Abstract
The ability to form an accurate map of sensory input to the brain is an essential aspect of interpreting functional brain signals. Here, we consider the somatotopic map of vibrissa-based touch in the primary somatosensory (vS1) cortex of mice. The vibrissae are represented by a Manhattan-like grid of columnar structures that are separated by inter-digitating septa. The development, dynamics and plasticity of this organization is widely used as a model system. Yet, the exact anatomical position of this organization within the vS1 cortex varies between individual mice. Targeting of a particular column in vivo therefore requires prior mapping of the activated cortical region, for instance by imaging the evoked intrinsic optical signal (eIOS) during vibrissa stimulation. Here, we describe a procedure for constructing a complete somatotopic map of the vibrissa representation in the vS1 cortex using eIOS. This enables precise targeting of individual cortical columns. We found, using C57BL/6 mice, that although the precise location of the columnar field varies between animals, the relative spatial arrangement of the columns is highly preserved. This finding enables us to construct a canonical somatotopic map of the vibrissae in the vS1 cortex. In particular, the position of any column, in absolute anatomical coordinates, can be established with near certainty when the functional representations in the vS1 cortex for as few as two vibrissae have been mapped with eIOS.
This article is part of the themed issue ‘Interpreting BOLD: a dialogue between cognitive and cellular neuroscience’.
Keywords: blood oxygen level-dependent (BOLD) imaging, functional imaging, intrinsic optical signal, vibrissa, whisker
1. Introduction
The body surface is topographically represented point-for-point in the somatosensory cortex. This somatotopic organization is particularly striking in the case of the long mystacial sinus hairs, or vibrissae, in rodents. Here, each vibrissa is represented in the primary somatosensory (vS1) cortex by a column of neurons that responds exclusively to some aspect of motion of that hair. The columns for different vibrissa are arranged in a Manhattan-like lattice that matches the spatial distribution of vibrissae in the mystacial pad [1]; reviewed in [2]. This special geometry is historically referred to as the ‘barrel field’ and has enabled studies that depend on the contrasting effect of manipulating one sensory input, or vibrissa, relative to control conditions for neighbouring vibrissae. As examples, these include neuronal development and plasticity [3–7], electrical dynamics among neighbouring columns [8–14], the transference of learning between columns [15], microstrokes to single columns [16] and local blood-flow dynamics [17,18]. Further studies involve multi-columnar reconstructions of neuron architectonics [19–21] and large-scale neural network simulations [21].
The detailed location of cortical columns within the vS1 cortex varies between animals [22]. Thus, measurements to ascertain the precise spatial geometry of the vibrissa field are necessary when measurements, stimulation or injections in specific columns are required. A common and non-invasive method to map the vibrissa field is to image the evoked intrinsic optical signal (eIOS) [23–25] through a cranial window [26] or thinned transcranial window [27,28]. The eIOS, like the blood oxygen level-dependent (BOLD) functional magnetic resonant image (fMRI) signal [29,30], exploits the change in electronic structure of haemoglobin that occurs when heme transitions from the oxidized to reduced, or deoxygenated, state [31]. This transition results in a change in the absorption spectrum [23,32]. Thus a wavelength of the light can be selected to predominantly measure either a relative increase or decrease in blood oxygen concentration [33,34]; the absorption at so-called ‘isobestic points’ are sensitive only to the total concentration of haemoglobin. Concurrent with the change in absorption is a change in the magnetic state of haemoglobin. Oxyhaemoglobin is weakly diamagnetic while deoxyhaemoglobin is a strong paramagnet; the later state leads to a gradient in the local magnetic field of blood vessels and may be detected via dephasing of the magnetization of water protons.
Here, we focus on mapping with the eIOS and consider single wavelength measurements in the relatively broad near infrared range of 620–780 nm, where the absorption of deoxyhaemoglobin exceeds that of oxyhaemoglobin. Thus an increase in metabolism leads to an increase of absorption and a concurrent decrease in the reflectance of light. In response to vibrissa stimulation, the eIOS follows a tri-phasic time course [35]. Relative to the pre-stimulus light reflectance, the eIOS is initiated by a brief, approximately 1 s net-negative reflectance, historically labelled the ‘initial dip’, followed by 1–2 s net-positive reflectance and concluded by a prolonged, i.e. 5 s or longer net-negative reflectance. The initial net-negative phase of the eIOS is presumed to reflect oxygen consumption as a result of heightened neural activity, while the following net-positive phase results from an active vasodynamic response that increases the diameter of vessels within the activated cortical column, as measured optically [36,37] and by fMRI [38]. While the initial deoxygenation related component co-localizes closely with the activated cortical column, the vasodynamic response may not [35,39]. This limits the precision of eIOS, and by inference BOLD fMRI, in mapping studies.
Early studies that used eIOS to map the representation of individual vibrissae in the vS1 cortex were limited to mapping between five and nine vibrissae in the same animal [40–42]. More recently, we described a protocol, motivated by the prescient fMRI work of Kim and co-workers [43], for mapping of the entire vibrissae field during isoflurane anaesthesia [17]. We used this protocol to demonstrate that eIOS maps are precisely co-localized with the anatomically described vibrissa columns [17]. Anaesthesia generally suppresses the amplitude of haemodynamic responses [44–52]. Strong vasodilators such as isoflurane [43,53], however, specifically suppress the vasodynamic component and isolate the localized haemodynamic eIOS component that is caused by tissue deoxygenation, i.e. the build-up of deoxyhaemoglobin. This leads to improved signal-to-noise in eIOS images [17,54,55]. Here, we describe further improvements in the technique of eIOS mapping under isoflurane and generate complete maps of the vibrissa field across a large cohort of C57BL/6 mice. We use this data to complete a canonical map of the vibrissa field, along with the variability in that map.
2. Material and methods
(a). Subjects
Twenty-nine female C57BL/6 Thy1-ChR2 transgenic mice (JAX strain B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J) [56], of 22–24 g in mass and ages 5–18 weeks, were prepared for intrinsic optical imaging; the presence of channelrhodopsin is not relevant for this work and relates to subsequent manipulations performed with these animals. The weight range of these mice was similar to that reported for wild-type female C57BL/6 mice [57].
