Abstract
Introduction
Staphylococcus aureus remains one of the leading causes of morbidity and mortality worldwide. This is to a large extent due to antibiotic-resistant strains, in particular methicillin-resistant S. aureus (MRSA). While the toll of invasive MRSA infections appears to decrease in U.S. hospitals, the rate of community-associated MRSA infections remains constant and there is a surge of MRSA in many other countries. This situation calls for continuing if not increased efforts to find novel strategies to combat MRSA infections.
Areas covered
This review will provide an overview of current investigational antibiotics in clinical development (up to phase II), and of therapeutic antibodies and alternative drugs against S. aureus in preclinical and clinical development, including a short description of the mechanism of action and a presentation of microbiological and clinical data.
Expert opinion
Increased recent antibiotic development efforts and results from pathogenesis research have led to several new antibiotics and alternative drugs, as well as a more informed selection of targets for vaccination efforts against MRSA. This developing portfolio of novel anti-staphylococcal drugs will hopefully provide us with additional and more efficient ways to combat MRSA infections in the near future and prevent us from running out of treatment options, even if new resistances arise.
Keywords: antibiotics, antibiotic resistance, alternative therapeutics, quorum-sensing, quorum-sensing blockers, antibody, Staphylococcus aureus
1. Introduction
Staphylococcus aureus is a dangerous human pathogen known to cause numerous human diseases. In the U.S. alone, S. aureus infections cause more than 11,000 deaths annually, along with an estimated annual cost of ~ $ 14 billion [1]. This serious situation is predominantly due to antibiotic-resistant strains.
Antibiotic resistance (ABR) is a major threat to human society. Infections with methicillin-resistant S. aureus (MRSA) in the hospital and the recent rise of highly infectious community-associated MRSA (CA-MRSA) strains have been the premier cause of the immense morbidity, mortality, and the economic burden due to S. aureus infections in the U.S. and elsewhere [2].
Since the recognition of decreases in antibiotic efficacy, researchers have attempted to understand the basis of antibiotic resistance and tried to invent ways to overcome it. Antimicrobial resistance is genetically based and most commonly mediated by the acquisition of extra-chromosomal genetic elements via horizontal gene transfer (HGT). For example, S. aureus resistance to penicillin spread already in the 1940s, shortly after introduction of penicillin into clinical use, and is due to acquisition of plasmid-encoded beta-lactamase. The beta-lactamase-resistant antibiotic methicillin was then developed and introduced to combat penicillin-resistant strains, but methicillin-resistant strains arose quickly and spread worldwide. Methicillin resistance is due primarily to the mecA gene, which is part of the mobile genetic element, staphylococcal cassette chromosome mec (SCCmec) [3]. The product of the mecA gene, penicillin-binding protein 2a (PBP2a), is essential for cell wall synthesis. PBP2a’s active site is unique in that methicillin and other beta-lactams are unable to inhibit its cell wall synthesis reactions [4].
The spread of penicillin and methicillin resistance brought on the development of some new antibiotic classes; and while MRSA still represents a serious global threat, physicians now have more prospects to treat MRSA infections. The current availability of a broad portfolio of antibiotics against MRSA (e.g. linezolid, daptomycin, ceftaroline, ceftobibrole, tigecycline, telavancin, oritavancin, tedizolid, dalbavancin) is a welcome development, which may have resulted from continuous investments and commitments by major big pharmaceutical companies over the last two decades. However, strains of S. aureus that are resistant to many of the newly marketed drugs have already been reported. This includes S. aureus strains that are resistant to vancomycin, which is still the most commonly used antibiotic for MRSA and multi-drug-resistant S. aureus [5]. With the imminent crisis of antibiotic resistance, significant efforts have been made to treat staphylococcal disease through the discovery of new antimicrobial targets. In addition to antibiotics, research has focused on the use of alternate therapeutics, such as bacteriophages, quorum-sensing inhibitors and antibodies.
This review will provide an overview on the portfolio of current investigational drugs and therapeutic antibodies against S. aureus. As for investigational antibiotics, we focus on those in clinical development up to phase II. For alternative drugs, such as anti-virulence drugs and antibodies, we also include those in pre-clinical stages, as in most cases these have not yet progressed to clinical evaluation. A short summary of the mechanism of action and a brief overview of the microbiological data and/or clinical data for each of the compounds will be presented.
2. Investigational antibiotics against MRSA
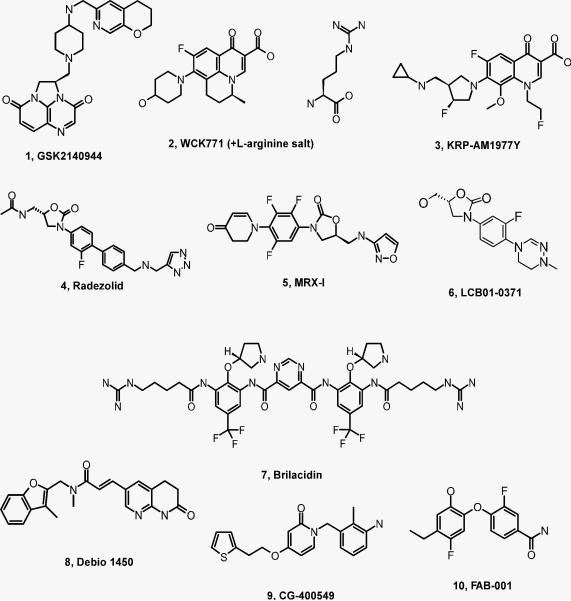
There are currently nine investigational antibiotics against S. aureus, including MRSA, in clinical phase I and II trials, one in phase I and eight in phase II, belonging to five different structural classes - quinolones, 2nd generation of oxazolidinone, peptidomimetic, 2-pyridone, and ene-amide class (Fig. 1).
Fig. 1. Structures of investigational drugs against MRSA.
1 – 3, quinolone class; 4 – 6, oxazolidone class; 7, peptidomimetic class; 8 – 10, fatty acid inhibitors.
2.1. Quinolones
Quinolones are potent antibacterial agents that have been known for over three decades. The clinically validated targets for quinolones - DNA gyrase and topoisomerase IV (type IIA) - are ubiquitous in bacteria and essential for bacterial survival. Importantly, the homologous human enzymes differ significantly in structure and drug affinities. The antibacterial mechanism of quinolones is based on the formation of a ternary complex with DNA and DNA replication enzymes. This antibiotic class is still very attractive for many research and development programs, because total chemical synthesis is feasible and the relatively small molecules are amenable to oral and parenteral administration. Furthermore, there is an extensive understanding of structure-activity-relationships (SAR) and relatively good pharmacokinetics, such as rapid distribution into the lung and soft tissues.
Three quinolones have entered the clinical development phase: GSK2140944 from GlaxoSmithKline plc (GSK) in phase II, WCK2349/ WCK 771 from Wockhardt Ltd. in phase I/II, and KRP-AM1977Y from Kyorin Pharmaceutical Co. Ltd. in phase II.
2.1.1. GSK2140944
GSK2140944, a piperidinyl-quinoline, is a novel non-fluoroquinolone bacterial type II topoisomerase inhibitor (NBTI) from GSK in clinical phase II with bactericidal activities against key respiratory pathogens, including MRSA. The antibacterial activity of GSK2140944 was assessed against a global collection of relevant clinical isolates. The minimum inhibitory concentration (MIC) to inhibit 90% of the tested S. aureus isolates, MIC90, was in the range of < 0.06 - 1 µg/ml [6, 7]. Target binding of GSK2140944 differs from that of fluoroquinolones by a distinct modus of binding to the type II topoisomerase, resulting in a lack of cross-resistance to fluoroquinolones [6]. Seven phase I studies were conducted to investigate the bioavailability of two formulations (oral and parenteral), safety, tolerability, pharmacokinetic profiles, and cardiac safety. A randomized, multi-center, dose-ranging study clinical phase II trial to evaluate efficacy against Gram-positive acute bacterial skin and skin structure infections (ABSSSI) is in the state of recruiting patients.
2.1.2. WCK2349/ WCK771
WCK2349 is the pro-drug form with oral availability of WCK771 from Wockhardt Ltd. WCK771 is an improved development of nadifloxacin, a topical fluoroquinolone antibiotic, with bactericidal activity against quinolone-susceptible methicillin-sensitive S. aureus (MSSA) and quinolone-resistant MRSA [8]. Both investigational drugs, WCK2349 and WCK771, have completed clinical phase II trials in India [9]. In the U.S., a phase I trial was reported to explore the food-effect and absolute bioavailability of WCK2349 and WCK771 in healthy volunteers. Three further phase I trials for WCK2349 are open for recruitment to assess plasma and intrapulmonary pharmacokinetics, supra-therapeutic dose and cardiac safety, and pharmacokinetic profiles in hepatic-impaired patients.
2.1.3. KRP-AM1977Y
KRP-AM1977Y from Kyorin Pharmaceutical Co. Ltd. is a further non-fluorinated quinolone NBTI with activity against MRSA. A randomized, multi-center, open-label, parallel-group, comparative clinical phase II study to investigate the clinical efficacy, safety, and pharmacokinetic profiles of KRP-AM1977Y in patients with acquired pneumonia was reported by Kyorin. Clinical trials outside Japan have not yet been initiated. Microbiological data for KRP-AM1977Y have not been disclosed.
