells and they will be used in our further investigations.
Keywords: HSPs, Aspirin, BAPTA-AM, Heat stress, Myocardial cell, Inhibitor
Introduction
Exposure to high temperatures leads to severe heat stress (HS) injury in animals. The damages caused by heat stress to breeding industries have been explained in our previous works, especially the considerable economic loss on poultry breeding (Wu et al. 2015, 2016). Previous research also has revealed that acute HS leads to cardiac dysfunction and other forms of myocardial cell damage (Tang et al. 2013), and these effects are responsible for sudden infant death in animals and even in humans after HS exposure (Gathiram et al. 1987, 1988; Rai and Ambwany 1980).
Heat stress leads to large amounts of changes in organisms, and different studies probably accept different HS indicators. HS exposure increases the permeability of myocardial cells, causing a series of enzymes such as isoenzyme creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) to be released from the myocardial cells (Ma et al. 2013; Xue et al. 2015). These enzymes are categorized as myocardial cell damage-related enzymes and are widely used as markers of myocardial cell injury (Saravanan et al. 2013; Tang et al. 2013; Wu et al. 2015). In addition to these enzymes, histopathological or cytopathological damages are also commonly observed in myocardial cells after HS; for example, the cells may be swollen in size, show uneven matrix staining in the cytoplasm, appear shrunken and dull-stained in the nucleus, or exhibit acute degeneration (Tang et al. 2013; Wu et al. 2015). Furthermore, HS has been reported to be closely associated with oxidative stress (Paul et al. 2009), and variations in superoxide dismutase (SOD) and methane dicarboxylic aldehyde (MDA), which are indicative of oxidative stress, were shown to be induced by HS in broiler chickens (Altan et al. 2003). Other reports have also suggested that apoptosis or necrosis can be induced by HS exposure (Green 2011; Masri and Chandrashekhar 2008). So many indicators respond to HS injury differently in different researches, sometimes even make confusions to researchers; therefore, in this experiment, we also attempted to find out a series of reliable indicators to evaluate heat stress injury in chicken myocardial cells in vitro for future studies.
To survive from HS, organisms from fungi to mammals have evolved indigenous anti-HS injury systems. Across both organs and species, these systems include heat shock proteins (HSPs) (Li and Srivastava 2004). Previous studies have asserted that some HSPs possess massive stress resistance activities, particularly HspB1 (Liu et al. 2007; Mearow et al. 2002), Hsp60 (Ranford et al. 2000), Hsp70 (Wegele et al. 2004; Yoshimune et al. 2002), and Hsp90 (Pearl and Prodromou 2001). A number of researchers believe that heat stress damage can be largely diminished if the HSPs are appropriately induced. Under natural circumstances, many stimuli such as oxidation (Calabrese et al. 2005; Das et al. 1995), ultraviolet radiation (Cao et al. 1999), and hyperthermy are capable of inducing HSPs. Scientists have attempted to develop various solutions to induce HSPs in stressed animals via physical, chemical, and biological stimulation; however, most of these attempts were either highly cost or produced limited benefits, and some even resulted in higher mortality rates than HS itself (Endo et al. 2007; Wischmeyer 2002).
Recent studies indicate that the protective expression of HSPs can be induced by aspirin (Amberger et al. 1999; Sandoval-Montiel et al. 2013). First developed as a painkiller, the cardiac-protective function of aspirin was discovered in subsequent investigations. The cardiac-protective function was initially attributed to the anti-coagulation effect of aspirin (Patrono et al. 2005); however, few non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) that possess anti-inflammatory and anti-platelet properties similar to those of aspirin exhibit cardiac-protective effects (Solomon et al. 2002). Even though the detailed mechanism of the cardiac-protective effect of aspirin remains unclear, further researches found that appropriate dosage of aspirin has been shown to induce the expression of different HSPs in different species (Ebert et al. 2005; Endo et al. 2007; Wischmeyer 2002). Since HSPs are widely known to impart stress resistance, ASA can be reasonably considered to protect against myocardial injury due to heat stress damage by inducing HSPs. In fact, previous studies have suggested that aspirin helps reduce stress injury, a function that is closely related to the induction of HSPs (Ghavami et al. 2002; Jurivich et al. 1992; Wu et al. 2015). Based on the above factors, in our recent studies, we inferred that the heat stress resistance function of aspirin in the myocardial cells of chickens is related to the expression of some HSPs, particularly HspB1 (also called Hsp27) (Wu et al. 2015, 2016). However, more evidence is required to support the association between aspirin-induced heat stress resistance and the expression of HSPs in myocardial cells.
To continue the investigation of the connection between aspirin-induced heat stress resistance and HSPs, HSP inhibitors such as BAPTA-AM have been employed experimentally. First used as a Ca2+-chelating agent, BAPTA-AM was recently shown to inhibit some HSPs (Kaida et al. 1999; Kiang et al. 2000; Tokuda et al. 2002). In some novel studies, BAPTA-AM was even used as a broad-spectrum HSP inhibitor, with good results being obtained (Kiang 2003; Nuss et al. 2014). Accordingly, in this study, we evaluated the heat stress injury and assessed the anti-injury effects of aspirin with and without the addition of BAPTA-AM as an HSP inhibitor. The results provide evidence for the important role of HSPs in aspirin-induced heat stress resistance in vitro.
Materials and methods
Cell culture and stress model
Primary cultures of chicken myocardial cells (provided by Shanghai Fu Meng Biological Technology Ltd, Shanghai, China) were cultured as we described before (Wu et al. 2016). Cells were cultured (37 °C and 5 % CO2) on the Petri dishes for 72 h until the fusion rate was higher than 90 %. The cell culture medium contained 20 % fetal bovine serum (FBS; SH30088.03, Hyclone, Logan, UT, USA), 100 IU penicillin, and streptomycin (SV30010, HyClone) in Dulbecco’s modified Eagle’s medium (NYH0954, Thermo Scientific, Waltham, MA, USA).
Four groups were designated: the heat stress challenge group (group HS), the group treated with aspirin before heat stress (group A), the group treated with BAPTA-AM before heat stress (group B), and the group treated with both aspirin and BAPTA-AM before heat stress (group A + B). The working concentrations of aspirin (1 mg/mL) and BAPTA-AM (18 μM) were measured by MTT assay in a preliminary experiment (data not shown). Aspirin powder (>98 % purity) and BAPTA-AM powder (>95 % purity) were purchased from Sigma, St. Louis, MO, USA.
