Abstract
GLUT1, and to a lesser extent, GLUT3, appear to be interesting targets in the treatment of glioblastoma multiforme. The current review aims to give a brief history of the scientific community’s understanding of these glucose transporters and to relate their importance to the metabolic changes that occur as a result of cancer. One of the primary changes that occurs in cancer, the Warburg Effect, is characterized by an extreme shift toward glycolysis from the usual reliance on oxidative phosphorylation and is currently being investigated to target the upstream and downstream factors responsible for Warburg-induced changes. Further, it aims to explain the differential expression of GLUT1 and GLUT3 in glioblastoma tissue, and how these modulations in expression can serve as targets to restore a more normal metabolism. Additionally, hypoxia-induced factor-1α’s (HIF1α) role in a number of transcriptional changes typical to GBM will be discussed, including its role in GLUT upregulation. Finally, the four known subtypes of GBM [proneural, neural, mesenchymal, and classical] will be characterized in order to discuss how metabolic changes differ in each subtype. These changes have the potential to be selectively targeted in order to provide specificity to the clinical treatment options in GBM.
Keywords: GLUT1, GLUT3, Warburg effect, glioblastoma, hypoxia
A brief overview of the glucose transporters
In 1948, Paul LeFavre first postulated the existence of a relatively vague mechanism for glucose uptake into metabolically active cells, and that this mechanism works via facilitated diffusion rather than by passive diffusion [1]. Later, in 1981, Baldwin and colleagues reported on a specific protein transporter, (what would eventually be characterized as GLUT1) that uptakes glucose into human erythrocytes [2]. Currently, 14 GLUT transporters have been characterized in various human tissues [3], all comprising the Solute Carrier 2 (SLC2) family of transporters.
The GLUT family exhibits two unique traits that helps us understand in order to understand how cancers can “hijack” these transporters in order to survive. First, the various GLUTs are expressed in differing amounts dependent on tissue type [4]. Second, the expression levels of each GLUT are under strict transcriptional control to a large degree, contingent on metabolic demands of the tissue [5]. This second characteristic of GLUT expression makes it a very logical target for any pathological condition that involves the metabolism of glucose, as glucose influx into a cell is the first rate-limiting process involved in glucose metabolism. Cancer in particular takes advantage of a cell’s ability to generate energy from numerous pathways, and this flexibility in energy derivation is also a leading target in the development of therapeutic agents.
Prior to the discovery of glucose transporters in 1927, Otto Warburg postulated the shift in cancer cells toward glycolysis as their primary method of energy derivation in what is now known as the Warburg Effect [6]. Glycolysis is the most time-effective and least energetically demanding process by which a cell can derive additional energy, despite its lower yield of ATP when compared to that of oxidative phosphorylation [7]. Additionally, cancer utilizes the fact that glycolysis happens in the cytosol in order to continue unchecked proliferation, migration, and invasion, bypassing mitochondrial transport in the process [8]. In order to engage in the Warburg effect, the cancer cells use much more glucose than a normal cell, which harvests most of its energy from aerobic metabolism [9], and so it follows that the ability for such cells to bring glucose into their cytoplasm needs to be increased.
The upregulation of various glucose transporters, primarily GLUTs 1, 3, and 4, is observed in endometrial cancer [10], gastric cancer [11], squamous cell carcinomas [12], ovarian cancer [13], meningiomas [13], and glioblastomas [14], and in many of these cell lines where the Warburg Effect is most apparent, efforts have been made to develop therapeutic strategies that involve halting the influx of glucose into the cell [15]. This mechanism of treatment deserves to be explored more extensively, particularly in difficult-to-treat cancers with poor prognoses such as glioblastoma, due to the fact that GLUT1 contributes to the maintenance of the blood-brain barrier [16]. Specifically, GLUTs 1 & 3 have been identified as proteins that are transcriptionally upregulated in glioblastoma multiforme (GBM), and the mechanism by which these transporters are upregulated in conjunction with directly or indirectly decreasing the amount of glucose that these transporters allow into cells can lend insight to the reversal of the Warburg Effect observed in GBM tissue.
Glucose transporter 1 upregulation in hypoxic microenvironments: promoting the Warburg effect in glioblastoma
As early as 1992, differential expression of glucose transporters has been noted in various grades of glioma, with an observed upregulation of GLUT1, the most prevalent type of GLUT [17]. There was contention as to whether GLUT1 was overexpressed [17] or underexpressed [18] in GBM, but it is now believed that there are areas of both over and underexpression of GLUT1 in GBM tissue [19]. The glucose transporter’s most significant overexpression is observed in the intermediate zone of the tumor [20]. This intermediate region of the tumor is characterized as both moderately hypoxic and exhibiting the highest amounts of HIF-1α within the tumor [20].
