Abstract
Few effective therapies exist for the treatment of neurodegenerative diseases that have been characterized as protein misfolding disorders. Upregulation of heat shock proteins (Hsps) mitigates against the accumulation of misfolded, aggregation-prone proteins and synaptic dysfunction, which is recognized as an early event in neurodegenerative diseases. Enhanced induction of a set of Hsps in differentiated human SH-SY5Y neuronal cells was observed following co-application of celastrol and arimoclomol, compared to their individual application. The dosages employed did not affect cell viability or neuronal process morphology. The induced Hsps included the little studied HSPA6 (Hsp70B’), a potentially neuroprotective protein that is present in the human genome but not in rat and mouse and hence is missing in current animal models of neurodegenerative disease. Enhanced induction of HSPA1A (Hsp70-1), DNAJB1 (Hsp40), HO-1 (Hsp32), and HSPB1 (Hsp27) was also observed. Celastrol activates heat shock transcription factor 1 (HSF1), the master regulator of Hsp gene transcription, and also exhibits potent anti-inflammatory and anti-oxidant activities. Arimoclomol is a co-activator that prolongs the binding of activated HSF1 to heat shock elements (HSEs) in the promoter regions of inducible Hsp genes. Elevated Hsp levels peaked at 10 to 12 h for HSPA6, HSPA1A, DNAJB1, and HO-1 and at 24 h for HSPB1. Co-application of celastrol and arimoclomol induced higher Hsp levels compared to heat shock paired with arimoclomol. The co-application strategy of celastrol and arimoclomol targets multiple neurodegenerative disease-associated pathologies including protein misfolding and protein aggregation, inflammatory and oxidative stress, and synaptic dysfunction.
Keywords: HSPA6 (Hsp70B’), HSPA1A (Hsp70-1), Human SH-SY5Y neuronal cells, Heat shock, Celastrol, Arimoclomol
Introduction
As average life expectancy increases worldwide, there has been an elevation in the prevalence of neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases (Martin 1999; Chen and Brown 2007; Lang 2010; Dunkel et al. 2012). Despite numerous clinical trials, few effective therapies for neurodegenerative diseases have been identified (Dunkel et al. 2012; Pratt et al. 2015). This may be due to deficiencies in current animal models to encompass the complexity of the human brain (Lang 2010; t Hart et al. 2012; McGonigle and Ruggeri 2014; Sasaki 2015). Furthermore, the progression of neurodegenerative diseases is multifactorial; hence, targeted inhibition of a single disease pathology is compensated by concurrent deleterious pathways (Cavalli et al. 2008; Lang 2010; Dunkel et al. 2012; Huang and Mucke 2012; Sheikh et al. 2013). Multidrug therapies that target several aspects of disease pathology are gaining attention and may provide more effective avenues for treating multipathology diseases (Cavalli et al. 2008; Lang 2010; Dunkel et al. 2012; Huang and Mucke 2012; Sheikh et al. 2013; Veloso et al. 2013a, b, 2014). Additionally, there is an urgent need for treatment strategies that impact early stages of disease progression, such as synaptic dysfunction (DeKosky and Marek 2003; Lang 2010; Dunkel et al. 2012; Stephan et al. 2012; Chung et al. 2015).
Neurodegenerative diseases have been characterized as “protein misfolding disorders” because a common underlying pathology is the accumulation of misfolded, aggregation-prone proteins that disrupt normal cell function leading to cell death (Muchowski and Wacker 2005; Westerheide and Morimoto 2005; Asea and Brown 2008; Richter et al. 2010). Heat shock proteins (Hsps) represent a line of defense against misfolded proteins (Westerheide and Morimoto 2005; Richter et al. 2010). Upregulating Hsps is a potential strategy to combat disease pathology and improve clinical outcome for neurodegenerative disease patients (Muchowski and Wacker 2005; Asea and Brown 2008; Pratt et al. 2015).
Overexpression of Hsps confers neuroprotective benefits in a number of animal models of neurodegenerative disease including Parkinson’s disease (Klucken et al. 2004; Shen et al. 2005), amyotrophic lateral sclerosis (ALS) (Gifondorwa et al. 2007; Kalmar et al. 2008), Alzheimer’s disease (Hoshino et al. 2011), and polyglutamine disease (Labbadia et al. 2012). In addition to mitigating against protein misfolding and aggregation, Hsps carry out a number of other neuroprotective functions that include inhibiting apoptosis (Kennedy et al. 2014), as well as protecting the functional integrity of synapses (Karunanithi et al. 1999, 2002; Asea and Brown 2008; Brown 2008; Karunanithi and Brown 2015), which are adversely affected in early stages of neurodegenerative diseases (Masliah et al. 2001; Scheff et al. 2006; Milnerwood and Raymond 2010; Stephan et al. 2012; Chung et al. 2015; Wu et al. 2015). Functional activities of Hsps, such as protein refolding (Fan et al. 2003; Goloubinoff and De Los Rios 2007; Mayer 2013; Mattoo and Goloubinoff 2014; Dekker et al. 2015) and disruption of protein aggregates (Nillegoda and Bukau 2015; Nillegoda et al. 2015), require co-operation between several different classes of Hsps. Therefore, upregulation of a set of Hsps by activation of heat shock transcription factor 1 (HSF1), the master regulator of heat shock gene transcription, is more effective than genetic manipulation of individual Hsps (Liu et al. 2005; Batulan et al. 2006; Asea and Brown 2008).
Celastrol induces Hsps in neuronal cells (Chow and Brown 2007) and is neuroprotective in a number of animal models of neurodegenerative disease including ALS (Kiaei et al. 2005), Parkinson’s disease (Cleren et al. 2005), polyglutamine expansion disease (Zhang and Sarge 2007), and Alzheimer’s disease (Paris et al. 2010). Celastrol also exhibits potent anti-inflammatory and anti-oxidant properties (Allison et al. 2001; Jung et al. 2007; Faust et al. 2009; Kim et al. 2009; Venkatesha et al. 2012; Wong et al. 2012; Yang et al. 2014; Sharma et al. 2015). This may provide additional benefits to neurodegenerative disease patients, as inflammation and oxidative stress are associated with disease pathology and are thought to exacerbate disease progression (Gao and Hong 2008; Amor et al. 2010, 2014).
Another compound that modulates the heat shock response is arimoclomol, which has been shown to improve motor performance and extend lifespan in SOD1G93A mice, a transgenic animal model of ALS (Kieran et al. 2004; Goloubinoff and De Los Rios 2007; Kalmar et al. 2008, 2014; McGoldrick et al. 2013; Poppe et al. 2014). Arimoclomol is a co-inducer of Hsps that prolongs the binding of activated HSF1 to heat shock elements (HSEs) in the promoter regions of heat shock genes (Hargitai et al. 2003; Kieran et al. 2004; Kalmar et al. 2014). Interestingly, arimoclomol is currently in human clinical trials for ALS (ClinicalTrials.gov identifier: NCT00706147) and is well tolerated in ALS patients up to 300 mg/day (Genc and Ozdinler 2013).
