Abstract
The generation of reactive oxygen species (ROS), a byproduct of aerobic energy metabolism, is maintained at physiological levels by the activity of antioxidant components. Insufficiently opposed ROS results in oxidative stress characterized by altered mitochondrial function, decreased protein activity, damage to nucleic acids, and induction of apoptosis. Elevated levels of inadequately opposed ROS induce autophagy, a major intracellular pathway that sequesters and removes damaged macromolecules and organelles. In early pregnancy, autophagy induction preserves trophoblast function in the low oxygen and nutrient placental environment. Inadequate regulation of the ROS-autophagy axis leads to abnormal autophagy activity and contributes to the development of preeclampsia and intrauterine growth restriction. ROS-autophagy interactions are altered at the end of gestation and participate in the initiation of parturition at term. The induction of high levels of ROS coupled with a failure to induce a corresponding increase in autophagy results in the triggering of preterm labor and delivery.
Keywords: Oxidative stress, Autophagy, Preeclampsia, Intrauterine growth restriction, Preterm birth, Pregnancy
Reactive oxygen species (ROS), such as superoxide radicals, hydroxyl radicals, alkoxy radicals as well as the non-radical intermediates hydrogen peroxide, ozone, and singlet oxygen, are byproducts of aerobic energy metabolism (Sies 1991; Finkel 2011). The concentration of these radicals is maintained at physiological levels by activity of the antioxidant enzymes superoxide dismutase, catalase, and peroxiredoxins as well as by concentrations of glutathione and vitamins C and E. When this system becomes out of balance, the generation of insufficiently opposed ROS results in altered mitochondrial function, a diminution of protein activity, damage to nucleic acids, and induction of apoptosis. The consequent tissue damage is manifested in a range of pathologies to different organ systems (Domingueti et al. 2015; Aluganti et al. 2016; Hernández et al. 2016). Recently, insufficiently regulated ROS has been recognized as a major contributor to disorders of pregnancy (Coppe et al. 2010; Redman and Sargent 2010; Menon 2014; Wu et al. 2015a, b; Zhang et al. 2015).
A major pathway for removing macromolecules and organelles that have been damaged by ROS is macroautophagy, hereafter referred to as autophagy. This catabolic process is responsible for eliminating altered proteins, mitochondria, and inflammasomes from the cytoplasm. Macromolecules and organelles marked for destruction are enclosed within a double-membrane structure called autophagosome. Its subsequent fusion with a lysosome results in degradation of the enclosed entity and release of the component amino acids, carbohydrates, nucleic acid degradation products, and lipids into the cytoplasm as building blocks for synthesis of new macromolecules or for the generation of energy by mitochondria. Autophagy is a constitutive process and functions at a low level under physiological conditions to maintain homeostasis. However, autophagy activity is greatly up-regulated in response to nutrient limitation or to an altered intracellular milieu. A dysregulation of autophagy activity contributes to the pathology of multiple diseases (Chang et al. 2015; Tai et al. 2016; Edens et al. 2016), including complications of pregnancy (Hung et al. 2012; Kanninen at el. 2013b; Gao et al. 2015; Brickle et al. 2015; Agrawal et al. 2015).
In this communication, we delineate the interactions between oxidative stress and autophagy during gestation and highlight how disturbances in this relationship contribute to pregnancy pathologies.