HS was applied by incubating the cells in water bath (40 ± 1 °C). The HS phase was initiated by gently transferring the cell dishes into the water bath (time = 0 h). All the administrations (aspirin and/or BAPTA-AM) in different groups were added into the cell culture medium 2 h before 0 h, and this time point was marked as −2 h. The vehicle, dimethyl sulfoxide, was added in group HS as a control. The cell samples were collected at four time points in total: −2, 0, 2, and 5 h. These four time points represent 2 h before HS, the beginning of HS, HS for 2 h, and for 5 h, respectively. All experiments were conducted in triplicate.
ELISA assays
Total proteins were extracted using RIPA lysis buffer (WB-0071, Dingguo Changsheng Biotechnology Co., Ltd., Nanjing, China). Concentrations of total proteins were measured using a Micro BCA assay kit (BCA01, Dingguo Changsheng Biotechnology Co., Ltd., Nanjing, China), and samples were diluted with lysis buffer to normalize the protein loading amounts. The concentrations of four HSPs (HspB1, Hsp60, Hsp90, and Hsp70) were measured by ELISA kits according to manufacturer’s instructions (MBS700383 for HspB1, MBS737544 for Hsp60, MBS704670 for Hsp90, and MBS740753 for Hsp70, MyBiosource, USA).
Enzymatic activities in the supernatants of primary cultured myocardial cells
The supernatants of primary cultures of myocardial cells were collected. The activities of CK-MB, LDH, T-SOD, and MDA were measured according to the kit manuals (E006 for CK-MB, A020-2 for LDH, A001-1 for T-SOD, and A003-2 for MDA, Nanjing Jiancheng Biochemical Reagent Co. Ltd., Nanjing, China) using a clinical biochemical indicator auto-analyzer (Infinite M200PRO, Switzerland).
Cytopathological studies
Myocardial cells were cultured on glass coverslips treated with poly-L-lysine (PLL; product ID: 1014318, Sigma, USA). The cell-cultured coverslips were fixed in paraformaldehyde overnight, washed twice with PBS (pH 7.4), and stained by hematoxylin and eosin (H&E). The cytopathological sections were viewed under a light microscope, and images were taken for cytopathological analysis.
Flow cytometry
Flow cytometry assays were used to detect cell apoptosis and necrosis using an Annexin V FITC Apoptosis Detection Kit I (KGA107, KeyGEN, Nanjing, China). After incubation and the administration of the different treatments, the myocardial cells (2 × 105) were harvested with EDTA-free trypsin (Gibco, USA). All steps were carried out according to the manufacturer’s protocols. A FACSCalibur flow cytometer (FACS101, Becton–Dickinson, USA) was used to detect cell apoptosis.
Statistical analysis
Curve Expert 1.3 software was used to generate standard curves for ELISAs. The HSP expression, enzyme activity, and flow cytometry data at 0, 2, and 5 h were compared with the baseline level (−2 h) using one-way analysis of variance followed by Fisher’s least significant difference test using the Statistical Package for Social Sciences software (SPSS version 20.0 for Windows). Significant differences (*) were indicated by P < 0.05, and highly significant differences (**) were indicated by P < 0.01 in this study. All the raw data presented in this paper are presented as mean ± SD.
Results
Differences caused by involving aspirin into heat stress response
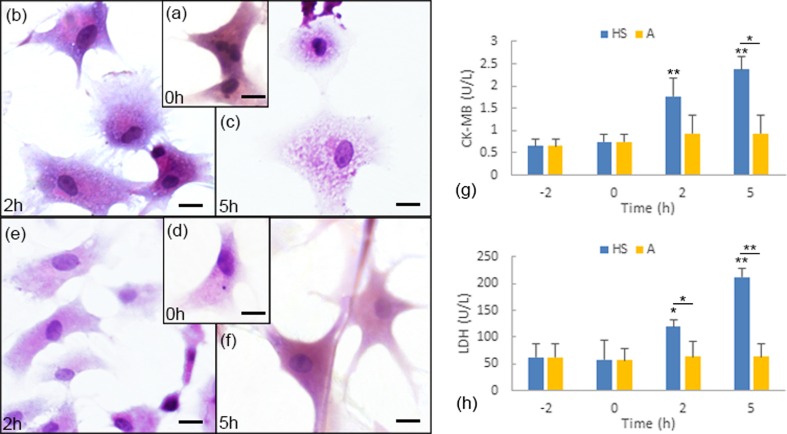
The protective function of aspirin against heat stress has been better presented in our former research (Wu et al. 2016). In this study, we focused on the differences between group HS (HS challenge group) and group A (with aspirin pretreatment before HS) in short-term heat stress response. The activities of CK-MB and LDH in the supernatants of primary cultured myocardial cells, the cytopathological observations, and the flow cytometry assay results clearly indicated the aspirin-induced HS resistance (Figs. 1 and 2). In group HS, compared to the cells before exposure to heat stress (Fig. 1a), the cytoplasm of the myocardial cells after 2 h of exposure to high temperature presented significant granular degeneration; meanwhile, the regionally uneven matrix stained by H&E also indicated acute degeneration in the cytoplasm (Fig. 1b). After 5 h of heat exposure, acute degeneration characterized by swelling, a severely uneven matrix, and numerous tiny granules and vacuoles were observed in the cytoplasm of the myocardial cells in vitro (Fig. 1c). In group A, which was treated with aspirin, no obvious pathological lesions were observed throughout the entire experiment (Fig. 1d, f), with the exception of slight vacuoles in the cytoplasm of the myocardial cells after 2 h of heat exposure (Fig. 1e).
Fig. 1.
