Abstract
Calcineurin is a calmodulin-stimulated phosphatase that regulates the nuclear translocation of nuclear factor of activated T cell (NFAT) c1-4 through dephosphorylation. We believe that this mechanism plays various roles in the remodeling and maintenance of Ictidomys tridecemlineatus skeletal muscle. During hibernation, bouts of torpor and arousal take place, and squirrels do not lose muscle mass despite being inactive. Protein expression of Ca2+ signaling proteins were studied using immunoblotting. A DNA-protein interaction ELISA technique was created to test the binding of NFATs in the nucleus to DNA probes containing the NFAT response element under environmental conditions reflective of those during hibernation. Calcineurin protein levels increased by 3.08-fold during torpor (compared to euthermic control), whereas calpain1 levels also rose by 3.66-fold during torpor. Calmodulin levels were elevated upon entering torpor. NFATc4 binding to DNA showed a 1.4-fold increase during torpor, and we found that this binding was further enhanced when 600 nM of Ca2+ was supplemented. We also found that decreasing the temperature of ELISAs resulted in progressive decreases in the binding of NFATs c1, c3, and c4 to DNA. In summary, calmodulin and calpain1 appear to activate calcineurin and NFATc4 during torpor. NFAT binding to target promoters is affected by intranuclear [Ca2+] and environmental temperatures. Therefore, Ca2+ signaling and temperature changes play key roles in regulation of the NFAT-calcineurin pathway in skeletal muscle of hibernating 13-lined ground squirrels over the torpor-arousal cycle, and they may contribute to the avoidance of disuse-induced muscle atrophy that occurs naturally in these animals.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-016-0713-5) contains supplementary material, which is available to authorized users.
Keywords: Nuclear factor of activated T cells, Calcium signaling, Hibernation, Ictidomys tridecemlineatus, Immunoblot, DPI-ELISA
Introduction
The 13-lined ground squirrel (Ictidomys tridecemlineatus) survives the cold winters in the prairies of North America by hibernating underground. In order to cope with extreme environmental stressors such as frigid temperatures and a lack of access to food, this animal has developed various survival mechanisms. During hibernation, these animals undergo cycles of torpor and arousal. During torpor, many of the above-mentioned survival mechanisms are activated, including metabolic rate depression (often to 2–4 % of normal conditions) and a decline in body temperature (Tb) to just 0–5 °C compared to a Tb of 35 to 38 °C when the animal is active and awake (Frerichs and Hallenbeck 1998; Storey 2010; Storey and Storey 2004; Wang and Lee 1996). During this process of metabolic rate depression within torpor, most physiological functions are reduced; respiration rates (approximately 2.5 % of euthermia), organ perfusion (<10 % of euthermia), and neuron firing are such examples (Storey and Storey 2004; McArthur and Milsom 1991; Buck and Barnes 2000). These long periods of torpor (often 1–2 weeks or more) are interspersed with short periods where metabolic rate and Tb return to euthermic values; this process is known as arousal. These cycles of torpor and arousal can save these hibernators as much as 88 % of the ATP required to otherwise sustain euthermic physiological conditions during the winter (Wang and Lee 1996).
The present study investigates how the skeletal muscle of 13-lined ground squirrels adapts on a molecular level to maintain function at low Tb to support long-term torpor. One issue affecting skeletal muscle during hibernation is the potential for disuse-induced muscle wasting, which occurs commonly after long periods of inactivity and results in reduced muscle mass, strength, and relative amount of slow oxidative muscle (Malatesta et al. 2009; Choi et al. 2009; Rourke et al. 2004; Bassel-Duby and Olson 2006). What makes hibernating mammals, specifically the thirteen-lined ground squirrel, interesting as a model to study muscle biology is that they demonstrate a lack of skeletal muscle atrophy despite prolonged periods of mechanical unloading that occur over long periods of hibernation (Cotton and Harlow 2015; Gao et al. 2012; Xu et al. 2013). Therefore, these animals have a natural ability to resist muscle wasting, which humans do not possess, and this makes hibernators fascinating as models to further our understanding of muscle preservation and regeneration mechanisms.
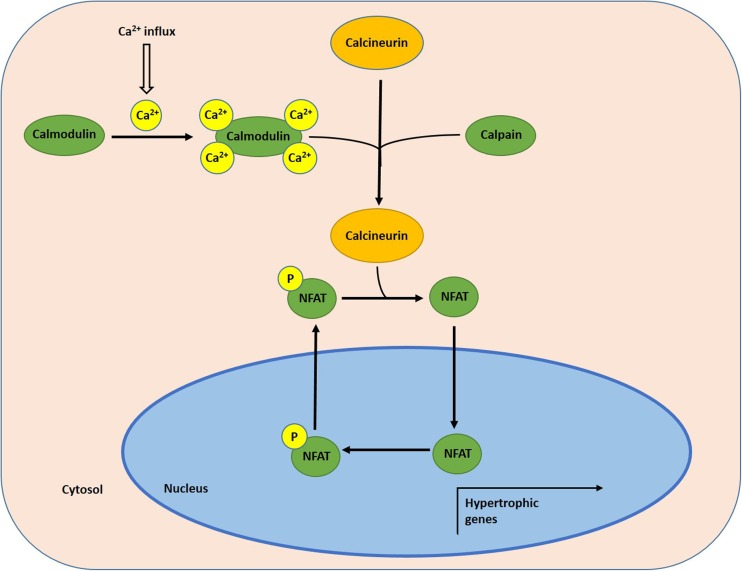
The present study focuses on the role and regulation of the nuclear factor of activated T cells (NFAT) family of transcription factors in I. tridecemlineatus, as they have been implicated as key regulators of skeletal muscle hypertrophy (Schiaffino et al. 2007; Hudson et al. 2014; Delling et al. 2000; Armand et al. 2008; Zhang and Storey 2015). The NFAT family contains five members named NFAT1-5 or NFATc1-4 and NFAT5, with NFATc1-4 being regulated primarily by calcineurin (Rao et al. 1997). Calcineurin is a calmodulin-stimulated protein phosphatase that regulates NFATs through dephosphorylation, thereby activating and allowing NFATs to translocate to the nucleus and regulate gene transcription (Rusnak and Mertz 2000). Calmodulin is a ubiquitously expressed calcium (Ca2+)-binding protein that is involved in a variety of signaling pathways that are Ca2+-dependent. It contains four EF-hand motifs, each of which binds a Ca2+ ion (Kretsinger 1987). Calmodulin regulates calcineurin by binding to the regulatory domain of the calcineurin A subunit when it is exposed, due to conformational changes caused by activation of the calcineurin B subunit, when there is an increase in intracellular Ca2+ levels (Yang and Klee 2000; Klee et al. 1979). When calcineurin B binds to Ca2+ ions, a conformational change occurs in its C-terminal autoinhibitory domain. The Ca2+-dependent cysteine protease - calpain, specifically calpain1/calpain-μ, cleaves the autoinhibitory domain, thus activating calcineurin (Burkard 2005; Lee et al. 2014; Shioda et al. 2006). Therefore, both calmodulin and calpain1 are important regulators of the NFAT-calcineurin pathway (Fig. 1).
Fig. 1.
