Abstract
KRAS mutant lung cancers have long been considered as untreatable with drugs. Transforming growth factor-β-activated kinase 1 (TAK1) appears to play an anti-apoptotic role in response to multiple stresses and has been reported to be a responsive kinase that regulates cell survival in KRAS-dependent cells. In this study, in order to find a useful approach to treat KRAS mutant lung cancer, we focused on the combined effects of 5Z-7-oxozeaenol, a TAK1 inhibitor, with hyperthermia (HT) in KRAS mutant lung cancer cell line A549. Annexin V-FITC/PI assay, cell cycle analysis, and colony formation assay revealed a significant enhancement in apoptosis induced by HT treatment, when the cells were pre-incubated with 5Z-7-oxozeaenol in a dose-dependent manner. The enhanced apoptosis by 5Z-7-oxozeaenol was accompanied by a significant increase in reactive oxygen species (ROS) generation and loss of mitochondrial membrane potential (MMP). In addition, western blot showed that 5Z-7-oxozeaenol enhanced HT-induced expressions of cleaved caspase-3, cleaved caspase-8, and HSP70 and decreased HT-induced expressions of Bcl-2, p-p38, p-JNK, and LC3. Moreover, 5Z-7-oxozeaenol pre-treatment resulted in a marked elevation of intracellular calcium level which might be associated with endoplasmic reticulum (ER) stress-related pathway. Taken together, our data provides further insights of the mechanism of action of 5Z-7-oxozeaenol and HT treatment, and their potential application as a novel approache to treat patients with KRAS mutant lung cancer.
Keywords: 5Z-7-oxozeaenol, TAK1, Hyperthermia, Apoptosis, Mitochondria, Calcium
Introduction
Lung cancer is the leading cause of cancer death in both men and women around the world (Siegel et al. 2014). Lung cancer is classified into three main groups: non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and carcinoids. Activating KRAS mutations are found in 15–30 % of patients with NSCLC; unfortunately, no effective treatment is currently available for this sub-type of NSCLC (Ku et al. 2015). KRAS mutations are generally related with treatment-refractory tumors (Downward 2003). Therefore, finding an effective treatment for the patients with KRAS mutant lung cancer is urgently required.
Transforming growth factor-β-activated kinase 1 (TAK1) was first identified as a mitogen-activated protein kinase kinase kinase (MAP3K) and found to be activated by TGFβ and bone morphologic protein (Yamaguchi et al. 1995). Since this original finding, TAK1 also has been shown to be activated by numerous stressors such as cytokines and bacterial ligands and then transmits the signal-transduction pathway leading to cellular responses. TAK1 stimulates activation of IkB kinase (IKK)-NF-kBNF-κB and MAPK pathway, which regulate cell survival, cell growth, tumorigenesis, and metabolism (Hayden and Ghosh 2008; Mihaly et al. 2014; Sakurai 2012). TAK1 has been reported to be a responsive kinase that regulates cell survival in KRAS-dependent cells, in which KRAS regulates TAK1 and Wnt signaling through BMP-7 receptor activation (Singh et al. 2012). It is also known that the NF-κ B pathway is a potential target in KRAS mutant cancer cells (Cordero et al. 2010). Together, these findings identify TAK1 inhibition as a potential therapeutic strategy for the treatment-refractory cancers exhibiting aberrant KRAS activation. 5Z-7-oxozeaenol, a resorcyclic acid lactone, first reported as a highly potent chemical inhibitor of TAK1, can inhibit picryl chloride-induced ear swelling (Ninomiya-Tsuji et al. 2003). Moreover, 5Z-7-oxozeaenol can suppress tumor growth of KRAS-dependent cell lines in vivo (Sakurai 2012). However, very often chemotherapy alone is not sufficient for the patients due to increasing drug resistance and side effects. To overcome this limitation, chemotherapy is usually combined with physical treatments such as hyperthermia (HT). HT has been known to enhance the drug delivery and thermosensitizes cancer cells to certain chemotherapeutic drugs at their low doses (Engelhardt 1987). Previously, our group has already reported that TAK1 plays a role in cell death induced by HT in HeLa cells (Li et al. 2013). In the present study, we investigated the effects of 5Z-7-oxozeaenol, a TAK1 inhibitor on human NSCLC A549 cells in combination with HT. A549 cells were treated with low doses of 5Z-7-oxozeaenol in combination with HT, and we tried to investigate the associated molecular mechanisms involved in the enhancement of apoptosis by combined treatment.
Materials and methods
Chemicals
5Z-7-oxozeaenol was purchased from R&D Systems (Minneapolis, MN, USA). Stock solutions were prepared using dimethyl sulfoxide (DMSO) as a solvent and further dissolved to make the desired concentrations for experimental use.
Cell line and culture
Human alveolar adenocarcinoma cell line, A549, was obtained from Human Sciences Research Resource Bank (Japan Human Sciences Foundation, Tokyo, Japan) and maintained in RPMI1640 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS) at 37 °C in humidified air with 5 % CO2.