Aspirin-induced HS resistance reflected by CK-MB and LDH levels and pathological observation. a–f Representative images of hematoxylin and eosin (H&E) staining of myocardial cells. Scale bar = 10 μm. a Myocardial cells at 0 h in group HS act as the control, and the cells have not yet been exposed to heat stress. b Myocardial cells after 2 h of heat exposure in group HS. c Myocardial cells after 5 h of heat exposure in group A. d Myocardial cells at 0 h in group A; the cells have been treated with aspirin for 2 h but have not been exposed to high temperature. e Myocardial cells after 2 h of heat exposure in group A (treated with aspirin 2 h prior to heat exposure). f Myocardial cells after 5 h of heat exposure in group A (treated with aspirin 2 h prior to heat exposure). g Variations in CK-MB activities in group HS and group A. h Variations in LDH activities in group HS and group A. Enzyme activity at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
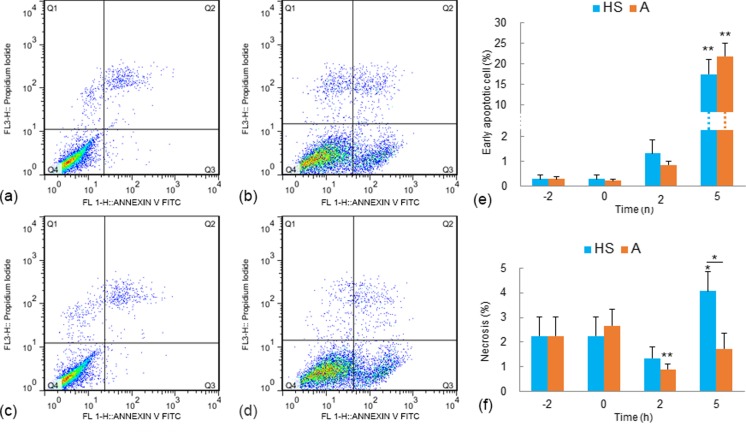
Fig. 2.
Analysis of apoptosis and necrosis in myocardial cells with Annexin V FITC and PI staining. a–d The X-axis concerns cells with positive Annexin V FITC signals, and the Y-axis concerns cells with positive PI signals. a Baseline level (0 h) of myocardial cells in group HS (the cells were treated with neither aspirin nor heat stress). b Myocardial cells after heat exposure for 5 h in group HS. c Myocardial cells at 0 h in group A (the cells were treated with aspirin for 2 h but not exposed to HS). d Myocardial cells after heat exposure for 5 h in group A (cells were treated with aspirin 2 h prior to heat exposure). e The rates of early apoptotic cells in group HS and group A. **P < 0.01; *P < 0.05. f The necrosis rates in group HS and group A. Flow cytometry data at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
The activities of the myocardial cell damage-related enzymes (i.e., CK-MB and LDH) were tested, and the results are shown in Fig. 1g, h. In group HS, comparing to those at −2 h (CK-MB, 0.6502 ± 0.1609 U/L; LDH, 61.7695 ± 26.5847 U/L), the activities of both CK-MB and LDH in the supernatant of the cultured cells increased significantly after the myocardial cells were exposed to high temperature for 2 h (CKMB, 1.7649 ± 0.4257 U/L, P < 0.01; LDH, 119.6110 U/L, P < 0.05) and 5 h (2.3780 ± 0.2927, P < 0.01; 212.6674 U/L, P < 0.01). However, when the cells were treated with aspirin prior to HS (group A), the activities of both CK-MB and LDH did not change significantly from the baseline levels (Fig. 1g, h). Flow cytometry tests were used to assess myocardial cell apoptosis using Annexin V FITC and PI staining (Fig. 2). In both group HS and group A, no obvious sign of apoptosis was detected until the cells were exposed to heat stress for 5 h. The early apoptotic rate of the myocardial cells increased to 17.43 ± 3.656 % from the baseline level (0.2787 ± 0.1690 %) after 5 h of heat exposure in group HS. In group A, the early apoptotic rate of the myocardial cells increased to 21.77 ± 5.661 % after 5 h of heat exposure. The apoptotic rates of group HS and group A after 5 h of heat exposure were not statistically different. The increase in apoptotic cells was limited to the early stage; and no statistically significant changes were detected in the percentages of late apoptotic cells. In addition to the fluctuations in apoptotic rates, the necrosis rates of the tested myocardial cells also varied. In group HS, the percentage of necrosis cells after 5 h of exposure to heat stress was onefold higher than the baseline level (−2 h; Fig. 2f). In contrast, with aspirin pretreatments in group A, the percentage of necrosis cells decreased after 2 h of heat exposure, and the necrosis rate at 5 h remained .unchanged comparing to the baseline levels (Fig. 2f).
Inhibition of aspirin-induced HS resistance by BAPTA-AM
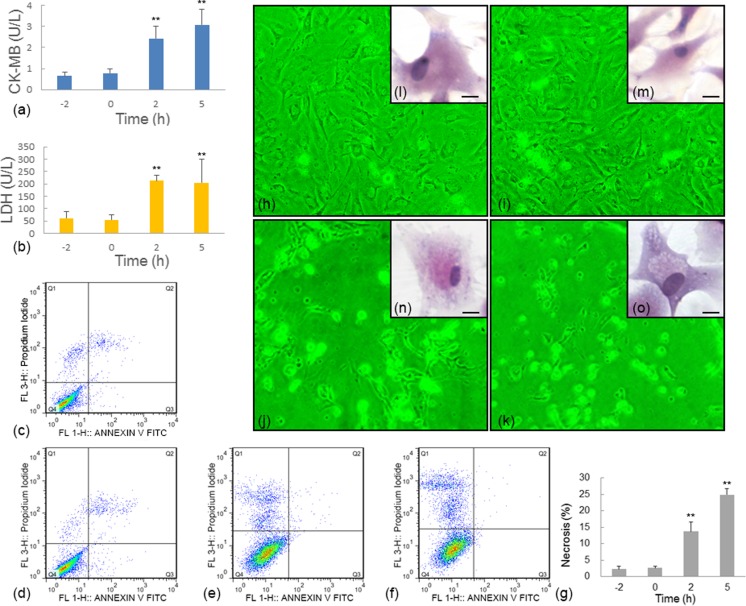
When BAPTA-AM was added, serious HS injury was observed in the myocardial cells, even when the myocardial cells were pretreated with aspirin (group A + B; Fig. 3).
Fig. 3.