Oxidative stress and autophagy during pregnancy and parturition
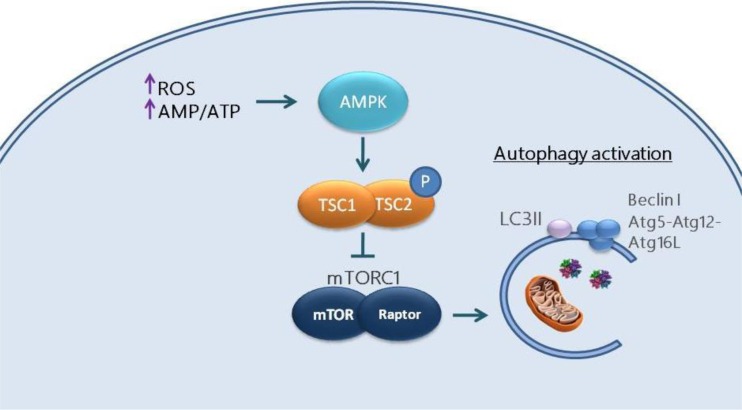
Trophoblast growth, differentiation, and migration are central to formation of the placenta. Under physiological conditions, extravillous trophoblast (EVT) invades the maternal decidua and facilitates vascular remodeling of the spiral arteries. A low availability of energy and nutrients in the first trimester placenta due to a limited blood supply at the maternal-fetal interface (Lyall 2002) results in an elevated ratio of intracellular adenosine monophosphate to adenosine triphosphate (AMP/ATP). Under these conditions, production of the enzyme 5′-AMP-activated protein kinase (AMPK) is up-regulated. AMPK phosphorylates and prevents activation of the intracellular complex, mammalian target of rapamycin complex 1 (mTORC1), which is the major inhibitor of autophagy (Kim and Guan 2015). In addition, AMPK also phosphorylates tuberous sclerosis complex 1 and 2 (TCS1, 2), converting it into an inhibitor of mTORC1. This mechanism is outlined in Fig. 1. Thus, autophagy is activated under conditions of low nutrient and oxygen availability that favor increased ROS production to promote survival of the EVT and preserving its ability to invade into the myometrium. After about the 12th week of gestation, EVT infiltrates deeper into the endometrium and myometrium, and the increased maternal blood flow results in a greatly elevated oxygen level. The subsequent increased production of ROS stimulates a further elevation in autophagy to maintain EVT homeostasis. Culture-based experiments have confirmed that oxygen deficiency induces autophagy in primary human trophoblast cells (Chen et al. 2012). In addition, hypoxia-induced autophagy has been shown to enhance the invasion capacity of EVT (Nakashima et al. 2013). However, it has also been reported that inhibition of production of autophagy-related proteins by siRNA transfection had no effect on invasion activity of a trophoblast cell line (Hung et al. 2013). The role of autophagy in the initiation and persistence of trophoblast invasion in vivo remains to be further clarified (Saito and Nakashima 2014).
Fig. 1.
Regulation of autophagy by oxidative stress in first trimester placenta. Elevated levels of reactive oxygen species (ROS) and an increased AMP/ATP ratio (low ATP levels) activate AMP-activated protein kinase (AMPK) which phosphorylates Tsc2 and activates the Tsc1-Tsc2 complex. Activated Tsc inhibits mammalian target of rapamycin complex (mTORC1) thereby stimulating production of the autophagy-related proteins LC3II, beclin 1, and Atg5-Atg12-Atg16L and induction of autophagy
Few clinical studies have evaluated oxidative stress and autophagy at later stages of pregnancy, due primarily to the difficulty in obtaining and evaluating second trimester placentas and fetal membranes from women with normal gestations. One study (Hung et al. 2013) identified autophagy activity in placentas from early, mid, and late gestations from uncomplicated pregnancies by transmission electron microscopy, immunofluorescence, and detection of mRNA coding for autophagy-related proteins. Recent experiments from our laboratory demonstrated that autophagy activity in peripheral blood mononuclear cells increases as gestation progresses (Kanninen et al. 2013a). Regarding oxidative stress, Basu et al. (2015) investigated oxidative balance in healthy pregnant women throughout gestation by measuring a byproduct of lipid peroxidation, malondialdehyde (MDA), in 201 placentas from women who underwent elective termination of pregnancy between 6- and 26-week gestation and from women with term deliveries. Placental levels of MDA, and hence oxidative stress, were the highest in first trimester placenta and decreased as gestation progressed. Concomitantly, there was an increase in total antioxidant capacity as gestation progressed. However, towards the end of pregnancy, levels of ROS in maternal-fetal compartments again become elevated due to higher fetal metabolic demands, depletion of antioxidants, and a reduced maternal supply of metabolic substrates (Myatt and Cui 2004; Agarwal et al. 2005; Paamoni-Keren et al. 2007).