Drug treatment and hyperthermia
A cell suspension containing 1 × 106 cells/dish was pretreated with 5Z-7-oxozeaenol for 1 h at 37 °C.
For hyperthermia treatment, A549 cells were seeded 24 h before treatment at a density of 0.2 × 106/ml in 35 mm × 10 mm dishes, then were sealed with paraffin film, and placed in a hot water bath at 44 °C for 20 min. After the treatment, cells were then transferred to an incubator at 37 °C until analysis.
Apoptosis assay by flow cytometry
Flow cytometry was performed using propidium iodide (PI) and fluorescein isothiocyanate (FITC)-labeled annexin V (Immunotech, Marseille, France) to detect phosphatidylserine externalization (on the surface of cell membrane) as an endpoint indicator of early apoptosis (Sakurai 2012). After the treatments, the remaining intact cells were incubated at 37 °C for 6 h, collected, washed with cold PBS at 4 °C, and centrifuged at 500×g for 5 min. FITC-labeled Annexin V (5 μl) and PI (5 μl) were added to 490 μl of the cell suspension and mixed gently. After incubation at 4 °C for 30 min in the dark, the cells were analyzed by flow cytometry (Epics XL, Beckman-Coulter, Miami, FL).
Analysis of cell cycle
Cells were harvested and washed with PBS. The cells were re-suspended in 100 μl of PBS then fixed in 1 ml of 70 % cold ethanol (-20 °C), stored overnight at 4 °C, washed with PBS, and incubated for 20 min at 37 °C in the dark with RNase solutions (1 × 106/0.25 mg/ml of RNase) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing propidium iodide (50 μg/ml) (Wako) followed by flow cytometry (Epics XL, Beckman-Coulter, FL).
Colony formation assay
Cell survival after HT treatment was measured by colony formation assay. Cells were seeded into 60-mm dishes on day 0 and allowed to attach for 24 h at 37 °C. Cells were then treated with HT exposure (44 °C, 20 min). Cells were incubated in the incubator for 12 days. Colonies were examined by Giemsa staining, and visible colonies containing approximately 50 or more cells were counted. Survival fraction was calculated relative to the number of control cells to determine the plating efficiency in each experiment (number of HT-treated colonies/number of colonies in control).
Measurement of intracellular ROS production
To detect intracellular reactive oxygen species (ROS) production, the cells were incubated at 37 °C for 15 min with 5 μM Hydroxyphenyl fluorescein (HPF, Sekisui medical co., Tokyo, Japan) to detect intracellular hydroxyl radical and peroxynitrite; with 5 μM Hydroethidine (HE, Molecular Probes, Eugene, OR) to detect intracellular superoxide. For all of them, the fluorescence emission was analyzed using flow cytometry.
Measurement of mitochondrial membrane potential (MMP)
To measure changes in MMP, A549 cells were stained with 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3)) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in 0.5 ml of PBS plus 1 % FBS for 15 min at 37 °C. The fluorescence of DiOC6(3) was analyzed using a flow cytometer, with excitation and emission settings at 484 and 500 nm, respectively. Because DiOC6(3) is a lipophilic cationic fluorochrome that accumulates in mitochondrial matrix proportionally to the transmembrane potential, cells that showed low MMP were estimated as the fraction of cells with weak fluorescence intensity of DiOC6(3).
Western blot analysis of proteins
The cells were collected and lysed in lysis buffer (1 M Tris–HCl, 5 M NaCl, 1 % Nonidet P-40 (v/v), 1 % sodium deoxycholate, 0.05 % SDS, 1 mM phenylmethylsulfonyl fluoride) for 20 min. After brief sonication, the lysates were centrifuged at 12,000×g 10 min at 4 °C, and the protein content in the supernatant was measured using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA). The protein lysates were denatured at 96 °C for 5 min, after mixing with SDS-loading buffer, applied on an SDS polyacrylamide gel (Daiichi Pure Chemicals Co., Ltd, Tokyo, Japan) for electrophoresis, and transferred to nitrocellulose membranes (Amersham Biosciences, Buchinghamshire, UK). Western blot analysis was performed using primary antibodies (1:1000) to NF-kB p65 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) (sc-109), caspase-3 (#9662), Bcl-2(#2876), JNK(#9252), phospho-JNK(#9255), p38(#9212), phospho-p38(#4631), A20(#5630), phospho-NF-kB p65(#3033) (Cell Signaling Technology), and β-actin (Sigma-Aldrich, St. Louis, MO). The secondary horseradish peroxidase (HRP)-conjugated antibodies (1:1000) were purchased from Cell Signaling Technology. The band signals were visualized using a luminescent image analyzer (LAS4000, Fujifilm Co., Tokyo, Japan) with chemi-luminescence ECL system (Amersham Biosciences).
