Abstract
Drug resistance is one of the main hurdles for the successful treatment of hepatic carcinoma. However, the detailed mechanisms underlying resistance remain largely unknown and therapeutic approaches are limited. In the present study, we show that miR-122 confers resistance to 5-fluorouracil induced hepatocellular carcinoma cell apoptosis in vitro and reduces the potency of 5-fluorouracil in the inhibition of tumor growth in a mouse xenograft model in vivo. Further studies indicate that miR-122 modulates drug resistance through down-regulation of expression of PCDH20, which belongs to the protocadherin gene family and negatively regulates Akt activation. Knockdown of PCDH20 expression increases Akt phosphorylation, which leads to elevated mTOR activity and enhanced 5-fluorouracil resistance; whereas rescue of PCDH20 expression in miR-122-expressing cells decreases Akt and mTOR phosphorylation, re-sensitizing hepatocellular carcinoma cell to 5-fluorouracil induced apoptosis. Moreover, a specific and potent Akt inhibitor reverses miR-122-conferred 5-fluorouracil resistance. These findings indicate that the miR-122/PCDH20/Akt/mTOR signaling axis has an important role in mediating response to chemotherapy in human hepatocellular carcinoma. A major implication of our study is that inhibition of miR-122 or restoration of PCDH20 expression may have significant therapeutic potential to overcome drug resistance in hepatocellular carcinoma and that the combined use of an Akt inhibitor with 5-fluorouracil may increase efficacy in liver cancer treatment.
Keywords: PCDH20, microRNA-122, 5-FU, hepatic carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver, and the leading cause of cancer-related mortality around the world [1]. Although the main etiologies of HCC are now well defined, the molecular mechanisms involved in tumor initiation and progression have yet to be fully characterized. During the initiation and progression of hepatocarcinogenesis, multiple genetic and epigenetic events accumulated, leading to deregulated expression of various genes [2]. 5-Fluorouracil (5-FU) is one of the chemotherapeutic drugs most widely used alone or combined with other drugs in liver cancer treatment [3]. 5-FU primarily interrupts synthesis of the pyrimidine thymidine, a nucleoside required for DNA replication, by blocking the activity of thymidylate synthase. Consequently, 5-FU induces cell cycle arrest and/or apoptosis in cancer cells [4]. Although adjuvant 5-FU treatment has yielded a good success rate, the failure of treatment in bulk of patients with HCC is due to drug resistance [5]. Many mechanisms have been suggested to be responsible for drug resistance, including blocking apoptosis. Although resistance to chemotherapy is one of the biggest obstacles for effective cancer therapy, no significant advance has been made to identify targets overcoming drug resistance [6].
MicroRNAs (miRNAs), which are a class of small (about 22 bps) non-coding regulatory RNA molecules, provide functions essential for diverse biological processes in cancer [7]. Currently, a number of miRNAs has been described which may have a specific role in cancer pathogenesis, biological and clinical disease behavior as well as in modulating response to anti-cancer treatments [8]. To date, more and more reports have indicated that alternations in miRNA expression have been associated with 5-FU drug resistance and may function as crucial mediators of resistance (for example, the miR-200 family, miR-124, and miR-122) [9]. In fact, miRNA-based anti-cancer therapies are being developed, either alone or in combination with targeted therapies, with the goal to improve disease response and increase patient survival. MiR-122 is the most abundant miRNA (constituting 70% of the total miRNA population) in the liver and is implicated in different physiological and pathological processes in the liver, including hepatitis C virus replication, lipid metabolism, and HCC development [10]. For examples, miR-122 represses hepatitis B virus (HBV) replication, and is decreased in the livers of HBV-positive patients [11]. More recently, it has been reported that miR-122 regulates tumorigenesis in hepatocellular carcinoma by targeting serine/threonine kinase (Akt) [12]. However, little is known about the role of miR-122 in hepatocellular carcinoma patients’ drug resistance. Because of many important roles of miR-122, it is necessary to further understand its regulatory mechanisms in drug resistance.
Protocadherin-20 (PCDH20), also known as PCDH13, is located at 13q21.2 and belongs to the δ subgroup of nonclustered protocadherin gene family [13]. PCDH20 has attracted attention for their implications in the tumourigenesis. It has been reported that PCDH20 was frequently silenced by promoter methylation in non-small cell lung cancers [14]. Restoration of PCDH20 expression in NSCLC cells reduced cell numbers in colony formation and anchorage-independent assays. PCDH20 was frequently silenced by promoter methylation in non-small-cell carcinoma (NSCLC) cancers, suggested that it might function as a candidate tumor suppressor. Furthermore, PCDH20 functions as a tumor-suppressor gene through suppressing Akt activities and promoting glycogen synthesis kinase-3β (GSK-3β) signaling activities in hepatocellular carcinoma [15]. Till now, the functions of PCDH20 in HCC remain elusive.
In this study, we have discovered a novel miR-122/PCDH20/Akt/mTOR signaling axis that mediates the response of hepatoma carcinoma cell to 5-FU treatment. Our results show that miR-122 expression is suppressed by 5-FU treatment in the sensitive but not resistant hepatoma carcinoma cells. MiR-122 confers resistance to 5-FU-induced apoptosis through the inhibition of PCDH20 expression, which is a direct target of miR-122. Knock-down of PCDH20 confers 5-FU resistance in liver cancer cells, mimicking miR-122 effect. Inhibition of miR-122 expression or rescue of PCDH20 expression in hepatoma cells increases their sensitivity to 5-FU treatment. Additionally, an Akt inhibitor re-sensitizes miR-122 over-expressing cells to 5-FU. Moreover, experiments in tumor xenograft mouse models reveal that miR-122 significantly reduces the effectiveness of 5-FU in the inhibition of tumor growth. Our studies have identified miR-122 as a potential target for drug resistance in hepatic cancer and suggest that modulating the PCDH20/Akt/mTOR axis may have benefits against 5-FU resistance.
Materials and methods
Cell lines and reagents
The human hepatoma carcinoma cell SNU-449, MHCC97, HepG2 and SMMC-7721 cell lines were cultured in indicated medium. Cells were maintained at 37°C in a humidified incubator with 5% CO2. 5-FU and GSK690693 were purchased from Selleckchem (Houston, TX, USA), respectively. Anti-hsa-miR-122 miScript miRNA inhibitor was purchased from Qiagen Inc. (Valencia, CA, USA).
One solution cell proliferation assay and apoptosis assays
Tumor cells viability was determined by CellTiter 96® Aqueous One Solution cell proliferation assay (Promega, Madison, USA). Briefly, cells were seeded in 96-well cell culture plates and treated with indicated agents. After incubation for indicated time, 20 μL of One Solution reagent were added to each well and incubation was continued for 4 h. The absorbance was measured at 490 nm using Synergy™ HT Multi-Mode Microplate Reader (Bio-Tek, Winooski, VT, USA). The effect of indicated agents on cell viability was assessed as the percent of cell viability compared with vehicle-treated control cells, which were arbitrarily assigned 100% viability [16]. Apoptosis was detected using a DNA fragmentation ELISA kit (Roche, Indianapolis, IN, USA) [17].
