Abstract
According to the National Institutes of Health and the Center for Disease and Prevention, it is estimated that 65 to 80 percent of the human infectious are caused by biofilms. Antibiotics and drainage of purulent discharge are the main treatment measures. But in chronic infections sometimes these findings are not easy to recognize. Biofilm is an extracellular polymeric conglomeration generally composed of extracellular DNA, proteins, and polysaccharides, which help bacteria to survive into the host. The following case is the example of chronic wound in burn patient, where biofilm gets involved and special attention is given to the relationship biofilm–chronic wound injury in a burn patient.
Key Words: Chronic infection, Burn, Biofilm
INTRODUCTION
Burn is still the most devastating condition in emergency settings in developed and developing countries, leading to physical and psychological injuries.1 Various factors influence the morbidity and mortality. Inhalation injury and development of other significant secondary complications also affect the outcome.2 For survivors, the most important problem is scarring, therefore, wound healing and the outcome has always been under investigation. In burn wound healing, inflammation, granulation, and remodeling of the tissue are the most prominent processes.3 Factors such as oxygen free radicals, oxidative stress contribute to delay the wound repair process.4 Advances in wound healing and improvements in the technology have driven the control of infections and antibiotic resistances.5 Silver sulfadiazine was reported as the gold standard in topical burn therapy with antibacterial properties.6 Several reports are available on the resistance of bacteria to silver sulfadiazine.5 So there is a need for new agents for treatment of burn wounds in health care practice with less adverse problems and better efficacy.
In Mexico, as in many undeveloped countries, burns injuries remain a serious and devastating problem. Annually in the “Nuevo Hospital Civil de Guadalajara Juan I. Menchaca” our team receives many children with these types of injuries. Attention and care is coordinated with other centers inside and outside the country. In this setting a patient with chronic wound that is not uncommon, biofilm gets involved and special attention is given to the relationship between biofilm and chronic wound injury in a burn patient.
CASE REPORT
The patient is a 15 years old male with past history of substance abuse. Six months ago, he presented to the emergency room with burn injury caused by fighter while he was intoxicated with non-specified illegal drug. This injury caused second and third grade burns in arms, thorax and abdomen. The estimated corporal burn surface was estimated as 40%. Initial treatment and hydration with fluid using modified Galveston scheme was administrated in the emergency room. After two month of care in the burn unit, several surgical scrubbing, skin grafts and other procedures the patient was discharged. Due economic and social problems, the patient had an irregular follow up in the clinic and he missed appointments. The patient could not take a shower and proper care was not possible at home.
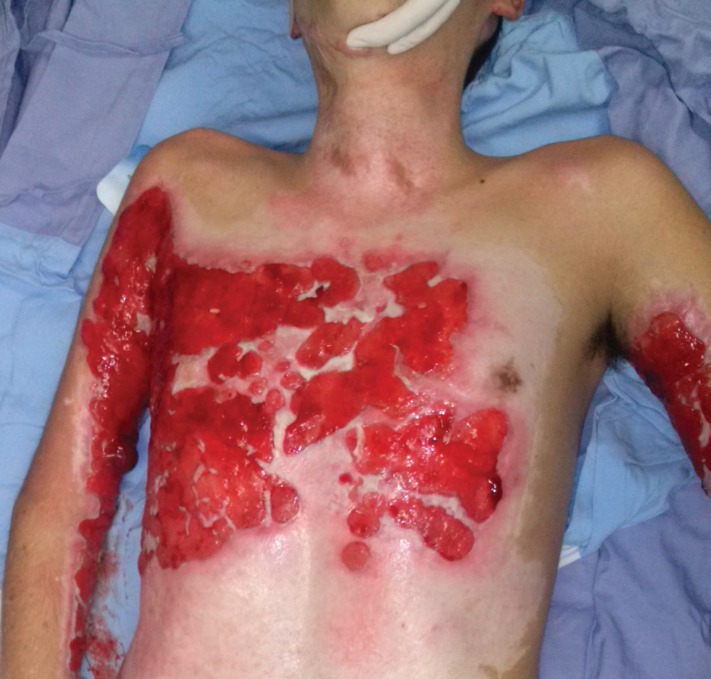
At the sixth month the patient came back to the clinic. We found in arms, thorax and abdomen, discolored granulation tissue and impressive bad odor. After discussing the case with his family, we proposed to readmit the patient and continue wound care. Figure 1 shows the condition at the sixth month at the time of the readmission. This is best example of chronic wound in a burn patient, where infection and inflammation are the hallmark. In this scenario biofilm, is getting more attention.