Measurement of intercellular free calcium ions
To monitor the effect of 5Z-7-oxozeaenol treatment on intracellular calcium homeostasis, intracellular free Ca2+ was measured using calcium probe Fluo-3/AM (Dojindo Laboratories Co., Ltd., Kumamoto, Japan). Cells were treated with 5Z-7-oxozeaenol for 1 h, then were exposed to HT (44 °C, 20 min). After 12 h incubation, the cells were harvested and then loaded with 5 μM Fluo-3/AM for 30 min at 37 °C. Excess Fluo-3/AM was removed by washing three times with PBS. The fluorescence intensity of free Ca2+ levels was measured by flow cytometry.
Statistical analysis
Data are expressed as the means ± SD. Statistical analysis was carried out using the Student’s t test. P values <0.05 were regarded as significant. All the experiments were performed in triplicate.
Results
Effects of 5Z-7-oxozeaenol on HT-induced cell death
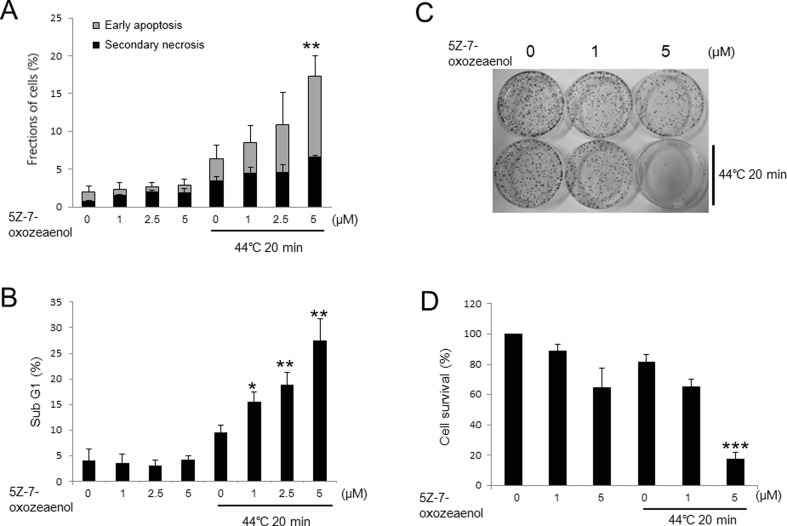
To investigate the effects of 5Z-7-oxozeaenol on HT-induced cell death, PI/annexin V-FITC assay was carried out following the treatment of 5Z-7-oxozeaenol at different concentrations and HT in A549 cells. The percentage of early apoptotic and secondary necrotic cells was significantly enhanced in a dose-dependent manner by 5Z-7-oxozeaenol 24 h after HT exposure (Fig. 1a). The increased percentage of subG1 fraction was also observed in 5Z-7-oxozeaenol-treated cells in a dose-dependent manner after HT exposure (Fig. 1b). To further examine the effects of 5Z-7-oxozeaenol on cell survival in response to HT exposure, colony formation assay was performed in the presence of 5Z-7-oxozeaenol at the dose of 1 μM and 5 μM. The results showed that 5Z-7-oxozeaenol notably decreased cell survival at the dose of 5 μM 12 days after HT treatment (Fig. 1c, d). Taken together, all the data showed that 5Z-7-oxozeaenol remarkably increased HT-induced cell death in A549 cells.
Fig. 1.
5Z-7-oxozeaenol increased HT-induced cell death in A549 cells. The cells were treated with HT (44 °C, 20 min) combined with or without 5Z-7-oxozeaenol (1, 2.5, and 5 μM) for 1 h and incubated at 37 °C. a Cells were harvested after incubation for 24 h and stained with Annexin V-FITC and PI for flow cytometry. b Cells were collected 24 h after the treatment and incubated with RNase solution and PI at 37 °C for 30 min. Cell cycle distribution was determined by flow cytometry. Cells were treated with indicated concentrations of 5Z-7-oxozeaenol (1, 2.5 and 5 μM) for 1 h followed by HT treatment (44 °C, 20 min). c Colony formation assay was performed after 12 days incubation. d Percentage of colonies was calculated. The results are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 compared with HT-treated cells
Effects of 5Z-7-oxozeaenol on HT-induced ROS generation
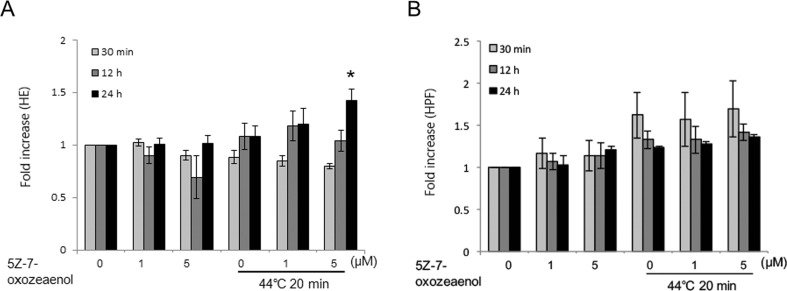
Excess generation of intracellular ROS causes oxidative stress, which is an important signal in apoptosis (Tripathi and Hildeman 2004; Ott et al. 2007). To address the role of ROS in enhanced apoptosis by 5Z-7-oxozeaenol in response to HT treatment, we measured ROS using HPF and HE staining by flow cytometry. HT alone increased the intensity of HE fluorescence from 12 to 24 h after treatment, which was further enhanced in the presence of 5 μM 5Z-7-oxozeaenol at 24 h (Fig. 2a). Intensity of HPF fluorescence was increased 30 min after HT exposure and further become decrease at 12 and 24 h HT treatment (Fig. 2b). However, 5Z-7-oxozeaenol had no effects on the intensity of HPF fluorescence after the HT exposure at any indicated time point.