Western blot analysis
Cell lysates were prepared in RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), 5 mM EDTA and a protease inhibitor cocktail (Sigma)). Equivalent amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). Subsequently, the membranes were blocked with 5% fat-free dry milk in Tris-buffered saline containing 0.1% Tween-20, and then incubated separately with primary antibodies at 4°C overnight. After incubation with horseradish peroxidase-conjugated secondary antibodies for 2 h, proteins were detected using chemiluminescence detection reagents (Pierce, Rockford, IL). Antibodies were purchased as indicated: anti-PCDH20 (Santa Cruz, CA, USA); anti-PARP (Santa Cruz, CA, USA), anti-cleaved PARP (Santa Cruz, CA, USA), anti-Akt (Santa Cruz, CA, USA), anti-pAkt (T308) (Santa Cruz, CA, USA), anti-pAkt (S473) (Santa Cruz, CA, USA), anti-mTOR (Santa Cruz, CA, USA), anti-pmTOR (Ser2481) (Santa Cruz, CA, USA), anti-pmTOR (Ser2448) (Santa Cruz, CA, USA) and β-tublin (Abcam, Cambridge, MA, USA).
RT-PCR and real-time Q-PCR
For RT-PCR, 2 μg of RNA was reverse-transcribed with M-MLV reverse transcriptase (Promega, Madison, WI, USA) using a random primer. cDNA (2 ul) product was used to amplify human pri-miR-122, pre-miR-122 and β-actin. Primer sequences for pri-miR-122 were 5’-GCAGATAAGGAGGAGCTTCA-3’ (forward) and 5’-AAGAGTCACCGGTCACAGGA-3’ (reverse), for pre-miR-122 were 5’-TGGAAAGTCCATTCCTCTGC-3’ (forward) and 5’-TGGACTTTCCAATCTTGCTG-3’ (reverse), and for β-actin were 5’-TGACGGGGTCACCCACACTGTGCCCAT-3’ (forward) and 5’-CTAGAAGCATTTGCGGTGGACGATGGAGG-3’ (reverse). Expression of miR-122 was determined by miScript primer assays and miScript SYBR Green PCR Kit from Qiagen Inc. The U6 endogenous control was used for normalization.
Luciferase assays
Oligos with sequences of PCDH20 3’-UTR containing predicted recognition sites of miR-122 were synthesized and cloned into a Promega psiCHECK-2 vector downstream of Renilla reporter gene to generate psiCHECK2-PCDH20. The seed sequences of miR-122 recognition sites were mutated to generate psiCHECK2-PCDH20-MUT as negative controls. The psiCHECK2 vector contains a second reporter gene, firefly luciferase, and is designed for endpoint lytic assays [18]. The reporters were transfected into cells using Lipofectamine LTX (Life Technologies). Luciferase activity was measured 48 h later using Dual-Luciferase Reporter Assay (Promega). Values were normalized with firefly luciferase activity.
Plasmid construction and lentiviral infection
cDNA encoding human miR-122 precursor (~300 bp) was cloned into pCDH-CMV lentiviral vector purchased from System Biosciences (SBI, Mountain View, CA, USA). PCDH20 lentiviral expression vector was purchased from Cell Biolabs, Inc. (San Diego, CA, USA). Packaging cells (293T) were co-transfected with pPackH1 packaging plasmid mix (SBI) and the lentiviral vectors using Fugene HD (Promega). Viruses were harvested 48 h later to infect target cells.
Knockdown assay
siRNAs used to knock down PCDH20 expression were purchased from RiboBio Co., Ltd. (Guangzhou, China). The target sequences for siRNAs are GGAAGCUAGTCAGCUTAACA (siRNA #1) and ACCCGTGGUATTGAUAUUCAA (siRNA #2). Transfections were performed using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instruction [19].
In vivo xenograft model
Experiments involving animals were approved by the Anhui Medical University Animal Care and Use Committee. HepG2 cells (2 × 106) expressing miR-122 or empty vector were injected into the flank of male athymic nude mice (10 mice per group). On the seventh day, mice with appropriate size (100-150 mm3) of tumors were divided randomly into four groups including vehicle-treated group and 5-FU dosage groups. Tumor volume and mice body weight were measured every 3 days. Tumor volumes were calculated from caliper measurements by using the following ellipsoid formula: (D × d2)/2, in which D represents the large diameter of the tumor, and d represents the small diameter [20].
Immunohistochemical staining
After sacrificing mice on day 25, formalin-fixed paraffin-embedded tissue blocks of tumors were incubated with the primary antibody against TUNEL and Ki67 (ZSGB-BIO, Beijing, China), followed by a peroxidase-conjugated secondary antibody. Three tumors from each group were analyzed. The histologically similar fields were randomly selected from each slide for analysis. Apoptosis and proliferation of tumor cells was determined quantitatively by counting the numbers and calculating the percentage of positively stained cells for TUNEL and Ki67 at × 20 magnification, respectively [21].
Statistical analysis
The data were presented as mean ± SD. Differences in the results of two groups were evaluated using either two-tailed Student’s t test or one-way ANOVA followed by post hoc Dunnett’s test. The differences with P < 0.05 were considered statistically significant.
Results
MiR-122 mediates resistance to 5-FU-induced apoptosis in hepatic carcinoma cells
Although 5-FU based adjuvant chemotherapy is one of the most important chemotherapeutic agents for liver cancer treatment, the intrinsic or acquired 5-FU resistance in the course of treatment has been a major obstacle. To exploration the molecular basis of 5-FU resistance, we determined 5-FU effect in a panel of hepatic cancer cell lines by one solution cell proliferation assay, in which 5-FU decreased cell viability both in a dose-dependent and time-dependent manner. As shown in Figure 1A, 5-FU had a much more pronounced effect on SNU-449 and MHCC97 cells proliferation than on HepG2 and SMMC-7721 cells. Similarly, SNU-449 and MHCC97 cells displayed much higher sensitivity to 5-FU induced apoptosis than HepG2 and SMMC-7721 cells as demonstrated by PARP cleavage (Figure 1B) and DNA fragmentation assays (Figure 1C). To identify the molecular determinants of differential 5-FU sensitivity, we evaluated the contribution of miR-122 owing to the relationship between miR-122 dys-regulation and cancer resistance has attracted increasing attention. As expected, the expression of miR-122 was significantly suppressed by 5-FU in highly sensitive SNU-449 and MHCC97 cells, but not in relatively resistant HepG2 and SMMC-7721 cells (Figure 1D). In addition, the expression of both pri-miR-122 and pre-miR-122 were inhibited by 5-FU in SNU-449 and MHCC97 cells (Figure 1E), suggesting that 5-FU might regulate miR-122 expression at the transcriptional level.