In the present report, we evaluate Hsp expression and cell viability in differentiated human SH-SY5Y neuronal cells following co-application of celastrol and arimoclomol. Neurodegenerative diseases are protein misfolding disorders of the adult nervous system that affect differentiated neurons. SH-SY5Y cells were therefore differentiated to induce formation of neuronal processes (Jacobs et al. 2006; Ross and Spengler 2007; Cheung et al. 2009). Our results demonstrate that co-application of celastrol and arimoclomol, at concentrations that do not affect cell viability or neuronal process morphology, enhances expression of a set of Hsps that includes the little studied HSPA6 that is present in the human genome but not in rat and mouse genomes. This co-application strategy targets multiple aspects of disease pathology including protein misfolding and protein aggregation, inflammatory and oxidative stress, and synaptic dysfunction that occurs at early stages of neurodegenerative diseases.
Materials and methods
Cell culture and neuronal differentiation
Human SH-SY5Y cells (American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and cultured at 37 °C in a humidified 5 % CO2 atmosphere. Cells, plated at 4.5 × 104 cells per square centimeter, were allowed to settle onto the growth surface for 24 h. Culture plates (10 cm) were used for Western blotting experiments or 22 × 22-mm coverslips placed inside six-well plates for immunofluorescence experiments. Neuronal differentiation was induced by a treatment with 10 μM all-trans-retinoic acid (R2625; Sigma-Aldrich, St. Louis, MO, USA) for 72 h under serum-free conditions.
Induction of Hsps
Following 72 h of differentiation, media containing all-trans-retinoic acid were removed and replaced with fresh serum-free DMEM with or without celastrol and arimoclomol. Celastrol (70950; Cayman Chemical, Ann Arbor, MI, USA) dissolved in DMSO was added directly to the media. Arimoclomol (gift from Professor Michael Cheetham, Institute of Ophthalmology, University College London, UK) was prepared fresh for each experiment by dissolving in an appropriate volume of serum-free DMEM and filtering (0.2-μm pore size). The cells were incubated at the indicated concentrations for 24 h unless otherwise specified. DMSO treatment alone was used as a vehicle control for celastrol.
Heat shock
Cells were immersed in a circulating water bath calibrated at 43 °C ± 0.1 °C for 20 min (Chow et al. 2010) and returned to a humidified 5 % CO2 atmosphere at 37 °C for 24 h before being harvested for Western blotting. The start of the 20-min heat shock represents the zero time point (t = 0). Arimoclomol was added before (HS1; t = 0) or after (HS2; t = 20 min) heat shock.
Viability assay
For qualitative analysis of cell viability by fluorescence microscopy, cells were stained with 10 μg/mL propidium iodide (P4864; Sigma-Aldrich) to identify compromised cells (pink) and then fixed with 4 % paraformaldehyde (PFA) for 30 min. PFA was purchased from Canemco Inc. (0173; Canton de Gore, QC, CA). Hoechst 33258 (94403; Sigma-Aldrich) at 0.5 μg/mL was used as a counterstain for nuclei. For analysis of neuronal process morphology, cells were fixed as above and permeabilized in 0.1 % Triton X-100 with 100 mM glycine in PBS. The cells were blocked for 1 h in 5 % FBS in PBS and incubated with primary antibody against α-tubulin (ab18251; Abcam, Toronto, ON, CA) in 1 % FBS in PBS overnight at 4 °C. Donkey anti-rabbit AlexaFluor488-conjugated secondary antibody (Molec. Probes, Life Tech., Burlington, ON, CA) was used. DNA was stained with 300 nM DAPI (Invitrogen, Life Technologies). Cells were imaged by structured illumination microscopy using an AxioCam HRm camera with an ApoTome module on an AxioVert 200M microscope (Carl Zeiss, Toronto, ON, CA).
For quantitative analysis, cells were harvested at the indicated time point after single or co-application of celastrol and arimoclomol. An equal volume of cell suspension was mixed with 0.4 % trypan blue (T10282; Life Technologies) and incubated for 2 min. The cell suspension was loaded into a disposable Countess® cell counting chamber slide, and the percent of cells stained by trypan blue was quantified using a Countess® automated cell counter (C10281; Invitrogen, Life Technologies).
Western blotting and densitometry
At the indicated time points following the application of celastrol and/or arimoclomol, the cells were harvested, dissolved in Laemmli buffer, and boiled for 10 min. Protein quantification was carried out using the RC DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Thirty micrograms of protein was loaded into each lane and separated on a 12 % SDS-PAGE gel using the Mini-PROTEAN 3 Electrophoresis Module (Bio-Rad Laboratories) with a 4 % stacking gel. Proteins were transferred to nitrocellulose membranes using the Mini Trans-Blot® Module (Bio-Rad Laboratories). Primary antibodies for HSPA1A (SPA-810), HSPA6 (SPA-754), HSPA8 (SPA-815), HSPH1 (SPA-1101), HSPC1 (SPS-771), DNAJB1 (SPA-400), HSPB1 (SPA-803), and Hsp32 (OSA-110) were purchased from Enzo Life Sciences (Farmingdale, NY, USA). Primary antibody for β-tubulin (MAB3408) was purchased from EMD Millipore (Billerica, MA, USA). Horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) were detected using enhanced chemiluminescence (Amersham, Piscataway, NJ, USA). Densitometry was performed using Quantity One® 1-D Analysis software (Bio-Rad Laboratories).
Statistical analysis
GraphPad Prism 5 software was employed for data analysis. Student’s t test was used to test for statistical significance. A p value of less than 0.05 (or 0.01 where indicated) was considered statistically significant. Data represent the mean ± the standard error of the mean (SEM) for three independent replicates. For Hsp protein levels, optical densities were normalized and plotted as the difference relative to the vehicle control condition.
