Abstract
Stress, a state of perceived threat to homeostasis, regulates a panel of important physiological functions. The human mind and body respond to stress by activating the sympathetic nervous system and secreting the catecholamines epinephrine and norepinephrine in the “fight-or-flight” response. However, the protective mechanism of acute stress is still unknown. In the present study, an acute stress mouse model was constructed by intraperitoneal injection of epinephrine (0.2 mg kg−1) for 4 h. Epinephrine treatment induced heat shock 70(Hsp70) expression in the stress responsive tissues, such as the cortex, hippocampus, thymus, and kidney. Further, the expression of thioredoxin-1(Trx-1), a cytoprotective protein, was also upregulated in these stress responsive tissues. In addition, the phosphorylation of cAMP-response element binding protein (CREB), a transcription factor of Trx-1, was increased after treatment with epinephrine. The block of CREB activation by H89 inhibited the acute epinephrine stress-induced Trx-1 and Hsp70 expression. Taken together, our data suggest that acute stimuli of epinephrine induced Trx-1 expression through activating CREB and may represent a protective role against stress.
Keywords: Acute stress, Epinephrine, Hsp70, Trx-1, CREB
Introduction
Whenever an endogenous or exogenous challenge is perceived as unpleasant, aversive or threatening, a series of systems and processes are activated that generates a coordinated response to that particular challenge, or stressor. This so-called stress response, an integral part of any adaptive biological system, is conserved throughout evolution. Many factors can initiate a stress response (Lucassen et al. 2014), enhancing the secretion of catecholamines epinephrine and norepinephrine (Faraut et al. 2015; Vandael et al. 2015). These hormones can promote the intracellular expression of oxidative stress genes (Graziano et al. 2014) and result in proto-oncogene activation, tumor suppressor gene inactivation, DNA damage, and genomic instability (Calvani et al. 2015; Pacak 2011). Our previous results showed that chronic epinephrine stress induced DNA damage via activating the β-adrenergic receptors/β-arrestin-1(Jia et al. 2014). Acute stress is considered to protect against harmful environmental stimuli. However, the protective mechanism of acute stress is largely unknown.
Thioredoxin-1(Trx-1) is a small multifunctional protein with a redox-active disulfide/dithiol in its active site (Cys-Gly-Pro-Cys) and operates together with NADPH and thioredoxin reductase as an efficient reducing system for exposed protein disulfides. Trx-1 has various biological activities and is upregulated in response to a wide variety of stresses, including viral infections and ultraviolet and X-ray irradiation (Masutani et al. 2004). Our previous findings showed that Trx-1 played neuroprotective roles in oxidative stress, neurodegenerative disorders, cerebral ischemia, chronic stress, and morphine addiction (Jia et al. 2014; Luo et al. 2013; Zeng et al. 2014a; Zeng et al. 2015; Zeng et al. 2014b). However, it is unclear whether Trx-1 is involved in acute stress.
Here, we constructed a mouse model of acute stress by treatment with epinephrine and explored the expression of Trx-1 in the stress responsive tissues. The mechanism was further investigated. Trx-1 was induced by acute stress through activating CREB.
Materials and methods
Chemicals
Epinephrine hydrochloride was obtained from Shenyang First Pharmaceutical Factory (Shenyang, China). The primary antibodies against Hsp70, p-CREB, CREB, and β-actin were supplied by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-mouse Trx-1 was obtained from Cell Signaling Technology (Boston, MA, USA). The horse radish peroxidase labeled anti-Rabbit IgG (H+L) antibody and anti-mouse IgG (H+L) antibody were purchased from KPL, Inc. (Gaithersburg, MD, USA). H-89 was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA).
Animal experiments and treatment
Male C57BL/6 mice, 8 weeks of age, were used in the experiments. Mice were housed in plastic cages and maintained on a 12 h light-dark cycle and had free access to food and water. Mice were randomly divided into two groups (n = 10 per group). Mice of epinephrine group were intraperitoneally administered with epinephrine (0.2 mg kg−1) for 4 h and mice of control group were treated with equivalent saline. After administration, animals were killed and the cortex, hippocampus, thymus and kidney were rapidly dissected out, frozen, and stored in a deep freezer at −80 °C until analyzed. All animal care and use were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local Committee on Animal Use and Protection.