Figure 1.
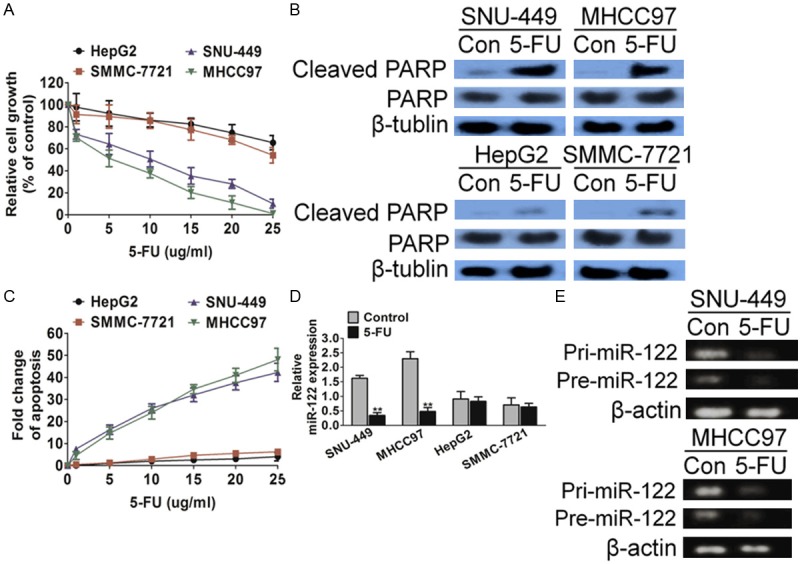
Hepatic carcinoma cells show differential responses to 5-FU treatment. A. SNU-449, MHCC97, HepG2 and SMMC-7721 cells were treated with 5-FU. MTT assays showed different sensitivity of those cells to 5-FU treatment. B. SNU-449, MHCC97, HepG2 and SMMC-7721 cells were treated with 5-FU. DNA fragmentation assays showed different sensitivity of those cells to 5-FU treatment. C. Western blot analysis showed that cleaved PARP was higher in SNU-449 and MHCC97 cells than in HepG2 and SMMC-7721 cells after 5-FU treatment. D. Determined by real time Q-PCR analysis, miR-122 expression in SNU-449 and MHCC97 cells, which are relatively sensitive to 5-FU-induced apoptosis, was significantly reduced after exposure to 5-FU. E. RT-PCR analysis showed 5-FU inhibited the expression of pri-miR-122 and pre-miR-122. The data are presented as the Mean ± S.D. of triplicate experiments. *P < 0.05, **P < 0.01 compared to control cells.
Confirming miR-122 confers drug resistance to 5-FU-induced apoptosis
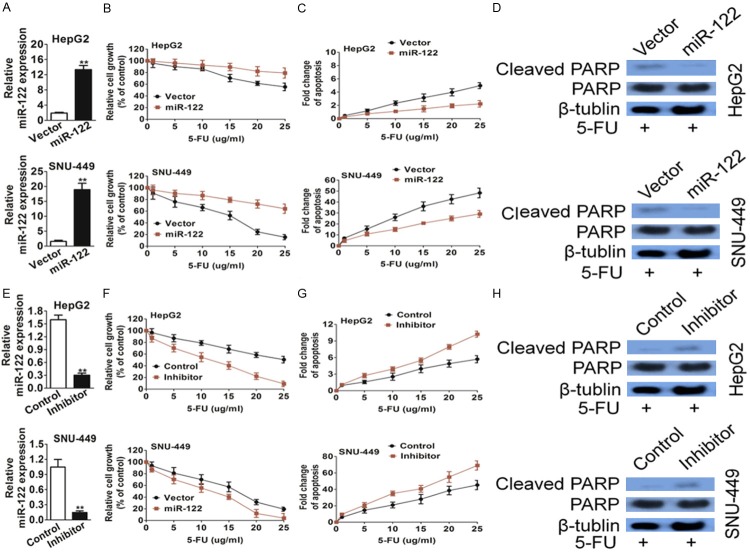
To affirm whether miR-122 contributes to 5-FU resistance, miR-122 was ectopically expressed in HepG2 and SNU-449 cells, which resulted in miR-122 expression increasing (Figure 2A). Consequently, miR-122 over-expressing cells shown increased cell viability (Figure 2B) and decreased apoptosis (Figure 2C) in response to 5-FU compared with vector transfection cells. Consistently, 5-FU-induced PARP cleavage was reduced in miR-122 over-expressing cells as compared with vector control cells (Figure 2D).
Figure 2.
MiR-122 confers resistance to 5-FU treatment in hepatic carcinoma cells in vitro. (A) miR-122 expression was increased in HepG2 and SNU-449 cells as compared with vector control, and ectopically expressing miR-122 as determined by quantitative real-time PCR. The data are presented as the Mean ± S.D. of triplicate experiments. **P < 0.01 compared to vector cells. (B) miR-122- and vector-expressing cells were treated with increasing concentrations of 5-FU for 72 h. MTT assays showed sensitivity of cells to 5-FU treatment. (C) DNA fragmentation assays showed sensitivity of those cells to 5-FU treatment. (D) Western blot analysis showed that cleaved PARP was higher in miR-122-expressing cells than vector control cells after 5-FU treatment. (E) An miR-122 inhibitor decreased miR-122 expression in HepG2 and SNU-449 cells as determined by quantitative real-time PCR. The data are presented as the Mean ± S.D. of triplicate experiments. **P < 0.01 compared to control cells. (F and G) The miR-122 inhibitor-treated or control cells were subjected to increasing concentrations of 5-FU. MTT assays (F) and DNA fragmentation assays (G) showed increased sensitivity to 5-FU-induced apoptosis in the inhibitor-treated cells as compared with the control cells. (H) Western blot analysis showed that cleaved PARP was lower in miR-122-expressing cells than control cells after 5-FU treatment.
Complementarily, an anti-hsa-miR-122 miScript miRNA inhibitor was used to inhibit miR-122 expression. As shown in Figure 2E, the inhibitor decreased miR-122 effectively in both HepG2 and SNU-449 cells. As a result, the inhibitor sensitized HepG2 and SNU-449 cells to 5-FU (Figure 2F) and increased apoptosis (Figure 2G). Consistently, 5-FU-induced PARP cleavage was increased in miR-122 down-expressing cells as compared with control cells (Figure 2H). Taken together, these results indicated that miR-122 confers resistance to 5-FU-induced apoptosis in hepatic carcinoma cells.