2.2. Oxazolidinone class
Oxazolidinones are small synthetic molecules with good activities against multi-drug-resistant (MDR) Gram-positive isolates, including MRSA and vancomycin-resistant enterococci (VRE). The first member of this novel antibacterial class, linezolid, was approved in 2000 for the treatment of complicated and uncomplicated bacterial skin and skin structure infections, pneumonia, including hospital-acquired pneumonia (HAP), osteomyelitis, and invasive bloodstream infections. Linezolid is used off-label to treat serious MDR Gram-positive infections. The molecular target of oxazolidinones is the P site at the 50S ribosome subunit of bacteria; inhibition results in interruption of translation. Access to total chemical synthesis, broad activity against Gram-positive pathogens, good tissue distribution, and very low resistance rates may attract further research and development of this bacteriostatic antibacterial class. Of note, myelo-suppression and monoamino oxidase inhibition, potentially leading to toxic serotonin levels in patients taking specific antidepressants, is a common problem for almost all oxazolidinone antibiotics, including linezolid [10].
Three 2nd-generation oxazolidinone antibacterials have entered the clinical development phase. Radezolid from Melinta Therapeutics and MRX-1 from MicuRx Pharmaceuticals Inc. are in phase II, and LCP01-0371 from LegoChem Bioscience is in phase I.
2.2.1. Radezolid
Radezolid (RX-01_0667, RX-174, RibX Pharmaceuticals) from Melinta Therapeutics is a biaryl-oxazolidinone antibiotic with improved activity against Gram-positive isolates compared to linezolid [11]. Key advantages of radezolid over linezolid are an extended spectrum against community-acquired pneumonia (CAP) pathogens and improved potency against cfr-containing linezolid-resistant S. aureus strains [12]. Two multi-center, randomized clinical phase II trials were successfully completed for CAP and uncomplicated skin and skin structure infections. No phase III trial has been reported yet. Notably, Milenta Therapeutics reported the development of a topical formulation for Radezolid in phase I by a licensee.
2.2.2. MRX-I
MRX-I from MicuRx Pharmaceuticals Inc. is a further 2nd-generation oxazolidinone with a 2,3-dihydropyridin-4-one (DHPO) ring replacing the morpholine ring found in linezolid [13]. It is active against methicillin-resistant Staphylococcus epidermidis, other coagulase-negative staphylococci, and MRSA [14, 15]. Although structurally related to linezolid, MRX-I showed improved antibacterial activity and reduced potential for hemolytical toxicity, myelo-suppression, and monoamine oxidase inhibition in preclinical toxicology studies [16]. The safety profile, tolerability, and pharmacokinetic of MRX-I was investigated in clinical phase I trials in China and Australia with no obvious evidence of myelo-suppression. A phase II double-blind, multi-center, clinical trial with linezolid as active comparator, is under investigation in China enrolling patients with ABSSSI. A second multi-center, randomized clinical phase II trial to assess the safety and efficacy of MRX-I versus linezolid in adult subjects with ABSSI was reported in the U.S.
2.2.3. LCB01-0371
LCB01-0371 from LegoChem Bioscience is another 2nd-generation oxazolidinone containing a cyclic amidrazone rather than the morpholine ring of linezolid. The antibacterial activity of LCB01-0371 is good against Gram-positive isolates [17]. In a murine model of systemic infection, the in-vivo efficacy of LCB01-0371 was 2-4 fold greater than that of linezolid, although the in-vitro MIC90 data of the two compounds were in close range [17]. LCB01-0371 has been reported to exhibit high solubility in water and good absorption, distribution, metabolism, excretion, toxicity, and pharmakokinetic profiles [17]. Two clinical phase I trials have been completed to explore the safety, tolerability, and pharmacokinetics of LCB01-0371.
2.3. Peptidomimetics
Peptidomimetic compounds are designed to mimic naturally occurring peptides or proteins. These compounds still act on biological targets but with improved properties that are critical for drug design (such as, bioavailability and stability against proteolysis). Several peptidomimetics have been designed to introduce antibacterial activities by mimicking the amphiphilic features of antimicrobial host defense peptides, while precluding the unfavorable cytotoxic properties of positively charged defensins [18].
2.3.1. Brilacidin
Brilacidin (formely PMX-30063 – Polymedix) from Cellceutix Corp. is a new, rapidly bactericidal antibiotic. As of July 2015, it is advancing into clinical phase III. The MIC90 data against a collection of MDR S. aureus and CoNS isolates were 2 µg/ml and 1 µg/ml, respectively [19]. Two clinical phase II trials have been completed to investigate safety, tolerability, pharmacokinetic profiles, and safety for cardiac toxicity in two different indications, ABSSSI and serious skin infections [20].
2.4. Fatty Acid Synthesis (FAS) Inhibitor
The pathways for the biosynthesis of fatty acids (FAS) are distinct in bacteria (FASII) and mammals (FASI). This biosynthesic pathway is essential in bacteria and therefore appealing for antibiotic drug discovery. The prokaryotic FAS pathway involves mono-functional enzymes in a complex with an enoyl-acyl-carrier (ACP) reductase (FabI). Three representative, small-molecule FabI inhibitors have advanced to clinical development to investigate safety, tolerability, pharmacokinetic profiles, and safety for cardiac toxicity for the treatment of MRSA/MSSA infections: Debio 1450, previously owned by Affinium Pharmaceuticals and now by Debiopharm International SA, which is in clinical phase I, CG-400549 from CrystalGenomics Inc., and FAB-001.
2.4.1. Debio 1450/ Debio 1452
Debio 1450 (formerly, AFN-1720, for oral administration) from Debiopharm International SA is the pro-drug of Debio 1452 (formerly, AFN-1252, for i.v. administration; Debiopharm International SA). Both investigational drugs display narrow-spectrum bacteriostatic activities against MRSA [21, 22]. A multi-center, randomized clinical phase IIa trial to explore the safety, tolerability, and efficacy of AFN-1252 in the treatment of staphylococcal ABSSSI has been completed and some adverse effects were reported [23]. The sponsor of this study was Affinium Pharmaceuticals. In 2014, the Swiss Debiopharm Group acquired the clinical assets and platform of Affinium Pharmaceuticals. A randomized, double-blind, multi-center phase II study to investigate the safety, tolerability, and efficacy of Debio 1450, as compared to vancomycin and linezolid, for ABSSSI caused by MSSA or MRSA has been recently initiated.
2.4.2. CG-400549
CG-400549, a 2-pyridone from CrystalGenomics Inc., is a further investigational drug directed against FabI of S. aureus [24, 25]. Crystal Genomics completed a clinical phase IIa, repeated-dose, open-label study for the treatment of ABSSSI caused by MRSA in 2012 and has announced further clinical development of CG-400549.
3. Alternatives to conventional antibiotics
3.1. Phage therapies
A bacteriophage, also referred to as phage, is a specific virus capable to invade and replicate within bacteria. Binding of a bacteriophage to its host depends on specific receptor/ligand interactions. Phage therapy has been successfully used to treat infection before the discovery of penicillin [26]. Nowadays, phage therapy is still routinely in used in several Eastern countries for the treatment of bacterial skin infections. Approval of phage therapy for humans in Western countries is rare, although phage therapy has been described by the FDA in 2006 as “Generally regarded as safe”. The worldwide rise of multi-resistant bacteria and the decreasing commitment of the pharmaceutical industry to antibiotic drug discovery may open a pathway for phage therapy. For example, the European Medicines Agency (EMA) has recently called for a workshop to open the dialog with academia, industry, and regulatory bodies on the use of phages for the treatment of bacterial infections. Currently, there are two anti-staphylococcal phage therapies in phase I trials: P128 from GangaGen and CF-301 from Contrafect [27]. Of note, phage therapy against S. aureus is predominantly directed toward decolonization, based on the fact that infection with S. aureus is associated with asymptomatic nasal colonization [28].
3.1.1. P128 (StaphTAME)
A tail-associated muralytic enzyme (TAME) was identified from bacteriophage K with potent activity against staphylococcal cells. The catalytic domain of TAME belongs to the N-terminal amidohydrolase/peptidase family; the C-terminal domain belongs to the SH3b family of cell wall–binding proteins. P128 from GangaGen was created by fusing the truncated smaller portion of TAME (C-terminus), harboring the anti-staphylococcal activity, with a staphylococcal cell wall-binding SH3b domain. Preclinical studies showed that the killing of S. aureus by P128 is dose-dependent. Studies in rats showed that clearance of nasally colonized S. aureus was achieved after 3 days of treatment with P128 protein [29]. A clinical phase I trial has been reported for P128 to investigate the safety, tolerability, immunogenicity and pharmacokinetics for S. aureus nasal decolonization, including MRSA, in carriers. P128 is applied to the anterior nares of healthy volunteers and patients with chronic kidney diseases who are on dialysis.