Fig. 1.
Chronic wound healing in a patient
DISCUSSION
According to the National Institutes of Health and the Center for Disease and Prevention, it is estimated that 65 to 80 percent of the human infectious are caused by biofilms.7-9 Traditionally, infections are divided in two types of acute and chronic. Taking in count Koch principles, acute infections are recognized for the following features: isolation of the causative microbe from an afflicted animal, propagating of the organism in pure culture, reestablishing the original disorder by reintroducing the putative pathogen and re-isolation of the pathogen from the newly affected host. In the clinic some features are easy to recognize, as for example cellulitis, rubor, swelling, fever and other systemic findings. Antibiotics and drainage of purulent discharge are the main treatment.10
Some bacteria have the capacity to resist and attach to surface in the body; this is the scenario where biofilm gets involved in a chronic infection. At this level, bacteria population can adapt to the host and survive.11-13 Pseudomonas aeruginosa is the best example to show how through different mechanism bacteria can cause acute infection and later attach and persist in the organism.14 At the begging P. aeruginosa expresses multiple virulence factors in order to adapt and survive in an adverse environment, as for example pyocyanin (small cidal molecule), pyoverdines (family of protein siderophores), exotoxin A, phospolipases (A to C), rhamnolipids, proteases (elastase, alkaline protease, Las A proteinase, protease IV) and T3SS effector proteins including ExoU (phospholipase 2) all of which can easily breech host epithelial or mucosal barriers.7
Once P. aeruginosa broke the mucosa or epithelium, it has the capacity to initiate a process to live for a longer period into what we called biofilm. P. aeruginosa has been studied in cystic fibrosis that causes significant morbidity and mortality in children, where biofilm keeps an important role as matrix or glue, keeping bacteria together.8 This fact is potentiated by the capacity of P. aeruginosa to create resistance against antibiotics. Three polysaccharides: Psl, Pel and alginate; are important to maintain biofilm and help to resist medical treatment.15
Psl has an important scaffold and signaling role. It stimulates two diguanylate cyclase: SiaD and SadC, to produce more of the intracellular second messenger molecule c-di-GMP and increase the production of Psl by itself to form and unique positive feedback. Pel has glucose rich matrix material and cellulose-sensitive extracellular matrix. Pel is important in making solid surface-associated with biofilms. Alginates has the capacity to protect the bacteria from the neutrophils and macrophages by scavenge free radicals.15
Not only the polysaccahrides help P. aeruginosa, eDNA to have an important role in biofilm process because it helps to keep cation gradients, genomic DNA and antibiotic resistances. Also eDNA facilitates the twitching motility mediated biofilm expansion maintaining coherent cell alignments and coordinating cells movement. P. aeruginosa has another important part in biofilm process regulated by the extracellular proteins and several proteinaceous components, which includes type IV pili, flagella and fimbriae. They work as adhesion factors and structural support in biofilm structure.15-17
All these process cannot be done without help of gene regulation.18,19 Biofilm has multicellular process involving environmental signals and a concerted regulation combining both environment signals and regulatory networks. P. aeruginosa has a Bis-(3´-5´)-cyclic dimeric guanosine monosphosphate (c-di-GMP), that is intracellular second messenger widely distributed in bacteria.15 c-di-GMP stimulates biosynthesis of adhesins and exopolysaccharide mediated biofilm formation and inhibits bacterial motilites, important in the motile planktonic and sessile biofilm associated lifestyle of bacteria. In the case of pel and psl genes of exopolysaccharide production are regulate by the expression of regulatory RNAs that is under the control of GacA/GacS two component system.15
It looks like bacteria can live in a community environment instead of unicellular and self-dependent organisms. They can organize into groups, form well organized communities and communicate for coordinated activities or social life that was once believed to be restricted to multicellular organisms.17,19 These findings are different from the initial though that bacteria behaved as self-sufficient individuals and maintained a strictly unicellular life-style. We can tell that biofilm is the home where bacteria can live in a long peace, harmony and well organized lifestyle.