In these regions of unchecked proliferation and low-functioning apoptotic regulation, GLUT1 is recruited via increased transcription by a number of contributing oncogenes to provide energy to regions where oxidative phosphorylation is not as viable due to a decreased availability of oxygen and/or the modulation of mitochondria by the cancerous cells. Therefore, an increase of GLUT1 is largely observed in tumor-associated vessel endothelium and within the cells proper, with a decrease in expression observed in tumor-associated fibroblasts [21]. An added result of this hypoxic environment that is created at the tumor’s core and intermediate zone is the upregulation of hypoxia-induced factor-1α (HIF-1α), which will now be discussed in relation to its cascade of effects on hypoxic tissue such as that which is seen in glioblastoma.
HIF1α’s downstream effects on GLUT transcription & GBM metabolism
Hypoxia-induced factor-1α (HIF-1α) is a protein that is highly transcribed for during times of hypoxic stress (i.e., at a tumor’s inner regions), and has wide-ranging control over how these hypoxic cells derive and convert energy in order to survive, and whether or not they differentiate from their stem-like origins. Thus, the downstream products of HIF-1α, namely, GLUT1, is also important in determining the frequency at which these tumor cells differentiate and proliferate. HIF-1α has four major effects on GBM cells. Primarily, and perhaps most importantly to this review, HIF-1α increases GLUT1 expression. GLUT1 and OCT4 transcription are activated as a response to HIF-1α in hypoxic conditions seen most highly expressed in the intermediate zone of the tumor [22]. This activation also contributes to the stem-like phenotype observed in the core zone of GBM, as GLUT1 blockades have been shown to inhibit self-renewal of cancer stem cells [23]. Additionally, the HIF-1α cascade contributes to the epithelial-to-mesenchymal transition observed in glioblastoma cells [24]. This transition is essential in the phenotypic hyperplasticity that GBM tissue exhibits. Second, HIF-1α activates transcription of vascular endothelial growth factor (VEGF) [25]. VEGF is expressed in the peripheral and intermediate zones of GBM, and have been a target in therapeutic efforts [20]. VEGF/VEGFR2 inhibitors have been shown to normalize vessels and decrease angiogenesis, but not tumorigenesis; however, the inhibition of angiogenesis in the peripheral zone of these tumors should attenuate the hyper-vascularity observed in this more normoxic region [26]. VEGF receptors have also been related to GLUT1 activity in stem cells, as VEGF receptor and calcium channel transactivation upregulate GLUT1 synthesis and trafficking to the cellular membrane, demonstrating the redundancy that exists in HIF’s ability to upregulate a cell’s glucose intake via GLUT1 [27]. Finally, HIF-1α up-regulates expression of pyruvate dehydrogenase kinase 1 (PDK1). PDK1 normally increases ATP production via glycolysis, decreases reactive oxygen species production, and decreases programmed cell death. It also typically inhibits pyruvate dehydrogenase (PDH) inhibiting the conversion of pyruvate to acetyl-coenzyme A [28]. Therefore, down-regulation of PDK1 will decrease energy availability to the cancer cells, initiating a cascade leading ultimately to apoptosis. PDK1 down-regulation is a contributing factor to the ultimate attenuation of tumorigenesis in HIF-targeted therapies. Due to these three factors, an interesting and novel approach to treating GBM would involve the inactivation of GLUT1, in conjunction with the already-established downregulation of PDK1 transcription, which has potential in inhibiting tumorigenesis, leading to an impaired cancer growth and apoptosis [29] (Figure 1).
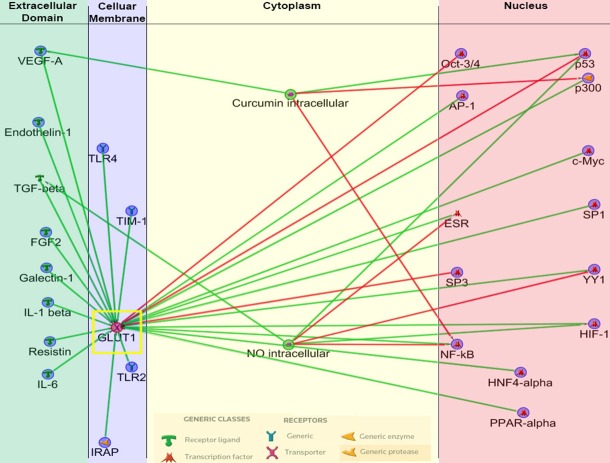
Figure 1.
Interplay of Effectors and Inhibitors on GLUT1. This is a map that depicts both GLUT1 effectors and inhibitors as well as downstream targets that GLUT1 effects or inhibits. HIF-1α and VEGF-A are notably present again, emphasizing the interplay that exists between these molecules, in addition to the tumor suppressor p53 as well as the proto-oncogene c-MYC. (Modified from Metacore, a Thomas-Reuters product).