(b). Surgery
Each animal was anaesthetized with 1.5–2.0% isoflurane (v/v) in oxygen, and maintained at a constant body temperature of 37°C using a temperature control system (no. 40–90–8; FHC Inc.). The bone overlying the vS1 cortex was thinned [28]. Briefly, the bone was skim-cut with a drill (EXL-M40, Osada, CA, USA) and a no. 005 bit (Hager & Meisinger GmbH) past the cancellous layer to a final thickness of no more than 100 µm. Two to four fiducial marks were painted close to the corners of the thinned-skull window with a fine-point black ink pen (Sakura, Pigma Micron 005, Japan). The coordinates of the fiducial marks were measured relative to bregma with a stereotactic device with an accuracy of about 10 µm. The bone was left to air-dry and covered with a thin layer of cyanoacrylate glue over which a piece of #0 cover glass was placed. The edges of the window were covered with dental cement (Grip Cement, Denstply #675571) and a custom made titanium-alloy head-bar attached for head-fixation during imaging experiments. The mice were given analgesics (Buprenex, Reckitt Benckiser Pharmaceuticals; 7 µg per mouse) at the conclusion of surgery.
(c). Intrinsic optical signal imaging
During imaging sessions, animals were either awake or anaesthetized with isoflurane at 1.8–2.0% (v/v) in oxygen. Imaging with acclimated and head-fixed mice was performed as described and illustrated [36,58]. Anaesthetized mice were maintained at a constant body temperature of 37°C using a temperature control system (no. 40–90–8; FHC Inc.) and continuously infused with 5% (v/v) glucose in 0.9% (w/v) NaCl subcutaneously at a rate of 10 ml kg−1 h−1 using a syringe pump (no. 780101; Harvard Apparatus), as previously described [17]. Awake mice were administered 5% (v/v) glucose in water orally before and after imaging sessions. Imaging experiments in anaesthetized animals lasted 3–5 h.
We followed previously described protocols for intrinsic optical imaging [17,24]. Briefly, a pair of front-to-front camera lenses imaged an approximately 4 × 4 mm region on the cortical surface onto a 512 × 512 pixel region of a CCD camera (Pantera TF 1M60; Teledyne Dalsa) at a resolution of 7.87 µm per pixel. Prior to eIOS imaging, a reference vessel image was recorded with illumination at 455 nm (no. M455L2; ThorLabs). Throughout functional imaging, the cortical surface was illuminated at 625 nm (no. M625L2; Thorlabs). Frames were acquired as averages over 0.5 s intervals using a custom-written LabView application (National Instruments, Austin, TX, USA). For activation of the cortical representation of the vibrissae, individual vibrissae were trimmed to 70–90% of their full length, placed inside a quartz capillary attached to a piezoelectric element, and displaced 0.5 mm in both rostral and caudal directions with a 10 Hz sinusoidal pattern for 4 s at a distance of 10% of the original length. Four seconds of baseline activity was acquired prior to stimulation, 4 s during stimulation, and 16 s after the end of stimulus, for a total of 24 s of data per trial. The inter-trial interval was 10 s. Five to thirty trials were acquired and averaged per vibrissa; the exact number was decided by inspecting the data on-the-fly. This procedure was repeated for 12–30 vibrissae in the same animal. A total of 635 vibrissae were mapped across 28 different mice.
(d). Image analysis
Raw image frames were loaded into Matlab (MathWorks, Natick, MA, USA) and processed with custom-written code. A single baseline image (Rbase) was created by averaging over eight, 0.5 s frames that preceded stimulus onset. Signal frames (ΔIf/If) were generated by subtracting and normalizing individual frames (Rf) by this baseline using the formula ΔIf/If = (Rf − Rbase)/Rbase [59]. The median pixel value across the entire field was subtracted from all pixels of each signal frame to balance the effects of large, spontaneous fluctuations caused by isoflurane induced burst suppression [60]. Finally, we averaged each frame across multiple trials.
Maps of the entire field of the macro-vibrissae in the vS1 cortex were computed as follows (figures 1–4):
1. The stimulus-evoked map of a single-vibrissa's cortical representation was generated by averaging signal frames in the range 0.5–5.0 s after stimulus onset (figure 1c). This procedure was repeated for all vibrissa mapped in the same mouse.
2. Individual vibrissa maps were cropped by a circular region of interest, 0.5–1.5 mm in diameter (0.90 ± 0.22 mm; mean ± s.d.), that was manually centred on the evoked region. This was necessary because of an occasional presence of motion artefacts, associated in particular with the edges of the imaging windows and fiducial marks on bone under the window. Motion artefacts were readily distinguished from evoked activity by their dark-bright appearance (figure 1c).
3. Vibrissae maps were next convolved with a 0.25 × 0.25 mm Gaussian window with width σ = 0.08 mm.
4. Vibrissa maps were normalized and thresholded at 20% of the maximum signal amplitude.
5. All vibrissae maps obtained from the same animal were combined by taking the maximum value, pixel by pixel, across all maps. With one exception (figure 4c), this maximum projection was computed before any spatial transformations (figure 3).
Figure 1.
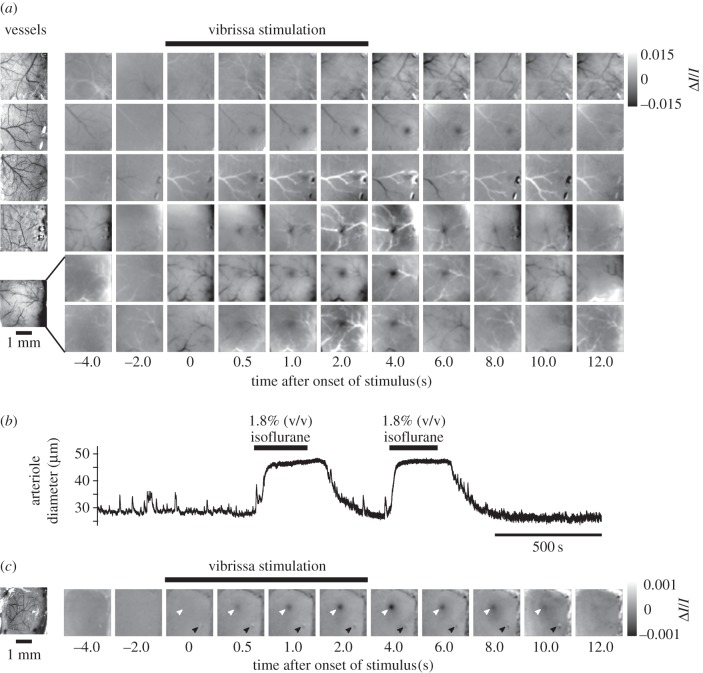
(a) Evoked IOS measured in the vS1 cortex of awake, head-fixed mice during stimulation of the C2 vibrissa. Time points indicate the onset of 0.5 s frames. The images in each row are the average across 20 sequential trials. The data in the top five rows are from different animals while that in the sixth row is a second set of trials from the same animal and field as used for the fifth row. The left-most images were taken under 455 nm illumination and show the layout of dural and pial vessels. (b) Vasodilatory effects of isoflurane on pial arteriole diameter measured in an awake, head-fixed mouse transitioning in and out of anaesthetized state induced by administration of 1.8% (v/v) isoflurane. (c) Intrinsic optical signals measured in an isoflurane anaesthetized mouse before, during and after stimulation of the C4 vibrissa; 20 trial average. White arrows point to the region of evoked activity. Black arrows point to the location of a motion artefact rendering a fiducial on the bone under the window visible in the signal images.