Cell culture and treatment
PC12 cells of the rat pheochromocytoma tumor cell line were purchased from Kunming Institute of Zoology, Chinese Academy of Sciences (Kunming, China) and maintained in RPMI1640 medium supplementary with a 10 % heat-inactivated horse serum and 5 % heat-inactivated fetal bovine serum and antibiotics (100 IU/ml penicillin and 100 mg/ml streptomycin) at 37 °C in a humid atmosphere containing 5 % CO2. PC12 cells that were plated in a 6-well plates at density of 4 × 105 and allowed to adhere for 12 h. PC12 cells were pretreated with H89(20 μM) for 30 min followed by incubation with epinephrine (10 μM) for 2 h and then harvested for immunoblot assay.
Immunoblot
Protein lysates were prepared using the solubilizing solution (20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 % NP-40, 1 mM EDTA, 1 mM PMSF, 1 mM EGTA, 1 % Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM β-glycerolphosphate and 1 mg/ml leupeptin). Protein concentration was determined using Bio-Rad protein assay reagent (Hercules, CA, USA). An equal quantity of proteins was separated by 10 %(for Hsp70), 12 % (for p-CREB) or 15 % (for Trx-1) SDS-PAGE and transferred to a PVDF membrane (Millipore Corporation, Billerica, MA, USA). The membranes were soaked in 5 % bovine serum albumin (in TBS, pH 7.2, containing 0.1 % Tween-20) for 2 h at room temperature, then incubated with primary antibodies(1:1000) overnight followed by peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:10,000). The immunoreactive proteins were visualized by an ECL immunoblot detection kit (Millipore Corporation, Billerica, MA, USA). Densitometry analysis was performed by using ImageJ software.
Data analysis
Data were expressed as means ± SD values. Statistical analysis was performed by using SPSS software. The one-way ANOVA followed by a post hoc multiple comparison test was used to compare control and treated groups. P values less than 0.05 were considered statistically significant. All blots are representative of experiments that were performed at least three times.
Results
Acute epinephrine stress induced Hsp70 expression
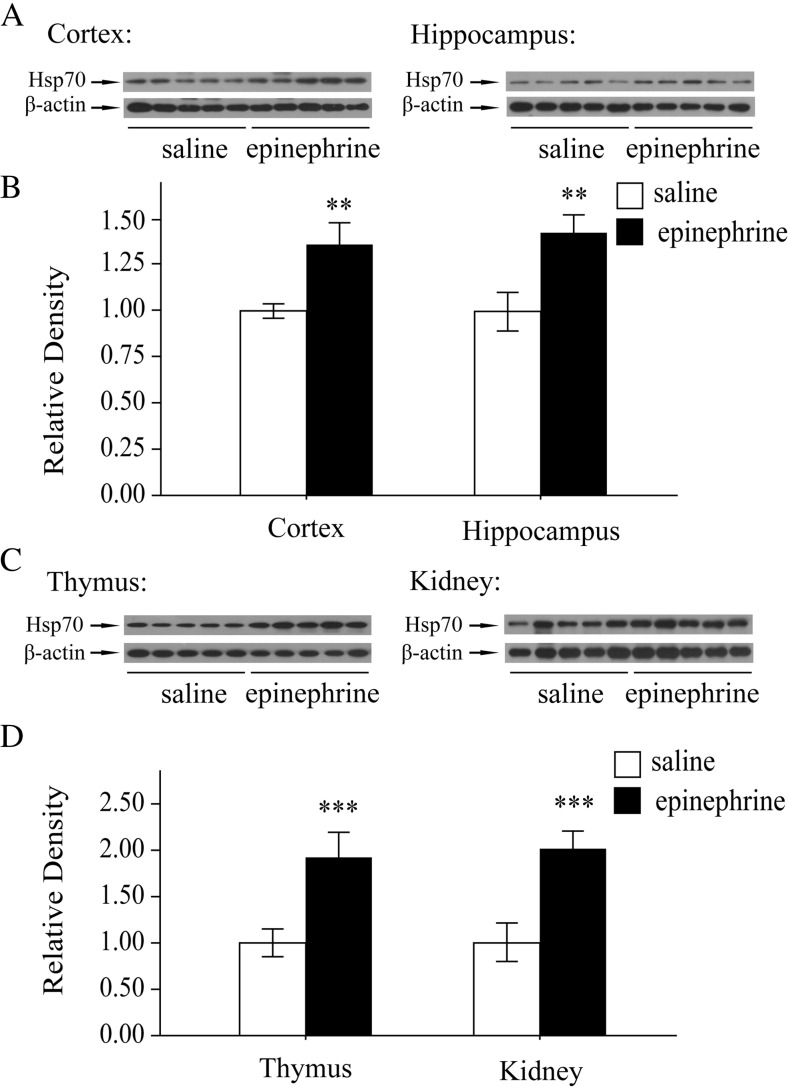