Results
Co-application of celastrol and arimoclomol enhances induction of a set of Hsps in differentiated human neuronal cells
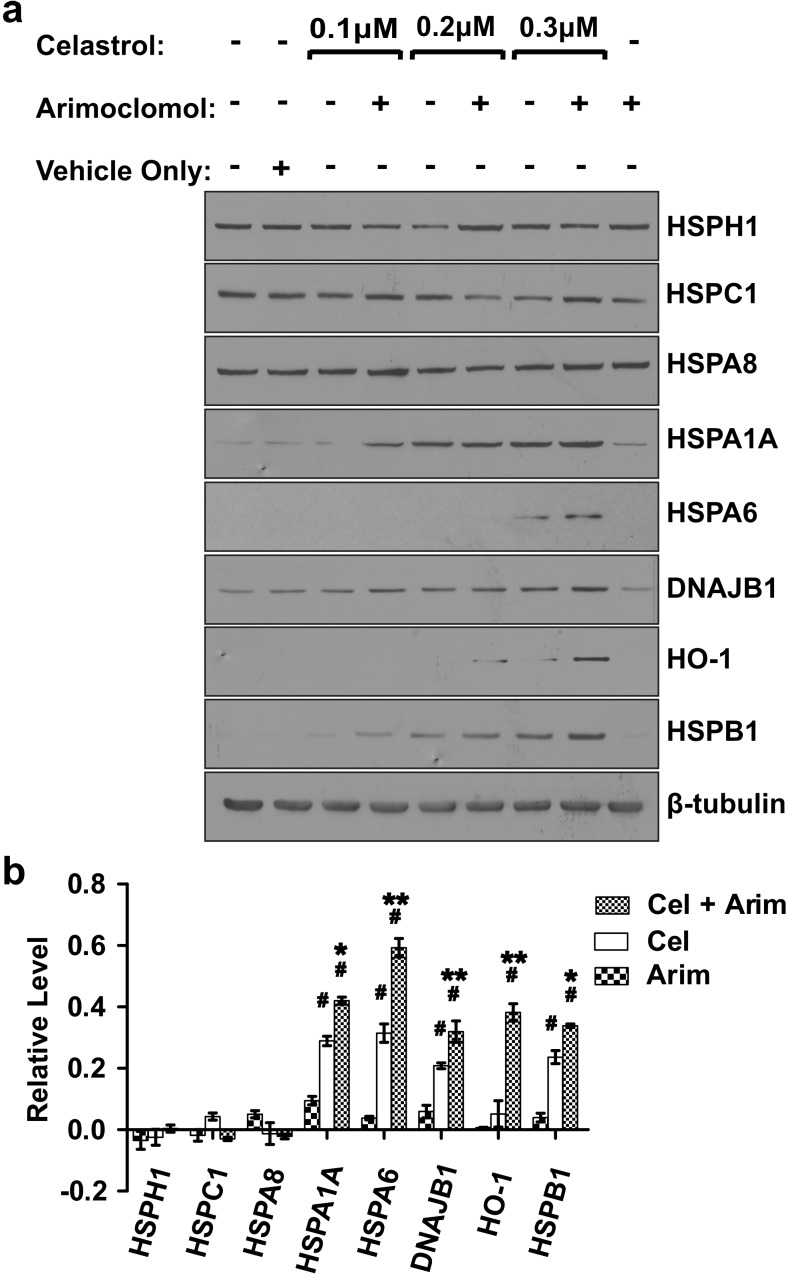
Differentiated human SH-SY5Y neuronal cells were treated with celastrol, plus or minus arimoclomol. As shown in Fig. 1a, inclusion of arimoclomol with celastrol enhanced the induction of several Hsps, compared to celastrol alone. This included the little studied HSPA6 that is found in the human genome but not in the genomes of rat and mouse and hence is lacking in current animal models of neurodegenerative diseases (Chow and Brown 2007; Noonan et al. 2007a, b; Chow et al. 2010). In addition to HSPA6, enhanced induction was also observed following co-application of celastrol and arimoclomol for HSPA1A (Hsp70-1), DNAJB1 (Hsp40), HO-1 (Hsp32), and HSPB1 (Hsp27). Induction of HSPA1A and HSPB1 was detected at 0.1 μM celastrol plus arimoclomol, whereas induction of HSPA6 was observed at 0.3 μM celastrol plus arimoclomol. Induction was not observed for constitutively expressed HSPH1 (Hsp105), HSPC1 (Hsp90a), and HSPA8 (Hsc70). As shown in Fig. 1b, statistically significant enhanced induction of HSPA6, HSPA1A, HSPB1, DNAJB1, and HO-1 was observed following co-application of 0.3 μM celastrol and 250 μM arimoclomol, compared to either celastrol or arimoclomol alone.
Fig. 1.
Enhanced induction of a set of Hsps by co-application of celastrol and arimoclomol to differentiated human neuronal cells. a Differentiated SH-SY5Y neuronal cells were treated with celastrol (0.1–0.3 μM) with or without arimoclomol (250 μM). Cells were harvested after 24 h and Hsps examined by Western blotting. β-Tubulin was used as a loading control. b Quantification of Hsp levels relative to vehicle control for co-application of 0.3 μM celastrol plus 250 μM arimoclomol (Cel + Arim), 0.3 μM celastrol (Cel), and 250 μM arimoclomol (Arim). Induction (#p < 0.01) of HSPA6, HSPA1A, HO-1, HSPB1, and DNAJB1 was seen following application of celastrol alone or in combination with arimoclomol. Significant enhanced induction (*p < 0.05; **p < 0.01) was observed following co-application of celastrol and arimoclomol, compared to celastrol alone. Levels of constitutively expressed HSPH1, HSPC1, and HSPA8 did not change with celastrol and arimoclomol application alone or in combination
Enhanced induction of Hsps is maintained using a fivefold lower concentration of arimoclomol
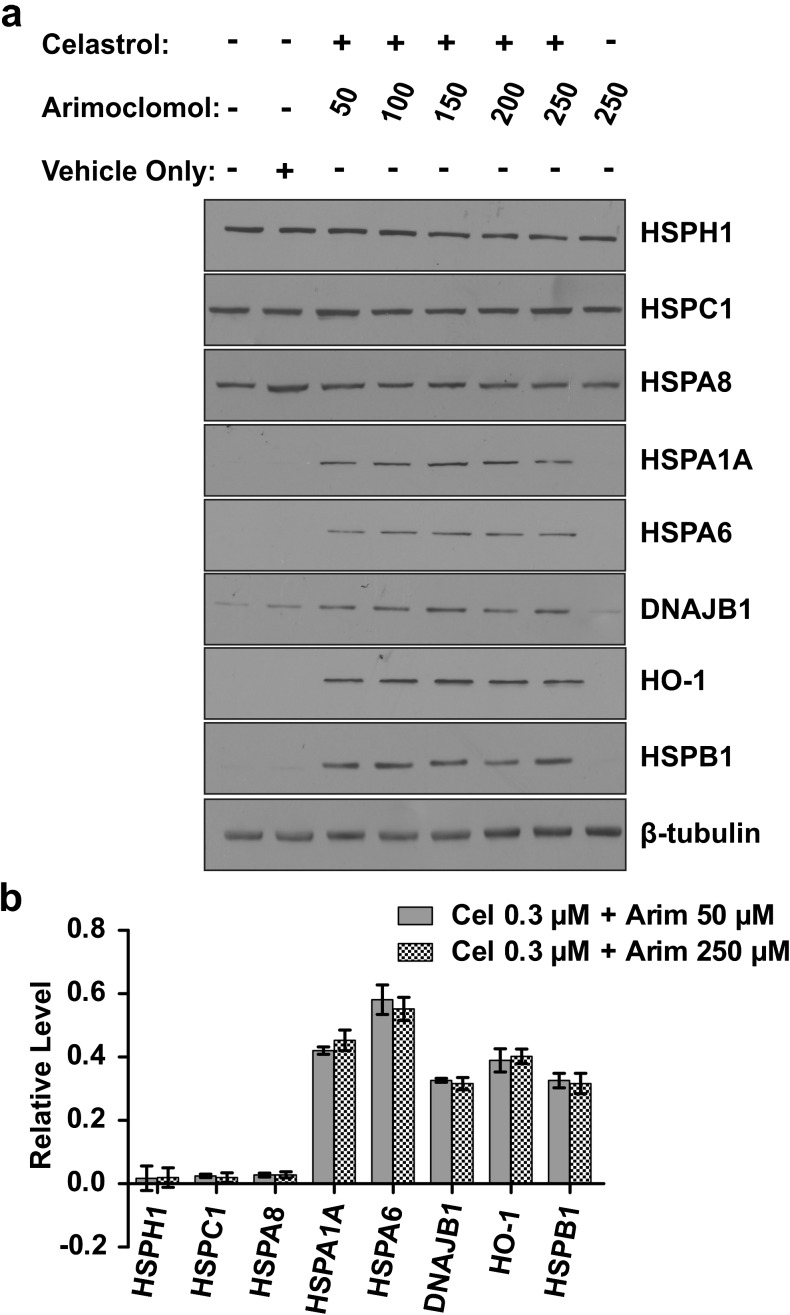
To determine whether enhanced induction of Hsps could be achieved using a lower concentration of arimoclomol, differentiated human neuronal cells were co-treated with 0.3 μM celastrol plus or minus arimoclomol at concentrations ranging from 50 to 250 μM. As shown in Fig. 2, quantitative analysis demonstrated that levels of induction of HSPA6, HSPA1A, DNAJB1, HO-1, and HSPB1 were maintained when the arimoclomol concentration is lowered fivefold from 250 to 50 μM. Levels of constitutively expressed HSPH1, HSPC1, and HSPA8 were not affected.