Schematic diagram of the calcineurin-NFAT pathway and its regulation by Ca2+ signaling in myocytes. Ca2+ uptake by myocytes activates calmodulin and calpain1, which activate calcineurin as a result. Activated calcineurin removes the phosphate group on NFAT transcription factors, allowing for nuclear translocation, where it can regulate the expression of genes essential for muscle growth. When intracellular Ca2+ levels decrease and calcineurin becomes inactive, and NFAT is phosphorylated and exported by several different kinases including GSK3β, PKA, and Dyrk1a. Abbreviations: NFAT nuclear factor of activated T cells (NFAT)
Since activation of the NFAT-calcineurin pathway ultimately leads to increased binding of NFAT transcription factors to its target promoters, we used a DNA-protein interaction-enzyme-linked immunosorbent assay (DPI-ELISA) to quantitatively assess the binding of NFATs to its consensus binding sequence. This technique has been used previously to analyze transcription factor binding activity to DNA because of its simplicity and robustness (Brand et al. 2010, 2013; Jagelská et al. 2002). Given the extreme environmental stressors confronting 13-lined ground squirrels during hibernation, we suspected that environmental factors such as temperature could potentially affect the binding ability of NFATs and potentially other transcription factors, to DNA. Recent literature has begun to show that gene expression could be affected by temperature, but no study has directly investigated the temperature dependence of transcription factor binding to DNA (Novák et al. 2015; Chen et al. 2015; Swindell et al. 2007; Riehle et al. 2003). Most of these studies use DNA microarrays to study the global changes in gene expression when temperature stress is induced on an organism (Swindell et al. 2007; Riehle et al. 2003). However, although this approach identifies targets that may be involved in stress-response, it does not directly elucidate mechanisms such as transcription factor binding affinity. In addition to the ground squirrel’s ability to thermoregulate during torpor-arousal cycles, they also show enhanced capabilities to maintaine intracellular Ca2+ and urea concentrations in comparison with non-hibernating animals under the same temperature stress (Chilian and Tollefson 1976; Kristofferson 1963; Wang and Zhou 1999; Wang et al. 1999, 2002; Liu et al. 1991). Therefore, we adapted our DPI-ELISA protocol in order to run these environmental ELISAs that allow us to characterize the effects of temperature and different cellular metabolites such as Ca2+, and urea on transcription factor-DNA binding.
We sought to identify the role of Ca2+ signaling factors such as calmodulin, calpain1, and calcineurin on NFAT transcriptional regulation in the skeletal muscle of 13-lined ground squirrels. To do this, we quantified relative protein levels via immunoblotting and utilized the DPI-ELISA technique to measure changes in transcription factor binding to an oligonucleotide containing the NFAT response element. We predicted that the NFAT-calcineurin pathway would be activated through upregulation of Ca2+ signaling proteins during torpor. The secondary objective of this study was to identify the impact of environmental conditions on NFAT transcription factor binding to target genes. We tested this theory using a modified, environmental DPI-ELISA that allowed us to adjust the temperature and concentration of metabolites within the assay.
Materials and methods
Animal ethics statement and experimental conditions
Thirteen-lined ground squirrels (I. tridecemlineatus), which weighed 150–300 g, were wild-captured by the United States Department of Agriculture (USDA)-licensed trappers (TLS Research, Bloomingdale, IL). Animals were then transported to the Animal Hibernation Facility at the National Institute of Neurological Disorders and Stroke (NINDS, Bethesda, MD), where all experiments were conducted by the laboratory of Dr. J.M. Hallenbeck as previously described (McMullen and Hallenbeck 2010). All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke (NIH; animal protocol no. ASP 1223–05). Male and female ground squirrels were sampled equally in the study with a mixture of genders in each experimental condition, and all the animals were between 1–3 years of age, although the exact age of the animals is unknown since animals were wild-captured. At NINDS, the animals were housed individually in cages in a holding room with a constant ambient temperature of 21 °C under a 12-h light:12-h dark cycle. The animals were fitted with a sterile programmable temperature transponder (IPTT-300; Bio Medic Data Systems) injected subcutaneously in the intrascapular area while the squirrels were anesthetized with 5 % isofluorane. The animals were fed water and standard rodent chow ad libitum until they gained sufficient lipid stores to enter hibernation.
To enable a natural transition into torpor, the animals were transferred to constant darkness in an environmental chamber at 4–5 °C at the end of October. To not disturb the torpid squirrels, a red safe light (3–5 lux) was used when entering the chamber, and a heavy dark curtain was used to shield the shelves containing the cages and block the light and sound resulting from opening and closing the door to the environmental chamber. Body temperature (Tb), time elapsed, and respiration rates were monitored and used to determine the stage of torpor-arousal cycle. All the animals had been through torpor-arousal bouts prior to sampling. Four different animals were euthanized, and tissue samples were collected at six different sampling points: (1) EC designates euthermic in the cold room. These squirrels had a stable Tb of 37 °C for at least 3 days and were capable of entering torpor, but had not re-entered hibernation in the past 72 h. These euthermic animals displayed slow-wave sleep characteristics that were observed in all the sampling animals, and thus were chosen as the reference group to eliminate compounding variables of environmental light, temperature, feeding, in addition to time/season. (2) EN designates entrance into hibernation; entrance into the torpor-arousal cycle is characterized by falling Tb with sampling occurring between 31 and 18 °C. (3) ET designates early torpor; squirrels had entered torpor with a stable Tb at 5–8 °C for ~24 h. (4) LT designates late torpor; animals maintained a Tb at 5–8 °C for >5 days. (5) EA designates early arousal; animals with a Tb rising to at least ~12 °C with increasing respiration to at least 60 breaths/min after torpor, (6) LA designates late arousal; animals with increased respiration rate and Tb of 28–32 °C. A diagram of these torpor-arousal cycle stages is shown in a recent review written by Tessier and Storey (Tessier and Storey 2016).
Total protein extract preparation
Total soluble protein extracts were prepared as previously described (Zhang and Storey 2015) for samples of frozen skeletal muscle from four animals for each of the six stages in the torpor-arousal cycle (EC, EN, ET, LT, EA, and LA). Frozen samples of ~0.5 g tissue were quickly weighed, powdered into small pieces under liquid nitrogen, and then homogenized (using a Polytron PT10) 1:3 w:v in ice-cold homogenizing buffer (20 mM Hepes, 200 mM NaCl, 0.1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate at a pH of 7.5) with 1 mM phenylmethylsulfonyl fluoride (Bioshop) and 1 μL/mL protease inhibitor cocktail (Bioshop) added. Samples were centrifuged at 10,000 rpm for 10 min at 4 °C, and supernatants were removed. Soluble protein concentration was assayed using the Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA; Cat #500-0006) at 595 nm on a MR5000 microplate reader. The samples were then adjusted to a final protein concentration of 10 μg/μL by the addition of a small volume of homogenizing buffer, and then aliquots were combined 1:1 v:v with 2× SDS loading buffer (100 mM Tris-base, pH 6.8, 4 % w:v SDS, 20 % v:v glycerol, 0.2 % w:v bromophenol blue, 10 % v:v 2-mercaptoethanol) and then boiled. The final protein samples at a concentration of 5 μg/μL were stored at −20 °C until use.
Preparation of nuclear protein extracts
Nuclear protein extracts were prepared as previously described (Zhang and Storey 2015) and were separately extracted from the skeletal muscle of the four animals for each of the six experimental stages. Frozen skeletal muscle samples were homogenized 1:2 w:v using a Dounce homogenizer (five piston strokes) in lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 10 mM EDTA, 20 mM β-glycerophosphate), with 10 μL of 100 mM DTT, 10 μL of protease inhibitor cocktail added immediately before homogenization. The samples were centrifuged for 10 min at 10,000 rpm and 4 °C, and the supernatants were removed as the cytoplasmic fraction. Pellets were resuspended in 147 μL of nuclear extraction buffer (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 10 % v/v glycerol, 20 mM β-glycerophosphate) with 1.5 μL of 100 mM DTT, and 1.5 μL of protease inhibitor cocktail was added. The samples were incubated on ice with gentle rocking for 1 h and then centrifuged for 10 min at 10,000 rpm at 4 °C. Protein concentrations were determined with the Bio-Rad protein assay, adjusted to 5 μg/μL, and the samples were stored at −80 °C until use.