3.1.2. CF-301.
CF-301, also referred to as PlySs2, is an anti-staphylococcal lysin encoded within a prophage of Streptococcus suis. The catalytic domain belongs to the N-terminal amidohydrolase/peptidase family; the C-terminal domain belongs to the SH3b family of cell wall–binding proteins. CF-301, developed by Contrafect, showed rapid killing activity by degradation of the peptidoglycan of S. aureus, including MRSA. In combination with vancomycin, CF-301 proved successful in the treatment of bacteremia in mice with superiority over antibiotic monotherapy [30]. A phase I dose-escalating study is currently recruiting patients to investigate safety and tolerability in healthy subjects. According to Contracfect, CF-301 will be developed for the treatment of bacteremia.
3.2. Quorum sensing inhibitors
Quorum-sensing (QS), or sensing of cell density, is an important mechanism that bacteria use to communicate with one another. The accessory gene regulator (Agr) is the premier QS system in S. aureus [31]. It controls expression of a number of cytolytic bacterial toxins and cell surface antigens. The extracellular QS signal of Agr is a post-translationally modified peptide, auto-inducing peptide (AIP). Nucleotide variation within the agr operon generates AIPs with different amino acid sequences but a conserved thiolactone (or, rarely, lactone) ring structure. This accounts for the sub-classification of four Agr types (I-IV) in S. aureus. While there is agonistic activity of AIPs towards their own subgroups, natural competitive cross-inhibition is observed between most of the AIPs with non-cognate AgrC receptors. This phenomenon also occurs across staphylococcal species [32]. The molecular mechanism of Agr interference is dependent on differences in AIP amino acid composition and the N-terminal tail length [33, 34]. A large repertoire of modified AIP molecules, with various degrees of Agr inhibition and Agr specificity in vitro, has been created with the aim of reducing the severity of staphylococcal disease. Indeed, one study convincingly showed that a modified S. aureus type II AIP significantly reduced the size and severity of dermonecrosis in a skin abscess infection model in mice when co-injected with Agr group I S. aureus compared to injection with bacteria alone [35]. Similarly, systematic approaches, based on crystal structure topology and drug libraries, have been used to discover molecules that interfere with Agr. One such molecule, S. aureus virulence inhibitor (savirin), is an S. aureus specific Agr inhibitor that exerts its activity through direct interaction between AgrA and target DNA sequences [36]. Co-injection of savirin with S. aureus USA300 reduced the severity and size of dermonecrosis in mice. Additionally, delayed administration of savirin reduced the severity of dermonecrosis, but abscess size was not affected. Furthermore, in a murine air pouch infection model, a reduction in bacterial load was observed in the air pouch and kidneys, when savirin was administered at the same time as the bacteria [36].
Interestingly, other bacteria, fungi, plants, and herbs naturally produce molecules with demonstrated Agr inhibitory activity against S. aureus in vitro, but few have been tested in animal models of S. aureus infection [37].
3.3. Therapeutic antibodies
Understanding the molecular mechanisms of staphylococcal pathogenesis is crucial for selecting appropriate target antigens for the development of therapeutic antibodies. S. aureus produces an arsenal of cytolytic toxins that contribute to many facets of staphylococcal disease. These toxins include alpha-hemolysin, beta-hemolysin, multiple superantigens, leukotoxins, and phenol-soluble modulins (PSMs). Active immunization with single [38-42] or multiple [43-46] staphylococcal antigen(s) repeatedly showed induction of protective immune responses that abolished or reduced the severity of many different forms of S. aureus disease in animals.
On the other hand, antibodies to many staphylococcal antigens are readily detected in healthy individuals [47, 48]. Although the antibody responses to staphylococcal antigens in infected individuals are generally greater than in non-infected individuals [49, 50], humoral immune responses between individuals with the same S. aureus disease can vary [51]. However, there is evidence that greater levels of anti-staphylococcal antibodies contribute to more favorable clinical outcomes [49, 52-55], which may be attributed to the potent toxin-neutralizing activities of the antibodies. These findings have galvanized efforts to explore the use of polyclonal and monoclonal antibodies (mAbs) as therapeutics to neutralize the cell-damaging properties of staphylococcal toxins and enhance recognition of S. aureus by interfering with its ability to evade the immune system (Tab. 1).
Tab. 1.
Investigational anti-S. aureus antibodies.
|
Antibody
name |
Description |
Challenge
agent |
Dosage | Model | Species | Reference |
|---|---|---|---|---|---|---|
| Alpha-hemolysin | ||||||
| 7B8 (IgG2a) | Mouse mAb; |
S. aureus
Newman, LAC |
24 h prior to infection (10 mg/kg) IP |
Pneumonia | Mouse | [59] |
| 1A9 (IgG2b) | Mouse mAb; |
S. aureus
Newman, LAC |
24 h prior to infection (10 mg/kg) IP |
Pneumonia | Mouse | [59] |
| LTM14 | Human mAb; converted to full IgG from a single-chain variable fragment (scFv) phage library built from human donors |
S. aureus
LAC |
24 h prior to infection (30 mg/kg) IP |
Pneumonia | Mouse | [60] |
|
S. aureus
LAC |
24 h prior to infection (50 mg/kg) IP |
Skin abscess | Mouse | [60] | ||
|
S. aureus
LAC |
24 h prior to infection (30 mg/kg) IP |
Bacteremia | Mouse | [60] | ||
| LTM14 Fab | Human mAb; Fab fragment of LTM14 |
S. aureus
8325-4 |
9 h post infection, every 30 min (495 μg/drop/eye) |
Keratitis | Rabbit | [129] |
| 2A3 (IgG1) | Humanized mouse mAb; precursor to LC10 (MEDI4893*) |
S. aureus
NRS261, NRS382, SF8300 |
24 h prior to infection (5 mg/kg) IP |
Skin abscess | Mouse | [58] |
| 2A3 (IgG1) | Humanized mouse mAb; precursor to LC10 (MEDI4893*) |
S. aureus
SF8300 |
24 h prior to infection (10 mg/kg) IP |
Skin abscess | Mouse | [130] |
| LC10 (MEDI4893*) (IgG1κ) |
Human affinity-optimized mAb variant of 2A3; |
S. aureus
NRS261, NRS382, SF8300, FPR3757 |
24 h prior to infection (15, 20, 45 mg/kg) IP |
Pneumonia | Mouse | [131] |
| LC10 (MEDI4893*) (IgG1κ) |
Human affinity-optimized mAb variant of 2A3; |
S. aureus
SF8300 |
1 h post infection (1, 2.5, 5, 10 mg/kg) IP |
Skin abscess | Mouse | [132] |
| LC10 (MEDI4893*) (IgG1κ) |
Human affinity-optimized mAb variant of 2A3; |
S. aureus
SF8300 |
24 h prior to infection (5, 15, 45 mg/kg) IP |
Pneumonia | Mouse (immu nocom promis ed) |
[133] |
|
SEB
20B1, 14G8 and 6D31 |
Mouse mAb; | SEB | 10 min prior to toxin challenge (500 μg) IP |
SEB- induced lethal shock |
Mouse (HLA- DR3) |
[134] |
| 20B1 (IgG1) | Mouse mAb; |
S. aureus
38 and 45 |
30, 60, 120 min post infection (500 μg) IV |
Bacteremia | Mouse | [68] |
|
S. aureus
38 |
24 h prior to infection (500 μg) IP |
Superficial skin Infection |
Mouse | [68] | ||
|
S. aureus
38 |
24 h prior to infection (500 μg) IV |
Deep tissue implant Infection |
Mouse | [68] | ||
| 20B1 (IgG1, IgG2a, IgG2b2) |
Mouse mAb; | SEB | 10 min prior to toxin challenge (50, 100, 250, 500 μg) IP |
SEB- induced lethal shock |
Mouse | [66] |
|
S. aureus
38 |
120 min prior to infection (300 μg) IV |
Bacteremia | Mouse | [66] | ||
| Hu-1.6/1.1 | Humanized mouse mAb of 20B1 |
SEB | 10 min prior to toxin challenge (500 μg) IP |
SEB- induced lethal shock |
Mouse | [67] |
| 120 min prior to infection (500 μg) IV |
Deep tissue implant Infection |
Mouse | [67] | |||
|
S. aureus
38 |
24 h prior to infection (500 μg) IV |
Bacteremia | Mouse | [67] | ||
| Ch 82M, Ch 63 |
Humanized mouse mAb; | SEB | At time of toxin challenge (1 mg) IP |
SEB- induced lethal shock |
Mouse | [135] |
| Multiple mAbs described |
Human Fabs and mAbs; isolated from recombinant library by panning against SEB vaccine |
SEB | Co-injection of pre-incubated mAb (10 μg) + SEB |
SEB- induced lethal shock |
Mouse | [64] |
| Multiple mAbs described |
Human Fabs and mAbs; Phage library |
SEB | 60 min post infection (200 μg) IP |
SEB- induced lethal shock |
Mouse | [63] |
| PVL | ||||||
| Tetravalent bispecific antibody |
Humanized heavy chain-only antibody | 300 ng LukS-PV + 300 ng LukF-PV |
Co-injection (0.2 - 20 μg) intraocular |
Endophthal mitis |
Rabbit | [72] |
| Protein A | ||||||
| 5A10 (IgG1), 3F6 (IgG2a), 3D11 (IgG2b) |
Mouse mAb |
S. aureus
Newman |
4 h prior to infection (5 mg/kg) IP |
Renal abscess |
Mouse | [80] |
| Mouse mAb |
S. aureus
MW2 |
24 h prior to infection (15 mg/kg) IP |