As pregnancy advances to term, profound uterine stretching to accommodate the growing fetus and greatly increased fetal nutritional demands lead to a progressive elevation in oxidative stress. In response to ROS and increased hydrogen peroxide production, the transcription factor nuclear factor kappa B (NF-kB) is activated resulting in the up-regulation of genes coding for pro-inflammatory cytokines (Menon 2014). Elevated ROS and inflammation also result in induction of the stress-induced 70-kDa heat shock protein (HSPA1A, Kampinga et al. 2009). This protein binds to intracellular proteins maintaining their proper conformation and function under stressful conditions. Both NF-kB (Djavaheri-Mergny et al. 2006) and HSPA1A (Dokladny et al. 2013; Sisti et al. 2015) are inhibitors of autophagy. A decreased level of autophagy is a characteristic of placentas evaluated following a vaginal delivery but is not a feature of placentas obtained by cesarean section from women who were not in labor (Signorelli et al. 2011). Moreover, it was recently shown that oxidative stress at term is also associated with a decrease in chromosome telomere length and induced senescence of cells in the chorioamniotic membranes (Behnia et al. 2015; Polettini et al. 2015). Senescence, an irreversible arrest of cell growth and subsequent cellular and/or tissue aging, is a process that occurs under both physiologic and pathologic conditions (Campisi and Robert 2014). It has been hypothesized that senescence enhances inflammation through promotion of the so-called senescence-associated secretory phenotype in the uterus, and participates in labor induction (Coppe et al. 2010; Behnia et al. 2015). Thus, while ROS results in autophagy induction to maintain trophoblast viability and activity in the first trimester, its increased intensity near term coupled with activation of pro-inflammatory mediators and release of danger signals results in its contribution to the inhibition of autophagy near term. This is illustrated in Fig. 2. The intracellular accumulation of abnormal proteins and reduced availability of amino acids for synthesis of new functional proteins due to impaired autophagy activity would limit production in myometrial cells of pro-quiescence factors such as progesterone receptor B and caspase 3 and thus promote induction of myometrial contractions (Sisti et al. 2016).
Fig. 2.
Regulation of autophagy by oxidative stress near term. Near term, highly elevated ROS results in induction of 70-kDa heat shock protein (HSPA1A) and transcription factor nuclear factor kappa B (NF-kB). HSPA1A inhibits autophagy through activation of the protein kinase, Akt, that leads to phosphorylation of mammalian target of rapamycin (mTOR), which, in turn, binds to raptor to form the mTORC1 complex that inhibits autophagy. Prolonged NF-kB activation is an additional inhibitor of autophagy through mTORC1 activation. Impaired autophagy leads to intracellular accumulation of abnormal proteins coupled with limited production of the pro-quiescence factors progesterone receptor B (PRB) and caspase 3, thus promoting myometrial contractions. Concomitantly, withdraw of autophagy induces decidual senescence that leads to membrane weakening and production of the senescence-associated secretory phenotype (SASP), further inducing labor
A third mechanism by which ROS-autophagy interaction contributes to initiation of labor is by activation of inflammasomes. A Toll-like receptor located in the cytoplasm, NOD-like receptor protein (NLRP) 3, becomes activated in response to an altered intracellular milieu and binds to both an apoptosis-associated speck-like protein (ASC) and procaspase 1 to form the inflammasome. This complex then cleaves procaspase 1 to form an active caspase 1 protein which binds to the inactive precursor forms of both interleukin (IL)-1 and IL-18. These pro-inflammatory cytokines thus become activated and are released from cells (Petrilli et al. 2007). The concentration of inflammasomes within a cell is normally regulated by their ingestion by autophagy to prevent excessive pro-inflammatory cytokine release (Abdelaziz et al. 2015). ROS generated from aged and defective mitochondria is a major activator of NLRP3 (Kepp et al. 2011). Since altered mitochondria that release elevated levels of ROS are also normally removed from the cytoplasm by autophagy, a decrease in autophagy activity, such as occurs at the end of a term gestation, can be seen to promote inflammasome formation as well as result in the enhanced release of pro-inflammatory cytokines that contribute to the triggering of parturition. The involvement of inflammasome activation in initiation of labor and delivery has recently been proposed (Brickle et al. 2015; Romero et al. 2016). This pathway is outlined in Fig. 3.