Therapeutic strategies that target HIF-1α inhibition rely on minimizing hyper-vascularization in the periphery of the tumor via reduced VEGF transcription [30]. Simultaneously, they attempt to eliminate the Warburg Effect modulations occurring at the core and intermediate zones of the tumor by decreasing glucose availability via GLUT1 down-regulation and glycolytic activity via PDK1 down-regulation [31]. Upstream targets to stop HIF-induced transcriptional changes have also been largely tested [32]. As an example, the Ras pathway has been targeted in attempts to stop these HIF-triggered changes [33]. More recent therapies to combat the transcriptional changes induced by HIF include the silencing of the MCT4 gene, which has reduced the capacity to survive in some forms of glioblastoma, especially those with large CD133+ cell populations, to proliferate in vitro and reduced the ability of GBM to form intracranial xenografts in vivo [34]. Another gene targeted in this attempt to impair cellular responses to hypoxia is the c-MYC gene, which has been well-characterized as a major player in the HIF-mediated changes that cancer cells undergo [35]. This HIF pathway also is affected by ERK1/2, PKM2, and MDM2, which have been targeted in GBM therapies that focus on hypoxia-induced phenotypic plasticity as well [36]. HIF-1α also appears to affect transcription of GLUT3, and so there is utility in discussing this transporter as well when exploring therapeutic targets for GBM [36].
GLUT3 expression in brain tumor-initiating cells
Although much of the discussion in glucose transport in GBM revolves around GLUT1, GLUT3 also has interesting transcriptional modulation in brain cancers. GLUT3 has historically been characterized as the “brain type” glucose transporter, as it is most expressed in neurons [37]. In comparing the expression levels of GLUT1 and GLUT3 within brain tumor-initiating cells (BTICs), researchers found a positive increase of 300% from non-BTICs to BTICs in GLUT3 expression, and a lesser increase of 20% in GLUT1 [38]. Additionally, GLUT3 expression levels have been found to serve as a highly correlated factor in the determination of the clinical outcomes of those afflicted with classical and proneural subtypes of glioblastoma [38]. Moreover, the correlation between upregulation of GLUT3 and acquisition of a stem cell state has been supported by a significant increase of GLUT3 expression in induced pluripotent cells, as opposed to parental fibroblasts [38]. GLUT3 expression is upregulated during hypoxic conditions, and those cells cultured under 20% oxygen had significant reductions in GLUT3 expression when compared with cells that experienced hypoxic conditions [39]. However, this work has been met with some contradictory research, as other labs working on similar tissue showed that GLUT3 transcription is affected by neither hypoxia nor oxidative phosphorylation inhibition [40]. Finally, GLUT3 expression is found to be correlated with that of OCT4, a protein which exhibits cooperative interactions with HIF-1α and acts as a pluripotency marker [39]. Although immunocytochemistry studies from Christensen’s work suggest that GLUT3 leads to higher expression of OCT4, hinting at the possible existence of a positive feedback cycle between the proteins, more quantitative measures should be performed to confirm these results [39]. Now that both GLUT1 and GLUT3, the two main glucose transporters implicated in Warburg-resultant changes, have been well-established, it is important to look to current trends in GBM subtyping in order to determine whether GLUT-specific targeting can be accomplished to provide specificity in the disease’s patients.
Subtyping glioblastoma multiforme to create specificity in treatment
Current literature recognizes four primary subtypes of GBM: namely, proneural, neural, classical, and mesenchymal, and, as previously stated, the identification and further characterization of specific subtypes may assist with further understanding and developing treatment methods for this devastating cancer. To determine relative localizations of the various subtypes, Steed and colleagues sought to quantitatively evaluate the distance from the subventricular zone (SVZ) that each subtype tends to lie. The reason for using this zone as an origin of sorts is that within the adult brain, the SVZ is the area wherein both neural stem cells and astrocyte precursors reside [41-43]. It was found that proneural and neural subtypes of GBM are found to be in relative proximity with the SVZ, and exhibit expression patterns paralleling those of neural stem cells. In comparison, the classical and mesenchymal subtype locations are found dispersed more evenly throughout the brain and farther from the SVZ [44].