MiR-122 reduces the efficiency of 5-FU-induced inhibition of tumor growth in vivo
To determine whether miR-122 conferred resistance to 5-FU-induced apoptosis in vivo, a xenograft model of hepatic carcinoma was conducted. HepG2 cells transfected with the control vector or miR-122 were implanted subcutaneously into athymic nude mice for tumor growth and mice were randomly segregated into five groups when tumor grown to reach a size larger than 100 mm3. Two group was treated with 5-FU (40 mg/kg per day) administered by intraperitoneal injection (i.p.) injection and the other with vehicle. Compared with vector control, miR-122 tumors showed considerably smaller reduction of tumor size (Figure 3A and 3B) after 5-FU administration. In addition, tumor growth-curves showed that miR-122 conferred HepG2 hepatocellular carcinoma growth advantage as compared with vector control (Figure 3C). Consistent with in vitro results, miR-122 triggered a marked difference in the response to 5-FU. Tumors with vector-transfection cells were sensitive to 5-FU and failed to growth, whereas tumors with miR-122-expressing cells were relatively resistant to 5-FU and continued to grow at a steady rate during drug treatment. These results demonstrated that 5-FU was much less potent in the inhibition of miR-122-expressing hepatoma carcinoma tumor growth.
Figure 3.
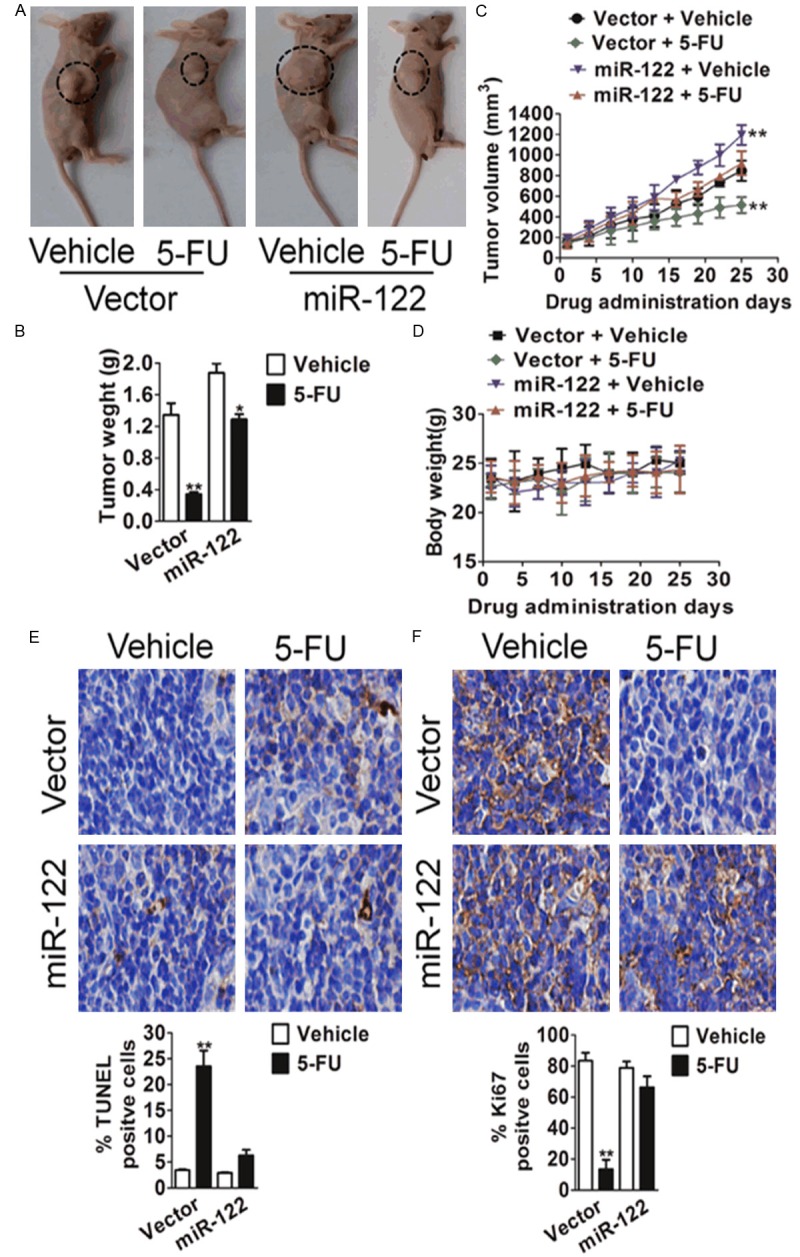
MiR-122 decreases the effectiveness of 5-FU in the inhibition of tumor growth in vivo. (A) Representative mice with tumor xenografts. Scale bars, 25 μm. (B) MiR-122 decreases the effectiveness of 5-FU in the inhibition of tumor weight in vivo. Values represent means ± SD, n = 6, *P < 0.05, **P < 0.01 versus vehicle group. (C) Xenograft tumor growth curves. Values represent means ± SD, n = 6, **P < 0.01 versus vector + vehicle group. (D) Body weight changes in 5-FU and vehicle treated mice. There was no significant difference in body weight between 5-FU and vehicle treated group. Values represent means ± SD, n = 6. (E and F) Images of TUNEL (E) and Ki67 (F) staining of tumors are shown in the left panels. The images are representative of multiple fields of tumor sections from each group. Percentage of positive TUNEL and Ki67 staining cells were determined. The data are presented as the mean ± S.E. *P < 0.05, **P < 0.01 versus vehicle group.
To future determine miR-122-mediated resistance to 5-FU induced apoptosis in vitro was associated with 5-FU inhibited tumor growth in vivo, TUNEL method was performed to detect the apoptotic index of tumors. TUNEL staining showed that the proportion of apoptotic cells was similar in miR-122 and vector tumors from mice un-treated with 5-FU. However, the increase of apoptotic cells triggered by 5-FU treatment was considerably lower in miR-122 tumors than that in vector tumors (Figure 3E). Moreover, Ki67 staining indicated that miR-122 tumors have slightly more proliferative cells than vector tumors in the absence of 5-FU (Figure 3F). In addition, 5-FU treatment decreased the percentage of proliferative cells in miR-122 tumors by 17% and in vector tumors by 66%. Taken together, these findings in vitro and in vivo reveal the critical role of miR-122 in drug resistance of hepatic carcinoma.