HS damage in group A + B was reflected by the activities of myocardial cell damage-related enzymes (CK-MB and LDH), cytopathological observations, and flow cytometry results. a, b Variation in CK-MB (a) and LDH (b) activities in group A + B. c–f Flow cytometry results for myocardial cells with Annexin V FITC and PI staining in group A + B at various time points: −2 (c), 0 (d), 2 (e), and 5 h (f). The X-axis is Annexin V FITC, and the Y-axis is PI. g Rates of necrosis at different time points in group A + B. h–k Representative contrast microscopy images (×40) for group A + B at −2 (h), 0 (i), 2 (j), and 5 h (k). l–o Representative images of hematoxylin and eosin (H&E) staining of myocardial cells in group A + B at −2 (l), 0 (m), 2 (n), and 5 h (o). Scale bar = 10 μm. Enzyme activity and flow cytometry data at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
After treatment with both aspirin and BAPTA-AM for 2 h without HS, the myocardial cells presented no sign of injury. After exposure to HS, the presence of myocardial cell damage-related enzymes along with the flow cytometry and cytopathological results indicated that the cells were seriously damaged. Very significant (P < 0.01) increases in the activities of CK-MB and LDH were observed after heat exposure for 2 h (CK-MB, 2.4151 ± 0.5801 U/L; LDH, 213.4312 ± 22.3136 U/L) and 5 h (CK-MB, 3.0653 ± 0.7373 U/L; LDH, 204.3548 ± 94.7702 U/L) (Fig. 3a, b).
The flow cytometry results revealed that after 2 h of heat exposure, myocardial cell necrosis increased to 5.0-fold and 10.1-fold higher than the baseline level (−2 h), respectively (Fig. 3c–g). Meanwhile, high non-adherent rates were clearly observed after 2 and 5 h of heat exposure (Fig. 3j, k); such high non-adherent rates were not found in group HS and group A. An enhanced apoptotic rate was not detected in group A + B.
Cytopathological studies after H&E staining also indicated that no obvious changes occurred before HS (Fig. 3l, m), while serious cell damage including acute degeneration characterized by granules, uneven matrix staining, and swelling was observed after 2 h of exposure, and structural damage occurred after 5 h (Fig. 3n, o).
Myocardial cell injury with BAPTA-AM pretreatment before HS exposure
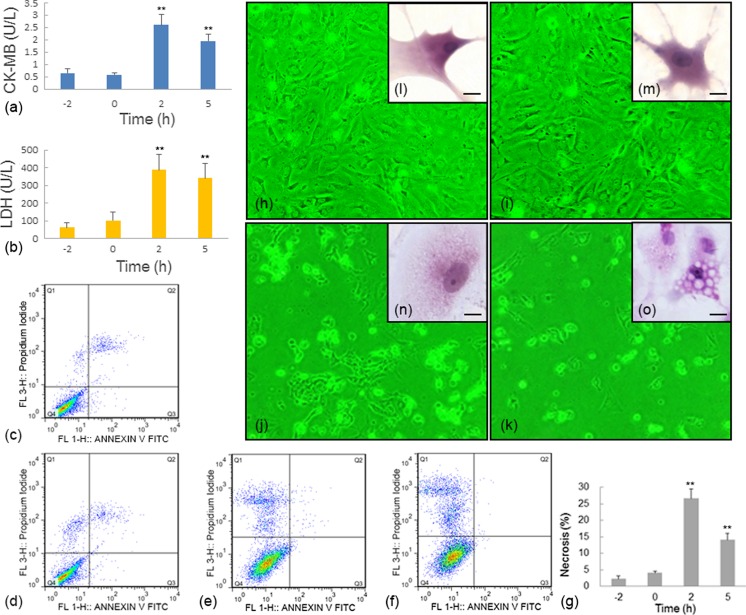
The HS damage in group B, in which BAPTA-AM was administered before HS, was reflected by multiple indicators, including the activities of myocardial cell damage-related enzymes (CK-MB and LDH), cytopathological observations, and flow cytometry assays (Fig. 4).
Fig. 4.
HS damage in group B was reflected by the activities of myocardial cell damage-related enzymes (CK-MB and LDH), cytopathologic observations, and flow cytometry studies. There were no detectable acute changes when the myocardial cells were treated by BAPTA-AM but not exposed to HS (a, b). Variation of CK-MB and LDH in group B, respectively. c–f Flow cytometry studies in myocardial cells with Annexin V FITC and PI staining; X-axis was Annexin V FITC, and Y-axis was PI. c–f The conditions at time points −2, 0, 2, and 5 h in group B, respectively. g Rate of necrosis at different time points in group B. h–k Representative contrast microscopy images (×40). h–k present the myocardial cells at time point −2 h, 0 h, 2 h and 5 h in group B. l–o Representative images of hematoxylin and eosin (H & E) staining of myocardial cells. Scale bar = 10 μm. l–o present the myocardial cells at time points −2, 0, 2, and 5 h in group B. Enzyme activity and flow cytometry data at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
Pretreatment with only BAPTA-AM was harmless to myocardial cells, at least in the short term, and there were no detectable acute changes when the myocardial cells were treated by BAPTA-AM for 2 h but not exposed to HS.
In contrast, compared to the baseline level before HS exposure, the activities of CK-MB and LDH in the supernatants of the cultured myocardial cells increased significantly (P < 0.01) after 2 h (CK-MB, 2.6009 ± 0.4257 U/L; LDH, 388.0273 ± 87.4072 U/L) and 5 h (CK-MB, 1.9507 ± 0.2787 U/L; LDH, 340.3633 ± 85.7462 U/L) of heat exposure in group B (Fig. 4a, b).
Flow cytometry tests with Annexin V FITC and PI staining showed no significant change in the rates of apoptosis or necrosis after pretreatment with BAPTA-AM alone for 2 h (time point 0 h) (Fig. 4c, d, g). However, a very significant increase in the percentage of necrosis cells was observed after HS exposure. The necrosis rates were 10.8-fold and 5.28-fold higher than the baseline level (−2 h) after heat exposure for 2 and 5 h, respectively (Fig. 4e–g). Interestingly, as mentioned above, such high necrosis rates were not observed in group HS and group A. Moreover, when the cells were treated with BAPTA-AM, changes in apoptotic cells were no longer detected in group B.