Heat shock proteins (Hsps) are an evolutionarily conserved family of proteins, with individual members named according to their molecular weight. Intracellular Hsps are expressed both constitutively and inducibly after exposure to a wide array of physiological and psychological stressors (Lee et al. 2015). Hsp70 remains the most widely studied member of the Hsp family due to its multiple functions related to protein folding (Kampinga et al. 2009). In the present study, the levels of Hsp70 in the cortex and hippocampus, the stress responsive tissues of mice treated with acute epinephrine were detected by western blot. As shown in Fig. 1a, b, Hsp70 was increased after acute epinephrine stress. We also examined the expression of Hsp70 in another two stress responsive tissues. The immunoblot results showed that acute epinephrine stimuli induced the expression of Hsp70 in the thymus and kidney (Fig. 1c, d). These results suggest that the acute stress model was constructed successfully.
Fig. 1.
Hsp70 expression was induced by acute epinephrine stress. a Changes in Hsp70 expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the cortex and hippocampus of mouse brain. b Quantitative analyses of epinephrine-induced Hsp70 expression in the cortex and hippocampus. c Changes in Hsp70 expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the thymus and kidney of mouse brain. d Quantitative analyses of epinephrine-induced Hsp70 expression in the thymus and kidney. Each bar represents the mean ± SD (n = 10). Asterisks indicate statistical significance (**P < 0.01, ***P < 0.001)
Acute epinephrine stress induced Trx-1 expression
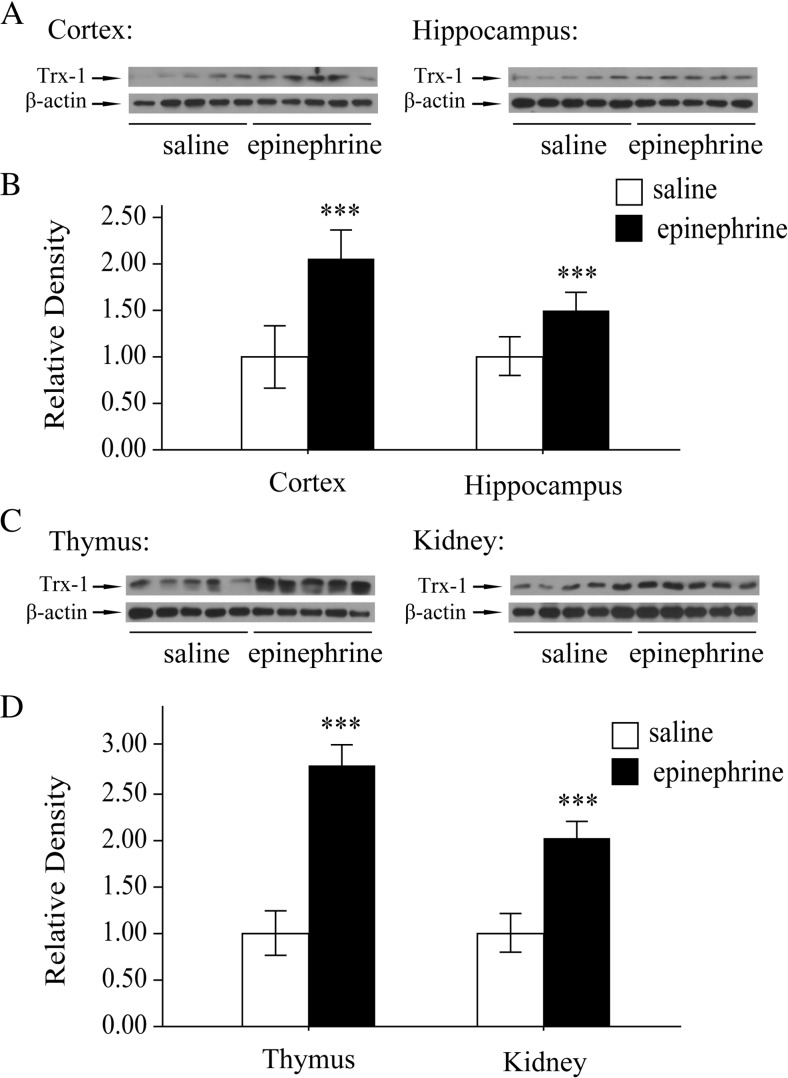
Trx-1 is an inducible redox protein. Our previous study suggested that chronic epinephrine stress induced Trx-1 expression. Here we tested whether acute epinephrine treatment increases the expression of Trx-1. As shown in Fig. 2a, b, Western blot analysis clearly showed upregulation of Trx-1 expression in the cortex and hippocampus after mice were treated with epinephrine compared with those treated with saline only. Similarly, the expression of Trx-1 in the thymus and kidney was enhanced by acute epinephrine treatment (Fig. 2c, d). These data suggest that acute stress induced the Trx-1 expression.