MiR-122 mediates 5-FU resistance by targeting gene PCDH20
To search for target genes of miR-122 and further understand molecular mechanism of miR-145 mediates 5-FU resistance, several algorithms including, TargetScan, PicTar and miRanda-mirSVR were applied to predict the targets of miR-122. The candidate target genes were predicted based on the representation of miR-122 recognition sites in their 3’-UTRs. Among those examined, we found that protocadherin 20 (PCDH20), which acting as a candidate tumor-suppressor gene could be the potential targets of miR-122 (Figure 4A). To determine whether PCDH20 is the direct target of miR-122, oligos representing two predicted recognition sites of miR-122 in the 3’-UTR of PCDH20 were synthesized and cloned into the luciferase reporter construct psiCHECK2 (designated psiCHECK2-PCDH20-WT), whereas oligos with seed sequences of the recognition sites mutated were also cloned into the same construct (designated psiCHECK2-MUT), which were used as controls. Luciferase assays showed that the luciferase activity of psiCHECK2-PCDH20-WT was decreased in miR-122-expressing cells as compared with vector control cells, whereas that of the control plasmid, psiCHECK2, and of the mutant construct, psiCHECK2-MUT, remained unchanged (Figure 4B). In addition, PCDH20 showed decreased expression in HepG2 and SNU-449 cells expressing miR-122 as compared with vector cells (Figure 4C). On the other hand, the miR-122 inhibitor increased PCDH20 expression in both cell types (Figure 4D). These results indicate that miR-122 represses the 3’-UTR of PCDH20 and that the expression of PCDH20 is directly regulated by miR-122.
Figure 4.

PCDH20 is a direct target of miR-122. A. Sequence of the miR-122 binding sites within the human PCDH20 3’-UTR and a schematic of the reporter construct shown the PCDH20 3’-UTR sequence and the mutated PCDH20 3’-UTR sequence. B. Luciferase activity of the PCDH20 reporter in the presence of miR-122 or scramble. Data were collected from three independent experiments and were average ± SD. values. **P < 0.01, compared to scramble transfected cells. C. PCDH20 expression was decreased and Akt phosphorylation and mTOR activity were increased by miR-122 expression in HepG2 and SNU-449 cells as determined by western blot analysis. D. The miR-122 inhibitor increased PCDH20 expression and suppressed Akt phosphorylation and mTOR activity, determined by western blot analysis.
Confirming PCDH20 is required for 5-FU-induced apoptosis
PCDH20 has been characterized as a tumor suppressor. PCDH20 is a novel protocadherin which is expressed predominantly in nervous system and has also attracted attention for their implications in the neurological disorders and tumourigenesis. To determine whether PCDH20 modulates 5-FU-induced apoptosis, its expression was knocked down by two independent siRNAs, which demonstrated robust effect in HepG2 and SNU-449 cells and resulting in the reduction of PCDH20 expression (Figure 5A). Knockdown of PCDH20 expression enhanced the tolerance of HepG2 and SNU-449 cells to 5-FU-induced apoptosis as demonstrated by increased cell viability (Figure 5B) and reduced apoptosis (Figure 5C). Consistently, 5-FU-induced PARP cleavage was reduced in PCDH20 down-expressing cells as compared with control cells (Figure 5D). These results indicated that PCDH20 is required for 5-FU-induced apoptosis, suggesting that miR-122 may increase 5-FU resistance through the inhibition of PCDH20 expression.
Figure 5.
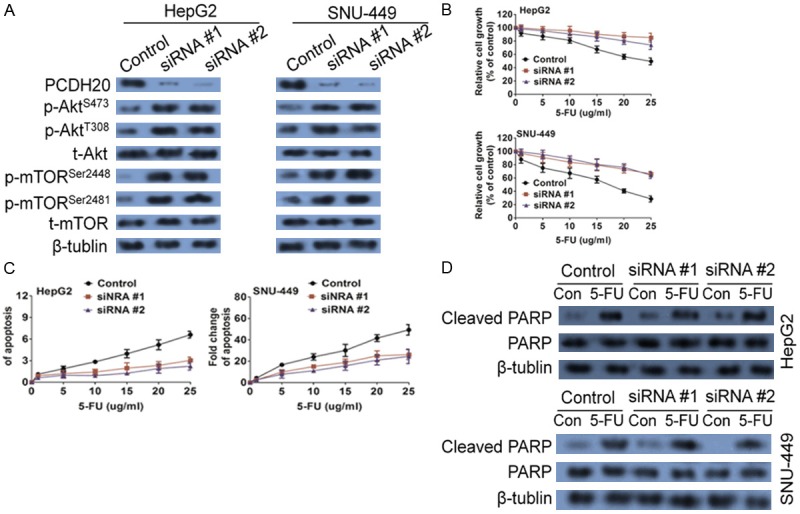
PCDH20 expression is required for 5-FU-induced apoptosis. (A) Expression of PCDH20 was knocked down by siRNAs in HepG2 and SNU-449 cells, resulting in the up-regulation of Akt phosphorylation and mTOR phosphorylation. PCDH20 knockdown and control cells were treated with 5-FU. MTT assays (B), DNA fragmentation assays (C) and western blot analysis of cleaved PARP (D) were performed, all of which indicated that knockdown of PCDH20 expression conferred resistance to 5-FU-induced apoptosis.
To determine whether miR-122 mediated resistance to 5-FU-induced apoptosis could be reversed by restoration of PCDH20. PCDH20 cDNA was introduced into miR-122-expressing HepG2 and SNU-449 cells. Ectopically expressed PCDH20 is resistant to down-regulation mediated by miR-122 owing to the lack of 3’-UTR (Figure 6A). Restoration of PCDH20 expression almost completely restored the sensitivity of HepG2 and SNU-449 cells to 5-FU-induced apoptosis (Figure 6B) and apoptosis (Figure 6C). These results indicate that miR-122 enhances 5-FU resistance through the down-regulation of PCDH20 expression.
Figure 6.
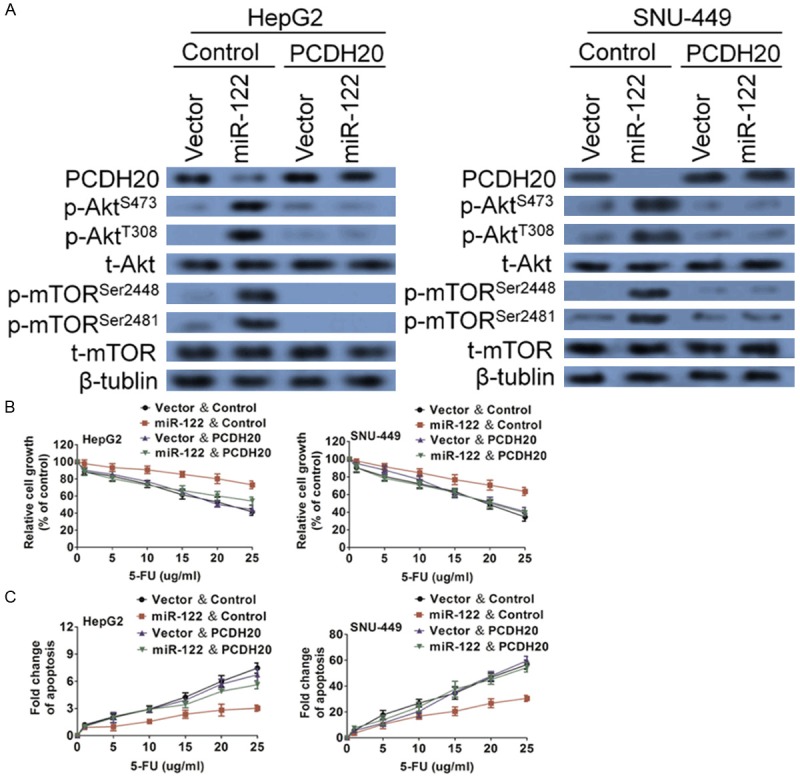
Confirming PCDH20 is required for 5-FU-induced apoptosis. (A) PCDH20 was ectopically expressed in vector- and miR-122-expressing cells. Western blot analysis was performed to confirm the rescued expression of PCDH20. As a result, Akt and mTOR phosphorylation were inhibited. Cells were treated with increasing concentrations of 5-FU. MTT assays (B) and DNA fragmentation assays (C) showed that restoration of PCDH20 expression re-sensitized miR-122-expressing cells to 5-FU treatment. The data are presented as the mean ± S.D. of triplicate experiments.