Large amounts of cells became non-adherent after 2 and 5 h of heat exposure (Fig. 4h–k); this effect also was not observed in group HS and group A. Meanwhile, cytopathological studies also indicated serious cell damage after exposure to high temperature. After 2 h of heat exposure, the myocardial cells were swollen and exhibited acute granular degeneration (Fig. 4n). After 5 h of heat exposure, serious vacuoles were observed in the cell cytoplasm, indicating that structural damage had occurred (Fig. 4o).
Detection of HSPs induced by aspirin pretreatment and inhibited by BAPTA-AM
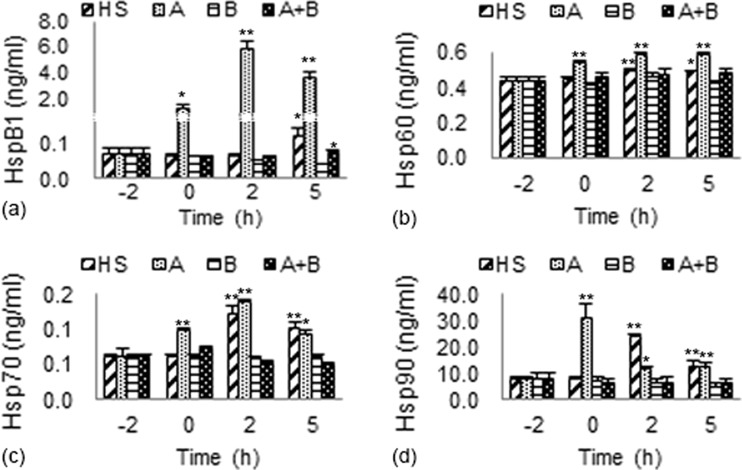
The variations in the levels of the four tested HSPs (HspB1, Hsp60, Hsp70, and Hsp90) in myocardial cells of different treatment groups compared to the baseline levels (−2 h) are shown in Fig. 5.
Fig. 5.
The expression of HSPs (HspB1, Hsp60, Hsp70, and Hsp90) in different groups. a Variations of HspB1 expression in different groups. b Variations of Hsp60 expression in different groups. c Variations of Hsp70 expression in different groups. d Variations of Hsp90 expression in different groups. HSP expressions at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
After exposure to heat stress, the expressions of all the four tested HSPs in myocardial cells of group HS displayed increasing tendencies. A significant increase of HspB1 expression (P < 0.05) was detected only after 5 h of heat exposure. The levels of Hsp60, Hsp70, and Hsp90 in group HS increased significantly at 2 h (P < 0.01, P < 0.01, and P < 0.01, respectively) and 5 h (P < 0.05, P < 0.01, and P < 0.01, respectively).
In group A, after 2 h pretreatment with aspirin prior to the heat stress, the expressions of all the four tested HSPs increased greatly, especially the level of HspB1. After aspirin administration, the expression level of HspB1 in the myocardial cells at 0 h was 20-fold greater than the baseline level (−2 h, 0.06790 ± 0.01502 ng/mL). After 2 h of heat exposure, the HspB1 expression remained at least one order of magnitude higher than the baseline level and reached a maximum of 5.857 ± 0.5844 ng/mL (86-fold greater than the baseline level). The levels of Hsp60 and Hsp70 reached to their maximum values at 2 h and remained significantly higher (at least P < 0.05) than the baseline levels after 5 h of HS. The expression of Hsp90 was also significantly higher (at least P < 0.05) than the baseline level, although the highest expression was observed at 0 h.
However, the changes in the expressions of the four HSPs in group HS and group A were no longer detected when BAPTA-AM was added. The expressions of HspB1, Hsp60, Hsp70, and Hsp90 were not significantly different from their baseline levels at all the time points in group B and group A + B.
Variation of T-SOD and MDA in this experiment
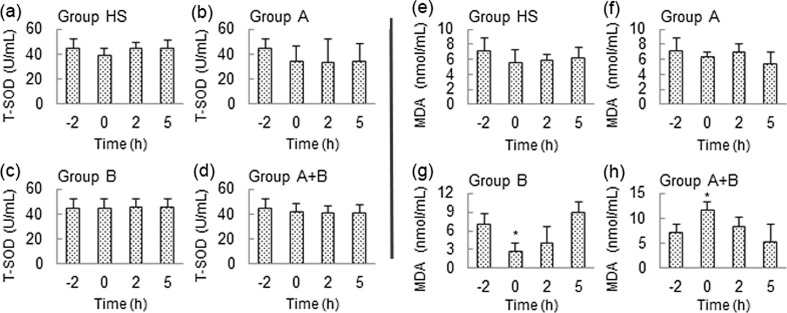
The levels of T-SOD and MDA in the supernatant of the primary cultured myocardial cells in different groups were measured and are shown in Fig. 6. The activities of T-SOD and MDA were mostly the same before and after HS exposure in the different groups, the only exceptions being a reduction in the MDA level at 0 h of HS in group B and an induction at 0 h of HS in group A + B.
Fig. 6.
T-SOD and MDA levels were unchanged before and after HS exposure in the different groups. a–d Variations in T-SOD levels in different groups. e–h Variations in MDA levels in different groups. Data at 0, 2, and 5 h were compared with the baseline level (−2 h). **P < 0.01; *P < 0.05
Discussion
According to a series of markers for injury evaluation, aspirin-induced HS resistance, which has been reported before (Wu et al. 2015, 2016), was also observed in this study. Similar to the former studies, aspirin pretreatment inhibited the increased levels of the myocardial cell damage-related enzymes CK-MB and LDH and the pathological changes. These findings were also supported by the decreased necrosis rate found by the flow cytometry assays. All these demonstrate the potential to develop aspirin as a treatment to prevent HS injury.
A series of biological factors were measured as candidates to evaluate HS injury. In addition to the expression of myocardial cell damage-related enzymes and pathological changes, which have been validated by previous studies in our laboratory (Tang et al. 2013, 2014; Wu et al. 2015), oxidative stress factors like SOD and MDA (Altan et al. 2003; Paul et al. 2009) along with the rates of apoptosis and necrosis (Green 2011; Masri and Chandrashekhar 2008) have also been suggested to respond to HS. However, in the present study, the oxidative stress indicators and apoptosis rate did not accurately reflect the myocardial cell damage. Although the oxidative system is thought to be highly connected to the HS system (Paul et al. 2009), the oxidative stress injury characterized by increases in T-SOD and MDA expression (Chang et al. 2007; Wang et al. 2014) remained unchanged in this study, even when serious injury was clearly observed by microscopy in the myocardial cells. These results suggest that HS alone, or at least a short duration of HS, may not be a sufficient trigger of oxidative stress in chicken’s myocardial cells. Alternatively, the reported changes in T-SOD and MDA found by others in animal models may not be of cardiac origin (Altan et al. 2003). Activation of in vivo oxidation/anti-oxidation systems could be a multiple organ-related consequence of long duration stress exposure.