Fig. 2.
Hsp induction maintained when the concentration of arimoclomol decreased fivefold. a Hsp induction profile following co-application of celastrol (0.3 μM) and arimoclomol (50, 100, 150, 200, or 250 μM). b Comparable levels of Hsp induction were observed when arimoclomol concentration was reduced from 250 to 50 μM for HSPA6, HSPA1A, DNAJB1, HO-1, and HSPB1
Time course of neuronal induction of Hsps after co-application of celastrol and arimoclomol
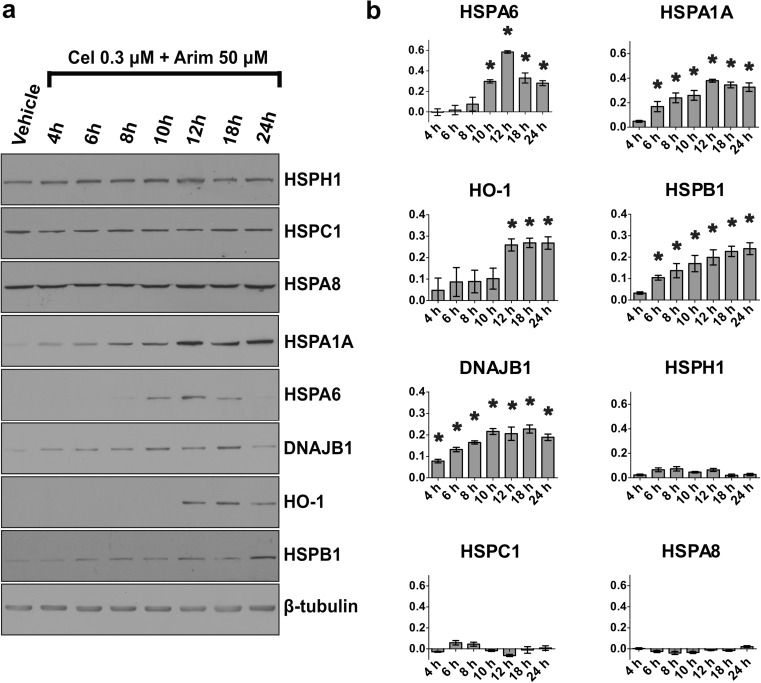
Inducible members of the HSPA family, HSPA6 and HSPA1A, demonstrated maximal levels at 12 h after co-application of 0.3 μM celastrol with 50 μM arimoclomol. As shown quantitatively in Fig. 3, HSPA6 levels declined at 18 h, whereas HSPA1A levels were maintained. HO-1 attained maximal levels at 12 to 24 h. HSPB1 increased progressively from 6 to 24 h, while DNAJB1 reached maximal levels at 10 h. Levels of constitutively expressed HSPH1, HSPC1, and HSPA8 did not show a significant change.
Fig. 3.
Time course of neuronal induction of Hsps after co-application of celastrol and arimoclomol. a Co-application of celastrol (0.3 μM) and arimoclomol (50 μM) to differentiated human SH-SY5Y neuronal cells. b Quantification of Hsp levels relative to vehicle control (*p < 0.05). Peak induction of HSPA6, HSPA1A, DNAJB1, and HO-1 was observed at 10–12 h. Levels of constitutively expressed HSPH1, HSPC1, and HSPA8 did not change. Y-axis = relative Hsp level
Comparison of celastrol and heat shock to induce neuronal Hsps when paired with arimoclomol
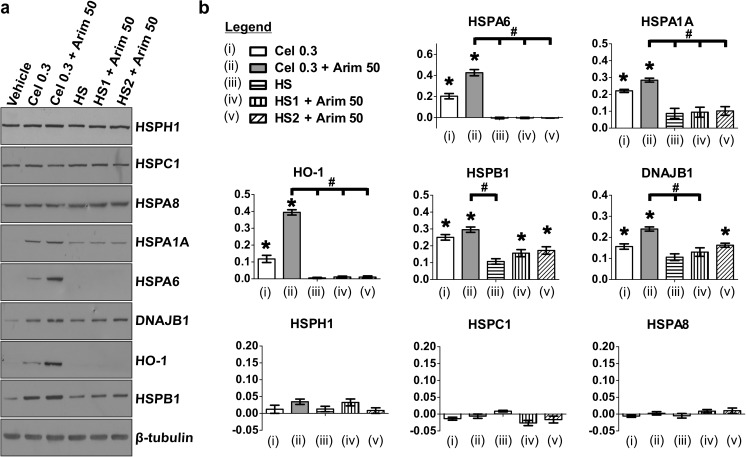
Celastrol activates HSF1 monomers to a trimerized form that binds to HSEs in the promoter regions of inducible heat shock genes resulting in their upregulation (Westerheide et al. 2004; Salminen et al. 2010). Heat shock is the classical activator of HSF1, the master regulator of Hsp induction (Morimoto 1993; Sarge et al. 1993). Celastrol and heat shock were compared in their ability to induce Hsps in differentiated human neuronal cells when coupled with arimoclomol as co-inducer. As shown quantitatively in Fig. 4, co-application of celastrol and arimoclomol at 0.3 and 50 μM, respectively, resulted in an enhanced induction of HSPA6 compared to celastrol alone. However, heat shock applied concurrently with arimoclomol (HS1), or immediately preceding arimoclomol (HS2), resulted in no detectable induction of HSPA6. Similarly, co-application of celastrol and arimoclomol resulted in a greater induction of HSPA1A, HO-1, HSPB1, and DNAJB1 compared to heat shock paired with arimoclomol. Hence, celastrol co-applied with arimoclomol is a more efficient strategy for Hsp upregulation in differentiated human neuronal cells compared to heat shock paired with arimoclomol. Upregulation was not observed of constitutively expressed HSPH1, HSPC1, or HSPA8 following celastrol plus arimoclomol or heat shock plus arimoclomol.