Western blotting
Equal amounts of protein from each sample (25 μg) were loaded onto 8 % (calcineurin, calpain1) polyacrylamide gels and were run at 180 V for 60–90 min. For calmodulin, 15 % polyacrylamide gels were used and loaded with 35 μg of protein for each sample. Proteins were then transferred to PVDF membranes by electroblotting at 320 mA for 90 min (calcineurin, calpain1) or at 30 V for 100 min (calmodulin) using a transfer buffer containing 25 mM Tris (pH 8.5), 192 mM glycine, and 10 % v:v methanol at room temperature. Membranes were then blocked for 30 min with 5 % w:v milk in 1× TBST (20 mM Tris base, pH 7.6, 140 mM NaCl, 0.05 % v:v Tween-20, 90 % v:v ddH2O). After washing for 3 × 5 min again with 1× TBST, membranes were probed with specific primary antibodies at 4 °C overnight. Antibodies specific for mammalian calmodulin (06–396) from Upstate Biotechnology (Lake Placid, NY), as well as calcineurin A (GTX111039) and calpain1 (GTX102340) antibodies from Genetex (Irving, CA), were purchased and used at a 1:1000 v:v dilution in 1× TBST. After probing with primary antibody, membranes were washed for 3 × 5 min with 1× TBST and then incubated with HRP-linked anti-rabbit IgG secondary antibody (Bioshop: 1:6000 v:v dilution) for 30 min at room temperature. After a second set of three washes, bands were visualized by enhanced chemiluminescence (H2O2 and Luminol). Then, blots were restained using Coomassie blue (0.25 % w/v Coomassie brilliant blue, 7.5 % v/v acetic acid, 50 % methanol) to visualize total protein levels. Immunoblot bands for ground squirrel proteins corresponded to the molecular weights indicated on the respective antibody specification sheets or the amino acid sequence of the I. tridecemlineatus isoform, as confirmed by running PINK Plus Prestained Protein Ladder (FroggaBio) for calpain1 (82 kDa), calcineurin (59 kDa), and calmodulin (19 kDa). For calmodulin, the molecular weight was determined using the I. tridecemlineatus protein sequence from the NCBI protein database (NP_001182569.1) and calculated using the ExPASy Mw/pI tool.
DNA-protein interaction-ELISA
DNA oligonucleotides were designed based on the DNA-binding elements of NFATc1-4 and were produced by Sigma Genosys (Oakville, ON, Canada). The biotinylated probe (NFAT 5′-Biotin-GGGAAGGAAAGTGCGGGTGG-3′) and the complement probe (NFAT 5′-Biotin CCACCCGCACCCTTTTTCCC-3′) were first diluted in sterile water (500 pmol/μL), and the two probes were mixed 1:1 v:v for a total of 20 μL. The probes were then placed in a thermocycler for 10 min at 94 °C and gradually cooled to room temperature. Double stranded DNA probes were diluted in 1 × PBS (137 mM NaCl, 2.7 mM KCL, 10 mM Na2HPO4, pH 7.4), and 50 μL of diluted DNA probe was added (40 pmol DNA/well) to streptavidin-coated wells on a microplate. Following a 1-h incubation, unbound probe was discarded, and wells were rinsed twice with 1× wash buffer (1× PBS containing 0.1 % Tween-20), and a third time with 1× PBS. Transcription factor binding buffer (10 mM HEPES, 50 mM KCL, 0.5 mM EDTA, 3 mM MgCl2, 10 % v/v glycerol, 0.5 mg/mL bovine serum albumin, 0.05 % NP-40, 0.5 mM DTT, 20 pg/μL Salmon Sperm DNA, 44 mM NaCl, pH 7.9) was added to each well containing the DNA probe along with 27.5 μg of the nuclear protein extract. Two negative control wells were loaded with transcription factor binding buffer but no protein. Following another 1-h incubation with gentle shaking, protein mixtures were discarded, and the wells were washed three times with 1× wash buffer.
Diluted primary antibody (1:500) was then added (60 μL/well) for 1 h. Primary antibodies specific for mammalian NFATc1 (sc-13033), c3 (sc-8321), and c4 (sc-13036) were purchased from Santa Cruz Biotechnologies and used for the primary antibody incubations. Primary antibodies were then discarded, and wells were rinsed three times with 1× wash buffer before incubation with diluted secondary (1:1000, 60 μL/well) for 1 h. This antibody was then discarded, and wells were rinsed three times with wash buffer. The secondary antibodies used were the same as those used for immunoblots. After secondary antibody incubation and washing, bound antibody was detected using tetramethylbenzidine (TMB) (Bioshop). A 60-μL aliquot of TMB was added to each well, color was developed for 10–15 min, and then the reaction was stopped with 60 μL of 1 M HCl. Absorbance was measured at 450 nm (reference wavelength of 655 nm) using a Multiskan spectrophotometer. To control for background absorbance and non-specific binding, test strip ELISA experiments were run with negative controls containing no probe or no protein or no primary antibody added being run in duplicates using a pooled sample of multiple sampling points. Conditions were optimized such that negative control wells showed >50 % decreases in absorbance relative to sample wells before quantification runs of sampling points were conducted.
Environmental DPI-ELISA
To assess how transcription factor-DNA binding is altered when environmental conditions (temperature, [CA2+], [urea]) are altered, the DPI-ELISA protocol described above was modified. To test for the effect of temperature on transcription factor-DNA binding, the initial DNA probe synthesis, incubation, and washing steps were carried out as previously described. Afterwards, transcription factor binding buffer was added to each well containing the DNA probe, plus one other well that is used to monitor solution temperature. Buffer temperature was monitored using a digital thermometer with two probes, one placed outside the solution to monitor ambient temperature and the other placed inside the well to monitor solution temperature. The ELISA plate was placed in either a 4 °C fridge, a 37 °C incubator, or left at room temperature. When the solution temperature has matched and stabilized to the ambient temperature inside the fridge or incubator, 27.5 μg of nuclear extracts of EC and LT samples were added to wells containing the DNA probe with the exception of the duplicate negative controls, and the plates were placed on shakers. Following the 1-h incubation, all plates were placed at room temperature, and the rest of the procedure was performed as described above. Temperature DPI-ELISAs were performed to evaluate (1) the effect of temperature (37, 21, and 4 °C) on NFAT-DNA binding for the EC samples, (2) the effect of temperature (37, 21, and 4 °C) on binding for the LT samples, and (3) the difference in binding between the EC and LT sampling points using their physiological temperatures, 37 and 4 °C, respectively.
In order to test for the effect of Ca2+ and urea on transcription factor-DNA binding, the DPI-ELISA protocol described above was followed; adjusting the transcription factor binding buffer by adding Ca2+ or urea. To assess the effect of Ca2+ on transcription factor-DNA binding, quantification runs were performed on four biological replicates of the LT samples with no protein and no Ca2+ (negative controls), no Ca2+, 100 nM Ca2+, and 600 nM Ca2+ added to the transcription factor binding buffer during the protein incubation step; 100 and 600 nM of Ca2+ were selected because they represent the minimum and maximum concentrations of nuclear Ca2+ that have been identified mathematically and experimentally (Brière et al. 2006; Xiong et al. 2012; Luan 2011; Dobi and Agoston 1998). A similar experiment was performed to assess for the effect of urea on transcription factor-DNA binding, with quantification runs being conducted for the LT samples (n = 4) with no protein and no urea (negative controls), no urea, 5 mM urea, and 100 mM urea added to the transcription factor binding buffer during the protein incubation step. These two concentrations were tested as 5 mM is approximately the normal physiological concentrations of serum urea in hibernating mammals (Chilian and Tollefson 1976; Kristofferson 1963; Stenvinkel et al. 2013). Also, 100 mM was shown experimentally as the maximum concentration of urea that could be supplemented to media before cell culture growth and survival was inhibited (Yancey and Burg 1990).
Comparative sequence analysis
The amino acid sequences of calcineurin (rabbit: XP_008248097.1, squirrel: XP_005325804.1, mouse: NP_032939.1, human: AAB23769.1), calmodulin (rabbit: NP_001182569.1, squirrel: XP_005226620.1, mouse: NP_031615.1, human: AAD45181.1), and calpain1 (rabbit: XP_008250016.1, squirrel: XP_005333505.1, mouse: NP_031626.1, human: NP_001185797.1) from the NCBI protein database were compared using the Clustal Omega multiple alignment tool at EMBL-EBI using default multiple sequence alignment and pairwise alignment options. Pairwise alignment scores (percent identity) between the two sequences are calculated by taking the number of identities between the two sequences, dividing by the length of the alignment, and representing this ratio as a percentage (Larkin et al. 2007; Goujon et al. 2010; McWilliam et al. 2013).
Quantification and statistics
Band densities on chemiluminescent immunoblots were visualized using a Chemi-Genius BioImaging system (Syngene, Frederick, MD) and quantified using the Gene Tools software. Immunoblot band density in each lane was standardized against the summed intensity of a group of Coomassie-stained protein bands in the same lane; this group of bands was chosen because they were not located close to the protein band of interest but were prominent and constant across all the samples. This method of standardizing against a total protein loading control has been suggested to be more accurate in comparison with standardizing against housekeeping proteins such as tubulin (Eaton et al. 2013). For DPI-ELISA quantification runs, absorbance readings were corrected by subtracting values for each sampling point from negative controls containing no protein, and these values were normalized relative to EC. Similarly, western blot band densities were also normalized at each other sampling point relative to EC. Immunoblotting and absorbance data are expressed as means ± SEM, n = 4 independent samples from different animals. Statistical testing used the one-way ANOVA and the Tukey post hoc functions from the GraphPad Prism software (San Diego, CA).