Figure 2.
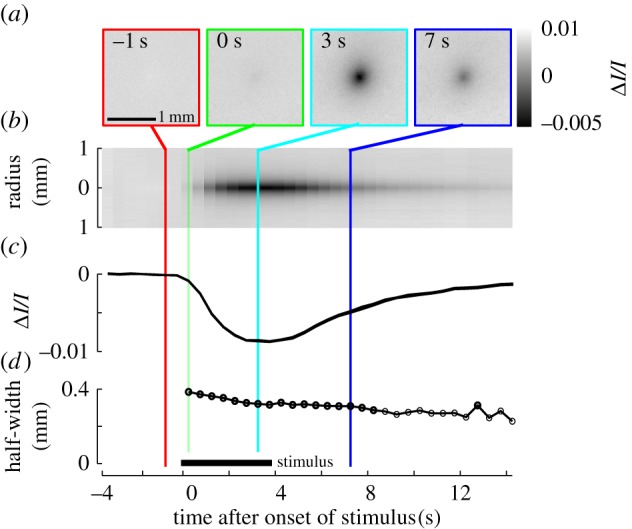
Evoked IOSs measured in isoflurane anaesthetized mice. We averaged across a subset of 268 vibrissae, whose eIOS centroids were located within the centre 2 × 2 mm region in images. (a) Evoked IOS maps obtained during single-vibrissa stimulation, centred and averaged at different time points relative to stimulation onset. Each frame is the average eIOS over 0.5 s. (b) Average eIOS maps collapsed and averaged in all radial directions, illustrating the spatial extent and time course of the eIOS. The panel is mirror symmetric with respect to the horizontal axis. The crossing coloured lines correspond to the time points of frames in panel (a). (c) Average amplitude of the eIOS as a function of time, measured at the negative peak. The trace fluctuates in thickness corresponding to its standard error. (d) Half-width of the eIOS as a function of time.
Figure 3.
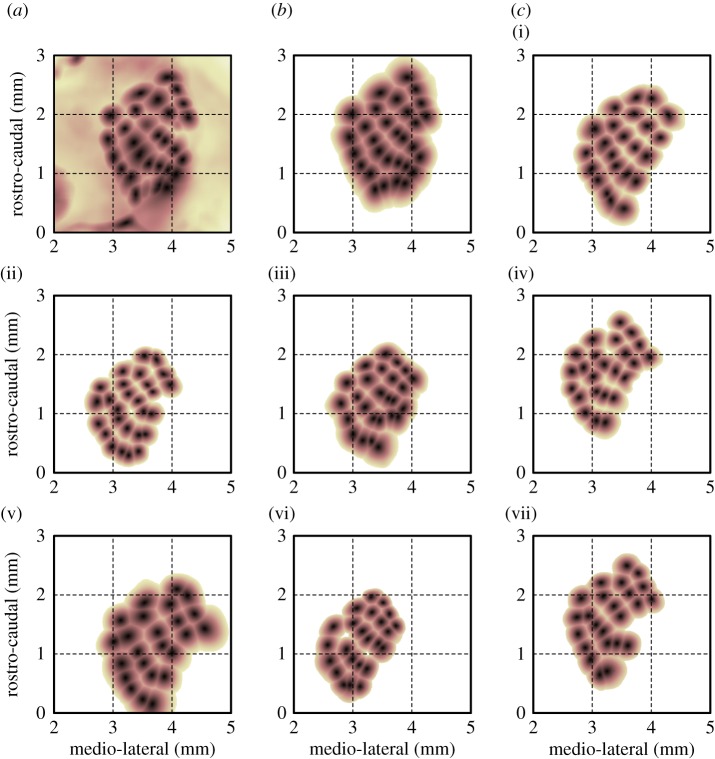
(a) Maximum projection of 30 single-vibrissa eIOS maps measures in the same mouse. The maps were normalized and smoothed (σ = 80 µm) prior to projection. (b) The same combined map as in panel (a), except that individual maps were first cropped by a circular ROI centred on the negative peak of the eIOS, then normalized and thresholded at 20%. This particular map served as the reference map for later alignment of maps across different mice. (c)(i–vii). Multi-vibrissa eIOS maps obtained from seven additional mice, pre-processed and projected as in panel (b).
Figure 4.