Fig. 2.
Trx-1 expression was induced by acute epinephrine stress
a Changes in Trx-1 expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the cortex and hippocampus of mouse brain. b Quantitative analyses of epinephrine-induced Trx-1 expression in the cortex and hippocampus. c Changes in Trx-1 expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the thymus and kidney of mouse brain. d Quantitative analyses of epinephrine-induced Trx-1 expression in the thymus and kidney. Each bar represents the mean ± SD (n = 10). Asterisks indicate statistical significance (***P < 0.001).
Effect of acute epinephrine stress on CREB activation
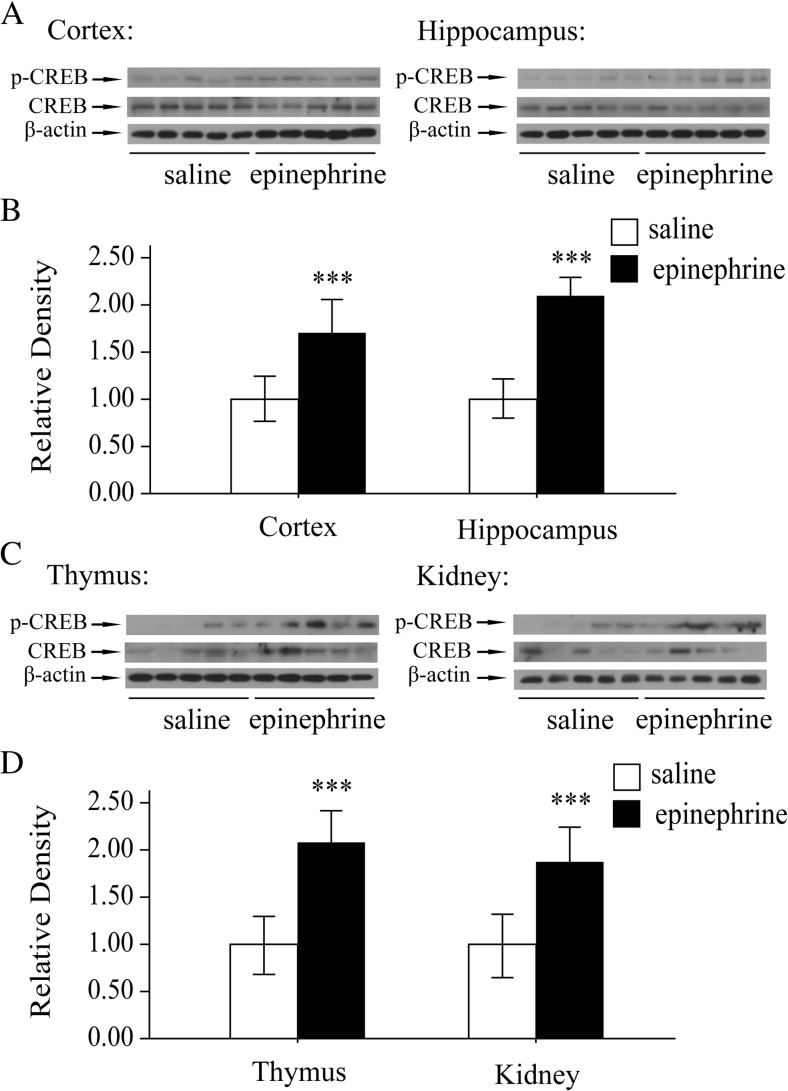
The 5′-upstream sequence of Trx-1 gene contains cyclic AMP responsive element (CRE) (Masutani et al. 2004). The augmentation of Trx-1 expression upon acute stress was hypothesized to be by activating its transcription factor, cAMP-response element binding protein (CREB). So the phosphorylation of CREB was examined. The phosphorylation of CREB (p-CREB, Ser-133) was significantly increased in the cortex and hippocampus by epinephrine compared with the control mice (Fig. 3a, b). Also, the levels of p-CREB (Ser-133) in the thymus and kidney was upregulated markedly by acute epinephrine treatment (Fig. 3c, d).