Results
Calcineurin, calmodulin, and calpain protein levels
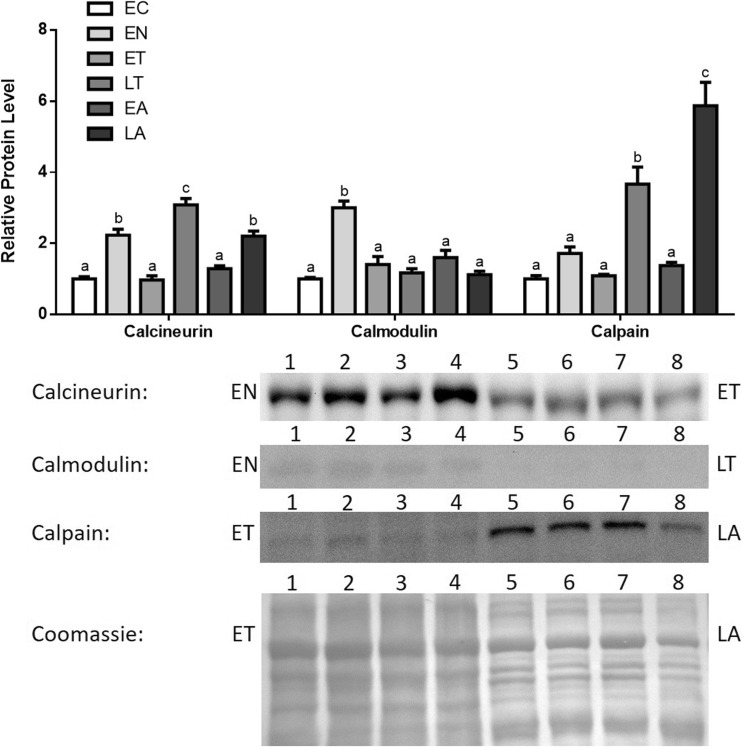
Immunoblotting was used to assess changes in the relative protein levels of the Ca2+-signaling factors, calcineurin, calmodulin, and calpain1 levels in ground squirrel skeletal muscle over the torpor-arousal cycle (Fig. 2.). Calcineurin protein levels increased upon entering torpor (EN) by 1.19-fold in comparison with those at euthermic control (EC), p < 0.05. Protein levels then returned to baseline levels at EC during early torpor (ET), but it spiked once more by 2.08-fold (in comparison with those at EC, p < 0.05) and reached its highest level during late torpor (LT). Upon entering arousal, calcineurin levels decreased once again to baseline at early arousal (EA) and then increased once more by 1.2-fold (compared to those at EC, p < 0.05) at late arousal (LA). Calpain1 showed a similar pattern of expression, where spikes in protein levels occurred at EN, LT, and LA. Calpain levels increased modestly at EN by 0.72-fold relative to EC. At LT, there was a greater increase of 2.37-fold relative to EC (p < 0.05). The final spike at LA was even greater, where protein levels increased by 4.4-fold in comparison with that at EC (p < 0.05). Calmodulin protein levels remained fairly constant throughout the torpor-arousal cycle with the exception of EN, where levels increased by two-fold relative to those of EC (p < 0.05) (Fig. 2).
Fig. 2.
Changes in calcineurin, calmodulin, and calpain1 total protein levels in skeletal muscle over the torpor-arousal cycle in I. tridecemlineatus. Calcineurin, calmodulin, and calpain1 total protein expression levels were visualized at the six sampling points: euthermic cold (EC), entrance (EN), early torpor (ET), late torpor (LT), early arousal (EA), and late arousal (LA). See “Materials and methods” section for more extensive definitions of the sampling points. Westerns blots and Coomassie total protein loading controls representative of the results are shown for pairs of selected time points, which are labeled to the left and right of the immunoblot picture. Lane numbers labeled along the top indicate replicates (n = 4) of one sample (e.g., for calcineurin: EN lanes 1, 2, 3, and 4) and four of another sample (e.g., for calcineurin: ET lanes 5, 6, 7, and 8). Also shown are histograms with mean standardized band densities (±S.E.M., n = 4 independent protein isolations from different animals). Data was analyzed using a one-way analysis of variance with a post hoc Tukey’s test (p < 0.05); for each parameter measured, values that are not statistically different from each other share the same letter notation
NFATc4 relative binding to DNA throughout the torpor-arousal cycle
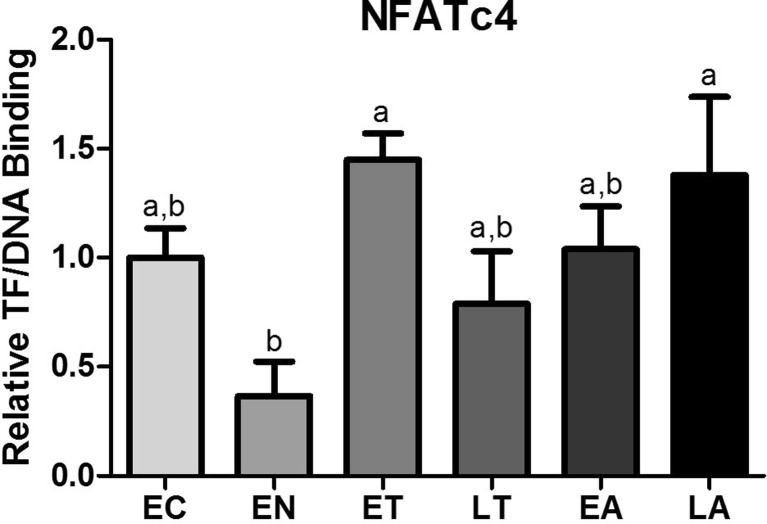
Using the rVista program and the conserved NFAT-binding sequence from the literature (GGAAA), DNA probes used to analyze NFAT binding were created as previously described (Zhang and Storey 2015; Rao et al. 1997; Hung et al. 2008). A DNA-protein interaction (DPI)-ELISA was used to study the binding ability of transcription factors in nuclear extracts of 13-lined ground squirrel skeletal muscle with the DNA probes targeting the promoter regions of these transcription factors. DPI-ELISAs were used because of its simplicity and robustness (Brand et al. 2010, 2013; Jagelská et al. 2002). We identified in this study that NFATc4 was able to bind to the DNA oligonucleotide (Fig. 3). Relative binding to DNA was measured for six time points: EC, EN, ET, LT, EA, and LA. NFATc4 binding levels decreased modestly by 63 % at EN from EC, then binding increased dramatically by 3.96-fold relative at ET relative to EN (p < 0.05). Following ET, binding activity decreased slightly at LT, then it increased slowly during EA and LA, with binding at LA being 3.77-fold greater than that at EN (p < 0.05).
Fig. 3.