Fig. 2.
5Z-7-oxozeaenol enhanced HT-induced ROS generation in A549 cells. Cells were pre-incubated with indicated concentrations of 5Z-7-oxozeaenol (1 and 5 μM) for 1 h and then were treated with HT (44 °C, 20 min). a After incubated for indicated time, cells were collected and loaded with HE for 15 min. Fluorescence intensity was detected by flow cytometry at indicated time after HT exposure. b After incubated for indicated time, cells were collected and loaded with HPF for 15 min. Fluorescence intensity was detected by flow cytometry at indicated time after HT exposure. The results are presented as mean ± SD (n = 3). *P < 0.05 compared with HT treatment alone
Effects of 5Z-7-oxozeaenol on mitochondrial pathway after HT exposure
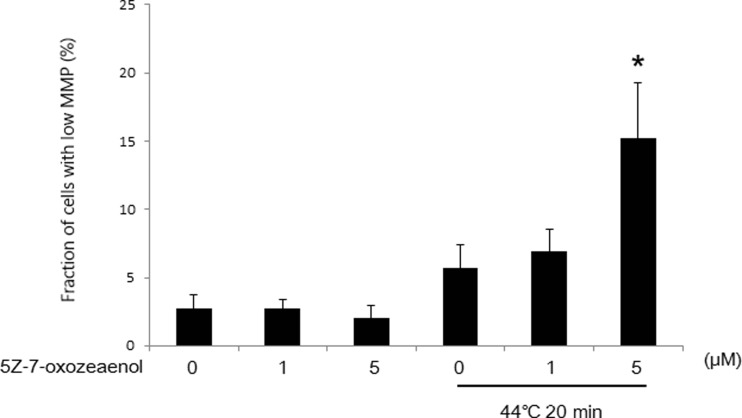
To explore the effects of 5Z-7-oxozeaenol on mitochondrial membrane potential (MMP) in response to HT treatment, the cells treated with HT and/or 5Z-7-oxozeaenol were detected by DiOC6(3) staining. Loss of MMP induced by HT was not significant, but significant promotion of MMP loss was observed in the presence of 5Z-7-oxozeaenol at high dose of 5 μM (Fig. 3).
Fig. 3.
5Z-7-oxozeaenol increased HT-induced MMP loss in A549 cells. Cells were pretreated with two doses of 5Z-7-oxozeaenol (1 and 5 μM), further treated with HT (44 °C, 20 min). After 18 h incubation, cells were collected and subjected to flow cytometry using DiOC6(3) staining. The results are presented as mean ± SD (n = 3). *P < 0.05 compared with HT treatment alone
Effects of 5Z-7-oxozeaenol on cell death-related proteins
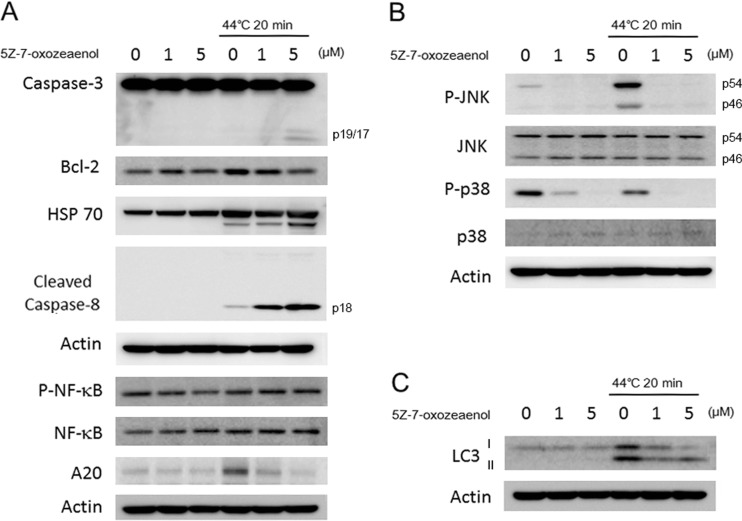
To further investigate the molecular mechanism of 5Z-7-oxozeaenol in response to HT, we also evaluated expressions of cell death-related proteins by western blot analysis. Caspases are the important executioners of apoptosis induced by various apoptotic stimuli. The results showed that 5Z-7-oxozeaenol in combination with HT markedly increased the expression of cleaved caspase-3 and cleaved caspase-8 (Fig. 4a). Furthermore, suppression of Bcl-2 was observed in 5Z-7-oxozeaenol-treated cells 12 h after HT treatment (Fig. 4a). Western blot analysis also showed that 5Z-7-oxozeaenol slightly increased HT-induced HSP 70 expression (Fig. 4a). Since, TAK1 is essential for activating the IκB kinase (IKK)/NF-κB pathway and MAPK pathway in response to diverse stimuli (Hayden and Ghosh 2008; Mihaly et al. 2014; Sakurai 2012). The expression of NF-κB and p-NF-κB in A549 cells treated with HT with/without 5Z-7-oxozeaenol was detected; changes in expressions of p-NF-κB and NF-κB were not observed between HT-treated cells and in combined treatment (Fig. 