The capacity to communicate between bacteria it is not restricted to the same species, for example, dental plaque is well recognized biofilm community characterized by its biodiversity and high cell density, where different species of bacteria can live in harmony.16 Biofilm is an extracellular polymeric conglomeration generally composed of extracellular DNA, proteins, and polysaccharides. Biofilm has basic and important features. First it has the capacity to attach to an inanimate surface.8 On the other hand, in the case of an organism, it can attach to a wound bed, suture or implanted medical device.
Another feature is that bacteria secrete substances to protect their home “biofilm”, from environment dangers such as bacteriophage, ultraviolet light and desiccation in the natural world.7,8 Biofilm has the strength to mix its components to suit its needs or changed its composition to confront different treatments or threats.17 This complex interaction in a complex and well organized mechanism is known as quorum sensum. It regulates growth, interaction between bacterias and even death.17
This process of intercellular communication, called quorum sensing was first described in the marine bioluminescent bacterium vibrio fisheri, which lives in symbiotic associations with a number of marine animal hosts. In these partnerships, the host uses light produced by V. fisheri for specific purposes such as attracting prey, avoiding predators or finding a mate. In exchange for the light it provides, V. fischeri obtains a nutrient-rich environment where it resides.19
Quorum sensing relies upon the interaction of a small diffusible signal molecule with a sensor or transcriptional activator to initiate gene expression for coordinated activities. Quorum sensing systems in bacteria have been generally divided into a three classes: Lux/LuxR-type quorum sensing in gram negative bacteria, which use acyl-homoserine lactones (AHL) as signal molecules, oligopeptide-two-component-type quorum sensing in gram positive bacteria, which use small peptides as signal molecules, and luxS-encoded auntoinducer 2 (AI-2) quorum sensing in both Gram-negative and Gram-positive bacteria.18,19
In the case of P. aeruginosa, it seems that there is a well-organized network that makes simple the communication, where several virulence factors interact such as exoproteases, siderophores, exotoxins and rhamnolipids. P. aeruginosa has three quorum sensing signaling system (LasR/LasI, Rh1R/Rh1I and PQS), that allow the control of cellular process in the production of extracellular virulence factors and biofilm formation.15
The quorum sensing controlled virulence expression in this case has been demonstrated in vitro and in vivo models. In the case of Staphylococcus aureus and various Streptococci, it used signal peptide-mediated systems for quorum sensing. Many infections caused by S. aureus such as a endocarditis, osteomielitis and foreign-body related infections are not caused by free-living cells but rather by biofilms, where many virulence factors are regulated by quorum sensing via the accessory gene regulator (agr) system.21 This system influences in the attachment of cells to surfaces and dispersion of the biofilm (bacteria). These findings are strong related with the chronic nature of biofilm-associated infections.