The proneural subtype, most common in younger GBM patients, is most commonly characterized by the high amounts of the proteins coded by the mutated platelet-derived growth factor receptor alpha (PDGFR) and isocitrate dehydrogenase 1 (IDH1) genes [45]. However, in samples used by Verhaak and colleagues, the PDGFR mutation was less prevalent when the IDH1 point mutations were present, suggesting IDH1’s relative importance in the determination of the proneural subtype [45]. The mutated IDH1 gene causes the production of D-2-hydroxyglutarate instead of α-ketoglutarate, preventing the continuation of cell development, and causing underdeveloped cells to accumulate and form tumors [46,47]. The normal PDGFR gene creates its namesake receptor protein, which, in turn, regulates cell growth and division. However, the mutation common in proneural cells causes overproduction of this receptor and division of immature cells [48]. Additional data analyzed by Verhaak and colleagues showed that proneural patients, when exposed to concomitant chemo-radiation treatment, showed no change in their survival rate, implying that different therapies must be used [45]. However, perhaps in part due to the younger age of the proneural patients, the survival rate was longer than the other three subtypes [45].
Neural GBM cells are most often identified by the presence of the markers: NEFL, GABRA1, SYT1 and SLC12A5 [45]. SLC12A5 codes for another member of the solute-like carrier family of transporters of which the GLUTs are members, but this particular transporter is a potassium-chloride cotransporter. These cells also contain similar mutations as the other three types, with no specific gene currently identified as having a higher or lower mutation rate than the others. According to data analyzed by Verhaak, neural GBM patients were, on average, the oldest, and displayed lower mortality rates after given aggressive treatment compared to those given non-aggressive treatment. However, the difference between the treatment groups was not adequately significant, and therefore, more data must be collected [45].
The classical subtype is identified by mutation differences in two major genes. Primarily, the EGFR gene is mutated at a higher rate than it is in the other subtypes. Secondly, there is a lack of mutation in the TP53 gene, which is mutated in the other three subtypes [45]. EGFR creates the epidermal growth factor receptor, a protein. In classical GBM, the mutation causes an increase in the expression of the gene, leading to increased division of immature cells [49]. Perhaps most relevant to the scope of this review, in other cancer types, GLUT1 upregulation appears to be coupled with increases in EGFR expression [50], which leads to the interesting question: can GLUT1 in particular be targeted in the classical subtype? The TP53 gene is a tumor suppressor, inducing apoptosis in cells with DNA that is damaged beyond repair. However, the mutation leads to a decrease in expression of the gene and lack of apoptotic behavior in cells [51]. Fortunately, current data indicates that classical subtype cell viability in vitro decreased and in vivo models in the lab showed a significant decrease in mortality after receiving aggressive treatment [45].
Finally, the mesenchymal subtype is characterized by the mutations of the NF1 and PTEN genes. Both genes are tumor suppressors, with their mutations inhibiting their expressions [45]. NF1 suppresses the tumors by regulating RAS and adenyl cyclase, both involved in cell growth and division [52]. Unexpectedly, GLUT1 was found to be “potently suppressed” when these pathways were simultaneously targeted in NF1 tumors, which has significant implications for the merits of targeting GLUT1 in this subtype [53]. PTEN tumor suppression consists of preventing activation of PDK and AKT, which, due to the lack of cell growth promoters, eventually causes apoptosis [54]. With both genes having mutations, the cell can continue to divide and proliferate uncontrollably, creating the tumors. Similar to the classical subtype, the patients analyzed by Verhaak and colleagues displayed a lower mortality rate when exposed to the more aggressive treatment, implying that this treatment should be used if the patient has a mesenchymal tumor [45].
In understanding that glioblastoma multiforme exists due to a number of different types of gene mutations as described above, it seems reasonable to believe that any given treatment targeting GLUT1 will have varying effects on the different subtypes of GBM that exist. As an example, one study looked to see whether IDH-1-mutated gliomas exhibited higher levels of GLUT1, finding that there is a very slight, but not significant, increase in GLUT1 expression in IDH-1-mutated tumors [55]. Similarly, TP53, one of the genes found to be mutated in classical subtype GBM, is heavily involved in the regulation of GLUT1 transcription as well as other glycolytic molecules involved in the Warburg-type phenotype seen in GBM [56]. In understanding the varying impact of glucose transport on treatment efficacy, GLUT inhibitors can now be introduced and explained in order to shed light on how these treatments might look from a clinical perspective.
Inhibition of glucose transporters in GBM
In order to properly target those glucose transporters found to be overexpressed in glioblastoma, it is critical that GLUT inhibitors are identified and described. Fasentin, a small-molecule inhibitor of GLUT1 characterized by Wood & colleagues in 2008, [57], has been found to directly bind to GLUT1 and thus inhibit the uptake of glucose in prostate cancer cells [58]. Furthermore, fasentin was shown to inhibit glucose uptake even when simultaneously administered with 2-deoxyglucose (2-DG), an analogue of glucose. Those patients afflicted with cerebral glioma responded well to an oral dose of 200 mg per kg of bodyweight [70]. However, 2-DG was also found to exhibit side effects similar to those found in hypoglycemia through interruption of glucose transporters. Fasentin has been shown to work by both blocking glucose influx into the cell and sensitizing the death ligand on the cell, pushing the cell toward FAS-induced apoptosis [57]. Although no literature appears to exist on fasentin being used in GBM treatment, it certainly poses as a potential therapy of interest in a combinatory treatment strategy (Table 1).