4a). Further, we also evaluate the expression of A20 in HT with/without 5Z-7-oxozeaenol-treated cells. Expression of A20 was evoked by HT exposure alone but decreased in the presence of 5Z-7-oxozeaenol in a dose-dependent manner in A549 cells (Fig. 4a). In order to investigate whether MAPKs signaling pathway was involved in 5Z-7-oxozeaenol-mediated enhancement of apoptosis, we measured the activities of JNK and p38 by western blot at 1 h after HT exposure with or without 5Z-7-oxozeaenol pre-treatment. 5Z-7-oxozeaenol itself significantly suppressed expressions of p-JNK and p-p38 before HT treatment and abrogated phosphorylation of JNK and p38 markedly after HT treatment (Fig. 4b), while the expression of JNK and p38 remained unchanged (Fig. 4b). Interestingly, expression of LC3 II, the autophagy marker, markedly increased by HT treatment and this elevation by HT treatment was suppressed in the presence of 5Z-7-oxozeaenol (Fig. 4c).
Fig. 4.
Assessment of cell death related proteins. After 12-h incubation, protein was extracted from the cells and western blot analysis was performed to detect the expressions of diverse proteins. a Changes in expressions of cell death related proteins such as caspase-3, Bcl-2, HSP 70, and cleaved caspase-8 were detected by western blot analysis. b Changes in expressions of MAPK pathway-related proteins such as p-JNK, JNK, p-p38, and p38 were detected by western blot analysis. c Changes in expression of LC3 was detected by western blot analysis
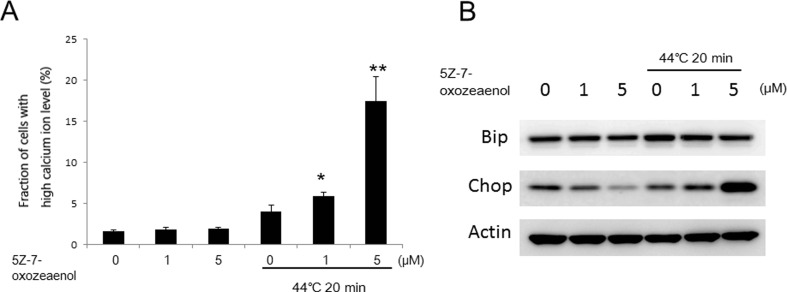
Effects of 5Z on intracellular Ca2+ levels and ER stress-mediated pathway
Calcium homeostasis is essential for varied cellular functions, such as protein folding, processing, transport, and signal transduction (Giorgi et al. 2012). To measure the role of intracellular Ca2+ in the enhancement of apoptosis by 5Z-7-oxozeaenol, we assessed intracellular Ca2+ levels by flow cytometry. The result showed that HT-induced intracellular Ca2+ levels were significantly enhanced by 5Z-7-oxozeaenol in a dose-dependent manner (Fig. 5a). Further, to investigate the reason of this [Ca2+]i increase, effects on ER stress were determined. Cells were harvested 12 h after treatment with 5Z-7-oxozeaenol and/or HT for western blot analysis. Results showed that HT-induced Bip expression was slightly suppressed by 5Z-7-oxozeaenol in A549 cells (Fig. 5b). Although the expression of CHOP was decreased at 5Z-7-oxozeaenol 5 μM treatment alone, the expression of CHOP was markedly increased in the combined treated cells (Fig. 5b), indicating that ER stress might associate with 5Z-7-oxozeaenol-mediated apoptotic enhancement in response to HT.
Fig. 5.
