Abstract
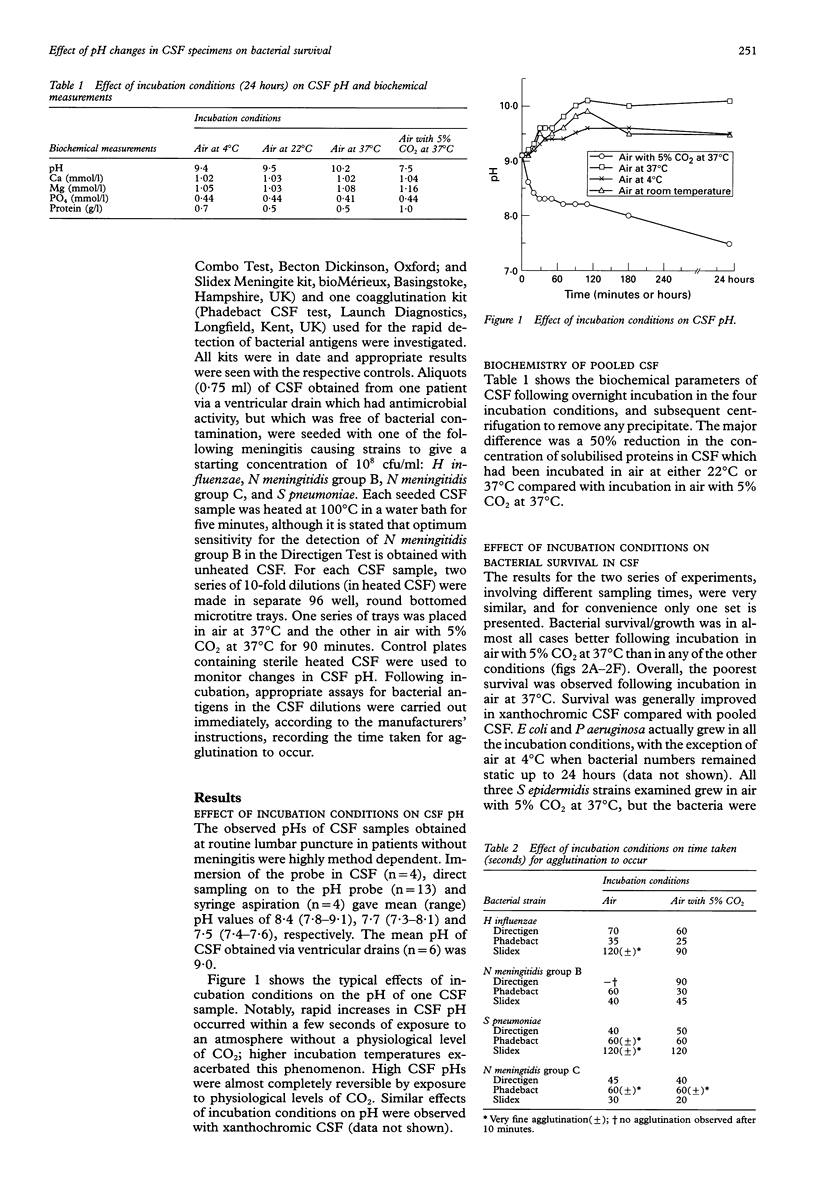
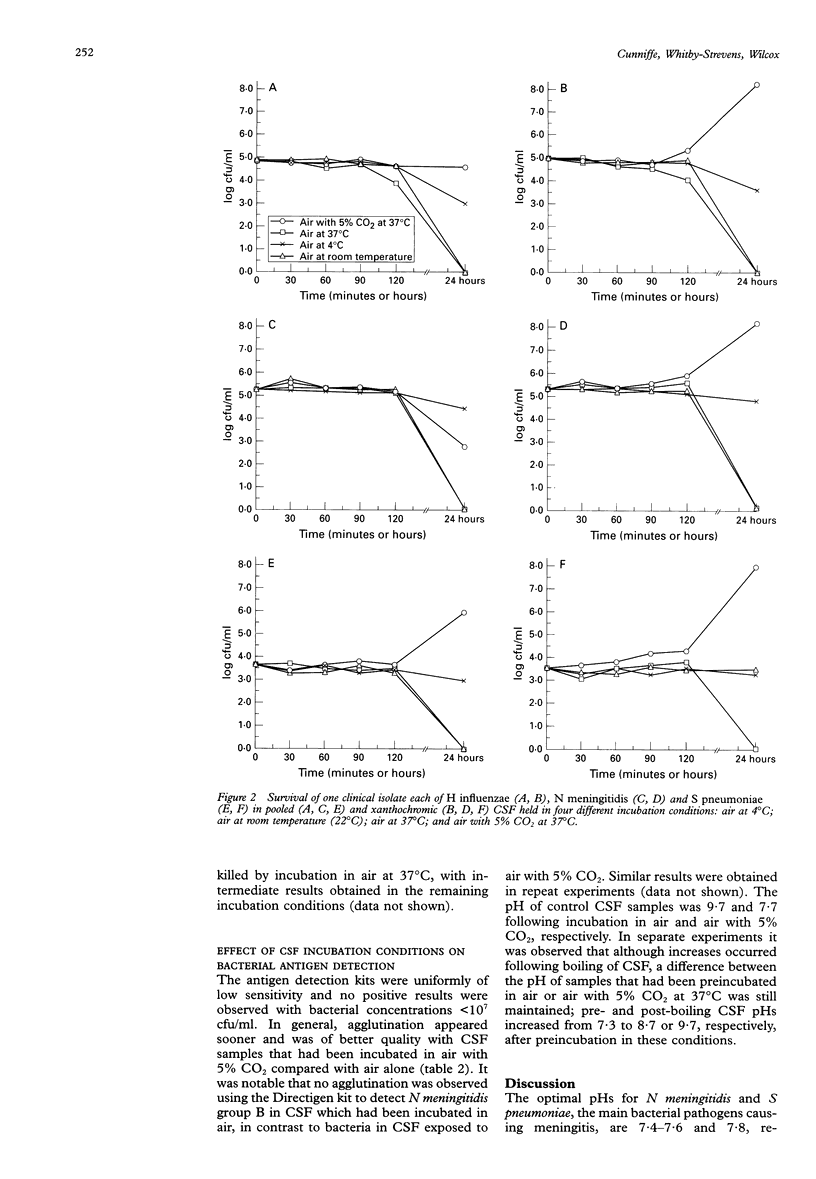
AIMS: To determine the effect of pH changes occurring in cerebrospinal fluid (CSF) after sampling on the viability of meningitis causing bacteria, and on the performance of agglutination assays used for the rapid detection of bacterial antigens. METHODS: The pH of CSF collected via lumbar puncture was measured by various methods, and the effects of the following different incubation conditions on subsequent changes were determined: air at 4 degrees C; air at room temperature (22 degrees C); air at 37 degrees C; and air with 5% CO2 at 37 degrees C. The growth/survival in pooled CSF of 15 bacterial isolates collected from 74 patients with meningitis was assessed in these incubation conditions over 24 hours. The effects of pH changes in the CSF on the sensitivity of two latex agglutination and one co-agglutination kits for detecting Haemophilus influenzae, Neisseria meningitidis groups B and C, and Streptococcus pneumoniae were determined. RESULTS: The measured pH of CSF was highly affected by the method used and particularly the time delay between patient sampling and assay. Measured pH values at the time of sampling (mean 7.5) increased rapidly within 60 seconds by about one unit. CSF pH continued to increase during incubation in all tested conditions (up to approximately pH 10), with the exception of in air with 5% CO2 at 37 degrees C where pH changes were reversible and near physiological values were attained. Bacterial survival for all species tested was poorest in CSF incubated in air at 37 degrees C and best following exposure to air with 5% CO2 at 37 degrees C. Agglutination in rapid antigen detection kits with CSF incubated in air as opposed to air with 5% CO2 generally took longer to occur and in some instances was less prominent. In one case a false negative result was obtained with CSF seeded with N meningitidis group B incubated in the former but not the latter conditions. CONCLUSIONS: CSF pH increases after patient sampling are minimised and/or mostly reversed by incubation in an atmosphere containing 5% CO2. CSF samples should ideally be placed in such an atmosphere as soon as possible after collection, and left there until laboratory processing occurs, to reduce the detrimental effects of pH stress on bacterial survival. pH increases may also reduce the likelihood of obtaining a positive result in rapid antigen detection assays.