Changes in binding of the transcription factor NFATc4 to a DNA-binding element designed for the NFAT consensus sequence in the skeletal muscle of I. tridecemlineatus over the torpor-arousal cycle. DNA-protein interaction (DPI)-ELISA absorbance readings were corrected by subtraction of negative controls containing no protein, and values were expressed relative to EC. Histograms show mean relative values ± S.E.M., n = 4 independent biological replicates for each of the six experimental conditions. Data was analyzed using a one-way analysis of variance with a post hoc Tukey’s test (p < 0.05); for each parameter measured, values that are not statistically different from each other share the same letter notation
Effect of temperature on NFATc1, c3, and c4 relative binding to DNA
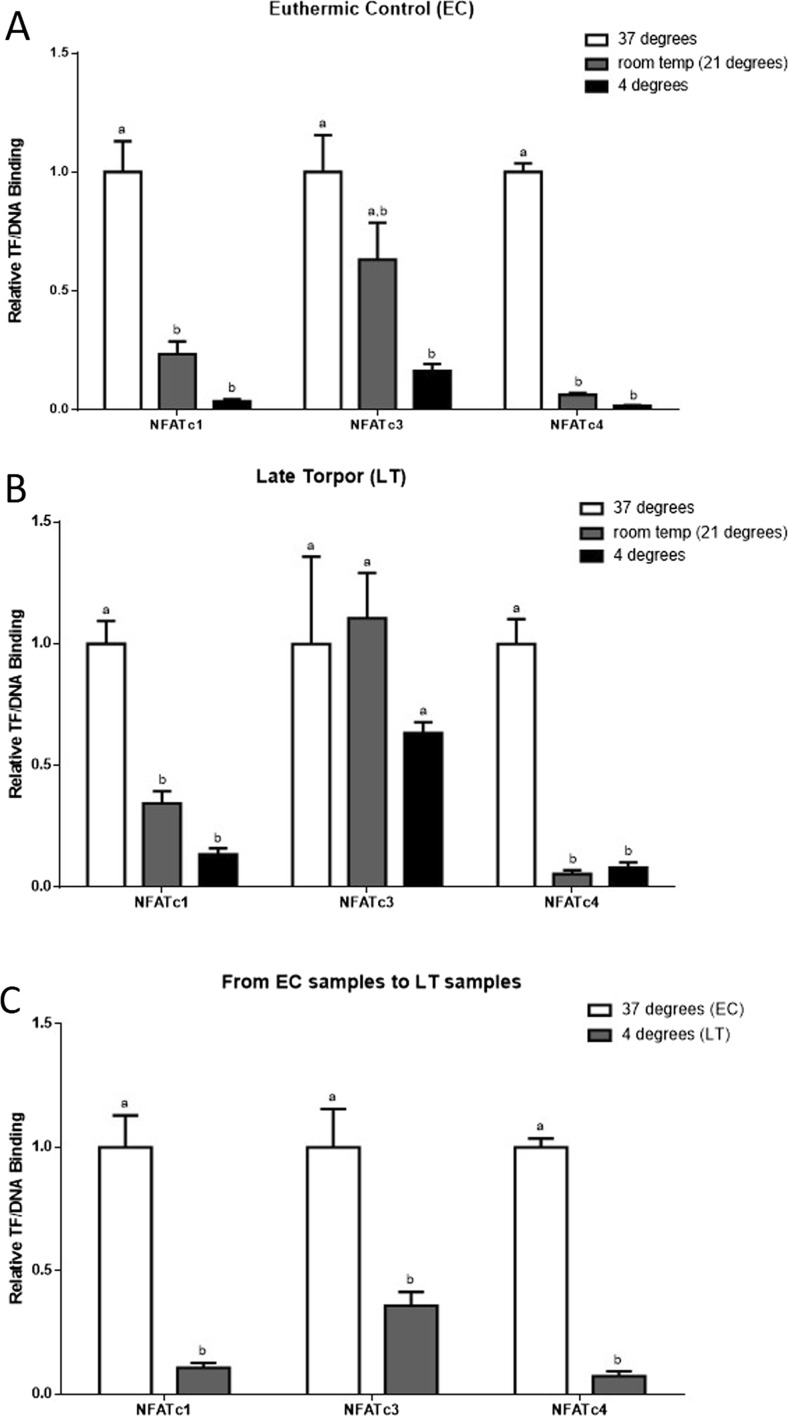
Due to the drastic changes in Tb when ground squirrels enter torpor, we modified the DPI-ELISA in order to study the effect of temperature on NFATc1, c3, and c4 transcription factor binding to DNA. Three temperatures (37, room temperature −21, and 4 °C) were studied at the EC and LT sampling points. As mentioned previously, the EC animals had not entered hibernation yet, so their Tb remained at 37 °C. The LT animals were at the deepest part of torpor, where Tb was 4–5 °C (McMullen and Hallenbeck 2010).
The temperature DPI-ELISA experiments performed on the EC time point showed that NFATc1 and NFATc4 binding to DNA decreased dramatically (p < 0.05) by 77 and 94 %, respectively, from 37 °C to room temperature. NFATc3, on the other hand, showed a modest decrease of 37 % in binding activity. However, when we compared changes in binding for all the three NFATs between 37 and 4 °C, they all showed significant decreases in binding (p < 0.05) by at least 84 % (Fig. 4a). Therefore, all the three NFATs showed progressive declines in binding activity at the EC time point as temperature was decreased. The temperature DPI-ELISA using the LT sampling point showed a similar pattern for NFATc1 and c4, where binding decreased by 66 and 95 %, respectively, from 37 °C to room temperature (Fig. 4b). NFATc1 binding levels decreased further from 37 to 4 °C by 86 % (p < 0.05). On the other hand, NFATc4 binding levels stabilized from room temperature to 4 °C. For the LT sampling point, NFATc3 binding levels did not seem to be affected much by the changes in temperature, as there was only a modest decline in binding by 46 % when comparing 37 to 4 °C (Fig. 4b). While analyzing the difference in transcription factor binding to DNA at physiological conditions from EC at 37 °C to LT at 4 °C, we observed sharp declines in binding for both NFATc1 and c4 by 89 and 93 %, respectively (p < 0.05). The decline in binding from EC to LT for NFATc3 was 64 %, which is less compared to the differences observed for NFATc1 and c4, but this difference was still significant (p < 0.05) (Fig. 4c).
Fig. 4.
Effect of adjusting temperature on transcription factor-DNA binding of NFATc1, c3, and c4. a) Transcription factor-DNA binding was measured at 37, 24 (room temperature), and 4 °C at the EC sampling point before hibernation is initiated. b) Transcription factor-DNA binding was measured at 37, 24 (room temperature), and 4 °C at the LT sampling point deep within hibernation. c) Changes in the binding of NFAT c1, c3, and c4 transcription factors to DNA at physiological temperatures from EC (37 °C) and LT (4 °C). Modified DPI-ELISA absorbance readings were corrected by subtraction of negative controls containing no protein, and values were expressed relative to 37 °C for both EC and LT. Data in panel (a) and (b) were analyzed using a one-way analysis of variance with a post hoc Tukey’s test (p < 0.05), for each parameter measured, and the data in panel (c) were analyzed using a paired T test (p < 0.05). Values that are not statistically different from each other share the same letter notation
Effect of Ca2+ and urea on NFATc1, c3, and c4 relative binding to DNA
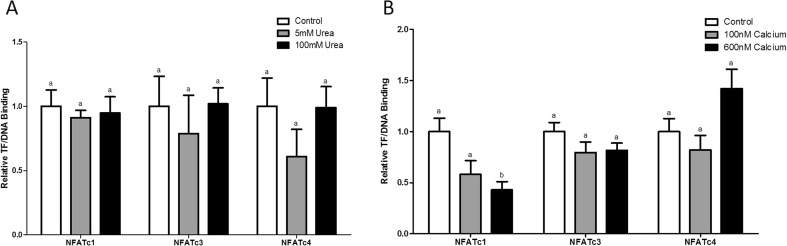
We tested for the effect of adding Ca2+ and urea to the DPI-ELISA assay in an attempt to discover how these two metabolites/substrates affect NFATc1, c3, and c4 transcription factor-DNA binding. Urea and Ca2+ are of particular interest due to the unique changes in the animal’s regulation of the urea cycle and Ca2+ signaling, which occur during mammalian hibernation (Chilian and Tollefson 1976; Wang et al. 1999, 2002; Stenvinkel et al. 2013; Lee et al. 2012; Epperson et al. 2011). The addition of 5 and 100 mM of urea seemed to have no effect on the binding of NFATc1, c3, or c4 to DNA, with binding levels remaining stable throughout the different conditions during torpor (Fig. 5a). When 100 nM of Ca2+ was added to the protein incubation, the binding of NFATc1 to DNA showed a sizeable difference (42 % decrease relative to the no Ca2+ control) out of the three NFATs tested at LT. When 600 nM of Ca2+ was added however, NFATc1-DNA binding continued to decrease (57 % decrease relative to the control, p < 0.05) with NFATc4 binding showing a 0.42-fold increase relative to the no Ca2+ control. Throughout the conditions, NFATc3 binding to DNA did not change appreciably during torpor (Fig. 5b).
Fig. 5.