Assessment of intracellular Ca2+ levels and ER stress. Cells were pretreated with 5Z-7-oxozeaenol (1 and 5 μM), further treated with HT (44 °C, 20 min), and then harvested 12 h after treatment. a Cells were loaded with 5 μM calcium probe Fluo-3/AM for 30 min, and intracellular Ca2+ level was measured by flow cytometry. The results are presented as mean ± SD (n = 3). *P < 0.05 compared with HT-treated cells. b Changes in expressions of CHOP and Bip were detected by western blot analysis
Discussions
TAK1 has a potent pro-survival role in activating the Ikb kinase (IKK)-NF-κB pathway, which has numerous target genes, many of which block apoptosis, promote cell proliferation, and stimulate inflammatory responses (Hayden and Ghosh 2008; Mihaly et al. 2014; Sakurai 2012). Our previous study has already shown that TAK1 promotes cell survival in irradiated HeLa cells and HT-exposed HeLa cells (Li et al. 2013). Thus, inhibition of TAK1 may sensitize cells to death through many of the myriad pathways. 5Z-7-oxozeaenol was identified as the potent TAK1 inhibitor and sensitized both HeLa and MEF cells to TNF-α, doxorubicin, and etoposide-induced cell death (Fan et al. 2013). In this study, we investigated the effects of 5Z-7-oxozeaenol on HT-induced cell death in A549 cells.
Oxidative stress plays an established role in apoptosis (Tripathi and Hildeman 2004; Ott et al. 2007). Heat stress to cells induces ROS such as superoxide anion, hydrogen peroxide, hydroxyl radical, nitric oxide, and peroxynitrite (Zhao et al. 2006). Several reports have linked oxidative stress to heat stress and revealed a synergistic augmentation of cell death as increased ROS generation was observed in HT-exposed cells (McAnulty et al. 2005; Gu et al. 2015). Superoxide anion is the precursor of most ROS and a mediator in oxidative chain reactions may react with other radicals including nitric oxide (Turrens 2003). In the present study, pre-treatment with 5 μM of 5Z-7-oxozeaenol does not enhance superoxide anion generation at 30 min and 12 h after HT treatment, however, markedly increased superoxide anion generation at 24 h after HT treatment in A549 cells (Fig. 2a, b). Although, the involvement of TAK1 in oxidative stress has been reported, its contribution to the generation of ROS is controversial. It was initially reported that TAK1 deficiency causes elevated ROS generation in TNF-stimulated keratinocytes (Schober 2008). Consistent with this study, TAK1 blockage formed more ROS in response to LPS stimulation in neutrophils, indicating that TAK1 is a negative regulator of ROS production (Omori et al. 2008). However, recent studies have shown that pharmacological blockade or genetic ablation of TAK1 causes a drastic reduction in ROS production such as superoxide formation in vascular smooth muscle cells and neurons (Song et al. 2014; Neubert et al. 2011). Herein, the enhancement of superoxide formation by 5Z-7-oxozeaenol might be essential for the apoptotic enhancement after HT treatment.
Mitochondria are the target and source of ROS, which play a vital role in the regulation of apoptotic and autophagy pathways (Hou et al. 2014). Enhanced ROS generation or decreased Bcl-2 open the mitochondria permeability transition pore (MPTP) through the outer mitochondrial voltage-dependent anion conductance channel to release cytochrome C into cytosol (Chien et al. 2014). This will eventually lead to the activation of caspase-3, followed by DNA fragmentation and cell death (Li et al. 2014). The Bcl-2 family proteins exist in the mitochondrial membrane and regulate mitochondria-dependent apoptosis (Adams and Cory 2001). In the current study, treatment with 5Z-7-oxozeaenol dramatically increased HT-induced MMP loss in a dose-dependent manner and decreased Bcl-2 expression after HT exposure (Figs. 3 and 4a). Eventually, treatment with 5Z-7-oxozeaenol notably enhanced HT-induced cleaved caspase-3 expression which was one of the central executioners of apoptosis (Fig. 4a). Furthermore, Fas/TNF-R1 can trigger apoptosis via the direct activation of caspase cascade or via the mitochondria by activating caspase-8 and Bid (Yin 2000; Kim et al. 2006). Our results revealed that 5Z-7-oxozeaenol could activate caspase-8 after HT treatment (Fig. 4a). Taken together, all the data indicated that 5Z-7-oxozeaenol could increase HT-induced apoptosis in a dose-dependent manner via mitochondria-dependent pathway. In addition, induction of heat shock proteins is the most well characterized heat shock response. HSP 70 has been shown to play a critical role in cell survival and thermo-tolerance possibly through preventing recruitment of procaspase-9 to the apoptosome by binding to Apaf-1, thereby inhibiting the activation of caspase-3 (Beere et al. 2000). Our results showed that HSP 70 may not involve in the apoptotic enhancement by 5Z-7-oxozeaenol in response to HT treatment (Fig. 4a).