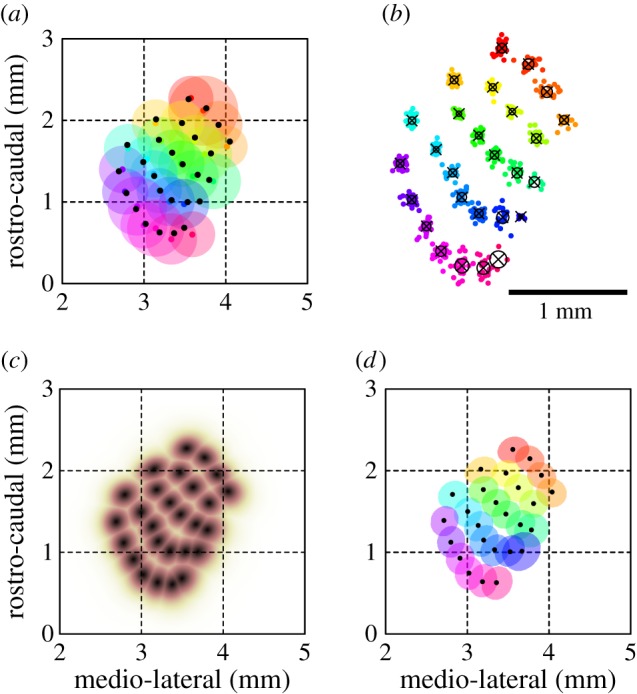
Construction of a canonical map in the vS1 cortex. (a) Distributions of eIOS centroids, in absolute anatomical coordinates, represented by the mean (black dots) and standard deviation (circles colour coded by vibrissa identity) of the geometric position of eIOS centroids (541 vibrissae from 25 mice). (b) Distributions of eIOS centroids, in relative anatomical coordinates (629 vibrissae from 28 mice). The locations of map centroids obtained from the same mouse were maintained in fixed relative coordinates and rotated, translated and re-scaled to optimally match, in the least-squared sense, the location, scale, and orientation of centroids of a reference eIOS map (figure 3b). Centroids of individual eIOS maps are plotted and colour coded by vibrissae identity (as in panel (a)), and additionally represented by the average (black x's) and standard deviations (black circles) of their relative geometric positions. (c) Canonical somatotopic map of the vibrissa representation in the vS1 cortex. Individual eIOS maps were aligned as in part (b) and then averaged, according to vibrissa identity, across all 28 mice and combined through normalization and maximal projection to yield an average map in relative coordinates. This map was then transformed to absolute coordinates by finding the affine transform (in the least-squares sense) that matched the distribution of eIOS centroids in relative coordinates (x's in in panel (b)) onto the distribution of eIOS centroids in absolute coordinates (black dots in in panel (a)). The result shown is thus the combined eIOS map of all individual vibrissa, averaged across mice, at its average spatial location and orientation in absolute anatomical coordinates relative to bregma. We term this map the canonical map of vibrissa representation in the vS1 cortex. (d) Spatial locations of individual vibrissa representations in the vS1 cortex in terms of the centroids and half-widths of the eIOS of the canonical vibrissa map.
The location and orientation of an eIOS map combined from many single-vibrissa maps was found from fiducial marks placed on the bone outside the thinned part of the imaging window during surgery. These marks, which had known anatomical coordinates relative to bregma, were manually selected in images taken with illumination at 455 nm (figure 1c). The pair of fiducial marks with the largest spatial separation was used to compute the location of bregma, in units of pixels, and to align an anatomical grid onto the acquired frames. All eIOS maps were then rotated and reflected such that the medio-lateral and rostro-caudal axes were coincident with the abscissa and ordinate, respectively (figure 3).
The relative organization of the vibrissa field across mice was compared by aligning all combined eIOS maps with the map that contained the largest number of mapped vibrissae (figure 3a), as follows:
1. For each vibrissa eIOS map the pixel coordinate of the peak amplitude was obtained, yielding a set of control coordinates with known vibrissae IDs for each animal. The set of coordinates obtained in the reference animal are referred to as the ‘reference coordinates’.
2. For each set of control coordinates, vibrissae ID and coordinates that did not exist in the reference set were removed.
3. The ‘control coordinates’ were then spatially registered with the reference coordinates, based on vibrissae IDs, through a least-squares affine transformation optimization routine using the ‘cp2tform’ function in the Matlab Image Processing Toolbox. We used the ‘similarity’ transformation type that includes rotation, scaling, translation and reflection.
A canonical vibrissa representation in the vS1 cortex was obtained by averaging all maps across mice, as follows:
1. For each unique vibrissa, e.g. C2, the pixel coordinate of the peak eIOS amplitude was obtained across all animals, yielding a spatial distribution of eIOS peak-amplitude locations in absolute anatomical coordinates with a mean and standard deviation (figure 4a). A new reference set of vibrissa representation coordinates was defined as the set of mean peak-amplitude locations, across animals.
2. Each set of control coordinates, as defined above, was then spatially registered to this new reference set of average coordinates in absolute anatomical coordinates. This transformation resulted in a data structure containing the spatial transformation.
3. The spatial transformation was then applied to the each of the individual maps of the raw eIOS, averaged for the 0.5–5 s time-span after stimulation onset. The spatially transformed maps were next averaged by vibrissa ID across all animals, yielding an average eIOS map in absolute anatomical coordinates per vibrissa.
4. Each average eIOS map was then normalized and thresholded at 20% of the maximum amplitude, as described above, before the maximum projection was computed across all vibrissae. This maximum projection map resulted in what we term a ‘canonical map’ of the vibrissa representation in the vS1 cortex (figure 4c). Additional statistics of this canonical map were computed, such as the half-width (figure 4d) and average locations of individual vibrissa representations (table 1).
Table 1.
Location and spatial distribution of individual vibrissa intrinsic map peaks. ML: lateral distance of map peak relative to the horizontal midline passing through bregma point. RC: caudal distance of map peak relative to bregma. s.d.rel: standard deviation of the spread of mapped eIOS centroids after affine transformation (cf. Figure 4b). s.d.abs: standard deviation of the spread of mapped eIOS centroid in absolute coordinates (cf. Figure 4a). Half-width: width of the eIOS at 50% peak amplitude.