Table 1.
Direct and Indirect GLUT1 Inhibitors in Clinic
| Drug | Clinical Use | Mechanism of Action | Paper |
|---|---|---|---|
| WZB117 | Colon cancer treatment | Small-molecular inhibitor of GLUT1 | [65] |
| STF31 | Renal cell carcinoma treatment | Small-molecular inhibitor of GLUT1 | [66] |
| Resveratrol | Ovarian cancer treatment | Interrupts GLUT1 trafficking to membrane | [67] |
| Fasentin | N/A | Selective GLUT1 inhibitor/sensitizes cell to death ligand | [57] |
| Quercetin | Flavonoid (No validated clinical use) | Competitive inhibitor of GLUT1 | [59] |
| Forskalin | Flavonoid (No validated clinical use) | Competitive inhibitor of GLUT1 | [68] |
| Cytochalain B | Actin polymerization inhibitor | Competitive inhibitor of GLUT1 | [57] |
| Verapamil | Ca++ Channel Blocker | Decreases stress-induced transport activity of GLUT1 | [69] |
Above is a table of notable GLUT1 inhibitors and their general mechanisms of action, as well as where papers on their utility as GLUT1 inhibitors can be found. There are a number of flavonoids on this list, as well as already-established chemotherapeutic agents for cancer.
Additionally, flavonoids, a class of 15-carbon ringed secondary metabolites of plants and fungi, act as inhibitors of the GLUT1 transporter [59]. Quercetin is one such example of a flavonoid that inhibits the efflux of glucose from human red blood cells [59], and it has been used in the treatment of a number of cancer types, including in the treatment glioblastoma, where is has been shown to facilitate mitochondrial-mediated apoptosis [60]. Additionally, a number of labs have demonstrated that GBM cells respond more to temozolomide treatment when administered simultaneously with quercetin [61-63]. As a more potent alternative, cytochalasin B also works as an inhibitor to GLUT1 [57]. Its potency is too strong, though, as researchers found cytochalasin B to be directly toxic towards all cell lines tested.
There are other organic molecules derived from plant metabolism that seem to have similar inhibitory function on GLUT transporters. Rubusoside, a component of Chinese sweet leaf tea, proves to be one such inhibitor [64]. Recent studies have also elucidated the fact that Rubus leaf extract may provide antiangiogenic effects through a mechanism of its gallic acid. In fact, when injected intraperitoneally with 0.1% sweat tea leaf extract, researchers found a 41% inhibition of angiogenesis. The inhibition enacted, however, only partially and did not exceed 50% at non-cytotoxic doses (Figure 2).
Figure 2.
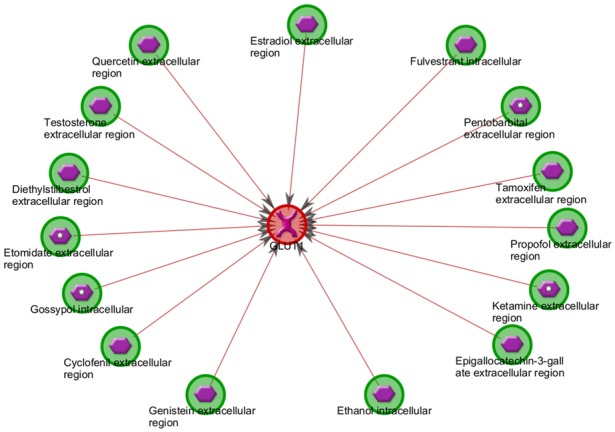
GLUT1 Inhibitors. This figure depicts known inhibitors of GLUT1 pulled from existent literature. Of note is quercetin, a flavonoid that has been used clinically in GBM. Additionally, estradiol, testosterones are notable sex hormones that inhibit GLUT1 transcription, and tamoxifen is an estrogen modulator that is used in the treatment of breast cancer. (Modified from Metacore, a Thomas-Reuters product).
Conclusion
Understanding the tenants of how glucose enters cells via facilitated diffusion is central to creating better targets for therapeutic agents in many cancer lines. In glioblastoma multiforme in particular, but certainly in many other cancers as well, the Warburg Effect plays a central role in the pleiotropic changes that occur in affected tissue. The hypoxic environment that exists at the inner regions of the tumor causes a cascade, initiated by HIF-1α, which leads to an noted increase in GLUT1 expression amongst a number of other transcriptional changes that allow the cells at this core to shift largely to glycolysis as their primary source of ATP synthesis. GLUT3 also appears to perhaps be upregulated in the hypoxic environment, but this fact has been met with contention. Regardless, the degree to which this transporter is upregulated is largely predictive of patient outcomes in clinic. Additionally, the subtyping of GBM and other cancers is essential now more than ever because of researchers and clinicians alike now have the ability to develop and implement increasingly specific therapies. By better understanding the nuanced variations in metabolism and phenotype that exist between subtypes of a given cancer, prognoses for patients will continue to improve.