Likewise, the apoptotic rate detected by flow cytometry assays also did not react reliably to HS injury in this study. Although the protective function of aspirin was clearly demonstrated by the unchanging levels of CK-MB and LDH, the cytopathological results, and the necrosis rates, the myocardial cells exhibited enhanced early apoptotic stages after exposure to HS. This paradoxical phenomenon suggests that the early apoptotic rate is not an appropriate indicator for serious HS injury. Although apoptosis was initially thought to be irreversible (Masri and Chandrashekhar 2008), later evidence indicated otherwise (Dash et al. 2011). In fact, early apoptosis is a relatively moderate symptom and may even confer advantages to the organism’s lifecycle, which is quite different from necrosis or other serious cellular reactions (Green 2011; Keith et al. 2008). The phenomenon is called apoptosis interrupts, which has been widely reported (Narula et al. 2001) and is believed to lead to a range of possibilities other than cell death (Masri and Chandrashekhar 2008). For example, treatment with camptothecin caused cells in Trypanosoma cruzi to be arrested at an early stage instead of progressing to late apoptosis and cell death. Some studies have indicated that DNA repair can be activated in p53-induced apoptosis, leading to the reverse of apoptosis (Zuma et al. 2014). Additionally, another explanation for this seeming contradiction concerns the deficiency of flow cytometry assays with Annexin V staining. This method detects early apoptosis based on the binding between Annexin V and phosphatidylserines, which are present on the cell surface in high levels during early apoptosis. However, many cells that stain positive for Annexin V are viable and can resume growth and reestablish phospholipid asymmetry (Hammill et al. 1999). Therefore, although this technology is widely used to measure apoptosis, using it to demonstrate early apoptosis or heat stress injury is questionable. At the very least, it should not be used as the primary evidence.
Given the above findings, simply measuring T-SOD and MDA and performing the flow cytometry test for the apoptotic rate using Annexin V staining cannot be considered to provide reliable indicators of short-term HS injury in vitro. Instead, the results of this study suggest that the activities of CK-MB and LDH, the results of cytopathological studies, and necrosis rates are more reliable markers.
In addition to the above injury markers, the expressions of HSPs also showed variations after the different treatments in this study. Remarkably, accompanying the resistance to HS injury, the expression of HspB1 increased by over one order of magnitude compared to the baseline level when aspirin was administered prior to HS. This suggests that HspB1 is related to aspirin’s myocardial cell protective function. In fact, the overexpression of HspB1 has been reported to attenuate cardiac dysfunction in transgenic mice (Liu et al. 2007). Although aspirin caused a series of variations in HSP expression, all the effects of HSPs (both under heat stress alone and under aspirin administration before heat stress) were inhibited by treatment with BAPTA-AM. It should be noted that BAPTA-AM suppressed increases of HSP expression rather than totally diminishing HSP expression. Since basic amounts of HSPs were still present to maintain cell survival, the myocardial cells pretreated with BAPTA-AM but not exposed to heat stress (0 h in group B) remained unharmed. And, treatment with BAPTA-AM alone is not a stress factor like high temperature.
In this study, BAPTA-AM inhibited the four tested HSPs as expected. Meanwhile, it also suppressed the aspirin-induced heat stress resistance, suggesting an association between HSP expression and aspirin-induced heat stress resistance. BAPTA-AM is known as an intracellular Ca2+-chelating agent (Kuwabara et al. 2003; Rcom-H’cheo-Gauthier et al. 2014). This leads to a novel but reasonable hypothesis that intracellular Ca2+ might be involved in HSP regulation and helps aspirin-induced HS resistance. Although the complex relations between aspirin and intracellular Ca2+ remain unclear (De Cristobal et al. 2002; Flescher et al. 1991; Rodemann et al. 1982), quite a few studies have indicated that aspirin acts at sites beyond the adenylate cyclase/cAMP system and before the proton pump (Levine et al. 1990). And in fact, many biological effects may be related to such aspirin-induced increases in Ca2+ (Dragomir et al. 2004; Fiorucci et al. 1998). Calcium ion is known to have multiple effects on heat stress response and HSP functions. Intracellular free Ca2+ increased by heat stress could increase the complex formation between heat shock transcriptional factor (HSF) and heat shock elements (HSEs), enhancing the production of the heat-inducible form of Hsp70 (Ding et al. 1996; Kiang et al. 1994). As the HSF-HSE complex is an important upstream trigger for the expression of other HSPs (Morimoto 1993), it is easy to understand how Ca2+ might induce the expression of other HSPs. This proposed mechanism might explain the significant increases in HspB1, Hsp60, Hsp70, and Hsp90 expression observed after aspirin administration in this study. These highly expressed HSPs, particularly HspB1, which was strongly induced by aspirin, exerted their anti-heat stress activities in the chicken myocardial cells. Moreover, increased Ca2+ levels have also been reported to activate kinase or phosphatase, which could lead to the phosphorylation of a series of proteins including HspB1 (Landry et al. 1988). Phosphorylated HspB1 could then transfer to the nucleus, conferring more anti-HS effects (Benn et al. 2002; de Graauw et al. 2005; Geum et al. 2002) as we described in our previous work (Wu et al. 2015).