Akt activation mediated by PCDH20 contributes to miR-122-conferred 5-FU resistance
It has been shown that Akt is an anti-apoptotic factor and that its activation is negatively regulated by the PP2A complex through de-phosphorylation of Akt. One of the anti-apoptotic effectors regulated by Akt is mTOR. To determine the underlying mechanisms of miR-122-mediated 5-FU resistance, we examined Akt/mTOR signaling and found that miR-122 increased Akt phosphorylation and mTOR phosphorylation in both cell types (Figure 4C). Moreover, the miR-122 inhibitor suppressed Akt phosphorylation and reduced mTOR activity (Figure 4D). In addition, the knockdown of PCDH20 expression significantly increased Akt phosphorylation and Akt activation, as reflected by the phosphorylation of Akt and enhanced mTOR activity (Figure 5A). In contrast, restoration PCDH20 expression inhibited Akt phosphorylation and mTOR activation (Figure 6A). These results support the possible involvement of Akt/mTOR in miR-122 mediated 5-FU resistance.
To confirm that Akt contributes to miR-122-mediated 5-FU resistance, a specific and potent Akt inhibitor, GSK690693, was used to block Akt activation and function. Although GSK690693 had little effect on both Akt and mTOR phosphorylation in vector control cells, it significantly repressed Akt phosphorylation and mTOR phosphorylation in miR-122-expressing cells (Figure 7A). As a result, it diminished miR-122-conferred 5-FU resistance in both HepG2 and SNU-449 cells, as demonstrated by cell viability (Figure 6B) and apoptosis (Figure 7C) assays. Taken together, these results indicate that miR-122 mediates resistance to 5-FU-induced apoptosis through regulating the PCDH20/Akt/mTOR signaling axis.
Figure 7.
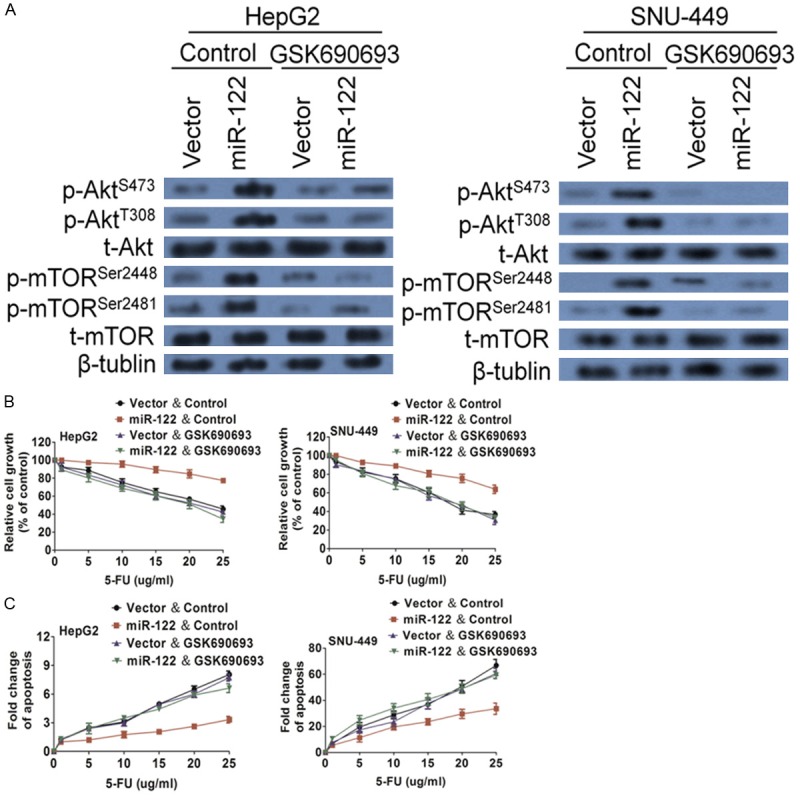
Akt inhibitor sensitizes miR-122-expressing cells to 5-FU-induced apoptosis. (A) Akt phosphorylation and mTORphosphorylation were downregulated by GSK690693 in miR-122-expressing cells as examined by western blot analysis. HepG2 and SNU-449 cells were treated with GSK690693 together with 5-FU. MTT assays (B) and DNA fragmentation assays (C) were performed, which showed that GSK690693 re-sensitized miR-122-expressing cells to 5-FU treatment. The data are presented as the mean ± S.D.
Discussion
Hepatocellular carcinoma (HCC) is one of the most common highly lethal tumors, and the prognosis of HCC patients is startling low unless the disease is diagnosed early [22]. Beyond physical approaches such as radiation and surgery, few therapies are recognized, and a short list of expensive candidate drugs that display a high failure rate exists. 5-FU is a common first-line chemotherapeutic drug for the treatment of HCC [23]. However, the development of acquired resistance to 5-FU confines its clinical usages. In this study, we have identified a novel miR-122/PCDH20/Akt/mTOR signaling axis that regulates the response of liver cancer cells to 5-FU treatment. Ectopic expression of miR-122 enhances 5-FU resistance in vitro and in tumor xenografts in vivo. An miR-122 inhibitor sensitizes hepatic cancer cells to 5-FU treatment. MiR-122 promotes drug resistance by the inhibition of PCDH20 expression, which leads to increased Akt and mTOR activation, and PCDH20 is required for 5-FU-induced apoptosis. Despite advances in chemotherapy, drug resistance is still one of the biggest obstacles for treating advanced hepatic carcinoma effectively. Therefore, the discovery of miR-122 as a contributing factor of drug resistance and the identification of the regulatory miR-122/PCDH20/Akt/mTOR signaling axis may facilitate to design strategies to increase the overall efficacy of chemotherapy of HCC.