Fig. 3.
Promotion of inflammasome formation by inhibition of autophagy and increased ROS. A decrease in autophagy activity leads to the accumulation of defective mitochondria which generate unopposed ROS. This leads to activation of NLRP 3, its binding to apoptosis-associated speck-like protein (ASC) and procaspase 1 and formation of the inflammasome. The inflammasome complex catalyzes caspase 1 activation which then acts on the inactive precursor forms of interleukin (IL)-1 and IL-18 and culminates in the formation and release of the active pro-inflammatory cytokines
Oxidative stress and autophagy in preeclampsia and intrauterine growth restriction
Excessive levels of inadequately opposed oxidative stress as well as increased or impaired autophagy are major contributors to pregnancy complications. Preeclampsia (PEC) is a syndrome marked by hypertension and proteinuria that occurs in 3–6 % of pregnancies and is responsible for about 18 % of maternal mortalities worldwide (Duley 2009; Ghulmiyyah and Sibai 2012; Anderson et al. 2012). PEC is often associated with intrauterine growth restriction (IUGR), defined as a fetal weight below the 10 % percentile for a given gestational age (Brodsky and Christou 2004). Rates of IUGR vary widely from 5 to 20 % in different geographical locations (Scifres and Nelson 2009; Saleem et al. 2011). IUGR is associated with increased risk for preterm birth, stillbirth, and short- and long-term morbidities such as hypoglycemia, cardiac dysfunction, and neuro-developmental disorders (Smith and Fretts 2007; Longo et al. 2013; Salam et al. 2014; Gaccioli and Lager 2016).
While knowledge of the pathogenesis of PEC and IUGR remains incomplete, it is clear that abnormal placentation and oxidative stress are involved in their development (Wu et al. 2015a). The extent of EVT invasion of the spiral arteries in women who develop PEC is shallow and inadequate. This leads to episodes of hypoxia/reoxygenation and enhanced levels of ROS at the maternal-fetal interface as well as in the circulation (Hung and Burton 2006). Concomitantly, antioxidant capacity has been shown to be compromised in PEC (Beauséjour et al. 2007; Burton and Jauniaux 2011). This oxidative imbalance induces NF-kB activation in the placenta and release of pro-inflammatory cytokines, chemokines, and anti-angiogenic factors such as soluble endoglin and soluble Fms-like tyrosine kinase-1 (sFLT-1) (Lazdam et al. 2012). Increased levels of these compounds result in systemic inflammation and placental and endothelial damage resulting in the PEC phenotype (Molvarec et al. 2010; Szarka et al. 2010; Redman and Sargent 2010; Wu et al. 2015b). As mentioned above, autophagy is also inhibited under these conditions. It has been demonstrated that while sera from women with normotensive pregnancies progressively induce higher levels of autophagy as gestation advances, this is not the case for sera from women with PEC (Kanninen et al. 2014).
The pathophysiology of IUGR is similar to that of PEC. Placental insufficiency results in hypoxia/reoxygenation and synthesis of ROS leading to an inadequate nutrient supply to the growing fetus (Zhang et al. 2015; Wu et al. 2015b). The degree of defective placentation may differentiate between cases of PEC-mediated IUGR from IUGR that occurs in the absence of concomitant hypertension (Myatt and Webster 2009). Elevated levels of oxidative stress have been detected in placental villi from women with IUGR when compared to healthy pregnancies (Mert et al. 2012) and increased concentration of peroxidation products—MDA and 8-hydroxydeoxyguanosine—have been associated with growth-restricted newborns (Kim et al. 2005; Maisonneuve et al. 2014). Despite the elevated levels of oxidative stress in PEC and IUGR, the administration of antioxidants to alleviate these conditions is controversial and reports regarding the positive effects of such intake are inconclusive (Basaran et al. 2010; Thorne-Lyman and Fawzi 2012). While the balance between ROS and antioxidants is essential for cell homeostasis, it is important to consider that oxidative stress seems to be a consequence and not a primary cause of PEC and IUGR, and thus, a decrease in PEC and IUGR is not likely to be reversed by antioxidants (Menon 2014). Moreover, antioxidant administration may hinder the physiological activity of ROS (Efimova et al. 2011).