Renal abscess |
Mouse | [80] | |
| 3F6 (IgG2a) | Mouse mAb |
S. aureus
LAC |
24 h prior to infection (5 mg/kg) IP |
Renal abscess |
Mouse | [136] |
| 3F6 (IgG2a) | Mouse mAb |
S. aureus
LAC |
24 h prior to infection (5 mg/kg) IP |
Bacteremia | Mouse neonat es |
[81] |
| IsdB | ||||||
| 2H2 (IgG1 and IgG2b) |
Mouse mAb |
S. aureus
Becker |
18-24 h prior to infection (0.3 - 0.5 mg) IP |
Bacteremia | Mouse | [85] |
|
S. aureus
MCL8538 |
24 h prior to infection (600 μg) IP |
Central venous catheter |
Mouse | |||
| CS-D7 (IgG1) | Human mAb |
S. aureus
SA025 |
4 h prior to infection (400 μg) IP |
Bacteremia | Mouse | [86, 87] |
|
S. aureus
SA025 |
1-2 h prior to infection (4 mg) IP |
Central venous catheter |
Rat | [86] | ||
| ClfA | ||||||
| 12-9 (IgG1) | Mouse mAb | MRSA clinical isolate 67- 0 (clfA+ clfB+ fnb+), Newman |
1 day prior to infection (0.3 mg) IP |
Bacteremia | Mouse | [137] |
| T1-2 (IgG1) (Aurexis tefibazumab) |
Humanized mouse mAb | MRSA clinical isolate 67- 0 (clfA+ clfB+ fnb+) |
24 h prior to infection (30 mg/kg) IP |
Infective endocarditis |
Rabbit | [91] |
| IsaA | ||||||
| UK-66P (IgG1) |
Mouse mAb |
S. aureus
USA300 |
0 h and 24 h post infection (15 mg/kg) IV |
Bacteremia | Mouse | [124] |
|
S. aureus
MA12 |
0 h and 24 h post infection (15 mg/kg) IV |
Central venous catheter |
Mouse | [124] | ||
| 1D9 (IgG1) | Human mAb |
S. aureus
USA300 |
3 h prior to infection (5 mg/kg) IV |
Bacteremia | Mouse | [126] |
| PNAG/PIA | ||||||
| F598 (IgG1) | Human mAb |
S. aureus
strains Mn8 and Reynolds |
4 h prior to infection (600 μg) IP |
Bacteremia | Mouse | [113] |
| Glucosaminidase | ||||||
| 1C11 (IgG1) | Mouse mAb |
S. aureus
Xen 29 |
1 day prior to infection (40 mg/kg) IP |
Implant-associated osteomyelitis |
Mouse | [123] |
| Deacylated peptidoglycan | ||||||
| ZBIA5H | Mouse mAb |
S. aureus
MW2 |
0 h (1 mg) IP | Bacteremia | Mouse | [120] |
|
S. aureus
MW2 |
3 days post infection (1 mg) IP |
Pneumonia | Mouse | [120] | ||
| AIP | ||||||
| AP4-24H11 (IgG) |
Mouse mAb |
S. aureus
RN4850 |
Co-injection (0.6 mg) SQ |
Skin abscess | Mouse | [127] |
|
S. aureus
RN4850 |
2 h prior to infection (1 mg) IP |
Bacteremia | Mouse | [127] | ||
| Multi-valent | ||||||
| Hla-F#5 ( IgG1) |
Human mAb |
S. aureus
TCH1516 |
24 h prior to infection (5 mg/kg) IP |
Bacteremia | Mouse | [138] |
|
S. aureus
TCH1516 |
24 h prior to infection (5 mg/kg) IP |
Pneumonia | Mouse | [138] | ||
114G8 and 6D3 were not protective when given alone in the HLA DR3 mice but their efficacy of protection could be greatly enhanced when mAbs were co-administered simultaneously.
IgG2b isotype showed superior protection.
3.3.1. Antibodies against staphylococcal toxins
3.3.1.1. Alpha-hemolysin (Hla)
Alpha-hemolysin, a major virulence factor in staphylococcal pathogenesis, has been the subject of intense research, both as a vaccine antigen and mAb. It exerts its activity by binding to its cognate receptor, the zinc-dependent metalloprotease A disintegrin and metalloproteinase 10 (ADAM10), which is found on the surface of many eukaryotic cells. Oligomerization of monomeric toxin molecules follows interaction with the receptor that ultimately leads to pore formation in the cytoplasmic membrane [56].
Several research groups have produced mAbs that interfere with at least two aspects of alpha-hemolysin functionality: heptamer formation and interaction with ADAM10 [57-60]. Importantly, when administered prophylactically, these mAbs show remarkable protective efficacies in passive immunization experiments and reduce the severity of disease in animal against models of S. aureus infection [58-60].
Of particular interest is mAb MEDI4893 (MedImmune). MEDI4893, a derivative of MEDI4893* [58], was designed to increase serum half-life through the introduction of three amino acid substitutions in the heavy-chain CH2 constant region of the Fc domain [61]. Unlike the other anti-alpha-hemolysin mAbs, MEDI4893 has dual inhibitory activity, interfering with both oligomerization and interaction with ADAM10 [57]. Recently, a phase I clinical trial with MEDI1893 was completed in the U.S. that evaluated the safety, tolerability, and pharmacokinetics in healthy adults after intravenous administration of escalated doses. An ongoing phase II study, which initiated in multiple European cities, will assess the safety of different intravenous doses of MEDI4893 in mechanically-ventilated adult patients and assess the prevention of S. aureus pneumonia.
AR-301 (KBSA301/Salvecin™), from Aridis Pharmaceuticals, was discovered by screening B-cell lymphocytes of a patient with confirmed S. aureus infection. No data is available describing its mechanism of action. However, this anti-alpha-hemolysin mAb is currently being assessed in phase I/II clinical trials in Spain, France and Belgium. The randomized, double-blind, placebo-controlled, single ascending dose study will evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical outcome of patients who have severe pneumonia caused by S. aureus after intravenous administration of 1, 3, 10 and 20 mg/kg of AR-301 mAb in addition to antibiotic regimens.
3.3.1.2. Staphylococcal enterotoxin B
The staphylococcal superantigens (SAgs) are a large family of protein toxins that can cause food poisoning and severe diseases such as toxic shock syndrome (TSS). The activity of SAgs is largely attributed to their T-cell stimulating activity [62]. Toxic shock syndrome toxin 1 (TSST-1) and staphylococcal enterotoxin B (SEB) belong to this group. Even though the SAg expression profile differs considerably among different S. aureus strains, there is major interest in protection against SEB because of its bio-warfare potential. A number of potent neutralizing anti-SEB mAbs have recently been described (Tab. 1). While the neutralizing activities of the antibodies are comparable, there are considerable differences regarding cross-reactivity with other SAgs produced by S. aureus [63] or other bacteria [64, 65].
All classes of anti-SEB mAbs reduce SEB-induced lethal shock in vivo (Tab. 1). However, only one mouse mAb, 20B1, and its humanized derivatives, have been tested in mouse models of S. aureus infection [66-68]. 20B1 and its derivatives significantly improved the survival of animals in a sepsis model and reduced the dissemination of bacteria. Furthermore, isotype switching of the original IgG1 mAb 20B1 backbone, to IgG2a, showed significantly greater protection than IgG1 or IgG2b isotypes in murine SEB intoxication and S. aureus sepsis models [66]. Interestingly, a highly thermally stable anti-SEB mAb was produced but it was not tested in animal models of SAg-mediated disease [69]. Furthermore, MORAb-048 is an investigational fully human IgG monoclonal antibody that targets SEB and is being developed by Morphotek and the United States Army Medical Research Institute. There are no reports of any anti-SEB mAbs in clinical trials. However, it is noteworthy that a clinical trial of an investigational vaccine against TSS based on a recombinant mutated form of SEB has been completed. Safety and production of neutralizing Abs were tested, but results were not published.
3.3.1.3. Leukotoxins
Leukotoxins, including Panton-Valentine Leukocidin (PVL), γ-hemolysin, LukDE, and LukGH (LukAB), are important virulence factors that target and lyse cells of myeloid lineage. Even though there is significant controversy over whether PVL is a decisive virulence determinant in S. aureus [70], there is still strong interest in creating a vaccine against PVL. Polyclonal and mAbs to PVL are neutralizing [71-73] and protective in passive immunization experiments in a rabbit model of S. aureus infection [72, 74]. Furthermore, humanized heavy chain-only antibodies against PVL neutralized toxin activity and inhibited inflammatory reactions and tissue destruction in a rabbit endophthalmitis model [72]. However, it was recently shown S. aureus disease was exacerbated if antibodies to PVL were given to mice prior to infection with a PVL-expressing strain of S. aureus [75] suggesting that pre-existing antibodies to PVL may be harmful to the human host.
3.3.2. Antibodies against staphylococcal surface proteins
A number of staphylococcal surface proteins are anchored to the cell wall via an LPXTG sorting signal [76]. These 17 proteins, which include surface protein A (SpA) and clumping factor A (ClfA), are involved in staphylococcal adherence to host tissues through binding to components of the extracellular matrix. They play key roles in immune evasion and are crucial for pathogenesis [77]. As such, several of these proteins have been used as targets for mAb production.