| vibrissa | ML (mm) | RC (mm) | s.d.rel (µm) | s.d.abs (µm) | half-width (µm) |
|---|---|---|---|---|---|
| α | 3.56 | 2.26 | 38 | 287 | 348 |
| β | 3.16 | 2.02 | 30 | 324 | 354 |
| γ | 2.81 | 3.48 | 27 | 329 | 350 |
| δ | 2.71 | 1.39 | 31 | 297 | 348 |
| A1 | 3.77 | 2.15 | 41 | 359 | 334 |
| A2 | 3.91 | 1.95 | 43 | 379 | 336 |
| A3 | 4.04 | 1.74 | 32 | 270 | 179 |
| B1 | 3.47 | 1.97 | 28 | 269 | 358 |
| B2 | 3.62 | 1.79 | 25 | 323 | 358 |
| B3 | 3.81 | 1.60 | 37 | 297 | 340 |
| C1 | 3.19 | 1.77 | 21 | 269 | 340 |
| C2 | 3.35 | 1.61 | 27 | 278 | 340 |
| C3 | 3.47 | 1.47 | 33 | 280 | 340 |
| C4 | 3.65 | 1.34 | 40 | 306 | 348 |
| C5 | 3.79 | 1.27 | 42 | 336 | 414 |
| D1 | 3.00 | 1.50 | 22 | 281 | 348 |
| D2 | 3.13 | 1.33 | 31 | 275 | 354 |
| D3 | 3.20 | 1.15 | 37 | 304 | 360 |
| D4 | 3.33 | 1.03 | 34 | 326 | 366 |
| D5 | 3.52 | 1.00 | 49 | 353 | 366 |
| D6 | 3.67 | 1.01 | 16 | 461 | 464 |
| E1 | 2.79 | 1.12 | 33 | 322 | 358 |
| E2 | 2.91 | 0.92 | 35 | 351 | 364 |
| E3 | 3.02 | 0.74 | 41 | 333 | 368 |
| E4 | 3.18 | 0.63 | 56 | 284 | 374 |
| E5 | 3.36 | 0.62 | 51 | 306 | 386 |
| E6 | 3.48 | 0.69 | 60 | 276 | 530 |
(e). Single vessel imaging
The effect of isoflurane on vasodynamics of individual arterioles was ascertained with two-photon imaging of thinned-skull, head-fixed animals [58].
3. Results
Cortical activation during sensory stimulation is typically much stronger in awake compared with anaesthetized rodents [46]. Thus, we initially assessed the suitability of using an awake preparation for precise somatotopic mapping. We mechanically stimulated one vibrissa at a time at 10 Hz for 4 s [17]. In awake, head-fixed mice we found that the trial-to-trial variability in the eIOS measured with 625 nm illumination was generally high and persisted when comparing blocks of averaged trials (20 trials) (figure 1a). Although we were able to reproduce blocks of trials with tri-phasic response, these features were not seen in every block of averaged trials. In some blocks the ‘initial dip’ persisted for the duration of the stimulus. More generally, there was significant overlap in time and space between large amplitude vasodynamic responses and the smaller amplitude responses associated with direct neuronal activation by vibrissa stimulation. All told, the rich vasodynamics observed with eIOS in awake animals complicates its use as a tool to rapidly map individual columns in the vS1 cortex.
Isoflurane induces vasodilation of cortical blood vessels (figure 1b) via activation of ATP-sensitive K+ channels [43,53] and thus suppresses the potential for vasodynamic events during somatosensory stimulation [17,54]. We found that intrinsic signals, void of vasodynamic signatures evoked by vibrotactile stimulation, could be obtained for up to 5 h when isoflurane anaesthesia was combined with thermal regulation and continuous subcutaneous administration of 5% (w/v) glucose in NaCl (figure 1c). This allowed us to precisely locate the somatotopic representation of each vibrissa, one-by-one, in the vS1 cortex. The exact concentration of isoflurane in oxygen was critical for this result. If the isoflurane level was too low, vasodynamic responses associated with vessels re-appeared even though the animal remained anaesthetized. If the isoflurane level was too high, the amplitude of the eIOS was suppressed or not observed at all. An isoflurane level close to 1.8% (v/v) in oxygen overall provided the best results. The data in figure 2 shows the average evoked spatial signature and time course of the intrinsic signal before, during, and after a 4 s vibrotactile stimulus. The amplitude of the eIOS response grew throughout stimulation, peaked at about 4 s, and then receded to baseline beyond 10 s after stimulation ceased (figure 2a–c). The area, or half-width, of the evoked response decreased monotonically with time following stimulation onset (figure 2d). The half-width decreased faster during the 4 s stimulation period, at −20.7 µm s−1, and slower after vibrissa stimulation ceased, at −6.8 µm s−1.
The appearance of only a single feature in the eIOS response for each vibrissa enabled us to combine individual responses into a single somatotopic map (figure 3a) (Material and methods); we cropped the individual maps within a circle, if required, to remove movement or vasodynamic artefacts (figure 3b). The stereotypic layout of the vibrissae representation in the vS1 cortex was apparent in the combined somatotopic maps of all mapped mice; eight representative maps are shown in figure 3. The absolute location and orientation of the vibrissae map, however, varied between animals. The centroids of the eIOS clustered in anatomical coordinates according to the identity of mapped vibrissae (figure 4a and table 1). On average, across all mapped vibrissae, the standard deviation of eIOS centroids averaged by vibrissa identity (circles in figure 4a) was 0.31 ± 0.04 mm (mean ± s.d.), or approximately two columnar radii. By comparison, the average inter-row distance between nearest-neighbour eIOS centroids was 0.16 ± 0.04 mm (mean ± s.d.). Thus the absolute location of the vibrissae relative to the bregma point is quite variable between animals.
Qualitatively, the relative layout of the vibrissa representation in the vS1 cortex was well preserved across mice (figure 3). To quantify the relative configuration of the vibrissa representation across animals, the eIOS map from each mouse was aligned to a reference map, chosen as the map with the largest number of vibrissae (figure 3b). The alignment was solely through an affine transformation, found by least-squares optimization of distances to eIOS centroids in the reference map. The re-scaled and aligned maps, with respect to the reference map, are shown in figure 4b; the centroids of individual eIOS vibrissa maps have been colour coded according to vibrissa identity. In these relative coordinates, the eIOS centroids were more tightly clustered than in absolute coordinates. Averaged across all vibrissae, the standard deviation of centroid positions was 0.036 ± 0.010 mm (mean ± s.d.), as grouped by vibrissa identity, averaged across the horizontal and vertical axes (figure 4b and table 1). Thus the relative spatial configuration of the somatotopic vibrissae eIOS map is highly stereotyped across mice (figure 4b).
Evoked eIOS responses during vibrissa stimulation are well co-localized with the position of the underlying columns but do not stop at the boundaries of these structures [17] (figure 2). Averaged across all vibrissae, the half-width of the eIOS (figure 3d) was 0.36 ± 0.06 mm (mean ± s.d.) (table 1), which is more than the average diameters of vibrissa columns in mice [17]. The eIOS thus extends into septa and neighbouring columns. Figure 4d shows the contours of where the eIOS for each vibrissa was greater than the half-maximal response. This measure is an approximation of the half-width, but also highlights the asymmetries and orientation of the eIOS, e.g. compare the eIOS orientation of straddlers versus row E vibrissae.