Our discovery of miR-122 as a mediator of 5-FU response in hepatic cancer provides a potential therapeutic target. Inhibition of miR-122 expression could increase liver cancer cells 5-FU sensitivity. Interestingly, we observed that the basal level of miR-122 expression is higher in SNU-449 and MHCC97 cells than in HepG2 and SMMC-7721 cells in the absence of 5-FU. In fact, miR-122-expressing cells displayed a higher growth rate both in vitro and in vivo than vector-expressing cells without 5-FU treatment. These results indicate that, in addition to 5-FU treatment, miR-122 has a pro-proliferative and pro-survival role. However, 5-FU decreases miR-122 expression in 5-FU-sensitive SNU-449 and MHCC97 cells but not in its resistant HepG2 and SMMC-7721 cells, which leads to lower miR-122 expression in SNU-449 and MHCC97 cells than in HepG2 and SMMC-7721 cells after 5-FU treatment. All this contributes to cells differential 5-FU response. Therefore, the sensitivity to 5-FU-induced apoptosis is dependent upon the ability of 5-FU to suppress miR-122 expression.
Protocadherin (PCDH) gene functions have predominantly been studied in neuronal cells, although PCDH expression is also observed in other cells including gut, lung and kidney [24]. PCDHs have extracellular cadherin domains involved in cell adhesion and intracellular domains, which can translocate to the nucleus and regulate gene expression [25]. Although little is known about protocadherin molecular functions in relation to tumor biology, the diverse and complex cellular activities they mediate likely impinge on key cellular pathways involved in cell death and proliferation. A few members of the protocadherin family have been suspected of involvement in the carcinogenesis of several tumors, such as colon cancer, hepatocellular carcinoma, renal cancer, and prostate cancer, through their over-expression or inactivation [26]. However, the association between protocadherin proteins and the pathogenesis of many other cancers remains unclear. PCDH20 has been identified as a tumor suppressor in colorectal and lung cancer [27]. It has been reported that PCDH20 expression is regulated by multiple transcriptional factors including Ets-1, SP1/SP3 and RXRα/β [28]. Our current findings that miR-122 suppresses PCDH20 expression indicate that miRNA-mediated posttranscriptional regulation could be a novel mechanism for suppressing PCDH20 expression in colorectal cancer. This is important because cancer cells often develop multiple mechanisms to reduce the expression of or inactivate tumor-suppressor genes. Understanding of the mechanisms would help design strategies to restore the expression or activity of tumor suppressors.
Akt is a major survival factor in liver cancer cells. Activated Akt mediates cell survival through different mechanisms including blockage of the release of cytochrome c from the mitochondria, and phosphorylation mediated inactivation of the proapoptotic factors BAD, procaspase-953 and FOXO (forkhead box class O) transcription factors [29]. In addition, activated Akt also activate mTOR. mTOR has been shown to be a key determinant for chemo-resistance in different types of cancer [30]. On the basis of the results of this study, we report that the inhibition of Akt activation by GSK690693 results in reduced activity of mTOR and increased sensitivity to 5-FU-induced apoptosis, implicating Akt as a contributor to 5-FU resistance through up-regulation of mTOR activity. Our studies indicate that knockdown of PCDH20 expression increases phosphorylation of Akt at both Thr308 and Ser473, which leads to increased resistance to 5-FU treatment. Taken together, these results suggest that miR-122 functions through suppression of PCDH20 expression, thereby leading to increased Akt activation and elevated mTOR phosphorylation, which enhances the downstream anti-apoptotic pathway. Therefore, the combination of an Akt inhibitor with 5-FU may potentially increase efficacy of 5-FU treatment in liver cancer patients.
Acknowledgements
This research was partly supported by the National Natural Science Foundation of China (Nos. 81201906 and 81272398), and Anhui Provincial Natural Science Foundation (No. 1408085MKL71).
Disclosure of conflict of interest
None.
References
- 1.Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: Surgeon’s view on latest findings and future perspectives. World J Hepatol. 2015;7:1168–1183. doi: 10.4254/wjh.v7.i9.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng L, Ren Z, Jia Q, Wu W, Shen H, Wang Y. Schedule-dependent antitumor effects of 5-fluorouracil combined with sorafenib in hepatocellular carcinoma. BMC Cancer. 2013;13:363. doi: 10.1186/1471-2407-13-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakabe T, Tsuchiya H, Kanki K, Azumi J, Gonda K, Mizuta Y, Yamada D, Wada H, Shomori K, Nagano H, Shiota G. Identification of the genes chemosensitizing hepatocellular carcinoma cells to interferon-alpha/5-fluorouracil and their clinical significance. PLoS One. 2013;8:e56197. doi: 10.1371/journal.pone.0056197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao S, Zhang PJ, Guo JH, Chen H, Xu HF, Liu P, Yang RJ, Zhu X. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:10443–10452. doi: 10.3748/wjg.v21.i36.10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Yu J, Brock MV, Tao Q, Herman JG, Liang P, Guo M. Epigenetic silencing of BCL6B inactivates p53 signaling and causes human hepatocellular carcinoma cell resist to 5-FU. Oncotarget. 2015;6:11547–11560. doi: 10.18632/oncotarget.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyuga S, Shiraishi M, Hori A, Hyuga M, Hanawa T. Effects of Kampo medicines on MDR-1-mediated multidrug resistance in human hepatocellular carcinoma HuH-7/PTX cells. Biol Pharm Bull. 2012;35:1729–1739. doi: 10.1248/bpb.b12-00371. [DOI] [PubMed] [Google Scholar]
- 7.Mansoori B, Mohammadi A, Shirjang S, Baradaran B. Micro-RNAs: The new potential biomarkers in cancer diagnosis, prognosis and cancer therapy. Cell Mol Biol (Noisy-le-grand) 2015;61:1–10. [PubMed] [Google Scholar]