Fig. 4.
Neural induction of Hsps by co-application of celastrol and arimoclomol compared to heat shock paired with arimoclomol. a Hsp induction profile following co-application of celastrol (0.3 μM) and arimoclomol (50 μM) or 43 °C heat shock for 20 min (HS) plus or minus arimoclomol (50 μM). Arimoclomol was added either concurrently (HS1) or subsequent to (HS2) heat shock. b Quantification of Hsp levels relative to vehicle control (*p < 0.01). Higher levels (#p < 0.05) of HSPA6, HSPA1A, HO-1, HSPB1, and DNAJB1 were observed following co-application of celastrol and arimoclomol compared to heat shock plus arimoclomol. Y-axis = relative Hsp level
Effect of celastrol and arimoclomol co-application on the viability of differentiated human neuronal cells
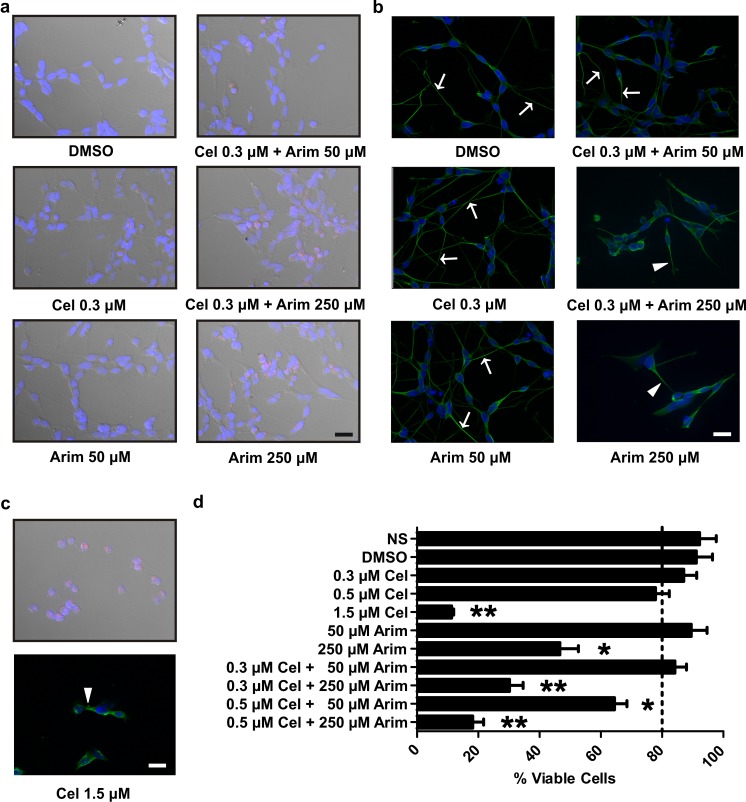
Viability assays were conducted in order to determine the effect of co-application of celastrol and arimoclomol on differentiated human neuronal cells at dosages that resulted in enhanced Hsp induction. Propidium iodide (PI) dye exclusion microscopy showed that neuronal cell viability was observed following co-application of celastrol and arimoclomol at 0.3 and 50 μM, respectively, but severely impacted if arimoclomol was increased to 250 μM in combination with 0.3 μM celastrol (Fig. 5a, cells with PI uptake indicated in pink). Arimoclomol alone at 250 μM, or increasing celastrol to 1.5 μM (Fig. 5c), resulted in cellular uptake of PI, whereas comparatively little PI uptake was observed for arimoclomol or celastrol alone at 50 and 0.3 μM (Fig. 5a). As shown in Fig. 5d, quantification employing cellular uptake of trypan blue revealed that 84.2 % ± 3.77 cell viability is maintained following application of celastrol and arimoclomol at the optimized concentrations (0.3 and 50 μM, respectively), alone or in combination. Cell viability decreased slightly as celastrol concentration was increased from 0.3 to 0.5 μM with a major decrease at 1.5 μM celastrol and at 250 μM arimoclomol.
Fig. 5.
Effect of co-application of celastrol and arimoclomol on cellular viability and neuronal process morphology of differentiated human SH-SY5Y neuronal cells. a Propidium iodide (pink) staining indicated that co-application of celastrol and arimoclomol did not induce neuronal cell death. Hoechst 33258 (blue) was used to identify neuronal nuclei by DNA staining. Differential interference contrast (DIC) imaging was employed to view neuronal morphology. b Neuronal processes were assessed by staining with α-tubulin (green). Nuclei are stained with DAPI (blue). Arrows—long neuronal processes; arrowheads—retracted neuronal processes. c High concentrations of celastrol (1.5 μM) compromised neuronal viability. Upper panel—rounding of neuronal cell bodies (observed by DIC) and uptake of propidium iodide; lower panel—retraction of neuronal processes (arrowhead). d Quantification of cell viability by trypan blue staining. After low-dose co-application of celastrol and arimoclomol for 24 h at the optimized concentrations (0.3 and 50 μM, respectively), 84.2 % ± 3.77 neuronal viability (dotted line) was maintained. Significant (*p < 0.05; **p < 0.01) loss of cell viability was observed at 250 μM arimoclomol or 1.5 μM celastrol. Scale bars represent 20 μm (Color figure online)
An important marker of neuronal stress is the integrity of neuronal processes which retract in challenged cells (Kroemer et al. 2009). As shown in Fig. 5b, extensive neuronal process morphology (visualized by α-tubulin staining) was apparent at 0.3 μM celastrol and 50 μM arimoclomol, alone or in combination, but severely impacted when arimoclomol was increased to 250 μM alone or in combination with celastrol. Elevation of celastrol to 1.5 μM resulted in the loss of neuronal process morphology (Fig. 5c).