We now come to the construction of a canonical somatotopic map of the vibrissa representation in the vS1 cortex. The location of eIOS centroids, averaged by vibrissa identity, was spatially registered from relative (figure 4b) to absolute (figure 4a) coordinates, in the least-squares sense, by affine transformation. The same transformation was then applied to every individual vibrissa eIOS map obtained, and the transformed eIOS maps were averaged across mice by vibrissa identity, normalized, and used to compute the maximum projection across all vibrissae. In total, the canonical somatotopic vibrissa map (figure 4d) encompassed 27 macro-vibrissae across all rows and arcs, including all straddlers.
4. Discussion
We obtained a total of 635 in vivo maps, across 28 different mice, of the somatotopic representation of macro-vibrissa in the vS1 cortex using an improved method for imaging the eIOS [17]. We compared eIOS responses in awake and isoflurane anaesthetized mice (figure 1). Consistent with previous studies showing that isoflurane has vasodilatory properties [43,53], we observed strong dilation of pial arterioles when isoflurane was administered at 1.8% (v/v) in oxygen (figure 1b). Although we observed that isoflurane anaesthesia suppressed the amplitude of the eIOS (figure 1a,c), isoflurane improved the signal-to-noise of the eIOS deoxygenation signal by suppressing vasodynamic responses (figures 1a,c and 2). Beyond a dependence on the concentration of isoflurane in oxygen, high quality imaging depended on the maintenance of normal body temperature and the continuous subcutaneous injection of glucose.
We observed that in the absence of a large vasodynamic ‘overshoot’, the deoxyhaemoglobin signal persisted at the same location in cortex throughout the 4 s time window during vibrissa stimulation (figure 2). This result suggests that changes in blood flow and volume, such as that associated with sensory-induced dilation of cortical blood vessels [36], normally mask haemodynamic signals in the eIOS attributed to increased neural activity. The persistence of a deoxyhaemoglobin signal enabled us to average acquisition frames over the entire stimulation time window, thereby improving the spatial specificity of the eIOS method. Previous studies within BOLD fMRI have reported similar improved spatial specificity with the vasodilator sodium nitroprusside [61]. Lastly, it is possible that the maps may be further refined though optimization of the stimulus interval and duty cycle [39,41].
While the absolute location and orientation of the vibrissa field varied between animals (figure 4a), we found that the relative arrangement of the field was preserved across mice (figure 4b), consistent with previous studies in mice [62] and rats [63]. Spatial spreads of the centroids of cortical columns for the same vibrissa averaged 0.036 mm (s.d.), or approximately less than one-fifth of the average columnar radius [62] and less than a quarter of the average inter-column distance. Thus, the location of any vibrissa column can be established with near certainty in coordinates relative to all other columns. Assuming a flat vertical projection, as used in this study, eIOS imaging of two or few vibrissae in a novel mouse may be used to find the orientation and scale between the new vibrissa map and the canonical map (figure 4c). Alternative methods may also be used to locate individual vibrissa representations in vS1, such as extracellular recording. The canonical map is then either formed manually, using figure 4b as a template, or by computationally finding an affine transformation that minimizes the offsets between the measured columnar locations and the average locations provided in table 1. Thus our canonical map should increase the speed and precision of mapping procedures, in support of physiological investigations of the vibrissa system [2,64].
It will be of interest to determine whether the mean position and variability of the centroids and boundaries of other sensory maps in the cortex have as reproducible a map as that for the vibrissae. This includes the retinotopic organization of the multitude of visual areas [65,66] and the tonotopic versus periodotopic (harmonic) organization of the primary auditory cortex [67–69].
Acknowledgements
We thank Pablo Blinder, Patrick Drew and Michael Pesavento for contributions to code used for eIOS imaging and analysis.
Ethics
All protocols were approved by the Institutional Animal Care and Use Committee at UCSD.
Authors' contributions
All authors planned the experiments. The data were obtained by P.M.K. and C.M., analysed by P.M.K., and the manuscript was written by P.M.K. and D.K. All reagents were contributed by D.K., who further attended to the myriad of university rules and forms that govern environmental health and safety, including the ethical use of animals as well as the use chemicals, controlled substances and hazardous substances.
Competing interests
We declare we have no competing interests.
Funding
Our work was supported by the United States National Institutes of Mental Health (grant no. MH108503), United States National Institutes of Biomedical Imaging and Bioengineering (grant no. EB003832), United States National Institutes of Neurological Disease and Stroke (grants nos. NS058668 and NS057198), and the United States National Science Foundation (grant no. PHY153264).