Fig. 3.
CREB was activated by acute epinephrine stress
a Changes in p-CREB expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the cortex and hippocampus of mouse brain. b Quantitative analyses of epinephrine-induced p-CREB expression in the cortex and hippocampus. c Changes in p-CREB expression after epinephrine treatment (0.2 mg kg−1, for 4 h) in the thymus and kidney of mouse brain. d Quantitative analyses of epinephrine-induced p-CREB expression in the thymus and kidney. Each bar represents the mean ± SD (n = 10). Asterisks indicate statistical significance (***P < 0.001).
PKA activity was required for Trx-1 and Hsp70 by acute epinephrine stress
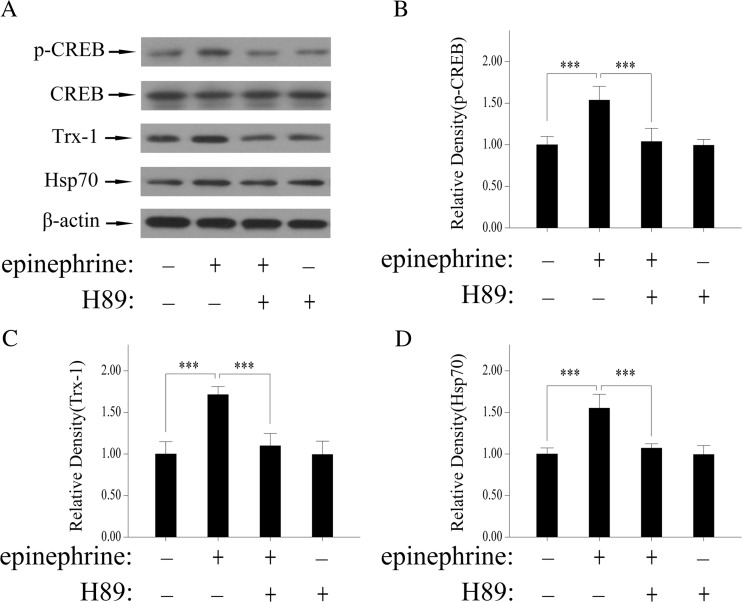
CREB phosphorylation and transcriptional activation are mediated by protein kinase A (PKA) (Avni et al. 2010; Kwok et al. 1994). To clarify that whether the CREB is related to acute epinephrine treatment-induced Trx-1 expression, PC12 cells were pretreated with H89, a PKA inhibitor, for 30 min followed by administration with epinephrine for 2 h. Epinephrine induced phosphorylation of CREB (Ser-133) compared with control, which was inhibited by H89(Fig. 4a, b). As expected, the expression of Trx-1 was enhanced by acute epinephrine treatment and attenuated by H-89(Fig. 4a, c). In addition, the expression of Hsp70 in PC12 cells was also detected with or without epinephrine and H89 and the variety is similar to Trx-1(Fig. 4a, d). These findings indicate that CREB activity was required for Trx-1 increase by acute epinephrine stress.
Fig. 4.
PKA activity was required for Trx-1and Hsp70 by acute epinephrine stress. PC12 cells were pretreated with H89(PKA inhibitor, 20 μM) for 30 min and then stimulated with epinephrine (10 μM) for 2 h. a The effects of H89 on CREB phosphorylation and increase of Trx-1 and Hsp70 by acute epinephrine stress. b Quantitative analyses of p-CREB expression. c Quantitative analyses of Trx-1 expression. d Quantitative analyses of Hsp70 expression. Each bar represents the mean ± SD. All experiments were repeated for three times. Asterisks indicate statistical significance (***P < 0.001)
Discussion
The present study aimed to examine the expression of Trx-1 in the various organs of the adult male mice following an acute epinephrine stress. As expected, acute epinephrine stress elicited significant elevation of Trx-1 levels in the cortex, hippocampus, thymus and kidney. And the activation of CREB is required for acute epinephrine stress-induced Trx-1 expression.