However, the detailed function of Ca2+ itself and the downstream effects of Ca2+ in aspirin-induced HS still require further investigation. What is beyond doubt is that since HSPs are dynamic anti-stress factors, the inhibition of HSPs by BAPTA-AM at least participates in the suppression of the anti-heat stress function of aspirin. This study revealed that BAPTA-AM is able to terminate aspirin-induced heat stress resistance, and that HSPs may contribute both to aspirin’s protective function and to BAPTA-AM’s inhibiting mechanism. Obviously, multiple HSPs are associated with aspirin’s protective functions, but HspB1 may be the one that contributes the most. This study also further demonstrated the potential value of aspirin pretreatment as an anti-HS measure in poultry breeding. However, more investigations are required before aspirin can be developed into commercial products.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31372403), the Natural Science Foundation of the Jiangsu Province (Grant No. BK20140107), the Postgraduate Student Research and Innovation Project of Jiangsu Province (KYLX15_0558), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Sino-German Agricultural Cooperation Project of the Federal Ministry of Food, the Agriculture and Consumer Production, Berlin, Germany.
References
- Altan O, Pabuccuoglu A, Altan A, Konyalioglu S, Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br Poult Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Amberger A, Hala M, Saurwein-Teissl M, Metzler B, Grubeck-Loebenstein B, Xu Q, Wick G. Suppressive effects of anti-inflammatory agents on human endothelial cell activation and induction of heat shock proteins. Mol Med. 1999;5:117–128. [PMC free article] [PubMed] [Google Scholar]
- Benn SC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/S0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Calabrese V, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci. 2005;233:145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ohwatari N, Matsumoto T, Kosaka M, Ohtsuru A, Yamashita S. TGF-beta1 mediates 70-kDa heat shock protein induction due to ultraviolet irradiation in human skin fibroblasts. Pflugers Arch - Eur J Physiol. 1999;438:239–244. doi: 10.1007/s004240050905. [DOI] [PubMed] [Google Scholar]
- Chang CK, Chang CP, Liu SY, Lin MT. Oxidative stress and ischemic injuries in heat stroke. Prog Brain Res. 2007;162:525–546. doi: 10.1016/S0079-6123(06)62025-6. [DOI] [PubMed] [Google Scholar]
- Das DK, Maulik N, Moraru II. Gene expression in acute myocardial stress. Induction by hypoxia, ischemia, reperfusion, hyperthermia and oxidative stress. J Mol Cell Cardiol. 1995;27:181–193. doi: 10.1016/S0022-2828(08)80017-X. [DOI] [PubMed] [Google Scholar]
- Dash R, et al. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Reson Med. 2011;66:1152–1162. doi: 10.1002/mrm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cristobal J, Cardenas A, Lizasoain I, Leza JC, Fernandez-Tome P, Lorenzo P, Moro MA. Inhibition of glutamate release via recovery of ATP levels accounts for a neuroprotective effect of aspirin in rat cortical neurons exposed to oxygen-glucose deprivation. Stroke; J Cerebr Circ. 2002;33:261–267. doi: 10.1161/hs0102.101299. [DOI] [PubMed] [Google Scholar]
- de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem. 2005;280:29885–29898. doi: 10.1074/jbc.M412708200. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Smallridge RC, Galloway RJ, Kiang JG. Increases in HSF1 translocation and synthesis in human epidermoid A-431 cells: role of protein kinase C and [Ca2+]i. J Invest Med : Off Publ Am Fed Clin Res. 1996;44:144–153. [PubMed] [Google Scholar]
- Dragomir E, Manduteanu I, Voinea M, Costache G, Manea A, Simionescu M. Aspirin rectifies calcium homeostasis, decreases reactive oxygen species, and increases NO production in high glucose-exposed human endothelial cells. J Diabetes Complicat. 2004;18:289–299. doi: 10.1016/j.jdiacomp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ebert MP, et al. Protective role of heat shock protein 27 in gastric mucosal injury. J Pathol. 2005;207:177–184. doi: 10.1002/path.1815. [DOI] [PubMed] [Google Scholar]
- Endo S, et al. Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol. 2007;72:1337–1348. doi: 10.1124/mol.107.039164. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Migliorati G, Santucci L, Morelli O, Federici B, Morelli A. TNFalpha processing enzyme inhibitors prevent aspirin-induced TNFalpha release and protect against gastric mucosal injury in rats. Aliment Pharmacol Ther. 1998;12:1139–1153. doi: 10.1046/j.1365-2036.1998.00409.x. [DOI] [PubMed] [Google Scholar]
- Flescher E, Fossum D, Gray PJ, Fernandes G, Harper MJ, Talal N. Aspirin-like drugs prime human T cells. J Immunol. 1991;146:2553–2559. [PubMed] [Google Scholar]
- Gathiram P, Gaffin SL, Brock-Utne JG, Wells MT. Time course of endotoxemia and cardiovascular changes in heat-stressed primates. Aviat Space Environ Med. 1987;58:1071–1074. [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Raidoo D, Brock-Utne JG, Gaffin SL. Portal and systemic plasma lipopolysaccharide concentrations in heat-stressed primates. Circ Shock. 1988;25:223–230. [PubMed] [Google Scholar]
- Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem. 2002;277:19913–19921. doi: 10.1074/jbc.M104396200. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Nutt MP, Hardy SP. Heat shock protein and high-dose aspirin: effects on random skin flap survival in a rat model. Ann Plast Surg. 2002;48:60–67. doi: 10.1097/00000637-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Green DR (2011) Means to an end: apoptosis and other cell death mechanisms. Cold Spring Harbor Laboratory Press
- Hammill AK, Uhr JW, Scheuermann RH. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res. 1999;251:16–21. doi: 10.1006/excr.1999.4581. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Kaida T, et al. Vasopressin stimulates the induction of heat shock protein 27 and alphaB-crystallin via protein kinase C activation in vascular smooth muscle cells. Exp Cell Res. 1999;246:327–337. doi: 10.1006/excr.1998.4277. [DOI] [PubMed] [Google Scholar]
- Keith A, Alexander J, Julian L, Martin R, Walter R (2008) Apoptosis: programmed cell death eliminates unwanted cells Molecular biology of the cell, 5th edn Garland Science, New York 1115