Discussion
As the prevalence of neurodegenerative diseases in the human population ramps up as average life span increases, few effective therapies for these neural diseases, particularly Alzheimer’s, have been identified to date despite a large number of clinical trials (Lang 2010; Dunkel et al. 2012; Pratt et al. 2015). This may be due to the multifactorial nature of neurodegenerative diseases, and targeted inhibition of a single disease pathology may be compensated by concurrent deleterious pathways (Cavalli et al. 2008; Lang 2010; Dunkel et al. 2012; Huang and Mucke 2012; Sheikh et al. 2013). Multidrug therapies that target several aspects of disease pathology are gaining attention and may provide more effective avenues for treating multipathology diseases (Cavalli et al. 2008; Lang 2010; Dunkel et al. 2012; Huang and Mucke 2012; Sheikh et al. 2013; Veloso et al. 2013a, b, 2014). There is also an urgent need for treatment strategies that impact early stages of disease progression (DeKosky and Marek 2003; Lang 2010; Dunkel et al. 2012). Synaptic dysfunction and loss are an early phenomenon in neurodegenerative diseases, which have been characterized as protein misfolding disorders (Stephan et al. 2012; Chung et al. 2015). Upregulation of Hsps has been shown to protect synapses at a functional level (Karunanithi et al. 1999, 2002; Brown 2008; Karunanithi and Brown 2015).
In the present report, we demonstrate that co-application of celastrol and arimoclomol enhances the induction of a set of Hsps in differentiated human SH-SY5Y neuronal cells at dosages that do not affect cell viability or neuronal process morphology. This includes upregulation of HSPA6 (Hsp70B’), a little studied member of the HSPA (Hsp70) family that is present in the human genome, but not in rat and mouse (Chow and Brown 2007; Noonan et al. 2007a, b, 2008a, b; Chow et al. 2010; Ramirez et al. 2014). Recently, we have demonstrated a unique feature of HSPA6, namely localization to transcription sites in human neuronal cells during recovery from stress-induced inhibition (Khalouei et al. 2014), a feature that is missing in current animal models of neurodegenerative diseases which lack HSPA6. The present problem of therapeutic compounds, identified in rodent models of disease, that fail to translate to effective treatments in the human clinical setting could be addressed by the generation of primate animal models using the common marmoset (Lang 2010; t Hart et al. 2012; McGonigle and Ruggeri 2014; Sasaki 2015). Rodent models fail to encompass the complexity of the human brain (Lang 2010; t Hart et al. 2012; McGonigle and Ruggeri 2014; Sasaki 2015). Interestingly, the HSPA6 gene is found in the marmoset genome (NCBI gene ID: 100411854). However, marmoset models are in early testing stages; hence, it is important to investigate the effects of potential therapeutic compounds in differentiated human neuronal cells.
The rationale for co-application of celastrol and arimoclomol to human neuronal cells is as follows. Celastrol was identified in an NIH-sponsored drug screen aimed at identifying potential therapeutic compounds that could suppress a hallmark of neurodegenerative diseases, namely protein misfolding resulting in aggregation (Abbott 2002; Heemskerk et al. 2002). Subsequently, celastrol was shown to be beneficial in a number of animal models of neurodegenerative diseases, including ALS (Kiaei et al. 2005), Parkinson’s disease (Cleren et al. 2005), polyglutamine disease (Zhang and Sarge 2007), and Alzheimer’s disease (Paris et al. 2010). Celastrol also exhibits potent anti-inflammatory and anti-oxidant properties in several animal models of inflammation and apoptosis to combat other aspects of neurodegenerative disease pathology (Allison et al. 2001; Faust et al. 2009; Kim et al. 2009; Sharma et al. 2015). In human SH-SY5Y neuronal cells, which are employed as a model system in the present study, it has been demonstrated that celastrol prevents the increase in reactive oxygen species that is observed following exposure to the mitochondrial toxin rotenone (Choi et al. 2014). The mechanism of action of celastrol involves activation of HSF1 monomers to a trimerized form that binds to HSEs in the promoter regions of inducible heat shock genes resulting in their upregulation (Westerheide et al. 2004; Salminen et al. 2010; Sharma et al. 2015).
Arimoclomol is a co-inducer that acts by prolonging the binding of heat shock transcription factor HSF1, the master regulator of the heat shock response, to HSE elements in the promoter regions of stress-inducible genes resulting in prolonged transcription of Hsp genes (Hargitai et al. 2003). In a mouse model of ALS, arimoclomol was found to improve motor performance and extend lifespan of SOD1G93A transgenic mice (Kieran et al. 2004; Kalmar et al. 2008, 2014; McGoldrick et al. 2013; Poppe et al. 2014). Interestingly, arimoclomol is now in phase IIb/III clinical trials (ClinicalTrials.gov identifier: NCT00706147) for the treatment of ALS (Genc and Ozdinler 2013).
By combining celastrol and arimoclomol at low dosages in differentiated human SH-SY5Y neuronal cells, enhanced protein expression was observed in a set of inducible Hsp genes, including HSPA6, relative to individual application of either compound. Neuronal cell viability and process morphology were maintained following co-application of these compounds at low-dose concentrations which result in enhanced Hsp induction. Higher doses of either arimoclomol or celastrol reduced cell viability, as has been reported for celastrol (Jantas et al. 2013). The ability of heat shock or celastrol to induce Hsps in differentiated human neuronal cells, when paired with arimoclomol, was analyzed. Co-application of celastrol and arimoclomol resulted in greater induction of Hsps compared to arimoclomol coupled with heat shock conditions that we have previously used on SH-SY5Y neuronal cells (Chow et al. 2010).
Therapy options that target multiple aspects of neurodegenerative disease pathology are gaining popularity and may provide more effective avenues for treatment (Cavalli et al. 2008; Lang 2010; Dunkel et al. 2012; Huang and Mucke 2012; Sheikh et al. 2013; Veloso et al. 2013a, b, 2014). Celastrol exhibits anti-inflammatory and anti-oxidant properties (Allison et al. 2001; Jung et al. 2007; Faust et al. 2009; Kim et al. 2009; Venkatesha et al. 2012; Wong et al. 2012; Yang et al. 2014; Sharma et al. 2015). In addition to inducing Hsp upregulation to prevent misfolding and aggregation of mutant, disease-associated proteins, celastrol also has the potential to reduce inflammatory and oxidative stress that accompanies neurodegeneration and is thought to exacerbate disease progression (Gao and Hong 2008; Amor et al. 2010, 2014).