References
- 1.Woolsey TA, Van Der Loos H. 1970. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 17, 205–242. ( 10.1016/0006-8993(70)90079-X) [DOI] [PubMed] [Google Scholar]
- 2.Petersen CC. 2007. The functional organization of the barrel cortex. Neuron 56, 339–355. ( 10.1016/j.neuron.2007.09.017) [DOI] [PubMed] [Google Scholar]
- 3.Simons DJ, Land PW. 1987. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 326, 694–697. ( 10.1038/326694a0) [DOI] [PubMed] [Google Scholar]
- 4.Fox K. 1992. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 12, 1826–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polley DB, Kvasnak E, Frostig RD. 2004. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature 429, 67–71. ( 10.1038/nature02469) [DOI] [PubMed] [Google Scholar]
- 6.Lendvai B, Stern EA, Chen B, Svoboda K. 2000. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881. ( 10.1038/35009107) [DOI] [PubMed] [Google Scholar]
- 7.Erzurumlu RS, Gaspar P. 2012. Development and critical period plasticity of the barrel cortex. Eur. J. Neurosci. 35, 1540–1553. ( 10.1111/j.1460-9568.2012.08075.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons DJ. 1985. Temporal and spatial integration in the rat SI vibrissa cortex. J. Neurophysiol. 54, 615–635. [DOI] [PubMed] [Google Scholar]
- 9.Mirabella G, Battiston S, Diamond ME. 2001. Integration of multiple-whisker inputs in rat somatosensory cortex. Cereb. Cortex 11, 164–170. ( 10.1093/cercor/11.2.164) [DOI] [PubMed] [Google Scholar]
- 10.Armstrong-James M, Fox K. 1987. Spatiotemporal convergence and divergence in the rat S1 ‘barrel’ cortex. J. Comp. Neurol. 263, 265–281. ( 10.1002/cne.902630209) [DOI] [PubMed] [Google Scholar]
- 11.Armstrong-James M, Fox K, Das-Gupta A. 1992. Flow of excitability within barrel cortex on striking a single vibrissa. J. Neurophysiol. 68, 1345–1358. [DOI] [PubMed] [Google Scholar]
- 12.Frostig RD, Xiong Y, Chen-Bee CH, Kvasnák E, Stehberg J. 2008. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J. Neurosci. 28, 13 274–13 284. ( 10.1523/JNEUROSCI.4074-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinfeld D, Delaney KR. 1996. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage sensitive dyes. J. Comp. Neurol. 375, 89–108. ( 10.1002/(SICI)1096-9861(19961104)375:1%3C89::AID-CNE6%3E3.0.CO;2-K) [DOI] [PubMed] [Google Scholar]
- 14.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CCH. 2010. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65, 422–435. ( 10.1016/j.neuron.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 15.Harris JA, Diamond ME. 2000. Ipsilateral and contralateral transfer of tactile learning. Neuroreport 11, 263–266. ( 10.1097/00001756-200002070-00008) [DOI] [PubMed] [Google Scholar]
- 16.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. 2013. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat. Neurosci. 16, 55–63. ( 10.1038/nn.3278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. 2013. The murine cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897. ( 10.1038/nn.3426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolsey TA, Rovainen CM, Cox SB, Henger MH, Liange GE, Liu D, Moskalenko YE, Sui J, Wei L. 1996. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb. Cortex 6, 647–660. ( 10.1093/cercor/6.5.647) [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter M, de Kock CP, Feldmeyer D, Bruno RM, Sakmann B. 2007. Reconstruction of an average cortical column in silico. Brain Res. Rev. 55, 193–203. ( 10.1016/j.brainresrev.2007.07.011) [DOI] [PubMed] [Google Scholar]
- 20.Narayanan RT, Egger R, Johnson AS, Mansvelder HD, Sakmann B, de Kock CP, Oberlaender M. 2015. Beyond columnar organization: cell type- and target layer-specific principles of horizontal axon projection patterns in rat vibrissal cortex. Cereb. Cortex 25, 4450–4468. ( 10.1093/cercor/bhv053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markram H, et al. 2015. Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492. ( 10.1016/j.cell.2015.09.029) [DOI] [PubMed] [Google Scholar]
- 22.Strominger RN, Woolsey TA. 1987. Templates for locating the whisker area in fresh flattened mouse and rat cortex. J. Neurosci. Methods 22, 113–118. ( 10.1016/0165-0270(87)90004-5) [DOI] [PubMed] [Google Scholar]
- 23.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. 1990. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc. Natl Acad. Sci. USA 87, 6082–6086. ( 10.1073/pnas.87.16.6082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grinvald A, Lieke EE, Frostig RD, Gilbert CD, Wiesel TN. 1986. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364. ( 10.1038/324361a0) [DOI] [PubMed] [Google Scholar]
- 25.Narayan SM, Santori EM, Toga AW. 1994. Mapping functional activity in rodent cortex using optical intrinsic signals. Cereb. Cortex 4, 195–204. ( 10.1093/cercor/4.2.195) [DOI] [PubMed] [Google Scholar]
- 26.Holtmaat A, et al. 2009. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144. ( 10.1038/nprot.2009.89) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih AY, Mateo C, Drew PJ, Tsai PS, Kleinfeld D. 2012. A polished and reinforced thinned skull window for long-term imaging and optical manipulation of the mouse cortex. J. Vis. Exp. 67, e3742 See http://www.jove.com/video/3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Davalos D, Blinder P, Akassoglou K, Tsai PS, Kleinfeld D. 2010. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984. ( 10.1038/nmeth.1530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa S, Lee T-M, Nayak AS, Glynn P. 1990. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high fields. Magn. Reson. Med. 14, 68–78. ( 10.1002/mrm.1910140108) [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Lee TM. 1990. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn. Reson. Med. 16, 9–18. ( 10.1002/mrm.1910160103) [DOI] [PubMed] [Google Scholar]
- 31.Pauling L, Coryell CD. 1936. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl Acad. Sci. USA 22, 210–216. ( 10.1073/pnas.22.4.210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. 2003. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39, 353–359. ( 10.1016/S0896-6273(03)00403-3) [DOI] [PubMed] [Google Scholar]
- 33.Hillman EMC. 2007. Optical brain imaging in vivo: techniques and applications from animal to man. J. Biomed. Opt. 12, 051402 ( 10.1117/1.2789693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boas DA, Dale AM, Franceschini MA. 2004. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 23, S275–S288. ( 10.1016/j.neuroimage.2004.07.011) [DOI] [PubMed] [Google Scholar]