Acknowledgements
We thank Christina Constantinidou for manuscript preparation. We also thank Mark Linder Walk for the Mind, Illinois Neurological Institute and OSF foundation, Peoria, IL for their funding support.
Disclosure of conflict of interest
None.
References
- 1.LeFEVRE PG. Evidence of active transfer of certain non-electrolytes across the human red cell membrane. J Gen Physiol. 1948;31:505–527. doi: 10.1085/jgp.31.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin JM, Gorga JC, Lienhard GE. The monosaccharide transporter of the human erythrocyte. Transport activity upon reconstitution. J Biol Chem. 1981;256:3685–3689. [PubMed] [Google Scholar]
- 3.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–5. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990;13:198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- 5.Neufer PD, Dohm GL. Exercise induces a transient increase in transcription of the GLUT-4 gene in skeletal muscle. Am J Physiol. 1993;265:C1597–603. doi: 10.1152/ajpcell.1993.265.6.C1597. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review) Oncol Lett. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 9.Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Hui Y, Lin L, Wu Y, Zhang X, Liu P. Clinical significance of COX-2, GLUT-1 and VEGF expressions in endometrial cancer tissues. Pak J Med Sci. 2015;31:280–284. doi: 10.12669/pjms.312.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Wen D, Fang X, Wang X, Liu T, Zhu J. p38MAPK Signaling Enhances Glycolysis Through the Up-Regulation of the Glucose Transporter GLUT-4 in Gastric Cancer Cells. Cell Physiol Biochem. 2015;36:155–165. doi: 10.1159/000374060. [DOI] [PubMed] [Google Scholar]
- 12.Abdou AG, Eldien MM, Elsakka D. GLUT-1 Expression in Cutaneous Basal and Squamous Cell Carcinomas. Int J Surg Pathol. 2015;23:447–453. doi: 10.1177/1066896915589968. [DOI] [PubMed] [Google Scholar]
- 13.van de Nes JA, Griewank KG, Schmid KW, Grabellus F. Immunocytochemical analysis of glucose transporter protein-1 (GLUT-1) in typical, brain invasive, atypical and anaplastic meningioma. Neuropathology. 2015;35:24–36. doi: 10.1111/neup.12148. [DOI] [PubMed] [Google Scholar]
- 14.Bache M, Rot S, Kessler J, Guttler A, Wichmann H, Greither T, Wach S, Taubert H, Soling A, Bilkenroth U, Kappler M, Vordermark D. mRNA expression levels of hypoxia-induced and stem cell-associated genes in human glioblastoma. Oncol Rep. 2015;33:3155–3161. doi: 10.3892/or.2015.3932. [DOI] [PubMed] [Google Scholar]
- 15.Hong SY, Hagen T. 2-Deoxyglucose induces the expression of thioredoxin interacting protein (TXNIP) by increasing O-GlcNAcylation - Implications for targeting the Warburg effect in cancer cells. Biochem Biophys Res Commun. 2015;465:838–844. doi: 10.1016/j.bbrc.2015.08.097. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–18040. [PubMed] [Google Scholar]
- 17.Nishioka T, Oda Y, Seino Y, Yamamoto T, Inagaki N, Yano H, Imura H, Shigemoto R, Kikuchi H. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992;52:3972–3979. [PubMed] [Google Scholar]
- 18.Tsukamoto H, Boado RJ, Pardridge WM. Differential expression in glioblastoma multiforme and cerebral hemangioblastoma of cytoplasmic proteins that bind two different domains within the 3'-untranslated region of the human glucose transporter 1 (GLUT1) messenger RNA. J Clin Invest. 1996;97:2823–2832. doi: 10.1172/JCI118738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pistollato F, Abbadi S, Rampazzo E, Persano L, Della Puppa A, Frasson C, Sarto E, Scienza R, D'avella D, Basso G. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28:851–862. doi: 10.1002/stem.415. [DOI] [PubMed] [Google Scholar]
- 20.Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, D’Avella D, Panchision DM, Della Puppa A, Scienza R, Basso G. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28:1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL Tumour Angiogenesis Research Group. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J. Clin. Oncol. 2006;24:4301–4308. doi: 10.1200/JCO.2006.05.9501. [DOI] [PubMed] [Google Scholar]