Another important finding is the capacity of bacteria to work as a community where through social cooperation, a portion of the population survives to starvation by forming the fruiting bodies, but most cells in the population, which provide cooperation, are sacrificed.7,8 Biofilm development process involved different stages, where an initial attachment is necessary. After time the biofilm get strength and the maturation process gets involved. At the end biofilm has the capacity to move to another place what is called dispersion.13
Unfortunately biofilm cannot be identified in traditional cultures and light and electron microscopy are necessary to make diagnoses.7,8 The polymicrobial nature of biofilms is identified in most of the studies. Species as Staphylococcus aureus, E. faecalis and P. aeuroginosa are the favorite of biofilm in the setting of chronic wounds. Propionibacterium acne is identified in the biofilm in implant associated infections as for example periprosthetic joint infection, cardiac devices or breast implant.20-23
In normal adult skin, estimated bacterial levels are up to 2 million bacteria per square centimeter.8 The variety of microorganism isolated from skin surfaces is highly dependent upon the culture techniques used and host characteristics (such as age, gender, ethnicity and anatomic location). Commonly isolated bacterial residents include Staphylococcus, Corynebacterium, Propionobacterium, Micrococcus, Brevibacterium and Acinetobacter species.20,22 Besides the bacteria species, fungi and yeast are also part of the skin microbiota. Not only infections or chronic infections are related with skinmicrobiota. Other inflammatory diseases as psoriasis, atopic dermatitis and acne are linked to alterations in the cutaneous microbial ecosystem. Unfortunately the precise mechanism remains unclear.22
The presence of bacteria can modified immunity. Several studies have identified modulation in case of chronic infection of the host in CD4 and CD8 T-cell responses. This finding can explain the adaptive immune responses can be regulated by alterations in the local microbe population.23 Not only biofilm has a role in chronic inflammatory conditions as for example capsular contracture in breast implant. CD4 cells are predominant lymphocytes population inside the capsule. CD4 causes a specific profibrotic cytokine profile which mediates the local immune response by means of activated TH1/TH17-cells. As the intracapsular T-cell ratio has been inversely proportional to the clinical stage of fibrosis, it has been hypothesized that profibrotic cytokines and growth factors stimulated capsular fibrosis in the retention of local regulatory T-cells.21-24
Most of these fascinating findings are still under research. Maybe more doors will be open in near future to treat the relationship biofilm-chronic wounds. Some of these options are: inhibition of signal generation, interference with signal dissemination, blocking signal receptors and inhibition of signaling response system.8,25,26 In this way quorum sensing inhibitors have been found to increase the susceptibility of antibiotics within biofilm in vitro and in vivo thereby increasing the success of treatment.8,9
It is important to check other factors that contribute to the problem in chronic wound, as for example the etiology of the wound, adequate blood supply, host factors (as coexisting diseases, drugs), past treatment and compliance of the patient with instructions.10 Morbidities as Diabetes Mellitus, atherosclerosis, vasculitis or other autoimmune diseases should be consider as possible cause of failure in treatment. Right control and treatment of these conditions make difference in wound healing. Medications as steroids delay healing and predispose to infections.10
In the clinical case the patient presented inability to have a good care due economic and social problems makes high risk of chronic infection with the consequent presence of biofilm. After been admitted, nutritional support was started and the general conditions of the patient were improved. Surgical scrubbing and removal of the granulation tissue helped to decrease the bacterial load and the presence of biofilm into the wound.
By removing the granulation tissue it was possible to decrease the amount of biofilm, improve the condition, and make another procedure to close the wound as skin graft. At this moment the patient continue follow up in clinic, where, with cooperation and help of different services as psychology, social work and physiotherapy, the patient is getting improvement of the consequences of burn contracture caused by the chronic wound.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Please cite this paper as:
Ramos-Gallardo G. Chronic Wounds in Burn Injury: A Case Report on Importance of Biofilms. World J Plast Surg 2016;5(2):175-180.