Increased autophagy in placentas from women with PEC and IUGR has also been noted (Oh et al. 2008; Hung et al. 2012; Curtis et al. 2013). This up-regulation may be an attempt to facilitate trophoblast cell survival in response to an altered placental microenvironment (Hung et al. 2013). In a recent study addressing early-onset PEC, treatment with the oxidative stress inducer glucose oxidase increased autophagosome formation, detected by transmission electron microscopy and transcription of genes coding for the autophagy-related proteins LC3 and Beclin-1 in both EVT and endothelial cells (Gao et al. 2015). EVT invasiveness was also reduced by glucose oxidase treatment, a phenotype that could be partially reversed by treatment with an inhibitor of autophagosome formation. Moreover, LC3 and Beclin-1 co-located with an oxidative stress marker, nitrotyrosine. These findings strongly indicate that elevated oxidative stress increases autophagy in preeclamptic placentas. An evaluation of the role of autophagy in the differential development of early- or late-onset PEC, and its association with IUGR, warrants further investigation.
The increased induction of autophagy in association with PEC and IUGR likely contributes to development of a growth-restricted fetus. As noted above, oxidative stress-induced autophagy activation results from blocking activity of the autophagy inhibitor, mTORC1. Growth of the fetus is dependent on the transport of nutrients across the placenta, and mTORC1 is central to this process (Jansson et al. 2012). An inhibition of mTORC1 activity in the placenta results in a decreased level of amino acid transporters on trophoblast cells and a subsequent deficiency in nutrient availability to the fetus.
Oxidative stress and autophagy in the pathophysiology of preterm birth and premature preterm rupture of membranes
Preterm birth (PTB), defined as birth before 37 complete weeks of gestation, is the leading cause of neonatal morbidity and mortality. Worldwide, 5 to 18 % of pregnancies reach their outcome prematurely, which translates into 15 million preterm newborns every year (Romero et al. 2014). These neonates are prone to more short- and long-term disabilities in distinct organ systems than their term counterparts and often present with infections, enterocolitis, pneumonia, and neurological developmental disorders. One out of six million deaths among children younger than 5 years old can be traced to prematurity (Nour 2012).
Approximately 40 % of PTB are medically induced due to maternal or fetal indications while the remainder are spontaneous following preterm labor (PTL) and/or preterm premature rupture of membranes (PPROM) (Romero et al. 2006; Goldenberg et al. 2008; Menon 2014). A multitude of risk factors are associated with PTL and PPROM, from behavioral—nutrition, stress, environment—to genetic—polymorphisms, ethnicity—and pathological conditions—intrauterine infection, hydramnios, decidual hemorrhage (Romero et al. 2014). Regardless of the risk factor(s) involved, inflammatory pathways are notably activated and culminate in PTB (Romero et al. 2014).
All the above risk factors can generate redox imbalance. Oxidative stress-induced damage has been proposed to activate signals that trigger PTL and PPROM (Menon 2014). Cigarette smoke extract has recently been shown to induce ROS, oxidative damage, and inflammation in cultured amnion cells. Interestingly, levels were higher than was concomitant treatment of the cells with the major cell wall component of gram-negative bacteria, lipopolysaccharide (LPS) (Behnia et al. 2016). This induction of oxidative stress was demonstrated to provoke telomere reduction, cell cycle arrest, and senescence via activation of the p38MAPK pathway. In contrast, LPS treatment mainly activated the classic NF-kB inflammatory pathway (Menon et al. 2013; Behnia et al. 2016). Elevated levels of oxidative stress markers have been described in the maternal-fetal compartment from women in PTL and in deliveries preceded by PPROM (Bredeson et al. 2014; Dutta et al. 2016). Perhaps, the timing of the onset of oxidative stress, and not only its intensity, in relation to antioxidant capacity is most critical to predispose to different premature gestational outcomes. Several behavior-related risk factors for PTL (obesity, stress, smoking) provoke the premature induction of elevated ROS levels.