3.3.2.1. Protein A (SpA)
The genome-encoded virulence determinant, SpA (protein A), subverts the host immune response through its ability to bind the immunoglobulin Fcγ domain, permitting S. aureus to avoid opsonophagocytic killing. Furthermore, SpA cross-links the Fab domain of VH3-type B cell receptors (IgM), which effectively cripples the ability of the host to mount adaptive immune responses [78]. Mutations in the immunoglobulin binding domains of SpA give rise to a non-toxigenic molecule, SpA(KKAA), that fails to bind Fcγ or VH3 [79]. When SpA(KKAA) is used as an immunogen in mice, the humoral response to S. aureus is alleviated, and protective immune responses against intravenous challenge with S. aureus are restored. Passive immunization with a murine mAb made against SpA(KKAA) reduced hematogenous seeding in mice against different strains of S. aureus [80] and increased survival in neonatal mice against S. aureus challenge [81].
There are reports of a humanized monoclonal antibody that neutralizes SpA, MVQ3 (Terravab), and of the development of an IgM isotype antibody that does not bind SpA (STROX Biopharmaceuticals). However, information on these molecules is scarce. Lastly, there are no reports of SpA-specific mAbs currently being investigated in clinical trials.
3.3.2.2. Iron-Regulated Surface determinant B (IsdB) protein
IsdB promotes S. aureus adhesion to platelets to mediate platelet aggregation [82]. Moreover, IsdB has recently been shown to mediate adhesion to non-phagocytic host cells [83]. Although there have been sobering experiences using IsdB as a target, because a heavily publicized active vaccination trial failed [84], several groups have developed mAbs to IsdB [85, 86] and have independently demonstrated therapeutic potential in mouse models. Interestingly, the mechanism of action of the human mAb (CS-D7) is unique, because enhanced survival in mice was FcγR-independent and did not involve classical complement activation, or direct inhibition of bacterial growth, but required complement, phagocytes and lymphocytes [87].
3.3.2.3. Clumping factor A (ClfA)
ClfA, which adheres to both fibrinogen and fibronectin, contributes significantly to staphylococcal pathogenesis [88]. Immunization with ClfA antigen induces humoral [89] and protective [90] responses. Furthermore, recombinant derivatives of ClfA are included in vaccine formulations (see below). An anti-ClfA mAb, Aurexis tefibazumab, which was protective in animal models of S. aureus infection after passive immunization [91] was tested in a phase II randomized, double-blinded, multi-center study in S. aureus bacteremia patients undergoing vancomycin therapy [92]. Dishearteningly, the study failed to demonstrate any significant differences in overall-adverse clinical outcomes in treated patients compared to the placebo group. Similarly, a phase III clinical trial, which investigated the therapeutic efficacy of a pooled human immunoglobulin preparation from donors with high antibody titers against S. aureus ClfA and S. epidermidis SdrG (INH-A21) in neonates, failed to demonstrate differences in rates of late-onset sepsis caused by S. aureus or coagulase-negative staphylococci compared to the control group [93].
3.3.3. Antibodies against staphylococcal non-protein antigens
3.3.3.1. Capsular polysaccharide and poly-N-acetyl-D-glucosamine
Antibodies to staphylococcal capsular polysaccharides, which are anti-phagocytic virulence factors crucial for persistence of S. aureus in the blood and tissues in animal models of infection, are often found in patients with S. aureus bacteremia. Between 70 to 80% of isolates from humans produce one of two serotypes, 5 (CP5) or 8 (CP8) [94]. Active vaccination with conjugated capsular vaccine formulations induced protective immunity in animal models of S. aureus infection and CP-specific antibodies reduced the severity of infection against S. aureus disease in passive immunization experiments [95-98]. However, these findings are not reflected in humans [99-101]. Furthermore, protection is not always observed with CP-specific antibodies in mouse models of S. aureus infection [102]. Most strikingly, a vaccine comprised of pooled human anti-capsular polysaccharide (CP) types 5 and 8 antibodies (Altastaph; Nabi) failed to show a significant difference in clinical outcomes among patients with complicated S. aureus bacteremia in a phase II clinical trial [103]. These remarkable differences in vaccine efficacy may be attributed to the fact that circulating clinical strains such as USA300 may not express capsule [104]. Despite failed clinical trials with single-component vaccines, capsular polysaccharides are included in multi-component antigen vaccines with the premise that targeting several antigens will be significantly more effective. Indeed, rats immunized with CP5- or CP8-conjugated to tetanus toxoid plus detoxified alpha-hemolysin or recombinant domain A of ClfA produced a robust humoral response to these antigens [105]. This study also demonstrated that inclusion of detoxified alpha-hemolysin better protected rats against S. aureus-induced osteomyelitis compared to control rats. Rats immunized with CP5- or CP8-conjugated to tetanus toxoid plus recombinant domain A of ClfA showed somewhat weaker protective responses. However, in a different study, mice passively immunized with antibodies to S. aureus CP5 plus an anti-ClfA mAb, had reduced bacterial loads in infected mammary glands after infection with an encapsulated bovine CP5-expressing strain [106].
The bacterial surface carbohydrate antigen, poly-N-acetylglucosamine (PNAG, or polysaccharide intercellular adhesion, PIA), which is synthesized by the proteins encoded in the ica locus [107], contributes to intercellular adhesion, biofilm formation, immune evasion [108], and is an important virulence determinant [109]. PNAG undergoes partial deacetylation, which is important for anchoring of PNAG to the cell wall [110, 111]. Furthermore, the deacetylated form of PNAG (dPNAG) induces opsonic antibodies, which when passively administered, protect mice against S. aureus-induced bacteremia and lethal challenge [112]. Lastly, a PNAG-specific mAb also demonstrated greater specificity to the deacetylated form of PNAG and conferred protection in a murine lethal challenge model with S. aureus by favoring opsonization of S. aureus and killing by human neutrophils [113]. Sanofi recently investigated a different PNAG-specific mAb, SAR279356, in a phase II clinical trial. However, the study was terminated and no results were reported.
3.3.4. Antibodies against S. aureus cell wall components
3.3.4.1. Lipoteichoic acids (LTA)
Anionic glycopolymers composed of many identical sugar-phosphate repeating units, called teichoic acids (TAs), are found on the cell wall of many Gram-positive bacteria. TAs may be linked to peptidoglycan (wall teichoic acids) or to the cytoplasmic membrane (lipoteichoic acids, LTA) [114, 115]. The accessibility of the highly conserved LTA polymer on the cell surface, its relatively uniform basic structure, and the fact that TAs are expressed by all S. aureus isolates, would place TAs high on the list of advantageous vaccine candidates. In support of targeting LTAs, passive immunization with polyclonal antibodies from synthetic LTA or LTA from Enterococcus faecalis-immunized animals are able to protect against S. aureus bacteremia [116, 117]. Unfortunately, these encouraging results were not duplicated in human clinical trials with a humanized mAb developed against LTA from S. aureus, called Pagibaximab [118, 119].
3.3.4.2. Deaceylated peptidoglycan
Similar to PNAG, deaceylated peptidoglycan is more immunogenic than its natural form and mAbs were made against the processed product [120]. Passive immunization with one mAb, ZBIA5H, protected mice against multiple S. aureus strains in bacteremia infection model.
3.3.4.3. Autolysins
Autolysin (Atl) is a protein with various functions; it is involved in the separation of the daughter cells by hydrolyzing specific bonds within the bacterial cell wall peptidoglycan [121], acts directly as an adhesin by binding to fibronectin and vitronectin, and is involved in biofilm formation [122]. 1C11, a mAb that targets the glucosaminidase subunit of Atl, was shown to reduce the severity of S. aureus implant-associated osteomyelitis by inhibiting S. aureus growth and binary fission, and facilitating opsonophagocytosis [123].
IsaA is an autolysin that is expressed by many S. aureus isolates. Passive immunization experiments with mouse mAb, UK-66P (IgG1), protected mice against bacteremia and central venous catheter infections with S. aureus [124]. UK-66P was later humanized and different isotypes were generated to evaluate their protective efficacies. In vitro, the IgG1 isotype demonstrated the best in-vitro opsonophagocytosis activity when compared to the IgG2 and IgG4 isotypes. Unfortunately, no further testing was conducted to substantiate whether these claims were reflected in vivo [125]. A human IgG1 mAb was recently created, and similar to UK-66P, it showed protection in a mouse model of S. aureus bacteremia [126].
3.3.5. Antibodies against AIPs
A murine mAb, AP4-24H11, which recognizes AIP IV [127] was shown to interfere with QS when incubated with an Agr group IV strain (RN4850) and, to a lesser extent, group I strain (Wood 46). Although co-injection of AP4-24H11 with strain RN4850 significantly abolished the severity of dermonecrosis in a mouse skin abscess model, an impressive 100% protection was documented in mice that were passively immunized with AP4-24H11 prior to a lethal intraperitoneal injection of RN4850 compared with a majority of mice that succumbed to death when given a control mAb.