References
- 1.Mohammadi AA, Amini M, Mehrabani D, Kiani Z, Seddigh A. A survey on 30 months electrical burns in Shiraz University of Medical Sciences Burn Hospital. Burns. 2008;34:111–13. doi: 10.1016/j.burns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Tanideh N, Haddadi MH, Rokni-Hosseini MH, Hossienzadeh M, Mehrabani D, Sayehmiri K, Koohi-Hossienabadi O. The healing effect of scrophularia striata on experimental burn wounds infected to pseudomonas aeruginosa in rat. World J Plast Surg. 2015;4:16–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. 2007;5:23–26. doi: 10.1016/j.ijsu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, Zaghi AH, Khalili N. Active immunization using exotoxin a confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC J Microbiol. 2009;9:19–23. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanideh N, Rokhsari P, Mehrabani D, Mohammadi Samani S, Sabet Sarvestani F, Ashraf MJ, Koohi Hosseinabadi O, Shamsian Sh, Ahmadi N. The healing effect of licorice on Pseudomonas aeruginosa infected burn wounds in experimental rat model. World J Plast Surg. 2014;3:99–106. [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrabani D, Farjam M, Geramizadeh B, Tanideh N, Amini M, Panjehshahin MR. The Healing Effect of Curcumin on Burn Wounds in Rat. World J Plast Surg. 2015;4:29–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Wolcott R, Dowd S. The role of biofilm: are we hitting the right target? Plast Recontr Surg. 2010;127:28s–35s. doi: 10.1097/PRS.0b013e3181fca244. [DOI] [PubMed] [Google Scholar]
- 8.Wong VW, Martindale RG, Longaker MT, Gurtner GC. From germ theory to germ therapy: skin microbiota, chronic wounds and probiotics. Plast Reconstr Surg. 2013;132:854e–61e. doi: 10.1097/PRS.0b013e3182a3c11e. [DOI] [PubMed] [Google Scholar]
- 9.Thomson CH. Biofilms: do they affect wound healing? Int Wound. 2011;8:63–7. doi: 10.1111/j.1742-481X.2010.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibbad RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage. 2003;49:24–51. [PubMed] [Google Scholar]
- 11.James GA, Swogger E, Wollcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilm in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 12.Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 13.Monroe D. Looking for Chinks in the arbor of bacterial biofilm. PLoS Biol. 2007;5:e307. doi: 10.1371/journal.pbio.0050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amini M, Kherad M, Mehrabani D, Azarpira N, Panjehshahin MR, Tanideh N. Effect of Plantago major on burn wound healing in rat. J Appl Anim Res. 2010;37:53–56. [Google Scholar]
- 15.Wei Q, Ma LZ. Biofilm Matrix and Its Regulation in Pseudomonas aeruginosa. Int J Mol Sci. 2013;14:20983–21005. doi: 10.3390/ijms141020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, Lamont RJ, Jenkinson HF. Microbial interactions in building of communities. Mol Oral Microbiol. 2013;28:83–101. doi: 10.1111/omi.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo H, Otto M. Molecular basis of in-vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–13. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YH, Tian X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors. 2012;12:2519–38. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–99. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 20.Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013:804391. doi: 10.1155/2013/804391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–9. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 22.Del Pozo JL, Tran NV, Petty PM, Johnson CH, Walsh MF, Bite U, Clay RP, Mandrekar JN, Piper KE, Steckelberg JM, Patel R. Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol. 2009;47:1333–7. doi: 10.1128/JCM.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiert AE, Boyce M, Sorg H. Capsular contracture by silicone breast implant: possible causes, biocompatibility and prophylactic strategies. Med Devices (Auckl) 2013;6:211–8. doi: 10.2147/MDER.S49522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieger UM J, Mesina J, Kalbermatten DF, Haug M, Frey HP, Pico R, Frei R, Pierer G, Lüscher NJ, Trampuz A. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100:768–74. doi: 10.1002/bjs.9084. [DOI] [PubMed] [Google Scholar]
- 25.Ammons MC, Copie V. Lactoferrin: Mini-review: Lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling. 2013;29:443–55. doi: 10.1080/08927014.2013.773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurler J, Sorense KK, Fallarero A, Vuorela P, Skalko-Basnet N. Liposomes-in-hydrogel delivery system with mupirocin: in vitro antibiofilm studies and in vivo evaluation in mice burn model. Biomed Res Int. 2013;2013:498485. doi: 10.1155/2013/498485. [DOI] [PMC free article] [PubMed] [Google Scholar]