- Kiang JG. Genistein inhibits herbimycin A-induced over-expression of inducible heat shock protein 70 kDa. Mol Cell Biochem. 2003;245:191–199. doi: 10.1023/A:1022836616452. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Carr FE, Burns MR, McClain DE. HSP-72 synthesis is promoted by increase in [Ca2+] i or activation of G proteins but not pHi or cAMP. Am J Phys Cell Phys. 1994;267:C104–C114. doi: 10.1152/ajpcell.1994.267.1.C104. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Gist ID, Tsokos GC. Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells. Mol Cell Biochem. 2000;204:169–178. doi: 10.1023/A:1007016822939. [DOI] [PubMed] [Google Scholar]
- Kuwabara M, Takahashi K, Inanami O. Induction of apoptosis through the activation of SAPK/JNK followed by the expression of death receptor Fas in X-irradiated cells. J Radiat Res. 2003;44:203–209. doi: 10.1269/jrr.44.203. [DOI] [PubMed] [Google Scholar]
- Landry J, Crete P, Lamarche S, Chretien P. Activation of Ca2+-dependent processes during heat shock: role in cell thermoresistance. Radiat Res. 1988;113:426–436. doi: 10.2307/3577240. [DOI] [PubMed] [Google Scholar]
- Levine RA, Nandi J, King RL. Aspirin potentiates prestimulated acid secretion and mobilizes intracellular calcium in rabbit parietal cells. J Clin Invest. 1990;86:400–408. doi: 10.1172/JCI114725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Srivastava P (2004) Heat-shock proteins Current protocols in immunology / edited by John E Coligan [et al.] Appendix 1:Appendix 1T doi:10.1002/0471142735.ima01ts58 [DOI] [PubMed]
- Liu L, et al. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:762–769. doi: 10.1016/j.ejheart.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang K, Li H, Han S, Ma Z, Tu P. Extracts from Astragalus membranaceus limit myocardial cell death and improve cardiac function in a rat model of myocardial ischemia. J Ethnopharmacol. 2013;149:720–728. doi: 10.1016/j.jep.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Masri C, Chandrashekhar Y. Apoptosis: a potentially reversible, meta-stable state of the heart. Heart Fail Rev. 2008;13:175–179. doi: 10.1007/s10741-007-9069-3. [DOI] [PubMed] [Google Scholar]
- Mearow KM, Dodge ME, Rahimtula M, Yegappan C. Stress-mediated signaling in PC12 cells—the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem. 2002;83:452–462. doi: 10.1046/j.1471-4159.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Narula J, Arbustini E, Chandrashekhar Y, Schwaiger M. Apoptosis and the systolic dysfunction in congestive heart failure. Cardiol Clin. 2001;19:113–126. doi: 10.1016/S0733-8651(05)70198-3. [DOI] [PubMed] [Google Scholar]
- Nuss JE, et al. Multi-faceted proteomic characterization of host protein complement of Rift Valley fever virus virions and identification of specific heat shock proteins, including HSP90, as important viral host factors. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–919. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure, function, and mechanism of the Hsp90 molecular chaperone. Adv Protein Chem. 2001;59:157–186. doi: 10.1016/S0065-3233(01)59005-1. [DOI] [PubMed] [Google Scholar]
- Rai UC, Ambwany P. Cardiovascular changes during varied thermal stress. Indian J Physiol Pharmacol. 1980;24:119–125. [PubMed] [Google Scholar]
- Ranford JC, Coates AR, Henderson B. Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev Mol Med. 2000;2:1–17. doi: 10.1017/S1462399400002015. [DOI] [PubMed] [Google Scholar]
- Rcom-H’cheo-Gauthier A, Goodwin J, Pountney DL. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules. 2014;4:795–811. doi: 10.3390/biom4030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann HP, Waxman L, Goldberg AL. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982;257:8716–8723. [PubMed] [Google Scholar]
- Sandoval-Montiel AA, Zentella-de-Pina M, Ventura-Gallegos JL, Frias-Gonzalez S, Lopez-Macay A, Zentella-Dehesa A. HSP-72 accelerated expression in mononuclear cells induced in vivo by acetyl salicylic acid can be reproduced in vitro when combined with H2O2. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S. Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol. 2013;165:494–498. doi: 10.1016/j.ijcard.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. 2002;162:1099–1104. doi: 10.1001/archinte.162.10.1099. [DOI] [PubMed] [Google Scholar]
- Tang S, et al. Localization and expression of Hsp27 and alphaB-crystallin in rat primary myocardial cells during heat stress in vitro. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Lv Y, Chen H, Adam A, Cheng Y, Hartung J, Bao E. Comparative analysis of alphaB-crystallin expression in heat-stressed myocardial cells in vivo and in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H, Kozawa O, Niwa M, Matsuno H, Kato K, Uematsu T. Mechanism of prostaglandin E2-stimulated heat shock protein 27 induction in osteoblast-like MC3T3-E1 cells. J Endocrinol. 2002;172:271–281. doi: 10.1677/joe.0.1720271. [DOI] [PubMed] [Google Scholar]
- Wang X, Yuan B, Dong W, Yang B, Yang Y, Lin X, Gong G. Induction of heat-shock protein 70 expression by geranylgeranylacetone shows cytoprotective effects in cardiomyocytes of mice under humid heat stress. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele H, Muller L, Buchner J. Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18:225–228. doi: 10.1016/S0899-9007(01)00796-1. [DOI] [PubMed] [Google Scholar]
- Wu D, et al. Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperones. 2015 doi: 10.1007/s12192-015-0596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. In vitro evaluation of aspirin-induced HspB1 against heat stress damage in chicken myocardial cells. Cell Stress Chaperones. 2016 doi: 10.1007/s12192-016-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue HC, Li ZX, Zheng WW, Guo YZ, Feng DY, Liu JW. Injuries of myocardial cells and changes of myocardial enzymes after firearm wound-induced intestinal perforation in porcine abdomen. Int J Clin Exp Med. 2015;8:2273–2278. [PMC free article] [PubMed] [Google Scholar]
- Yoshimune K, Yoshimura T, Nakayama T, Nishino T, Esaki N. Hsc62, Hsc56, and GrpE, the third Hsp70 chaperone system of Escherichia coli. Biochem Biophys Res Commun. 2002;293:1389–1395. doi: 10.1016/S0006-291X(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Zuma AA, Mendes IC, Reignault LC, Elias MC, de Souza W, Machado CR, Motta MC. How Trypanosoma cruzi handles cell cycle arrest promoted by camptothecin, a topoisomerase I inhibitor. Mol Biochem Parasitol. 2014;193:93–100. doi: 10.1016/j.molbiopara.2014.02.001. [DOI] [PubMed] [Google Scholar]