Stress is a response of the central nervous system to environmental stimuli perceived as a threat to homeostasis. The stress response involves a complex network of mechanisms essential for survival and triggers the release of neurotransmitters and hormones from the hypothalamic pituitary adrenal axis and the hypothalamic sympathetic axis (Lee et al. 2016). Chronic epinephrine stress is linked to lead to DNA damage (Jia et al. 2014). The heart muscle can be damaged by excessive epinephrine release (Paur et al. 2012). Acute stress induces a “fight-or-flight” reaction that promotes a rapid response to injury (Bigler et al. 2015). Acute stress causes the rapid release of stress hormones which bind to hippocampal receptors, continues for several hours following the stressful event, and has powerful effects on hippocampally dependent memory that generally promote acquisition and consolidation (Osborne et al. 2015). It is critical to determine the biological effects of acute stress if we are to understand the protective roles of acute stress. In the present study, we constructed an acute stress mouse model administrated with epinephrine. We showed that acute epinephrine treatment induced the expression of Hsp70 in the stress responsive tissues, such as the cortex, hippocampus, thymus, and kidney (Fig. 1). Hsp70 is a commonly used marker of the cellular stress response (Morris et al. 2015). An increase in expression of genes encoding Hsp70 may indicate an increase in intracellular macromolecular damage in the stress response (Feder and Hofmann 1999). The finding in our study suggests that the increase of Hsp70 expression may be a defensive response against acute stress.
Oxidative stress, which could be triggered by chronic stress, is considered as a principal contributor in the pathogenesis of many diseases (Rahal et al. 2014). Chronic mild stress increased the oxidative stress in mitochondrial particles in the brain of rats (Lucca et al. 2009). Acute epinephrine administration modulated α-adrenoceptors to increase the levels of the antioxidant glutathione and ameliorated severe gastric hemorrhage and decreased gastric mucosal lipid peroxidation (Hsu et al. 2005). Trx-1, another vital redox protein, plays a critical role against oxidative stress (Madrigal-Matute et al. 2015; Zeng et al. 2015). Our previous study suggests that Trx-1 protects PC12 cells and the several organs (thymus, cortex, and hippocampus) of mice against chronic epinephrine stress-induced DNA damage (Jia et al. 2014). Here, we detected the effect of acute epinephrine treatment on Trx-1 expression. As expected, the levels of Trx-1 in the above organs of mice were significantly increased after acute epinephrine stress (Fig. 2). The induction of Trx-1 could maintain the redox homeostasis in the mice under acute stress, which is consistent with the reduced lipid peroxidation in the brain of male albino rats subjected to acute administration of stress hormone (Han et al. 2015). Therefore, Trx-1 may be pivotal in the protection of acute stress, so the precise mechanism of Trx-1 will be investigated further.
Usually epinephrine plays various activities by binding the α- and β-adrenergic receptors (Jia et al. 2015). The increase of Trx-1 expression by acute epinephrine stress is in accord with our previous study, in which chronic epinephrine stress induced Trx-1 expression via a β-adrenergic receptors-mediated signaling pathway (Jia et al. 2014). Several studies suggested the important role of α1-adrenergic receptors in the norepinephrine-induced expression of 70-kDa heat shock-protein family (Chin et al. 1996; Johnson et al. 2005). However, there is no evidence suggesting that epinephrine increases Hsp70 expression through adrenergic receptors. We speculated that acute epinephrine stress induced Hsp70 via α1-adrenergic receptors and the precise signaling pathway will be researched in the future.