Ca2+ is a second messenger in cells that translates extracellular stimuli into intracellular activities that are important for regulation of diverse physiological processes (Speidel 2010). Excessive elevation or prolonged activation of Ca2+ signaling would cause cell death. It is reported that oxidative stress and intracellular Ca2+ overload are connected to each other in HT-mediated cell death (Wold et al. 2006; Yu et al. 2006). It is well established that intracellular Ca2+ overload can trigger apoptosis via the mitochondria-dependent pathway (Orrenius et al. 2003). In our study, all the data showed that the enhanced intracellular Ca2+ level was observed when cells were treated with HT and 5Z-7-oxozeaenol (Fig. 5a). Moreover, endoplasmic reticulum (ER) is by far the best studied releasable calcium store, possessing a well-defined repertoire of calcium channels, pumps, and buffers except mitochondria. Recent evidence has suggested that cytochrome c can bind to ER inositol 1,4,5-trisphosphate receptor (IP3R), rendering the channel insensitive to auto-inhibition by high intracellular Ca2+ and resulting in enhanced ER Ca2+ release. The close proximity between the ER and mitochondria assists this cross-talk (Brookes et al. 2004). To further investigate whether ER stress is associated with the pro-apoptotic function of 5Z-7-oxozeaenol in response to HT exposure, we assessed two major ER proteins: Bip and CHOP. Bip, the major ER-localized chaperone, is induced by ER stress. Activation of Bip is demonstrated to regulate toxicants- or stimulus-induced apoptotic pathways (Choi et al. 2010). CHOP also plays a key role in ER stress-induced apoptosis (Oyadomari and Mori 2004). Depletion of the CHOP gene could result in a reduction of ER stress-induced cancer cell death, and overexpression of CHOP gene promotes cell death via down-regulation of Bcl-2 expression, depletion of cellular glutathione, and exaggerated production of ROS (Miyazaki et al. 2011; McCullough et al. 2001). As shown in Fig. 5b, Bip did not involve in the enhanced intracellular Ca2+ level by 5Z-7-oxozeaenol; however, CHOP expression was markedly increased in the presence of 5Z-7-oxozeaenol after HT exposure (Fig. 5b). All the data suggested that 5Z-7-oxozeaenol might enhance intracellular Ca2+ level through ER stress-mediated pathway.
Activated TAK1 can phosphorylate IKK to activate NF-kB pathway and mitogen-activated protein kinase kinases (MKKs) to activate the MAPK kinase pathway (Hayden and Ghosh 2008; Mihaly et al. 2014; Sakurai 2012). Activated NF-kB mediates the expression of diverse target genes that promote cell proliferation, regulate apoptosis, facilitate angiogenesis, and stimulate invasion and metastasis (Lee et al. 2007) and thus serves as a key transcriptional regulator of anti-apoptotic and antioxidant molecules. A20 can negatively regulate IKK and NF-κB signaling pathway which is activated by TAK1 (Li et al. 2013). Overexpression of A20 can protect cells from TNF-mediated apoptosis by blocking the activation of the apical caspase-8 and 2, preventing subsequent activation of downstream effectors and maintain the mitochondrial integrity (Daniel et al. 2004). Activation of MAPKs plays an essential role in apoptosis induced by many cellular stresses. To examine the role of NF-κB, MAPKs, and A20 in the enhanced apoptosis by 5Z-7-oxozeaenol, we detected the expressions of NF-κB, MAPKs, and A20. The results showed that 5Z-7-oxozeaenol alone slightly decreased p-NF-κB expression due to its own NF-κB inhibition activity but had no effects on p-NF-κB expression in combined treatment, indicating that NF-κB was not involved in the enhanced apoptosis by 5Z-7-oxozeaenol after the HT treatment (Fig. 4a). Expressions of p-JNK and p-38 were significantly inhibited by 5Z-7-oxozeaenol before and after HT exposure (Fig. 4b). However, HT-induced A20 expression was remarkably prevented by 5Z-7-oxozeaenol after HT exposure, suggesting that A20 may be involved in the enhancement of apoptosis (Fig. 4a).
In the present study, LC3-II bands were clearly observed in HT-treated cells, indicating that autophagy was induced by HT exposure (Fig. 4c). Previous studies have already demonstrated that HT-triggered autophagy was associated with increased cell survival in most cases (Zhang and Calderwood 2011; Swanlund et al. 2008; Chen et al. 2008). Therefore, the reduction of LC3-II expression by 5Z-7-oxozeaenol revealed that cell survival may be abrogated, and cell death may be increased after the HT exposure in the presence of 5Z-7-oxozeaenol (Fig. 4c).
In conclusion, this study demonstrates that 5Z-7-oxozeaenol enhances apoptosis in response to HT in A549 cells. Treatment with 5Z-7-oxozeaenol resulted in mitochondria-dependent apoptotic pathway via increase of ROS generation, MMP loss, intracellular Ca2+ level, and reduction of Bcl-2 expression. In addition, blockade of LC3-II expression by 5Z-7-oxozeaenol may also participate in the enhanced apoptosis following the HT treatment. Our findings may help the potential application of the agent with HT treatment as a future strategy for KRAS mutant lung cancer.
Acknowledgments
This work was supported in part by Grant-in-Aid for Challenging Exploratory Research (No. 26670551), Japan society for the Promotion of Science.