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blayo M. C., Bazin C., Gaudebout C. Etude comparative des pressions partielles gazeuses, des équilibres acido-basique et hydroélectrolytique du liquide céphalo-rachidien des méningites bactériennes de l'adulte. Rev Eur Etud Clin Biol. 1971 Mar;16(3):224–232. [PubMed] [Google Scholar]
- Davies D. G. Cerebrospinal fluid sampling technique and Astrup pH and PCO2 values. J Appl Physiol. 1976 Jan;40(1):123–125. doi: 10.1152/jappl.1976.40.1.123. [DOI] [PubMed] [Google Scholar]
- Gray L. D., Fedorko D. P. Laboratory diagnosis of bacterial meningitis. Clin Microbiol Rev. 1992 Apr;5(2):130–145. doi: 10.1128/cmr.5.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. D., Mirrett S., Reller L. B. Rapid bacterial antigen detection is not clinically useful. J Clin Microbiol. 1995 Jun;33(6):1486–1491. doi: 10.1128/jcm.33.6.1486-1491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon F., Biscoping J., Hempelmann G. Säure-Basen-Befunde im Liquor cerebrospinalis bei prämedizierten Patienten. Anasth Intensivther Notfallmed. 1985 Oct;20(5):266–268. [PubMed] [Google Scholar]
- Steele R. W., Marmer D. J., O'Brien M. D., Tyson S. T., Steele C. R. Leukocyte survival in cerebrospinal fluid. J Clin Microbiol. 1986 May;23(5):965–966. doi: 10.1128/jcm.23.5.965-966.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M. H., Schumacher-Perdreau F. Lack of evidence for increased adherent growth in broth or human serum of clinically significant coagulase-negative staphylococci. J Hosp Infect. 1994 Apr;26(4):239–250. doi: 10.1016/0195-6701(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Smith D. G., Evans J. A., Denyer S. P., Finch R. G., Williams P. Influence of carbon dioxide on growth and antibiotic susceptibility of coagulase-negative staphylococci cultured in human peritoneal dialysate. J Clin Microbiol. 1990 Oct;28(10):2183–2186. doi: 10.1128/jcm.28.10.2183-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