Effect of adding free urea and Ca2+ on transcription factor-DNA binding of NFATc1, c3, and c4. a) Transcription factor-DNA binding was measured during the LT sampling point with no urea added (control), 5 mM urea added, and 100 mM urea added. b) Transcription factor-DNA binding was measured during the LT sampling point with no Ca2+ added, 100 nM Ca2+ added, and 600 nM Ca2+ added. Modified DPI-ELISA absorbance readings were corrected by subtraction of negative control containing no protein, and values were expressed relative to the control (no Ca2+ or no urea added). Data was analyzed using a one-way analysis of variance with a post hoc Tukey’s test (p < 0.05); for each parameter measured, values that are not statistically different from each other share the same letter notation
Comparative amino acid sequence analysis of calmodulin, calpain1, and calcineurin
The amino acid sequences of calmodulin, calpain1, as well as calcineurin in rabbit, 13-lined ground squirrel, mouse, and human were compared and tested for sequence conservation using the multiple-alignment tool, Clustal Omega (Larkin et al. 2007; Goujon et al. 2010; McWilliam et al. 2013). Alignment of the calmodulin sequences from the different animals showed very strong conservation as the amino acid sequences were exactly the same between all the four animals (Fig. S1a). Analysis done on the degree of conservation for calmodulin, calpain1, and calcineurin using pairwise alignments between the four animals indicated that the percent identity for all proteins, especially calmodulin and calpain1, were strong (>78 %) (Fig. S1b).
Discussion
The purpose of this study was to explore the molecular mechanisms underlying skeletal muscle remodeling and preservation that occur during hibernation in a well-researched model hibernator, the 13-lined ground squirrel. Recently, we have shown that the NFAT transcription factors play a key role in skeletal muscle during torpor to promote hypertrophy and maintain muscle mass (Zhang and Storey 2015). This occurs despite a predisposition for disuse-induced muscle atrophy that was shown in torpor through upregulation and activation of forkhead box O subclass (Foxo) transcription factors, which regulate many ubiquitin ligases in the ubiquitin proteasomal system (Wu and Storey 2014; Sandri et al. 2004). In the present study, we were interested in further characterizing the role of not only the NFAT transcription factors but also its regulation through calcineurin and other Ca2+ signaling proteins, within skeletal muscle. Calmodulin and calpain1 are two such proteins that contribute to activation of the NFAT-calcineurin pathway, thereby leading to increased gene expression of hypertrophic targets (Yang and Klee 2000; Klee et al. 1979; Burkard 2005; Lee et al. 2014; Shioda et al. 2006). Due to the improved Ca2+-handling abilities of ground squirrels in comparison with non-hibernating mammals, [Ca2+] changes very little from euthermia to the 0–5 °C Tb seen during torpor (Frerichs and Hallenbeck 1998; Wang and Lee 1996; Wang and Zhou 1999; Wang et al. 1999, 2002; Liu et al. 1991). However, the amplitude of Ca2+ transients following excitation is actually increased following excitation at low temperatures, and as a result, stronger contractions with higher amplitudes are seen at lower temperatures (Wang et al. 1997, 2000; Liu et al. 1990, 1993). Therefore, greater spikes in intracellular [Ca2+] following an action potential may lead to an activation of NFAT-calcineurin pathway, thus allowing for a maintenance of muscle mass during hibernation.
Our results show that indeed, there is an upregulation of Ca2+ signaling proteins like calmodulin and calpain1 during torpor, where protein levels increased by 2-fold and 0.72-fold relative to the euthermic control (EC), respectively, upon entering torpor (EN). These increases are accompanied by a 1.19-fold rise (compared to EC) in calcineurin levels downstream (Fig. 2). As a result of this increase in calcineurin levels and activity, NFATc4 translocates to the nucleus and shows an increase in binding to DNA during early torpor (ET) (Fig. 3). A similar pattern is seen during late arousal (LA), where calpain1 levels increased by 4.4-fold relative to EC, and there was an accompanying increase in calcineurin levels as well (1.2-fold compared to EC) (Fig. 2). Once more, there was a 3.77-fold increase in NFATc4 binding activity (relative to EN) accompanying the upregulation and activation of calcineurin at LA (Fig. 3). This upregulation of the NFAT-calcineurin pathway during LA is somewhat unexpected and could reflect the role that NFATc4 plays in not only muscle remodeling, but the generation of reactive oxygen species, which are produced rapidly due to oxidative thermogenesis in squirrels during arousal (Kalivendi et al. 2005). During late torpor (LT), 2.08-fold and 2.37-fold rises in calcineurin and calpain1 levels respective, relative to EC, were observed (Fig. 2). Furthermore, these changes correlate with the dramatic increase in NFATc3 binding activity that was seen at LT in a previous study (Zhang and Storey 2015). The regulation of NFATc3 activity through Ca2+ signaling provides an explanation for the preservation of muscle mass during torpor given the vital role that NFATc3 plays in regulation muscle remodeling (Hudson et al. 2014; Delling et al. 2000; Armand et al. 2008; Demonbreun et al. 2010).
Having established the important role of Ca2+ in regulating the NFAT-calcineurin pathway during hibernation through Ca2+-binding proteins, we became interested in determining whether Ca2+ can directly affect NFAT binding to target promoters during torpor. Several studies have previously shown that intranuclear Ca2+ can regulate gene expression by directly binding to DNA or through regulation of transcription factors and their co-factors (Dobi and Agoston 1998; Chawla et al. 1998; Pusl et al. 2002; Thompson et al. 2003). We identified using a modified environmental DPI-ELISA that Ca2+ did indeed affect the binding of NFAT transcription factors to DNA during torpor. It was observed that progressively increasing [Ca2+] decreased the binding of NFATc1, whereas NFATc4 showed increased binding to DNA when [Ca2+] was increased to 600 nM (Fig. 5b). The differential regulation of NFAT transcription factors by intranuclear calcium may be due in part to the different roles of each NFAT. For example, NFATc3 is known to regulate targets like myoferlin that are important for muscle preservation during torpor (Zhang and Storey 2015; Demonbreun et al. 2010). As a result, NFATc3 binding to DNA was not decreased by Ca2+ to the same extent as NFATc1 after increases in intracellular [Ca2+] were observed from the sharp Ca2+ transients seen at lower temperatures during torpor (Wang et al. 1997, 2000; Liu et al. 1990, 1993). Due to the specificity of intranuclear Ca2+ on NFAT-DNA binding during torpor, this effect is likely not due to the binding and blocking of DNA by intranuclear Ca2+ (Dobi and Agoston 1998). This effect is most likely due to Ca2+ regulation of specific export kinases like calmodulin-dependent protein kinase IV (CAMKIV) or through specific coactivators of individual NFATs, such as CREB-binding protein (CBP) (Chawla et al. 1998; Yang et al. 2001). Given that urea is another key metabolite that is crucial during hibernation, specifically torpor, we created an environmental DPI-ELISA to test whether urea could affect NFAT binding activity as well (Stenvinkel et al. 2013; Epperson et al. 2011). Urea did not have a significant effect on NFAT binding to DNA in any of the tested conditions during LT (Fig. 5a). Theoretically, the nuclear membrane allows compounds of 60 kDa or less to pass through into the nucleus, and urea is just over that cut-off (Gerace and Burke 1988). Therefore, it would not be able to translocate passively into the nucleus without the aid of channel proteins or transporters. Aquaporin proteins are a family of water-channel proteins that are known to transport urea, and they have been shown to reside partially on the nuclear membrane (Srivastava et al. 2014; Sato et al. 2011). Therefore, it is possible for urea to accumulate inside the nucleus, but as we have shown, the amount of nuclear urea is insufficient to significantly affect NFAT binding to DNA.