3.3.6. Multivalent antibody therapy
Several vaccines that contain multiple staphylococcal antigens have recently been described. For instance, the protective efficacy of a multi-component vaccine, 4C-Staph, which comprises four antigens, non-toxic alpha-hemolysin (Hla), a fusion of the EsxA and EsxB proteins, and the two surface proteins, ferric hydroxamate uptake D2 (FhuD2) and conserved staphylococcal antigen 1A (CSA1A), was evaluated in mice [43]. In addition to conferring protection in mouse models of abscess and peritonitis, the authors showed that protection by 4C-Staph was predominantly antibody-dependent.
A different group evaluated the efficacy of a vaccine formulation consisting of only staphylococcal toxins in rabbits [128]. Depending on the S. aureus challenge, rabbits were immunized with one or a combination of biologically inactive staphylococcal toxins [(TSST-1 toxoid G31S/S32P (MHC II binding deficient), SEB toxoid Q210A and SEC toxoid N23A (both T-cell receptor binding deficient), and alpha-hemolysin toxoid H35A, γ-toxin Β chain and β-toxin] and then challenged with a variety of strains of S. aureus belonging to different clonal lineages, including USA300. Most excitingly, all vaccinated rabbits survived from lethal pneumonia. Furthermore, immunized rabbits survived S. aureus challenge in an infective endocarditis model. Finally, delayed administration with the hyperimmune serum also protected against SEB-induced intrapulmonary lethality.
Two different tri-component vaccines consisting of different antigen components have recently been assessed. The first study investigated the protective efficacy of a vaccine formulation with recombinant ClfA, IsdB, and Hlg against intraperitoneal challenge in mice [44]. With this antigen combination, there was significant, although marginal, protection in immunized mice compared to the control group. The authors noted that high levels of opsonic IgG antibodies were produced in immunized animals. However, antigen-specific antibody data were not shown and the mechanism of protection was not evaluated. In contrast, the tri-component, SA3Ag, which consists of CP5 and CP8 individually conjugated to a non-toxic mutant form of diphtheria toxin (cross-reactive material 197) plus a recombinant mutant form of ClfA, was recently tested in a phase I clinical trial [45]. Increased immune responses to all three staphylococcal antigens were observed in patients who received the vaccine and the antibodies demonstrated potent opsonophagocytic activities. The vaccine was well tolerated after one dose. Additionally, a four-component vaccine, SA4Ag [46], includes the three aforementioned antigens plus the manganese transporter C (MntC) [38]. SA4Ag was recently assessed in several Phase I trials and is currently being investigated in two Phase II trials, but details from these studies have yet to be made public.
4. Conclusion
Research on anti-staphylococcal drugs and alternative therapeutics is advancing in all areas. Investigational drugs include several new antibiotics that are in clinical trials. Even some alternative drugs have made it to that level, although most of them are still in pre-clinical development. The most notable development, seen in particular in anti-staphylococcal vaccine research and anti-virulence drug development, is that researchers now rely on the considerable advances in pathogenesis research that have been achieved in recent years.
5. Expert Opinion
Hospital-associated MRSA infections have recently been reported to decline in the U.S. This is most likely due to increased hygiene precautions. However, the death toll due to S. aureus is still huge and S. aureus remains one of the most frequent causes of death due to an infectious agent worldwide. Furthermore, in many countries, MRSA infections are at a considerable rise. In contrast to some Gram-negative pathogens that have acquired resistance to all available antibiotics, S. aureus is still treatable by at least some antibiotics; and some novel anti-Gram-positive antibiotics are in the pipeline. However, many of those are not optimal in terms of efficacy or have considerable side effects; furthermore, history has shown that resistance always develops against any novel drug. It is thus vital to continue the evaluation and development of investigational drugs directed against S. aureus, despite the recent shift of pharmaceutical companies to the development of anti-Gram-negative therapeutics.
As we have discussed in here, there are several investigational antibiotics with efficacy against S. aureus that are currently in clinical trials. This is a much better situation as compared to about 10 years ago, when there was a distinct paucity of such agents. Whether any of those that are currently under evaluation will result in an efficient major new treatment option for MRSA remains to be seen.
The lack of potential treatment options for MRSA that people envisaged in the early 2000s also prompted the search for alternatives, such as drugs to treat virulence instead of vital cell processes. However, many of those alternative drugs have intrinsic problems. QS blockers for example will only work against acute forms of disease, while they may even enhance chronicity of other types of infection – this “double edged sword” problem is characteristic of many attempts to target bacterial regulatory systems. The efficacy of toxin-directed mAbs is, naturally, limited to disease types whose pathogenesis relies on these specific toxins. With S. aureus pathogenesis in different infection types being extremely multi-factorial, there are only a very limited number of toxins that may be targeted by such a therapeutic in a marketable fashion. Owing to research demonstrating its importance in a series of infection scenarios, especially alpha-hemolysin presents itself as such a marketable target.
Combining targets in multi-valent vaccines may hold some promise. For example, the induction of high antigen-specific antibody titers from active immunization with multi-valent vaccines against staphylococcal antigens is an encouraging outcome. However, whether these antibodies play a significant role in protection in humans requires further investigation. In general, the evaluation of combinatory therapeutics is a relatively recent approach in antibacterial drug development and it remains to be seen whether it will lead to improved treatment options.
Altogether, the future of anti-staphylococcal therapy remains a concern, but appears less grim than only some years ago, with considerable advances being made in all areas, including novel antibiotics, anti-virulence approaches, and even vaccines. While a broadly protective vaccine against staphylococcal infections is currently out of reach, these new approaches will help us control staphylococcal infections in the future, with staphylococcal pathogenesis research enabling us to produce better, efficient, and possibly combinatorial novel drugs.
Article highlights.
Nine novel investigational antibiotics with efficacy against MRSA are in phase I- or phase-II development.
Current alternative efforts to combat acute S. aureus infections include strategies to target virulence.
Blocking quorum-sensing represents an elegant way to inhibit production of aggressive virulence determinants of S. aureus.
Anti-toxin monoclonal antibodies offer an alternative way to reduce virulence, but many are still in pre-clinical development.
Combinatorial approaches, such as the combination of several anti-toxin mAbs, or the combination of virulence drugs with traditional antibiotics are being explored.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis. 2007;13:1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–21. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–93. doi: 10.1016/s0966-842x(01)02175-8. [DOI] [PubMed] [Google Scholar]
- 4.Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clinical microbiology reviews. 1997;10:781–91. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiramatsu K. Vancomycin resistance in staphylococci. Drug Resist Updat. 1998;1:135–50. doi: 10.1016/s1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 6.Ross JE, Scangarella-Oman NE, Flamm RK, Jones RN. Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent. J Clin Microbiol. 2014;52:2629–32. doi: 10.1128/JCM.00656-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.So W, Crandon JL, Nicolau DP. Pharmacodynamic Profile of GSK2140944 against Methicillin-Resistant Staphylococcus aureus in a Murine Lung Infection Model. Antimicrob Agents Chemother. 2015;59:4956–61. doi: 10.1128/AAC.00625-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MV, De Souza NJ, Gupte SV, et al. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob Agents Chemother. 2004;48:4754–61. doi: 10.1128/AAC.48.12.4754-4761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagwat SS, Mundkur LA, Gupte SV, et al. The anti-methicillin-resistant Staphylococcus aureus quinolone WCK 771 has potent activity against sequentially selected mutants, has a narrow mutant selection window against quinolone-resistant Staphylococcus aureus, and preferentially targets DNA gyrase. Antimicrob Agents Chemother. 2006;50:3568–79. doi: 10.1128/AAC.00641-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang V, Gortney JS. Risk of serotonin syndrome with concomitant administration of linezolid and serotonin agonists. Pharmacotherapy. 2006;26:1784–93. doi: 10.1592/phco.26.12.1784. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence L, Danese P, DeVito J, et al. In vitro activities of the Rx-01 oxazolidinones against hospital and community pathogens. Antimicrob Agents Chemother. 2008;52:1653–62. doi: 10.1128/AAC.01383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales G, Picazo JJ, Baos E, et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis. 2010;50:821–5. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 13.Meng J, Zhong D, Li L, et al. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-Dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43:646–59. doi: 10.1124/dmd.114.061747. [DOI] [PubMed] [Google Scholar]
- 14.Li CR, Zhai QQ, Wang XK, et al. In vivo antibacterial activity of MRX-I, a new oxazolidinone. Antimicrob Agents Chemother. 2014;58:2418–21. doi: 10.1128/AAC.01526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu DM, Wang W, Huang YQ, Zhang YY. Antibacterial spectrum of oxazolidinone MRX-I: potent activity against multidrug-resistant Gram-positive pathogens; International Conference on Antimicrobial Agents and Chemotherapy; San Francisco: American Society for Microbiology. 2012. [Google Scholar]
- 16.Gordeev MF, Yuan ZY. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J Med Chem. 2014;57:4487–97. doi: 10.1021/jm401931e. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JW, Jung SJ, Lee HH, et al. In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Antimicrob Agents Chemother. 2010;54:5359–62. doi: 10.1128/AAC.00723-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scorciapino MA, Rinaldi AC. Antimicrobial peptidomimetics: reinterpreting nature to deliver innovative therapeutics. Front Immunol. 2012;3:171. doi: 10.3389/fimmu.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott R, Korczak B, Jorgensen D, et al. Activity of PMX-30063 against drug resistant Staphylococci; International Conference on Antimicrobial Agents and Chemotherapy; San Francisco: American Society for Microbiology. 2012. [Google Scholar]