Another requirement for the advancement of effective therapies for the treatment of neurodegenerative diseases is for treatment options that can impact early stages of disease progression (DeKosky and Marek 2003; Lang 2010; Dunkel et al. 2012). Synaptic dysfunction and loss have been recognized as an early phenomenon in neurodegenerative diseases (Masliah et al. 2001; Scheff et al. 2006; Shankar and Walsh 2009; Milnerwood and Raymond 2010; Stephan et al. 2012; Wu et al. 2015). Our studies have demonstrated that overexpression of Hsp70 and heat shock preconditioning protects synapses at a functional level (Karunanithi et al. 1999, 2002; Brown 2008; Karunanithi and Brown 2015). Using biochemical isolation of synaptic fractions and electron microscopy immunocytochemistry, we have demonstrated that Hsp70, Hsp32, and Hsp27 localize to synaptic components where they may be involved in synaptic repair and protective mechanisms (Bechtold and Brown 2000; Bechtold et al. 2000). Furthermore, Hsc70 forms a complex with Hsp40 at synapses following hyperthermia in the cerebral cortex (Chen and Brown 2007). Other studies indicate that overexpression of Hsp70 induces synaptic plasticity in Drosophila neuromuscular junctions that results in higher levels of neurotransmitter release and improved locomotor performance (Xiao et al. 2007). Chen et al. (2014) observed upregulation of pre- and post-synaptic proteins through an HSF1-dependent transcriptional mechanism and reduction of amyloid-β-induced synaptic toxicity and memory impairment.
During normal human brain development, synaptic connections are overproduced and selective elimination (i.e., synaptic pruning) subsequently takes place to shape the developing brain based on activity levels at individual synapses (Stephan et al. 2012; Schafer and Stevens 2015; Wu et al. 2015; Hong et al. 2016). Aberrant reactivation of synaptic pruning mechanisms during neuronal aging has recently been proposed to contribute to early synapse dysfunction and loss that is seen in many neurodegenerative diseases (Stephan et al. 2012; Chung et al. 2015; Wu et al. 2015). Upregulation of Hsps could have beneficial effects on mitigating against synaptic loss and dysfunction during aberrant reactivation of synaptic pruning at early stages in the progression of neurodegenerative diseases.
Co-application of celastrol and arimoclomol resulting in upregulation of a set of Hsps, including HSPA6 which is present in the human genome and not in current animal models of neurodegenerative diseases, has the potential to influence several aspects of disease pathology including protein misfolding and protein aggregation, inflammatory and oxidative stress, and synaptic dysfunction. Celastrol has shown therapeutic potential in a number of animal models of neurodegenerative diseases including ALS (Kiaei et al. 2005), Parkinson’s disease (Cleren et al. 2005), polyglutamine disease (Zhang and Sarge 2007), and Alzheimer’s disease (Paris et al. 2010) and has the added benefit of being a potent anti-inflammatory and anti-oxidant (Allison et al. 2001; Jung et al. 2007; Faust et al. 2009; Kim et al. 2009; Venkatesha et al. 2012; Wong et al. 2012; Yang et al. 2014; Sharma et al. 2015). Arimoclomol is a co-inducer that amplifies the induction of Hsps, prolongs life and motor performance in an animal model of ALS (Hargitai et al. 2003; Kieran et al. 2004; Kalmar et al. 2008, 2014), and is currently in clinical trials on human patients (ClinicalTrials.gov identifier: NCT00706147). Co-application of celastrol and arimoclomol represents a potential multitarget therapeutic strategy for the treatment of neurodegenerative diseases that could impact disease mechanisms, such as synaptic dysfunction, at early stages of disease progression.
Acknowledgments
This study is supported by grants from NSERC to I.R.B.
References
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a. [DOI] [PubMed] [Google Scholar]
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/S0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM. Inflammation in neurodegenerative diseases—an update. Immunology. 2014;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea AA, Brown IR (2008) Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. Springer Science+Business Media B.V. pp. 1–373
- Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J, Durham HD. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis. 2006;24:213–225. doi: 10.1016/j.nbd.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Brain Res Mol Brain Res. 2000;75:309–320. doi: 10.1016/S0169-328X(99)00323-X. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Rush SJ, Brown IR. Localization of the heat-shock protein Hsp70 to the synapse following hyperthermic stress in the brain. J Neurochem. 2000;74:641–646. doi: 10.1046/j.1471-4159.2000.740641.x. [DOI] [PubMed] [Google Scholar]
- Brown IR (2008) Heat Shock Proteins at the synapse: implications for functional protection of the nervous system. In: Brown IR, Asea AA (eds) Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection, vol 3. Springer Science+Business Media B.V., pp 239–254
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Chen S, Brown IR. Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J Neurosci Res. 2007;85:402–409. doi: 10.1002/jnr.21124. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang B, Liu D, Li JJ, Xue Y, Sakata K, Zhu LQ, Heldt SA, Xu H, Liao FF (2014) Hsp90 chaperone inhibitor 17-AAG attenuates A beta-induced synaptic toxicity and memory impairment. J Neurosci 34(7):2464--2470. doi:10.1523/JNEUROSCI.0151-13.2014 [DOI] [PMC free article] [PubMed]
- Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Choi BS, Kim H, Lee HJ, Sapkota K, Park SE, Kim S, Kim SJ. Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson’s disease. Neurochem Res. 2014;39:84–96. doi: 10.1007/s11064-013-1193-y. [DOI] [PubMed] [Google Scholar]
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones. 2007;12:237–244. doi: 10.1379/CSC-269.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Mok P, Xiao D, Khalouei S, Brown IR. Heteromeric complexes of heat shock protein 70 (HSP70) family members, including Hsp70B’, in differentiated human neuronal cells. Cell Stress Chaperones. 2010;15:545–553. doi: 10.1007/s12192-009-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Dekker SL, Kampinga HH, Bergink S. DNAJs: more than substrate delivery to HSPA. Front Mol Biosci. 2015;2:35. doi: 10.3389/fmolb.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Dunkel P, Chai CL, Sperlagh B, Huleatt PB, Matyus P. Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2012;21:1267–1308. doi: 10.1517/13543784.2012.703178. [DOI] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:MFROHF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009;10:109. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Ozdinler PH. Moving forward in clinical trials for ALS: motor neurons lead the way please. Drug Discov Today. 2013 doi: 10.1016/j.drudis.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifondorwa DJ, et al. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2007;27:13173–13180. doi: 10.1523/JNEUROSCI.4057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P, De Los RP. The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem Sci. 2007;32:372–380. doi: 10.1016/j.tibs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Hargitai J, et al. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun. 2003;307:689–695. doi: 10.1016/S0006-291X(03)01254-3. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, Tobin AJ, Bain LJ (2002) Teaching old drugs new tricks. Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA Trends Neurosci 25:494–496 doi:10.1016/S0166-2236(02)02236-1 [DOI] [PubMed]
- Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, et al. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantas D, Roman A, Kusmierczyk J, Lorenc-Koci E, Konieczny J, Lenda T, Lason W. The extent of neurodegeneration and neuroprotection in two chemical in vitro models related to Parkinson’s disease is critically dependent on cell culture conditions. Neurotox Res. 2013;24:41–54. doi: 10.1007/s12640-012-9374-z. [DOI] [PubMed] [Google Scholar]
- Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J Neurochem. 2008;107:339–350. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Lu CH, Greensmith L. The role of heat shock proteins in amyotrophic lateral sclerosis: the therapeutic potential of Arimoclomol. Pharmacol Ther. 2014;141:40–54. doi: 10.1016/j.pharmthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Karunanithi S, Brown IR. Heat shock response and homeostatic plasticity. Front Cell Neurosci. 2015;9:68. doi: 10.3389/fncel.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19:4360–4369. doi: 10.1523/JNEUROSCI.19-11-04360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Brown IR, Robertson RM, Atwood HL. Enhancement of presynaptic performance in transgenic Drosophila overexpressing heat shock protein Hsp70. Synapse. 2002;44:8–14. doi: 10.1002/syn.10048. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Jager R, Mosser DD, Samali A. Regulation of apoptosis by heat shock proteins. IUBMB Life. 2014;66:327–338. doi: 10.1002/iub.1274. [DOI] [PubMed] [Google Scholar]
- Khalouei S, Chow AM, Brown IR. Localization of heat shock protein HSPA6 (HSP70B’) to sites of transcription in cultured differentiated human neuronal cells following thermal stress. J Neurochem. 2014;131:743–754. doi: 10.1111/jnc.12970. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Investig. 2009;39:819–827. doi: 10.1111/j.1365-2362.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Novoselov SS, Bett JS, Weiss A, Paganetti P, Bates GP, Cheetham ME. Suppression of protein aggregation by chaperone modification of high molecular weight complexes. Brain. 2012;135:1180–1196. doi: 10.1093/brain/aws022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE. Clinical trials of disease-modifying therapies for neurodegenerative diseases: the challenges and the future. Nat Med. 2010;16:1223–1226. doi: 10.1038/nm.2220. [DOI] [PubMed] [Google Scholar]
- Liu J, Shinobu LA, Ward CM, Young D, Cleveland DW. Elevation of the Hsp70 chaperone does not effect toxicity in mouse models of familial amyotrophic lateral sclerosis. J Neurochem. 2005;93:875–882. doi: 10.1111/j.1471-4159.2005.03054.x. [DOI] [PubMed] [Google Scholar]
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/WNL.56.1.127. [DOI] [PubMed] [Google Scholar]
- Mattoo RU, Goloubinoff P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci. 2014;71:3311–3325. doi: 10.1007/s00018-014-1627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38:507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- McGoldrick P, Joyce PI, Fisher EM, Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832:1421–1436. doi: 10.1016/j.bbadis.2013.03.012. [DOI] [PubMed] [Google Scholar]
- McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87:162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington’s disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Nillegoda NB, Bukau B. Metazoan Hsp70-based protein disaggregases: emergence and mechanisms. Front Mol Biosci. 2015;2:57. doi: 10.3389/fmolb.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda NB, et al. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524:247–251. doi: 10.1038/nature14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B’ regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Rasoulpour RJ, Giardina C, Hightower LE. Cell number-dependent regulation of Hsp70B’ expression: evidence of an extracellular regulator. J Cell Physiol. 2007;210:201–211. doi: 10.1002/jcp.20875. [DOI] [PubMed] [Google Scholar]
- Noonan E, Giardina C, Hightower L. Hsp70B’ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–2476. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Fournier G, Hightower LE. Surface expression of Hsp70B’ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones. 2008;13:105–110. doi: 10.1007/s12192-007-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, et al. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe L, Rue L, Robberecht W, Van Den Bosch L. Translating biological findings into new treatment strategies for amyotrophic lateral sclerosis (ALS) Exp Neurol. 2014;262(Pt B):138–151. doi: 10.1016/j.expneurol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353–371. doi: 10.1146/annurev-pharmtox-010814-124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VP, Stamatis M, Shmukler A, Aneskievich BJ. Basal and stress-inducible expression of HSPA6 in human keratinocytes is regulated by negative and positive promoter regions. Cell Stress Chaperones. 2014;20:95–107. doi: 10.1007/s12192-014-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ross RA, Spengler BA. Human neuroblastoma stem cells. Semin Cancer Biol. 2007;17:241–247. doi: 10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/MCB.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res. 2015;93:110–115. doi: 10.1016/j.neures.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Microglia function in central nervous system development and plasticity cold spring. Cold Spring Harb Perspect Biol. 2015;7:a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, et al. Celastrol, an oral heat shock activator, ameliorates multiple animal disease models of cell death. Cell Stress Chaperones. 2015;20:185–201. doi: 10.1007/s12192-014-0536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S, Safia, Haque E, Mir SS. Neurodegenerative diseases: multifactorial conformational diseases and their therapeutic interventions. J Neurodegener Dis. 2013;2013:563481. doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- t Hart BA, Abbott DH, Nakamura K, Fuchs E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov Today. 2012;17:1160–1165. doi: 10.1016/j.drudis.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso AJ, et al. Biological activity of sym-triazines with acetylcholine-like substitutions as multitarget modulators of Alzheimer’s disease. ACS Chem Neurosci. 2013;4:924–929. doi: 10.1021/cn400028w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso AJ, et al. sym-Triazines for directed multitarget modulation of cholinesterases and amyloid-beta in Alzheimer’s disease. ACS Chem Neurosci. 2013;4:339–349. doi: 10.1021/cn300171c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso AJ, et al. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal Chem. 2014;86:4901–4909. doi: 10.1021/ac500424t. [DOI] [PubMed] [Google Scholar]
- Venkatesha SH, Astry B, Nanjundaiah SM, Yu H, Moudgil KD. Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines. Bioorg Med Chem. 2012;20:5229–5234. doi: 10.1016/j.bmc.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Wong KF, Yuan Y, Luk JM. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin Exp Pharmacol Physiol. 2012;39:311–320. doi: 10.1111/j.1440-1681.2011.05586.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Mileva-Seitz V, Seroude L, Robertson RM. Targeting HSP70 to motoneurons protects locomotor activity from hyperthermia in Drosophila. Dev Neurobiol. 2007;67:438–455. doi: 10.1002/dneu.20344. [DOI] [PubMed] [Google Scholar]
- Yang L, Li Y, Ren J, Zhu C, Fu J, Lin D, Qiu Y. Celastrol attenuates inflammatory and neuropathic pain mediated by cannabinoid receptor type 2. Int J Mol Sci. 2014;15:13637–13648. doi: 10.3390/ijms150813637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Sarge KD. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J Mol Med (Berl) 2007;85:1421–1428. doi: 10.1007/s00109-007-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]