We further investigated the inducing mechanism of Trx-1 by epinephrine. Stimulation of the adrenergic receptors leads to Gs-dependent adenylyl cyclase activation and Cyclic AMP production, followed by the activation of PKA (Lefkowitz 2007). CREB is a PKA substrate and one of the transcription factors activated by multiple extracellular signals. The transcription of many target genes is activated by the phosphorylation of CREB after it is affected by extracellular signals. The 5′-upstream sequence of Trx-1 gene contains the cAMP response element, the binding site of CREB (Masutani et al. 2004). Our previous study also showed that Trx-1 expression is induced by ephedrine through the PKA-CREB cascades (Jia et al. 2013). In the present study, acute epinephrine stress activated CREB (Fig. 3). CREB phosphorylation and transcriptional activation are mediated by PKA (Avni et al. 2010; Kwok et al. 1994). So H89, the PKA inhibitor, was used to clarify whether the activation of CREB was involved with the upregulation of Trx-1 by acute epinephrine stress. As shown in Fig. 4a, b, the epinephrine-induced activation of CREB was blocked by H89 pretreatment. The increase of Trx-1 was also suppressed by H89(Fig. 4a, c). Hsp70 was usually induced in heat shock response through the activation of its transcription factor heat shock factor 1(HSF1), while the administration of a PKA inhibitor suppressed exercise-induced Hsp70 expression, suggesting a role for PKA in the regulation of HSF1 activation (Melling et al. 2006). HSF1 was activated by PKA through regulating the phosphorylation of HSF1 at Ser320(Murshid et al. 2010). Here, we also confirmed that acute epinephrine stress-induced Hsp70 expression was blocked by the PKA inhibitor H89(Fig. 4A and D). These results suggest that PKA plays an important role in acute epinephrine stress-induced expression of Trx-1 and Hsp70.
In conclusion, the acute epinephrine stress induces Trx-1 expression. And Trx-1 may have an important role in the protection of acute epinephrine stress. The precise role should be researched further.
Acknowledgments
This study was supported by the Key scientific research projects of the University in Henan Province (16 A180017, 16 A180018), the PhD. early development program and Nanhu Scholars Program for Young Scholars of XYNU.
Abbreviations
- CRE
cyclic AMP responsive element
- CREB
cAMP-response element binding protein
- HSF1
Heat shock factor 1
- Hsp70
Heat shock protein
- PKA
Protein kinase A
- Trx-1
Thioredoxin-1
Compliance with ethical standards
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Jin-Jing Jia and Xian-Si Zeng contributed equally to this work.
References
- Avni D, Philosoph A, Meijler MM, Zor T. The ceramide-1-phosphate analogue PCERA-1 modulates tumour necrosis factor-alpha and interleukin-10 production in macrophages via the cAMP-PKA-CREB pathway in a GTP-dependent manner. Immunology. 2010;129:375–385. doi: 10.1111/j.1365-2567.2009.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler MB, et al. Stress-induced in vivo recruitment of human cytotoxic natural killer cells favors subsets with distinct receptor profiles and associates with increased epinephrine levels. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani M, et al. Norepinephrine promotes tumor microenvironment reactivity through beta3-adrenoreceptors during melanoma progression. Oncotarget. 2015;6:4615–4632. doi: 10.18632/oncotarget.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JH, Okazaki M, Hu ZW, Miller JW, Hoffman BB. Activation of heat shock protein (hsp)70 and proto-oncogene expression by alpha1 adrenergic agonist in rat aorta with age. J Clin Invest. 1996;97:2316–2323. doi: 10.1172/JCI118674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt JP, Leger D. Napping reverses the salivary interleukin-6 and urinary norepinephrine changes induced by sleep restriction. J Clin Endocrinol Metab. 2015;100:E416–E426. doi: 10.1210/jc.2014-2566. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Graziano TS, Closs P, Poppi T, Franco GC, Cortelli JR, Groppo FC, Cogo K. Catecholamines promote the expression of virulence and oxidative stress genes in Porphyromonas gingivalis. J Periodontal Res. 2014;49:660–669. doi: 10.1111/jre.12148. [DOI] [PubMed] [Google Scholar]
- Han LL, Chen L, Dong ZL. Impact of acute and chronic stress hormone on male albino rat brain. Int J Clin Exp Pathol. 2015;8:6841–6846. [PMC free article] [PubMed] [Google Scholar]
- Hsu DZ, Wang ST, Deng JF, Liu MY. Epinephrine protects against severe acute gastric bleeding in rats: role of nitric oxide and glutathione. Shock. 2005;23:253–257. [PubMed] [Google Scholar]