A growing body of evidence indicates that a decreased level of autophagy is associated with premature delivery. Insufficiently opposed ROS (Bredeson et al. 2014; Dutta et al. 2016) may initiate the aforementioned activation of HSPA1A and NF-kB that impairs autophagy and culminates in PTB. The link between inhibited autophagy, decidual senescence, and PTB was first described in animal studies. From the observation that increased mTORC1 signaling was implicated in advancing physiological aging (Sengupta et al. 2010), Hirota et al. (2011) demonstrated that autophagy withdrawal led to induction of decidual senescence and preterm delivery. Furthermore, induction of autophagy in pregnant mice by rapamycin treatment prevented this occurrence. A recent in vitro study observed that both term and preterm labors were associated with a reduced expression of autophagy-related proteins in chorioamniotic membranes (Brickle et al. 2015). Membranes obtained from women with PPROM who were not in labor had a decreased expression of the autophagy-related proteins Beclin 1, Atg 3, and Atg 7 when compared to samples from women with intact membranes. Additional studies corroborated decreased expression of genes coding for autophagy components in the uterus and placenta in animal models of experimentally induced inflammation-mediated preterm labor (Agrawal et al. 2015). This alteration was shown to be partially mediated by decreased levels of a crucial molecule for activation of the lysosomal enzyme, transport of small molecules, and protein processing (a2V), necessary for the breakdown of components transported by autophagosomes (Zoncu et al. 2011; Agrawal et al. 2015). Moreover, recent experimental data using mice knocked out for the BECN1 gene that codes for an autophagy protein suggest that autophagy may also play a role in maintaining uterine quiescence by participating in progesterone synthesis and that withdraw of autophagy culminates in PTL (Gawriluk and Rucker 2015). Nevertheless, the literature in this field is still limited, and more studies, especially in humans, are needed to clarify whether there is a difference in autophagy levels between term and preterm labor and PPROM.
In summary, while PE and IUGR are marked by a disruption in intensity of both autophagy and oxidative stress, PTL and PPROM are characterized by premature triggering of heightened oxidative stress and impaired autophagy in fetal membranes and the placenta.
Conclusion
It is becoming increasingly clear that autophagy and oxidative stress are inter-related activities that are essential for fetal development and parturition. Further investigations of their inter-relationships at different gestational stages will lead to an improved understanding of the mechanisms by which alterations in their interactions contribute to placental pathology, disorders of pregnancy, and premature delivery. With this increased knowledge, novel protocols can then be developed to improve pregnancy outcomes.
References
- Abdelaziz DHA, Khalil H, Cormet-Boyaka E, Amer AO. The cooperation between the autophagy machinery and the inflammasome to implement an appropriate innate immune response: do they regulate each other? Immunol Rev. 2015;265:194–204. doi: 10.1111/imr.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal V, Jaiswal MK, Mallers T, Katara GK, Gilman-Sachs A, Beaman KD, et al. Altered autophagic flux enhances inflammatory responses during inflammation-induced preterm labor. Sci Rep. 2015;5:9410. doi: 10.1038/srep09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluganti Narasimhulu C, Fernandez-Ruiz I, Selvarajan K, Jiang X, Sengupta B, Riad A, et al. Atherosclerosis—do we know enough already to prevent it? Curr Opin Pharmacol. 2016;27:92–102. doi: 10.1016/j.coph.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Anderson UD, Olsson MG, Kristensen KH, Akerstr€om B, Hansson SR. Review: biochemical markers to predict preeclampsia. Placenta. 2012;33:S42–47. doi: 10.1016/j.placenta.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta-analysis. Obstet Gynecol Surv. 2010;65:653–667. doi: 10.1097/OGX.0b013e3182095366. [DOI] [PubMed] [Google Scholar]
- Basu J, Bendek B, Agamasu E, Salafia CM, Mishra A, Benfield N, et al. Placental oxidative status throughout normal gestation in women with uncomplicated pregnancies. Obstet Gynecol Int. 2015 doi: 10.1155/2015/276095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauséjour A, Bibeau K, Lavoie JC, St-Louis J, Brochu M. Placental oxidative stress in a rat model of preeclampsia. Placenta. 2007;28:52–58. doi: 10.1016/j.placenta.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Behnia F, Sheller S, Menon R. Mechanistic differences leading to infectious and sterile inflammation. Am J Reprod Immunol. 2016;75:505–518. doi: 10.1111/aji.12496. [DOI] [PubMed] [Google Scholar]
- Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014 doi: 10.1371/journal.pone.0113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickle A, Tran HT, Lim R, Liong S, Lappas M. Autophagy, which is decreased in labouring fetal membranes, regulates IL-1β production via the inflammasome. Placenta. 2015;36:1393–1404. doi: 10.1016/j.placenta.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004;19:307–319. doi: 10.1177/0885066604269663. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Robert L. Cell senescence: role in aging and age‐related diseases. Interdiscip Top Gerontol. 2014;39:45–61. doi: 10.1159/000358899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Hee SW, Hsieh ML, Jeng YM, Chuang LM. The role of organelle stresses in diabetes mellitus and obesity: implication for treatment. Anal Cell Pathol (Amst) 2015 doi: 10.1155/2015/972891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Longtine MS, Nelson DM. Hypoxia induces autophagy in primary human trophoblasts. Endocrinology. 2012;153:4946–4954. doi: 10.1210/en.2012-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S, Jones CJ, Garrod A, Hulme CH, Heazell AE. Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26:339–346. doi: 10.3109/14767058.2012.733764. [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288:14959–14972. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingueti CP, Dusse LM, Carvalho MD, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2015;30:738–45. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovský M, et al. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22:143–157. doi: 10.1093/molehr/gav074. [DOI] [PubMed] [Google Scholar]
- Edens BM, Miller N, Ma YC. Impaired autophagy and defective mitochondrial function: converging paths on the road to motor neuron degeneration. Front Cell Neurosci. 2016;10:44. doi: 10.3389/fncel.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova O, Szankasi P, Kelley TW. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS One. 2011 doi: 10.1371/journal.pone.0016013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaccioli F, Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. 2016;7:40. doi: 10.3389/fphys.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H, Baker PN. Excessive autophagy induces the failure of trophoblast invasion and vasculature: possible relevance to the pathogenesis of preeclampsia. J Hypertens. 2015;33:106–117. doi: 10.1097/HJH.0000000000000366. [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Rucker EB. BECN1, corpus luteum function, and preterm labor. Autophagy. 2015;11:183–184. doi: 10.4161/15548627.2014.984269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández JA, López-Sánchez RC, Rendón-Ramírez A. Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxidative Med Cell Longev. 2016 doi: 10.1155/2016/1543809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci U S A. 2011;108:18073–18078. doi: 10.1073/pnas.1108180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol. 2006;45:189–200. doi: 10.1016/S1028-4559(09)60224-2. [DOI] [PubMed] [Google Scholar]
- Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PLoS One. 2012 doi: 10.1371/journal.pone.0040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Hsieh TT, Chen SF, Li MJ, Yeh YL. Autophagy in the human placenta throughout gestation. PLoS One. 2013 doi: 10.1371/journal.pone.0083475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012 doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen TT, Ramos BR, Jaffe S, Bongiovanni AM, Linhares IM, Di Renzo GC, et al. Inhibition of autophagy by sera from pregnant women. Reprod Sci. 2013;20:1327–1331. doi: 10.1177/1933719113485301. [DOI] [PubMed] [Google Scholar]
- Kanninen TT, Ramos Ribeiro de Andrade B, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. Eur J Obstet Gynecol Reprod Biol. 2013;171:3–8. doi: 10.1016/j.ejogrb.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Kanninen TT, Jayaram A, Jaffe Lifshitz S, Witkin SS. Altered autophagy induction by sera from pregnant women with pre-eclampsia: a case-control study. BJOG. 2014;121:958–964. doi: 10.1111/1471-0528.12755. [DOI] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011;12:199–200. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, et al. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lazdam M, Davis EF, Lewandowski AJ, Worton SA, Kenworthy Y, Kelly B, et al. Prevention of vascular dysfunction after preeclampsia: a potential long-term outcome measure and an emerging goal for treatment. J Pregnancy. 2012;2012:704146. doi: 10.1155/2012/704146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo S, Bollani L, Decembrino L, DiComite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) J Matern Fetal Neonatal Med. 2013;26:222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- Lyall F. The human placental bed revisited. Placenta. 2002;23:555–562. doi: 10.1053/plac.2002.0850. [DOI] [PubMed] [Google Scholar]