- 20.Jorgensen D, Scott RW, O'Riordan WA, Tack KJ. A randomized, double-blind study comparing single-dose and short-course brilacidin to daptomycin in the treatment of acute bacterial skin & skin structure infections (ABSSSI); 25th European Congress of Clinical Microbiology and Infectious Diseases; Copenhagen: European Society of Clinical Microbiology and Infectious Diseases. 2015. [Google Scholar]
- 21.Karlowsky JA, Kaplan N, Hafkin B, et al. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcus-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–8. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlowsky JA, Laing NM, Baudry T, et al. In vitro activity of API-1252, a novel FabI inhibitor, against clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2007;51:1580–1. doi: 10.1128/AAC.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pucci MJ, Bush K. Investigational antimicrobial agents of 2013. Clin Microbiol Rev. 2013;26:792–821. doi: 10.1128/CMR.00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yum JH, Kim CK, Yong D, et al. In vitro activities of CG400549, a novel FabI inhibitor, against recently isolated clinical staphylococcal strains in Korea. Antimicrob Agents Chemother. 2007;51:2591–3. doi: 10.1128/AAC.01562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HS, Yoon YM, Jung SJ, et al. Antistaphylococcal activities of CG400549, a new bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. J Antimicrob Chemother. 2007;60:568–74. doi: 10.1093/jac/dkm236. [DOI] [PubMed] [Google Scholar]
- 26.Sulakvelidze A, Alavidze Z, Morris JG., Jr. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–59. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingwell K. Bacteriophage therapies re-enter clinical trials. Nat Rev Drug Discov. 2015;14:515–6. doi: 10.1038/nrd4695. [DOI] [PubMed] [Google Scholar]
- 28.von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 29.Paul VD, Rajagopalan SS, Sundarrajan S, et al. A novel bacteriophage Tail-Associated Muralytic Enzyme (TAME) from Phage K and its development into a potent antistaphylococcal protein. BMC Microbiol. 2011;11:226. doi: 10.1186/1471-2180-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuch R, Lee HM, Schneider BC, et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis. 2014;209:1469–78. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–64. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 32.Otto M, Echner H, Voelter W, Gotz F. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 2001;69:1957–60. doi: 10.1128/IAI.69.3.1957-1960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon GJ, Wright JS, Muir TW, Novick RP. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry. 2002;41:10095–104. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 34.Mayville P, Ji G, Beavis R, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96:1218–23. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JS, 3rd, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A. 2005;102:1691–6. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sully EK, Malachowa N, Elmore BO, et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014;10:e1004174. doi: 10.1371/journal.ppat.1004174. • Describes an S. aureus-specific QS inhibitor with in-vivo activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan BA, Yeh AJ, Cheung GY, Otto M. Investigational therapies targeting quorum-sensing for the treatment of Staphylococcus aureus infections. Expert Opin Investig Drugs. 2015;24:689–704. doi: 10.1517/13543784.2015.1019062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson AS, Scully IL, Timofeyeva Y, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205:1688–96. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–8. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra RP, Mariotti P, Fiaschi L, et al. Staphylococcus aureus FhuD2 is involved in the early phase of staphylococcal dissemination and generates protective immunity in mice. J Infect Dis. 2012;206:1041–9. doi: 10.1093/infdis/jis463. [DOI] [PubMed] [Google Scholar]
- 41.Mocca CP, Brady RA, Burns DL. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of Staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol. 2014;21:622–7. doi: 10.1128/CVI.00051-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi A, Pancari G, Cope L, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother. 2012;8:336–46. doi: 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagnoli F, Fontana MR, Soldaini E, et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A. 2015;112:3680–5. doi: 10.1073/pnas.1424924112. •• Describes the protective efficacy of a vaccine comprised of 4 staphylococcal antigens. Antibodies from vaccinated animals provided protection against S. aureus infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delfani S, Mohabati Mobarez A, Imani Fooladi AA, et al. Protection of mice against Staphylococcus aureus infection by a recombinant protein ClfA-IsdB-Hlg as a vaccine candidate. Med Microbiol Immunol. 2015 doi: 10.1007/s00430-015-0425-y. [DOI] [PubMed] [Google Scholar]
- 45.Nissen M, Marshall H, Richmond P, et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33:1846–54. doi: 10.1016/j.vaccine.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Anderson AS, Miller AA, Donald RG, et al. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8:1585–94. doi: 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colque-Navarro P, Jacobsson G, Andersson R, et al. Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin Vaccine Immunol. 2010;17:1117–23. doi: 10.1128/CVI.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus - The anti-S. aureus antibody response. Int J Med Microbiol. 2010;300:176–92. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsson G, Colque-Navarro P, Gustafsson E, et al. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2010;29:715–25. doi: 10.1007/s10096-010-0919-x. [DOI] [PubMed] [Google Scholar]
- 50.Verkaik NJ, de Vogel CP, Boelens HA, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis. 2009;199:625–32. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 51.den Reijer PM, Lemmens-den Toom N, Kant S, et al. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One. 2013;8:e53391. doi: 10.1371/journal.pone.0053391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma-Kuinkel BK, Wu Y, Tabor DE, et al. Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol. 2015;53:227–36. doi: 10.1128/JCM.02023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calderwood MS, Desjardins CA, Sakoulas G, et al. Staphylococcal enterotoxin P predicts bacteremia in hospitalized patients colonized with methicillin-resistant Staphylococcus aureus. J Infect Dis. 2014;209:571–7. doi: 10.1093/infdis/jit501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fritz SA, Tiemann KM, Hogan PG, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis. 2013;56:1554–61. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adhikari RP, Ajao AO, Aman MJ, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 56.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5:1140–66. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oganesyan V, Peng L, Damschroder MM, et al. Mechanisms of neutralization of a human anti-alpha-toxin antibody. J Biol Chem. 2014;289:29874–80. doi: 10.1074/jbc.M114.601328. • Discusses the dual mechanism of alpha-hemolysin interference by mAb MEDI4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tkaczyk C, Hua L, Varkey R, et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol. 2012;19:377–85. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77:2712–8. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foletti D, Strop P, Shaughnessy L, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus alpha-hemolysin. J Mol Biol. 2013;425:1641–54. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 62.Stach CS, Herrera A, Schlievert PM. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 2014;59:177–81. doi: 10.1007/s12026-014-8539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karauzum H, Chen G, Abaandou L, et al. Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J Biol Chem. 2012;287:25203–15. doi: 10.1074/jbc.M112.364075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larkin EA, Stiles BG, Ulrich RG. Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PLoS One. 2010;5:e13253. doi: 10.1371/journal.pone.0013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dutta K, Varshney AK, Franklin MC, et al. Mechanisms mediating enhanced neutralization efficacy of staphylococcal enterotoxin B by combinations of monoclonal antibodies. J Biol Chem. 2015;290:6715–30. doi: 10.1074/jbc.M114.630715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varshney AK, Wang X, Aguilar JL, et al. Isotype switching increases efficacy of antibody protection against staphylococcal enterotoxin B-induced lethal shock and Staphylococcus aureus sepsis in mice. MBio. 2014;5:e01007–14. doi: 10.1128/mBio.01007-14. • Isotype switching of mAbs directed towards SEB can alter potency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varshney AK, Wang X, MacIntyre J, et al. Humanized staphylococcal enterotoxin B (SEB)-specific monoclonal antibodies protect from SEB intoxication and Staphylococcus aureus infections alone or as adjunctive therapy with vancomycin. J Infect Dis. 2014;210:973–81. doi: 10.1093/infdis/jiu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varshney AK, Wang X, Scharff MD, et al. Staphylococcal Enterotoxin B-specific monoclonal antibody 20B1 successfully treats diverse Staphylococcus aureus infections. J Infect Dis. 2013;208:2058–66. doi: 10.1093/infdis/jit421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graef RR, Anderson GP, Doyle KA, et al. Isolation of a highly thermal stable lama single domain antibody specific for Staphylococcus aureus enterotoxin B. BMC Biotechnol. 2011;11:86. doi: 10.1186/1472-6750-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otto M. A MRSA-terious enemy among us: end of the PVL controversy? Nat Med. 2011;17:169–70. doi: 10.1038/nm0211-169. [DOI] [PubMed] [Google Scholar]
- 71.Hermos CR, Yoong P, Pier GB. High levels of antibody to panton-valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin Infect Dis. 2010;51:1138–46. doi: 10.1086/656742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laventie BJ, Rademaker HJ, Saleh M, et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc Natl Acad Sci U S A. 2011;108:16404–9. doi: 10.1073/pnas.1102265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gauduchon V, Cozon G, Vandenesch F, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–53. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 74.Karauzum H, Adhikari RP, Sarwar J, et al. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS One. 2013;8:e65384. doi: 10.1371/journal.pone.0065384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–6. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schneewind O, Model P, Fischetti VA. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–81. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 77.Mazmanian SK, Liu G, Jensen ER, et al. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci U S A. 2000;97:5510–5. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falugi F, Kim HK, Missiakas DM, Schneewind O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. MBio. 2013;4:e00575–13. doi: 10.1128/mBio.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HK, Cheng AG, Kim HY, et al. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 2010;207:1863–70. doi: 10.1084/jem.20092514. •• Description of a non-toxigenic variant of protein A that when used as an immunogen in mice, allows the host to produce a humoral response to S. aureus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim HK, Emolo C, DeDent AC, et al. Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice. Infect Immun. 2012;80:3460–70. doi: 10.1128/IAI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thammavongsa V, Rauch S, Kim HK, et al. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine. 2015;33:523–6. doi: 10.1016/j.vaccine.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miajlovic H, Zapotoczna M, Geoghegan JA, et al. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology. 2010;156:920–8. doi: 10.1099/mic.0.036673-0. [DOI] [PubMed] [Google Scholar]