- 8.Kumarswamy R, Mudduluru G, Ceppi P, Muppala S, Kozlowski M, Niklinski J, Papotti M, Allgayer H. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130:2044–2053. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 9.Brozovic A, Duran GE, Wang YC, Francisco EB, Sikic BI. The miR-200 family differentially regulates sensitivity to paclitaxel and carboplatin in human ovarian carcinoma OVCAR-3 and MES-OV cells. Mol Oncol. 2015;9:1678–1693. doi: 10.1016/j.molonc.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Liu HO, Liu YD, Liu WS, Pan D, Zhang WJ, Yang L, Fu Q, Xu JJ, Gu JX. Decreased expression of hepatocyte nuclear factor 4alpha (Hnf4alpha)/microRNA-122 (miR-122) axis in hepatitis B virus-associated hepatocellular carcinoma enhances potential oncogenic GALNT10 protein activity. J Biol Chem. 2015;290:1170–1185. doi: 10.1074/jbc.M114.601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottosen S, Parsley TB, Yang L, Zeh K, van Doorn LJ, van der Veer E, Raney AK, Hodges MR, Patick AK. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassirpour R, Mehta PP, Yin MJ. miR-122 regulates tumorigenesis in hepatocellular carcinoma by targeting AKT3. PLoS One. 2013;8:e79655. doi: 10.1371/journal.pone.0079655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Chen T, Long B, Ren G, Xiang T, Li L, Wang Z, He Y, Zeng Q, Hong S, Hu G. Protocadherin20 Acts as a Tumor Suppressor Gene: Epigenetic Inactivation in Nasopharyngeal Carcinoma. J Cell Biochem. 2015;116:1766–1775. doi: 10.1002/jcb.25135. [DOI] [PubMed] [Google Scholar]
- 14.Imoto I, Izumi H, Yokoi S, Hosoda H, Shibata T, Hosoda F, Ohki M, Hirohashi S, Inazawa J. Frequent silencing of the candidate tumor suppressor PCDH20 by epigenetic mechanism in non-small-cell lung cancers. Cancer Res. 2006;66:4617–4626. doi: 10.1158/0008-5472.CAN-05-4437. [DOI] [PubMed] [Google Scholar]
- 15.Lv J, Zhu P, Yang Z, Li M, Zhang X, Cheng J, Chen X, Lu F. PCDH20 functions as a tumour-suppressor gene through antagonizing the Wnt/beta-catenin signalling pathway in hepatocellular carcinoma. J Viral Hepat. 2015;22:201–211. doi: 10.1111/jvh.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Ling Y, Qi Q, Zhu M, Wan M, Zhang Y, Zhang C. Trastuzumab increases the sensitivity of HER2-amplified human gastric cancer cells to oxaliplatin and cisplatin by affecting the expression of telomere-associated proteins. Oncol Lett. 2015;9:999–1005. doi: 10.3892/ol.2014.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adaramoye O, Erguen B, Oyebode O, Nitzsche B, Hopfner M, Jung K, Rabien A. Antioxidant, antiangiogenic and antiproliferative activities of root methanol extract of Calliandra portoricensis in human prostate cancer cells. J Integr Med. 2015;13:185–193. doi: 10.1016/S2095-4964(15)60175-3. [DOI] [PubMed] [Google Scholar]
- 18.Dhar S, Kumar A, Rimando AM, Zhang X, Levenson AS. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget. 2015;6:27214–27226. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei ZJ, Tao ML, Zhang W, Han GD, Zhu ZC, Miao ZG, Li JY, Qiao ZB. Up-regulation of microRNA-302a inhibited the proliferation and invasion of colorectal cancer cells by regulation of the MAPK and PI3K/Akt signaling pathways. Int J Clin Exp Pathol. 2015;8:4481–4491. [PMC free article] [PubMed] [Google Scholar]
- 20.Fattore L, Malpicci D, Marra E, Belleudi F, Noto A, De Vitis C, Pisanu ME, Coluccia P, Camerlingo R, Roscilli G, Ribas A, Di Napoli A, Torrisi MR, Aurisicchio L, Ascierto PA, Mancini R, Ciliberto G. Combination of antibodies directed against different ErbB3 surface epitopes prevents the establishment of resistance to BRAF/MEK inhibitors in melanoma. Oncotarget. 2015;6:24823–24841. doi: 10.18632/oncotarget.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Cao F, Li X, Miao H, E J, Xing J, Fu CG. miR-320b suppresses cell proliferation by targeting c-Myc in human colorectal cancer cells. BMC Cancer. 2015;15:748. doi: 10.1186/s12885-015-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis. 2006;26:385–390. doi: 10.1055/s-2006-951606. [DOI] [PubMed] [Google Scholar]
- 23.Pan J, Zhang YF, Yang HY, Xu HF, Lu X, Sang XT, Zhong SX, Mao YL. The response of Golgi protein 73 to transcatheter arterial chemoembolization in patients with hepatocellular carcinoma may relate to the influence of certain chemotherapeutics. Hepatobiliary Pancreat Dis Int. 2015;14:406–412. doi: 10.1016/s1499-3872(15)60403-9. [DOI] [PubMed] [Google Scholar]
- 24.Terry S, Maille P, Baaddi H, Kheuang L, Soyeux P, Nicolaiew N, Ceraline J, Firlej V, Beltran H, Allory Y, de la Taille A, Vacherot F. Cross modulation between the androgen receptor axis and protocadherin-PC in mediating neuroendocrine transdifferentiation and therapeutic resistance of prostate cancer. Neoplasia. 2013;15:761–772. doi: 10.1593/neo.122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke C, Li C, Huang X, Cao F, Shi D, He W, Bu H, Gao F, Cai T, Hinton AO Jr, Tian Y. Protocadherin20 promotes excitatory synaptogenesis in dorsal horn and contributes to bone cancer pain. Neuropharmacology. 2013;75:181–190. doi: 10.1016/j.neuropharm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Haruki S, Imoto I, Kozaki K, Matsui T, Kawachi H, Komatsu S, Muramatsu T, Shimada Y, Kawano T, Inazawa J. Frequent silencing of protocadherin 17, a candidate tumour suppressor for esophageal squamous cell carcinoma. Carcinogenesis. 2010;31:1027–1036. doi: 10.1093/carcin/bgq053. [DOI] [PubMed] [Google Scholar]
- 27.Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW, Putti T, Murray P, Chan AT, Tao Q. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene. 2006;25:1070–1080. doi: 10.1038/sj.onc.1209154. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Ji W, Sun S, Zhang L, Chen HG, Mao Y, Liu L, Zhang X, Gong L, Deng M, Chen L, Han WJ, Chen PC, Hu WF, Hu X, Woodward Z, Liu WB, Xiao YM, Liang SP, Liu Y, Liu SJ, Li DW. The PP2A-Abeta gene is regulated by multiple transcriptional factors including Ets-1, SP1/SP3, and RXRalpha/beta. Curr Mol Med. 2012;12:982–994. doi: 10.2174/156652412802480916. [DOI] [PubMed] [Google Scholar]
- 29.Xie G, Li J, Chen J, Tang X, Wu S, Liao C. Knockdown of flotillin-2 impairs the proliferation of breast cancer cells through modulation of Akt/FOXO signaling. Oncol Rep. 2015;33:2285–2290. doi: 10.3892/or.2015.3826. [DOI] [PubMed] [Google Scholar]
- 30.Iacovelli S, Ricciardi MR, Allegretti M, Mirabilii S, Licchetta R, Bergamo P, Rinaldo C, Zeuner A, Foa R, Milella M, McCubrey JA, Martelli AM, Tafuri A. Co-targeting of Bcl-2 and mTOR pathway triggers synergistic apoptosis in BH3 mimetics resistant acute lymphoblastic leukemia. Oncotarget. 2015;6:32089–32103. doi: 10.18632/oncotarget.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]