Given the extreme variations in temperature that occurs during torpor-arousal cycles from euthermia (37 °C) to torpor (0–4 °C), we were interested in knowing whether temperature could potentiate or inhibit the binding of transcription factors, such as NFATs (Frerichs and Hallenbeck 1998; Storey 2010; Storey and Storey 2004; Wang and Lee 1996). We carried out a modified DPI-ELISA, adjusting for the ambient temperature during the protein incubation step where binding between transcription factors and the DNA oligonucleotide occurs. We found that there were dramatic differences in transcription factor binding of NFATc1, c3, and c4 as the temperature was progressively decreased from euthermic (EC) Tb (37 °C) to the depressed Tb seen during LT (4 °C) (Fig. 4a–c). When comparing the declines in NFAT-DNA binding from EC to LT, we can see that there was a lesser decrease in the binding activity of NFATc3 (64 %) relative to NFATc1 (89 %) and NFATc4 (93 %) (Fig. 4c). This relatively smaller decrease in binding activity could partly explain the preservation of skeletal muscle mass during torpor, as NFATc3 plays the most vital role in coordinating muscle remodeling out of the four NFATs (Hudson et al. 2014; Delling et al. 2000; Armand et al. 2008; Demonbreun et al. 2010). Due to metabolic rate depression and the need to conserve ATP during torpor, the expression of nonessential genes is likely halted; therefore, NFATc1 and c4 activities show a greater decline compared to NFATc3. This study is the first to identify changes in transcription factor-DNA binding affinity that are temperature-dependent, although further studies need to be conducted to determine whether our findings are specific for NFAT transcription factors or if it reflects a greater number of transcription factors. More importantly, further studies need to determine whether the temperature-sensitivity of NFAT transcription factors are due to conformational changes that occur at lower temperatures to the protein itself, to DNA, or if it has to do with interactions with temperature-sensitive co-factors. For example, NFATc2 has been shown to cooperate with heat shock transcription factor 1 (HSF1), which is responsible for regulating the gene expression of other heat shock proteins (Hayashida et al. 2010).
In conclusion, our findings demonstrate that Ca2+ signaling plays a key role in regulating the NFAT-calcineurin pathway in skeletal muscle of hibernating 13-lined ground squirrels over the torpor-arousal cycle. Activation of NFATc4 occurs during ET and LA as a result of upregulation and activation of calcineurin through calmodulin-binding and calpain1 cleavage. Also, activation of NFATc3 occurs during LT as a result of upregulation in calcineurin and calpain1 so that NFATc3 can regulate the expression of genes to maintain muscle mass despite disuse-induced muscle atrophy during torpor (Zhang and Storey 2015; Wu and Storey 2014). In addition, this study developed and used a novel technique, the environmental DPI-ELISA, to study the effects of environmental stimuli such as temperature, [urea], and [Ca2+] on NFAT binding to target promoters, which was the secondary objective of this study. We found that [urea] has little effect on NFAT binding, but intranuclear [Ca2+] seems to decrease the DNA-binding affinity of NFATc1, possibly through Ca2+ regulation of export kinases and coactivators. NFATc3 and c4 binding activity were not decreased with increasing [Ca2+] as they play more significant roles in the regulation of targets necessary for the maintenance of muscle mass (Zhang and Storey 2015; Demonbreun et al. 2010). Furthermore, we determined that temperature differences from euthermia (37 °C) to torpor (4 °C) have profound effects on the binding of NFATc1, c3, and c4 although the effects are more pronounced for NFATc1 and c4. The novel finding that transcription factor binding to DNA is temperature-dependent should be explored further for other transcription factors and to identify potential mechanisms. These findings contribute to our understanding of muscle remodeling, and these mechanisms involved in preserving ground squirrel skeletal muscle throughout the torpor-arousal cycle make studying the ground squirrel biologically relevant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 12 kb)
Acknowledgments
We thank Dr. J.M. Hallenbeck and Dr. D.C. McMullen (NINDS, NIH, Bethesda) for providing the tissue samples for this study. This work was supported by a discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (# 6793) to Kenneth B. Storey. Kenneth B. Storey holds the Canada Research Chair in Molecular Physiology; Yichi Zhang held a postgraduate Queen Elizabeth II Graduate Scholarship in Science and Technology.
Reference
- Armand AS, Bourajjaj M, Martínez-Martínez S, el Azzouzi H, da Costa Martins PA, Hatzis P, Seidler T, Redondo JM, De Windt LJ. Cooperative synergy between NFAT and MyoD regulates myogenin expression and myogenesis. J Biol Chem. 2008;283:29004–29010. doi: 10.1074/jbc.M801297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Brand LH, Kirchler T, Hummel S, Chaban C, Wanke D. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods. 2010;6:25. doi: 10.1186/1746-4811-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand LH, Henneges C, Schüssler A, Kolukisaoglu HÜ, Koch G, Wallmeroth N, Hecker A, Thurow K, Zell A, Harter K, Wanke D. Screening for protein-DNA interactions by automatable DNA-protein interaction ELISA. PLoS One. 2013;8:e75177. doi: 10.1371/journal.pone.0075177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brière C, Xiong TC, Mazars C, Ranjeva R. Autonomous regulation of free Ca2+ concentrations in isolated plant cell nuclei: a mathematical analysis. Cell Calcium. 2006;39:293–303. doi: 10.1016/j.ceca.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000;279:R255–R262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Burkard N. Targeted proteolysis sustains calcineurin activation. Circulation. 2005;111:1045–1053. doi: 10.1161/01.CIR.0000156458.80515.F7. [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- Chen J, Nolte V, Schlötterer C. Temperature related reaction norms of gene expression: regulatory architecture and functional implications. Mol Biol Evol. 2015;32(9):2393–2402. doi: 10.1093/molbev/msv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilian WM, Tollefson D. Blood urea levels and erythrocyte fragility to isosmotic urea during hibernation and activity of Spermophilus tridecimlineatus. Comp Biochem Physiol A Physiol. 1976;54:433–435. doi: 10.1016/0300-9629(76)90045-1. [DOI] [PubMed] [Google Scholar]
- Choi H, Selpides PJI, Nowell MM, Rourke BC. Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol. 2009;297:R578–R586. doi: 10.1152/ajpregu.00236.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton CJ, Harlow HJ. Avoidance of skeletal muscle atrophy in spontaneous and facultative hibernators. Physiol Biochem Zool. 2015;83:551–560. doi: 10.1086/650471. [DOI] [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HAEW, Windt LJDE, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/MCB.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Lapidos KA, Heretis K, Levin S, Dale R, Pytel P, Svensson EC, McNally EM. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J Cell Sci. 2010;123:2413–2422. doi: 10.1242/jcs.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Agoston DV. Submillimolar levels of calcium regulates DNA structure at the dinucleotide repeat (TG/AC)n. Proc Natl Acad Sci U S A. 1998;95:5981–5986. doi: 10.1073/pnas.95.11.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One. 2013;8:e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Technology development for physiological genomics metabolic cycles in a circannual hibernator. Physiol Genomics. 2011;80045:799–807. doi: 10.1152/physiolgenomics.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Gao YF, Wang J, Wang HP, Feng B, Dang K, Wang Q, Hinghofer-Szalkay HG. Skeletal muscle is protected from disuse in hibernating dauria ground squirrels. Comp Biochem Physiol A Mol Integr Physiol. 2012;161:296–300. doi: 10.1016/j.cbpa.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Gerace L, Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:695–699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida N, Fujimoto M, Tan K, Prakasam R, Shinkawa T, Li L, Ichikawa H, Takii R, Nakai A. Heat shock factor 1 ameliorates proteotoxicity in cooperation with the transcription factor NFAT. EMBO J. 2010;29:3459–3469. doi: 10.1038/emboj.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MB, Woodworth-Hobbs ME, Zheng B, Rahnert JA, Blount MA, Gooch JL, Searles CD, Price SR. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol. 2014;306:C551–558. doi: 10.1152/ajpcell.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung HF, Wang BW, Chang H, Shyu KG. The molecular regulation of resistin expression in cultured vascular smooth muscle cells under hypoxia. J Hypertens. 2008;26:2349–2360. doi: 10.1097/HJH.0b013e328311fa30. [DOI] [PubMed] [Google Scholar]