- 22.Rooj AK, Bronisz A, Godlewski J. The role of octamer binding transcription factors in glioblastoma multiforme. Biochim Biophys Acta. 2016;1859:805–811. doi: 10.1016/j.bbagrm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibuya K, Okada M, Suzuki S, Seino M, Seino S, Takeda H, Kitanaka C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015;6:651–661. doi: 10.18632/oncotarget.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Jiang C, Han H, Liu H, Tang M, Liu L, Ji W, Lu X, Yang X, Zhang Y, Liu Y. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKt/HIF-1alpha signalling. Clin Exp Pharmacol Physiol. 2015;42:1296–1307. doi: 10.1111/1440-1681.12488. [DOI] [PubMed] [Google Scholar]
- 25.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 26.Navis AC, Bourgonje A, Wesseling P, Wright A, Hendriks W, Verrijp K, van der Laak JA, Heerschap A, Leenders WP. Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PLoS One. 2013;8:e58262. doi: 10.1371/journal.pone.0058262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh HN, Han HJ. Fibronectin-induced VEGF receptor and calcium channel transactivation stimulate GLUT-1 synthesis and trafficking through PPARgamma and TC10 in mouse embryonic stem cells. Stem Cell Res. 2013;10:371–386. doi: 10.1016/j.scr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Velpula KK, Bhasin A, Asuthkar S, Tsung AJ. Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect. Cancer Res. 2013;73:7277–7289. doi: 10.1158/0008-5472.CAN-13-1868. [DOI] [PubMed] [Google Scholar]
- 30.Padfield E, Ellis HP, Kurian KM. Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang B, Zhu J, Wang Y, Geng F, Li G. MiR-454 inhibited cell proliferation of human glioblastoma cells by suppressing PDK1 expression. Biomed Pharmacother. 2015;75:148–152. doi: 10.1016/j.biopha.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Liu J, Mi X, Liang Y, Li J, Huang C. The N-terminal region of p27 inhibits HIF-1alpha protein translation in ribosomal protein S6-dependent manner by regulating PHLPP-Ras-ERK-p90RSK axis. Cell Death Dis. 2014;5:e1535. doi: 10.1038/cddis.2014.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum R, Jacob-Hirsch J, Amariglio N, Rechavi G, Kloog Y. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1alpha, causing glycolysis shutdown and cell death. Cancer Res. 2005;65:999–1006. [PubMed] [Google Scholar]
- 34.Lim KS, Lim KJ, Price AC, Orr BA, Eberhart CG, Bar EE. Inhibition of monocarboxylate transporter-4 depletes stem-like glioblastoma cells and inhibits HIF transcriptional response in a lactate-independent manner. Oncogene. 2014;33:4433–4441. doi: 10.1038/onc.2013.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mongiardi MP, Savino M, Falchetti ML, Illi B, Bozzo F, Valle C, Helmer-Citterich M, Ferre F, Nasi S, Levi A. c-MYC inhibition impairs hypoxia response in glioblastoma multiforme. Oncotarget. 2016 doi: 10.18632/oncotarget.8921. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagamatsu S, Sawa H, Kamada K, Nakamichi Y, Yoshimoto K, Hoshino T. Neuron-specific glucose transporter (NSGT): CNS distribution of GLUT3 rat glucose transporter (RGT3) in rat central neurons. FEBS Lett. 1993;334:289–295. doi: 10.1016/0014-5793(93)80697-s. [DOI] [PubMed] [Google Scholar]
- 38.Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, Weil RJ, Nakano I, Sarkaria JN, Stringer BW, Day BW, Li M, Lathia JD, Rich JN, Hjelmeland AB. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci. 2013;16:1373–1382. [Google Scholar]
- 39.Christensen DR, Calder PC, Houghton FD. GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic culture of human embryonic stem cells. Sci Rep. 2015;5:17500. doi: 10.1038/srep17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, Zhu M, Weldon R, Wu G, Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol. 2003;163:1873–1885. doi: 10.1016/S0002-9440(10)63546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 43.Quinones-Hinojosa A, Sanai N, Gonzalez-Perez O, Garcia-Verdugo JM. The human brain subventricular zone: stem cells in this niche and its organization. Neurosurg Clin N Am. 2007;18:15–20. vii. doi: 10.1016/j.nec.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Steed TC, Treiber JM, Patel K, Ramakrishnan V, Merk A, Smith AR, Carter BS, Dale AM, Chow LM, Chen CC. Differential localization of glioblastoma subtype: implications on glioblastoma pathogenesis. Oncotarget. 2016;7:24899–907. doi: 10.18632/oncotarget.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma S, Jiang B, Deng W, Gu ZK, Wu FZ, Li T, Xia Y, Yang H, Ye D, Xiong Y, Guan KL. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015;6:8606–8620. doi: 10.18632/oncotarget.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci U S A. 2001;98:9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K, Yamamoto H, Chin JR, Werb Z, Vu TH. Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment. J Biol Chem. 2004;279:53848–53856. doi: 10.1074/jbc.M403114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song YD, Zhang KF, Liu D, Guo YQ, Wang DY, Cui MY, Li G, Sun YX, Shen JH, Li XG, Zhang L, Shi FJ. Inhibition of EGFR-induced glucose metabolism sensitizes chondrosarcoma cells to cisplatin. Tumour Biol. 2014;35:7017–7024. doi: 10.1007/s13277-014-1902-4. [DOI] [PubMed] [Google Scholar]