- 35.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. 2007. The triphasic intrinsic signal: implications for functional imaging. J. Neurosci. 27, 4572–4586. ( 10.1523/JNEUROSCI.0326-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drew PJ, Shih AY, Kleinfeld D. 2011. Fluctuating and sensory-induced vasodynamics in rodent cortex extends arteriole capacity. Proc. Natl Acad. Sci. USA 108, 8473–8478. ( 10.1073/pnas.1100428108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devor A, et al. 2007. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signaling. J. Neurosci. 27, 4452–4459. ( 10.1523/JNEUROSCI.0134-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zong X, Kim T, Kim S-G. 2012. Contributions of dynamic venous blood volume versus oxygenation level changes to BOLD fMRI. Neuroimage 60, 2238–2246. ( 10.1016/j.neuroimage.2012.02.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JE. 2008. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J. Neurophysiol. 99, 787–798. ( 10.1152/jn.00658.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frostig RD, Dory Y, Kwon MC, Masino SA. 1993. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc. Natl Acad. Sci. USA 90, 9998–10002. ( 10.1073/pnas.90.21.9998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheth BR, Moore I, Sur M. 1998. Temporal modulation of spatial borders in rat barrel cortex. J. Neurophysiol. 79, 464–470. [DOI] [PubMed] [Google Scholar]
- 42.Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, Frostig RD. 2000. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J. Neurosci. 20, 5841–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez AL, Fukuda M, Kim SG. 2012. Evolution of the dynamic changes in functional cerebral oxidative metabolism from tissue mitochondria to blood oxygen. J. Cereb. Blood Flow Metab. 32, 745–758. ( 10.1038/jcbfm.2011.198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. 2000. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J. Neurosci. 20, 8111–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai M, et al. 2011. Mapping brain networks in awake mice using combined optical neural control and fMRI. J. Neurophysiol. 105, 1393–1405. ( 10.1152/jn.00828.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berwick J, Martin C, Martindale J, Jones M, Johnston D, Zheng Y, Redgrave P, Mayhew J. 2002. Hemodynamic response in the unanesthetized rat: intrinsic optical imaging and spectroscopy of the barrel cortex. J. Cereb. Blood Flow Metab. 33, 670–679. ( 10.1097/00004647-200206000-00005) [DOI] [PubMed] [Google Scholar]
- 47.Chen LM. 2005. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J. Neurosci. 25, 7648–7659. ( 10.1523/JNEUROSCI.1990-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcar VL, Schwarz U, Martin E, Loenneker T. 2006. How depth of anesthesia influences the blood oxygenation level-dependent signal from the visual cortex of children. Am. J. Neuroradiol. 27, 799–805. [PMC free article] [PubMed] [Google Scholar]
- 49.Martin C, Martindale J, Berwick J, Mayhew J. 2006. Investigating neural–hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32, 33–48. ( 10.1016/j.neuroimage.2006.02.021) [DOI] [PubMed] [Google Scholar]
- 50.Goense JBM, Logothetis NK. 2008. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 18, 631–640. ( 10.1016/j.cub.2008.03.054) [DOI] [PubMed] [Google Scholar]
- 51.Pisauro MA, Dhruv NT, Carandini M, Benucci A. 2013. Fast hemodynamic responses in the visual cortex of the awake mouse. J. Neurosci. 33, 18 343–18 351. ( 10.1523/JNEUROSCI.2130-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu M, Ramani R, Swetye M, Rajeevan N, Constable RT. 2008. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn. Reson. Med. 60, 987–996. ( 10.1002/mrm.21759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iida H, Ohata H, Iida M, Watanabe Y, Dohi SI. 1998. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology 89, 954–960. ( 10.1097/00000542-199810000-00020) [DOI] [PubMed] [Google Scholar]
- 54.Kim T, Kim SG. 2011. Temporal dynamics and spatial specificity of aterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J. Cereb. Blood Flow Metab. 31, 1211–1222. ( 10.1038/jcbfm.2010.226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda M, Wang P, Moon C-H, Tanifuji M, Kim S-G. 2006. Spatial specificity of the enhanced dip inherently induced by prolonged oxygen consumption in cat visual cortex: implication for columnar resolution functional MRI. Neuroimage 30, 70–87. ( 10.1016/j.neuroimage.2005.09.026) [DOI] [PubMed] [Google Scholar]
- 56.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. 2007. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218. ( 10.1016/j.neuron.2007.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babbitt JT, Kharazi AI, Taylor JMG, Bonds CB, Zhuang D, Mirell SG, Frumkin E, Hahn T. 2001. Increased body weight in C57BL/6 female mice after exposure to ionizing radiation or 60 Hz magnetic fields. Int. J. Radiat. Biol. 77, 875–882. ( 10.1080/09553000110055790) [DOI] [PubMed] [Google Scholar]
- 58.Shih AY, Driscoll JD, Drew PJ, Nishimura N, Schaffer CB, Kleinfeld D. 2012. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cereb. Blood Flow Metab. 32, 1277–1309. ( 10.1038/jcbfm.2011.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohn A, Metz C, Quibrera M, Tommerdahl MA, Whitsel BL. 2000. Functional neocortical microcircuitry demonstrated with intrinsic signal optical imaging in vitro. Neuroscience 95, 51–62. ( 10.1016/S0306-4522(99)00385-1) [DOI] [PubMed] [Google Scholar]
- 60.Akrawi WP, Drummond JC, Kalkman CJMPP. 1996. A comparison of the electrophysiologic characteristics of EEG burst-suppression as produced by isoflurane, thiopental, etomidate, and propofol. J. Neurosurg. Anesthesiol. 8, 40–46. ( 10.1097/00008506-199601000-00010) [DOI] [PubMed] [Google Scholar]
- 61.Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, Kim SG. 2005. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J. Cereb. Blood Flow Metab. 26, 1043–1051. ( 10.1038/sj.jcbfm.96000251) [DOI] [PubMed] [Google Scholar]
- 62.Welker E, Van der Loos H. 1986. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J. Neurosci. 6, 3355–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Egger R, Narayanan RT, Helmstaedter M, de Kock CPJ. 2012. 3D reconstruction and standardization of the rat vibrissal cortex for precise registration of single neuron morphology. PLoS Comput. Biol. 8, e1002837 ( 10.1371/journal.pcbi.1002837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleinfeld D, Deschênes M. 2011. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72, 455–468. ( 10.1016/j.neuron.2011.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47. ( 10.1093/cercor/1.1.1) [DOI] [PubMed] [Google Scholar]
- 66.Wang Q, Burkhalter A. 2007. Area map of mouse visual cortex. J. Comp. Neurol. 502, 339–357. ( 10.1002/cne.21286) [DOI] [PubMed] [Google Scholar]
- 67.Reale RA, Imig TJ. 1980. Tonotopic organization in auditory cortex of the cat. J. Comp. Neurol. 192, 265–291. ( 10.1002/cne.901920207) [DOI] [PubMed] [Google Scholar]
- 68.Langner GM, Sams M, Heil P, Schulze H. 1997. Frequency and periodicity are represented in orthogonal maps in the human auditory cortex: evidence from magnetoencephalography. J. Comp. Physiol. A 181, 665–676. ( 10.1007/s003590050148) [DOI] [PubMed] [Google Scholar]
- 69.Stiebler I, Neulist R, Fichtel I, Ehret G. 1997. The auditory cortex of the house mouse: left–right differences, tonotopic organization and quantitative analysis of frequency representation. J. Comp. Physiol. A 181, 559–571. ( 10.1007/s003590050140) [DOI] [PubMed] [Google Scholar]