- Jagelská E, Brázda V, Pospisilová S, Vojtesek B, Palecek E. New ELISA technique for analysis of p53 protein/DNA binding properties. J Immunol Methods. 2002;267:227–235. doi: 10.1016/S0022-1759(02)00182-5. [DOI] [PubMed] [Google Scholar]
- Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. Calcium coordination and the calmodulin fold: divergent versus convergent evolution. Cold Spring Harb Symp Quant Biol. 1987;52:499–510. doi: 10.1101/SQB.1987.052.01.057. [DOI] [PubMed] [Google Scholar]
- Kristofferson R. Urea-levels in blood and tissues of hibernating and non-hibernating hedgehogs. Nature. 1963;197:402–403. doi: 10.1038/197402a0. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lee TN, Buck CL, Barnes BM, O’Brien DM. A test of alternative models for increased tissue nitrogen isotope ratios during fasting in hibernating arctic ground squirrels. J Exp Biol. 2012;215:3354–3361. doi: 10.1242/jeb.068528. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi J, Kim H, Lee DH, Roh GS, Kim HJ, Kang SS, Choi WS, Cho GJ. FK506 reduces calpain-regulated calcineurin activity in both the cytoplasm and the nucleus. Anat Cell Biol. 2014;47:91–100. doi: 10.5115/acb.2014.47.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wohlfart B, Johansson BW. Effects of low temperature on contraction in papillary muscles from rabbit, rat, and hedgehog. Cryobiology. 1990;27:539–546. doi: 10.1016/0011-2240(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang LC, Belke DD. Effect of low temperature on the cytosolic free Ca2+ in rat ventricular myocytes. Cell Calcium. 1991;12:11–18. doi: 10.1016/0143-4160(91)90080-X. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang LCH, Belke DD. Effects of temperature and pH on cardiac myofilament Ca-2+ sensitivity in rat and ground squirrel. Am J Physiol. 1993;264:R104–R108. doi: 10.1152/ajpregu.1993.264.1.R104. [DOI] [PubMed] [Google Scholar]
- Luan S (2011) Coding and decoding of calcium signals in plants. Springer:41–61. doi: 10.1007/978-3-642-20829-4
- Malatesta M, Perdoni F, Battistelli S, Muller S, Zancanaro C. The cell nuclei of skeletal muscle cells are transcriptionally active in hibernating edible dormice. BMC Cell Biol. 2009;10:19. doi: 10.1186/1471-2121-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur MD, Milsom WK. Changes in ventilation and respiratory sensitivity associated with hibernation in Columbian (Spermophilus columbianus) and golden-mantled (Spermophilus lateralis) ground squirrels. Physiol Zool. 1991;64:940–959. doi: 10.1086/physzool.64.4.30157950. [DOI] [Google Scholar]
- McMullen DC, Hallenbeck JM. Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Comp Physiol B Biochem Syst Environ Physiol. 2010;180:927–934. doi: 10.1007/s00360-010-0468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41:597–600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák A, Boldizsár Á, Ádám É, Kozma-Bognár L, Majláth I, Båga M, Tóth B, Chibbar R, Galiba G (2015) Light-quality and temperature-dependent CBF14 gene expression modulates freezing tolerance in cereals. J Exp Bot:1102–1105 [DOI] [PubMed]
- Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Bennett AF, Lenski RE, Long AD. Evolutionary changes in heat-inducible gene expression in lines of Escherichia coli adapted to high temperature. Physiol Genomics. 2003;14:47–58. doi: 10.1152/physiolgenomics.00034.2002. [DOI] [PubMed] [Google Scholar]
- Rourke BC, Yokoyama Y, Milsom WK, Caiozzo VJ. Myosin isoform expression and MAFbx mRNA levels in hibernating golden- mantled ground squirrels (Spermophilus lateralis) Physiol Biochem. 2004;77:582–593. doi: 10.1086/421753. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Nakakura T, Ogushi Y, Akabane G, Kurabuchi S, Suzuki M, Tanaka S. Expression of a mammalian aquaporin 3 homolog in the anterior pituitary gonadotrophs of the tree frog, Hyla japonica. Cell Tissue Res. 2011;343:595–603. doi: 10.1007/s00441-010-1122-1. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Penna S, Van Nguyen D, Tran LSP. Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit Rev Biotechnol. 2014;8551:1–10. doi: 10.3109/07388551.2014.973367. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P, Fröbert O, Anderstam B, Palm F, Eriksson M, Bragfors-Helin AC, Qureshi AR, Larsson T, Friebe A, Zedrosser A, Josefsson J, Svensson M, Sahdo B, Bankir L, Johnson RJ. Metabolic changes in summer active and anuric hibernating free-ranging brown bears (Ursus arctos) PLoS One. 2013;8:e72934. doi: 10.1371/journal.pone.0072934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB. Out cold: biochemical regulation of mammalian hibernation—a mini-review. Gerontology. 2010;56:220–230. doi: 10.1159/000228829. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Camb Philos Soc. 2004;79:207–233. doi: 10.1017/S1464793103006195. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Huebner M, Weber AP. Plastic and adaptive gene expression patterns associated with temperature stress in Arabidopsis thaliana. Heredity. 2007;99:143–50. doi: 10.1038/sj.hdy.6800975. [DOI] [PubMed] [Google Scholar]
- Tessier SN, Storey KB. Lessons from mammalian hibernators: molecular insights into striated muscle plasticity and remodeling. Biomol Concepts. 2016;7(2):69–92. doi: 10.1515/bmc-2015-0031. [DOI] [PubMed] [Google Scholar]
- Thompson M, Andrade VA, Andrade SJ, Pusl T, Ortega JM, Goes AM, Leite MF. Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem Biophys Res Commun. 2003;301:267–274. doi: 10.1016/S0006-291X(02)03024-3. [DOI] [PubMed] [Google Scholar]
- Wang LCH, Lee TF. Torpor and hibernation in mammals: metabolic, physiological, and biochemical adaptations. In: Fregley MJ, Blatteis CM, editors. Handbook of physiology: environmental physiology, section 4. New York: Oxford University Press; 1996. pp. 507–532. [Google Scholar]
- Wang SQ, Zhou ZQ. Alpha-stat calibration of indo-1 fluorescence and measurement of intracellular free calcium in rat ventricular cells at different temperatures. Life Sci. 1999;65:871–877. doi: 10.1016/S0024-3205(99)00317-3. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Huang YH, Liu KS, Zhou ZQ. Dependence of myocardial hypothermia tolerance on sources of activator calcium. Cryobiology. 1997;35:193–200. doi: 10.1006/cryo.1997.2040. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhou Z, Qian H. Temperature dependence of intracellular free calcium in cardiac myocytes from rat and ground squirrel measured by confocal microscopy. Sci China C Life Sci. 1999;42:293–299. doi: 10.1007/BF03183606. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhou Z, Qian H. Recording of calcium transient and analysis of calcium removal mechanisms in cardiac myocytes from rats and ground squirrels. Sci China C Life Sci. 2000;43:191–199. doi: 10.1007/BF02879128. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Lakatta EG, Cheng H, Zhou ZQ. Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J Exp Biol. 2002;205:2957–2962. doi: 10.1242/jeb.205.19.2957. [DOI] [PubMed] [Google Scholar]
- Wu CW, Storey KB. FoxO3a-mediated activation of stress responsive genes during early torpor in a mammalian hibernator. Mol Cell Biochem. 2014;390:185–195. doi: 10.1007/s11010-014-1969-7. [DOI] [PubMed] [Google Scholar]
- Xiong X, Tao R, DePinho RA, Dong XC. The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J Biol Chem. 2012;287:39107–39114. doi: 10.1074/jbc.M112.412569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Andres-Mateos E, Mejias R, MacDonald EM, Leinwand LA, Merriman DK, Fink RHA, Cohn RD. Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp Neurol. 2013;247:392–401. doi: 10.1016/j.expneurol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Burg MB. Counteracting effects of urea and betaine in mammalian cells in culture. Am J Physiol. 1990;258:R198–204. doi: 10.1152/ajpregu.1990.258.1.R198. [DOI] [PubMed] [Google Scholar]
- Yang S, Klee CB. Low affinity Ca2+-binding sites of calcineurin B mediate conformational changes in calcineurin A. Biochemistry. 2000;39:16147–16154. doi: 10.1021/bi001321q. [DOI] [PubMed] [Google Scholar]
- Yang T, Davis RJ, Chow CW. Requirement of two NFATc4 transactivation domains for CBP potentiation. J Biol Chem. 2001;276:39569–39576. doi: 10.1074/jbc.M102961200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Storey KB. Expression of nuclear factor of activated T cells (NFAT) and downstream muscle-specific proteins in ground squirrel skeletal and heart muscle during hibernation. Mol Cell Biochem. 2015;412:27–40. doi: 10.1007/s11010-015-2605-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)