- Maisonneuve E, Delvin E, Ouellet A, Morin L, Dubé J, Boucoiran I, et al. Oxidative conditions prevail in severe IUGR with vascular disease and Doppler anomalies. J Matern Fetal Neonatal Med. 2014;15:1–5. doi: 10.3109/14767058.2014.957670. [DOI] [PubMed] [Google Scholar]
- Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One. 2013 doi: 10.1371/journal.pone.0083416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert I, Oruc AS, Yuksel S, Cakar ES, Buyukkagnici U, Karaer A, et al. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J Obstet Gynaecol Res. 2012;38:658–664. doi: 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigó J., Jr Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res. 2010;33:892–898. doi: 10.1038/hr.2010.92. [DOI] [PubMed] [Google Scholar]
- Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7:375–384. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, et al. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour N. Preterm delivery and the millennium development goal. Rev Obstet Gynecol. 2012;5:100e5. [PMC free article] [PubMed] [Google Scholar]
- Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15:912–920. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- Paamoni‐Keren O, Silberstein T, Burg A, Raz I, Mazor M, Saphier O, et al. Oxidative stress as determined by glutathione (GSH) concentrations in venous cord blood in elective cesarean delivery versus uncomplicated vaginal delivery. Arch Gynecol Obstet. 2007;276:43–46. doi: 10.1007/s00404-006-0304-2. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One. 2015 doi: 10.1371/journal.pone.0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2016 doi: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 2014;101–102:80–88. doi: 10.1016/j.jri.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Salam RA, Das JK, Bhutta ZA. Impact of intrauterine growth restriction on long-term health. Curr Opin Clin Nutr Metab Care. 2014;17:249–254. doi: 10.1097/MCO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Saleem T, Sajjad N, Fatima S, Habib N, Ali SR, Qadir M. Intrauterine growth retardation-small events, big consequences. Ital J Pediatr. 2011;37:41. doi: 10.1186/1824-7288-37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol. 2009;587:3453–3458. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Avagliano L, Virgili E, Gagliostro V, Doi P, Braidotti P, et al. Autophagy in term normal human placentas. Placenta. 2011;32:482–485. doi: 10.1016/j.placenta.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Sisti G, Kanninen TT, Ramer I, Witkin SS. Interaction between the inducible 70-kDa heat shock protein and autophagy: effects on fertility and pregnancy. Cell Stress Chaperones. 2015;20:753–758. doi: 10.1007/s12192-015-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti G, Kanninen TT, Witkin SS. Maternal immunity and pregnancy outcome: focus on preconception and autophagy. Genes Immun. 2016;17:1–7. doi: 10.1038/gene.2015.57. [DOI] [PubMed] [Google Scholar]
- Smith GC, Fretts RC. Stillbirth Lancet. 2007;370:1715–1725. doi: 10.1016/S0140-6736(07)61723-1. [DOI] [PubMed] [Google Scholar]
- Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S, Hu XQ, Peng DQ, Zhou SH, Zheng XL. The roles of autophagy in vascular smooth muscle cells. Int J Cardiol. 2016;211:1–6. doi: 10.1016/j.ijcard.2016.02.128. [DOI] [PubMed] [Google Scholar]
- Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Pediatr Perinat Epidemiol. 2012;26:36–54. doi: 10.1111/j.1365-3016.2012.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Tian FJ, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. 2015 doi: 10.1155/2015/293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Tian FJ, Lin Y, Xu WM. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. 2015 doi: 10.1111/aji.12454. [DOI] [PubMed] [Google Scholar]
- Zhang S, Regnault TR, Barker PL, Botting KJ, McMillen IC, McMillan CM, et al. Placental adaptations in growth restriction. Nutrients. 2015;7:360–389. doi: 10.3390/nu7010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]