- 51.Anonymous Intervertebral disc disease. Probl Vet Med. 1989;1:337–490. [PubMed] [Google Scholar]
- 52.Vizcaino MA, Shah S, Eberhart CG, Rodriguez FJ. Clinicopathologic implications of NF1 gene alterations in diffuse gliomas. Hum Pathol. 2015;46:1323–1330. doi: 10.1016/j.humpath.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malone CF, Fromm JA, Maertens O, DeRaedt T, Ingraham R, Cichowski K. Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer Discov. 2014;4:1062–1073. doi: 10.1158/2159-8290.CD-14-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 55.Metellus P, Colin C, Taieb D, Guedj E, Nanni-Metellus I, de Paula AM, Colavolpe C, Fuentes S, Dufour H, Barrie M, Chinot O, Ouafik L, Figarella-Branger D. IDH mutation status impact on in vivo hypoxia biomarkers expression: new insights from a clinical, nuclear imaging and immunohistochemical study in 33 glioma patients. J Neurooncol. 2011;105:591–600. doi: 10.1007/s11060-011-0625-2. [DOI] [PubMed] [Google Scholar]
- 56.Zawacka-Pankau J, Grinkevich VV, Hunten S, Nikulenkov F, Gluch A, Li H, Enge M, Kel A, Selivanova G. Inhibition of glycolytic enzymes mediated by pharmacologically activated p53: targeting Warburg effect to fight cancer. J Biol Chem. 2011;286:41600–41615. doi: 10.1074/jbc.M111.240812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546–3555. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 58.Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24:650–654. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin HJ, Kornmann F, Fuhrmann GF. The inhibitory effects of flavonoids and antiestrogens on the Glut1 glucose transporter in human erythrocytes. Chem Biol Interact. 2003;146:225–235. doi: 10.1016/j.cbi.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Kim H, Moon JY, Ahn KS, Cho SK. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496. doi: 10.1155/2013/596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sang DP, Li RJ, Lan Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014;35:832–838. doi: 10.1038/aps.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jakubowicz-Gil J, Langner E, Badziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34:2367–2378. doi: 10.1007/s13277-013-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pozsgai E, Bellyei S, Cseh A, Boronkai A, Racz B, Szabo A, Sumegi B, Hocsak E. Quercetin increases the efficacy of glioblastoma treatment compared to standard chemoradiotherapy by the suppression of PI-3-kinase-Akt pathway. Nutr Cancer. 2013;65:1059–1066. doi: 10.1080/01635581.2013.810291. [DOI] [PubMed] [Google Scholar]
- 64.George Thompson AM, Iancu CV, Nguyen TT, Kim D, Choe JY. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci Rep. 2015;5:12804. doi: 10.1038/srep12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Fang Y, Wang XT, Liu J, Dan X, Sun LL. Overcoming 5-Fu resistance of colon cells through inhibition of Glut1 by the specific inhibitor WZB117. Asian Pac J Cancer Prev. 2014;15:7037–7041. doi: 10.7314/apjcp.2014.15.17.7037. [DOI] [PubMed] [Google Scholar]
- 66.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gwak H, Haegeman G, Tsang BK, Song YS. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol Carcinog. 2015;54:1529–1540. doi: 10.1002/mc.22227. [DOI] [PubMed] [Google Scholar]
- 68.Sergeant S, Kim HD. Inhibition of 3-O-methylglucose transport in human erythrocytes by forskolin. J Biol Chem. 1985;260:14677–14682. [PubMed] [Google Scholar]
- 69.Louters LL, Stehouwer N, Rekman J, Tidball A, Cok A, Holstege CP. Verapamil inhibits the glucose transport activity of GLUT1. J Med Toxicol. 2010;6:100–105. doi: 10.1007/s13181-010-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh D, Banerji AK, Dwarakanath BS. Optimizing cancer radiotherapy with 2-deoxy-D-glucose. Strahlenther Onkol. 2005;181:507–514. doi: 10.1007/s00066-005-1320-z. [DOI] [PubMed] [Google Scholar]