- 83.Zapotoczna M, Jevnikar Z, Miajlovic H, et al. Iron-regulated surface determinant B (IsdB) promotes Staphylococcus aureus adherence to and internalization by non-phagocytic human cells. Cell Microbiol. 2013;15:1026–41. doi: 10.1111/cmi.12097. [DOI] [PubMed] [Google Scholar]
- 84.Fowler VG, Allen KB, Moreira ED, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 85.Brown M, Kowalski R, Zorman J, et al. Selection and characterization of murine monoclonal antibodies to Staphylococcus aureus iron-regulated surface determinant B with functional activity in vitro and in vivo. Clin Vaccine Immunol. 2009;16:1095–104. doi: 10.1128/CVI.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebert T, Smith S, Pancari G, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies. 2010;19:113–28. doi: 10.3233/HAB-2010-0235. [DOI] [PubMed] [Google Scholar]
- 87.Pancari G, Fan H, Smith S, et al. Characterization of the mechanism of protection mediated by CS-D7, a monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) Front Cell Infect Microbiol. 2012;2:36. doi: 10.3389/fcimb.2012.00036. •• Describes the unique mechanism of action of a mAb that is directed to iron regulated surface determinant B (IsdB) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreillon P, Entenza JM, Francioli P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–43. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hawkins J, Kodali S, Matsuka YV, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol. 2012;19:1641–50. doi: 10.1128/CVI.00354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Josefsson E, Hartford O, O'Brien L, et al. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–80. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 91.Domanski PJ, Patel PR, Bayer AS, et al. Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infect Immun. 2005;73:5229–32. doi: 10.1128/IAI.73.8.5229-5232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weems JJ, Jr., Steinberg JP, Filler S, et al. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:2751–5. doi: 10.1128/AAC.00096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeJonge M, Burchfield D, Bloom B, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–5. doi: 10.1016/j.jpeds.2007.04.060. 65 e1. [DOI] [PubMed] [Google Scholar]
- 94.O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–65. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JC, Park JS, Shepherd SE, et al. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–51. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park S, Gerber S, Lee JC. Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates. Infect Immun. 2014;82:5049–55. doi: 10.1128/IAI.02373-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wacker M, Wang L, Kowarik M, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis. 2014;209:1551–61. doi: 10.1093/infdis/jit800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 100.Fattom A, Matalon A, Buerkert J, et al. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother. 2015;11:632–41. doi: 10.4161/hv.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16. doi: 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cook J, Hepler R, Pancari G, et al. Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Hum Vaccin. 2009;5:254–63. doi: 10.4161/hv.5.4.6765. [DOI] [PubMed] [Google Scholar]
- 103.Rupp ME, Holley HP, Jr., Lutz J, et al. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:4249–54. doi: 10.1128/AAC.00570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyle-Vavra S, Li X, Alam MT, et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio. 2015:6. doi: 10.1128/mBio.02585-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lattar SM, Noto Llana M, Denoel P, et al. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun. 2014;82:83–91. doi: 10.1128/IAI.01050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tuchscherr LP, Buzzola FR, Alvarez LP, et al. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun. 2008;76:5738–44. doi: 10.1128/IAI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heilmann C, Schweitzer O, Gerke C, et al. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–91. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 108.Otto M. Staphylococcus epidermidis--the 'accidental' pathogen. Nat Rev Microbiol. 2009;7:555–67. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kropec A, Maira-Litran T, Jefferson KK, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–76. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vuong C, Kocianova S, Voyich JM, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279:54881–6. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 111.Cerca N, Jefferson KK, Maira-Litran T, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2007;75:3406–13. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly-Quintos C, Cavacini LA, Posner MR, et al. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74:2742–50. doi: 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010;300:148–54. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Percy MG, Grundling A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu Rev Microbiol. 2014;68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- 116.Laverde D, Wobser D, Romero-Saavedra F, et al. Synthetic teichoic acid conjugate vaccine against nosocomial Gram-positive bacteria. PLoS One. 2014;9:e110953. doi: 10.1371/journal.pone.0110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Theilacker C, Kropec A, Hammer F, et al. Protection against Staphylococcus aureus by antibody to the polyglycerolphosphate backbone of heterologous lipoteichoic acid. J Infect Dis. 2012;205:1076–85. doi: 10.1093/infdis/jis022. [DOI] [PubMed] [Google Scholar]
- 118.Weisman LE, Thackray HM, Garcia-Prats JA, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2009;53:2879–86. doi: 10.1128/AAC.01565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weisman LE, Thackray HM, Steinhorn RH, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271–9. doi: 10.1542/peds.2010-3081. [DOI] [PubMed] [Google Scholar]
- 120.Ohsawa H, Baba T, Enami J, Hiramatsu K. Successful selection of an infection-protective anti-Staphylococcus aureus monoclonal antibody and its protective activity in murine infection models. Microbiol Immunol. 2015;59:183–92. doi: 10.1111/1348-0421.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biswas R, Voggu L, Simon UK, et al. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett. 2006;259:260–8. doi: 10.1111/j.1574-6968.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 122.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–24. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 123.Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, et al. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res. 2014;32:1389–96. doi: 10.1002/jor.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lorenz U, Lorenz B, Schmitter T, et al. Functional antibodies targeting IsaA of Staphylococcus aureus augment host immune response and open new perspectives for antibacterial therapy. Antimicrob Agents Chemother. 2011;55:165–73. doi: 10.1128/AAC.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oesterreich B, Lorenz B, Schmitter T, et al. Characterization of the biological anti-staphylococcal functionality of hUK-66 IgG1, a humanized monoclonal antibody as substantial component for an immunotherapeutic approach. Hum Vaccin Immunother. 2014;10:926–37. doi: 10.4161/hv.27692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Berg S, Bonarius HP, van Kessel KP, et al. A human monoclonal antibody targeting the conserved staphylococcal antigen IsaA protects mice against Staphylococcus aureus bacteremia. Int J Med Microbiol. 2015;305:55–64. doi: 10.1016/j.ijmm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 127.Park J, Jagasia R, Kaufmann GF, et al. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem Biol. 2007;14:1119–27. doi: 10.1016/j.chembiol.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spaulding AR, Salgado-Pabon W, Merriman JA, et al. Vaccination against Staphylococcus aureus pneumonia. J Infect Dis. 2014;209:1955–62. doi: 10.1093/infdis/jit823. •• Describes the protective efficacies of different combinations of S. aureus toxoids against different S. aureus strains in rabbits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caballero A, Foletti D, Bierdeman M, et al. Effectiveness of Alpha-toxin Fab Monoclonal Antibody Therapy in Limiting the Pathology of Staphylococcus aureus Keratitis. Ocul Immunol Inflamm. 2014;1:7. doi: 10.3109/09273948.2014.920035. [DOI] [PubMed] [Google Scholar]
- 130.Tkaczyk C, Hamilton MM, Datta V, et al. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One. 2013;8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hua L, Hilliard JJ, Shi Y, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58:1108–17. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hilliard JJ, Datta V, Tkaczyk C, et al. Anti-alpha-toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother. 2015;59:299–309. doi: 10.1128/AAC.03918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hua L, Cohen TS, Shi Y, et al. MEDI4893* Promotes Survival and Extends the Antibiotic Treatment Window in a Staphylococcus aureus Immunocompromised Pneumonia Model. Antimicrob Agents Chemother. 2015;59:4526–32. doi: 10.1128/AAC.00510-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varshney AK, Wang X, Cook E, et al. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J Biol Chem. 2011;286:9737–47. doi: 10.1074/jbc.M110.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tilahun ME, Kwan A, Natarajan K, et al. Chimeric anti-staphylococcal enterotoxin B antibodies and lovastatin act synergistically to provide in vivo protection against lethal doses of SEB. PLoS One. 2011;6:e27203. doi: 10.1371/journal.pone.0027203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim HK, Emolo C, Missiakas D, Schneewind O. A monoclonal antibody that recognizes the E domain of staphylococcal protein A. Vaccine. 2014;32:464–9. doi: 10.1016/j.vaccine.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hall AE, Domanski PJ, Patel PR, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71:6864–70. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rouha H, Badarau A, Visram ZC, et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs. 2015;7:243–54. doi: 10.4161/19420862.2014.985132. •• Describes a mAb that cross reacts with multiple leukotoxins. [DOI] [PMC free article] [PubMed] [Google Scholar]