- Jia JJ, Zeng XS, Li Y, Ma S, Bai J. Ephedrine induced thioredoxin-1 expression through beta-adrenergic receptor/cyclic AMP/protein kinase A/dopamine- and cyclic AMP-regulated phosphoprotein signaling pathway. Cell Signal. 2013;25:1194–1201. doi: 10.1016/j.cellsig.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Jia JJ, Zeng XS, Yang LH, Bai J. The epinephrine increases tyrosine hydroxylase expression through upregulating thioredoxin-1 in PC12 cells. Biochimie. 2015;115:52–58. doi: 10.1016/j.biochi.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Jia JJ, Zeng XS, Zhou XS, Li Y, Bai J. The induction of thioredoxin-1 by epinephrine withdraws stress via interaction with beta-arrestin-1. Cell Cycle. 2014;13:3121–3131. doi: 10.4161/15384101.2014.949214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M (2005) Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol 99:1789–1795. doi:10.1152/japplphysiol.00390.2005 [DOI] [PubMed]
- Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RP, et al. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Lee BH, et al. Gintonin enhances performance of mice in rotarod test: involvement of lysophosphatidic acid receptors and catecholamine release. Neurosci Lett. 2016;612:256–260. doi: 10.1016/j.neulet.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Sukri NM, Ogden H, Vine C, Thake CD, Turner JE, Bilzon JL. A comparison of two commercially available ELISA methods for the quantification of human plasma heat shock protein 70 during rest and exercise stress. Cell Stress Chaperones. 2015;20:917–926. doi: 10.1007/s12192-015-0610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, et al. Neuropathology of stress. Acta Neuropathol. 2014;127:109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucca G, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res. 2009;43:864–869. doi: 10.1016/j.jpsychires.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Luo FC, Zhao L, Deng J, Liang M, Zeng XS, Liu H, Bai J. Geranylgeranylacetone protects against morphine-induced hepatic and renal damage in mice. Mol Med Rep. 2013;7:694–700. doi: 10.3892/mmr.2012.1217. [DOI] [PubMed] [Google Scholar]
- Madrigal-Matute J, et al. Thioredoxin-1/peroxiredoxin-1 as sensors of oxidative stress mediated by NADPH oxidase activity in atherosclerosis. Free Radic Biol Med. 2015;86:352–361. doi: 10.1016/j.freeradbiomed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Masutani H, Bai J, Kim YC, Yodoi J. Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol Neurobiol. 2004;29:229–242. doi: 10.1385/MN:29:3:229. [DOI] [PubMed] [Google Scholar]
- Melling CW, Krause MP, Noble EG. PKA-mediated ERK1/2 inactivation and Hsp70 gene expression following exercise. J Mol Cell Cardiol. 2006;41:816–822. doi: 10.1016/j.yjmcc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Morris JP, Thatje S, Ravaux J, Shillito B, Hauton C. Characterising multi-level effects of acute pressure exposure on a shallow-water invertebrate: insights into the kinetics and hierarchy of the stress response. J Exp Biol. 2015;218:2594–2602. doi: 10.1242/jeb.125914. [DOI] [PubMed] [Google Scholar]
- Murshid A, Chou SD, Prince T, Zhang Y, Bharti A, Calderwood SK. Protein kinase A binds and activates heat shock factor 1. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DM, Pearson-Leary J, McNay EC. The neuroenergetics of stress hormones in the hippocampus and implications for memory. Front Neurosci. 2015;9:164. doi: 10.3389/fnins.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65–90. doi: 10.4149/endo_2011_02_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paur H, et al. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126:697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandael DH, et al. Reduced availability of voltage-gated sodium channels by depolarization or blockade by tetrodotoxin boosts burst firing and catecholamine release in mouse chromaffin cells. J Physiol. 2015;593:905–927. doi: 10.1113/jphysiol.2014.283374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XS, Jia JJ, Kwon Y, Wang SD, Bai J. The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radic Biol Med. 2014;67:10–18. doi: 10.1016/j.freeradbiomed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Zeng XS, Jia JJ, Ma LF. Gensenoside Rb1 protects rat PC12 cells from oxidative stress-induced endoplasmic reticulum stress: the involvement of thioredoxin-1. Mol Cell Biochem. 2015;410:239–246. doi: 10.1007/s11010-015-2557-1. [DOI] [PubMed] [Google Scholar]
- Zeng XS, et al. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Can J Physiol Pharmacol. 2014;92:102–108. doi: 10.1139/cjpp-2013-0274. [DOI] [PubMed] [Google Scholar]