Abbreviations
- AIF
Apoptosis-inducing factor
- DMSO
Dimethyl sulfoxide
- DiOC6(3)
3,3′-dihexyloxacarbocyanine iodide
- FITC
Fluorescein isothiocyanate
- HE
Hydroethidine
- HPF
Hydroxyphenyl fluorescein
- HT
Hyperthermia
- IP3R
Inositol 1,4,5-trisphosphate receptor
- MAP3K
Mitogen-activated protein kinase kinase kinase
- MMP
Mitochondrial membrane potential
- MKKs
Mitogen-activated protein kinase kinases
- MPTP
Mitochondria permeability transition pore
- NSCLC
Non-small cell lung cancer
- PI
Propidium iodide
- ROS
Reactive oxygen species
- SCLC
Small cell lung cancer
- TAK1
Transforming growth factor-β-activated kinase 1
References
- Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/S0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol Int. 2008;32:715–723. doi: 10.1016/j.cellbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Chien CY, Chien CT, Wang SS. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J Thorac Cardiovasc Surg. 2014;148:705–713. doi: 10.1016/j.jtcvs.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Choi AY, Choi JH, Lee JY, Yoon KS, Choe W, Ha J, et al. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem Int. 2010;57:143–152. doi: 10.1016/j.neuint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Arvelo MB, Patel VI, Longo CR, Shrikhande G, Shukri T, et al. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood. 2004;104:2376–2384. doi: 10.1182/blood-2003-02-0635. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Engelhardt R. Rational for clinical application of hyperthermia and drugs. Rev Med Chir Soc Med Nat Iasi. 1987;91:347–352. [PubMed] [Google Scholar]
- Fan Y, Cheng J, Vasudevan SA, Patel RH, Liang L, Xu X, Zhao Y, Jia W, Lu F, Zhang H, Nuchtern JG, Kim ES, Yang J. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis. 2013;18:1224–1234. doi: 10.1007/s10495-013-0864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial Ca(2+) and apoptosis. Cell Calcium. 2012;52:36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu ZT, Li L, Wu F, Zhao P, Yang H, Liu YS, Geng Y, Zhao M, Su L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca(2+) dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci Rep. 2015;5:11497. doi: 10.1038/srep11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int J Mol Sci. 2014;15:17380–17395. doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Ku BM, Jho EH, Bae YH, Sun JM, Ahn JS, Park K, Ahn MJ. BYL719, a selective inhibitor of phosphoinositide 3-kinase α, enhances the effect of selumetinib (AZD6244, ARRY-142886) in KRAS-mutant non-small cell lung cancer. Invest New Drugs. 2015;33:12–21. doi: 10.1007/s10637-014-0163-9. [DOI] [PubMed] [Google Scholar]
- Lee CH, Jeon YT, Kim SH, et al. NF-kappaB as a potential molecular target for cancer therapy. BioFactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- Li P, Furusawa Y, Wei ZL, Sakurai H, Tabuchi Y, Zhao QL, Saiki I, Kondo T. TAK1 promotes cell survival by TNFAIP3 and IL-8 dependent and NF-kB independent pathway in HeLa cells exposed to heat stress. Int J Hyperthermia. 2013;29:688–695. doi: 10.3109/02656736.2013.828104. [DOI] [PubMed] [Google Scholar]
- Li P, Zhao QL, Wu LH, Jawaid P, Jiao YF, Kadowaki M, Kondo T. Isofraxidin, a potent reactive oxygen species (ROS) scavenger, protects human leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in p53-independent manner. Apoptosis. 2014;19:1043–1053. doi: 10.1007/s10495-014-0984-1. [DOI] [PubMed] [Google Scholar]
- McAnulty SR, McAnulty L, Pascoe DD, Gropper SS, Keith RE, Morrow JD, Gladden LB. Hyperthermia increases exercise-induced oxidative stress. Int J Sports Med. 2005;26:188–192. doi: 10.1055/s-2004-820990. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ. 2014;21:1667–1676. doi: 10.1038/cdd.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M. Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 2011;18:1521–1530. doi: 10.1038/cdd.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, Haber DA, Settleman J. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–650. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Zhu X, Jin R, Wang C, Yan J, Zheng Q, Nanda A, Granger DN, Li G. Roles of the kinase TAK1 in CD40-mediated effects on vascular oxidative stress and neointima formation after vascular injury. PLoS One. 2014;9:e101671. doi: 10.1371/journal.pone.0101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel D. Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol. 2010;20:14–24. doi: 10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Swanlund JM, Kregel KC, Oberley TD. Autophagy following heat stress: the role of aging and protein nitration. Autophagy. 2008;4:936–939. doi: 10.4161/auto.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Hildeman D. Sensitization of T cells to apoptosis—a role for ROS? Apoptosis. 2004;9:515–523. doi: 10.1023/B:APPT.0000038033.14925.02. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;555:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, Privratsky JR, Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Calderwood SK. Autophagy, protein aggregation and hyperthermia: a mini-review. Int J Hyperthermia. 2011;27:409–414. doi: 10.3109/02656736.2011.552087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QL, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med. 2006;40:1131–1143. doi: 10.1016/j.freeradbiomed.2005.10.064. [DOI] [PubMed] [Google Scholar]